Synthesis and Applications of Zeolite-Encapsulated Metal Catalysts
Abstract
1. Introduction
2. Preparation Method of Zeolite-Encapsulated Metal Catalyst
2.1. Impregnation
2.2. Ion Exchange Method
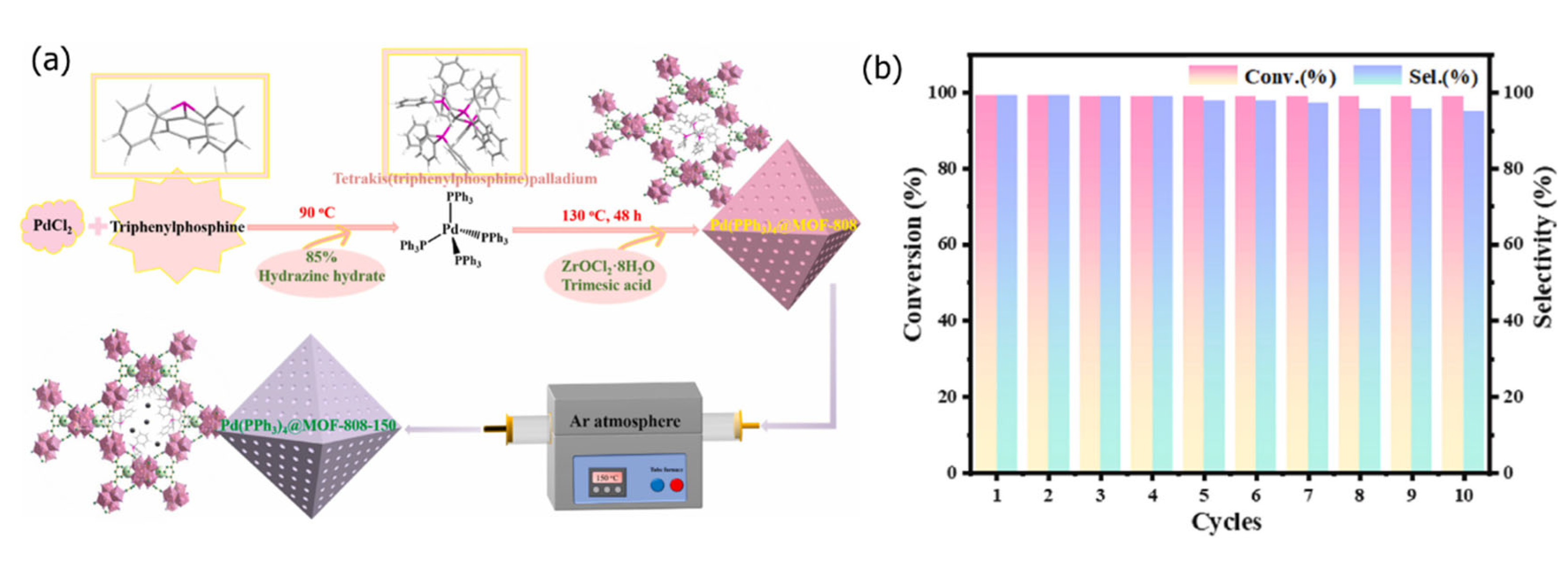
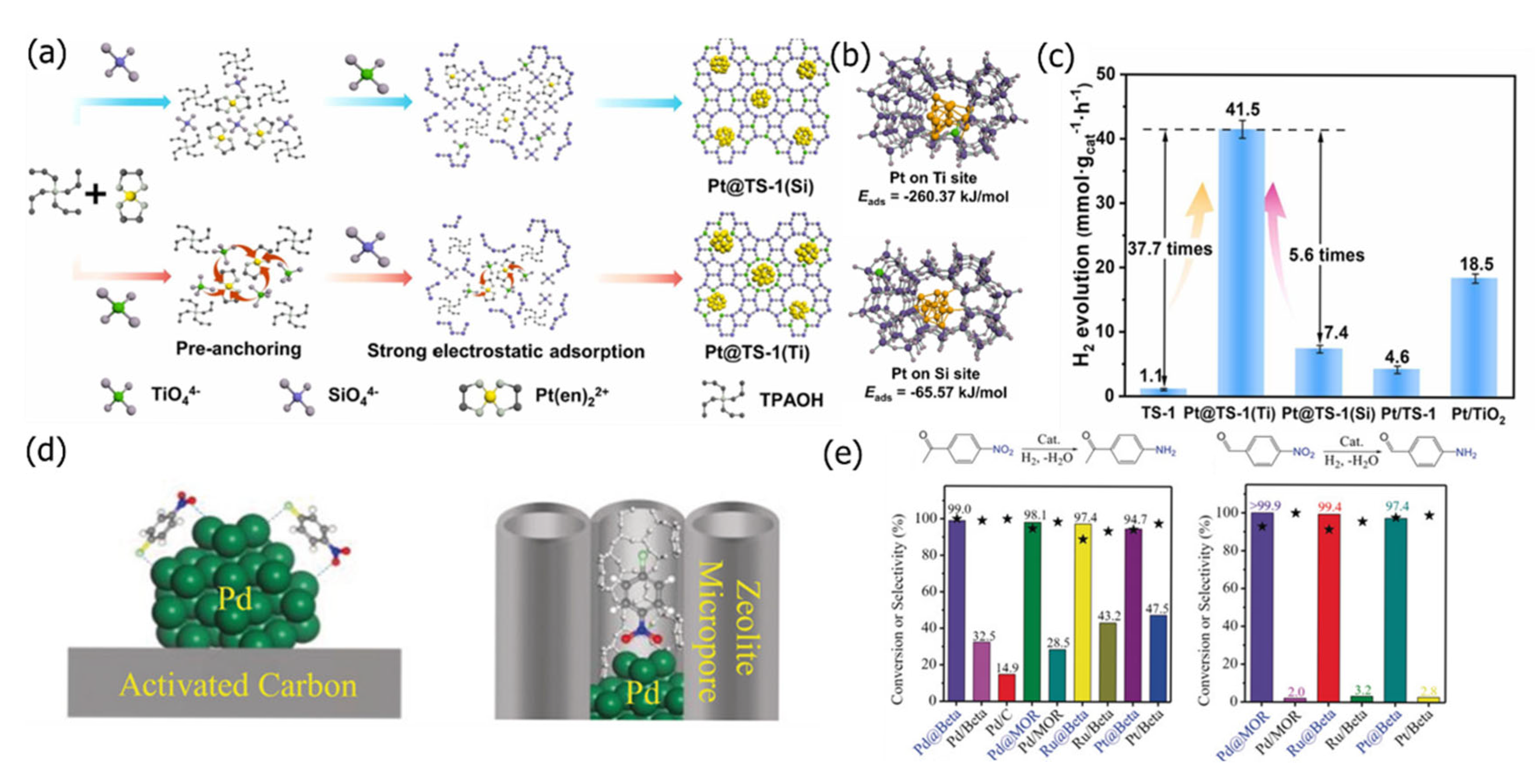
2.3. Ship-in-a-Bottle Approach
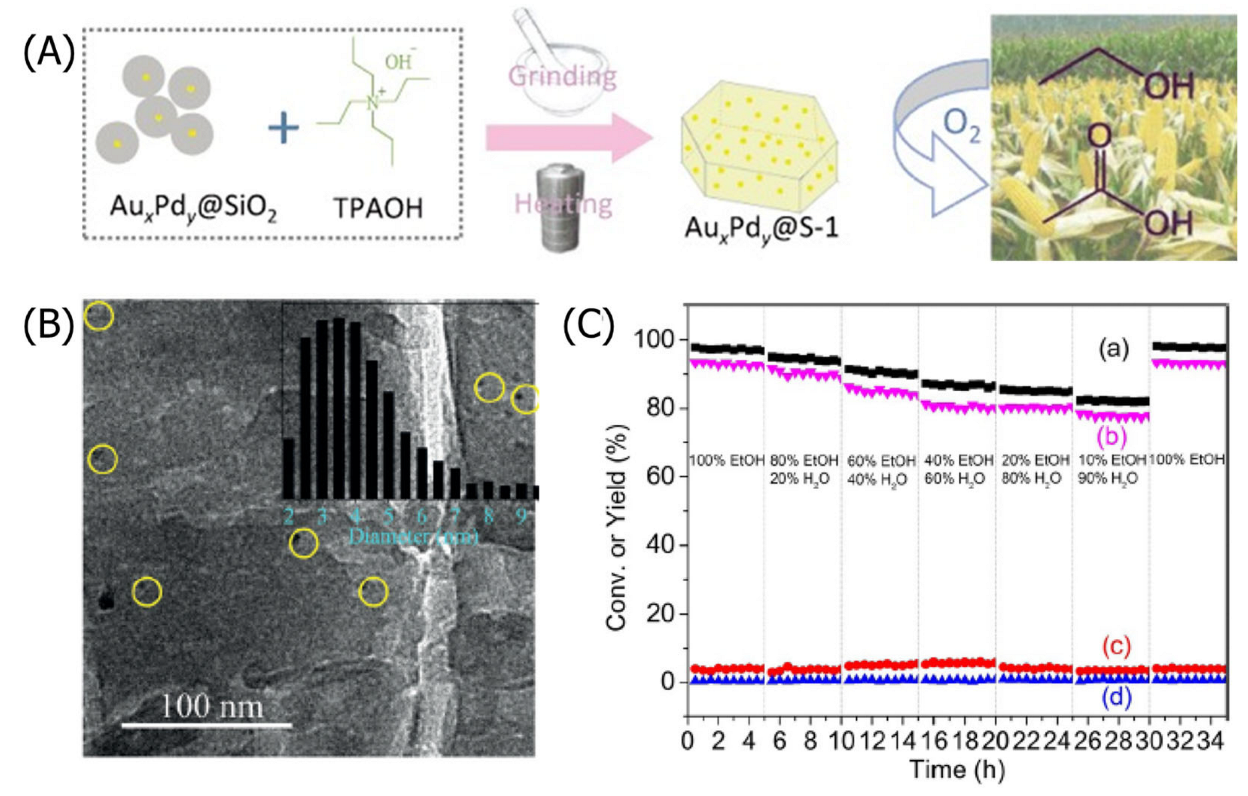
2.4. Solvent-Free Crystallization
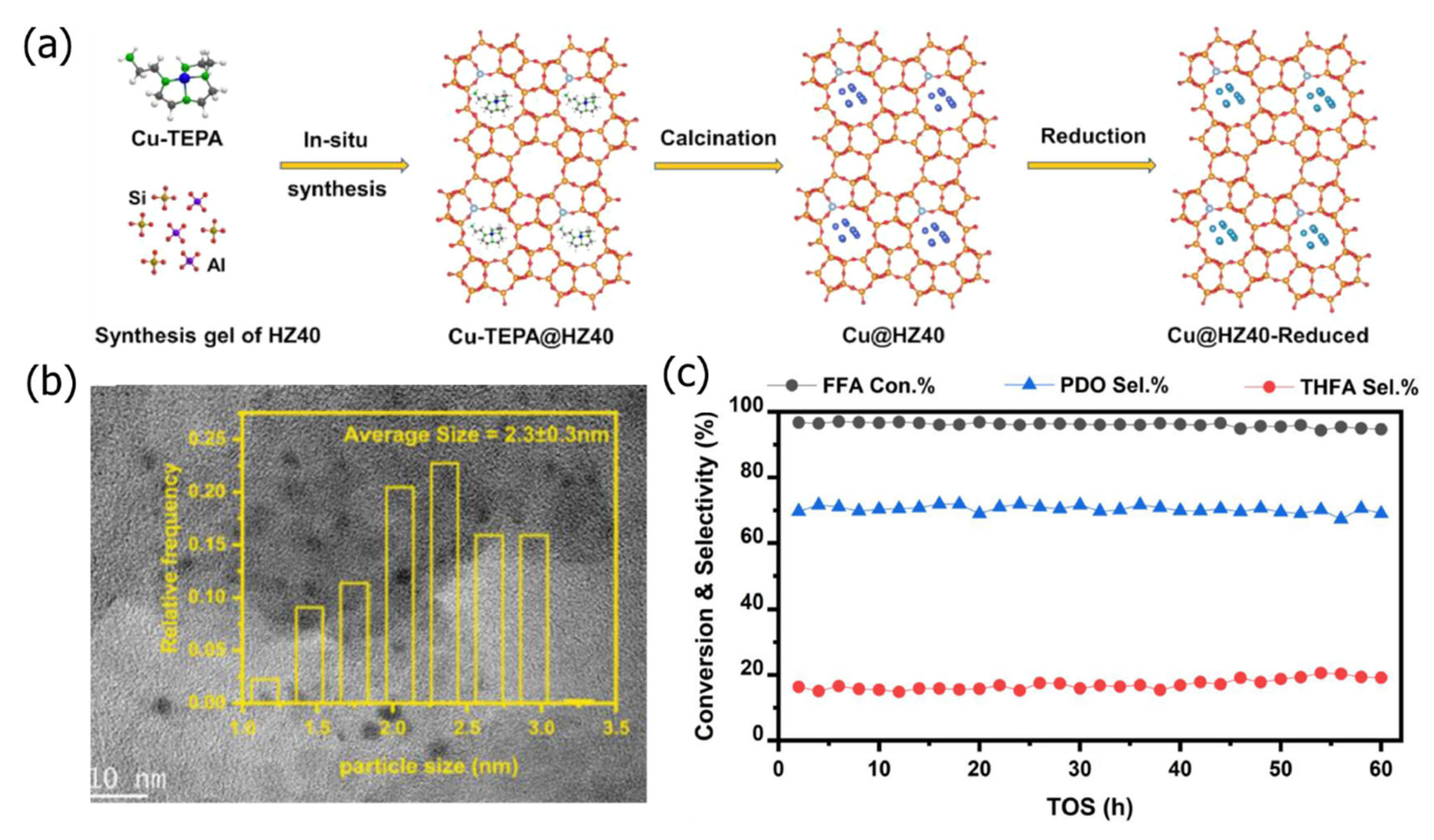
2.5. Hydrothermal Crystallization
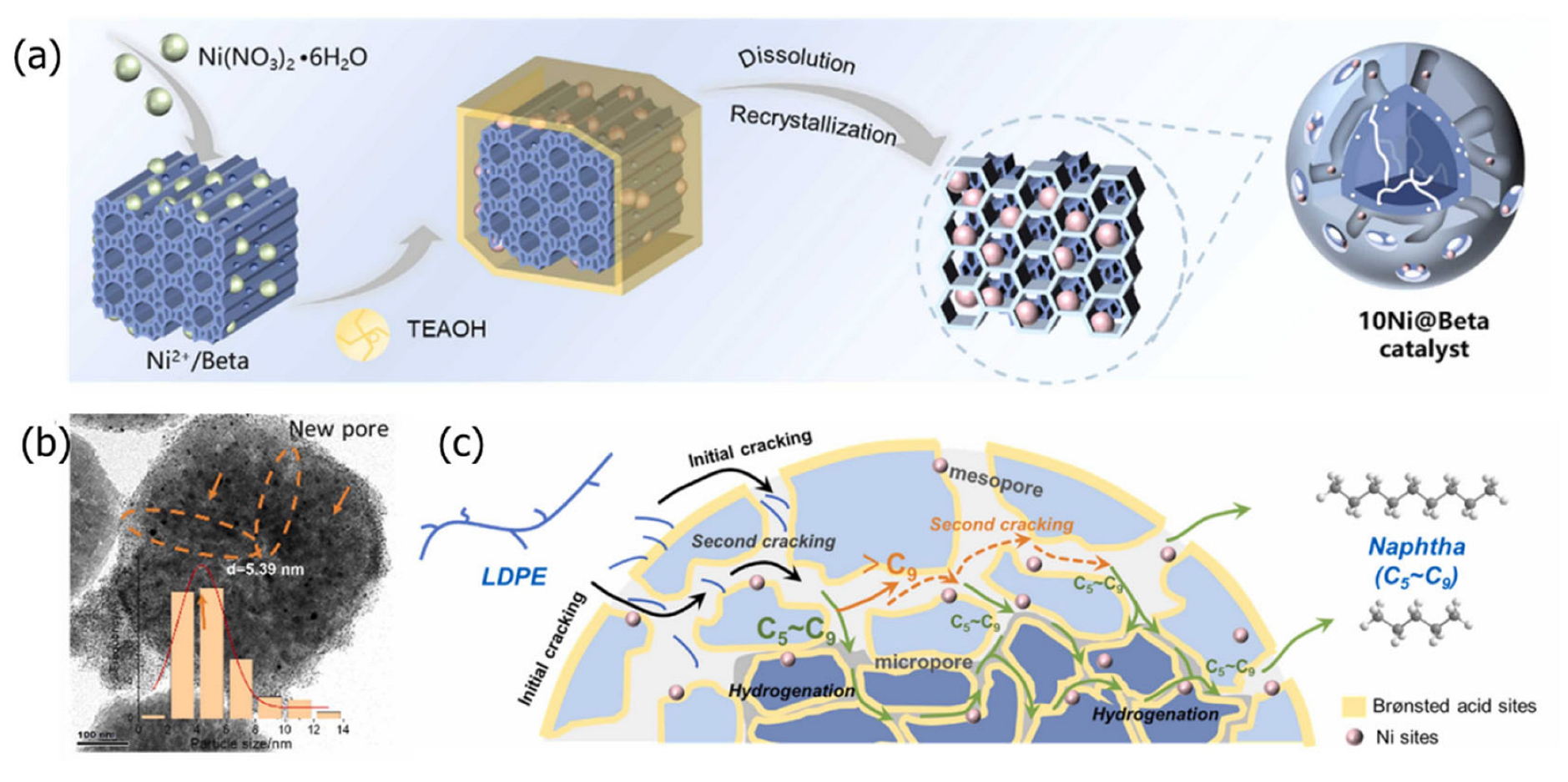
2.6. Dissolution–Recrystallization Method
3. Relationship Between the Structure and Performance of Zeolite-Encapsulated Metal Catalysts
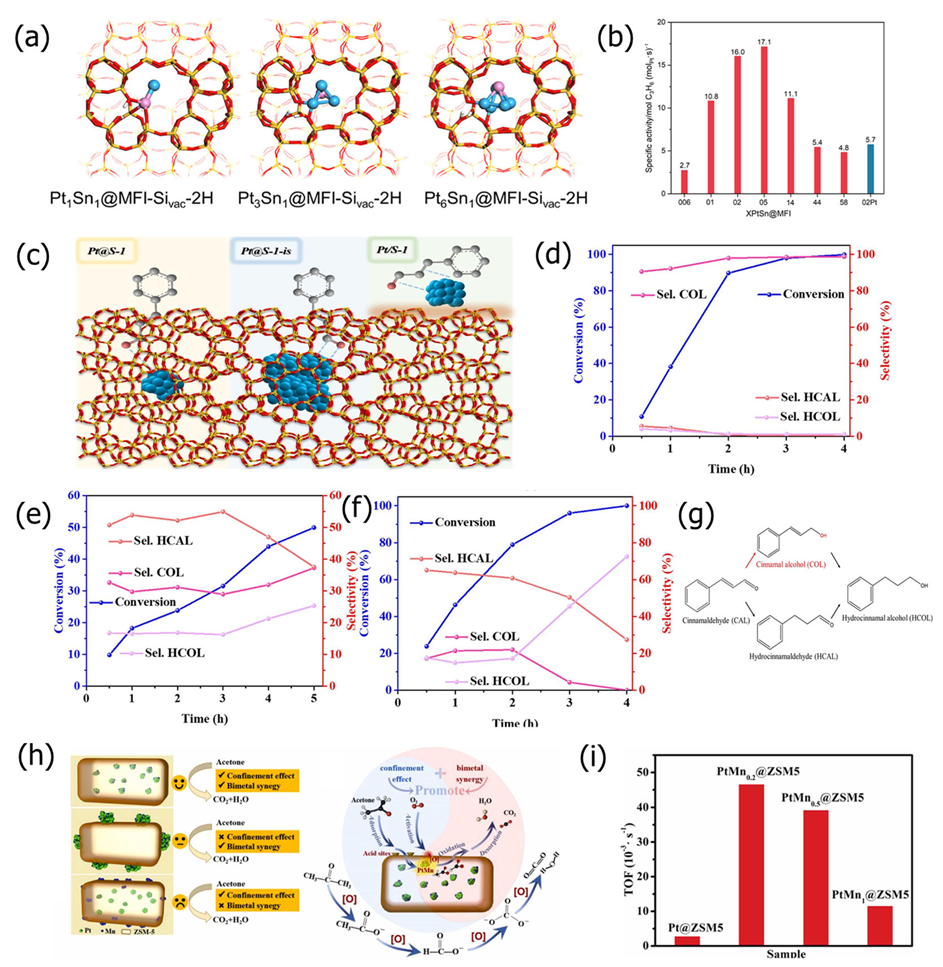
3.1. Effects of Metal Nanoparticle Size, Shape, and Alloy Composition on Catalytic Performance
3.1.1. Size Effect of Metal Nanoparticles
3.1.2. Shape Effects of Metal Nanoparticles
3.1.3. Synergistic Effect of Alloy Composition
3.2. Effects of Zeolite Structure and Properties on Catalysts
3.2.1. The Pore Structure of Zeolites
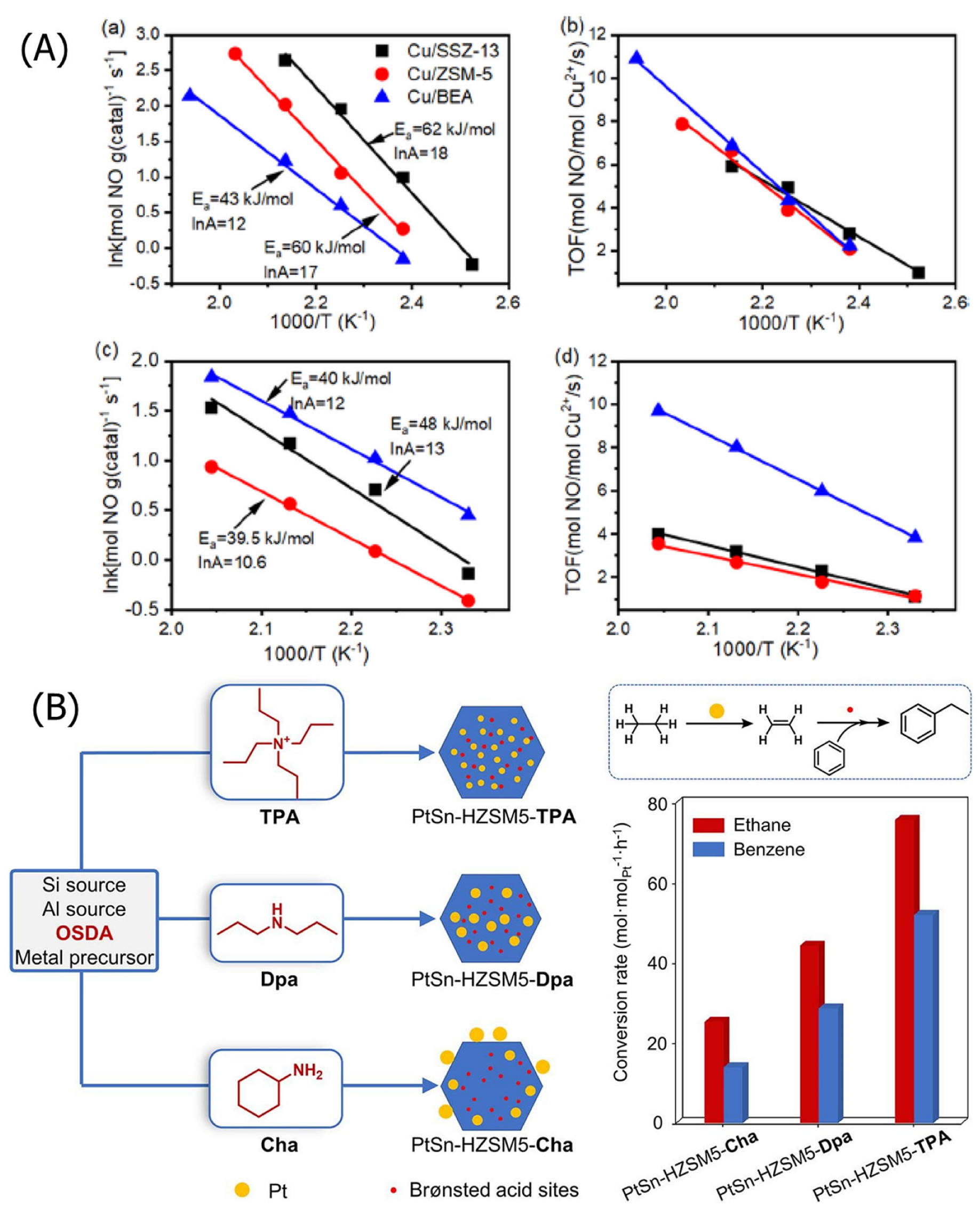
3.2.2. Acidity of Zeolites
3.3. Effect of the Interaction Between Metal and Zeolite on Performance
3.3.1. Electronic Interaction
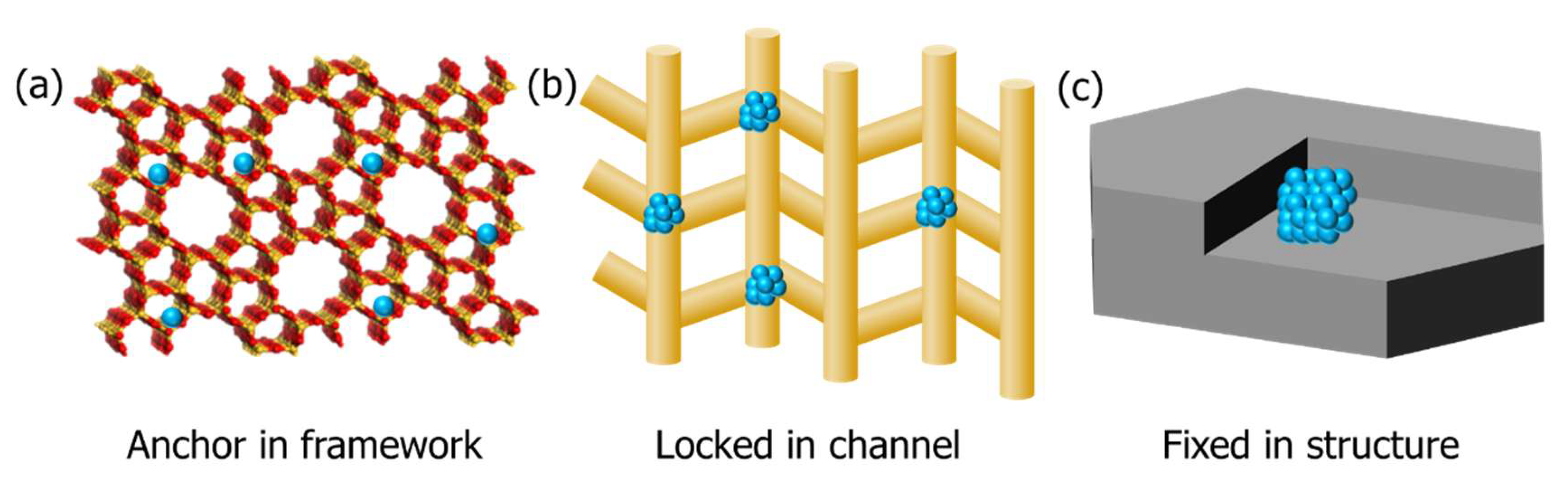
3.3.2. Geometric Constraint Effect
4. Application of Zeolite-Encapsulated Metal Catalysts
4.1. Hydrogenation Reaction
4.2. Oxidation Reaction
4.3. Reforming Reaction
4.4. Dehydrogenation Reaction
5. Summary and Outlook
5.1. Current Challenges
5.2. Future Development Trends and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Zhao, S.; Yang, W.; Huang, J. Hierarchical zeolite-encapsulated metal nanoparticles for heterogeneous catalysis. Nanoscale 2024, 16, 20842–20863. [Google Scholar] [CrossRef]
- Mohammadparast, F.; Halladj, R.; Askari, S. The Crystal Size Effect of Nano-Sized ZSM-5 in the Catalytic Performance of Petrochemical Processes: A Review. Chem. Eng. Commun. 2015, 202, 542–556. [Google Scholar] [CrossRef]
- Otun, K.O.; Liu, X.; Hildebrandt, D. Metal-organic framework (MOF)-derived catalysts for Fischer-Tropsch synthesis: Recent progress and future perspectives. J. Energy Chem. 2020, 51, 230–245. [Google Scholar] [CrossRef]
- Jia, L.; Liu, J.; Cheng, H.; Zhao, Z.; Liu, J. Review of Core-shell structure zeolite-based catalysts for NOx emission control. J. Environ. Sci. 2025, 150, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of Metal Nanoparticle Catalysts Within Mesoporous Zeolites and Their Enhanced Catalytic Performances: A Review. Front. Chem. 2018, 6, 00500. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, X.; Cui, R.; Han, W.; Zhang, G.; Tang, Z. Design of confined catalysts and applications in environmental catalysis: Original perspectives and further prospects. J. Clean. Prod. 2023, 390, 136125. [Google Scholar] [CrossRef]
- Farrusseng, D.; Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 2016, 40, 3933–3949. [Google Scholar] [CrossRef]
- Singh, B.K.; Kim, Y.; Kwon, S.; Na, K. Synthesis of Mesoporous Zeolites and Their Opportunities in Heterogeneous Catalysis. Catalysts 2021, 11, 1541. [Google Scholar] [CrossRef]
- Tao, Y.S.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparation, characterization, and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef]
- de Pietre, M.K.; Freitas, J.C.C. Fundamental studies on zeolite-adsorbate interactions: Designing a better aluminosilicate adsorbent for pollutants’ removal. Environ. Earth. Sci. 2022, 81, 17. [Google Scholar] [CrossRef]
- Cao, X.-Q.; Zhou, J.; Li, S.; Qin, G.-W. Ultra-stable metal nano-catalyst synthesis strategy: A perspective. Rare Met. 2020, 39, 113–130. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Wu, X.; Betancourt, L.E.; Tu, C.; Liao, M.; Cui, X.; Li, F.; Zheng, J.; Li, R. Ni/hierarchical ZSM-5 zeolites as promising systems for phenolic bio-oil upgrading: Guaiacol hydrodeoxygenation. Fuel 2020, 274, 117859. [Google Scholar] [CrossRef]
- Miah, A.; Bharadwaj, S.K.; Saikia, P. Surfactant free synthesis of gold nanoparticles within meso-channels of non-functionalized SBA-15 for its promising catalytic activity. Powder Technol. 2017, 315, 147–156. [Google Scholar] [CrossRef]
- Kockrick, E.; Krawiec, P.; Schnelle, W.; Geiger, D.; Schappacher, F.M.; Pöttgen, R.; Kaskel, S. Space-confined formation of FePt nanoparticles in ordered mesoporous silica SBA-15. Adv. Mater. 2007, 19, 3021–3026. [Google Scholar] [CrossRef]
- Guczi, L.; Kiricsi, I. Zeolite supported mono-and bimetallic systems: Structure and performance as CO hydrogenation catalysts. Appl. Catal. A 1999, 186, 375–394. [Google Scholar] [CrossRef]
- Moliner, M.; Gabay, J.E.; Kliewer, C.E.; Carr, R.T.; Guzman, J.; Casty, G.L.; Serna, P.; Corma, A. Reversible Transformation of Pt Nanoparticles into Single Atoms inside High-Silica Chabazite Zeolite. J. Am. Chem. Soc. 2016, 138, 15743–15750. [Google Scholar] [CrossRef]
- Kistler, J.D.; Chotigkrai, N.; Xu, P.H.; Enderle, B.; Praserthdam, P.; Chen, C.Y.; Browning, N.D.; Gates, B.C. A Single-Site Platinum CO Oxidation Catalyst in Zeolite KLTL: Microscopic and Spectroscopic Determination of the Locations of the Platinum Atoms. Angew. Chem. Int. Ed. 2014, 53, 8904–8907. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.; Tang, Y.; Li, Y.; Nguyen, L.; Li, Y.; Shan, J.; Xiao, D.; Gagne, R.; Frenkel, A.I.; et al. Low-Temperature Transformation of Methane to Methanol on PdO Single Sites Anchored on the Internal Surface of Microporous Silicate. Angew. Chem. Int. Ed. 2016, 55, 13441–13445. [Google Scholar] [CrossRef]
- Cai, J.; Ma, H.; Zhang, J.; Song, Q.; Du, Z.; Huang, Y.; Xu, J. Gold Nanoclusters Confined in a Supercage of Y Zeolite for Aerobic Oxidation of HMF under Mild Conditions. Chem.—Eur. J. 2013, 19, 14215–14223. [Google Scholar] [CrossRef]
- Kim, Y.; Sung, J.; Kang, S.; Lee, J.; Kang, M.-H.; Hwang, S.; Park, H.; Kim, J.; Kim, Y.; Lee, E.; et al. Uniform synthesis of palladium species confined in a small-pore zeolite via full ion-exchange investigated by cryogenic electron microscopy. J. Mater. Chem. A 2021, 9, 19796–19806. [Google Scholar] [CrossRef]
- Ichikawa, M.; Rao, L.; Ito, T.; Fukuoka, A. Ensemble and ligand effects in selective alkane hydrogenolysis catalysed on well characterised RhIr and RhFe bimetallic clusters inside NaY zeolite. Faraday Discuss. 1989, 87, 321–336. [Google Scholar] [CrossRef]
- Thabet, M.S.; Ahmed, A.H. Ship-in-a-bottle synthesis and physicochemical studies on zeolite encapsulated Mn(II), Mn(III)-semicarbazone complexes: Application in the heterogeneous hydroxylation of benzene. J. Porous Mater. 2013, 20, 319–330. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, A.; Li, J.; Hou, K.; Liu, M.; Song, C.; Guo, X. Synthesis of yolk-shell HPW@Hollow silicalite-1 for esterification reaction. Chem. Commun. 2014, 50, 4846–4848. [Google Scholar] [CrossRef]
- Okemoto, A.; Ueyama, K.; Taniya, K.; Ichihashi, Y.; Nishiyama, S. Direct oxidation of benzene with molecular oxygen in liquid phase catalysed by heterogeneous copper complexes encapsulated in Y-type zeolite. Catal. Commun. 2017, 100, 29–32. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Chen, X.; Guo, R.; Jiang, T.; Du, C.; Zhang, W.; Liu, Y.; Li, C.; Han, T.; et al. Constructing a synergistic catalyst by confining Pd within a MOF using an extended “bottle-around-ship” protocol. Microporous Mesoporous Mater. 2024, 363, 112828. [Google Scholar] [CrossRef]
- Sun, T.; Xu, S.; Xiao, D.; Liu, Z.; Li, G.; Zheng, A.; Liu, W.; Xu, Z.; Cao, Y.; Guo, Q.; et al. Water-Induced Structural Dynamic Process in Molecular Sieves under Mild Hydrothermal Conditions: Ship-in-a-Bottle Strategy for Acidity Identification and Catalyst Modification. Angew. Chem. Int. Ed. 2020, 59, 20672–20681. [Google Scholar] [CrossRef] [PubMed]
- Amooghin, A.E.; Sanaeepur, H.; Omidkhah, M.; Kargari, A. “Ship-in-a-bottle”, a new synthesis strategy for preparing novel hybrid host-guest nanocomposites for highly selective membrane gas separation. J. Mater. Chem. A 2018, 6, 1751–1771. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Sobhani, A. Ship-in-a-bottle synthesis, characterization and catalytic oxidation of cyclohexane by Host (nanopores of zeolite-Y)/guest (Mn(II), Co(II), Ni(II) and Cu(II) complexes of bis(salicyaldehyde)oxaloyldihydrazone) nanocomposite materials. J. Mol. Catal. A 2008, 285, 58–67. [Google Scholar] [CrossRef]
- Yuan, X.; Li, F.; Luo, H. Ship-in-bottle synthesis of NaY zeolite encapsulated Cosalen complex and its catalytic performance for cyclohexane oxidation. J. Cent. South Univ. Technol. 2007, 14, 78–83. [Google Scholar] [CrossRef]
- Liu, F.; Willhammar, T.; Wang, L.; Zhu, L.; Sun, Q.; Meng, X.; Carrillo-Cabrera, W.; Zou, X.; Xiao, F. ZSM-5 Zeolite Single Crystals with b-Axis-Aligned Mesoporous Channels as an Efficient Catalyst for Conversion of Bulky Organic Molecules. J. Am. Chem. Soc. 2012, 134, 4557–4560. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, A.; Liu, M.; Guo, X.; Song, C. Hollow ZSM-5 with Silicon-Rich Surface, Double Shells, and Functionalized Interior with Metallic Nanoparticles and Carbon Nanotubes. Adv. Funct. Mater. 2015, 25, 7479–7487. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Sun, Q.; Zhu, L.; Meng, X.; Xiao, F. Transesterification Catalyzed by Ionic Liquids on Superhydrophobic Mesoporous Polymers: Heterogeneous Catalysts That Are Faster than Homogeneous Catalysts. J. Am. Chem. Soc. 2012, 134, 16948–16950. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, J.; Wang, H.; Lewis, J.P.; Xiao, F.-S. Product Selectivity Controlled by Zeolite Crystals in Biomass Hydrogenation over a Palladium Catalyst. J. Am. Chem. Soc. 2016, 138, 7880–7883. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhu, L.; Wu, Q.; Chen, C.; Wang, X.; Ji, Y.; Meng, X.; Xiao, F.-S. Solvent-Free Synthesis of Zeolite Crystals Encapsulating Gold-Palladium Nanoparticles for the Selective Oxidation of Bioethanol. ChemSusChem 2015, 8, 2867–2871. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Sun, C.; Li, J.; Qi, L.; Quan, Y.; Gao, F.; Dong, L. An efficient strategy for highly loaded, well dispersed and thermally stable metal oxide catalysts. Catal. Commun. 2011, 12, 1075–1078. [Google Scholar] [CrossRef]
- Ye, J.; Tang, X.; Cheng, L.; Zhang, S.; Zhan, W.; Guo, Y.; Wang, L.; Cao, X.-M.; Wang, K.-W.; Dai, S.; et al. Solvent-Free Synthesis Enables Encapsulation of Subnanometric FeOx Clusters in Pure Siliceous Zeolites for Efficient Catalytic Oxidation Reactions. ACS Appl. Mater. Interfaces 2024, 16, 24691–24702. [Google Scholar] [CrossRef]
- Lo, K.-H.; Lin, J.-Y.; Hsieh, C.-K. Solvent-free synthesis of ZIF-67 thin films as the catalyst for the direct growth of carbon nanotubes for the fabrication of electrical double-layer capacitors. J. Chin. Chem. Soc. 2023, 70, 1656–1664. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, W.; Cheng, J.; Zhao, H.; Du, Y.; Yao, H.; Li, J. Pt@SAPO-11 bifunctional catalysts with spatial architecture constructed via a seed-directed solvent-free strategy for n-dodecane hydroisomerization. Microporous Mesoporous Mater. 2022, 330, 111607. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Wu, Q.; Wang, C.; Guo, X.; He, Y.; Zhang, P.; Yang, G.; Liu, G.; Wu, J.; et al. Capsule-like zeolite catalyst fabricated by solvent-free strategy for para-Xylene formation from CO2 hydrogenation. Appl. Catal. B Environ. 2022, 303, 120906. [Google Scholar] [CrossRef]
- Otto, T.; Ramallo-Lopez, J.M.; Giovanetti, L.J.; Requejo, F.G.; Zones, S.I.; Iglesia, E. Synthesis of stable monodisperse AuPd, AuPt, and PdPt bimetallic clusters encapsulated within LTA-zeolites. J. Catal. 2016, 342, 125–137. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.; Bai, R.; Li, X.; Guo, G.; Yu, J. In Situ Confinement of Ultrasmall Pd Clusters within Nanosized Silicalite-1 Zeolite for Highly Efficient Catalysis of Hydrogen Generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Zhang, Y.; Zhao, T.; Liu, D.; Feng, C.; Liu, Y. In-situ hydrothermal synthesis of Al-rich Cu@ZSM-5 catalyst for furfuryl alcohol upgrading to pentanediols. Mol. Catal. 2023, 547, 113381. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Yu, Q.; Chen, Y.; Chai, Z.; Zhao, G.; Liu, S.; Cheong, W.; Pan, Y.; Zhang, Q.; et al. A General Strategy for Fabricating Isolated Single Metal Atomic Site Catalysts in Y Zeolite. J. Am. Chem. Soc. 2019, 141, 9305–9311. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, B.; Hou, G.; Wang, L.; Liu, S.; Liu, G. Hydrothermal synthesis of highly cross-linked PtZn@Silicalite-1 structured catalysts for propane dehydrogenation. AIChE J. 2024, 70, 18444. [Google Scholar] [CrossRef]
- Ma, B.; Cui, H.; Wang, D.; Wu, P.; Zhao, C. Controllable hydrothermal synthesis of Ni/H-BEA with a hierarchical core-shell structure and highly enhanced biomass hydrodeoxygenation performance. Nanoscale 2017, 9, 5986–5995. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, A.; Li, L.; Hou, K.; Ding, F.; Li, J.; Mu, D.; Song, C.; Liu, M.; Guo, X. Synthesis of Hollow Nanocubes and Macroporous Monoliths of Silicalite-1 by Alkaline Treatment. Chem. Mater. 2013, 25, 4197–4205. [Google Scholar] [CrossRef]
- Dai, C.; Li, X.; Zhang, A.; Liu, C.; Song, C.; Guo, X. Pd and Pd-CuO nanoparticles in hollow silicalite-1 single crystals for enhancing selectivity and activity for the Suzuki-Miyaura reaction. RSC Adv. 2015, 5, 40297–40302. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow zeolite encapsulated Ni-Pt bimetals for sintering and coking resistant dry reforming of methane. J. Mater. Chem. A 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, A.; Luo, L.; Zhang, X.; Liu, M.; Wang, J.; Guo, X.; Song, C. Hollow zeolite-encapsulated Fe-Cu bimetallic catalysts for phenol degradation. Catal. Today 2017, 297, 335–343. [Google Scholar] [CrossRef]
- Zhan, J.; Li, L.; Dai, R.; Wei, B.; Luo, H.; Cong, R.; Zhou, H.; Xia, L.; Liu, X.; Zhang, S.; et al. Engineering porous Beta zeolite-encapsulated nickel catalyst for waste polyolefins upcycling. Appl. Catal. B Environ. Energy 2025, 373, 125359. [Google Scholar] [CrossRef]
- Guan, S.; Liu, Y.; Zhang, H.; Shen, R.; Wen, H.; Kang, N.; Zhou, J.; Liu, B.; Fan, Y.; Jiang, J.; et al. Recent Advances and Perspectives on Supported Catalysts for Heterogeneous Hydrogen Production from Ammonia Borane. Adv. Sci. 2023, 10, 202300726. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Y.; Han, J.; Guo, M.; Liu, Q. A Perspective on the Relationship Between Microstructure and Performance of Cu-Based Zeolites for the Selective Catalytic Reduction of NOx. Catal. Surv. Asia 2020, 24, 179–195. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.; Lu, J. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Li, W.; Zhang, K.; Hou, H.; He, Z.; Zhu, C.; Meira, D.M.; Lopez-Haro, M.; Xia, Z.; He, P.; et al. Size-Dependent Structural Features of Subnanometer PtSn Catalysts Encapsulated in Zeolite for Alkane Dehydrogenation. ACS Catal. 2024, 14, 2859–2871. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, P.; Wang, J.; Liu, H.; Zhang, X. Geometrically embedding dispersive Pt nanoparticles within silicalite-1 framework for highly selective α, β-unsaturated aldehydes hydrogenation via oriented C = O adsorption configuration. Chem. Eng. J. 2022, 446, 137064. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Han, R.; Fu, K.; Su, Y.; Zheng, Y.; Wu, X.; Song, C.; Ji, N.; Lu, X.; et al. Confinement and synergy effect of bimetallic Pt-Mn nanoparticles encapsulated in ZSM-5 zeolite with superior performance for acetone catalytic oxidation. Appl. Catal. B Environ. 2022, 309, 121224. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Bing, Q.; Si, R.; Liu, J.; Bai, R.; Zhang, P.; Jia, M.; Yu, J. Subnanometric Hybrid Pd-M(OH), M=Ni, Co, Clusters in Zeolites as Highly Efficient Nanocatalysts for Hydrogen Generation. Chem 2017, 3, 477–493. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, G.; Liu, W.; Li, G.; He, T.; Zhang, H.; Yu, Y.; Peng, H. Constructing highly active zeolite encapsulated PdAg alloy catalysts for typical VOCs deep oxidation: The role of electron-structure interaction. Appl. Catal. B Environ. Energy 2024, 352, 124051. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, P.; Tang, X.; Hagio, T.; Sato, K.; Nagaoka, K.; Li, X. Effects of the Topological Structure of Different Cu-Zeolites on the Relationship between Soot and NOx Removal on NH3-SCRF. Energy Fuels 2024, 38, 2212–2223. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Qiao, S.; Heng, Z.; Zhao, T.; Wu, H.; Xiong, T.; Li, J.; Yao, X.; Long, L.; et al. Ethane Ammoxidation over Sn/H-Zeolite Catalysts: Toward the Factors Contributing to the Yield of Acetonitrile. ACS Appl. Mater. Interfaces 2023, 15, 25604–25614. [Google Scholar] [CrossRef]
- Weissenberger, T.; Kapil, N.; Trogadas, P.; Coppens, M.-O. One-pot Synthesis of Hierarchical, Micro-macroporous Zeolites with Encapsulated Metal Particles as Sinter-resistant, Bifunctional Catalysts. ChemCatChem 2022, 14, 202200268. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; He, J.; Wang, X.; Xu, J.; Li, J.; Wu, M.; Jiang, J.; Chou, L.; Song, H. Synthesis and Catalytic Performance of Core-shell Micro-Mesoporous Composite Zeolite MCM-41@TS-1 Catalysts. J. Chin. Ceram. Soc. 2022, 50, 2611–2621. [Google Scholar]
- Kurbanova, A.; Zakutna, D.; Golabek, K.; Mazur, M.; Prech, J. Preparation of Fe@MFI and CuFe@MFI composite hydrogenation catalysts by reductive demetallation of Fe-zeolites. Catal. Today 2022, 390, 306–315. [Google Scholar] [CrossRef]
- Tian, J.; Tan, K.B.; Liao, Y.; Sun, D.; Li, Q. Hollow ZSM-5 zeolite encapsulating Pt nanoparticles: Cage-confinement effects for the enhanced catalytic oxidation of benzene. Chemosphere 2022, 292, 133446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, H.; Wang, X.; Wang, L.; Wang, R.; Jiang, H.; Jin, C. Spatial Configurational Construction of Pt-ZSM-5 Bifunctional Catalysts for Hydroisomerization of n-Heptane. Catal. Surv. Asia 2025, 29, 155–166. [Google Scholar] [CrossRef]
- Dou, X.; Yan, T.; Qian, L.; Hou, H.; Lopez-Haro, M.; Marini, C.; Agistini, G.; Meira, D.M.; Zhang, X.; Zhang, L.; et al. Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite. Nat. Catal. 2024, 7, 666–677. [Google Scholar] [CrossRef]
- Zhang, K.; Dou, X.; Zhou, Z.; Wang, Y.; Hou, H.; Meira, D.M.; Liu, L.; He, P. Tuning the size and spatial distribution of Pt in bifunctional Pt-zeolite catalysts for direct coupling of ethane and benzene. Chem. Eng. J. 2024, 497, 154874. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Chu, M.; Gao, D.; Wang, Y.; Lv, Y.; Wang, R.; Song, L.; Zhao, H.; Chen, J.; et al. Ultra-Narrow Alkane Product Distribution in Polyethylene Waste Hydrocracking by Zeolite Micro-Mesopore Diffusion Optimization. Angew. Chem. Int. Ed. 2024, 63, 202409288. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Wang, M.; Yuan, H. Synthesis of mesoporous Pt@KIT-6/SAPO-11 encapsulation to catalyze the decarboxylation of oleic acid to C8-C17 alkanes. J. Chem. Technol. Biotechnol. 2022, 97, 2055–2067. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Sun, S.; Ding, J.-J.; Zhang, Y.; Zhang, M.-M.; Gao, C.; Bao, J. Preparation, Structure and Performance of CuO-ZnO-Al2O3/HZSM-5 Core-Shell Bifunctional Catalysts for One-Step Synthesis of Dimethyl Ether from CO2+H2. Acta Phys.-Chim. Sin. 2012, 28, 1957–1963. [Google Scholar]
- Neylon, M.K.; Castagnola, M.J.; Castagnola, N.B.; Marshall, C.L. Coated bifunctional catalysts for NOx SCR with C3H6 Part I: Water-enhanced activity. Catal. Today 2004, 96, 53–60. [Google Scholar] [CrossRef]
- Castagnola, M.J.; Neylon, M.K.; Marshall, C.L. Coated bifunctional catalysts for NOx SCR with C3H6 Part II: In situ spectroscopic characterization. Catal. Today 2004, 96, 61–70. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Fu, X.; Gao, C.; Huang, J.; Li, B.; Yang, Y.; Gao, J.; Shen, Y.; Peng, Z.; Yang, J.-H.; et al. Enhancing the matching of acid/metal balance by engineering an extra Si-Al framework outside the Pd/HBeta catalyst towards benzene hydroalkylation. Catal. Sci. Technol. 2020, 10, 1467–1476. [Google Scholar] [CrossRef]
- Cao, P.; Lin, L.; Qi, H.; Chen, R.; Wu, Z.; Li, N.; Zhang, T.; Luo, W. Zeolite-Encapsulated Cu Nanoparticles for the Selective Hydrogenation of Furfural to Furfuryl Alcohol. ACS Catal. 2021, 11, 10246–10256. [Google Scholar] [CrossRef]
- Tian, S.; Guo, H.; Zhao, Q.; Liu, L.; Wang, H.; Wang, X.; Zhang, S.; Cui, W. TS-1 zeolite encapsulated Pt clusters with enhanced electronic metal-support interaction of Pt O(H) Ti for nearly-zero-carbon-emitting photocatalytic hydrogen production from methanol. Appl. Catal. B Environ. Energy 2024, 355, 124201. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Shao, Y.; Wang, Y.; Gates, B.C.; Xiao, F. A Pd@Zeolite Catalyst for Nitroarene Hydrogenation with High Product Selectivity by Sterically Controlled Adsorption in the Zeolite Micropores. Angew. Chem. Int. Ed. 2017, 56, 9747–9751. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Zhang, Q.; Zhou, L.; Zhang, Y. Nickel nano-particles encapsulated inside zeolite crystal for active and stable CO2 methanation. Fuel 2024, 371, 131909. [Google Scholar] [CrossRef]
- Liu, M.; Miao, C.; Xiao, P.; Wu, Z. Encapsulated Cu clusters in Zr-Beta zeolite as an efficient catalyst to promote the formation of methyl acetate from methanol. Chem. Eng. J. 2025, 511, 162210. [Google Scholar] [CrossRef]
- Wang, E.; Hu, D.; Xiao, C.; Song, S.; Zou, Y.; Fu, S.; Chi, K.; Liu, J.; Duan, A.; Wang, X. Highly dispersed Pt on core-shell micro-mesoporous composites assembled by mordenite nanocrystals for selective hydrogenation of polycyclic aromatics. Fuel 2023, 331, 125852. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Zhao, Y.; Moyo, P.S.; Zhao, Y.; Wang, S.; Ma, X. Confined high dispersion of Ni nanoparticles derived from nickel phyllosilicate structure in silicalite-2 shell for dry reforming of methane with enhanced performance. Microporous Mesoporous Mater. 2021, 313, 110842. [Google Scholar] [CrossRef]
- Zou, H.; Jin, Y.; Chen, L.; Chen, J. Encapsulating Ru Nanoclusters for Reductive Imination of Biomass-Based Furfural by Shape-Selective Catalysis. ACS Catal. 2025, 15, 2017–2032. [Google Scholar] [CrossRef]
- Kumar, A. Shape-Controlled Synthesis and Applications of One-Dimensional Palladium Nanostructures. Ph.D. Thesis, State University of New York, Buffalo, NY, USA, 2022. [Google Scholar]
- Shen, Z.; Ma, C.; Wang, D.; He, J.; Sun, H.; Zhu, Z.; Yang, W. Shape-selective alkylation of benzene with ethylene over a core-shell ZSM-5@MCM-41 composite material. Chin. J. Chem. Eng. 2021, 37, 64–71. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Z.; Hu, P.; Ding, L.; Xue, N.; Peng, L.; Guo, X.; Lin, M.; Ding, W. Platinum Nanoparticles Encapsulated in MFI Zeolite Crystals by a Two-Step Dry Gel Conversion Method as a Highly Selective Hydrogenation Catalyst. ACS Catal. 2015, 5, 6893–6901. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A. Designing of Highly Active and Sustainable Encapsulated Stabilized Palladium Nanoclusters as well as Real Exploitation for Catalytic Hydrogenation in Water. Catal. Lett. 2021, 151, 803–820. [Google Scholar] [CrossRef]
- Li, S.; Boucheron, T.; Tuel, A.; Farrusseng, D.; Meunier, F. Size-selective hydrogenation at the subnanometer scale over platinum nanoparticles encapsulated in silicalite-1 single crystal hollow shells. Chem. Commun. 2014, 50, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tuel, A.; Laprune, D.; Meunier, F.; Farrusseng, D. Transition-Metal Nanoparticles in Hollow Zeolite Single Crystals as Bifunctional and Size-Selective Hydrogenation Catalysts. Chem. Mater. 2015, 27, 276–282. [Google Scholar] [CrossRef]
- den Breejen, J.P.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Froseth, V.; Holmen, A.; de Jong, K.P. On the Origin of the Cobalt Particle Size Effects in Fischer-Tropsch Catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef] [PubMed]
- Hinchiranan, S.; Zhang, Y.; Nagamori, S.; Vitidsant, T.; Tsubaki, N. TiO2 promoted Co/SiO2 catalysts for Fischer-Tropsch synthesis. Fuel Process. Technol. 2008, 89, 455–459. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Chen, J.; Zhang, Y. Cobalt nanoparticles imbedded into zeolite crystals: A tailor-made catalyst for one-step synthesis of gasoline from syngas. Int. J. Hydrogen Energy 2016, 41, 21965–21978. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Zhao, Y.; Zhang, Y.; Hong, J. Effects of Ru nanoparticle sizes confined in cavities of SBA-16 on the catalytic performance of Fischer-Tropsch synthesis reaction. J. Nat. Gas. Chem. 2012, 21, 673–679. [Google Scholar] [CrossRef]
- Choi, M.; Wu, Z.; Iglesia, E. Mercaptosilane-Assisted Synthesis of Metal Clusters within Zeolites and Catalytic Consequences of Encapsulation. J. Am. Chem. Soc. 2010, 132, 9129–9137. [Google Scholar] [CrossRef]
- Shi, J.; Chen, L.; Ren, N.; Zhang, Y.; Tang, Y. Zeolitic microcapsule with encapsulated platinum nanoparticles for one-pot tandem reaction of alcohol to hydrazone. Chem. Commun. 2012, 48, 8583–8585. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Yadaw, D.; Tiwari, K.; Venkatesh, V.; Verma, S.; Pala, R.G.S.; Sivakumar, S. Hollow Zeolites Encapsulating Ultra-Low Noble Metal Nanoparticles for HMF Oxidation. Catal. Lett. 2021, 151, 921–931. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, Y.; Chen, D.; Li, L.; Li, G.; Wang, L.; Zhang, X.; Liu, G. Selective steam reforming of n-dodecane over stable subnanometric NiPt clusters encapsulated in Silicalite-1 zeolite. AIChE J. 2020, 66, 16917. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Zhang, X.; Liu, W.; Liu, H.; Qiu, J.; Yeung, K.L. New synthesis strategies for Ni/Al2O3-Sil-1 core shell catalysts for steam reforming of methane. Catal. Today 2014, 236, 34–40. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Tu, M.; Liu, W.; Liu, H.; Qiu, J.; Zhou, L.; Shao, Z.; Ho, H.L.; Yeung, K.L. Preparation of core (Ni base)-shell (Silicalite-1) catalysts and their application for alkali resistance in direct internal reforming molten carbonate fuel cell. J. Power Sources 2012, 198, 14–22. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Zhang, J.; Liu, H.; Zhou, L.; Yeung, K. Preparation of alkali-resistant, Sil-1 encapsulated nickel catalysts for direct internal reforming-molten carbonate fuel cell. Catal. Commun. 2009, 10, 1804–1807. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal-support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Xie, T.; Shi, L.; Zhang, J.; Zhang, D. Immobilizing Ni nanoparticles to mesoporous silica with size and location control a polyol-assisted route for coking- and sintering-resistant dry reforming of methane. Chem. Commun. 2014, 50, 7250–7253. [Google Scholar] [CrossRef]
- Shi, W.; Oda, A.; Yamamoto, Y.; Harada, S.; Ohtsu, T.; Sawabe, K.; Satsuma, A. Encapsulated Platinum-Tin Nanoparticles in Silicalite-1 Zeolite for Methylcyclohexane Dehydrogenation. ACS Sustain. Chem. Eng. 2025, 13, 3608–3621. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Niu, M.; Wu, J.; Han, Y.; Xu, Y.; Zhu, J.; Wang, Z. Platinum nanoparticles confined in Zn-S-1 for efficient propane dehydrogenation. Chem. Eng. J. 2025, 505, 159748. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Q.; Qin, Y.; Liu, H.; Hu, P.; Xiong, C.; Ji, H. Bimetallic CoCu-modified Pt species in S-1 zeolite with enhanced stability for propane dehydrogenation. J. Colloid Interface Sci. 2024, 663, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Suo, Y.; Lv, X.; Wang, Z.; Yuan, Z.-Y. Enhanced performances of bimetallic Ga-Pt nanoclusters confined within silicalite-1 zeolite in propane dehydrogenation. J. Colloid Interface Sci. 2021, 593, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, L.; Zhai, Z.; Li, G.; Liu, G. Subsurface-Regulated PtGa Nanoparticles Confined in Silicalite-1 for Propane Dehydrogenation. ACS Appl. Mater. Interfaces 2021, 13, 16259–16266. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.-P.; Lv, X.; Chen, L.; Yuan, Z.-Y. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation. J. Catal. 2020, 385, 61–69. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Liu, C.; Guo, H. Pt-Cu Alloy Nanoparticles Encapsulated in Silicalite-1 Molecular Sieve: Coke-Resistant Catalyst for Alkane Dehydrogenation. Catal. Lett. 2019, 149, 974–984. [Google Scholar] [CrossRef]
- Ding, R.; Li, Y.; Leng, F.; Jia, M.; Yu, J.; Hao, X.; Xu, J. PdAu Nanoparticles Supported by Diamine-Containing UiO-66 for Formic Acid Dehydrogenation. ACS Appl. Nano Mater. 2021, 4, 9790–9798. [Google Scholar] [CrossRef]
- Sofi, M.H.M.; Hamid, M.Y.S.; Jalil, A.A.; Alhebshi, A.; Hassan, N.S.; Bahari, M.B.; Mohamud, M.Y. Recent Advancements of SAPO-34 and ZSM-5 Zeolite in Converting Methanol to Olefin: A Review. Arab. J. Sci. Eng. 2025, 50, 3671–3697. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, X.; Deng, Z.; Shi, W.; Fan, W.; Wang, F. Review and Outlook of Confined Ni Catalysts for the Dry Reforming of Methane Reaction. Energy Fuels 2024, 38, 1633–1656. [Google Scholar] [CrossRef]
- Zhao, T.; Niu, B.; An, W.; Li, B.; Li, G.; Shui, Z.; Duan, X.; Zhang, Z.; Cheng, J.; Hao, Z. Highly efficient and stable single-atom Cu encapsulated in silicalite-1 zeolite for selective catalytic oxidation of nitrogen-containing VOCs. Appl. Catal. B Environ. Energy 2025, 371, 125277. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Shang, X.; Su, X.; Huang, Y. Boosting Pt atom efficiency by reinforcing the synergy with extra Sn sites encapsulated in MFI zeolite for the aromatization of n-hexane. Catal. Sci. Technol. 2025, 15, 2977–2987. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, M.; Wei, Y.; Yue, Y.; Bai, Z.; Yuan, P.; Fornasiero, P.; Basset, J.-M.; Mei, B.; Liu, Z.; et al. Pt migration-lockup in zeolite for stable propane dehydrogenation catalyst. Nature 2025, 643, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Peng, P.; Wang, H.; Xiong, H.; Xu, Z.; Chen, X.; Li, Y.; Zheng, A.; Wei, Y.; Yan, Z.; et al. Revealing the Crucial Roles of Pore Interconnectivity between Zeolitic and Nonzeolitic Components in Enhancing Diffusion and Catalytic Efficiency of Industrial Zeolite-Based Catalysts. J. Am. Chem. Soc. 2025, 147, 18550–18562. [Google Scholar] [CrossRef]
- Kirchner, T.; Shakhou, A.; Zeigermann, P.; Valiullin, R.; Kaerger, J. Probing mesopore connectivity in hierarchical nanoporous materials. Carbon 2012, 50, 4804–4808. [Google Scholar] [CrossRef]
| Preparation Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Impregnation | Metal precursors are mixed with zeolite, followed by drying and calcination. | Simple operation, controllable metal content, and complete pore filling. | Non-uniform metal distribution, pore blockage, and easy sintering. |
| Ion Exchange | Metal precursors replace exchangeable ions in zeolites, introducing metals into pores. | Intimate contact between metal ions and zeolite framework, achieving uniform dispersion and even atomic distribution. | Requires low-silica zeolites, limited metal loading, and potential poor thermal stability. |
| Ship-in-a-bottle approach | Metal complexes or clusters form within zeolite pores or supercages, too large to escape once formed. | Creates truly encapsulated, leak-resistant species with strong metal–support interaction and high stability. | Confined to large-cavity zeolites (mainly Y-type), low metal loading, and multi-step synthesis. |
| Solvent-free Crystallization | Amorphous silicate or aluminosilicate precursors and metal sources are mixed, with zeolite crystallizing around metal particles under heating without solvents. | Green process, uniform metal encapsulation within freshly grown zeolite crystals. | Requires strict control of solid-state heat and mass transfer; synthesis temperatures must exceed conventional hydrothermal limits. |
| Hydrothermal crystallization | Metal coordination complexes are used as precursors, and hydrothermal treatment forms dispersed nanocrystalline cores encapsulating metal components. | Simple operation, wide applicability, uniform particle size distribution, and high metal dispersion. | Long reaction times, high temperature and pressure requirements, and equipment dependency. |
| Dissolution–recrystallization | Parent zeolite or pre-impregnated metal/zeolite composite is partially dissolved in mild alkaline solution, followed by controlled re-crystallization to form a new zeolite shell encapsulating metal species. | Creates tunable core–shell and yolk–shell structures in one step, enhancing metal dispersion and sintering resistance. | Partial zeolite loss, alkaline effluent generation, and sensitivity of shell thickness and cavity size to base concentration, temperature, and time. |
| Parameter | Effect on Catalytic Performance |
|---|---|
| Metal nanoparticle size | Smaller size increases dispersion and surface energy, enhancing activity. Quantum effects alter electronic structure, influencing selectivity. |
| Metal nanoparticle shape | Determines exposed crystal planes and edge sites, affecting reactant adsorption and activation. Influences activity and selectivity. |
| Alloy composition | Synergistic effects between metals change activity and selectivity. Bimetallic/multimetallic systems combine advantages of different metals. |
| Zeolite pore structure | Micropores confine metal nanoparticles, preventing sintering. Hierarchical porosity improves mass transfer and accessibility. |
| Zeolite acidity | Acid sites synergize with metal sites. Spatial arrangement and acid strength affect activity and product distribution. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Zhang, T.; Xiao, L.; Zhang, C.; Li, Y. Synthesis and Applications of Zeolite-Encapsulated Metal Catalysts. Catalysts 2025, 15, 836. https://doi.org/10.3390/catal15090836
Zhu T, Zhang T, Xiao L, Zhang C, Li Y. Synthesis and Applications of Zeolite-Encapsulated Metal Catalysts. Catalysts. 2025; 15(9):836. https://doi.org/10.3390/catal15090836
Chicago/Turabian StyleZhu, Teng, Tianwei Zhang, Lei Xiao, Cunwei Zhang, and Yuming Li. 2025. "Synthesis and Applications of Zeolite-Encapsulated Metal Catalysts" Catalysts 15, no. 9: 836. https://doi.org/10.3390/catal15090836
APA StyleZhu, T., Zhang, T., Xiao, L., Zhang, C., & Li, Y. (2025). Synthesis and Applications of Zeolite-Encapsulated Metal Catalysts. Catalysts, 15(9), 836. https://doi.org/10.3390/catal15090836






