Computational Evaluation of Defects in Fe–N4-Doped Graphene for Electrochemical CO2 Reduction
Abstract
1. Introduction
2. Results and Discussion
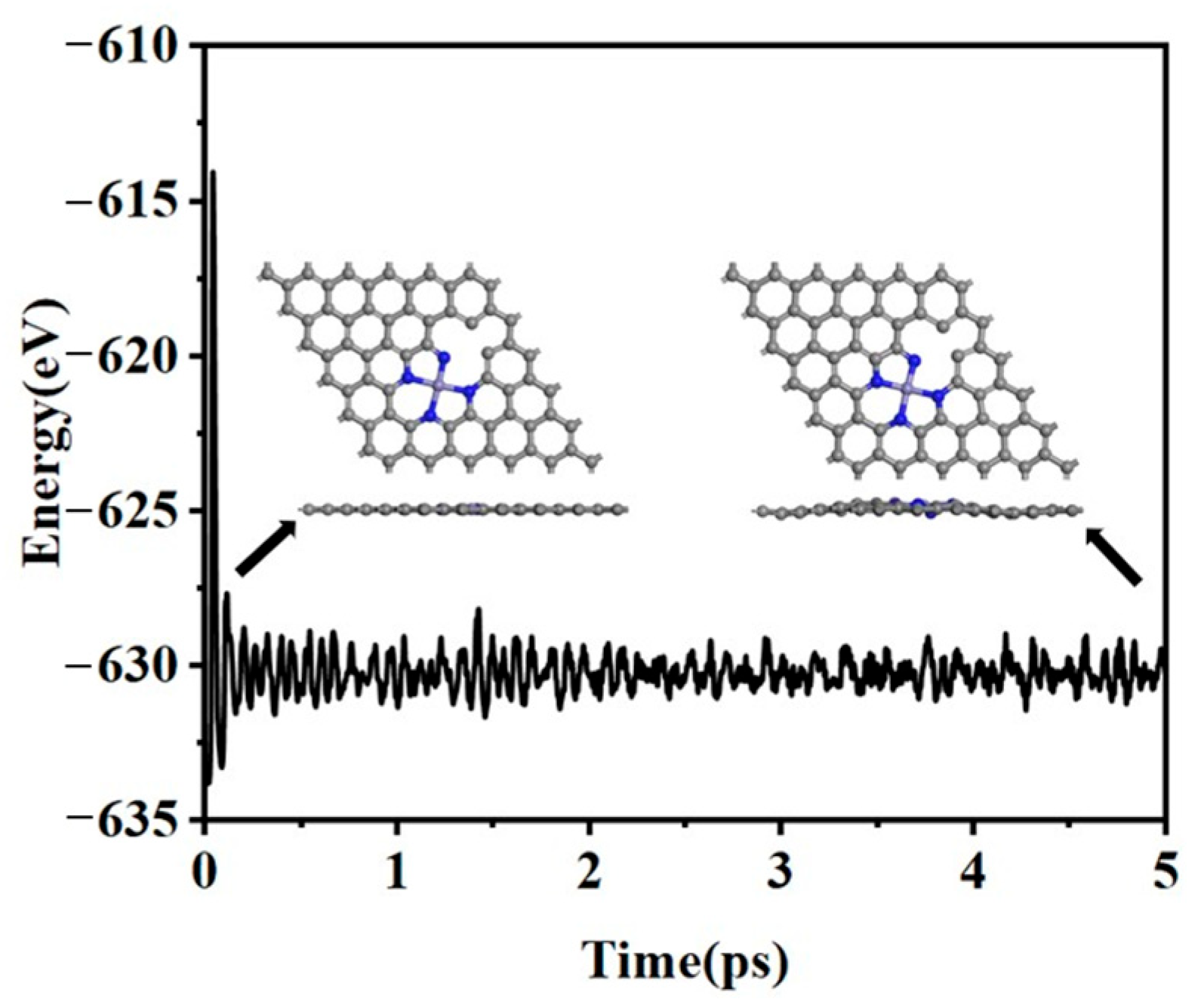
2.1. Structures and Stability
2.2. Electrocatalytic CO2 Reduction
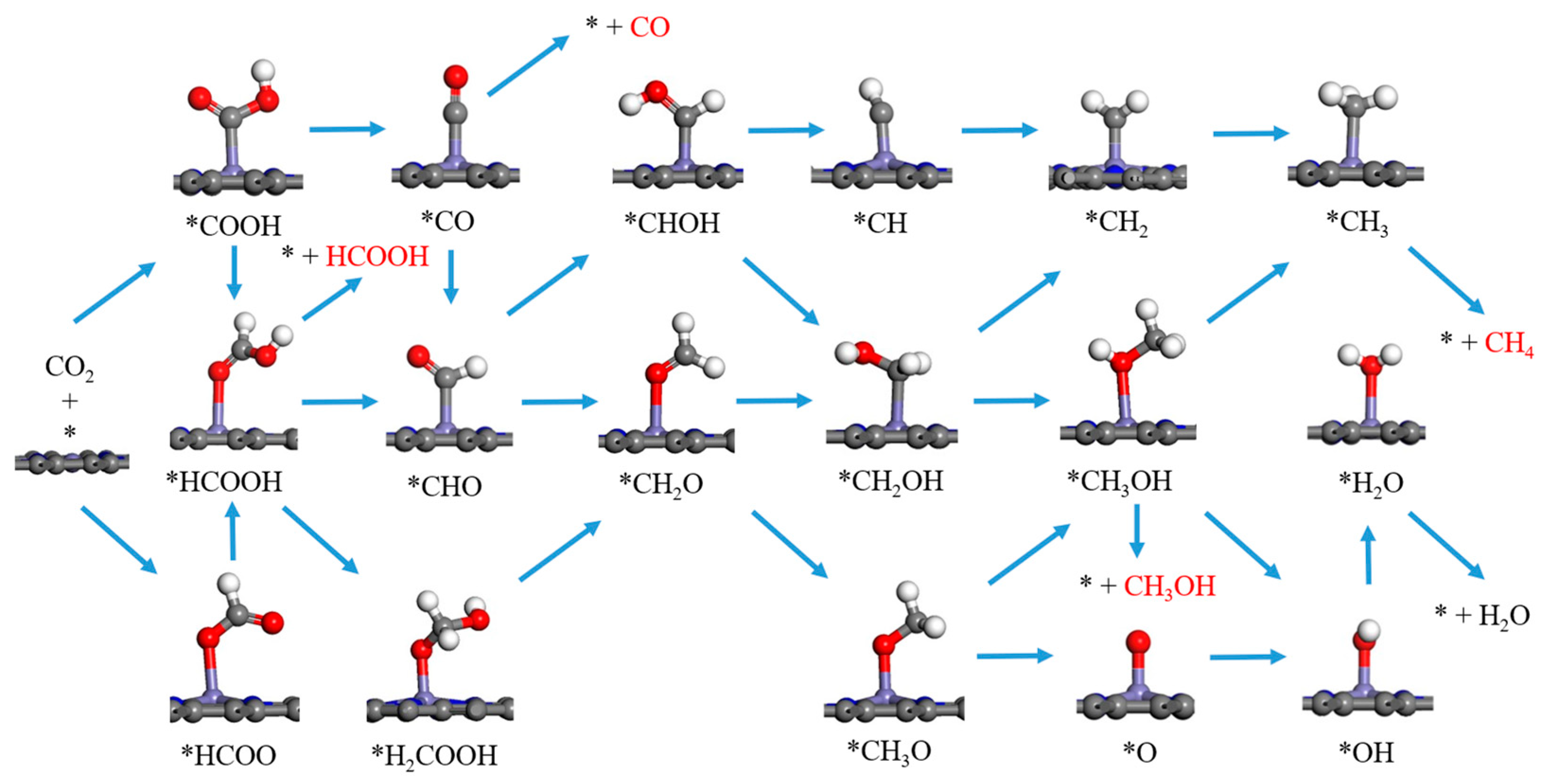
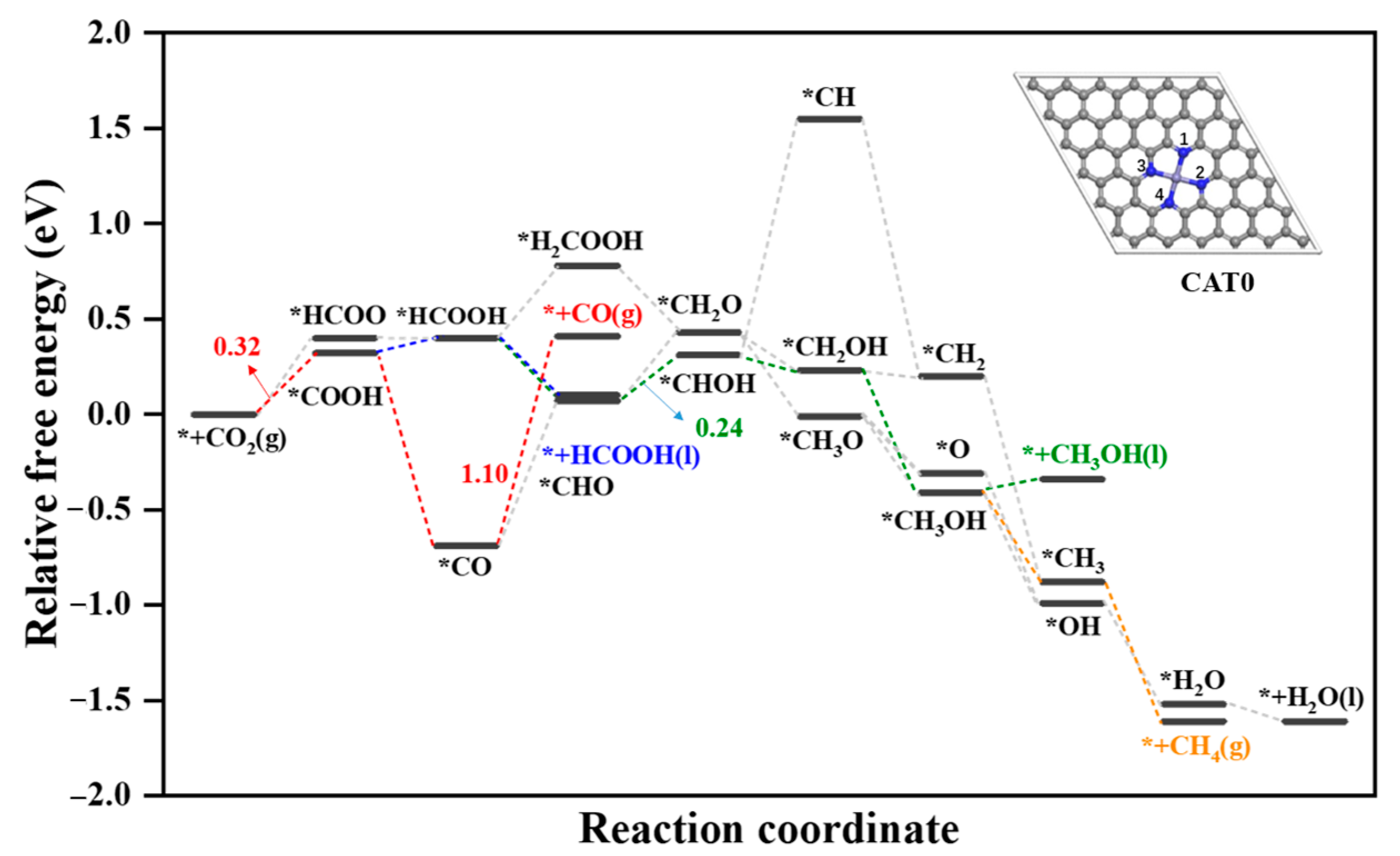
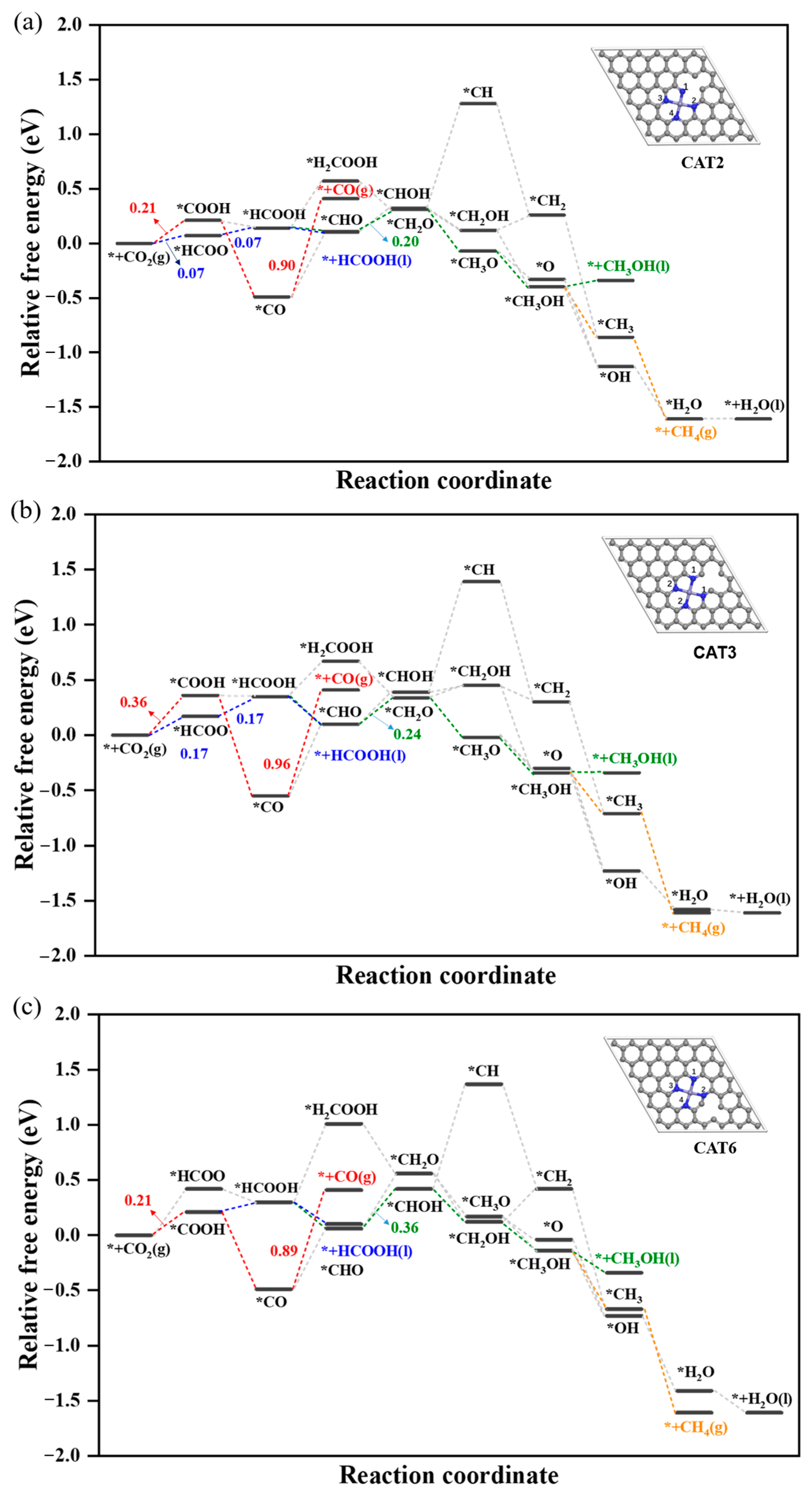
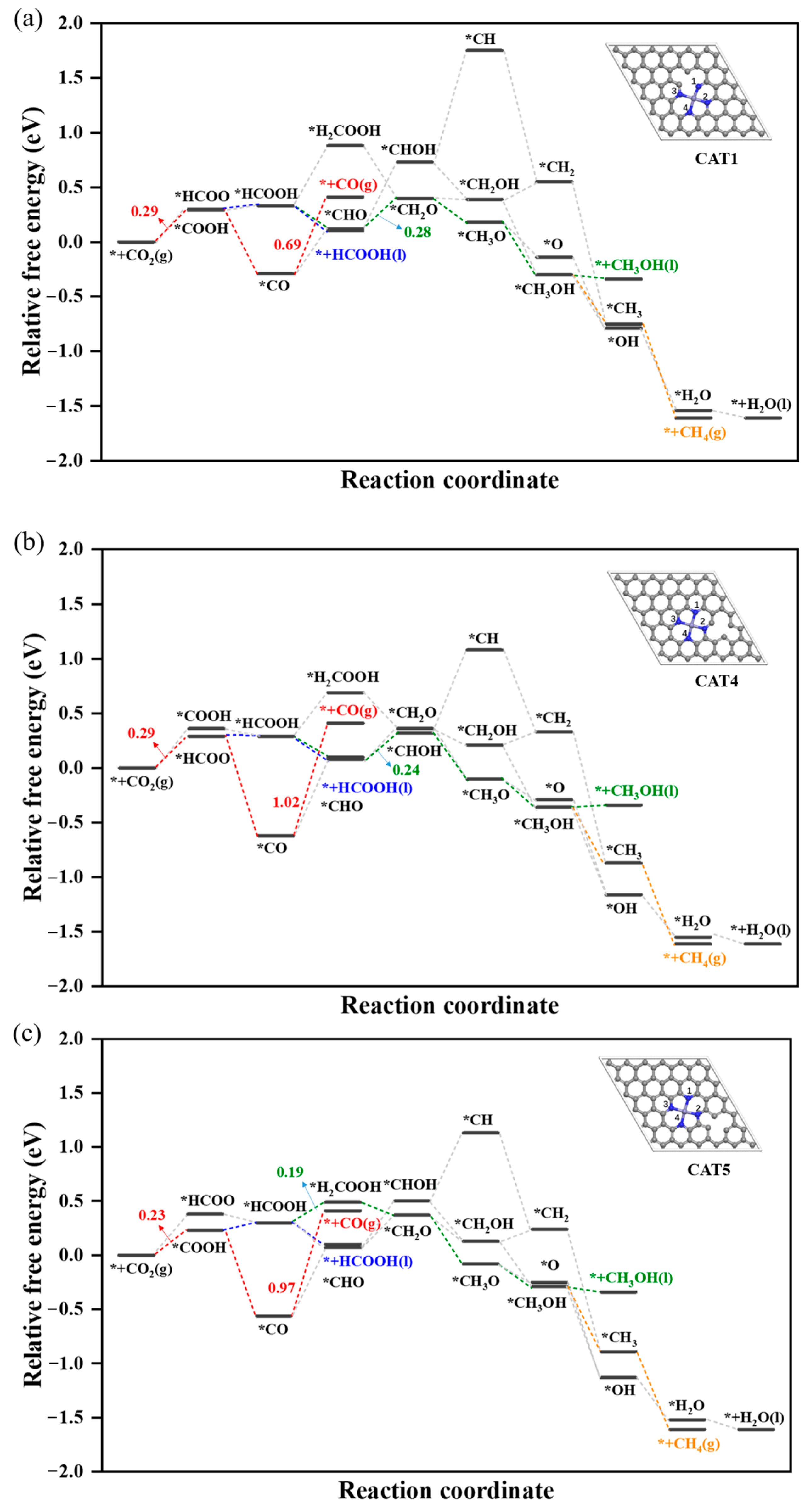
2.2.1. Reduction of CO2 to HCOOH
2.2.2. Reduction of CO2 to HCOOH, CH3OH and CH4
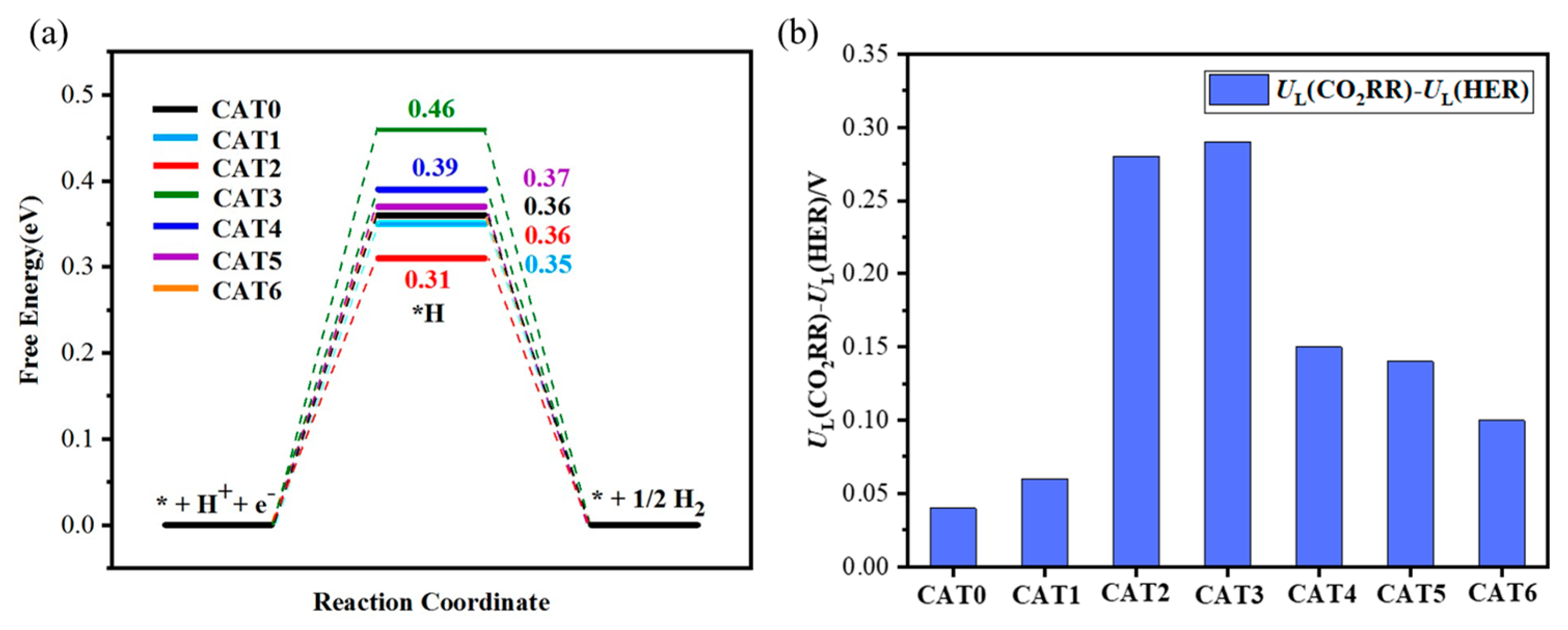
2.3. Comparison of CO2RR with HER
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, C.; Zhang, Y.; Jaroniec, M.; Qiao, S.-Z. Electrocatalytic Refinery for Sustainable Production of Fuels and Chemicals. Angew. Chem. Int. Ed. 2021, 60, 19572–19590. [Google Scholar] [CrossRef]
- Yang, P.-P.; Gao, M.-R. Enrichment of Reactants and Intermediates for Electrocatalytic CO2 Reduction. Chem. Soc. Rev. 2023, 52, 4343–4380. [Google Scholar] [CrossRef]
- Lu, T.; Xu, T.; Zhu, S.; Li, J.; Wang, J.; Jin, H.; Wang, X.; Lv, J.-J.; Wang, Z.-J.; Wang, S. Electrocatalytic CO2 Reduction to Ethylene: From Advanced Catalyst Design to Industrial Applications. Adv. Mater. 2023, 35, 2310433. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Zhang, D.; Du, Y.; Amal, R.; Qiao, S.; Wu, J.; Yin, Z. Surface Strategies for Catalytic CO2 Reduction: From Two Dimensional Materials to Nanoclusters to Single Atoms. Chem. Soc. Rev. 2019, 48, 5310–5349. [Google Scholar] [CrossRef]
- Deng, B.W.; Sun, D.M.; Zhao, X.Y.; Wang, L.L.; Ma, F.Y.; Li, Y.Z.; Dong, F. Accelerating Acidic CO2 Electroreduction: Strategies Beyond Catalysts. Chem. Sci. 2024, 15, 15087–15108. [Google Scholar] [CrossRef]
- Seger, B.; Kastlunger, G.; Bagger, A.; Scott, S.B. A Perspective on the Reaction Mechanisms of CO2 Electrolysis. ACS Energy Lett. 2025, 10, 2212–2227. [Google Scholar] [CrossRef]
- Xu, S.Z.; Carter, E.A. Theoretical Insights into Heterogeneous (Photo)electrochemical CO2 Reduction. Chem. Rev. 2019, 119, 6631–6669. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Pérez-Gallent, E.; Figueiredo, M.C.; Göttle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Sethuraman, V.; Michalsky, R.; Peterson, A.A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 2014, 4, 3742–3748. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Wang, T.; Cai, Y.-C.; Li, X.-Y.; Ye, J.-Y.; Zhou, Y.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Probing Electrolyte Effects on Cation-Enhanced CO2 Reduction on copper in acidic media. Nat. Catal. 2024, 7, 807–817. [Google Scholar] [CrossRef]
- Yohannes, A.G.; Lee, C.; Talebi, P.; Mok, D.H.; Karamad, M.; Back, S.; Siahrostami, S. Combined High-Throughput DFT and ML Screening of Transition Metal Nitrides for Electrochemical CO2 Reduction. ACS Catal. 2023, 13, 9007–9017. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wu, J.; Zhao, X.; Xiong, Y.; Luo, F.; Lei, Y. Asymmetric Atomic Sites Make Different: Recent Progress in Electrocatalytic CO2 Reduction. Nano Energy 2022, 103, 107815. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Wang, Y.; Pan, Z.; Chen, J.; Xu, Y.; Zhu, L.; Song, T.; Li, R.; Chen, L.; et al. Interfacial Microenvironment Effects on Electrochemical CO2 Reduction. Chem. Eng. J. 2024, 482, 148944. [Google Scholar] [CrossRef]
- Mariano, R.G.; McKelvey, K.; White, H.S.; Kanan, M.W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science 2017, 358, 1187–1192. [Google Scholar] [CrossRef]
- Sun, M.; Huang, B. Machine Learning Across Metal and Carbon Support for the Screening of Efficient Atomic Catalysts Toward CO2 Reduction. Adv. Energy Mater. 2023, 13, 2301948. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, Y.; Liu, W.; Wu, J.; Li, Q.; Feng, Q.G.; Chen, Z.Y.; Xiong, X.; Wang, D.S.; Lei, Y.P. Regulating the Coordination Structure of Metal Single Atoms for Efficient Electrocatalytic CO2 Reduction. Energy Environ. Sci. 2020, 13, 4609–4624. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, R.; Chen, Y.J.; Liu, S.J.; Zhu, W.; Cao, X.; Chen, W.X.; Wu, K.L.; Cheong, W.-C.; Wang, Y.; et al. Design of Single-Atom Co−N5 Catalytic Site: A Robust Electrocatalyst for CO2 Reduction with Nearly 100% CO Selectivity and Remarkable Stability. J. Am. Chem. Soc. 2018, 140, 4218–4221. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, J.; Wang, S.; Ren, X.; Tan, Y.; Lu, Y.-R.; Xi, S.; Wang, J.; Jaouen, F.; Li, X.; et al. Unraveling the Electronic Structure and Dynamics of the Atomically Dispersed Iron Sites in Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2023, 145, 15600–15610. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Li, Y. Single-atom catalysis for carbon neutrality. Carbon Energy. 2022, 4, 1021–1079. [Google Scholar] [CrossRef]
- Yang, F.; Han, H.; Duan, H.; Fan, F.; Chen, S.; Xia, B.Y.; He, Y.L. A Review on Single Site Catalysts for Electrochemical CO2 Reduction. Adv. Energy Mater. 2025, 15, 2405726. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; Arbiol, J. Metal–organic framework-derived single atom catalysts for electrocatalytic reduction of carbon dioxide to C1 products. Energy Adv. 2023, 2, 252–267. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Q.; Liu, H.; Xu, W.; Wang, Z.; Qiao, S.; Ding, H.; Chen, D.; Zhu, J.; Qi, Z.; et al. Asymmetric dinitrogen-coordinated nickel single-atomic sites for efficient CO2 electroreduction. Nat. Commun. 2023, 14, 3776. [Google Scholar] [CrossRef]
- Liu, S.-W.; Chen, H.-T. Mechanistic Understanding on Effect of Doping Nitrogen with Graphene Supported Single-Atom Fe toward Electrochemical CO2 Reduction: A Computational Consideration. Appl. Surf. Sci. 2023, 630, 157390. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Chiu, K.-Y.; Fan, C.-H.; Chen, H.-L. Electrocatalytic Carbon Dioxide Reduction on Graphene-Supported Ni Cluster and its Hydride: Insight from First-Principles Calculations. Appl. Surf. Sci. 2023, 629, 157418. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Wan, X.; Wang, J.; Kuai, C.; Zhang, Z.; Guo, Y. Identifying TM-N4 active sites for selective CO2-to-CH4 conversion: A computational study. Appl. Surf. Sci. 2022, 582, 152470. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Xiao, S.; Yang, Y.; Lai, L.; Chen, J.S.; Chen, Y. Axial chlorine coordinated iron-nitrogen-carbon single-atom catalysts for efficient electrochemical CO2 reduction. Chem. Eng. J. 2022, 430, 132882. [Google Scholar] [CrossRef]
- Adli, N.M.; Shan, W.; Hwang, S.; Samarakoon, W.; Karakalos, S.; Li, Y.; Cullen, D.A.; Su, D.; Feng, Z.; Wang, G.; et al. Engineering Atomically Dispersed FeN4 Active Sites for CO2 Electroreduction. Angew. Chem. Int. Ed. 2021, 60, 1022–1032. [Google Scholar] [CrossRef]
- Qiu, Y.-Z.; Liu, X.-M.; Li, W.; Li, J.; Xiao, H. Transient Dangling Active Sites of Fe(III)-N-C Single-Atom Catalyst for Efficient Electrochemical CO2 Reduction Reaction. Angew. Chem. Int. Ed. 2025, 64, e202424150. [Google Scholar] [CrossRef]
- Menisa, L.T.; Cheng, P.; Long, C.; Qiu, X.; Zheng, Y.; Han, J.; Zhang, Y.; Gao, Y.; Tang, Z. Insight into atomically dispersed porous M−N−C single-site catalysts for electrochemical CO2 reduction. Nanoscale 2020, 12, 16617–16626. [Google Scholar] [CrossRef]
- Zhou, D.; Li, X.; Shang, H.; Qin, F.; Chen, W. Atomic regulation of metal–organic framework derived carbon-based single-atom catalysts for the electrochemical CO2 reduction reaction. J. Mater. Chem. A 2021, 9, 23382–23418. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Yang, L.; Wang, X.; Wu, Q.; Hu, Z. Recent Advances in Non-Precious Metal–Nitrogen–Carbon Single-Site Catalysts for CO2 Electroreduction Reaction to CO. Electrochem. Energy Rev. 2022, 5, 11. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Wang, X.; Tsai, Y.; Luo, F.; Möller, T.; Wang, H.; Rossmeisl, J.; Varela, A.S.; Strasser, P. Unraveling Mechanistic Reaction Pathways of the Electrochemical CO2 Reduction on Fe−N−C Single-Site Catalysts. ACS Energy Lett. 2019, 4, 1663–1671. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Cui, L.; Gao, W.; Li, X.; Liu, H.; Zhou, W.; Wang, J. Coordination-based synthesis of Fe single-atom anchored nitrogen-doped carbon nanofibrous membrane for CO2 electroreduction with nearly 100% CO selectivity. Chin. Chem. Lett. 2024, 35, 108133. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and Interface Engineering for Aqueous Electrocatalytic CO2 Reduction. Joule 2018, 2, 2551–2582. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, Y.; Wang, D.; Li, Y. Defect engineering in earth-abundant electrocatalysts for CO2 and N2 reduction . Energy Environ. Sci. 2019, 12, 1730–1750. [Google Scholar]
- Lei, J.; Zhu, T. Impact of Potential and Active-Site Environment on Single-Iron-Atom-Catalyzed Electrochemical CO2 Reduction from Accurate Quantum Many-Body Simulations. ACS Catal. 2024, 14, 3933–3942. [Google Scholar] [CrossRef]
- Ni, W.; Liu, Z.; Zhang, Y.; Ma, C.; Deng, H.; Zhang, S.; Wang, S. Electroreduction of Carbon Dioxide Driven by the Intrinsic Defects in the Carbon Plane of a Single Fe−N4 Site. Adv. Mater. 2021, 33, 2003238. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Liu, T.; Wang, G. Unexpected effect of second-shell defect in iron-nitrogen-carbon catalyst for electrochemical CO2 reduction reaction: A DFT study. Chinese J. Catal. 2024, 66, 247–256. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, J.; Hu, L.; Li, J.; Li, S.; Gao, Y.; Zhang, Q.; Gu, L.; Yang, W.; Feng, X.; et al. Decarboxylation-Induced Defects in MOF-Derived Single Cobalt Atom@Carbon Electrocatalysts for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2021, 60, 21685–21690. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal-Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11185. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef]
- Tuckerman, M.; Laasonen, K.; Sprik, M.; Parrinello, M. Ab initio molecular dynamics simulation of the solvation and transport of hydronium and hydroxyl ions in water. J. Chem. Phys. 1995, 103, 150–161. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Perdersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How Copper Catalyzes the Electroreduction of Carbon Dioxide into Hydrocarbon Fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Yi, W.; Tang, G.; Chen, X.; Yang, B.; Liu, X. Qvasp: A Flexible Toolkit for VASP Users in Materials Simulations. Comput. Phys. Commun. 2020, 257, 107535. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]

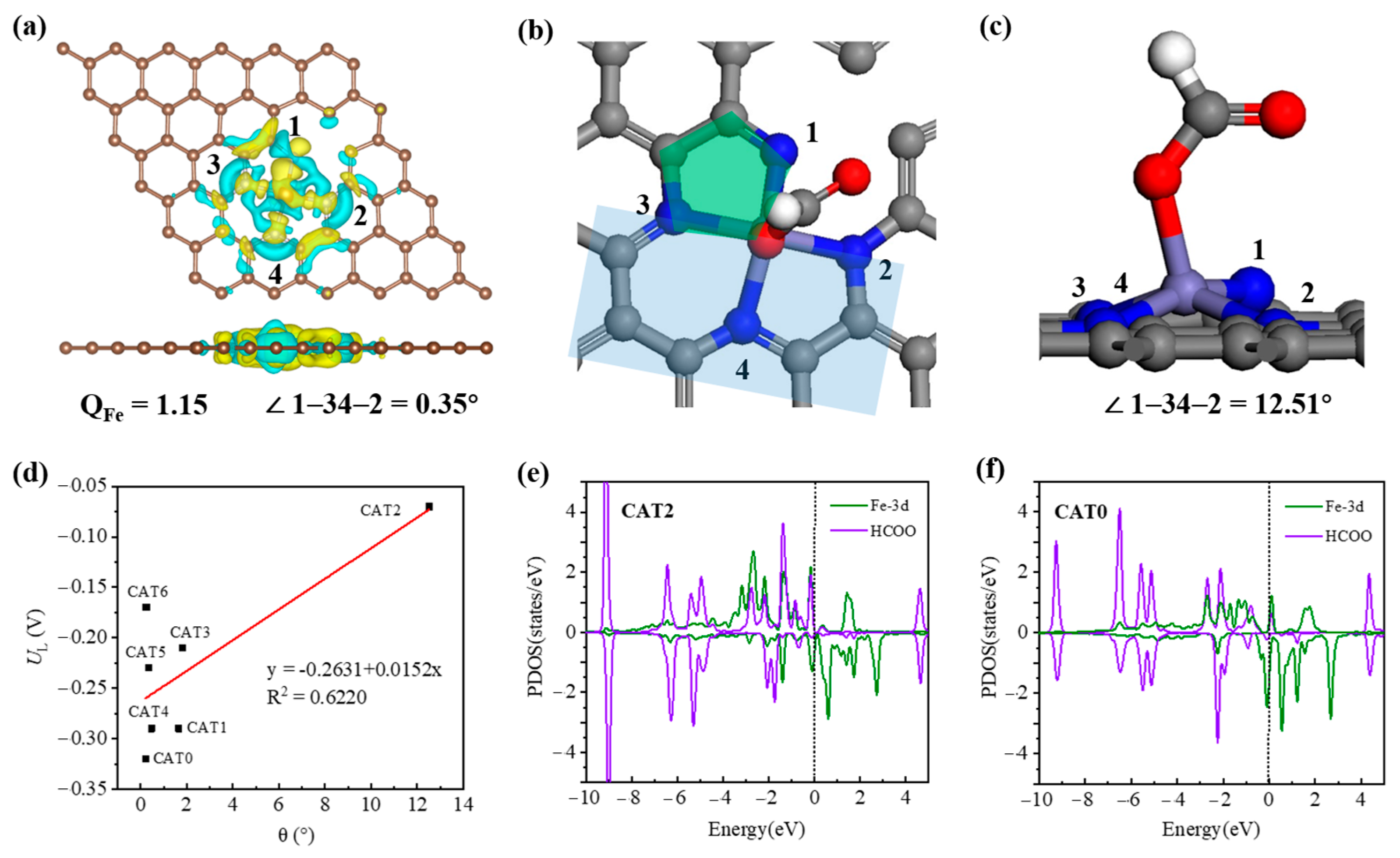
| Catalysts | Eb | Ef | Udiss |
|---|---|---|---|
| CAT0 | −7.94 | −2.59 | 0.85 |
| CAT1 | −8.58 | −3.23 | 1.17 |
| CAT2 | −8.50 | −3.15 | 1.12 |
| CAT3 | −6.91 | −1.56 | 0.33 |
| CAT4 | −7.07 | −1.72 | 0.41 |
| CAT5 | −8.39 | −3.04 | 1.07 |
| CAT6 | −7.03 | −1.68 | 0.39 |
| Catalysts | UL | ΔGCOOH | ΔGHCOO | QFe | θ |
|---|---|---|---|---|---|
| CAT0 | −0.32 | 0.32 | 0.40 | 1.10 | 0.21 |
| CAT1 | −0.29 | 0.29 | 0.30 | 1.18 | 1.65 |
| CAT2 | −0.07 | 0.21 | 0.07 | 1.15 | 12.51 |
| CAT3 | −0.17 | 0.36 | 0.17 | 1.08 | 0.24 |
| CAT4 | −0.29 | 0.36 | 0.29 | 1.09 | 0.47 |
| CAT5 | −0.23 | 0.38 | 0.23 | 1.10 | 0.34 |
| CAT6 | −0.21 | 0.21 | 0.42 | 1.05 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Liu, X.; Wang, M.; Liu, J. Computational Evaluation of Defects in Fe–N4-Doped Graphene for Electrochemical CO2 Reduction. Catalysts 2025, 15, 837. https://doi.org/10.3390/catal15090837
Yu K, Liu X, Wang M, Liu J. Computational Evaluation of Defects in Fe–N4-Doped Graphene for Electrochemical CO2 Reduction. Catalysts. 2025; 15(9):837. https://doi.org/10.3390/catal15090837
Chicago/Turabian StyleYu, Kewei, Xinyu Liu, Meiyan Wang, and Jingyao Liu. 2025. "Computational Evaluation of Defects in Fe–N4-Doped Graphene for Electrochemical CO2 Reduction" Catalysts 15, no. 9: 837. https://doi.org/10.3390/catal15090837
APA StyleYu, K., Liu, X., Wang, M., & Liu, J. (2025). Computational Evaluation of Defects in Fe–N4-Doped Graphene for Electrochemical CO2 Reduction. Catalysts, 15(9), 837. https://doi.org/10.3390/catal15090837






