Pt Nanoparticles Supported on Mesoporous Hollow TiO2@C Sphere Composite as Efficient Methanol Oxidation Reaction Electrocatalysts
Abstract
1. Introduction
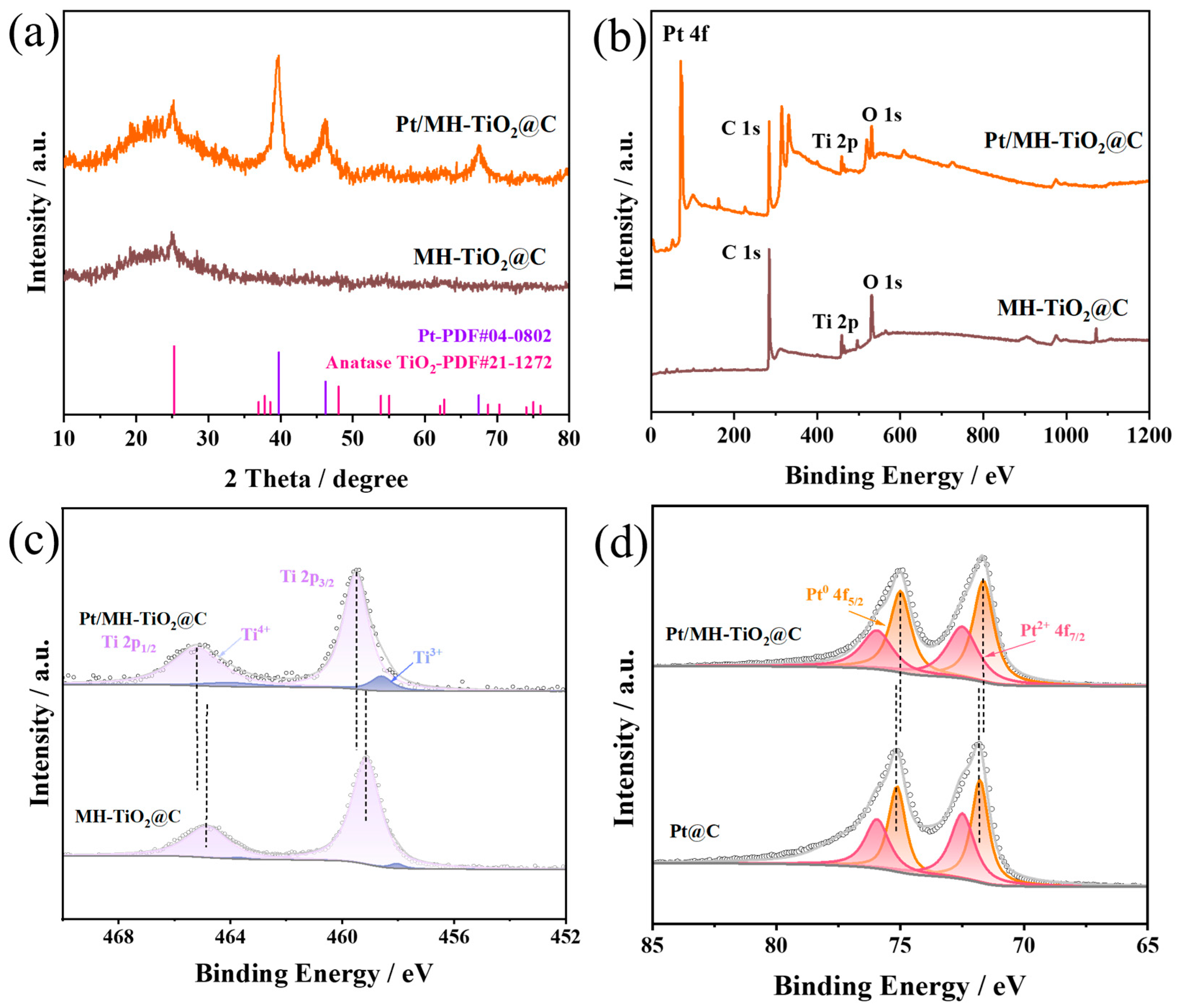
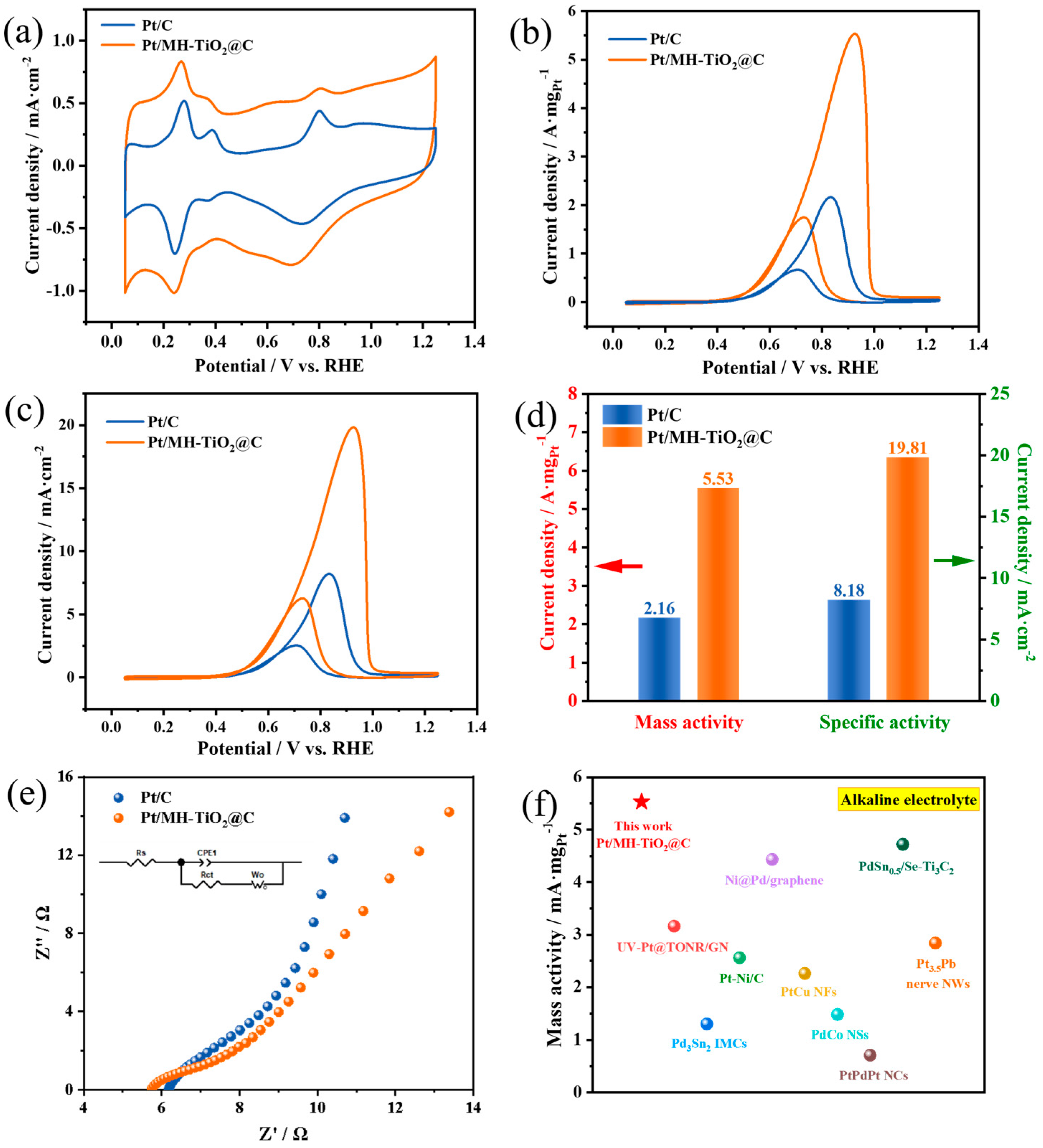
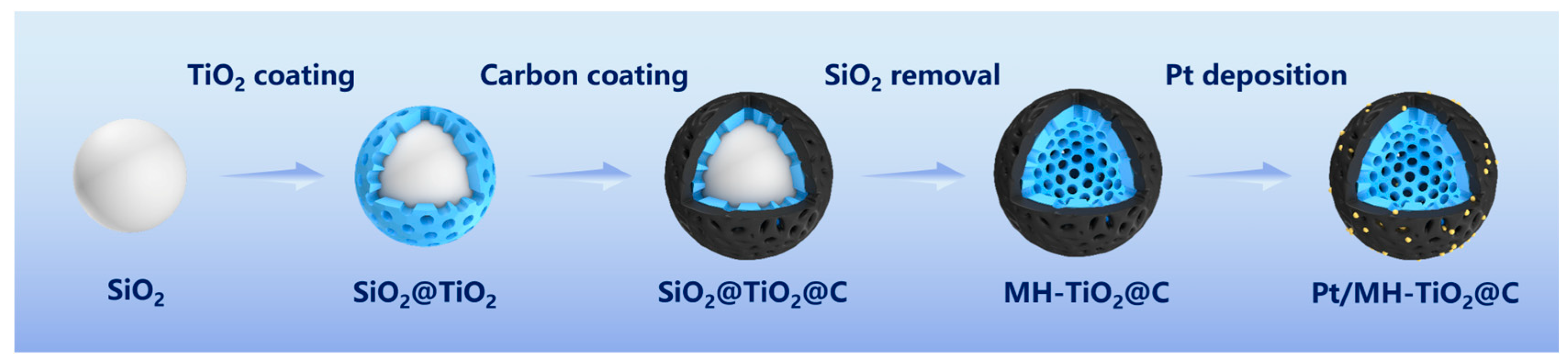
2. Results and Discussion
3. Experimental
3.1. Chemicals and Materials
3.2. Synthesis of Pt/MH-TiO2@C Electrocatalyst
3.3. Physical Characterization
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, K.; Zhang, W.; Bhuvanendran, N.; Ma, Q.; Xu, Q.; Xing, L.; Khotseng, L.; Su, H. Pt-based (Zn, Cu) nanodendrites with enhanced catalytic efficiency and durability toward methanol electro-oxidation via trace Ir-doping engineering. J. Colloid Interface Sci. 2021, 598, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Bhuvanendran, N.; Ravichandran, S.; Zhang, W.; Ma, Q.; Xu, Q.; Xing, L.; Khotseng, L.; Su, H. Bimetallic Pt3Mn nanowire network structures with enhanced electrocatalytic performance for methanol oxidation. Int. J. Hydrogen Energy 2020, 45, 30455–30462. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Li, W.; Bhuvanendran, N.; Liu, H.; Xu, Q.; Hooshyari, K.; Su, H. Ternary PtPdCo mesoporous nanospheres with superior electrocatalytic performance towards methanol oxidation reaction. J. Alloys Compd. 2023, 933, 167706. [Google Scholar] [CrossRef]
- Li, W.; Bhuvanendran, N.; Liu, H.; Zhang, W.; Hooshyari, K.; Lee, S.Y.; Xu, Q.; Su, H. In situ shaped PtPd nanocubes on common carbon powder for efficient methanol electrooxidation in practical fuel cells. Int. J. Hydrogen Energy 2024, 50, 1496–1506. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, S.; Xie, Y.; Ye, Y.; Zou, X.; Lin, S. Synthesis of Pt-Ni (trace)/GNs composite and its bi-functional electrocatalytic properties for MOR and ORR. J. Colloid Interface Sci. 2019, 554, 640–649. [Google Scholar] [CrossRef]
- Menshikov, V.; Paperzh, K.; Bayan, Y.; Beskopylny, Y.; Nikulin, A.; Pankov, I.; Belenov, S. The Development of High-Performance Platinum-Ruthenium Catalysts for the Methanol Oxidation Reaction: Gram-Scale Synthesis, Composition, Morphology, and Functional Characteristics. Catalysts 2022, 12, 1257. [Google Scholar] [CrossRef]
- Shang, H.; Xu, H.; Jin, L.; Chen, C.; Wang, C.; Song, T.; Du, Y. Three-dimensional palladium-rhodium nanosheet assemblies: Highly efficient catalysts for methanol electrooxidation. J. Colloid Interface Sci. 2019, 556, 360–365. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Maillard, F.; Silva, W.O.; Castanheira, L.; Dubau, L.; Lima, F.H.B. Carbon Corrosion in Proton-Exchange Membrane Fuel Cells: Spectrometric Evidence for Pt-Catalysed Decarboxylation at Anode-Relevant Potentials. ChemPhysChem 2019, 20, 3106–3111. [Google Scholar] [CrossRef]
- Tesfu-Zeru, T.; Sakthivel, M.; Drillet, J.F. Investigation of mesoporous carbon hollow spheres as catalyst support in DMFC cathode. Appl. Catal. B Environ. 2017, 204, 173–184. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Ren, Y.; Liu, Z.; Zhang, L.; An, Z.; Jia, Y.; Li, C.; Xu, M.; Zhang, N.; et al. Study on Attenuation Mechanism and Durability Improvement of Platinum-Carbon Catalysts for Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2024, 171, 094509. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Feng, L. Progress of supported Pt-based catalysts for electrochemical methanol energy conversion. Coord. Chem. Rev. 2025, 534, 216603. [Google Scholar] [CrossRef]
- Varela, H.; Paredes-Salazar, E.A.; Lima, F.H.B.; Eid, K. Renewable methanol and the energy challenge: The role of electrocatalysis. Curr. Opin. Electrochem. 2024, 46, 8. [Google Scholar] [CrossRef]
- Lu, Q.; Jin, R.; Huang, T.; Zhu, Y.; Sun, H.; Su, Z.; Ma, F.; Eid, K. Facile one-step synthesis of porous PtPb neural network-like nanowires for electrooxidation of methanol with a CO-poisoning tolerance. Int. J. Hydrogen Energy 2024, 91, 1127–1135. [Google Scholar] [CrossRef]
- Ma, F.; Jin, R.; Zhou, K.; Zhu, Y.; Huang, T.; Lu, Q.; Gai, L.; Liu, L.; Varma, R.S.; Eid, K. Rational one-step synthesis of porous PtAg nanowires for methanol oxidation with a CO-poisoning tolerance: An experimental and theoretical study. Chem. Eng. J. 2024, 492, 151988. [Google Scholar] [CrossRef]
- Qin, C.; Tian, S.; Wang, W.; Jiang, Z.-J.; Jiang, Z. Advances in platinum-based and platinum-free oxygen reduction reaction catalysts for cathodes in direct methanol fuel cells. Front. Chem. 2022, 10, 1073566. [Google Scholar] [CrossRef]
- Lee, J.-M.; Han, S.-B.; Kim, J.-Y.; Lee, Y.-W.; Ko, A.R.; Roh, B.; Hwang, I.; Park, K.-W. TiO2@carbon core-shell nanostructure supports for platinum and their use for methanol electrooxidation. Carbon 2010, 48, 2290–2296. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, T.; Zhou, W.; Huang, Z.; Duan, X.; Yu, M.; Zhou, W.; Lin, F.; Li, D.; Xu, J. Abundant oxygen vacancies Ce-doped TiO2 supported Pt nanoparticles for high-efficiency photoelectrocatalytic methanol oxidation. J. Alloys Compd. 2025, 1022, 179975. [Google Scholar] [CrossRef]
- Xi, J.; Wang, J.; Yu, L.; Qiu, X.; Chen, L. Facile approach to enhance the Pt utilization and CO-tolerance of Pt/C catalysts by physically mixing with transition-metal oxide nanoparticles. Chem. Commun. 2007, 1656–1658. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, W.; Liu, S.; Haruna, S.A.; Zareef, M.; Ahmad, W.; Hassan, M.M.; Li, H.; Chen, Q. A tailorable and recyclable TiO2 NFSF/Ti@Ag NPs SERS substrate fabricated by a facile method and its applications in prohibited fish drugs detection. J. Food Meas. Charact. 2022, 16, 2890–2898. [Google Scholar] [CrossRef]
- Antolini, E. Photo-assisted methanol oxidation on Pt-TiO2 catalysts for direct methanol fuel cells: A short review. Appl. Catal. B Environ. 2018, 237, 491–503. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Li, F.; Liu, C.; Chan, K.-Y.; Feng, X.; Lu, X. Core–shell TiO2/C nanofibers as supports for electrocatalytic and synergistic photoelectrocatalytic oxidation of methanol. J. Mater. Chem. 2012, 22, 4025–4031. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Han, G.; Du, C.; Sun, Y.; Du, L.; An, M.; Yin, G.; Gao, Y.; Song, Y. Superior catalytic performance and CO tolerance of Ru@Pt/C-TiO2 electrocatalyst toward methanol oxidation reaction. Appl. Surf. Sci. 2019, 473, 943–950. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Li, H.; Guo, P.; Li, Y.; Ji, D.; Zhao, X. Carbon-Supported PdCu Alloy as Extraordinary Electrocatalysts for Methanol Electrooxidation in Alkaline Direct Methanol Fuel Cells. Nanomaterials 2022, 12, 4210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, Y.; Tan, M.; Liu, H.; Ma, Q.; Xu, Q.; Su, H. TiO2 Nanolayer–Coated Carbon as Pt Support for Enhanced Methanol Oxidation Reaction. J. Electrochem. Energy Convers. Storage 2024, 21, 041006. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Z.; Liu, X.; Li, T. N Simultaneously Doped TiO2@Carbon Hollow Spheres with Enhanced Photocatalytic CO2 Reduction Activity. Catalysts 2025, 15, 39. [Google Scholar] [CrossRef]
- Qian, H.; Tang, J.; Hossain, M.S.A.; Bando, Y.; Wang, X.; Yamauchi, Y. Localization of platinum nanoparticles on inner walls of mesoporous hollow carbon spheres for improvement of electrochemical stability. Nanoscale 2017, 9, 16264–16272. [Google Scholar] [CrossRef]
- Han, S.; Xie, H.; Zhang, L.; Wang, X.; Zhong, Y.; Shen, Y.; Wang, H.; Hao, C. High-performance polyethylenimine-functionalized lignin/silica porous composite microsphere for the removal of hexavalent chromium, phosphate and Congo red from aqueous solutions. Ind. Crops Prod. 2023, 194, 116289. [Google Scholar] [CrossRef]
- Chao, Y.; Pang, J.; Bai, Y.; Wu, P.; Luo, J.; He, J.; Jin, Y.; Li, X.; Xiong, J.; Li, H.; et al. Graphene-like BN@SiO2 nanocomposites as efficient sorbents for solid-phase extraction of Rhodamine B and Rhodamine 6G from food samples. Food Chem. 2020, 320, 126666. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhang, M.; Yang, Y.; Din, Z.-u.; Chen, Q. Mesoporous silica-modified upconversion biosensor coupled with real-time ion release properties for ultrasensitive detection of Staphylococcus aureus in meat. Food Control 2023, 145, 109444. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Yan, Y. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 104427. [Google Scholar] [CrossRef]
- Yang, F.; Lv, K.; Zhao, X.; Kong, D.; Kong, N.; Luo, Z.; Tao, J.; Zhou, J.; Razal, J.M.; Zhang, J. Hierarchical heterostructures of MXene and mesoporous hollow carbon sphere for improved ion accessibility and rate performance. Chem. Eng. J. 2024, 494, 153246. [Google Scholar] [CrossRef]
- Wang, F.; Feng, L.; Qin, Y.; Zhao, T.; Luo, H.; Zhu, J. Dual functional SiO2@TiO2 photonic crystals for dazzling structural colors and enhanced photocatalytic activity. J. Mater. Chem. C 2019, 7, 11972–11983. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Tan, M.; Liu, H.; Ma, Q.; Xu, Q.; Pollet, B.G.; Su, H. Electrodeposited platinum with various morphologies on carbon paper as efficient and durable self-supporting electrode for methanol and ammonia oxidation reactions. Int. J. Hydrogen Energy 2023, 48, 2617–2627. [Google Scholar] [CrossRef]
- He, S.; Wu, C.; Sun, Z.; Liu, Y.; Hu, R.; Guan, L.; Zhan, H. Uniform Pt nanoparticles supported on urchin-like mesoporous TiO2 hollow spheres as stable electrocatalysts for the oxygen reduction reaction. Nanoscale 2020, 12, 10656–10663. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, E.; Lee, G.; Tak, Y. Superior durability and stability of Pt electrocatalyst on N-doped graphene-TiO2 hybrid material for oxygen reduction reaction and polymer electrolyte membrane fuel cells. Appl. Catal. B Environ. 2020, 268, 118414. [Google Scholar] [CrossRef]
- Hsieh, B.-J.; Tsai, M.-C.; Pan, C.-J.; Su, W.-N.; Rick, J.; Chou, H.-L.; Lee, J.-F.; Hwang, B.-J. Tuning metal support interactions enhances the activity and durability of TiO2-supported Pt nanocatalysts. Electrochim. Acta 2017, 224, 452–459. [Google Scholar] [CrossRef]
- Luo, Z.; Han, X.; Ma, Z.; Zhang, B.; Zheng, X.; Liu, Y.; Gao, M.; Zhao, G.; Lin, Y.; Pan, H.; et al. Unraveling the Unique Strong Metal-Support Interaction in Titanium Dioxide Supported Platinum Clusters for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2024, 63, e202406728. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Zheng, X.; Wu, R.; Song, J.; Chen, Y.; Cao, X.; Nie, Y. Renovating phase constitution and construction of Pt nanocubes for electrocatalysis of methanol oxidation via a solvothermal-induced strong metal-support interaction. Appl. Catal. B Environ. 2023, 325, 122383. [Google Scholar] [CrossRef]
- Ruiz-Camacho, B.; Medina-Ramirez, A.; Villicana Aguilera, M.; Minchaca-Mojica, J.I. Pt supported on mesoporous material for methanol and ethanol oxidation in alkaline medium. Int. J. Hydrogen Energy 2019, 44, 12365–12373. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Gao, J.; Zhang, Y.; Lu, Q.; Liu, M. Hollow porous carbon spheres with hierarchical nanoarchitecture for application of the high performance supercapacitors. Electrochim. Acta 2016, 211, 183–192. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Li, Z.; Huang, X.; Zou, X.; Tan, W.; Zhang, X.; Hu, X.; Wang, X.; et al. Impedimetric aptasensor based on highly porous gold for sensitive detection of acetamiprid in fruits and vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Zou, X.; Li, Y.; Tahir, H.E.; Huang, X.; Li, Z.; Zhai, X.; Hu, X. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem. 2017, 237, 423–430. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, P.; Huang, Y.; Zhang, G.; Lu, C.; Jiang, X. Application Status and Prospect of Impedance Spectroscopy in Agricultural Product Quality Detection. Agriculture 2022, 12, 1525. [Google Scholar] [CrossRef]
- Qin, C.; Guo, W.; Liu, Y.; Liu, Z.; Qiu, J.; Peng, J. A Novel Electrochemical Sensor Based on Graphene Oxide Decorated with Silver Nanoparticles–Molecular Imprinted Polymers for Determination of Sunset Yellow in Soft Drinks. Food Anal. Methods 2017, 10, 2293–2301. [Google Scholar] [CrossRef]
- Chen, S.; Liu, N.; Zhong, J.; Yang, R.; Yan, B.; Gan, L.; Yu, P.; Gui, X.; Yang, H.; Yu, D.; et al. Engineering Support and Distribution of Palladium and Tin on MXene with the Modulation d-Band Center for CO-resilient Methanol Oxidation. Angew. Chem. Int. Ed. 2022, 134, e202209693. [Google Scholar] [CrossRef]
- Huang, L.; Han, Y.; Zhang, X.; Fang, Y.; Dong, S. One-step synthesis of ultrathin PtxPb nerve-like nanowires as robust catalysts for enhanced methanol electrooxidation. Nanoscale 2017, 9, 201–207. [Google Scholar] [CrossRef]
- Li, L.; Gao, W.; Wan, X.; Wen, D. Pt Nanoparticles Dispersed on Ni/C Nanoflowers as Stable Electrocatalysts for Methanol Oxidation and Oxygen Reduction. ACS Appl. Nano Mater. 2021, 4, 10960–10968. [Google Scholar] [CrossRef]
- Sheng, G.; Chen, J.; Ye, H.; Hu, Z.; Fu, X.-Z.; Sun, R.; Huang, W.; Wong, C.-P. Hollow PdCo alloy nanospheres with mesoporous shells as high-performance catalysts for methanol oxidation. J. Colloid Interface Sci. 2018, 522, 264–271. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, W.; Zhang, F.; Xia, J.; Gong, S.; Xia, Y. Facile synthesis of PtPdPt nanocatalysts for methanol oxidation in alkaline solution. Electrochim. Acta 2016, 192, 400–406. [Google Scholar] [CrossRef]
- Xue, J.; Hu, Z.; Li, H.; Zhang, Y.; Liu, C.; Li, M.; Yang, Q.; Hu, S. Pd-Sn alloy nanoparticles for electrocatalytic methanol oxidation: Phase evolution from solid solution to intermetallic compounds. Nano Res. 2022, 15, 8819–8825. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Wu, J.; Deng, Y.; Wu, Y.; Yan, L. Morphological tuning engineering of Pt@TiO2 graphene catalysts with optimal active surfaces of support for boosting catalytic performance for methanol oxidation. J. Mater. Chem. A 2022, 10, 4254–4265. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Z.; Sun, Q.; Xie, J.; Jing, J. Synthetic core-shell Ni@Pd nanoparticles supported on graphene and used as an advanced nanoelectrocatalyst for methanol oxidation. New J. Chem. 2012, 36, 2533–2540. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Chen, B.; Wei, C.; Zhao, L.; Chen, J.; Zhang, X.; Lai, Z.; Fan, Z.; Tan, C.; et al. One-Pot Synthesis of Highly Anisotropic Five-Fold-Twinned PtCu Nanoframes Used as a Bifunctional Electrocatalyst for Oxygen Reduction and Methanol Oxidation. Adv. Mater. 2016, 28, 8712–8717. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Li, P.; Ibraheem, S.; Li, J.; Deng, J.; Wei, Z. Surface Ru enriched structurally ordered intermetallic PtFe@PtRuFe core-shell nanostructure boosts methanol oxidation reaction catalysis. Appl. Catal. B Environ. 2019, 252, 120–127. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.; Mao, Y.; Wang, Y. Fabrication of a core-shell-shell magnetic polymeric microsphere with excellent performance for separation and purification of bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, H.; Ma, Q.; Li, Z.; Lu, M.; Su, H.; Zhang, W.; Xu, Q. Pt Nanoparticles Supported on Mesoporous Hollow TiO2@C Sphere Composite as Efficient Methanol Oxidation Reaction Electrocatalysts. Catalysts 2025, 15, 834. https://doi.org/10.3390/catal15090834
Chen Y, Liu H, Ma Q, Li Z, Lu M, Su H, Zhang W, Xu Q. Pt Nanoparticles Supported on Mesoporous Hollow TiO2@C Sphere Composite as Efficient Methanol Oxidation Reaction Electrocatalysts. Catalysts. 2025; 15(9):834. https://doi.org/10.3390/catal15090834
Chicago/Turabian StyleChen, Yuan, Huiyuan Liu, Qiang Ma, Zhuo Li, Mengyue Lu, Huaneng Su, Weiqi Zhang, and Qian Xu. 2025. "Pt Nanoparticles Supported on Mesoporous Hollow TiO2@C Sphere Composite as Efficient Methanol Oxidation Reaction Electrocatalysts" Catalysts 15, no. 9: 834. https://doi.org/10.3390/catal15090834
APA StyleChen, Y., Liu, H., Ma, Q., Li, Z., Lu, M., Su, H., Zhang, W., & Xu, Q. (2025). Pt Nanoparticles Supported on Mesoporous Hollow TiO2@C Sphere Composite as Efficient Methanol Oxidation Reaction Electrocatalysts. Catalysts, 15(9), 834. https://doi.org/10.3390/catal15090834








