Algae to Biofuels: Catalytic Strategies and Sustainable Technologies for Green Energy Conversion
Abstract
1. Introduction
2. Algal Biomass: Composition and Biofuel Potential
2.1. Types of Algae
2.2. Advantages over Other Biomass Sources
3. Cultivation and Harvesting of Algae
3.1. Cultivation Systems
3.1.1. Open Raceway Ponds
3.1.2. Closed Photobioreactors
3.1.3. Wastewater-Based Cultivation
3.2. Growth Conditions and Nutrient Requirements
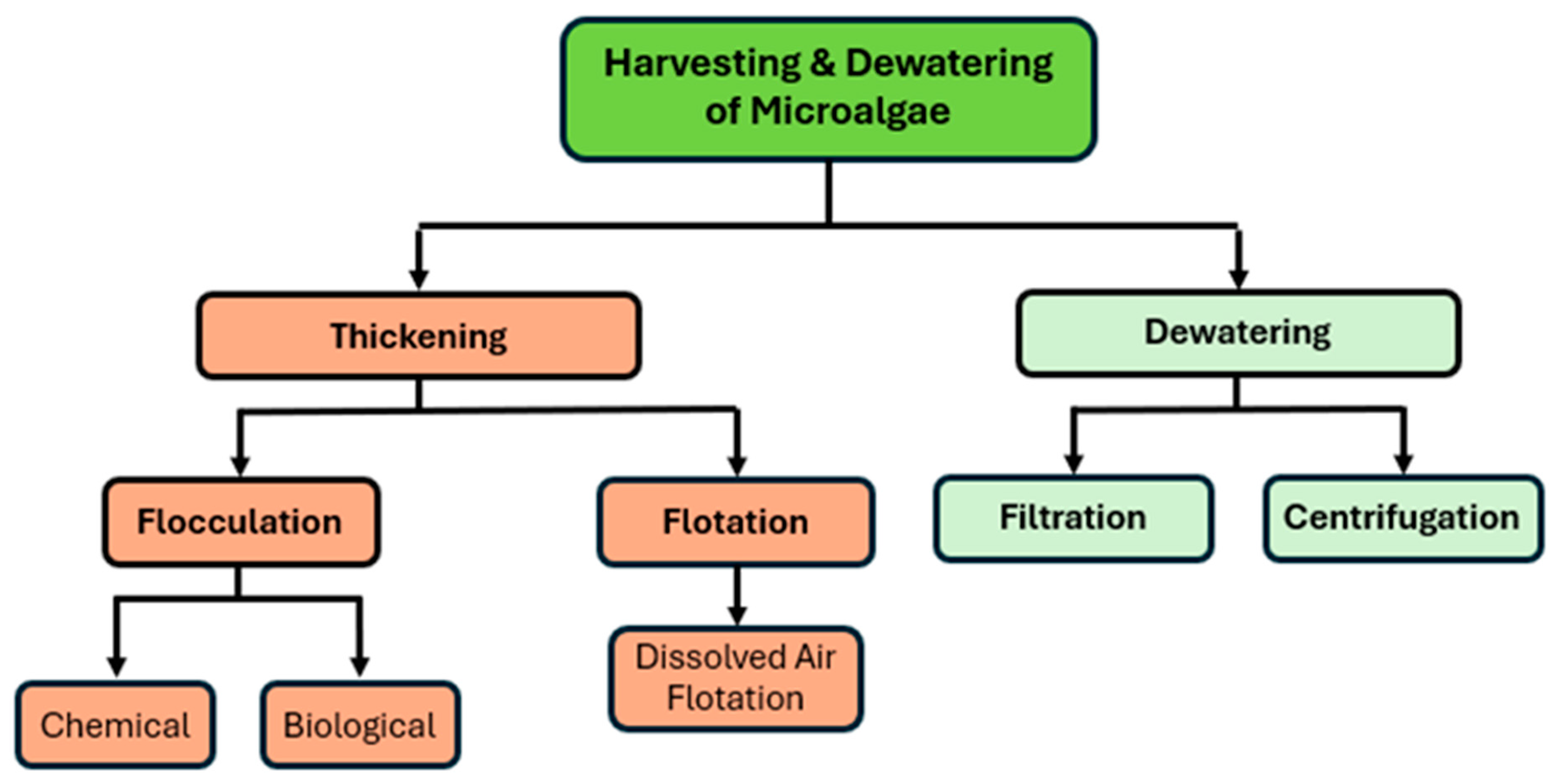
3.3. Harvesting and Dewatering Techniques
3.3.1. Filtration
3.3.2. Centrifuge
3.3.3. Flocculation
3.3.4. Flotation
| Method | TRL | Dryness (%Solids) | Typical Capacity | Process Limitations | References |
|---|---|---|---|---|---|
| Centrifugation | 8–9 | 10–25% | 1–100 m3/h | Energy-intensive, costly for large volumes, shear-sensitive | [62,63] |
| Flocculation | 6–8 | 1–5% | >100 m3/day | Requires bio-flocculants, risk of contamination | [64,65] |
| Filtration | 6–7 | 10–20% | 5–50 m3/h | Membrane fouling, limited to large cells, high maintenance | [59,60] |
| Dissolved Air Flotation | 6–7 | 1–5% | 10–50 m3/h | Need additional flocculants | [69] |
3.4. Process Set up and Integral Energy Balance
4. Conversion Pathways for Algal Biofuels
4.1. Lipid Extraction and Transesterification
4.2. Thermochemical Conversion
4.3. Biochemical Conversion
5. Catalytic Strategies in Algal Biofuel Production
5.1. Heterogeneous Catalysis
5.2. Homogeneous Catalysis
5.3. Emerging Trends
5.3.1. Photocatalysis
5.3.2. Electrocatalysis
5.4. Multicriteria Evaluation of Catalytic Pathways
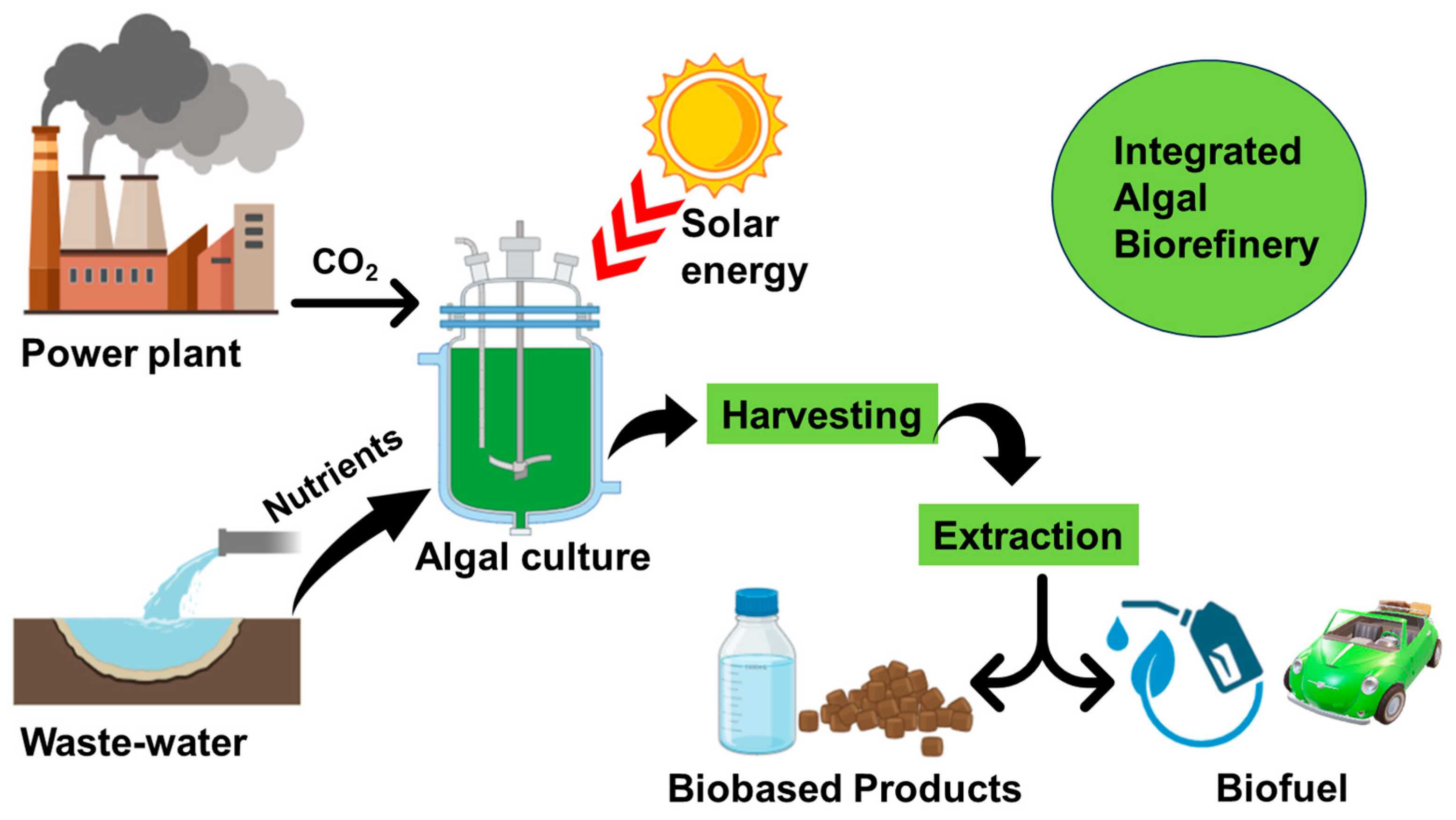
6. Integrated Algal Biorefineries
6.1. Concept and Design of Algal Biorefineries
6.2. Valorization of Co-Products (Proteins, Pigments, Fertilizers)
6.3. Energy and Economic Optimization
6.4. Life Cycle Assessment (LCA) and Sustainability Metrics
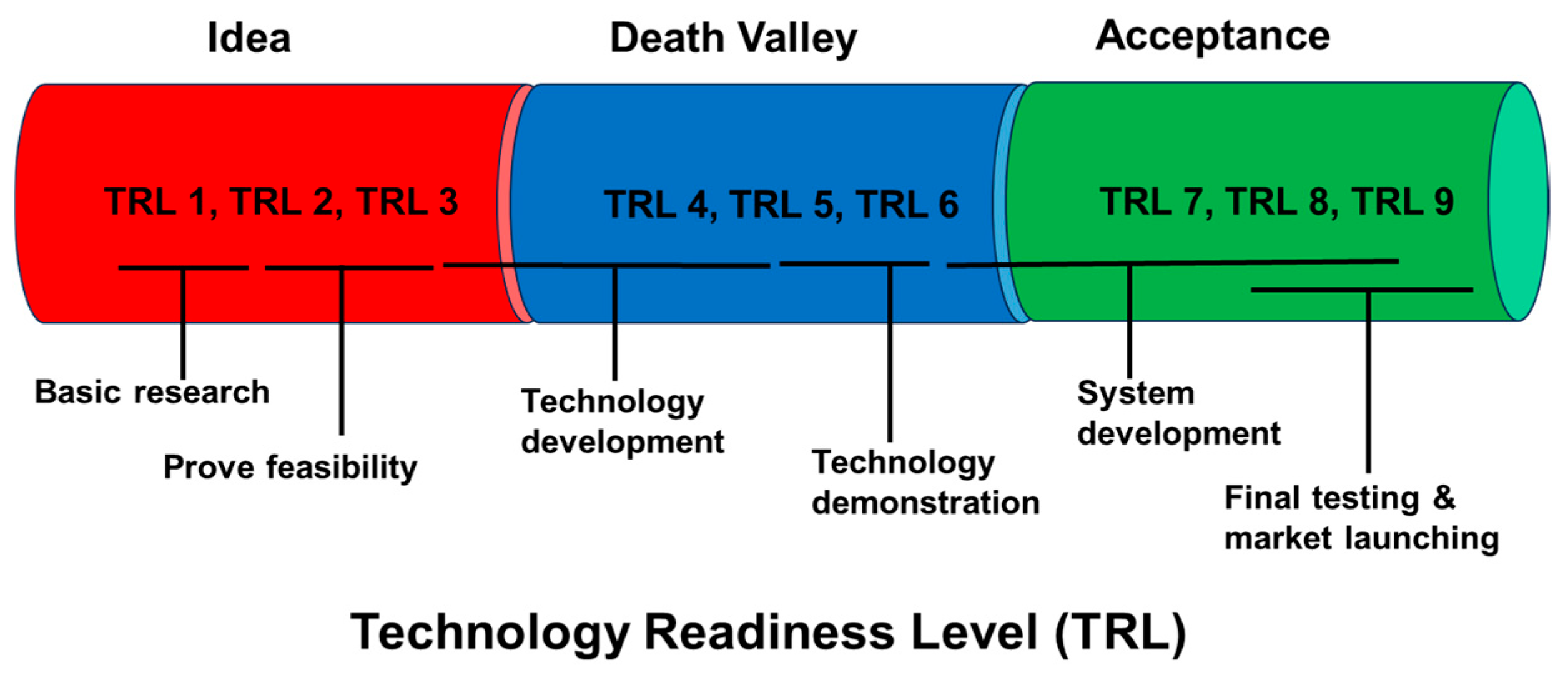
6.5. Pilot Installation, and Technology Readiness Level
7. Recent Advances in Sustainable Technologies
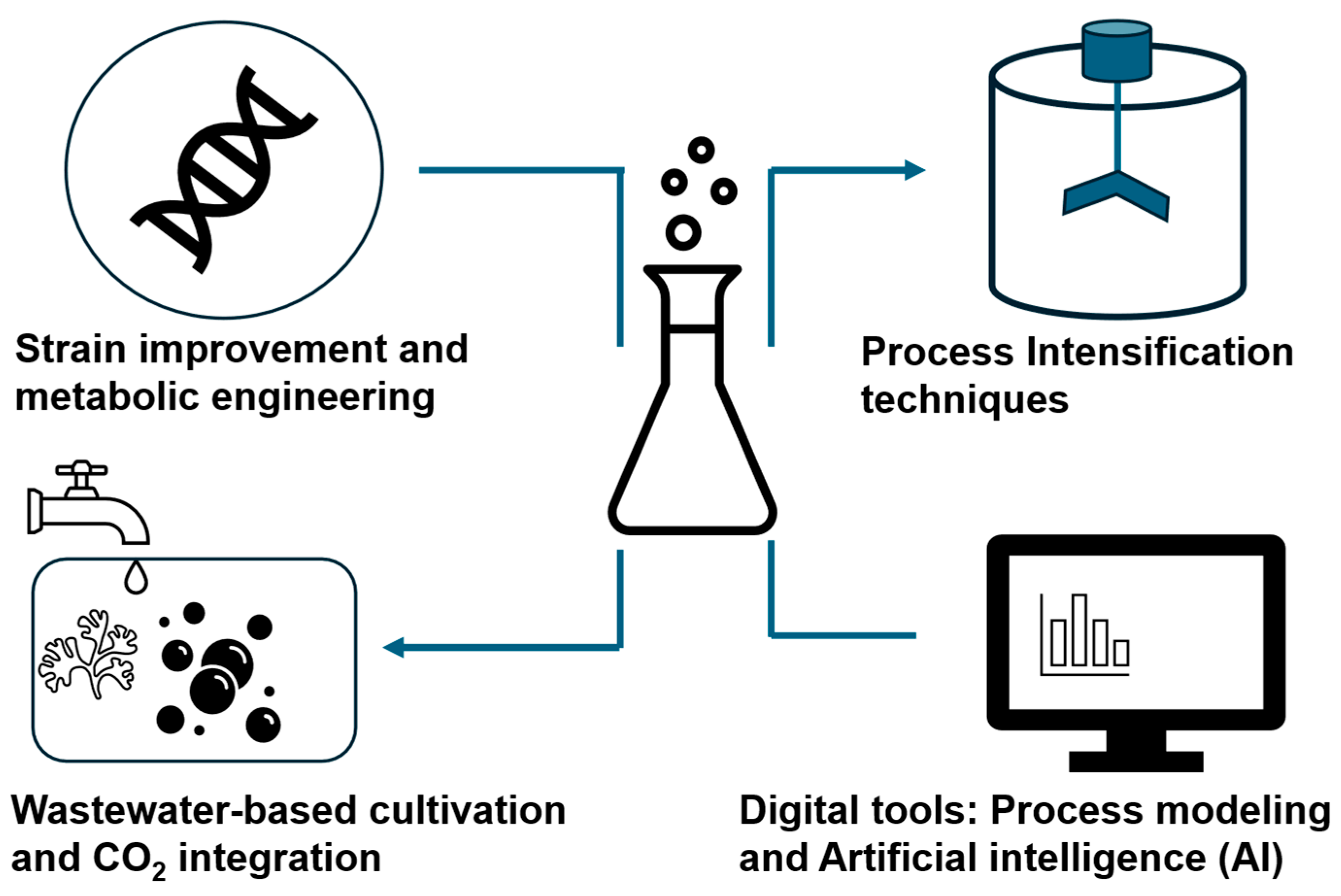
7.1. Strain Improvement and Metabolic Engineering
7.2. Process Intensification Techniques
7.3. Wastewater-Based Cultivation and CO2 Integration
7.4. Digital Tools: Process Modeling and Artificial Intelligence
8. Policy, Regulation, and Market Outlook
8.1. Global Policies Supporting Algal Biofuel Development
8.2. Subsidies, Incentives, and Carbon Credits
8.3. Market Trends and Commercialization Prospects
9. Challenges and Future Perspectives
9.1. Major Bottlenecks: Cost, Energy Input, and Scalability
9.2. Future R&D Directions: Synthetic Biology and Hybrid Technologies
9.3. Roadmap for Commercialization
10. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| ALA | Alpha Linolenic |
| ANN | Artificial Neural Network |
| BG-11 | Blue Green-11 |
| COD | Chemical Oxygen Demand |
| CI | Carbon Intensity |
| COE | Conventional Organic Solvent Extraction |
| DAF | Dissolved Air Flotation |
| DHA | Decosahexaenoic |
| ECH | Electrochemical Hydrogenation |
| EPA | Eicosapentaenoic |
| EPE | Electric Pulse Extraction |
| FAEE | Fatty Acid Ethyl Ester |
| FAME | Fatty Acid Methyl Ester |
| FFA | Free Fatty Acid |
| GA | Genetic Algorithm |
| GHG | Greenhouse Gas |
| GREET | Greenhouse Gases, Regulated Emissions and Energy Use in Transportation |
| GWP | Global Warming Potential |
| HDO | Hydrodeoxygenation |
| HMF | hydromehtylfurfural |
| HTL | Hyhdrothermal Liquefaction |
| HRAP | High-Rate Algal Pond |
| HZSM-5 | H-type Zeolite Socony Mobil-5 |
| IABR | Integrated Algal Biorefinery |
| LCA | Life Cycle Assessment |
| LCFS | Low-Carbon Fuel Standard |
| MAE | Microwave-Assisted Extraction |
| MCDA | Multicriteria Decision Analysis |
| MCE | Multicriteria Evaluation |
| ML | Machine Learning |
| MRV | Measurement, Reporting, and Verification |
| NER | Net Energy Ratio |
| PBR | Photobioreactor |
| PES | Polyethersulfone |
| PVDF | Polyvinylidene Fluoride |
| RFS | Renewable Fuel Standard |
| RIN | Renewable Identification Number |
| SAF | Sustainable Activation Fuel |
| SFE | Supercritical Fluid Extraction |
| TRL | Technology Readiness Level |
| UAE | Ultrasound-Assisted Extraction |
| VS | Volatile Solid |
References
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Banu, J.R.; Kumar, M.D.; Kumar, S.A.; Kumar, G. Algal Biorefinery towards Decarbonization: Economic and Environmental Consideration. Bioresour. Technol. 2022, 364, 128103. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Hussain, N.; Shahbaz, A.; Mulla, S.I.; Iqbal, H.M.N.; Bilal, M. Sustainable Production of Biofuels from the Algae-Derived Biomass. Bioprocess Biosyst. Eng. 2023, 46, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wołejko, E.; Ernazarovna, M.D.; Głowacka, A.; Sokołowska, G.; Wydro, U. Using Algae for Biofuel Production: A Review. Energies 2023, 16, 1758. [Google Scholar] [CrossRef]
- Feng, S.; Kang, K.; Salaudeen, S.; Ahmadi, A.; He, Q.S.; Hu, Y. Recent Advances in Algae-Derived Biofuels and Bioactive Compounds. Ind. Eng. Chem. Res. 2022, 61, 1232–1249. [Google Scholar] [CrossRef]
- Gautam, R.; Vinu, R. Reaction Engineering and Kinetics of Algae Conversion to Biofuels and Chemicals via Pyrolysis and Hydrothermal Liquefaction. React. Chem. Eng. 2020, 5, 1320–1373. [Google Scholar] [CrossRef]
- Hasnain, S.M.W.U.; Farooqi, A.S.; Singh, O.; Ayuni, N.H.; Ayodele, B.V.; Abdullah, B. Response Surface Optimization of Hydrogen-Rich Syngas Production by the Catalytic Valorization of Greenhouse Gases (CH4 and CO2) over Sr-Promoted Ni/SBA-15 Catalyst. Energy Convers. Manag. X 2023, 20, 100451. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Kim, W.; Karmegam, N.; Govarthanan, M. Advanced Thermochemical Conversion of Algal Biomass to Liquid and Gaseous Biofuels: A Comprehensive Review of Recent Advances. Sustain. Energy Technol. Assess. 2022, 52, 102211. [Google Scholar] [CrossRef]
- Sahu, S.; Kunj, P.; Kaur, A.; Khatri, M.; Singh, G.; Arya, S.K. Catalytic Strategies for Algal-Based Carbon Capture and Renewable Energy: A Review on a Sustainable Approach. Energy Convers. Manag. 2024, 310, 118467. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.K.; Mascarenhas, R.J.; Shetti, N.P.; Aminabhavi, T.M. Recent Advances and Viability in Biofuel Production. Energy Convers. Manag. X 2021, 10, 100070. [Google Scholar] [CrossRef]
- Chen, P.H.; Quinn, J.C. Microalgae to Biofuels through Hydrothermal Liquefaction: Open-Source Techno-Economic Analysis and Life Cycle Assessment. Appl. Energy 2021, 289, 116613. [Google Scholar] [CrossRef]
- Salles, B.D.C.; Souza, D.D. Interdisciplinary Approach to Microalgae Production in Partnerships Around the World. In Partnerships for the Goals; Filho, W.L., Azul, A.M., Brandli, L., Salvia, A.L., Wall, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 610–623. [Google Scholar] [CrossRef]
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-Term Anaerobic Digestion of Microalgae Grown in HRAP for Wastewater Treatment. Effect of Microwave Pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Amalapridman, V.; Ofori, P.A.; Abbey, L. Valorization of Algal Biomass to Biofuel: A Review. Biomass 2025, 5, 26. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D. Classification of Algae. In The Algae World; Sahoo, D., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 26, pp. 31–55. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Wychen, S.V.; Rowland, S.M.; Lesco, K.C.; Shanta, P.V.; Dong, T.; Laurens, L.M.L. Advanced Mass Balance Characterization and Fractionation of Algal Biomass Composition. J. Appl. Phycol. 2021, 33, 2695–2708. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Wychen, S.V.; McAllister, J.P.; Arrowsmith, S.; Dempster, T.A.; McGowen, J.; Pienkos, P.T. Strain, Biochemistry, and Cultivation-Dependent Measurement Variability of Algal Biomass Composition. Anal. Biochem. 2014, 452, 86–95. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Rosa, M.D.H.D.; Alves, C.J.; Santos, F.N.D.; Souza, A.O.D.; Zavareze, E.D.R.; Pinto, E.; Noseda, M.D.; Ramos, D.; Pereira, C.M.P.D. Macroalgae and Microalgae Biomass as Feedstock for Products Applied to Bioenergy and Food Industry: A Brief Review. Energies 2023, 16, 1820. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen Stress Triggered Biochemical and Morphological Changes in the Microalgae Scenedesmus Sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of Microalgae for Primary Metabolites Including β-Glucans and the Influence of Nitrate Starvation and Irradiance on β-Glucan Production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R.; Hvilsted, S. Comparison of Lignin, Macroalgae, Wood, and Straw Fast Pyrolysis. Energy Fuels 2013, 27, 1399–1409. [Google Scholar] [CrossRef]
- Langholtz, M.H.; Coleman, A.M.; Eaton, L.M.; Wigmosta, M.S.; Hellwinckel, C.M.; Brandt, C.C. Potential Land Competition between Open-Pond Microalgae Production and Terrestrial Dedicated Feedstock Supply Systems in the U.S. Renew. Energy 2016, 93, 201–214. [Google Scholar] [CrossRef]
- Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae Biomass and Lipids as Feedstock for Biofuels: Sustainable Biotechnology Strategies. Sustainability 2022, 14, 15070. [Google Scholar] [CrossRef]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae Cultivation in Wastewater. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–91. [Google Scholar] [CrossRef]
- Yen, H.; Ho, S.; Chen, C.; Chang, J. CO2, NOx and SOx Removal from Flue Gas via Microalgae Cultivation: A Critical Review. Biotechnol. J. 2015, 10, 829–839. [Google Scholar] [CrossRef]
- Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, F. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies 2018, 11, 664. [Google Scholar] [CrossRef]
- Anand, A.; Tripathi, K.; Kumar, A.; Gupta, S.; Raghuvanshi, S.; Verma, S.K. Bio-Mitigation of Carbon Dioxide Using Desmodesmus Sp. in the Custom-Designed Pilot-Scale Loop Photobioreactor. Sustainability 2021, 13, 9882. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Vu, M.T.; Vu, H.P.; Johir, M.A.H.; Labeeuw, L.; Ralph, P.J.; Mahlia, T.M.I.; Pandey, A.; Sirohi, R.; Nghiem, L.D. Microalgae-Based Carbon Capture and Utilization: A Critical Review on Current System Developments and Biomass Utilization. Crit. Rev. Environ. Sci. Technol. 2023, 53, 216–238. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Moheimani, N.R. Open Pond Culture Systems. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 133–152. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent Advancements in Photo-Bioreactors for Microalgae Cultivation: A Brief Overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of Light Intensity, Light Duration and Photoperiods in the Performance of an Outdoor Photobioreactor for Urban Wastewater Treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Park, J.; Ralph, P.J.; Craggs, R.J. Improved Microalgal Productivity and Nutrient Removal through Operating Wastewater High Rate Algal Ponds in Series. Algal Res. 2020, 47, 101850. [Google Scholar] [CrossRef]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative Energy Life-Cycle Analyses of Microalgal Biomass Production in Open Ponds and Photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Asadollahzadeh, M.J.; Ardjmand, M.; Seafkordi, A.A.; Heydarian, S.M. Efficient Storage and Utilization of CO2 in Open Raceway Ponds for Cultivation of Microalgae. Korean J. Chem. Eng. 2014, 31, 1425–1432. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Long Term Outdoor Operation of a Tubular Airlift Pilot Photobioreactor and a High Rate Algal Pond as Tertiary Treatment of Urban Wastewater. Ecol. Eng. 2013, 52, 143–153. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent Advances in CO2 Fixation by Microalgae and Its Potential Contribution to Carbon Neutrality. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef] [PubMed]
- Mehlitz, T.H. Temperature Influence and Heat Management Requirements of Microalgae Cultivation in Photobioreactors. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2009. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current Status and Challenges on Microalgae-Based Carbon Capture. Int. J. Greenh. Gas Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Wang, J.; Liu, T. Biomass Productivity of Scenedesmus dimorphus (Chlorophyceae) Was Improved by Using an Open Pond—Photobioreactor Hybrid System. Eur. J. Phycol. 2019, 54, 127–134. [Google Scholar] [CrossRef]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Vargas, J.E.O.; Weuster-Botz, D. Lab-Scale Photobioreactor Systems: Principles, Applications, and Scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef]
- Bender, A. Cost and Benefit Analysis of Implementing Photobioreactor System for Self-Sustainable Electricity Production from Algae. Master’s Thesis, University of Technology Sydney, Ultimo, Australia, 2017. Unpublished. [Google Scholar] [CrossRef]
- Hu, X.; Jalalah, M.; Wu, J.; Zheng, Y.; Li, X.; Salama, E.-S. Microalgal Growth Coupled with Wastewater Treatment in Open and Closed Systems for Advanced Biofuel Generation. Biomass Conv. Bioref. 2022, 12, 1939–1958. [Google Scholar] [CrossRef]
- Marchese, A.; Lima, S.; Cosenza, A.; Giambalvo, F.; Scargiali, F. Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design. Processes 2025, 13, 1159. [Google Scholar] [CrossRef]
- Wang, S.; Stiles, A.R.; Guo, C.; Liu, C. Microalgae Cultivation in Photobioreactors: An Overview of Light Characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Tian, X.; Lin, X.; Xie, Q.; Liu, J.; Luo, L. Effects of Temperature and Light on Microalgal Growth and Nutrient Removal in Turtle Aquaculture Wastewater. Biology 2024, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae Culture Quality Indicators: A Review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. The Effects of Light and Temperature on Microalgal Growth and Nutrient Removal: An Experimental and Mathematical Approach. RSC Adv. 2016, 6, 22896–22907. [Google Scholar] [CrossRef]
- Chantarasiri, A.; Ungwiwatkul, S. Effects of CO2 Aeration and Light Supply on the Growth and Lipid Production of a Locally Isolated Microalga, Chlorella Variabilis RSM09. Appl. Sci. 2024, 14, 10512. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P Ratio on Biomass Productivity and Nutrient Removal from Municipal Wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Rios, L.; Klein, B.C.; Luz, L., Jr.; Maciel, M.R.W.; Filho, R.M. Influence of Culture Medium on Desmodesmus Sp. Growth and Lipid Accumulation for Biodiesel Production. Chem. Eng. Trans. 2015, 43, 601–606. [Google Scholar] [CrossRef]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The Techno-Economic Potential of Renewable Energy through the Anaerobic Digestion of Microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; García-Depraect, O. Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes 2021, 11, 585. [Google Scholar] [CrossRef]
- Zou, S.; Gu, Y.; Xiao, D.; Tang, C.Y. The Role of Physical and Chemical Parameters on Forward Osmosis Membrane Fouling during Algae Separation. J. Membr. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Kim, K.; Shin, H.; Moon, M.; Ryu, B.-G.; Han, J.-I.; Yang, J.-W.; Chang, Y.K. Evaluation of Various Harvesting Methods for High-Density Microalgae, Aurantiochytrium Sp. KRS101. Bioresour. Technol. 2015, 198, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Dassey, A.J.; Theegala, C.S. Harvesting Economics and Strategies Using Centrifugation for Cost Effective Separation of Microalgae Cells for Biodiesel Applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef]
- Divakaran, R.; Sivasankara Pillai, V.N. Flocculation of Algae Using Chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Gerde, J.A.; Yao, L.; Lio, J.; Wen, Z.; Wang, T. Microalgae Flocculation: Impact of Flocculant Type, Algae Species and Cell Concentration. Algal Res. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Manheim, D.; Nelson, Y. Settling and Bioflocculation of Two Species of Algae Used in Wastewater Treatment and Algae Biomass Production. Environ. Prog. Sustain. Energy 2013, 32, 946–954. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A Comparative Study between Fungal Pellet- and Spore-Assisted Microalgae Harvesting Methods for Algae Bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, J.C.; Ju, Y.-H. Flotation Removal of Algae from Water. Colloids Surf. B Biointerfaces 1998, 12, 49–55. [Google Scholar] [CrossRef]
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. Surfactants as Bubble Surface Modifiers in the Flotation of Algae: Dissolved Air Flotation That Utilizes a Chemically Modified Bubble Surface. Environ. Sci. Technol. 2008, 42, 4883–4888. [Google Scholar] [CrossRef]
- Gao, S.; Yang, J.; Tian, J.; Ma, F.; Tu, G.; Du, M. Electro-Coagulation–Flotation Process for Algae Removal. J. Hazard. Mater. 2010, 177, 336–343. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Zittelli, G.C.; Sampietro, G. Energy Balance of Algal Biomass Production in a 1-Ha “Green Wall Panel” Plant: How to Produce Algal Biomass in a Closed Reactor Achieving a High Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Ghedini, E.; Taghavi, S.; Menegazzo, F.; Signoretto, M. A Review on the Efficient Catalysts for Algae Transesterification to Biodiesel. Sustainability 2021, 13, 10479. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild Disintegration of the Green Microalgae Chlorella Vulgaris Using Bead Milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Singh, R.K. Biofuel and Co-Products from Algae Solvent Extraction. J. Environ. Manag. 2019, 247, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of Carotenoids and Lipids from Algae by Supercritical CO2 and Subcritical Dimethyl Ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Maicaneanu, S.A.; Roman, C. Advances in Biodiesel Production from Microalgae. Energies 2023, 16, 1129. [Google Scholar] [CrossRef]
- Hariram, V.; John, J.G.; Seralathan, S. Spectrometric Analysis of Algal Biodiesel as a Fuel Derived through Base-Catalysed Transesterification. Int. J. Ambient Energy 2019, 40, 195–202. [Google Scholar] [CrossRef]
- Viegas, C.V.; Hachemi, I.; Mäki-Arvela, P.; Smeds, A.; Aho, A.; Freitas, S.P.; Gorgônio, C.M.D.S.; Carbonetti, G.; Peurla, M.; Paranko, J.; et al. Algal Products beyond Lipids: Comprehensive Characterization of Different Products in Direct Saponification of Green Alga Chlorella Sp. Algal Res. 2015, 11, 156–164. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Robles-Medina, A.; Hita-Peña, E.; Jiménez-Callejón, M.J.; Estéban-Cerdán, L.; González-Moreno, P.A.; Molina-Grima, E. Biodiesel Production from Wet Microalgal Biomass by Direct Transesterification. Fuel 2015, 150, 14–20. [Google Scholar] [CrossRef]
- Viêgas, C.V.; Hachemi, I.; Freitas, S.P.; Mäki-Arvela, P.; Aho, A.; Hemming, J.; Smeds, A.; Heinmaa, I.; Fontes, F.B.; Pereira, D.C.D.S.; et al. A Route to Produce Renewable Diesel from Algae: Synthesis and Characterization of Biodiesel via in Situ Transesterification of Chlorella Alga and Its Catalytic Deoxygenation to Renewable Diesel. Fuel 2015, 155, 144–154. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, W.; Chen, X.; Wang, J. Biodiesel Production from Algae Oil High in Free Fatty Acids by Two-Step Catalytic Conversion. Bioresour. Technol. 2012, 111, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Akbulut, A.; Ozalp, V.C.; Arica, M.Y. Immobilized Lipase on Micro-Porous Biosilica for Enzymatic Transesterification of Algal Oil. Chem. Eng. Res. Des. 2015, 95, 12–21. [Google Scholar] [CrossRef]
- Teo, C.L.; Jamaluddin, H.; Zain, N.A.M.; Idris, A. Biodiesel Production via Lipase Catalysed Transesterification of Microalgae Lipids from Tetraselmis Sp. Renew. Energy 2014, 68, 1–5. [Google Scholar] [CrossRef]
- Mota, G.F.; Sousa, I.G.D.; Oliveira, A.L.B.D.; Cavalcante, A.L.G.; Moreira, K.D.S.; Cavalcante, F.T.T.; Souza, J.E.D.S.; Falcão, Í.R.D.A.; Rocha, T.G.; Valério, R.B.R.; et al. Biodiesel Production from Microalgae Using Lipase-Based Catalysts: Current Challenges and Prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel Production through Lipase Catalyzed Transesterification: An Overview. J. Mol. Catal. B Enzym. 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Yanik, J.; Stahl, R.; Troeger, N.; Sinag, A. Pyrolysis of Algal Biomass. J. Anal. Appl. Pyrolysis 2013, 103, 134–141. [Google Scholar] [CrossRef]
- Piloni, R.V.; Daga, I.C.; Urcelay, C.; Moyano, E.L. Experimental Investigation on Fast Pyrolysis of Freshwater Algae. Prospects for Alternative Bio-Fuel Production. Algal Res. 2021, 54, 102206. [Google Scholar] [CrossRef]
- Wang, X.; Guo, F.; Li, Y.; Yang, X. Effect of Pretreatment on Microalgae Pyrolysis: Kinetics, Biocrude Yield and Quality, and Life Cycle Assessment. Energy Convers. Manag. 2017, 132, 161–171. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of Nitrogen and Evolution of N-Containing Species during Algae Pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef]
- Xu, W.; Ding, K.; Hu, L. A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals. Processes 2021, 9, 2042. [Google Scholar] [CrossRef]
- Díaz-Rey, M.R.; Cortés-Reyes, M.; Herrera, C.; Larrubia, M.A.; Amadeo, N.; Laborde, M.; Alemany, L.J. Hydrogen-Rich Gas Production from Algae-Biomass by Low Temperature Catalytic Gasification. Catal. Today 2015, 257, 177–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Eyk, P.J.V.; Boman, C.; Broström, M.; Kirtania, K.; Piotrowska, P.; Bostrom, D.; Nys, R.D.; Bhattacharya, S.; Gentili, F.G.; et al. Preliminary Understanding on the Ash Behavior of Algae during Co-Gasification in an Entrained Flow Reactor. Fuel Process. Technol. 2018, 175, 26–34. [Google Scholar] [CrossRef]
- Scopel, L.; Marcantonio, V. Gasification of Chlorella Vulgaris for Syngas Production and Energy Generation Through Gas Turbine. Energies 2024, 17, 6085. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, W.-H.; Lee, D.-J.; Chang, J.-S. Supercritical Water Gasification (SCWG) as a Potential Tool for the Valorization of Phycoremediation-Derived Waste Algal Biomass for Biofuel Generation. J. Hazard. Mater. 2021, 418, 126278. [Google Scholar] [CrossRef]
- Jena, U.; Eboibi, B.E.; Das, K.C. Co-Solvent Assisted Hydrothermal Liquefaction of Algal Biomass and Biocrude Upgrading. Fuels 2022, 3, 326–341. [Google Scholar] [CrossRef]
- Tian, C.; Li, B.; Liu, Z.; Zhang, Y.; Lu, H. Hydrothermal Liquefaction for Algal Biorefinery: A Critical Review. Renew. Sustain. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- Dong, S.; Liu, Z.; Yang, X. Exploration of Hydrothermal Liquefaction of Multiple Algae to Improve Bio-Crude Quality and Carbohydrate Utilization. Appl. Energy 2024, 361, 122861. [Google Scholar] [CrossRef]
- Naaz, F.; Samuchiwal, S.; Dalvi, V.; Bhattacharya, A.; Pant, K.K.; Malik, A. Hydrothermal Liquefaction Could Be a Sustainable Approach for Valorization of Wastewater Grown Algal Biomass into Cleaner Fuel. Energy Convers. Manag. 2023, 283, 116887. [Google Scholar] [CrossRef]
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Roles of Co-Solvents in Hydrothermal Liquefaction of Low-Lipid, High-Protein Algae. Bioresour. Technol. 2020, 310, 123454. [Google Scholar] [CrossRef]
- Deepika, C.; Mrinal; Pohrmen, C.B.; Jaiswal, K.S.; Sangmesh, B.; Jaiswal, K.K.; Ramasamy, A.P.; Jaiswal, A.K. Hydrothermal Liquefaction of Wet Microalgal Biomass for Biofuels and Platform Chemicals: Advances and Future Prospects. Discov. Appl. Sci. 2024, 6, 245. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy Recovery from Industrial Crop Wastes by Dry Anaerobic Digestion: A Review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel Highly Efficient Starch Producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Shibasaki, S.; Ueda, M. Utilization of Macroalgae for the Production of Bioactive Compounds and Bioprocesses Using Microbial Biotechnology. Microorganisms 2023, 11, 1499. [Google Scholar] [CrossRef]
- Sarris, D.; Papanikolaou, S. Biotechnological Production of Ethanol: Biochemistry, Processes and Technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, Y.; Sasmal, S. Perspectives of HMF and LA from Microalgal Biomass. Algal Res. 2023, 72, 103133. [Google Scholar] [CrossRef]
- Gencturk, E.; Ulgen, K.O. Understanding HMF Inhibition on Yeast Growth Coupled with Ethanol Production for the Improvement of Bio-Based Industrial Processes. Process Biochem. 2022, 121, 425–438. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic Digestion of Algae Biomass: A Review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Reshma, R.; Arumugam, M. Selective Degradation of the Recalcitrant Cell Wall of Scenedesmus Quadricauda CASA CC202. Planta 2017, 246, 779–790. [Google Scholar] [CrossRef]
- Banihani, Q.; Sierra-Alvarez, R.; Field, J.A. Nitrate and Nitrite Inhibition of Methanogenesis during Denitrification in Granular Biofilms and Digested Domestic Sludges. Biodegradation 2009, 20, 801–812. [Google Scholar] [CrossRef]
- Calicioglu, O.; Demirer, G.N. Carbon-to-Nitrogen and Substrate-to-Inoculum Ratio Adjustments Can Improve Co-Digestion Performance of Microalgal Biomass Obtained from Domestic Wastewater Treatment. Environ. Technol. 2019, 40, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Semi-Continuous Anaerobic Digestion of the Marine Micro-Algal Species I. Galbana and D. Salina Grown under Low and High Sulphate Conditions. Algal Res. 2019, 41, 101564. [Google Scholar] [CrossRef]
- Ehimen, E.A.; Sun, Z.F.; Carrington, C.G.; Birch, E.J.; Eaton-Rye, J.J. Anaerobic Digestion of Microalgae Residues Resulting from the Biodiesel Production Process. Appl. Energy 2011, 88, 3454–3463. [Google Scholar] [CrossRef]
- Batista, A.P.; Moura, P.; Marques, P.A.S.S.; Ortigueira, J.; Alves, L.; Gouveia, L. Scenedesmus obliquus as Feedstock for Biohydrogen Production by Enterobacter aerogenes and Clostridium butyricum. Fuel 2014, 117, 537–543. [Google Scholar] [CrossRef]
- Lacroux, J.; Llamas, M.; Dauptain, K.; Avila, R.; Steyer, J.-P.; Lis, R.V.; Trably, E. Dark Fermentation and Microalgae Cultivation Coupled Systems: Outlook and Challenges. Sci. Total Environ. 2023, 865, 161136. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Wang, X.; Zhang, B.; Tian, W.; Zhang, J. Hydrothermal Liquefaction of Microalgae over Transition Metal Supported TiO2 Catalyst. Bioresour. Technol. 2018, 250, 474–480. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M. Catalytic and solvent hydrothermal liquefaction of microalgae: A strategy for recovering fine chemicals. Stud. UBB Chem. 2024, 3, 109–129. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Biodiesel Production from Microalgal Biomass Using CaO Catalyst Synthesized from Natural Waste Material. Renew. Energy 2019, 136, 837–845. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A. Alkaline in Situ Transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Z.; Wu, X.; Miao, X. Direct Biodiesel Production from Wet Microalgae Biomass of Chlorella pyrenoidosa through In Situ Transesterification. BioMed Res. Int. 2013, 2013, 930686. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Chattanathan, S.A.; Gupta, R.B. Catalytic Pyrolysis of Green Algae for Hydrocarbon Production Using H+ZSM-5 Catalyst. Bioresour. Technol. 2012, 118, 150–157. [Google Scholar] [CrossRef]
- Lü, F.; Ji, J.; Shao, L.; He, P. Bacterial Bioaugmentation for Improving Methane and Hydrogen Production from Microalgae. Biotechnol. Biofuels 2013, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Babatabar, M.A.; Yousefian, F.; Mousavi, M.V.; Hosseini, M.; Tavasoli, A. Pyrolysis of Lignocellulosic and Algal Biomasses in a Fixed-bed Reactor: A Comparative Study on the Composition and Application Potential of Bioproducts. Intl. J. Energy Res. 2022, 46, 9836–9850. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, C. Catalytic Thermochemical Conversion of Algae and Upgrading of Algal Oil for the Production of High-Grade Liquid Fuel: A Review. Catalysts 2020, 10, 145. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Abdul Aziz, A.R. Solid Acid-Catalyzed Biodiesel Production from Microalgal Oil—The Dual Advantage. J. Environ. Chem. Eng. 2013, 1, 113–121. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Pace, R.; Morgan, T.; Behnke, C.; Sajkowski, D.J.; Lappas, A.; Crocker, M. Co-Processing of Hydrothermal Liquefaction Algal Bio-Oil and Petroleum Feedstock to Fuel-like Hydrocarbons via Fluid Catalytic Cracking. Fuel Process. Technol. 2019, 188, 164–171. [Google Scholar] [CrossRef]
- Bai, X.; Duan, P.; Xu, Y.; Zhang, A.; Savage, P.E. Hydrothermal Catalytic Processing of Pretreated Algal Oil: A Catalyst Screening Study. Fuel 2014, 120, 141–149. [Google Scholar] [CrossRef]
- Zheng, F.; Cho, H.M. Study on Biodiesel Production: Feedstock Evolution, Catalyst Selection, and Influencing Factors Analysis. Energies 2025, 18, 2533. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; Yan, Q.; Wu, X.; Zhang, H. Superparamagnetic Nanospheres with Efficient Bifunctional Acidic Sites Enable Sustainable Production of Biodiesel from Budget Non-Edible Oils. Energy Convers. Manag. 2023, 297, 117758. [Google Scholar] [CrossRef]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response Surface Methodology Based Optimization of in Situ Transesterification of Dry Algae with Methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Advances in Photocatalytic Biodiesel Production: Preparation Methods, Modifications and Mechanisms. Fuel 2024, 362, 130749. [Google Scholar] [CrossRef]
- Corro, G.; Pal, U.; Tellez, N. Biodiesel Production from Jatropha curcas Crude Oil Using ZnO/SiO2 Photocatalyst for Free Fatty Acids Esterification. Appl. Catal. B Environ. 2013, 129, 39–47. [Google Scholar] [CrossRef]
- Santoso, C.; Ratnawati; Slamet. Utilization of Glycerol Solution for Hydrogen Production by a Combination of Photocatalysis and Electrolysis Processes with Fe-TiO2 Nanotubes. Commun. Sci. Technol. 2023, 8, 208–215. [Google Scholar] [CrossRef]
- Page, J.R.; Manfredi, Z.; Bliznakov, S.; Valla, J.A. Recent Progress in Electrochemical Upgrading of Bio-Oil Model Compounds and Bio-Oils to Renewable Fuels and Platform Chemicals. Materials 2023, 16, 394. [Google Scholar] [CrossRef]
- Lam, C.H.; Deng, W.; Lang, L.; Jin, X.; Hu, X.; Wang, Y. Minireview on Bio-Oil Upgrading via Electrocatalytic Hydrogenation: Connecting Biofuel Production with Renewable Power. Energy Fuels 2020, 34, 7915–7928. [Google Scholar] [CrossRef]
- Kumar, M.; Meena, B.; Yu, A.; Sun, C.; Challapalli, S. Advancements in Catalysts for Glycerol Oxidation via Photo-/Electrocatalysis: A Comprehensive Review of Recent Developments. Green Chem. 2023, 25, 8411–8443. [Google Scholar] [CrossRef]
- Wei, C.; Xu, Y.; Li, Y.; Wei, W.; Feng, Y.; Li, Z.; Xu, L. Life-Cycle Assessment of Microalgae Liquid Biofuel Production in Biofilm Cultivation System via Conversion Technologies of Transesterification, Hydrothermal Liquefaction and Pyrolysis. J. Clean. Prod. 2024, 436, 140559. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Sadewo, B.R.; Haryanto, S.A.; Sudibyo, H. Selection of Oil Extraction Process from Chlorella Species of Microalgae by Using Multi-Criteria Decision Analysis Technique for Biodiesel Production. Open Chem. 2021, 19, 1029–1042. [Google Scholar] [CrossRef]
- Wu, W.; Chang, J.-S. Integrated Algal Biorefineries from Process Systems Engineering Aspects: A Review. Bioresour. Technol. 2019, 291, 121939. [Google Scholar] [CrossRef] [PubMed]
- Posada, J.A.; Brentner, L.B.; Ramirez, A.; Patel, M.K. Conceptual Design of Sustainable Integrated Microalgae Biorefineries: Parametric Analysis of Energy Use, Greenhouse Gas Emissions and Techno-Economics. Algal Res. 2016, 17, 113–131. [Google Scholar] [CrossRef]
- Pahl, S.L.; Lee, A.K.; Kalaitzidis, T.; Ashman, P.J.; Sathe, S.; Lewis, D.M. Harvesting, Thickening and Dewatering Microalgae Biomass. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 165–185. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of Biofuels from Microalgae—A Review on Cultivation, Harvesting, Lipid Extraction, and Numerous Applications of Microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Nejat Veziroglu, T.; Allakhverdiev, S.I. Review: Biofuel Production from Plant and Algal Biomass. Int. J. Hydrogen Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Monlau, F.; Suarez-Alvarez, S.; Lallement, A.; Vaca-Medina, G.; Giacinti, G.; Munarriz, M.; Urreta, I.; Raynaud, C.; Ferrer, C.; Castañón, S. A Cascade Biorefinery for the Valorization of Microalgal Biomass: Biodiesel, Biogas, Fertilizers and High Valuable Compounds. Algal Res. 2021, 59, 102433. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Li, M.; Hu, C. Algal Biomass Valorisation to High-Value Chemicals and Bioproducts: Recent Advances, Opportunities and Challenges. Bioresour. Technol. 2022, 344, 126371. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Huang, J.-M.; Tsai, T.-H.; Chang, J.-S.; Wu, W. Exploration of Algal Biorefinery Frameworks: Optimization, Quantification of Environmental Impacts and Economics. Algal Res. 2023, 69, 102903. [Google Scholar] [CrossRef]
- Pandey, A.; Shah, R.; Yadav, P.; Verma, R.; Srivastava, S. Harvesting of Freshwater Microalgae Scenedesmus Sp. by Electro-Coagulation-Flocculation for Biofuel Production: Effects on Spent Medium Recycling and Lipid Extraction. Environ. Sci. Pollut. Res. Int. 2020, 27, 3497–3507. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Barlow, J.; Sims, R.C.; Quinn, J.C. Techno-Economic and Life-Cycle Assessment of an Attached Growth Algal Biorefinery. Bioresour. Technol. 2016, 220, 360–368. [Google Scholar] [CrossRef]
- Somers, M.D.; Quinn, J.C. Sustainability of Carbon Delivery to an Algal Biorefinery: A Techno-Economic and Life-Cycle Assessment. J. CO2 Util. 2019, 30, 193–204. [Google Scholar] [CrossRef]
- Ubando, A.T.; Ng, E.A.S.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Khandelwal, N.; Kumari, S.; Poduval, S.; Behera, S.K.; Kumar, A.; Gedam, V.V. Life-Cycle Assessment of Three Biorefinery Pathways across Different Generations. Sci. Rep. 2025, 15, 13135. [Google Scholar] [CrossRef] [PubMed]
- Efroymson, R.A.; Dale, V.H. Environmental Indicators for Sustainable Production of Algal Biofuels. Ecol. Indic. 2015, 49, 1–13. [Google Scholar] [CrossRef]
- Reijnders, L. Environmental Sustainability Metrics and Indicators of Microalgae-Based Fuels. In 3rd Generation Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 813–833. [Google Scholar] [CrossRef]
- Bosma, R.; Vree, J.H.D.; Slegers, P.M.; Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Design and Construction of the Microalgal Pilot Facility AlgaePARC. Algal Res. 2014, 6, 160–169. [Google Scholar] [CrossRef]
- White, R.L.; Ryan, R.A. Long-Term Cultivation of Algae in Open-Raceway Ponds: Lessons from the Field. Ind. Biotechnol. 2015, 11, 213–220. [Google Scholar] [CrossRef]
- DE–EE0002867; Recovery Act—Integrated Pilot-Scale Biorefinery for Producing Ethanol from Hybrid Algae. Algenol Biotech LLC: Fort Myers, FL, USA, 2017. [CrossRef]
- Deprá, M.C.; Dias, R.R.; Zepka, L.Q.; Jacob-Lopes, E. Tackling Old Challenges in Microalgal Biotechnology: The Role of Photobioreactors to Advance the Technology Readiness Level. Processes 2024, 13, 51. [Google Scholar] [CrossRef]
- Aitken, D.; Antizar-Ladislao, B. Achieving a Green Solution: Limitations and Focus Points for Sustainable Algal Fuels. Energies 2012, 5, 1613–1647. [Google Scholar] [CrossRef]
- Samoraj, M.; Çalış, D.; Trzaska, K.; Mironiuk, M.; Chojnacka, K. Advancements in Algal Biorefineries for Sustainable Agriculture: Biofuels, High-Value Products, and Environmental Solutions. Biocatal. Agric. Biotechnol. 2024, 58, 103224. [Google Scholar] [CrossRef]
- Arora, N.; Yen, H.-W.; Philippidis, G.P. Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability 2020, 12, 5125. [Google Scholar] [CrossRef]
- Gupta, A.; Kang, K.; Pathania, R.; Saxton, L.; Saucedo, B.; Malik, A.; Torres-Tiji, Y.; Diaz, C.J.; Molino, J.V.D.; Mayfield, S.P. Harnessing Genetic Engineering to Drive Economic Bioproduct Production in Algae. Front. Bioeng. Biotechnol. 2024, 12, 1350722. [Google Scholar] [CrossRef]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.-S.; Chang, J.-S. Genetic Engineering of Microalgae for Enhanced Biorefinery Capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Mayfield, S.P. Exploiting Diversity and Synthetic Biology for the Production of Algal Biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef]
- Zafar, S.U.; Mehra, A.; Jutur, P.P. Synthetic Biology-Based Advanced Biotechnological Approach in Microalgal Biorefinery. In Micro-Algae: Next-Generation Feedstock for Biorefineries; Verma, P., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 205–230. [Google Scholar] [CrossRef]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef]
- Andersson, V.; Heyne, S.; Harvey, S.; Berntsson, T. Integration of Algae-based Biofuel Production with an Oil Refinery: Energy and Carbon Footprint Assessment. Int. J. Energy Res. 2020, 44, 10860–10877. [Google Scholar] [CrossRef]
- Joshi, S.; Gogate, P. Process Intensification of Biofuel Production from Microalgae. In Energy from Microalgae; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 59–87. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Davis, R.; Laurens, L.M.L.; Wychen, S.V.; Pienkos, P.T.; Nagle, N. Combined Algal Processing: A Novel Integrated Biorefinery Process to Produce Algal Biofuels and Bioproducts. Algal Res. 2016, 19, 316–323. [Google Scholar] [CrossRef]
- Mittal, R.; Ranade, V. Bioactives from Microalgae: A Review on Process Intensification Using Hydrodynamic Cavitation. J. Appl. Phycol. 2023, 35, 1129–1161. [Google Scholar] [CrossRef]
- Zhu, Y.; Albrecht, K.O.; Elliott, D.C.; Hallen, R.T.; Jones, S.B. Development of Hydrothermal Liquefaction and Upgrading Technologies for Lipid-Extracted Algae Conversion to Liquid Fuels. Algal Res. 2013, 2, 455–464. [Google Scholar] [CrossRef]
- Yadav, G.; Fabiano, L.A.; Soh, L.; Zimmerman, J.; Sen, R.; Seider, W.D. CO2 Process Intensification of Algae Oil Extraction to Biodiesel. AIChE J. 2021, 67, e16992. [Google Scholar] [CrossRef]
- Piemonte, V.; Di Paola, L.; Iaquaniello, G.; Prisciandaro, M. Biodiesel Production from Microalgae: Ionic Liquid Process Simulation. J. Clean. Prod. 2016, 111, 62–68. [Google Scholar] [CrossRef]
- Siegler, H.D.L.H. Process Intensification for Sustainable Algal Fuels Production. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 503–521. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Kumar, G.; Huy, M.; Bakonyi, P.; Bélafi-Bakó, K.; Kim, S.-H. Evaluation of Gradual Adaptation of Mixed Microalgae Consortia Cultivation Using Textile Wastewater via Fed Batch Operation. Biotechnol. Rep. 2018, 20, e00289. [Google Scholar] [CrossRef] [PubMed]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment With CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Imamoglu, E. Artificial Intelligence and/or Machine Learning Algorithms in Microalgae Bioprocesses. Bioengineering 2024, 11, 1143. [Google Scholar] [CrossRef]
- Nayak, M.; Dhanarajan, G.; Dineshkumar, R.; Sen, R. Artificial Intelligence Driven Process Optimization for Cleaner Production of Biomass with Co-Valorization of Wastewater and Flue Gas in an Algal Biorefinery. J. Clean. Prod. 2018, 201, 1092–1100. [Google Scholar] [CrossRef]
- Mafat, I.H.; Palla, S.; Surya, D.V. Machine Learning and Artificial Intelligence for Algal Cultivation, Harvesting Techniques, Wastewater Treatment, Nutrient Recovery, and Biofuel Production and Optimization. In Value Added Products From Bioalgae Based Biorefineries: Opportunities and Challenges; Arya, S.K., Khatri, M., Singh, G., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 463–487. [Google Scholar] [CrossRef]
- Glass, D.J. Government Regulation of the Uses of Genetically Modified Algae and Other Microorganisms in Biofuel and Bio-Based Chemical Production. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 23–60. [Google Scholar] [CrossRef]
- Behera, B.; Selvam, S.M.; Paramasivan, B. Research Trends and Market Opportunities of Microalgal Biorefinery Technologies from Circular Bioeconomy Perspectives. Bioresour. Technol. 2022, 351, 127038. [Google Scholar] [CrossRef]
- Haase, R.; Bielicki, J.; Kuzma, J. Innovation in Emerging Energy Technologies: A Case Study Analysis to Inform the Path Forward for Algal Biofuels. Energy Policy 2013, 61, 1595–1607. [Google Scholar] [CrossRef]
- DOE-CPC-0006317; Final Report: Scale-up of Algal Biofuel Production Using Waste Nutrients. California Polytechnic State University: San Luis Obispo, CA, USA, 2018. [CrossRef]
- Platt, R.; Bauen, A.; Reumerman, P.; Geier, C.; Ree, R.V.; Gursel, I.V.; Chavez, L.Y.G.; Behrens, M.; von Bothmer, P.; Howes, J.; et al. EU Biorefinery Outlook to 2030: Studies on Support to Research and Innovation in the Area of Bio-Based Products and Services; European Commission: Brussels, Belgium, 2021; Available online: https://data.europa.eu/doi/10.2777/103465 (accessed on 20 August 2025).
- Thornley, P.; Rathi, B.; Borah, A.J.; Saha, B.; Adams, J.; Hiloidhari, M.; Blanchard, R.; Dinsdale, R.; Nagarajan, S.; Kumar, S.; et al. Bioenergy Technologies for a Net Zero Transition: Outcomes of UK-India Bioenergy Research Scoping; Supergen Bioenergy Hub: Birmingham, UK, 2022; Available online: https://www.supergen-bioenergy.net/output/bioenergy-technologies-for-a-net-zero-transition-outcomes-of-uk-india-bioenergy-research-scoping (accessed on 20 August 2025).
- Knudsen, M.S.; Ferreira-Aulu, M.B.; Shabanova-Danielyan, E.; Wang, W.; Luukkanen, J.; Kaivo-oja, J. INTERNATIONAL ENERGY RESEARCH INFRASTRUCTURES: MAPPING THE GLOBAL LANDSCAPE OF ENERGY RIS (RISCAPE): Based on Finland Futures Research Centre’s Contribution to the Horizon 2020 Project European Research Infrastructures in the International Landscape (RISCAPE); UTUPub: Turky, Finland, 2022; Available online: https://urn.fi/URN:ISBN:978-952-249-582-2 (accessed on 20 August 2025).
- Usmani, R.A.; Mohammad, A.S.; Ansari, S.S. Comprehensive Biofuel Policy Analysis Framework: A Novel Approach Evaluating the Policy Influences. Renew. Sustain. Energy Rev. 2023, 183, 113403. [Google Scholar] [CrossRef]
- Manser, J.S.; Rollin, J.A.; Brown, K.E.; Rohlfing, E.A. ARPA-E: Accelerating U.S. Energy Innovation. ACS Energy Lett. 2016, 1, 987–990. [Google Scholar] [CrossRef]
- Sedighi, M.; Qhazvini, P.P.; Amidpour, M. Algae-Powered Buildings: A Review of an Innovative, Sustainable Approach in the Built Environment. Sustainability 2023, 15, 3729. [Google Scholar] [CrossRef]
- Kim, K.Y. Harnessing Seaweed Farming for Climate Mitigation in South Korea: Evaluating Carbon Dioxide Removal Potential and Future Research Directions. Algae 2024, 39, 329–347. [Google Scholar] [CrossRef]
- Falfushynska, H. Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology 2024, 4, 548–575. [Google Scholar] [CrossRef]
- Noor, A.; Naseer, F. History and Recent Advances of Algal Biofuel Commercialization. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 567–586. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Duraisamy, G.; Griffiths, G. Microalgal Biodiesel: A Challenging Route toward a Sustainable Aviation Fuel. Fermentation 2023, 9, 907. [Google Scholar] [CrossRef]
- Doshi, A.; Pascoe, S.; Coglan, L.; Rainey, T.J. Economic and Policy Issues in the Production of Algae-Based Biofuels: A Review. Renew. Sustain. Energy Rev. 2016, 64, 329–337. [Google Scholar] [CrossRef]
- Ebadian, M.; Dyk, S.V.; McMillan, J.D.; Saddler, J. Biofuels Policies That Have Encouraged Their Production and Use: An International Perspective. Energy Policy 2020, 147, 111906. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.; Murthy, G. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Hu, H.-Y.; Wu, Y.-H.; Zhuang, L.-L.; Xu, X.-Q.; Wang, X.-X.; Dao, G.-H. Promising Solutions to Solve the Bottlenecks in the Large-Scale Cultivation of Microalgae for Biomass/Bioenergy Production. Renew. Sustain. Energy Rev. 2016, 60, 1602–1614. [Google Scholar] [CrossRef]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid Production in Nannochloropsis Gaditana Is Doubled by Decreasing Expression of a Single Transcriptional Regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef]
- Ferreira, F.; Ortigueira, J.; Reis, A.; Lopes, T.F. Benchmarking Commercially Available Value-Added Fractions with Potential for Production via Microalgae-Based Biorefineries: Is It Worth It? Biotechnol. Biofuels Bioprod. 2025, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Alam, M.A.; Wang, Z. Open Pond Culture Systems and Photobioreactors for Microalgal Biofuel Production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 45–74. [Google Scholar] [CrossRef]
- Hoh, D.; Watson, S.; Kan, E. Algal Biofilm Reactors for Integrated Wastewater Treatment and Biofuel Production: A Review. Chem. Eng. J. 2016, 287, 466–473. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Liu, T.; Leng, S.; Yang, L.; Peng, H.; Jiang, S.; Zhou, W.; Leng, L.; Li, H. Machine Learning Prediction and Optimization of Bio-Oil Production from Hydrothermal Liquefaction of Algae. Bioresour. Technol. 2021, 342, 126011. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Gu, S. Commercialization Potential of Microalgae for Biofuels Production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Sarmah, A.K.; Sen, R. Zero-Waste Algal Biorefinery for Bioenergy and Biochar: A Green Leap towards Achieving Energy and Environmental Sustainability. Sci. Total Environ. 2019, 650, 2467–2482. [Google Scholar] [CrossRef]
- O’Connor, K.; Gaffey, J.; Gavin, E.; Stout, J.; Holden, N.M. Evidence Synthesis Report 2: Circular Bioeconomy Outlook Study 2030–2050 in Support of Climate Action, Sustainable Food and Biobased Systems; Environmental Protection Agency: San Francisco, CA, USA, 2023. [Google Scholar]

| Cultivation System | Biomass Productivity (gL−1day−1) | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Open Raceway Ponds | 0.01–0.12 | Low capital and operating costs | High risk of contamination Large land footprint | [32,33,34] |
| Closed Photobioreactors | 1.5–1.6 | Higher productivity and better control of contamination and condition | High installation and maintenance cost | [33,35,36] |
| Wastewater-Based Cultivation | 0.03–0.05 | Utilization of waste nutrients | Lower control overgrowth conditions | [34,37] |
| Energy output | GJ ha−1 | 1465 |
| Productivity | t ha−1 year−1 | 66.0 |
| Biomass energy content | MJ kg−1 | 22.2 |
| Energy inputs | GJ ha−1 | 848 |
| Eoperations | GJ ha−1 | 0 |
| Efertilizers | GJ ha−1 | 278 |
| Eembodied | GJ ha−1 | 570 |
| NER | 1.73 |
| Method | Strain | Catalyst | Biofuel | Condition | Biofuel Productivity | Refs |
|---|---|---|---|---|---|---|
| HTL | Nannochloropsis | Ni/TiO2 | Biocrude | 300 °C | 48.2 wt% | [116] |
| Chlorella vulgaris | Co/TiO2 | Biocrude | 290 °C | 57.8 wt% | [117] | |
| Spirulina maxima | Zeolite | Biocrude | 278 °C | 53.8 wt% | [117] | |
| Transesterification | Chlorella vulgaris | CaO | Biocrude | 70 °C, 180 min | 92.0 wt% | [118] |
| Chlorella vulgaris | NaOH | Biodiesel | 60 °C, 75 min | 77.6 wt% | [119] | |
| Chlorella pyrenoidosa | H2SO4 | Biodiesel | 120 °C, 120 min | 86.6 wt% | [120] | |
| Catalytic pyrolysis | Chlorella vulgaris | HZSM-5 | Bio-oil, aromatic | 500 °C | 52.7 wt% | [121] |
| Anaerobic digestion | Chlorella vulgaris | C. thermocellum | Methane | 52 °C | 403 mLg−1VS | [122] |
| Criteria | Transesterification | HTL | Pyrolysis |
|---|---|---|---|
| Treated Biomass (g/L) | 200 | 200 | 800 |
| NER | 2.18 | 0.88 | 2.06 |
| GHG Reduction | Moderate | High | Low |
| Biocrude Yield (m3d−1) | 0.96 | 0.79 | 0.72 |
| Cost | Low | Moderate | Moderate |
| Criteria | Weighted Factor | COE | UAE | MAE | EPE | SFE | HTL |
|---|---|---|---|---|---|---|---|
| Easy scalability | 0.53 | 4.27 | 3.71 | 3.71 | 2.93 | 2.75 | 2.93 |
| Extraction productivity | 0.17 | 0.20 | 0.91 | 1.67 | 1.05 | 0.17 | 0.34 |
| Energy input | 0.17 | 1.67 | 1.60 | 1.63 | 1.66 | 0.17 | 0.39 |
| Additional compound | 0.07 | 0.04 | 0.17 | 0.17 | 0.17 | 0.12 | 0.67 |
| Environmental impact | 0.07 | 0.33 | 0.40 | 0.40 | 0.40 | 0.53 | 0.67 |
| Total score | 1.00 | 6.50 | 6.79 | 7.57 | 6.21 | 3.73 | 4.99 |
| TRL | Description | Relevance to Algal Biorefineries |
|---|---|---|
| TRL 1 | Basic research | Lab-scale understanding of algal biology |
| TRL 2 | Conceptualization | Conceptual design of photobioreactors |
| TRL 3 | Proof of concept | Laboratory-scale experiments to understand the strain performance |
| TRL 4 | Technology validation in laboratory | Development of prototype for cultivation, harvesting, or conversion pathways |
| TRL 5 | Technology validation in open environment | Design of pilot photobioreactors and integrated systems with sunlight, flue gas, or wastewater |
| TRL 6 | Prototype demonstration in relevant environment | Design of full-scale algal biorefinery (e.g., harvesting + catalytic conversion of biomass) |
| TRL 7 | Prototype testing in operational environment | Example: Sapphire Energy using open pond cultivation with fuel upgrading |
| TRL 8 | Technology ready for transfer | Pre-commercial stage to understand efficiency, scalability, and environmental impact |
| TRL 9 | Actual transfer of technology and acceptance | Establishment of functional algal biorefineries and market acceptance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, S.K.; Kim, G.; Song, H. Algae to Biofuels: Catalytic Strategies and Sustainable Technologies for Green Energy Conversion. Catalysts 2025, 15, 806. https://doi.org/10.3390/catal15090806
Rai SK, Kim G, Song H. Algae to Biofuels: Catalytic Strategies and Sustainable Technologies for Green Energy Conversion. Catalysts. 2025; 15(9):806. https://doi.org/10.3390/catal15090806
Chicago/Turabian StyleRai, Shushil Kumar, Gyungmin Kim, and Hua Song. 2025. "Algae to Biofuels: Catalytic Strategies and Sustainable Technologies for Green Energy Conversion" Catalysts 15, no. 9: 806. https://doi.org/10.3390/catal15090806
APA StyleRai, S. K., Kim, G., & Song, H. (2025). Algae to Biofuels: Catalytic Strategies and Sustainable Technologies for Green Energy Conversion. Catalysts, 15(9), 806. https://doi.org/10.3390/catal15090806








