Progress in Post-Combustion Carbon Dioxide Capture, Direct Air Capture, and Utilization
Abstract
1. Introduction
2. CO2 Emissions: Sources and Their Impact on the Environment
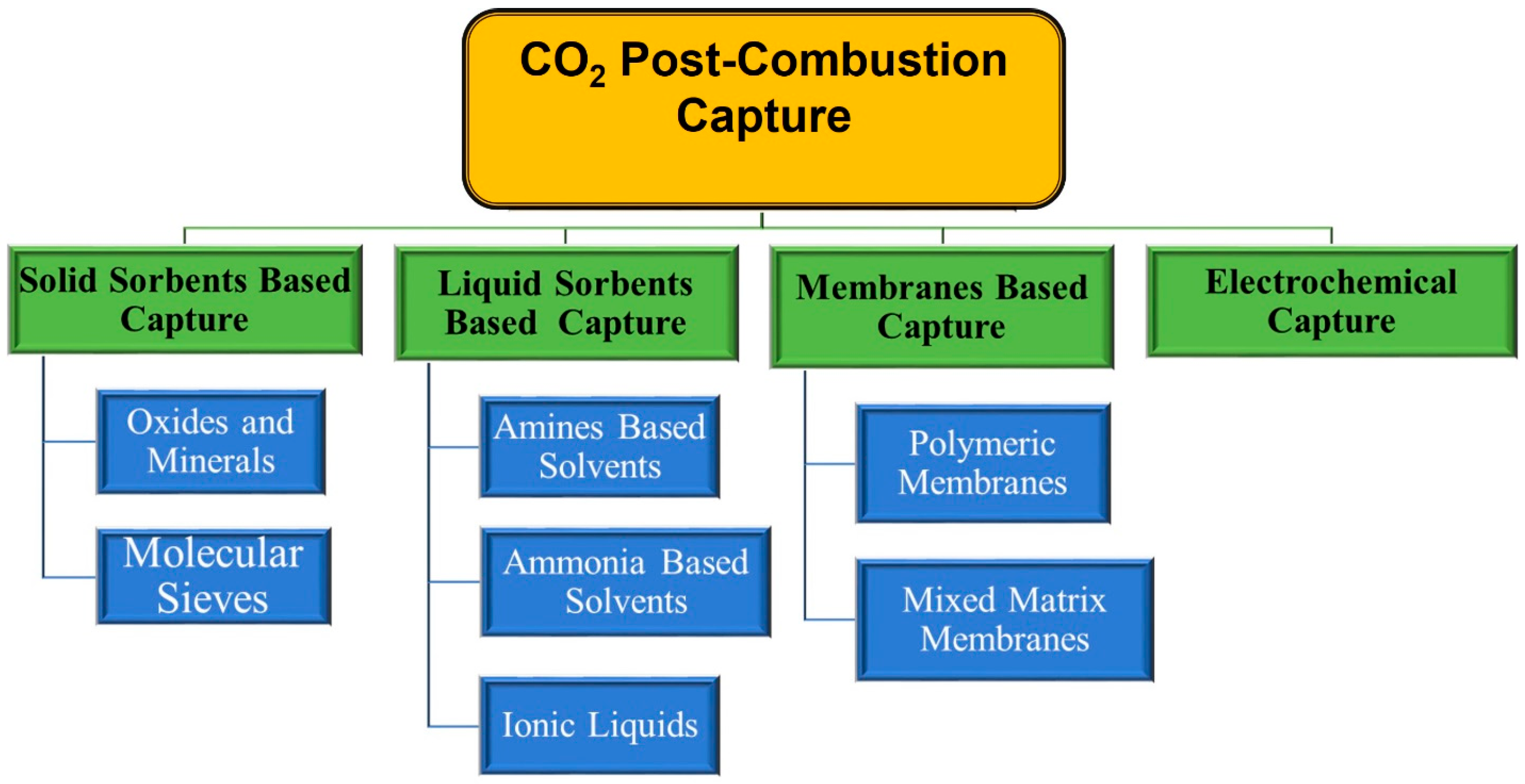
3. CO2 Capture Strategies
3.1. Post-Combustion Strategy
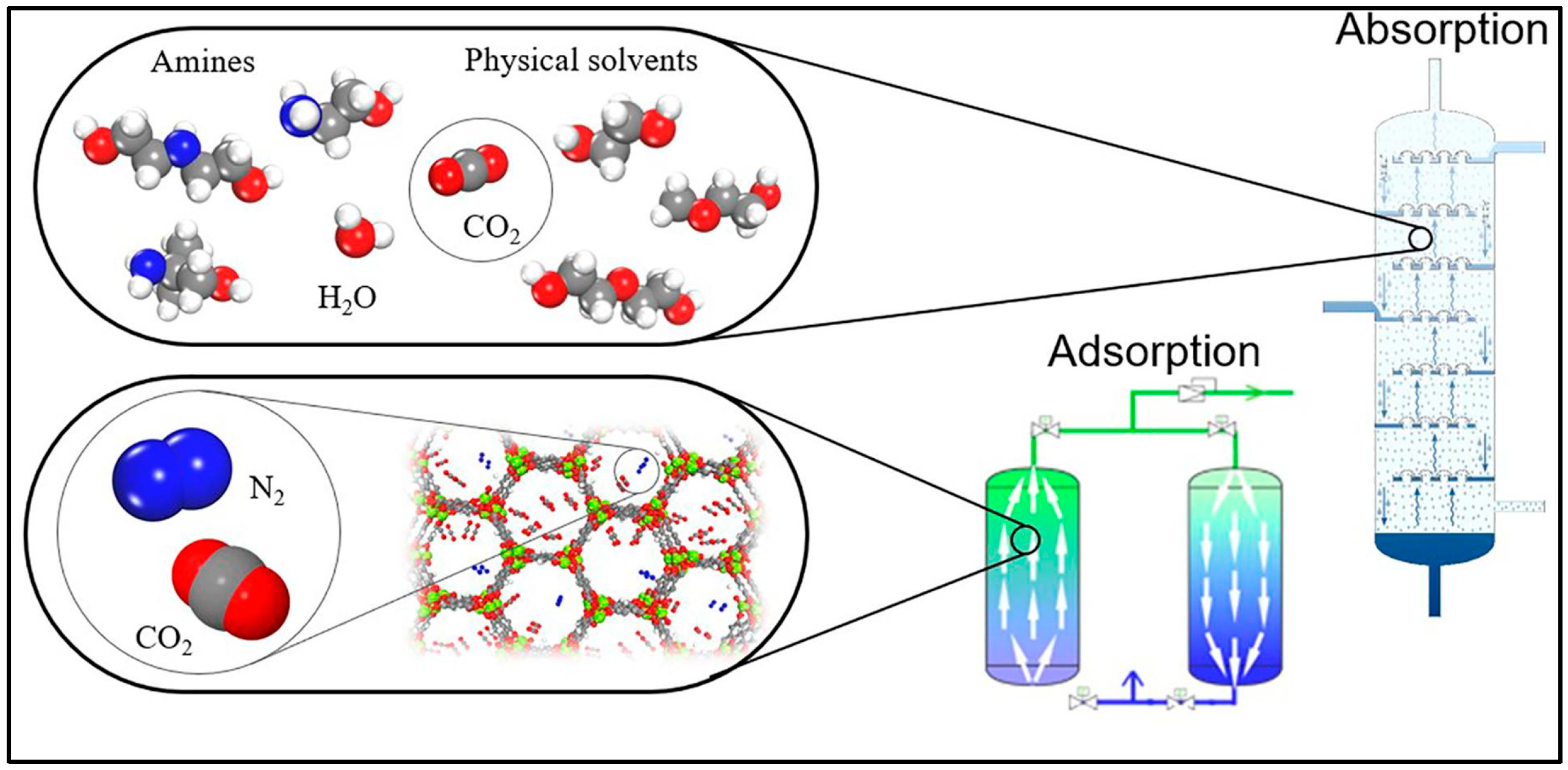
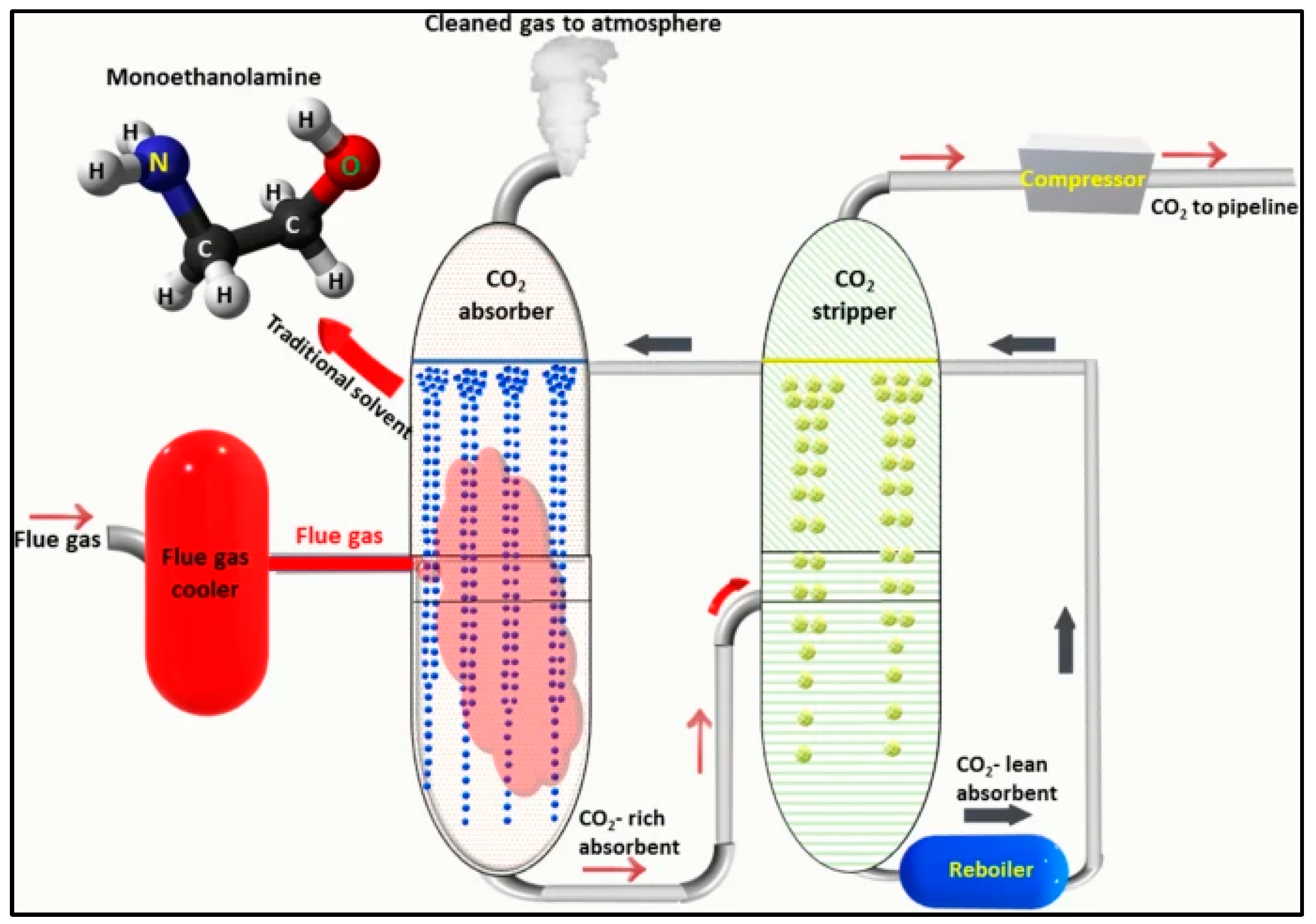
3.1.1. CO2 Capture via Liquid Sorbents
Amine-Based Liquid Sorbents
Ammonia-Based Sorbents
Ionic Liquid-Based Sorbents
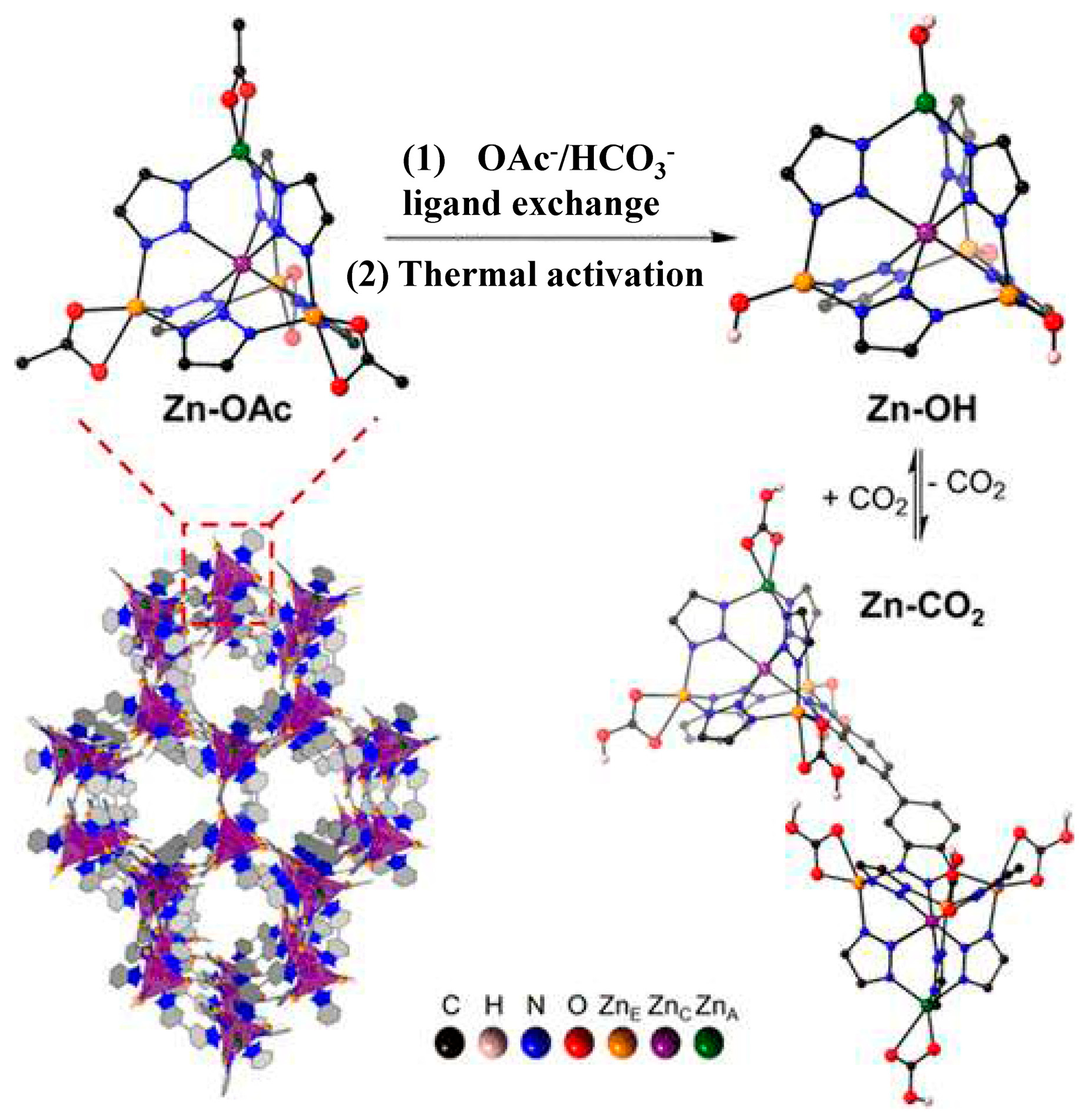
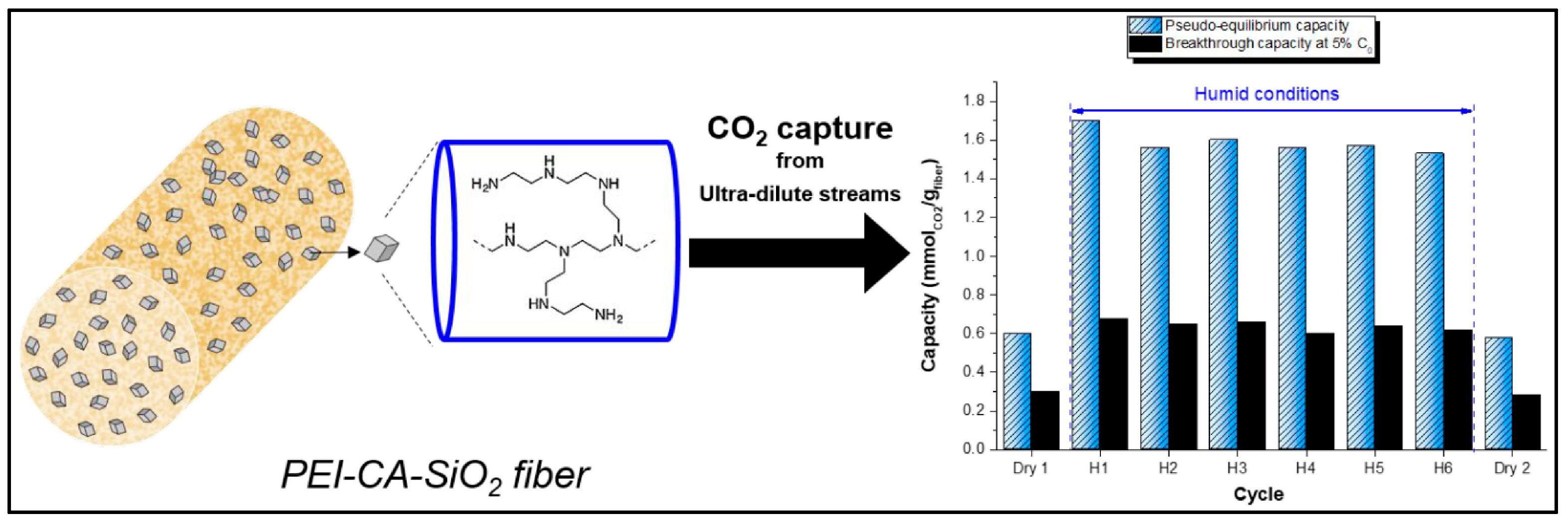
3.1.2. Solid Sorbents for CO2 Capture
- High selectivity toward CO2;
- Moderate renewal requirements;
- Low cost and quick desorption and adsorption kinetics;
- High CO2 operational capability.
Carbon-Based Materials
Silica-Based Materials
Alumina-Based Materials
Zeolite-Based Materials
Porous Crystalline Solids
3.2. Membranes to Capture CO2
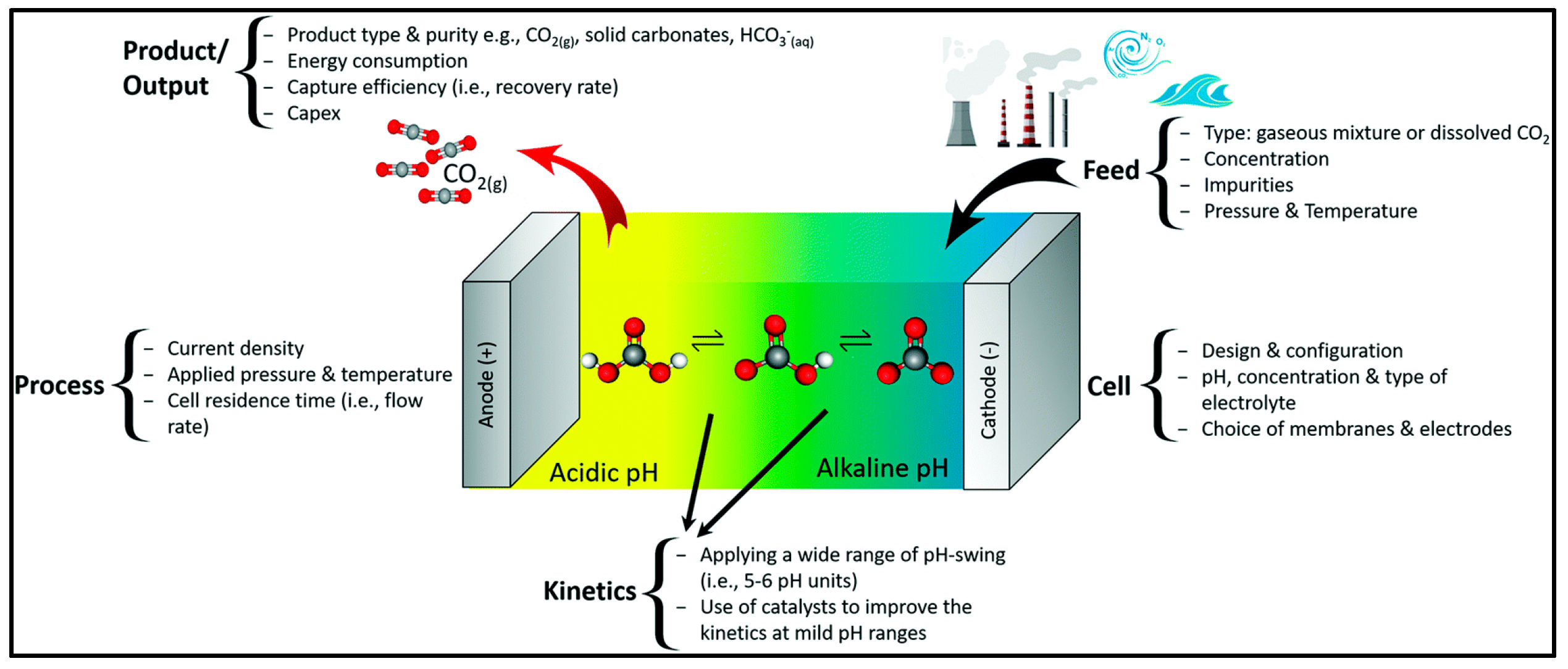
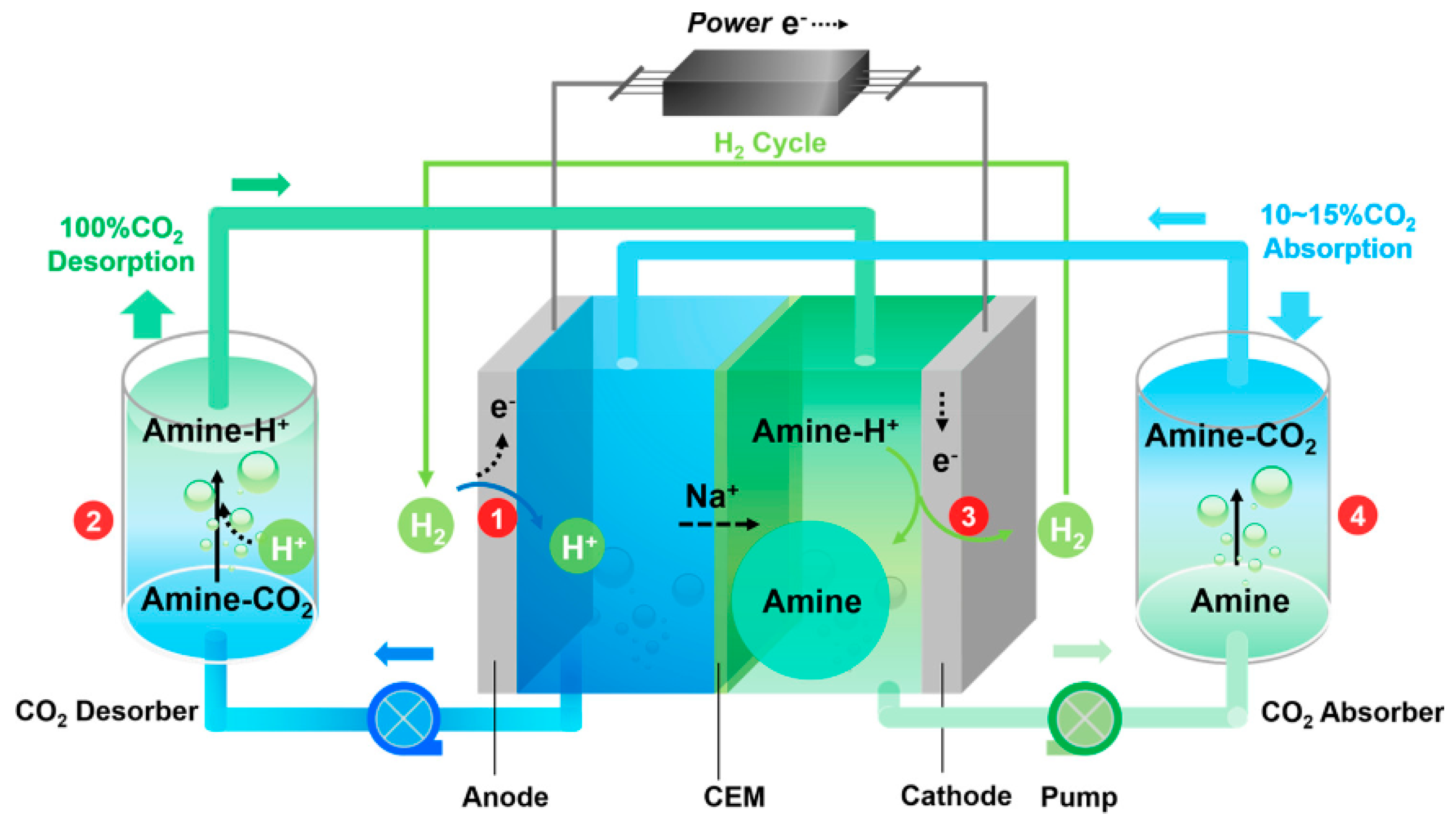
3.3. Electrochemical Techniques for CO2 Capture
3.4. Direct Capture of CO2
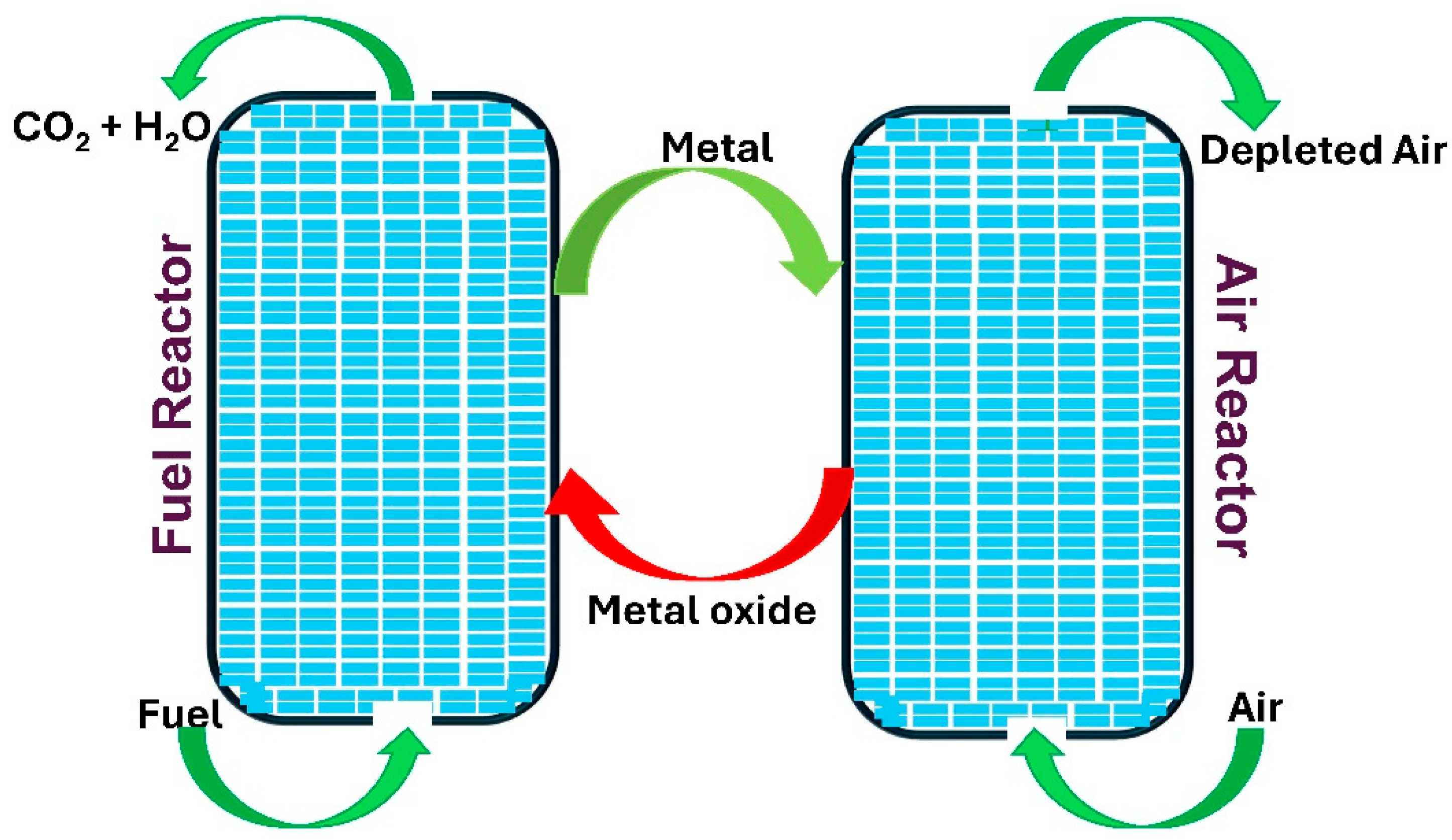
3.5. Chemical Looping Combustion
3.6. Calcium Looping Combustion
4. CO2 Utilization
4.1. Direct Utilization of CO2
4.2. Utilization of CO2 by Conversion
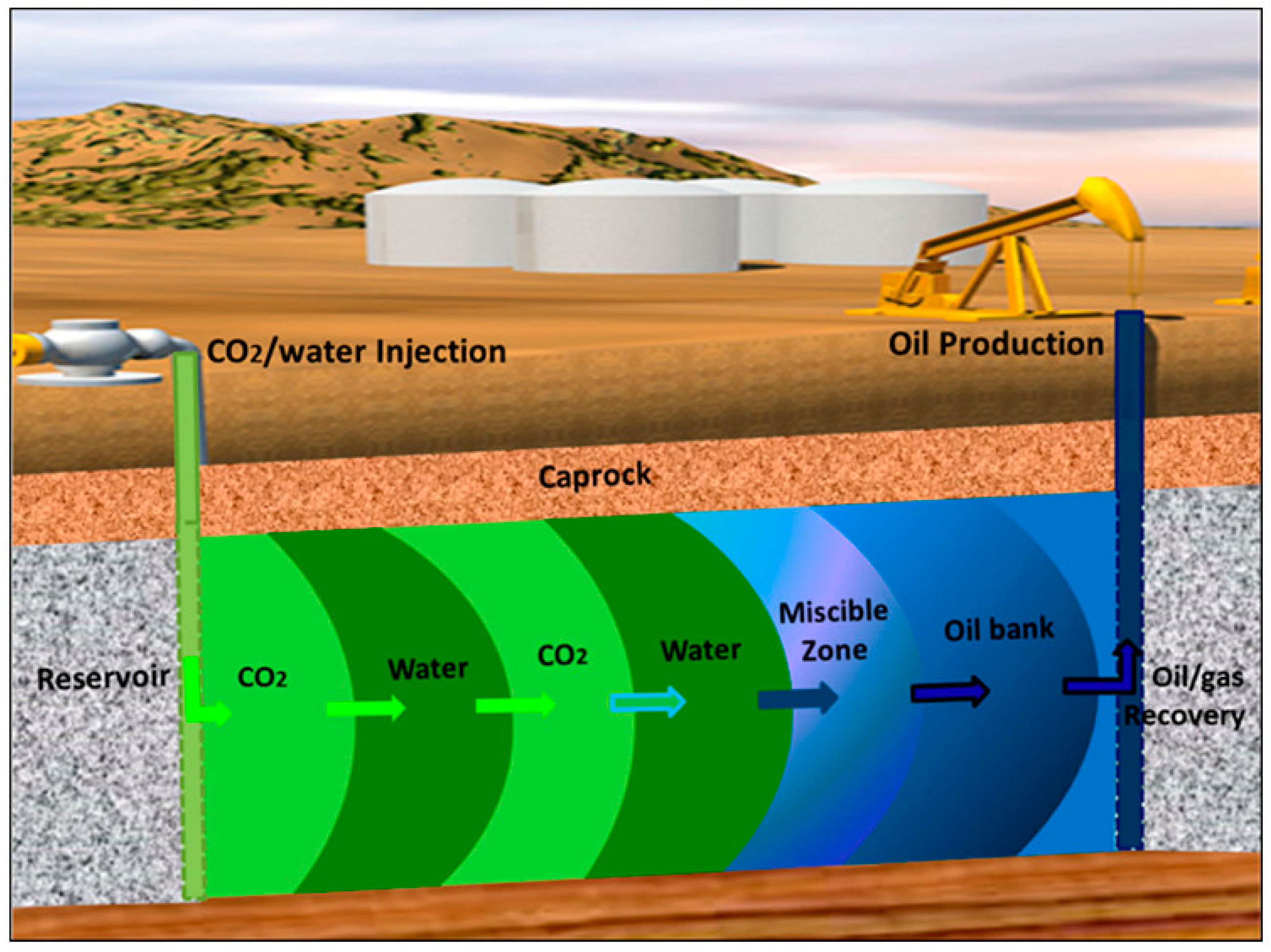
4.3. Enhanced Oil Recovery
5. Analysis and Outlooks
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erickson, P.; Lazarus, M.; Verkuijl, C.; Yehle, E. The Production Gap Report: The Discrepancy Between Countries’ Planned Fossil Fuel Production and Global Production Levels Consistent with Limiting Warming to 1.5 °C or 2 °C: 2020 Special Report. 2020. Available online: https://www.unep.org/resources/production-gap-report-2023 (accessed on 1 September 2020).
- Wuebbles, D.J.; Fahey, D.W.; Hibbard, K.A. Climate Science Special Report: Fourth National Climate Assessment; US Global Change Research Program: Washington, DC, USA, 2017; Volume I. [Google Scholar]
- Semenova, G. Global Environmental Problems in the World. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020. Available online: https://www.nrc.gov/docs/ML1900/ML19008A410.pdf (accessed on 14 December 2020).
- Consensus, S. Earth’s Climate is Warming; National Aeronautics and Space Administration (NASA): Washington, DC, USA, 2015. Available online: https://climate.nasa.gov/scientific-consensus (accessed on 20 June 2016).
- Wang, R.; Mirza, N.; Vasbieva, D.G.; Abbas, Q.; Xiong, D. The nexus of carbon emissions, financial development, renewable energy consumption, and technological innovation: What should be the priorities in light of COP 21 Agreements? J. Environ. Manag. 2020, 271, 111027. [Google Scholar] [CrossRef]
- Akimoto, K.; Sano, F.; Oda, J.; Kanaboshi, H.; Nakano, Y. Climate change mitigation measures for global net-zero emissions and the roles of CO2 capture and utilization and direct air capture. Energy Clim. Change 2021, 2, 100057. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Jelmy, E.J.; Thomas, N.; Mathew, D.T.; Louis, J.; Padmanabhan, N.T.; Kumaravel, V.; John, H.; Pillai, S.C. Impact of structure, doping and defect-engineering in 2D materials on CO2 capture and conversion. React. Chem. Eng. 2021, 6, 1701–1738. [Google Scholar] [CrossRef]
- Li, S.W.; Jiang, X.; Yang, Q.; Shao, L. Effects of amino functionalized polyhedral oligomeric silsesquioxanes on cross-linked poly(ethylene oxide) membranes for highly-efficient CO2 separation Chem. Eng. Res. Des. 2017, 122, 280–288. [Google Scholar] [CrossRef]
- Rana, A.; Andino, J.M. A Review of Materials for Carbon Dioxide Capture. Catalysts 2025, 15, 273. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Tebbiche, I.; Mocellin, J.; Huong, L.T.; Pasquier, L.-C. 27—Circular Economy and Carbon Capture, Utilization, and Storage. In Biomass, Biofuels, Biochemicals; Pandey, A., Tyagi, R.D., Varjani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 813–851. [Google Scholar]
- Raza, A.; Glatz, G.; Gholami, R.; Mahmoud, M.; Alafnan, S. Carbon mineralization and geological storage of CO2 in basalt: Mechanisms and technical challenges. Earth-Sci. Rev. 2022, 229, 104036. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Jiang, K.; Ashworth, P.; Zhang, S.; Liang, X.; Sun, Y.; Angus, D. China’s carbon capture, utilization and storage (CCUS) policy: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109601. [Google Scholar] [CrossRef]
- Nwabueze, Q.A.; Leggett, S. Advancements in the Application of CO2 Capture and Utilization Technologies—A Comprehensive Review. Fuels 2024, 5, 508–532. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Rehman, A.; Ma, H.; Ozturk, I.; Murshed, M.; Dagar, V. The dynamic impacts of CO2 emissions from different sources on Pakistan’s economic progress: A roadmap to sustainable development. Environ. Dev. Sustain. 2021, 23, 17857–17880. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. Chapter 1—CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar]
- Liu, Z.; Ciais, P.; Deng, Z.; Davis, S.J.; Zheng, B.; Wang, Y.; Cui, D.; Zhu, B.; Dou, X.; Ke, P.; et al. Carbon Monitor, a near-real-time daily dataset of global CO2 emission from fossil fuel and cement production. Sci. Data 2020, 7, 392. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- De Kleijne, K.; Hanssen, S.V.; Van Dinteren, L.; Huijbregts, M.A.J.; Van Zelm, R.; De Coninck, H. Limits to Paris compatibility of CO2 capture and utilization. One Earth 2022, 5, 168–185. [Google Scholar] [CrossRef]
- Karlsson, O.; Rocklöv, J.; Lehoux, A.P.; Bergquist, J.; Rutgersson, A.; Blunt, M.J.; Birnbaum, L.S. The human exposome and health in the Anthropocene. Int. J. Epidemiol. 2020, 50, 378–389. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials and hybrid nanocomposites for CO2 capture and utilization: Environmental and energy sustainability. RSC Adv. 2022, 12, 23869–23888. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Rownaghi, A.A.; Rezaei, F. Combined Capture and Utilization of CO2 for Syngas Production over Dual-Function Materials. ACS Sustain. Chem. Eng. 2018, 6, 13551–13561. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative Techno-Economic Analysis of Carbon Capture Processes: Pre-Combustion, Post-Combustion, and Oxy-Fuel Combustion Operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Biermann, M.; Langner, C.; Roussanaly, S.; Normann, F.; Harvey, S. The role of energy supply in abatement cost curves for CO2 capture from process industry—A case study of a Swedish refinery. Appl. Energy 2022, 319, 119273. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Lai, L.S.; Jusoh, N.; Tay, W.H.; Yeong, Y.F.; Kiew, P.L. Rapid Prototyping for the Formulation of Monolith and Membrane for CO2 Removal. Sep. Purif. Rev. 2021, 51, 503–520. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- de Riva, J.; Suarez-Reyes, J.; Moreno, D.; Díaz, I.; Ferro, V.; Palomar, J. Ionic liquids for post-combustion CO2 capture by physical absorption: Thermodynamic, kinetic and process analysis. Int. J. Greenh. Gas Control 2017, 61, 61–70. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A. Minimising the regeneration heat duty of post-combustion CO2 capture by wet chemical absorption: The misguided focus on low heat of absorption solvents. Int. J. Greenh. Gas Control 2010, 4, 36–43. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Xie, R.; Rutherford, M.; Peng, X. Formation of High-Quality I–III–VI Semiconductor Nanocrystals by Tuning Relative Reactivity of Cationic Precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Wang, Y.; Qi, C.; Zhang, S.; Wang, L.; Li, M. Development of biphasic solvent for CO2 capture by tailoring the polarity of amine solution. Fuel 2022, 325, 124885. [Google Scholar] [CrossRef]
- El-Said, W.A.; Choi, J.-H.; Hajjar, D.; Makki, A.A.; Choi, J.-W. Fabrication of Hollow Nanocones Membrane with an Extraordinary Surface Area as CO2 Sucker. Polymers 2022, 14, 183. [Google Scholar] [CrossRef]
- Mostafavi, E.; Ashrafi, O.; Navarri, P. Assessment of process modifications for amine-based post-combustion carbon capture processes. Clean. Eng. Technol. 2021, 4, 100249. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Wang, Y.; Shen, J.; Wang, Y.; Geng, Z.; Wang, Q.; Zhu, T. Progress of CCUS technology in the iron and steel industry and the suggestion of the integrated application schemes for China. Chem. Eng. J. 2022, 450, 138438. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. Identifying Cost-Effective CO2 Control Levels for Amine-Based CO2 Capture Systems. Ind. Eng. Chem. Res. 2006, 45, 2421–2429. [Google Scholar] [CrossRef]
- Santos, S.P.; Gomes, J.F.; Bordado, J.C. Scale-Up Effects of CO2 Capture by Methyldiethanolamine (MDEA) Solutions in Terms of Loading Capacity. Technologies 2016, 4, 19. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Peruzzini, M.; Barzagli, F.; Zhang, R. Investigating the Performance of Ethanolamine and Benzylamine Blends as Promising Sorbents for Postcombustion CO2 Capture through 13C NMR Speciation and Heat of CO2 Absorption Analysis. Energy Fuels 2022, 36, 9203–9212. [Google Scholar] [CrossRef]
- Liu, X.; Ao, Q.; Shi, S.; Li, S. CO2 capture by alcohol ammonia based deep eutectic solvents with different water content. Mater. Res. Express 2022, 9, 015504. [Google Scholar] [CrossRef]
- Xiao, M.; Cui, D.; Yang, Q.; Liang, Z.; Puxty, G.; Yu, H.; Li, L.; Conway, W.; Feron, P. Role of mono- and diamines as kinetic promoters in mixed aqueous amine solution for CO2 capture. Chem. Eng. Sci. 2021, 229, 116009. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H. Kinetics of CO2 absorption in aqueous N, N-diethylethanolamine and its blend with N-(2-aminoethyl) ethanolamine using a stirred cell reactor. Int. J. Greenh. Gas Control 2015, 37, 76–84. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Yu, H. Modelling analysis of solid precipitation in an ammonia-based CO2 capture process. Int. J. Greenh. Gas Control. 2014, 30, 133–139. [Google Scholar] [CrossRef]
- Wagaarachchige, J.D.; Idris, Z.; Arstad, B.; Halstensen, M.; Jens, K.-J. Low-Viscosity Nonaqueous Sulfolane–Amine–Methanol Solvent Blend for Reversible CO2 Capture. Ind. Eng. Chem. Res. 2022, 61, 5942–5951. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Atzori, F.; Barzagli, F.; Varone, A.; Cao, G.; Concas, A. CO2 absorption in aqueous NH3 solutions: Novel dynamic modeling of experimental outcomes. Chem. Eng. J. 2023, 451, 138999. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Behaein, S. Intensified biogas upgrading via various wastewater using microchannel. Chem. Eng. Process.-Process Intensif. 2022, 175, 108927. [Google Scholar] [CrossRef]
- Srivastava, R.K. Controlling SO2 Emissions—A Review of Technologies. 2000. Available online: http://purl.access.gpo.gov/GPO/LPS34639 (accessed on 10 June 2025).
- Chen, N. Morphology-Dependent Performance of Praseodymium-Based Materials via Controllable Synthesis for Energy Conversion and Storage. Ph.D. Thesis, UNSW Sydney, Sydney, Australia, 2020. Available online: http://hdl.handle.net/1959.4/67155 (accessed on 10 June 2025).
- Seddah, F.; Mustafa, D.; Bouziyah, A.; Battah, A. Carbon Dioxide Capture and Sequestration: Materials and Technology Potentials. 2020. Available online: https://hdl.handle.net/20.500.11888/16811 (accessed on 10 June 2025).
- Pragya, N.; Pandey, K.K.; Sahoo, P. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Li, L.; Conway, W.; Burns, R.; Maeder, M.; Puxty, G.; Clifford, S.; Yu, H. Investigation of metal ion additives on the suppression of ammonia loss and CO2 absorption kinetics of aqueous ammonia-based CO2 capture. Int. J. Greenh. Gas Control 2017, 56, 165–172. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Miao, H.; Zhao, J.; Zhang, H.; Yuan, J.; Yan, J. Current status and challenges of the ammonia escape inhibition technologies in ammonia-based CO2 capture process. Appl. Energy 2018, 230, 734–749. [Google Scholar] [CrossRef]
- Chu, F.; Jon, C.; Yang, L.; Du, X.; Yang, Y. CO2 Absorption Characteristics in Ammonia Solution inside the Structured Packed. Ind. Eng. Chem. Res. 2016, 55, 3696–3709. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Aryafard, M.; Minofar, B.; Cséfalvay, E.; Malinová, L.; Řeha, D. Novel room temperature ionic liquids and low melting mixtures based on imidazolium: Cheap ionic solvents for chemical and biological applications. J. Mol. Liq. 2021, 344, 117877. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, L.; Zeng, S.; Gao, H.; Zhang, S.; Fan, M. Ionic Liquids: Advanced Solvents for CO2 Capture. In Energy Efficient Solvents for CO2 Capture by Gas-Liquid Absorption: Compounds, Blends and Advanced Solvent Systems; Budzianowski, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–176. [Google Scholar]
- Jiang, W.; Li, X.; Gao, G.; Wu, F.; Luo, C.; Zhang, L. Advances in applications of ionic liquids for phase change CO2 capture. Chem. Eng. J. 2022, 445, 136767. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, N.; Wang, J.; Wang, C. Computer-assisted design of imidazolate-based ionic liquids for improving sulfur dioxide capture, carbon dioxide capture, and sulfur dioxide/carbon dioxide selectivity. Chem. Asian J. 2017, 12, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.M.; Zanatta, M.; Neumann, J.; Girard, A.-L.; Marin, G.; Stassen, H.; Dupont, J. Cation-Anion-CO2 interactions in imidazolium based ionic liquid sorbents. ChemPhysChem 2018, 19, 2879–2884. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Sridhar, V. Molecular understanding of carbon dioxide interactions with ionic liquids. J. Mol. Liq. 2021, 325, 115162. [Google Scholar] [CrossRef]
- Numpilai, T.; Pham, L.K.H.; Witoon, T. Advances in Ionic Liquid Technologies for CO2 Capture and Conversion: A Comprehensive Review. Ind. Eng. Chem. Res. 2024, 63, 19865–19915. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Morrow, T.I.; Brennecke, J.F.; Maginn, E.J. Why Is CO2 So Soluble in Imidazolium-Based Ionic Liquids? J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef]
- Hu, H.; Li, F.; Xia, Q.; Li, X.; Liao, L.; Fan, M. Research on influencing factors and mechanism of CO2 absorption by poly-amino-based ionic liquids. Int. J. Greenh. Gas Control 2014, 31, 33–40. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, R.; Liu, Z.; Liu, H.; Xu, C.; Meng, X.; Liang, M.; Liang, S. Absorption Performance and Mechanism of CO2 in Aqueous Solutions of Amine-Based Ionic Liquids. Energy Fuels 2015, 29, 6019–6024. [Google Scholar] [CrossRef]
- Kumar, S.; Cho, J.H.; Moon, I. Ionic liquid-amine blends and CO2BOLs: Prospective solvents for natural gas sweetening and CO2 capture technology—A review. Int. J. Greenh. Gas Control 2014, 20, 87–116. [Google Scholar] [CrossRef]
- Pinto, A.M.; Rodríguez, H.; Arce, A.; Soto, A. Carbon dioxide absorption in the ionic liquid 1-ethylpyridinium ethylsulfate and in its mixtures with another ionic liquid. Int. J. Greenh. Gas Control 2013, 18, 296–304. [Google Scholar] [CrossRef]
- Suleman, H.; Maulud, A.S.; Fosbøl, P.L.; Nasir, Q.; Nasir, R.; Shahid, M.; Nawaz, M.; Abunowara, M. A review of semi-empirical equilibrium models for CO2-alkanolamine-H2O solutions and their mixtures at high pressure. J. Environ. Chem. Eng. 2021, 9, 104713. [Google Scholar] [CrossRef]
- Alcantara, M.L.; de Carvalho, M.L.; Álvarez, V.H.; Ferreira, P.I.; Paredes, M.L.; Cardozo-Filho, L.; Silva, A.K.; Lião, L.M.; Pires, C.A.M.; Mattedi, S. High pressure vapor-liquid equilibria for binary carbon dioxide and protic ionic liquid based on ethanolamines+ butanoic acid. Fluid Phase Equilibria 2018, 460, 162–174. [Google Scholar] [CrossRef]
- Shaahmadi, F.; Shahraki, B.H.; Farhadi, A. The Solubility of Carbon Dioxide and Density for Binary Mixtures of 1-Butyl-3-methylimidazolium Acetate and 1-Butyl-3-methylimidazolium Tetrafluoroborate. J. Chem. Eng. Data 2019, 64, 584–593. [Google Scholar] [CrossRef]
- Murge, P.; Dinda, S.; Roy, S. Zeolite-based sorbent for CO2 capture: Preparation and performance evaluation. Langmuir 2019, 35, 14751–14760. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Wang, T.; Du, Y.; Yang, Y.; Jing, X.; Zhu, G. Imidazolium-Functionalized Ionic Porous Aromatic Frameworks for CO2 Capture and In Situ Conversion. Ind. Eng. Chem. Res. 2022, 61, 7284–7291. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Lan, J.; Xu, P.; Sun, J. Porous Zn(Bmic)(AT) MOF with Abundant Amino Groups and Open Metal Sites for Efficient Capture and Transformation of CO2. Inorg. Chem. 2019, 58, 13917–13926. [Google Scholar] [CrossRef]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hareb, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.L.; Srivastava, R. Trends in solid adsorbent materials development for CO2 capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Chakraborty, R.; Vilya, K.; Pradhan, M.; Nayak, A.K. Recent advancement of biomass-derived porous carbon based materials for energy and environmental remediation applications. J. Mater. Chem. A 2022, 10, 6965–7005. [Google Scholar] [CrossRef]
- Javed, M.; Zahoor, M.; Mazari, S.A.; Qureshi, S.S.; Sabzoi, N.; Jatoi, A.S.; Mubarak, N.M. An overview of effect of process parameters for removal of CO2 using biomass-derived adsorbents. Biomass Convers. Biorefin. 2021, 13, 4495–4513. [Google Scholar] [CrossRef]
- Mukherjee, A. Generation of Activated Carbon from Spent Coffee Grounds: Process Optimization, Kinetics and CO2 Capture. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2022. Available online: https://harvest.usask.ca/items/cd27abca-b48b-4e0a-a82f-54f5600757b7 (accessed on 10 June 2025).
- Hao, W.; Björnerbäck, F.; Trushkina, Y.; Bengoechea, M.O.; Salazar-Alvarez, G.; Barth, T.; Hedin, N. High-performance magnetic activated carbon from solid waste from lignin conversion processes. 1. Their use as adsorbents for CO2. ACS Sustain. Chem. Eng. 2017, 5, 3087–3095. [Google Scholar] [CrossRef]
- Pavlenko, V.; Khosravi H, S.; Żółtowska, S.; Haruna, A.B.; Zahid, M.; Mansurov, Z.; Supiyeva, Z.; Galal, A.; Ozoemena, K.I.; Abbas, Q.; et al. A comprehensive review of template-assisted porous carbons: Modern preparation methods and advanced applications. Mater. Sci. Eng. R Rep. 2022, 149, 100682. [Google Scholar] [CrossRef]
- Saleem, A.; Zhang, Y.; Usman, M.; Haris, M.; Li, P. Tailored architectures of mesoporous carbon nanostructures: From synthesis to applications. Nano Today 2022, 46, 101607. [Google Scholar] [CrossRef]
- Ghavipanjeh, F.; Yavari, M.; Beygzadeh, M.; Sheikholeslami, Z. Facile fabrication of flexible super hydrophilic activated carbon sponge for continuous capillary adsorption. J. Water Process Eng. 2022, 49, 102945. [Google Scholar] [CrossRef]
- Penchah, H.R.; Ghaemi, A.; Jafari, F. Piperazine-modified activated carbon as a novel adsorbent for CO2 capture: Modeling and characterization. Environ. Sci. Pollut. Res. 2022, 29, 5134–5143. [Google Scholar] [CrossRef]
- Machida, M.; Yoo, P.; Amano, Y. Adsorption of nitrate from aqueous phase onto nitrogen-doped activated carbon fibers (ACFs). SN Appl. Sci. 2019, 1, 323. [Google Scholar] [CrossRef]
- Prasad, R.; Desai, C.; Prakash, S.O.; Prasad, S.; Bhat, T.; Kamble, B.; Sarvalkar, P.; Kanthe, A.; Kale, R.; Banga, S.; et al. A Critical Review on Design and Development of Carbonaceous Materials for Veterinary Medicine. ES Food Agrofor. 2022, 9, 15–38. [Google Scholar]
- Saleh, M.; Chandra, V.; Kemp, K.C.; Kim, K.S. Synthesis of N-doped microporous carbon via chemical activation of polyindole-modified graphene oxide sheets for selective carbon dioxide adsorption. Nanotechnology 2013, 24, 255702. [Google Scholar] [CrossRef]
- Kemp, K.C.; Chandra, V.; Saleh, M.; Kim, K.S. Reversible CO2 adsorption by an activated nitrogen doped graphene/polyaniline material. Nanotechnology 2013, 24, 235703. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; He, L.; Tang, J.; Zhou, Y.; Li, J.; Ding, K. One-pot synthesis of multifunctional magnetic N-doped graphene composite for SERS detection, adsorption separation and photocatalytic degradation of Rhodamine 6G. Chem. Eng. J. 2017, 327, 694–704. [Google Scholar] [CrossRef]
- He, Y. Transition Metal Oxide Nanostructures in Heterogeneous Catalysis; Yale University: New Haven, CT, USA, 2020. [Google Scholar]
- Aguirre-Cruz, G.; Legorreta-Garcia, F.; Stanciu, L.; Aguirre-Alvarez, G. Synthesis of hierarchical silica zeolites for heterogenous catalysis and adsorption. Microporous Mesoporous Mater. 2022, 111, 112274. [Google Scholar] [CrossRef]
- Semeykina, V.; Zharov, I. Medium controlled aggregative growth as a key step in mesoporous silica nanoparticle formation. J. Colloid Interface Sci. 2022, 615, 236–247. [Google Scholar] [CrossRef]
- Qi, L.; Yang, W.; Zhang, L.; Liu, Q.; Fei, Z.; Chen, X.; Zhang, Z.; Tang, J.; Cui, M.; Qiao, X. Reinforced CO2 Capture on Amine-Impregnated Organosilica with Double Brush-like Additives Modified. Ind. Eng. Chem. Res. 2022, 61, 14859–14867. [Google Scholar] [CrossRef]
- Gebretatios, A.G.; Pillantakath, A.R.K.K.; Witoon, T.; Lim, J.-W.; Banat, F.; Cheng, C.K. Rice husk waste into various template-engineered mesoporous silica materials for different applications: A comprehensive review on recent developments. Chemosphere 2022, 310, 136843. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, X.; Chen, Y.; Chen, S. Preparation and structure tuning of CO2 adsorbent based on in-situ amine-functionalized hierarchical porous polymer. Microporous Mesoporous Mater. 2022, 330, 111585. [Google Scholar] [CrossRef]
- Li, T.; Yang, C.; Tantikhajorngosol, P.; Sema, T.; Tontiwachwuthikul, P. Experimental investigations of CO2 absorption and catalyst-aided CO2 desorption performance of several different amines blending with a promoter. Chem. Eng. Sci. 2022, 264, 118177. [Google Scholar] [CrossRef]
- Fan, Y.; Jia, X. Progress in Amine-Functionalized Silica for CO2 Capture: Important Roles of Support and Amine Structure. Energy Fuels 2022, 36, 1252–1270. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Q.; Wang, L.; Meng, F.; Dai, H. Mesoporous alumina-supported layered double hydroxides for efficient CO2 capture. J. CO2 Util. 2022, 60, 101982. [Google Scholar] [CrossRef]
- Silva, D.P.d.S.; Silva, D.C.M.; Ribeiro, T.R.S.; Solano, J.R.S.; da Silva, B.J.B.; Altino, S.A.; da Silva, A.O.S. Mesoporous Alumina Synthesis Using Carboxylic Acids to Enhance Performance in CO2 Adsorption. J. Environ. Chem. Eng. 2022, 10, 108928. [Google Scholar] [CrossRef]
- Feist, B.J.; Hill, J.M. CO2 and H2S adsorption on γ-Al2O3-supported lanthanum oxide. Energy Fuels 2015, 29, 6049–6056. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J. Cellulose derivatives and cellulose-metal-organic frameworks for CO2 adsorption and separation. J. CO2 Util. 2022, 64, 102163. [Google Scholar] [CrossRef]
- Fu, D.; Davis, M.E. Carbon dioxide capture with zeotype materials. Chem. Soc. Rev. 2022, 51, 9340–9370. [Google Scholar] [CrossRef] [PubMed]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Coudert, F.-X.; Kohen, D. Molecular insight into CO2 “trapdoor” adsorption in zeolite Na-RHO. Chem. Mater. 2017, 29, 2724–2730. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced Strategies in Metal-Organic Frameworks for CO2 Capture and Separation. Chem. Rec. 2022, 22, e202100230. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, J.; Mittal, A.; Kumari, K.; Maken, S.; Kumar, N. Carbon materials as CO2 adsorbents: A review. Environ. Chem. Lett. 2021, 19, 875–910. [Google Scholar] [CrossRef]
- Milner, P.J.; Siegelman, R.L.; Forse, A.C.; Gonzalez, M.I.; Runčevski, T.; Martell, J.D.; Reimer, J.A.; Long, J.R. A Diaminopropane-Appended Metal–Organic Framework Enabling Efficient CO2 Capture from Coal Flue Gas via a Mixed Adsorption Mechanism. J. Am. Chem. Soc. 2017, 139, 13541–13553. [Google Scholar] [CrossRef]
- Milner, P.J.; Martell, J.D.; Siegelman, R.L.; Gygi, D.; Weston, S.C.; Long, J.R. Overcoming double-step CO2 adsorption and minimizing water co-adsorption in bulky diamine-appended variants of Mg2(dobpdc). Chem. Sci. 2018, 9, 160–174. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Hu, X.; Liu, L.; Luo, X.; Xiao, G.; Shiko, E.; Zhang, R.; Fan, X.; Zhou, Y.; Liu, Y.; Zeng, Z.; et al. A review of N-functionalized solid adsorbents for post-combustion CO2 capture. Appl. Energy 2020, 260, 114244. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Adewuyi, Y.G. State-of-the-art review on capture of CO2 using adsorbents prepared from waste materials. Process Saf. Environ. Prot. 2020, 139, 1–25. [Google Scholar] [CrossRef]
- Zulys, A.; Yulia, F.; Muhadzib, N. Nasruddin Biological Metal–Organic Frameworks (Bio-MOFs) for CO2 Capture. Ind. Eng. Chem. Res. 2021, 60, 37–51. [Google Scholar] [CrossRef]
- Gutierrez-Ortega, A.; Montes-Morán, M.A.; Parra, J.B.; Sempere, J.; Nomen, R.; Gonzalez-Olmos, R. Comparative study of binderless zeolites and carbon molecular sieves as adsorbents for CO2 capture processes. J. CO2 Util. 2022, 61, 102012. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Flaig, R.W.; Popp, T.M.O.; Fracaroli, A.M.; Kapustin, E.A.; Kalmutzki, M.J.; Altamimi, R.M.; Fathieh, F.; Reimer, J.A.; Yaghi, O.M. The Chemistry of CO2 Capture in an Amine-Functionalized Metal–Organic Framework under Dry and Humid Conditions. J. Am. Chem. Soc. 2017, 139, 12125–12128. [Google Scholar] [CrossRef]
- Fracaroli, A.M.; Furukawa, H.; Suzuki, M.; Dodd, M.; Okajima, S.; Gándara, F.; Reimer, J.A.; Yaghi, O.M. Metal–Organic Frameworks with Precisely Designed Interior for Carbon Dioxide Capture in the Presence of Water. J. Am. Chem. Soc. 2014, 136, 8863–8866. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, R.; Pan, C.-Y.; Jiang, H.-L. Unprecedented Li+ Exchange in an Anionic Metal–Organic Framework: Significantly Enhanced Gas Uptake Capacity. Inorg. Chem. 2017, 56, 4263–4266. [Google Scholar] [CrossRef]
- Shi, Z.; Tao, Y.; Wu, J.; Zhang, C.; He, H.; Long, L.; Lee, Y.; Li, T.; Zhang, Y.-B. Robust Metal–Triazolate Frameworks for CO2 Capture from Flue Gas. J. Am. Chem. Soc. 2020, 142, 2750–2754. [Google Scholar] [CrossRef]
- Bien, C.E.; Chen, K.K.; Chien, S.-C.; Reiner, B.R.; Lin, L.-C.; Wade, C.R.; Ho, W.S.W. Bioinspired Metal–Organic Framework for Trace CO2 Capture. J. Am. Chem. Soc. 2018, 140, 12662–12666. [Google Scholar] [CrossRef] [PubMed]
- Bermeo, M.; Vega, L.F.; Abu-Zahra, M.R.M.; Khaleel, M. Critical assessment of the performance of next-generation carbon-based adsorbents for CO2 capture focused on their structural properties. Sci. Total Environ. 2022, 810, 151720. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, S.; Li, Z.; Wen, S.; Ning, P.; Lu, S.; Lu, P.; Huang, L.; Wang, Q. Comprehensive investigation of dynamic CO2 capture performance using Mg/DOBDC as precursor to fabricate a composite of metallic organic framework and graphene oxide. Chem. Eng. J. 2021, 415, 128859. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, D.; Wu, J.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Y.; Lv, Z.; Song, F.; Zhong, Q. Preparation and enhanced CO2 adsorption capacity of UiO-66/graphene oxide composites. J. Ind. Eng. Chem. 2015, 27, 102–107. [Google Scholar] [CrossRef]
- Zhao, H.; Bahamon, D.; Khaleel, M.; Vega, L.F. Insights into the performance of hybrid graphene oxide/MOFs for CO2 capture at process conditions by molecular simulations. Chem. Eng. J. 2022, 449, 137884. [Google Scholar] [CrossRef]
- Le, V.N.; Vo, T.K.; Yoo, K.S.; Kim, J. Enhanced CO2 adsorption performance on amino-defective UiO-66 with 4-amino benzoic acid as the defective linker. Sep. Purif. Technol. 2021, 274, 119079. [Google Scholar] [CrossRef]
- Wang, J.-X.; Liang, C.-C.; Gu, X.-W.; Wen, H.-M.; Jiang, C.; Li, B.; Qian, G.; Chen, B. Recent advances in microporous metal–organic frameworks as promising adsorbents for gas separation. J. Mater. Chem. A 2022, 10, 17878–17916. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Zhao, Y.; Sun, J. Hydrogen bond donor functionalized poly(ionic liquids)@MIL-101 for the CO2 capture and improving the catalytic CO2 conversion with epoxide. J. Colloid Interface Sci. 2022, 618, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ban, Y.; Yang, W. Assembly of ionic liquid molecule layers on metal–organic framework-808 for CO2 capture. Chem. Eng. J. 2022, 439, 135650. [Google Scholar] [CrossRef]
- Nguyen, H.T.D.; Tran, Y.B.N.; Nguyen, T.C.; Gándara, F.; Nguyen, P.T.K. A Series of Metal–Organic Frameworks for Selective CO2 Capture and Catalytic Oxidative Carboxylation of Olefins. Inorg. Chem. 2018, 57, 13772–13782. [Google Scholar] [CrossRef]
- Li, S.; Chang, S.-M.; Yin, M.-J.; Zhang, W.-H.; Sun, W.-S.; Shiue, A.; An, Q.-F. Build up ‘highway’ in membrane via solvothermal annealing for high-efficient CO2 capture. J. Membr. Sci. 2022, 652, 120444. [Google Scholar] [CrossRef]
- Hossain, I.; Park, S.; Husna, A.; Kim, Y.; Kim, H.; Kim, T.-H. PIM-PI-1 and Poly(ethylene glycol)/Poly(propylene glycol)-Based Mechanically Robust Copolyimide Membranes with High CO2-Selectivity and an Anti-aging Property: A Joint Experimental–Computational Exploration. ACS Appl. Mater. Interfaces 2021, 13, 49890–49906. [Google Scholar] [CrossRef]
- Nicotera, I.; Policicchio, A.; Conte, G.; Agostino, R.G.; Rehman, M.H.U.; Lufrano, E.; Simari, C. Quaternized polyepichlorohydrin-based membrane as high-selective CO2 sorbent for cost-effective carbon capture. J. CO2 Util. 2022, 63, 102135. [Google Scholar] [CrossRef]
- Sood, A.; Thakur, A.; Ahuja, S.M. Recent ameliorations in membrane based carbon capture technologies. Mater. Today Proc. 2022, 62, 6514–6529. [Google Scholar] [CrossRef]
- Wang, B.; Huang, W.; Zhu, Y.; Zhou, R.; Xing, W. Ultra-permeable high-selective SAPO-34 membranes for efficient CO2 capture. J. Membr. Sci. 2022, 650, 120420. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Qian, J.; Song, E.; Lian, H.; Jiang, J.; Wang, C.; Pan, Y. High-performance ZIF-302 mixed-matrix membranes for efficient CO2 capture. Korean J. Chem. Eng. 2022, 39, 1020–1027. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Sun, T.; Ruan, X.; Dai, Y.; Li, X.; Zhang, C.; He, G. PAN electrospun nanofiber skeleton induced MOFs continuous distribution in MMMs to boost CO2 capture. J. Membr. Sci. 2022, 650, 120330. [Google Scholar] [CrossRef]
- Bano, S.; Tariq, S.R.; Anjum, T.; Najam, M.; Usman, M.; Yasin, M.; Shafi, H.Z.; Khan, A.L. Development of highly permselective Mixed Matrix Membranes comprising of polyimide and Ln-MOF for CO2 capture. Chemosphere 2022, 307, 136051. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Z.; Ding, R.; Dai, Y.; Li, X.; Ruan, X.; He, G. Constructing continuous and fast transport pathway by highly permeable polymer electrospun fibers in composite membrane to improve CO2 capture. Sep. Purif. Technol. 2022, 285, 120332. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Yu, H.; Yang, S.; Li, K. Copper electrowinning-coupled CO2 capture in solvent based post-combustion capture. Appl. Energy 2022, 316, 119086. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, L.; Wang, Y.; Song, X.; Zhao, Z.; Lan, C.; Wu, Y.; Liu, T.; Xie, H. Electrochemical proton-coupled electron transfer driven amine regeneration for CO2 capture. Chem. Eng. J. 2025, 520, 166315. [Google Scholar]
- Sharifian, R.; Wagterveld, R.M.; Digdaya, I.A.; Xiang, C.; Vermaas, D.A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 2021, 14, 781–814. [Google Scholar] [CrossRef]
- Renfrew, S.E.; Starr, D.E.; Strasser, P. Electrochemical Approaches toward CO2 Capture and Concentration. ACS Catal. 2020, 10, 13058–13074. [Google Scholar] [CrossRef]
- Legrand, L.; Shu, Q.; Tedesco, M.; Dykstra, J.E.; Hamelers, H.V.M. Role of ion exchange membranes and capacitive electrodes in membrane capacitive deionization (MCDI) for CO2 capture. J. Colloid Interface Sci. 2020, 564, 478–490. [Google Scholar] [CrossRef]
- Wang, C.; Li, K.; Yu, H.; Yang, S.; Jiang, K. Electrochemical behavior of Cu-mediated electrowinning-coupled CO2 capture. Electrochim. Acta 2022, 422, 140571. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Y.; Liu, T.; Wang, F.; Chen, B.; Liang, B. Low-energy-consumption electrochemical CO2 capture driven by biomimetic phenazine derivatives redox medium. Appl. Energy 2020, 259, 114119. [Google Scholar] [CrossRef]
- Gao, N.; Quiroz-Arita, C.; Diaz, L.A.; Lister, T.E. Intensified co-electrolysis process for syngas production from captured CO2. J. CO2 Util. 2021, 43, 101365. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Vankani, C.; Sánchez-Martínez, C.; Anastasopol, A.; Goetheer, E. Integrating CO2 Capture with Electrochemical Conversion Using Amine-Based Capture Solvents as Electrolytes. Ind. Eng. Chem. Res. 2021, 60, 4269–4278. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Ahmad, M.Z.; Malankowska, M.; Coronas, J. A new relevant membrane application: CO2 direct air capture (DAC). Chem. Eng. J. 2022, 446, 137047. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Adamu, A.; Russo-Abegão, F.; Boodhoo, K. Process intensification technologies for CO2 capture and conversion—A review. BMC Chem. Eng. 2020, 2, 2. [Google Scholar] [CrossRef]
- Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2. Catalysts 2020, 10, 1293. [Google Scholar] [CrossRef]
- Sabatino, F.; Mehta, M.; Grimm, A.; Gazzani, M.; Gallucci, F.; Kramer, G.J.; Annaland, M. van S. Evaluation of a Direct Air Capture Process Combining Wet Scrubbing and Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2020, 59, 7007–7020. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, W.; Wu, J.; Miao, Y.; Xiang, C.; Chen, C.; Ge, B.; Gan, Z.; Yang, F.; Zhang, M.; et al. Recent Advances in Direct Air Capture by adsorption. Chem. Soc. Rev. 2022, 51, 6574. [Google Scholar] [CrossRef]
- Sodiq, A.; Abdullatif, Y.; Aissa, B.; Ostovar, A.; Nassar, N.; El-Naas, M.; Amhamed, A. A Review on Progress Made in Direct Air Capture of CO2. Environ. Technol. Innov. 2023, 29, 102991. [Google Scholar] [CrossRef]
- Kotowicz, J.; Niesporek, K.; Baszczeńska, O. Advancements and Challenges in Direct Air Capture Technologies: Energy Intensity, Novel Methods, Economics, and Location Strategies. Energies 2025, 18, 496. [Google Scholar] [CrossRef]
- Leonzio, G.; Mwabonje, O.; Fennell, P.S.; Shah, N. Environmental performance of different sorbents used for direct air capture. Sustain. Prod. Consum. 2022, 32, 101–111. [Google Scholar] [CrossRef]
- Custelcean, R. Direct air capture of CO2 via crystal engineering. Chem. Sci. 2021, 12, 12518–12528. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hua, C.; Madden, D.G.; O’Nolan, D.; Chen, K.-J.; Keane, L.-A.J.; Perry, J.J.; Zaworotko, M.J. Hybrid ultramicroporous materials (HUMs) with enhanced stability and trace carbon capture performance. Chem. Commun. 2017, 53, 5946–5949. [Google Scholar] [CrossRef]
- Piscopo, C.G.; Loebbecke, S. Strategies to Enhance Carbon Dioxide Capture in Metal-Organic Frameworks. ChemPlusChem 2020, 85, 538–547. [Google Scholar] [CrossRef]
- Bien, C.E.; Liu, Q.; Wade, C.R. Assessing the Role of Metal Identity on CO2 Adsorption in MOFs Containing M–OH Functional Groups. Chem. Mater. 2020, 32, 489–497. [Google Scholar] [CrossRef]
- Sujan, A.R.; Pang, S.H.; Zhu, G.; Jones, C.W.; Lively, R.P. Direct CO2 Capture from Air using Poly(ethylenimine)-Loaded Polymer/Silica Fiber Sorbents. ACS Sustain. Chem. Eng. 2019, 7, 5264–5273. [Google Scholar] [CrossRef]
- Williams, N.J.; Seipp, C.A.; Brethomé, F.M.; Ma, Y.-Z.; Ivanov, A.S.; Bryantsev, V.S.; Kidder, M.K.; Martin, H.J.; Holguin, E.; Garrabrant, K.A.; et al. CO2 capture via crystalline hydrogen-bonded bicarbonate dimers. Chem 2019, 5, 719–730. [Google Scholar] [CrossRef]
- Stamberga, D.; Einkauf, J.D.; Liu, M.; Custelcean, R. Direct Air Capture of CO2 via Reactive Crystallization. Cryst. Growth Des. 2024, 24, 4556–4562. [Google Scholar] [CrossRef]
- Wang, J.; Fu, R.; Wen, S.; Ning, P.; Helal, M.H.; Salem, M.A.; Xu, B.B.; El-Bahy, Z.M.; Huang, M.; Guo, Z.; et al. Progress and current challenges for CO2 capture materials from ambient air. Adv. Compos. Hybrid Mater. 2022, 5, 2721–2759. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, Y.; Sun, C.; Sun, Y.; Zhu, Y. Comparative Review for Enhancing CO2 Capture Efficiency with Mixed Amine Systems and Catalysts. Molecules 2024, 29, 4618. [Google Scholar] [CrossRef] [PubMed]
- Arias, B.; Criado, Y.A.; Méndez, A.; Marqués, P.; Finca, I.; Abanades, J.C. Pilot Testing of Calcium Looping at TRL7 with CO2 Capture Efficiencies toward 99%. Energy Fuels 2024, 38, 14757–14764. [Google Scholar] [CrossRef]
- Hack, J.; Maeda, N.; Meie, D.M. Review on CO2 Capture Using Amine-Functionalized Materials. ACS Omega 2022, 7, 39520–39530. [Google Scholar] [CrossRef]
- Mantripragada, H.C.; Rubina, E.S. Chemical Looping for Pre-combustion CO2 Capture Performance and Cost Analysis. Energy Procedia 2013, 37, 618–625. [Google Scholar] [CrossRef]
- Küng, L.; Aeschlimann, S.; Charalambous, C.; McIlwaine, F.; Young, J.; Shannon, N.; Strassel, K.; Maesano, C.; Kahsar, R.; Pike, D.; et al. A roadmap for achieving scalable, safe, and low-cost direct air carbon capture and storage. Energy Environ. Sci. 2023, 16, 4280–4304. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, P.; Yang, A.; Fennell, P. Energy and exergy analysis of chemical looping combustion technology and comparison with pre-combustion and oxy-fuel combustion technologies for CO2 capture. J. Environ. Chem. Eng. 2015, 3, 2104–2114. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef]
- Ritcher, H.; Knoche, K. Reversibility of Combustion Process, Efficiency and Costing. Second Law Anal. Process 1983, 235, 71. [Google Scholar]
- Galinsky, N.L.; Huang, Y.; Shafiefarhood, A.; Li, F. Iron oxide with facilitated O2− transport for facile fuel oxidation and CO2 capture in a chemical looping scheme. ACS Sustain. Chem. Eng. 2013, 1, 364–373. [Google Scholar] [CrossRef]
- Lisi, L.; Mancino, G.; Cimino, S. Chemical looping oxygen transfer properties of Cu-doped lanthanum oxysulphate. Int. J. Hydrog. Energy 2015, 40, 2047–2054. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, B.; Wang, G.P.; Li, Y.H. O−2 release of Mn-based oxygen carrier for chemical looping air separation (CLAS): An insight into kinetic studies. Aerosol Air Qual. Res. 2016, 16, 453–463. [Google Scholar] [CrossRef]
- Nazir, S.M.; Bolland, O.; Amini, S. Full plant scale analysis of natural gas fired power plants with pre-combustion CO2 capture and chemical looping reforming (CLR). In Proceedings of the 13th International Conference on Greenhouse Gas Control Technologies, Ghgt-13 2017, Lausanne, Switzerland, 14–18 November 2016; Dixon, T., Laloui, L., Twinning, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2146–2155. [Google Scholar]
- Aghabararnejad, M.; Patience, G.S.; Chaouki, J. TGA and kinetic modeling of CO, Mn and Cu oxides for chemical looping gasification (CLG). Can. J. Chem. Eng. 2014, 92, 1903–1910. [Google Scholar] [CrossRef]
- Skulimowska, A.; Felice, L.D.; Kaminska-Pietrzak, N.; Celinska, A.; Plawecka, M.; Hercog, J.; Krauz, M.; Aranda, A. Chemical looping with oxygen uncoupling (CLOU) and chemical looping combustion (CLC) using copper-enriched oxygen carriers supported on fly ash. Fuel Process. Technol. 2017, 168, 123–130. [Google Scholar] [CrossRef]
- Wang, W.J. Thermodynamic investigation on hydrogen production via self-sufficient chemical looping reforming of glycerol (CLRG) using metal oxide oxygen carriers. J. Energy Inst. 2014, 87, 152–162. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrog. Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Olaleye, A.K.; Wang, M.H. Techno-economic analysis of chemical looping combustion with humid air turbine power cycle. Fuel 2014, 124, 221–231. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent CO2 separations-a review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of calcium-looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Colelli, G.; Chacartegui, R.; Ortiz, C.; Carro, A.; Arena, A.P.; Verda, V. Life cycle and environmental assessment of calcium looping (CaL) in solar thermochemical energy storage, Energy Convers. Manage 2022, 257, 115428. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H. CO2 capturing methods: Chemical looping combustion (CLC) as a promising technique. Sci. Total Environ. 2021, 788, 147850. [Google Scholar] [CrossRef] [PubMed]
- Arias, B.; Diego, M.E.; Méndez, A.; Alonso, M.; Abanades, J.C. Calcium looping performance under extreme oxy-fuel combustion conditions in the calciner. Fuel 2018, 222, 711–717. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Xing, S.; Pang, C.; Hao, Y.; Song, C.; Liu, Q. Progress in reducing calcination reaction temperature of calcium-looping CO2 capture technology: A critical review. Chem. Eng. J. 2022, 450, 137952. [Google Scholar] [CrossRef]
- Tregambi, C.; Montagnaro, F.; Salatino, P.; Solimene, R. A model of integrated calcium looping for CO2 capture and concentrated solar power. Solar Energy 2015, 120, 208–220. [Google Scholar] [CrossRef]
- Duan, L.; Feng, T.; Jia, S.; Yu, X. Study on the performance of coal-fired power plant integrated with Ca-looping CO2 capture system with recarbonation process. Energy 2016, 115, 942–953. [Google Scholar] [CrossRef]
- Kumari, P.; Amoodi, N.A.; Dumée, L.F.; Hajaj, A.A. Integrated calcium looping technologies for enhanced CO2 valorisation—A critical review. Carbon Capture Sci. Technol. 2025, 14, 100371. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Integration of Calcium and Chemical Looping Combustion using Composite CaO/CuO-Based Materials. Environ. Sci. Technol. 2011, 45, 10750–10756. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Arenas, L.V.A.; Navarrete, B. Carbon capture and utilization technologies: A literature review and recent advances. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Gulzar, A.; Gulzar, A.; Ansari, M.B.; He, F.; Gai, S.; Yang, P. Carbon dioxide utilization: A paradigm shift with CO2 economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Nagireddi, S.; Agarwal, J.R.; Vedapuri, D. Carbon Dioxide Capture, Utilization, and Sequestration: Current Status, Challenges, and Future Prospects for Global Decarbonization. ACS Eng. Au 2024, 4, 22–48. [Google Scholar] [CrossRef]
- Zanatta, M. Materials for Direct Air Capture and Integrated CO2 Conversion: Advancement, Challenges, and Prospects. ACS Mater. Au 2023, 3, 576–583. [Google Scholar] [CrossRef]
- Podder, J.; Patra, B.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K. A Review of Carbon Capture and Valorization Technologies. Energies 2023, 16, 2589. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Abu-Reesh, I.M.; Mohanakrishna, G.; Bulut, M.; Pant, D. Advanced Routes of Biological and Bio-electrocatalytic Carbon Dioxide (CO2) Mitigation Toward Carbon Neutrality. Front. Energy Res. 2020, 8, 94. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Wang, L.-C.; Sugar, J.D.; Wu, W.; Ding, Y.; Li, J.; Ding, D. Switching of metal-oxygen hybridization for selective CO2 electrohydrogenation under mild temperature and pressure. Nat. Catal. 2021, 4, 274–283. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D. Carbon mineralization with concurrent critical metal recovery from olivine. Proc. Natl. Acad. Sci. USA 2022, 119, e2203937119. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Shibata, E. Carbon Capture and Utilization Technology without Carbon Dioxide Purification and Pressurization: A Review on Its Necessity and Available Technologies. Ind. Eng. Chem. Res. 2019, 58, 8941–8954. [Google Scholar] [CrossRef]
- Mikulčić, H.; Skov, I.R.; Dominković, D.F.; Alwi, S.R.W.; Manan, Z.A.; Tan, R.; Duić, N.; Mohamad, S.N.H.; Wang, X. Flexible Carbon Capture and Utilization technologies in future energy systems and the utilization pathways of captured CO2. Renew. Sustain. Energy Rev. 2019, 114, 109338. [Google Scholar] [CrossRef]
- Heeschen, K.U.; Deusner, C.; Spangenberg, E.; Priegnitz, M.; Kossel, E.; Strauch, B.; Bigalke, N.; Luzi-Helbing, M.; Haeckel, M.; Schicks, J.M. Production Method under Surveillance: Laboratory Pilot-Scale Simulation of CH4–CO2 Exchange in a Natural Gas Hydrate Reservoir. Energy Fuels 2021, 35, 10641–10658. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Khandelwal, H.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. CO2 hydrate stability in oceanic sediments under brine conditions. Energy 2022, 256, 124625. [Google Scholar] [CrossRef]
- Chauvy, R.; Meunier, N.; Thomas, D.; De Weireld, G. Selecting emerging CO2 utilization products for short-to mid-term deployment. Appl. Energy 2019, 236, 662–680. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Thakur, V.K.; Saini, V.; Khan, A.A.P.; Singh, N.; Singh, P. An overview on polymeric carbon nitride assisted photocatalytic CO2 reduction: Strategically manoeuvring solar to fuel conversion efficiency. Chem. Eng. Sci. 2021, 230, 116219. [Google Scholar] [CrossRef]
- Grim, R.G.; Huang, Z.; Guarnieri, M.T.; Ferrell, J.R.; Tao, L.; Schaidle, J.A. Transforming the carbon economy: Challenges and opportunities in the convergence of low-cost electricity and reductive CO2 utilization. Energy Environ. Sci. 2020, 13, 472–494. [Google Scholar] [CrossRef]
- Bragoni, V.; Gooßen, L.J. Development of Sustainable Catalytic Methods for the Chemical Utilisation of Carbon Dioxide and for the Valorisation of Cashew Nut Shell Liquid. Ph.D. Thesis, Ruhr-Universität Bochum, Bochum, Germany, 2020. [Google Scholar] [CrossRef]
- Lopes, E.J.; Ribeiro, A.P.; Martins, L.M. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Craven, M.; Wang, Y.; Kang, D.; Wu, C.; Tu, X. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renew. Sustain. Energy Rev. 2021, 135, 109702. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Beydoun, K.; Klankermayer, J. Recent Advances on CO2 Utilization as C1 Building Block in C-N and C-O Bond Formation. In Organometallics for Green Catalysis; Dixneuf, P.H., Soulé, J.-F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 39–76. [Google Scholar]
- Liu, Y.; Rui, Z. A storage-driven CO2 EOR for a net-zero emission target. Engineering 2022, 18, 79–87. [Google Scholar] [CrossRef]
- Mandal, A.; Ojha, K. Enhanced Oil Recovery; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Xiaolong, C.; Yiqiang, L.; Xiang, T.; Huan, Q.; Xuebing, S.; Jianghao, L. Effect of gravity segregation on CO2 flooding under various pressure conditions: Application to CO2 sequestration and oil production. Energy 2021, 226, 120294. [Google Scholar] [CrossRef]
- Kumar, N.; Sampaio, M.A.; Ojha, K.; Hoteit, H.; Mandal, A. Fundamental aspects, mechanisms and emerging possibilities of CO2 miscible flooding in enhanced oil recovery: A review. Fuel 2022, 330, 125633. [Google Scholar] [CrossRef]
- Ajoma, E.; Saira; Sungkachart, T.; Ge, J.; Le-Hussain, F. Water-saturated CO2 injection to improve oil recovery and CO2 storage. Appl. Energy 2020, 266, 114853. [Google Scholar] [CrossRef]
- Solbakken, J.S.; Aarra, M.G. CO2 mobility control improvement using N2-foam at high pressure and high temperature conditions. Int. J. Greenh. Gas Control 2021, 109, 103392. [Google Scholar] [CrossRef]
- Føyen, T.; Brattekås, B.; Fernø, M.A.; Barrabino, A.; Holt, T. Increased CO2 storage capacity using CO2-foam. Int. J. Greenh. Gas Control 2020, 96, 103016. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.-Y.; McPherson, B.; et al. CO2 Accounting and Risk Analysis for CO2 Sequestration at Enhanced Oil Recovery Sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, L.; Croiset, E.; Tan, Z.; Liu, Q.; Yang, J. Modeling study of the heat of absorption and solid precipitation for CO2 capture by chilled ammonia. RSC Adv. 2019, 9, 20075–20086. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, T.; Qiao, K.; Yang, L.; Du, X. Experimental study on carbon dioxide absorption by aqueous ammonia with nickel and chromium ions in bubbling tower at low temperatures. Chem. Eng. Res. Des. 2022, 179, 298–307. [Google Scholar] [CrossRef]
- Ali, S.A.; Mulk, W.U.; Ullah, Z.; Khan, H.; Zahid, A.; Shah, M.U.H.; Shah, S.N. Recent Advances in the Synthesis, Application and Economic Feasibility of Ionic Liquids and Deep Eutectic Solvents for CO2 Capture: A Review. Energies 2022, 15, 9098. [Google Scholar] [CrossRef]
- Gu, H.; Song, G.; Niu, M.; Zhao, S.; Gao, Y.; Li, F. Sr2CeO4 as a robust high temperature sorbent for CO2 capture with near 100% sorbent conversion efficiency. Chem. Eng. J. 2022, 441, 135942. [Google Scholar] [CrossRef]
- Ren, L.; Li, M.; Wang, S.; Li, L.; Wu, Y. Effects of Oxygen-Vacancy-Promoted Ion Diffusion on CO2 Capture Behavior of CaO-Based Sorbents. Ind. Eng. Chem. Res. 2022, 61, 5527–5535. [Google Scholar] [CrossRef]
- Choi, H.L.; Jeong, Y.; Lee, H.; Bae, T.-H. High-Performance Mixed-Matrix Membranes Using a Zeolite@MOF Core–Shell Structure Synthesized via Ion-Exchange-Induced Crystallization and Post-Synthetic Conversion. JACS Au 2024, 4, 253–262. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, W.; Wang, Y.; Zhao, Z.; Li, Q.; Wu, Y.; Liu, T.; Xie, H. Electrochemically Regenerated Amine for CO2 Capture Driven by a Proton-Coupled Electron Transfer Reaction. Ind. Eng. Chem. Res. 2022, 61, 13578–13588. [Google Scholar] [CrossRef]
- Rim, G.; Kong, F.; Song, M.; Rosu, C.; Priyadarshini, P.; Lively, R.P.; Jones, C.W. Sub-Ambient Temperature Direct Air Capture of CO2 using Amine-Impregnated MIL-101 (Cr) Enables Ambient Temperature CO2 Recovery. JACS Au 2022, 2, 380–393. [Google Scholar] [CrossRef] [PubMed]
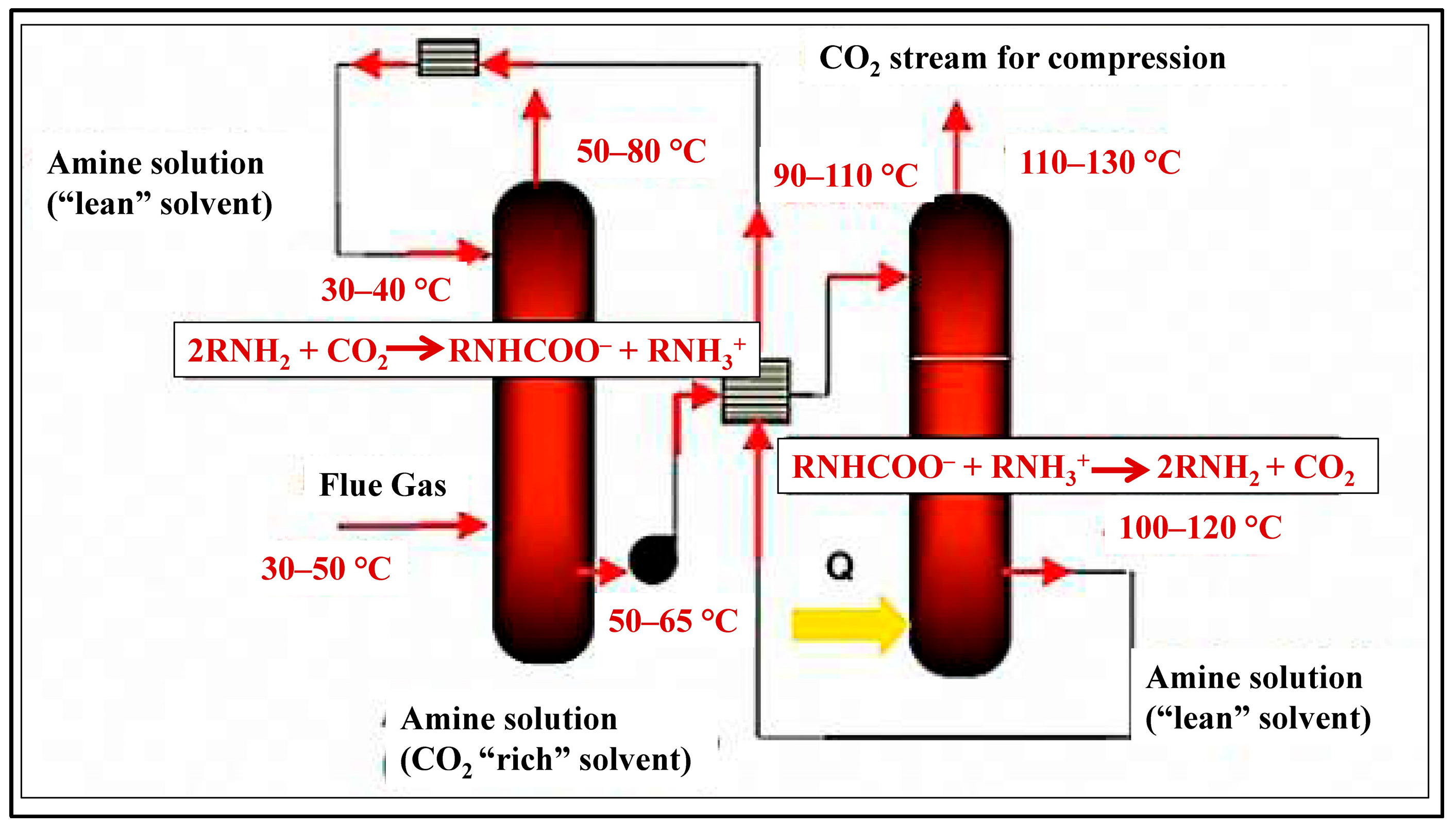

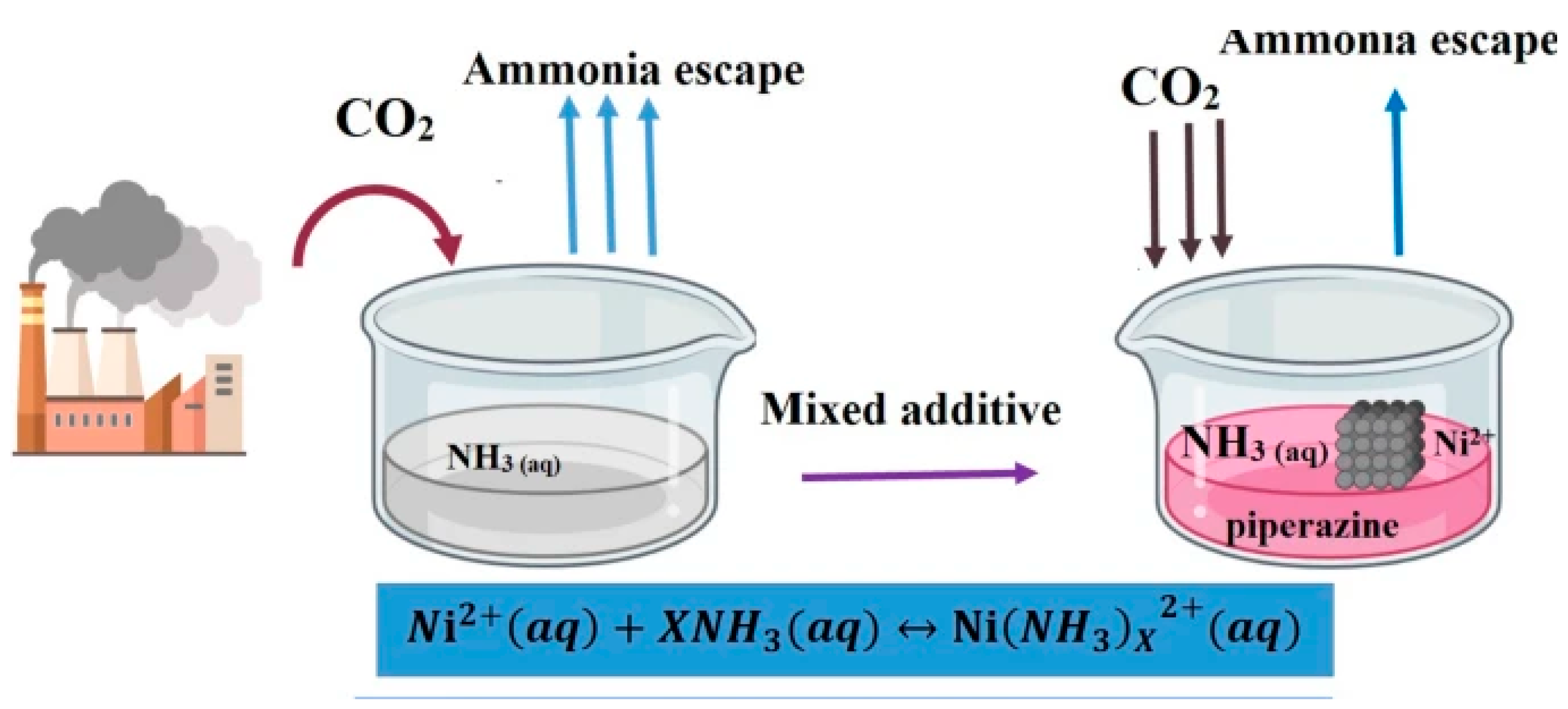
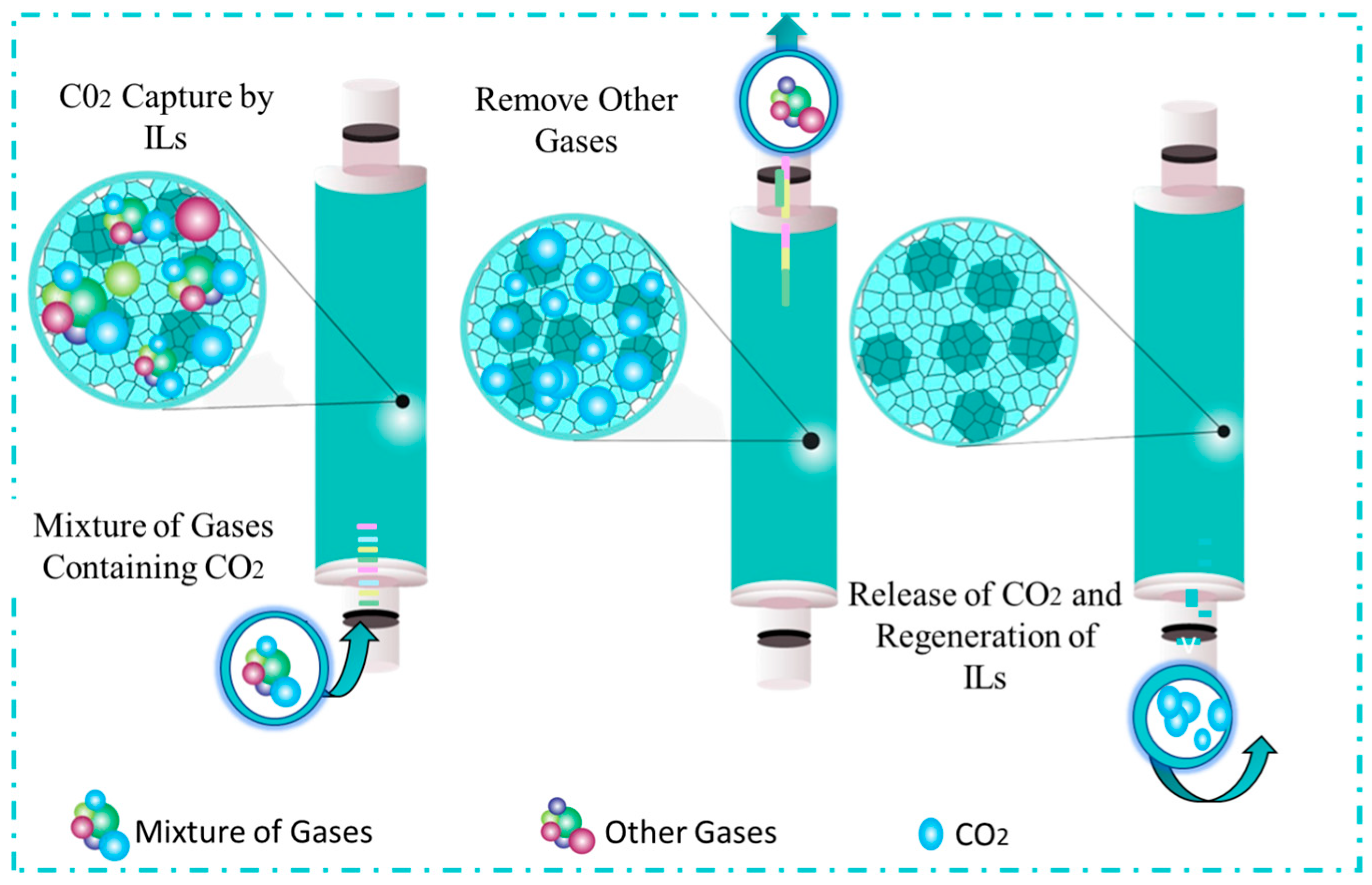

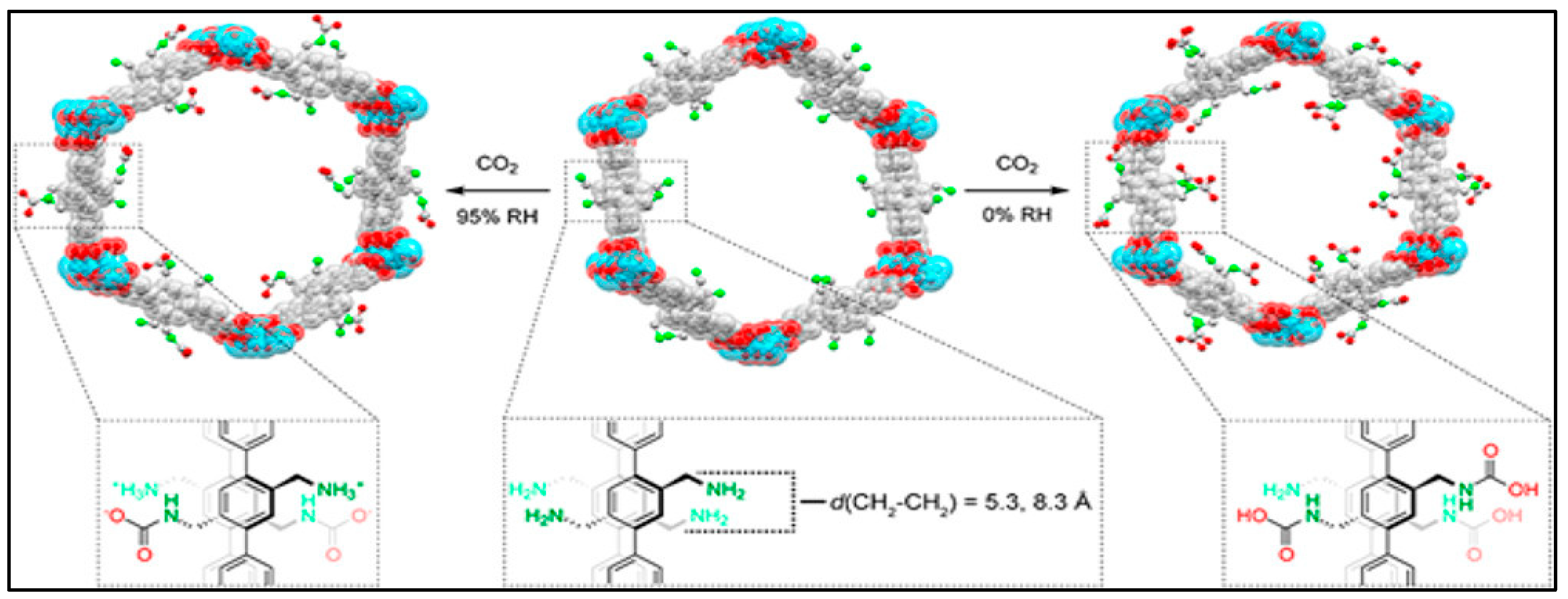


| Technology | CO2 Capture (%) | Energy (GJ/ton) | Cost (USD/ton) | TRL | Key Insight | Ref. |
|---|---|---|---|---|---|---|
| Amine Absorption (MEA) | 85–95 | 3–4 | 40–80 | 7–8 | Mature, widely deployed, but energy-intensive | [177] |
| Solid Sorbents (MOFs, ACs) | 70–90 | 2–3 | 50–100 | 3–4 | High selectivity, scalability challenges | [178] |
| Membrane Separation | 70–85 | 2–3 | 50–120 | 5–6 | Compact design, low flux/fouling issues | [179] |
| Calcium Looping (CaL) | 90–95 | 2.5–3 | 30–60 | 6–7 | Pilot-scale maturity, cost-effective potential | [180] |
| Direct Air Capture (DAC) | 50–70 | 5–8 | 300–600 | 4–5 | Negative emissions, very high cost | [181] |
| Technology | Advantages | Limitations | Development and Future Prospects | References |
|---|---|---|---|---|
| Amine-based sorbents | Most widely used technology for CO2 capture It has a high selectivity, a quick absorption rate, a big cycle capacity, and a high removal efficiency for CO2. Able to collect CO2 from the flue gas stream even at very low partial pressure | It demands significant regeneration energy due to thermal stripping at a higher temperature. Corrosion and deterioration of absorbents are other limiting factors. | Need to develop novel biphasic solvents and polyamine solvents for energy-efficient CO2 capture | [36] |
| Ammonia-based sorbents | It has a great capacity to collect CO2 with less energy consumption. | Absorption capacity declines because of ammonia leakage. | The application of chilled ammonia and the addition of transition metals can reduce ammonia leakage, but decrease efficiency. | [234,235] |
| Ionic liquids as adsorbents | It has extraordinarily high solubility for CO2, minimal vapor pressure, a low specific heat capacity, and strong thermal stability. It requires minimal regeneration energy. | Expensive solvents, high viscosity, and reduced fuel absorption at lower CO2 partial pressures | Phase change and blends with other solvents may reduce the effective cost. | [236] |
| Oxides and minerals | Chemically stable, more cost-effective, and requires low regeneration energy It can collect CO2 at an ambient temperature. They are widely available in nature. | Suffer from rapid loss of reactivity under cyclic operation due to agglomeration, pore structure collapse, and a decrease in active sites | Need to develop a highly stable novel sorbent | [237] |
| Molecular sieves | They have less energy consumption, lower enthalpy, and tunable structures. | Limited due to a lesser adsorption capacity at lower CO2 pressure | Surface modifications, building interaction between the adsorbent and CO2 | [238] |
| Membranes | Low maintenance and energy input requirements, a small environmental impact, excellent selectivity and dependability, and ease of operation | Require high-pressure gradients, the tradeoff between selectivity and permeability, and the low durability | Tuning and altering the characteristics of materials by surface treatment, pore size and porosity management, process engineering, and doping to enhance permeability | [239] |
| Electrochemical capturing | The low-temperature operation, excellent energy economy, and flexible plug-and-play operating mode | Still in the early stages of research and needs a lot of work to become widely used | A cost-effective system developed to capture CO2 and give valuable products | [240] |
| Direct air capture (DAC) | Can capture CO2 from a smaller and distributed point source, with no need for unnecessary transport | High setup cost, lack of experiments at ambient and sub-ambient temperatures | Developing materials that show promising results over a wide range of temperatures | [241] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhdhar, A.; Al-Bogami, A.S.; Akhtar, N.; El-Said, W.A. Progress in Post-Combustion Carbon Dioxide Capture, Direct Air Capture, and Utilization. Catalysts 2025, 15, 807. https://doi.org/10.3390/catal15090807
Akhdhar A, Al-Bogami AS, Akhtar N, El-Said WA. Progress in Post-Combustion Carbon Dioxide Capture, Direct Air Capture, and Utilization. Catalysts. 2025; 15(9):807. https://doi.org/10.3390/catal15090807
Chicago/Turabian StyleAkhdhar, Abdullah, Abdullah S. Al-Bogami, Naeem Akhtar, and Waleed A. El-Said. 2025. "Progress in Post-Combustion Carbon Dioxide Capture, Direct Air Capture, and Utilization" Catalysts 15, no. 9: 807. https://doi.org/10.3390/catal15090807
APA StyleAkhdhar, A., Al-Bogami, A. S., Akhtar, N., & El-Said, W. A. (2025). Progress in Post-Combustion Carbon Dioxide Capture, Direct Air Capture, and Utilization. Catalysts, 15(9), 807. https://doi.org/10.3390/catal15090807








