Metal-Free Cellulose Carbon Nanofiber Supported Graphitic Carbon Nitride for High-Efficient BPA Degradation by Photcatalytic Peroxymonosulfate Activation
Abstract
1. Introduction
2. Results and Discussion
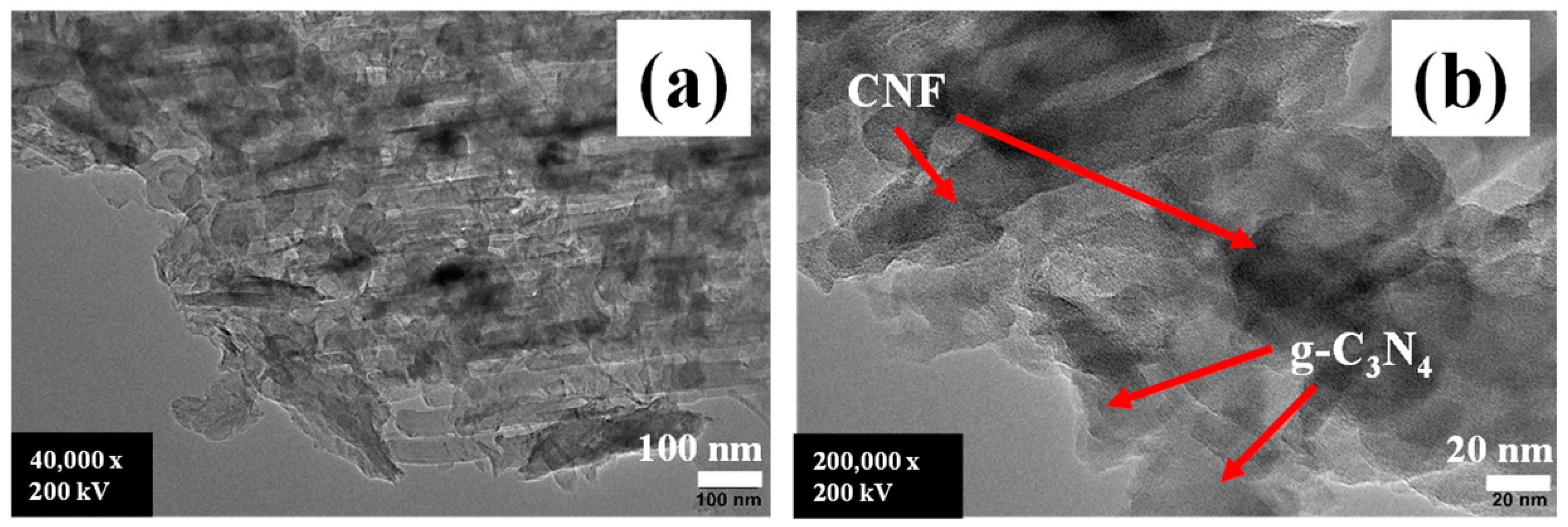
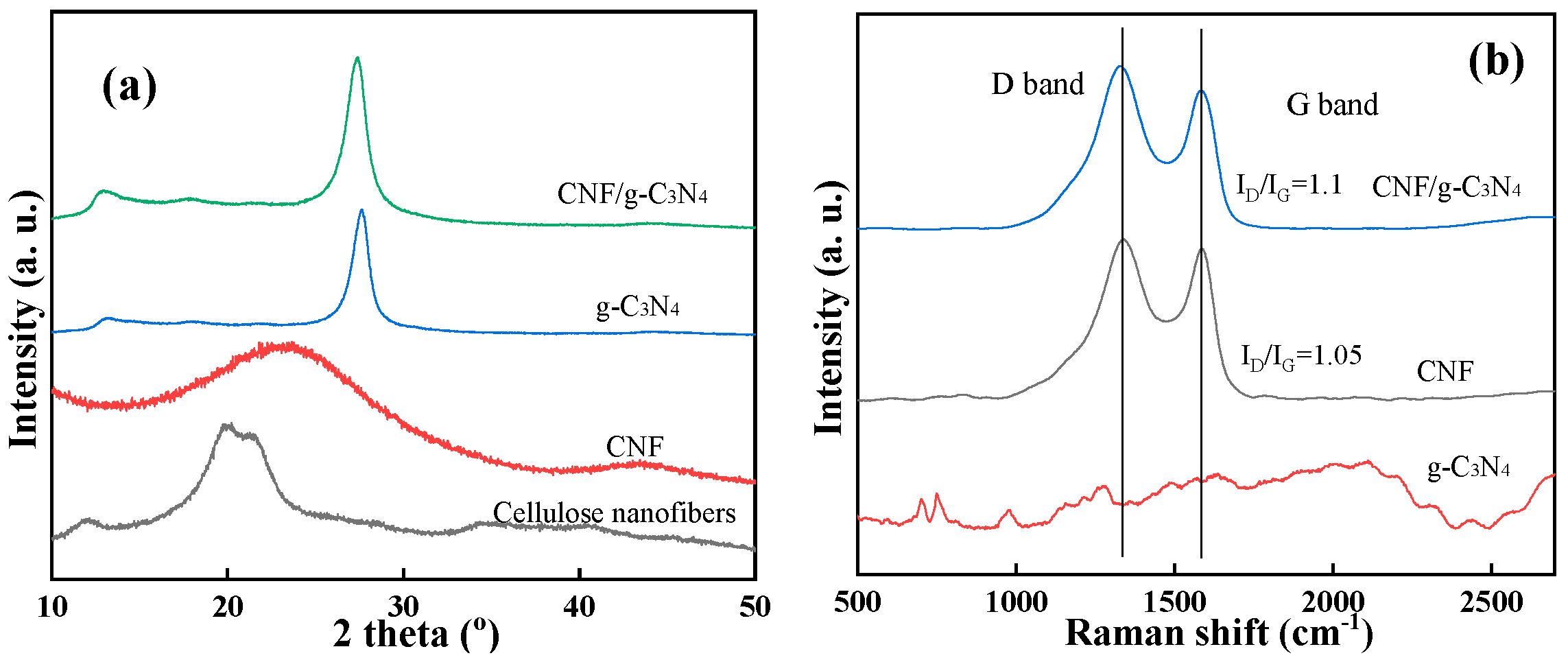
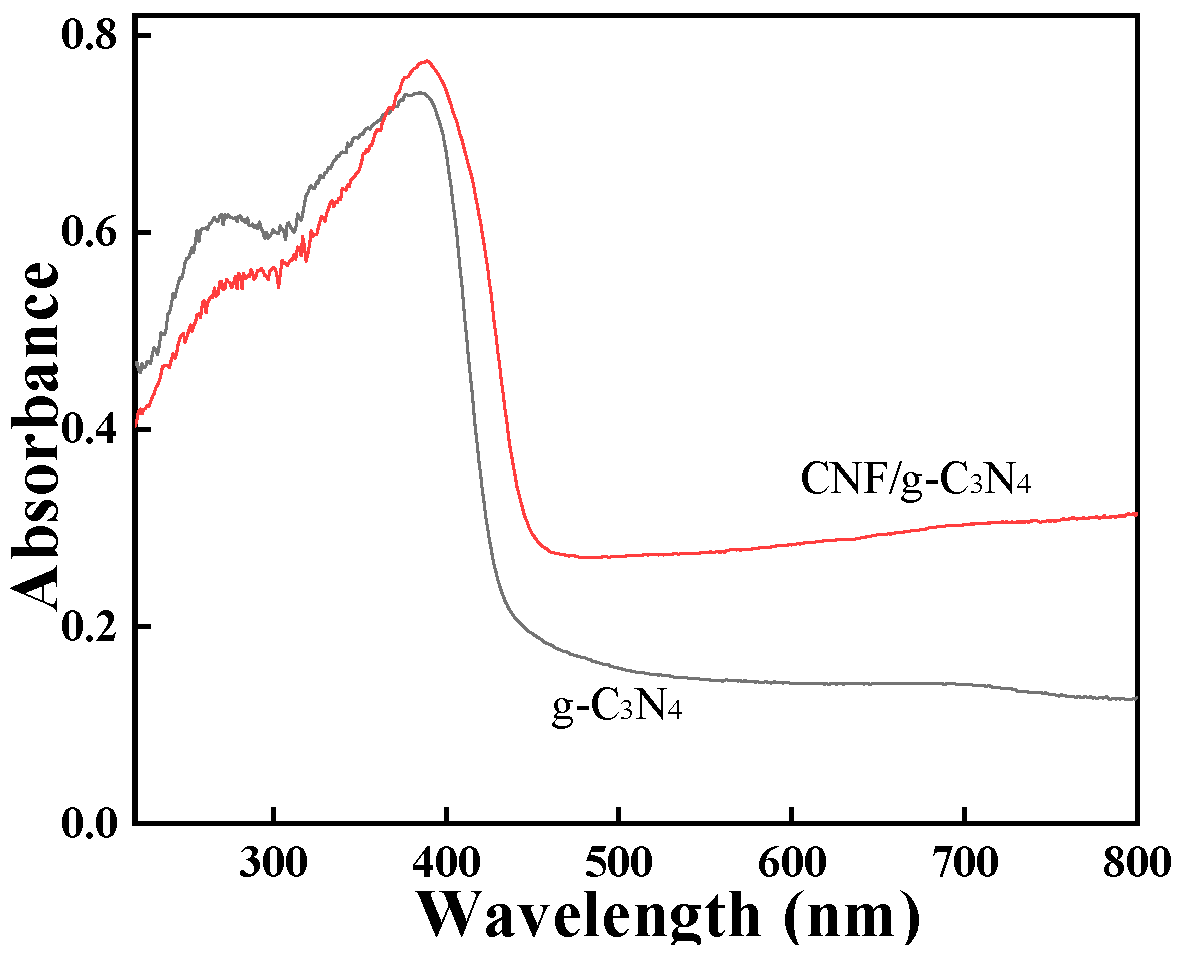
2.1. Characterization of g-C3N4/CNF
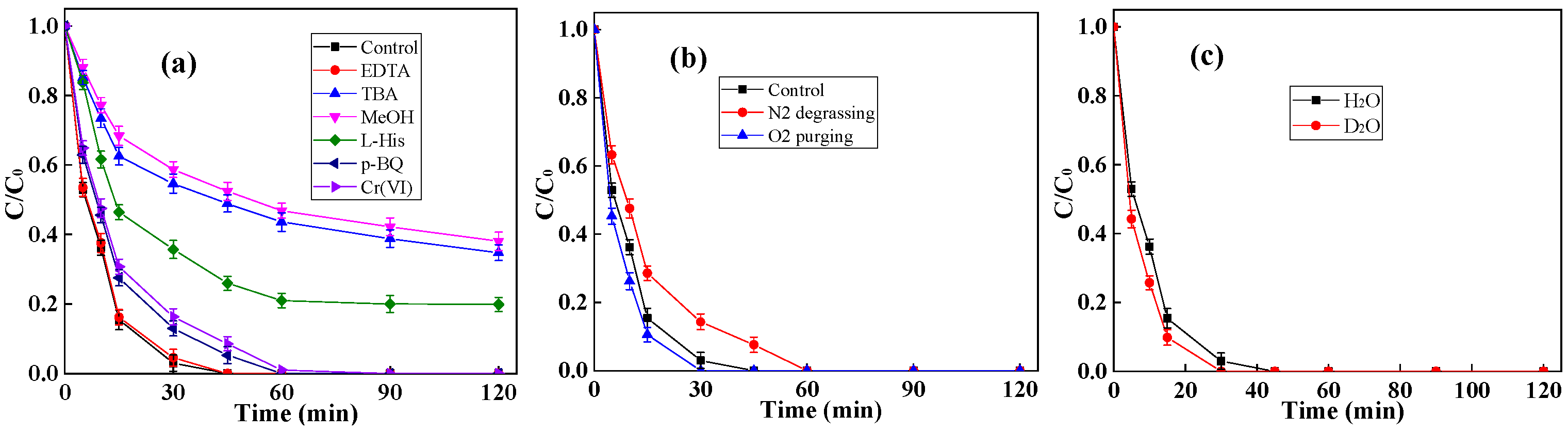
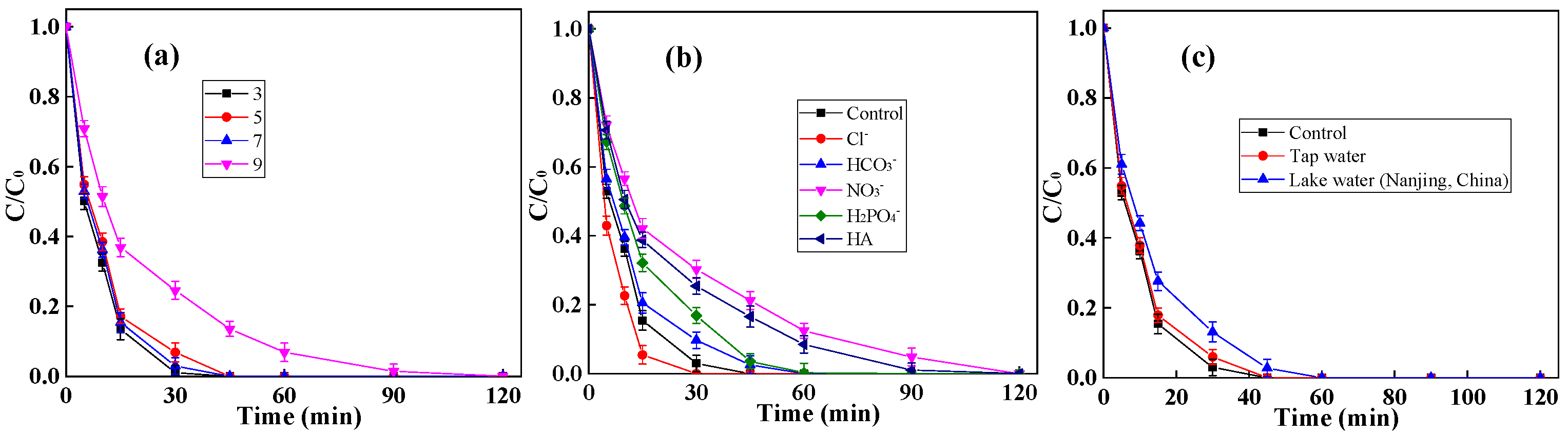
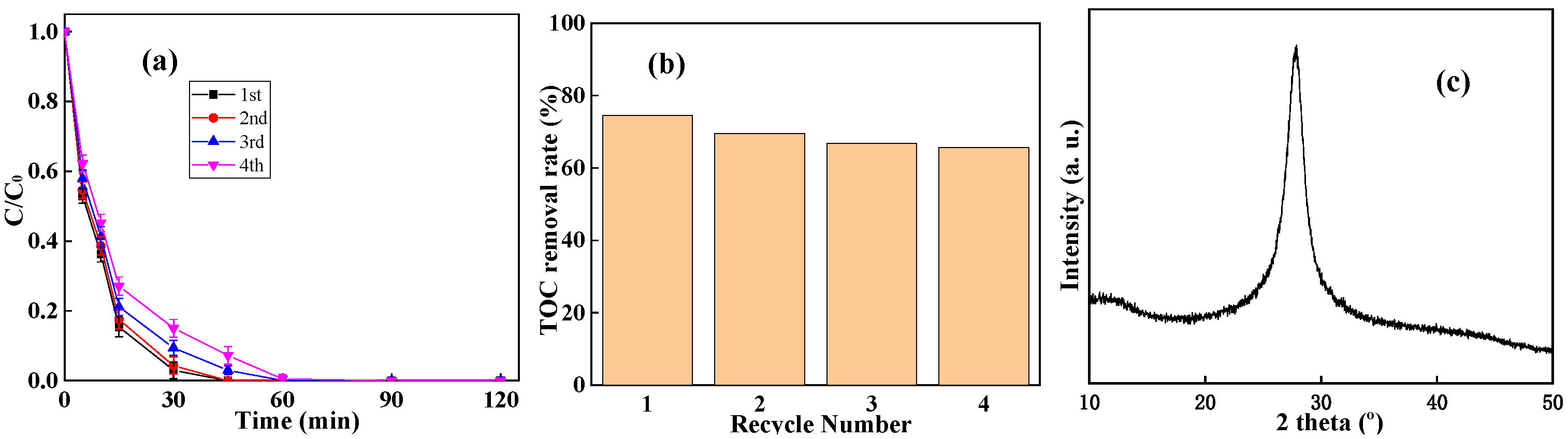
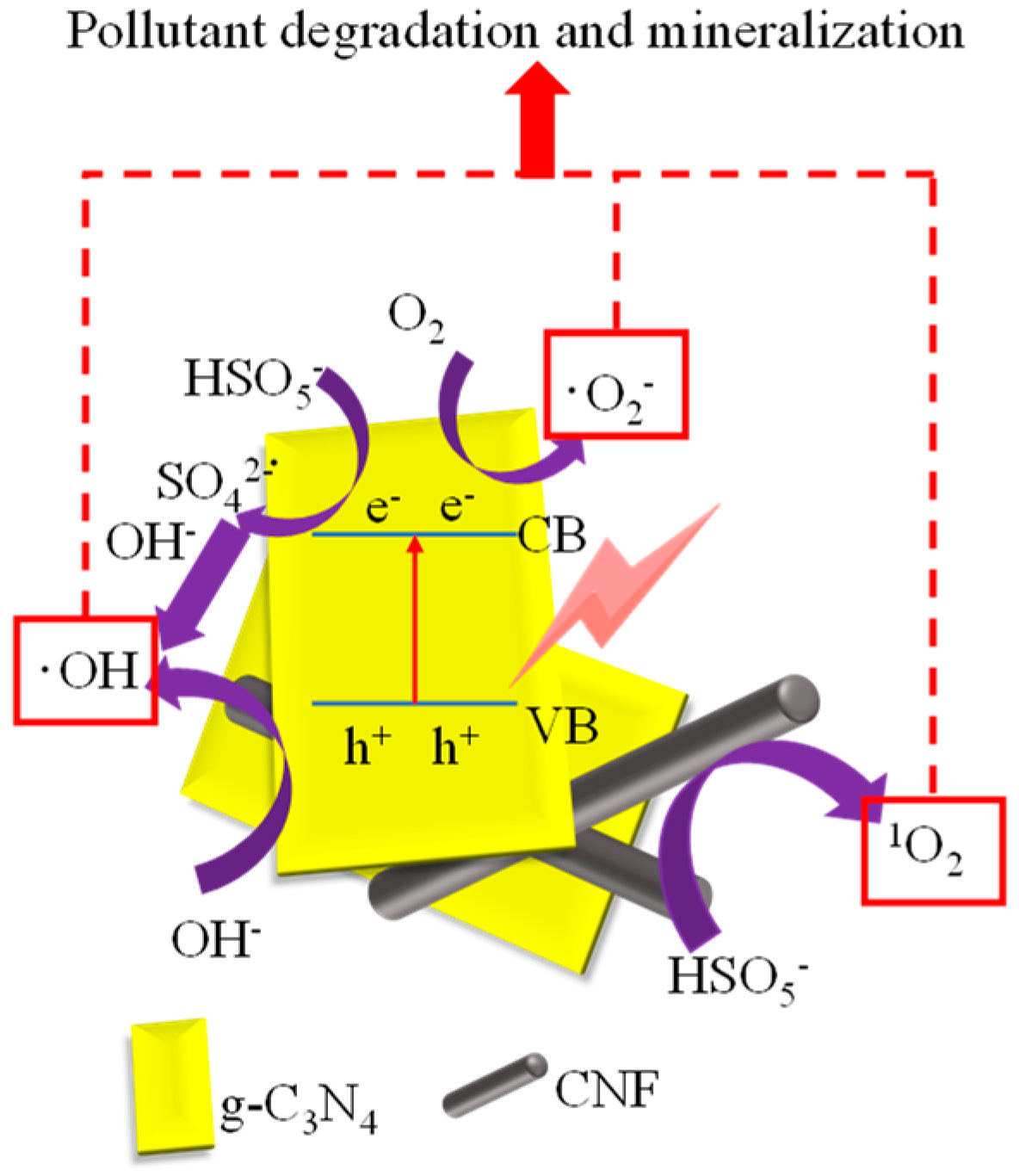
2.2. Performance of g-C3N4/CNF for BPA Degradation
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Preparation of CNF/g-C3N4
3.3. Characterizations
3.4. Degradation of BPA in the CNF/g-C3N4/Visible Light/PMS System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Qi, L.; Han, Y.; Lu, W.; Han, J.; Qiao, W.; Mei, X.; Pan, Y.; Song, K.; Ling, C.; et al. Improvement of Fe2+/peroxymonosulfate oxidation of organic pollutants by promoting Fe2+ regeneration with visible light driven g-C3N4 photocatalysis. Chem. Eng. J. 2022, 430, 132828. [Google Scholar] [CrossRef]
- Kim, K.-H.; Ihm, S.-K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef]
- Ruan, Y.; Kong, L.; Zhong, Y.; Diao, Z.; Shih, K.; Hou, L.a.; Wang, S.; Chen, D. Review on the synthesis and activity of iron-based catalyst in catalytic oxidation of refractory organic pollutants in wastewater. J. Clean. Prod. 2021, 321, 128924. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, L. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016, 103, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, X.; Shen, X.; Yu, X.; Xia, C.; Xu, L.; Zhang, Y.; Gan, L. Synergetic effect of photocatalytic oxidation plus catalytic oxidation on the performance of coconut shell fiber biochar decorated α-MnO2 under visible light towards BPA degradation. J. Environ. Manag. 2023, 345, 118911. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Kim, J.R.; Kan, E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wu, Y.; Fang, X.; Li, J.; Xu, L.; Han, S.; Cui, J.; Gan, L. One-step solvothermal synthesis of wood flour carbon fiber/BiOBr composites for photocatalytic activation of peroxymonosulfate towards sulfadiazine degradation: Mechanisms comparison between photo, chemical and photo-chemical oxidation processes. Sep. Purif. Technol. 2022, 297, 121399. [Google Scholar] [CrossRef]
- Lédé, J. Cellulose pyrolysis kinetics: An historical review on the existence and role of intermediate active cellulose. J. Anal. Appl. Pyrolysis 2012, 94, 17–32. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Shen, X.; Wu, Y.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. New insight into the S and N co-doped poplar biochar for efficient BPA removal via peroxymonosulfate activation: S for adsorptive removal and N for catalytic removal. Sep. Purif. Technol. 2025, 354, 128809. [Google Scholar] [CrossRef]
- Shi, J.; Dai, B.; Shen, X.; Xu, L.; Zhang, Y.; Gan, L. Wood induced preparation of Fe3C decorated biochar for peroxymonosulfate activation towards bisphenol a degradation with low ion leaching. J. Environ. Manag. 2023, 340, 117978. [Google Scholar] [CrossRef]
- Orooji, Y.; Ghanbari, M.; Amiri, O.; Salavati-Niasari, M. Facile fabrication of silver iodide/graphitic carbon nitride nanocomposites by notable photo-catalytic performance through sunlight and antimicrobial activity. J. Hazard. Mater. 2020, 389, 122079. [Google Scholar] [CrossRef]
- Zinin, P.V.; Ming, L.-C.; Sharma, S.K.; Khabashesku, V.N.; Liu, X.; Hong, S.; Endo, S.; Acosta, T. Ultraviolet and near-infrared Raman spectroscopy of graphitic C3N4 phase. Chem. Phys. Lett. 2009, 472, 69–73. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.; Shen, X.; Wu, Y.; Xu, L.; Zhang, Y.; Shi, J.; Gan, L. Lignin regulating the active sites and peroxymonosulfate activation capacities of iron incorporated 1D carbon nanofiber for efficient organic pollutant degradation. Sep. Purif. Technol. 2025, 354, 129156. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Song, C.; Xu, L.; Wang, L.; Fang, X.; Han, S.; Cui, J.; Mei, C. Simultaneous removal of rhodamine B and Cr(VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res. 2020, 185, 109414. [Google Scholar] [CrossRef]
- Yang, X.; Qian, F.; Zou, G.; Li, M.; Lu, J.; Li, Y.; Bao, M. Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation. Appl. Catal. B Environ. 2016, 193, 22–35. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Zhao, J. π-π stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Y.; Liu, H.; Liu, H.; Tao, J.; Cui, M.-H.; Zheng, Z.; Wen, D.; Zhan, X. FexN produced in pharmaceutical sludge biochar by endogenous Fe and exogenous N doping to enhance peroxymonosulfate activation for levofloxacin degradation. Water Res. 2022, 224, 119022. [Google Scholar] [CrossRef]
- Wu, M.; He, X.; Jing, B.; Wang, T.; Wang, C.; Qin, Y.; Ao, Z.; Wang, S.; An, T. Novel carbon and defects co-modified g-C3N4 for highly efficient photocatalytic degradation of bisphenol A under visible light. J. Hazard. Mater. 2020, 384, 121323. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lok, L.-W.; Veksha, A.; Giannis, A.; Lim, T.-T. Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C3N4 under solar irradiation: Performance and mechanistic studies. Chem. Eng. J. 2018, 333, 739–749. [Google Scholar] [CrossRef]
- Yan, S.; Shi, Y.; Tao, Y.; Zhang, H. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4/Fe2O3 heterojunction. Chem. Eng. J. 2019, 359, 933–943. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Li, X.; Li, L. The relation of interface electron transfer and PMS activation by the H-bonding interaction between composite metal and MCM-48 during sulfamethazine ozonation. Chem. Eng. J. 2020, 398, 125529. [Google Scholar] [CrossRef]
- Hua, L.-C.; OuYang, R.-C.; Zhao, Z.; Nguyen, T.N.A.; Huang, C. Homogeneous versus heterogeneous Mn(II) oxidation in peroxymonosulfate assisting chlorination: Synergistic role for enhanced Mn(II) oxidation in water treatment. Water Res. 2024, 265, 122265. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Y.; Lv, K.; Zhu, C.; Guan, X.; Xie, B.; Zou, X.; Luo, X.; Zhou, Y. N-doped biochar-Fe/Mn as a superior peroxymonosulfate activator for enhanced bisphenol a degradation. Water Res. 2025, 278, 123399. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Song, Y.; Yang, Y.; Guan, C.; Jiang, J. Theoretical investigation into activation of hydroperoxides by excited quinones under ultraviolet irradiation. Chem. Eng. J. 2023, 463, 142423. [Google Scholar] [CrossRef]
- Xie, J.; Pan, X.; Jiang, C.; Zhao, L.; Gong, X.; Liu, Y. Enhanced conversion of superoxide radical to singlet oxygen in peroxymonosulfate activation by metal-organic frameworks derived heteroatoms dual-doped porous carbon catalyst. Environ. Res. 2023, 236, 116745. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Gan, L. Catalytic Degradation of Bisphenol A in Water by Poplar Wood Powder Waste Derived Biochar via Peroxymonosulfate Activation. Catalysts 2022, 12, 1164. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of pH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, Y.; Wu, Y.; Zhang, J.; Deng, H.; Zheng, H.; Liu, Z.; Zhao, C. In Situ Regeneration of Phenol-Saturated Activated Carbon Fiber by an Electro-peroxymonosulfate Process. Environ. Sci. Technol. 2020, 54, 10944–10953. [Google Scholar] [CrossRef]
- Geng, A.; Meng, L.; Han, J.; Zhong, Q.; Li, M.; Han, S.; Mei, C.; Xu, L.; Tan, L.; Gan, L. Highly efficient visible-light photocatalyst based on cellulose derived carbon nanofiber/BiOBr composites. Cellulose 2018, 25, 4133–4144. [Google Scholar] [CrossRef]
- Han, Y.; Gan, L.; Gong, H.; Han, J.; Qiao, W.; Xu, L. Photoactivation of peroxymonosulfate by wood pulp cellulose biochar/g-C3N4 composite for diclofenac degradation: The radical and nonradical pathways. Biochar 2022, 4, 35. [Google Scholar] [CrossRef]



| Catalyst | Reaction System | k Value (min−1) | R2 |
|---|---|---|---|
| g-C3N4 | light | 0.018 | 0.98 |
| PMS | 0.001 | 0.88 | |
| light + PMS | 0.022 | 0.97 | |
| CNF | light | 0.001 | 0.96 |
| PMS | 0.013 | 0.92 | |
| Light + PMS | 0.014 | 0.97 | |
| CNF/g-C3N4 | light | 0.048 | 0.97 |
| PMS | 0.005 | 0.95 | |
| Light + PMS | 0.12 | 0.99 |
| Catalyst | Light Source | Catalyst Dosage | BPA Concentration | Removal Time | Reference |
|---|---|---|---|---|---|
| CNF/g-C3N4+PMS | 300 W Xenon lamp (CEL-PF300-T6, ZhongJiao jinyuan, Beijing, China) | 0.5 g/L | 0.05 mM | 100%@40 min | This work |
| Carbon/g-C3N4 | 300 W Xenon lamp (Perfect light, Beijing, China) | 0.2 g/L | 0.05 mM | 100%@75 min | [27] |
| Ag/S-g-C3N4 | 155 W Xe arc lamp (Newport 67005, Wuxi, China) | 0.4 g/L | 0.05 mM | 80%@180 min | [28] |
| Fe2O3/g-C3N4+PDS | LED lamp (Yaming, Shenzhen, China) | 0.5 g/L | 0.05 mM | 95%@60 min | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gao, G.; Gan, L. Metal-Free Cellulose Carbon Nanofiber Supported Graphitic Carbon Nitride for High-Efficient BPA Degradation by Photcatalytic Peroxymonosulfate Activation. Catalysts 2025, 15, 788. https://doi.org/10.3390/catal15080788
Liu J, Gao G, Gan L. Metal-Free Cellulose Carbon Nanofiber Supported Graphitic Carbon Nitride for High-Efficient BPA Degradation by Photcatalytic Peroxymonosulfate Activation. Catalysts. 2025; 15(8):788. https://doi.org/10.3390/catal15080788
Chicago/Turabian StyleLiu, Jingjing, Guilong Gao, and Lu Gan. 2025. "Metal-Free Cellulose Carbon Nanofiber Supported Graphitic Carbon Nitride for High-Efficient BPA Degradation by Photcatalytic Peroxymonosulfate Activation" Catalysts 15, no. 8: 788. https://doi.org/10.3390/catal15080788
APA StyleLiu, J., Gao, G., & Gan, L. (2025). Metal-Free Cellulose Carbon Nanofiber Supported Graphitic Carbon Nitride for High-Efficient BPA Degradation by Photcatalytic Peroxymonosulfate Activation. Catalysts, 15(8), 788. https://doi.org/10.3390/catal15080788








