Challenges and Prospects of TiO2-Based Photocatalysis for Wastewater Treatment: Keyword Analysis
Abstract
1. Introduction
2. Literature Search Process and Eligibility Criteria
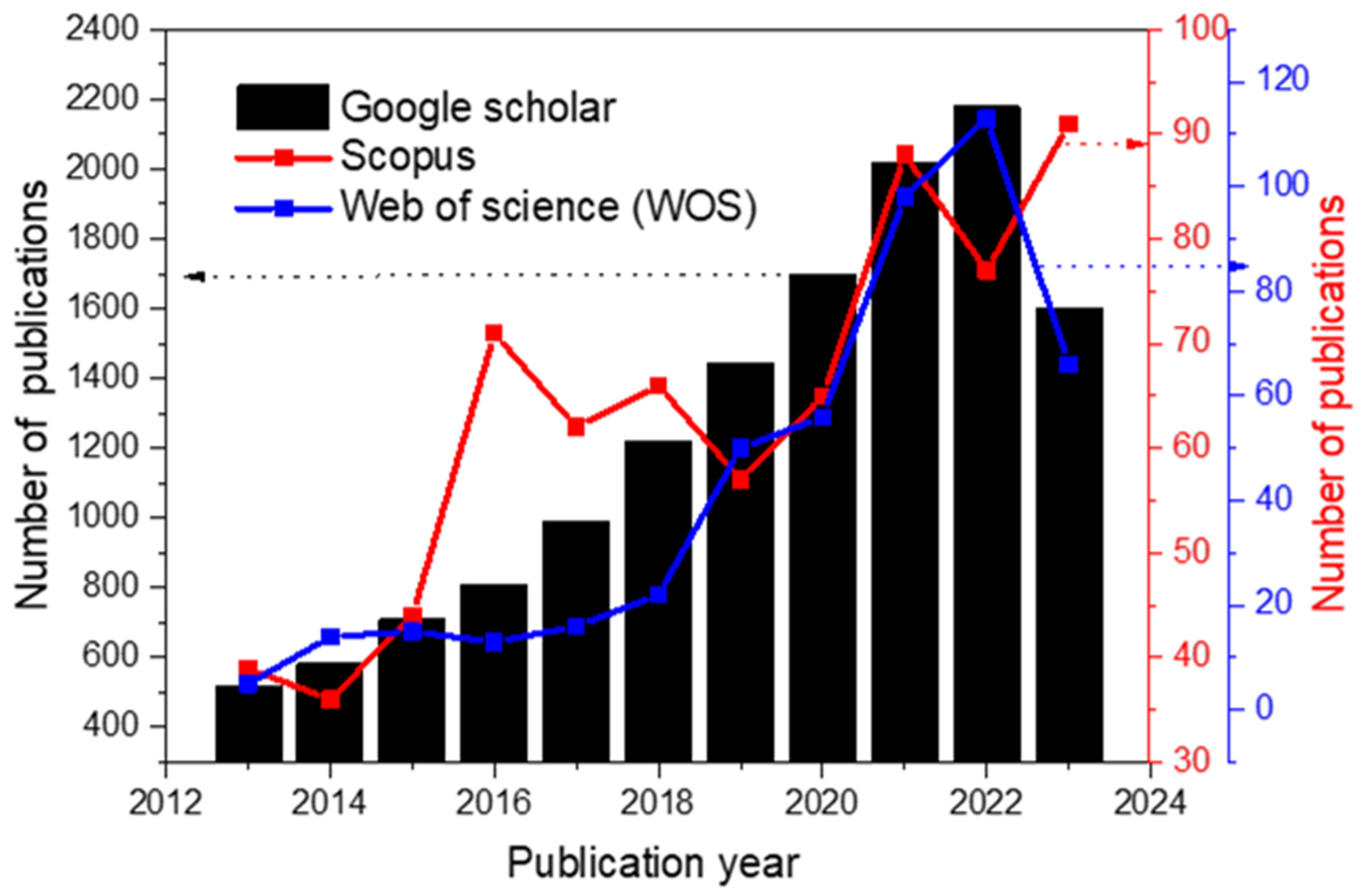
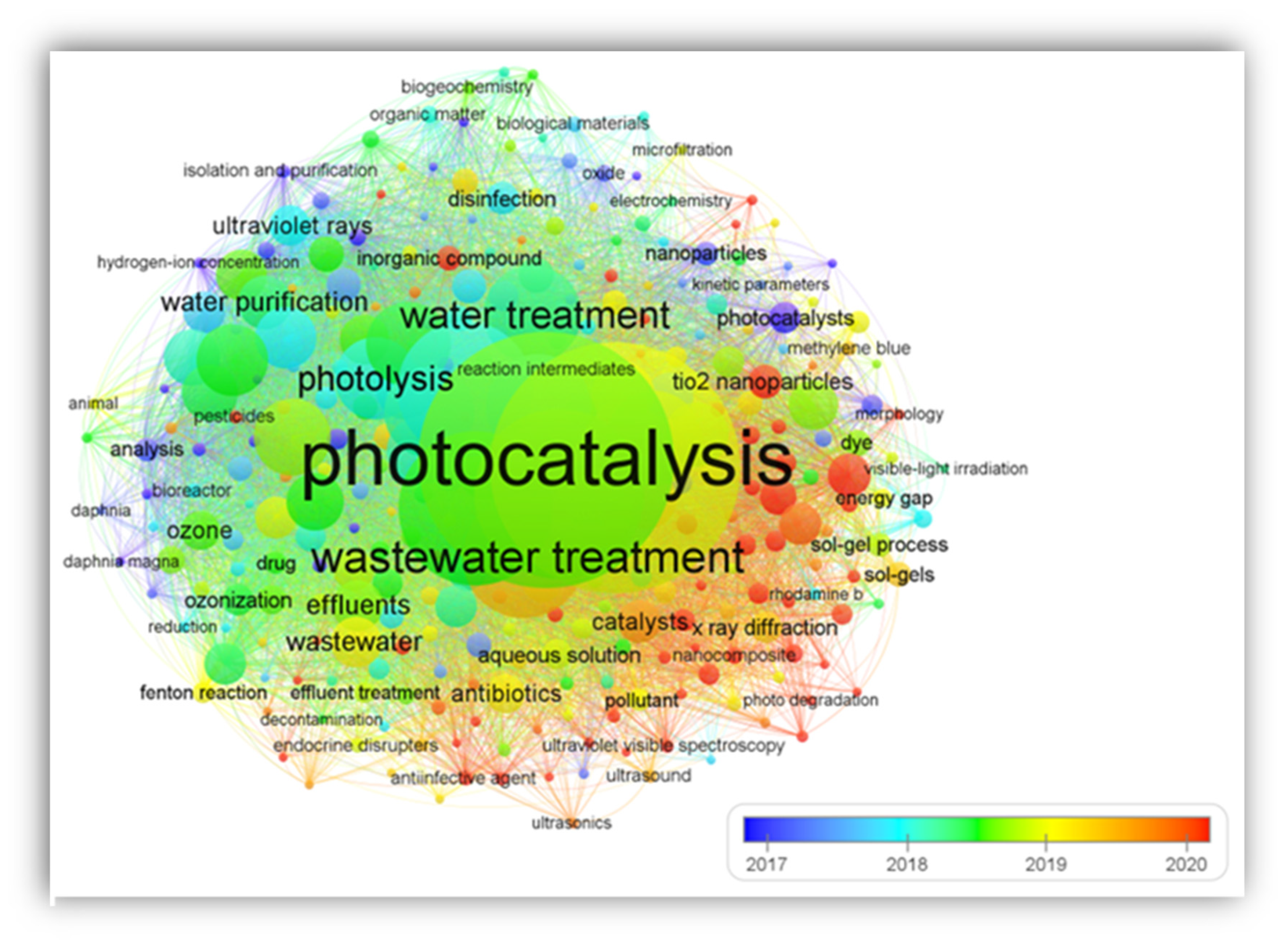
2.1. Research Development and Authors’ Keyword Occurrence Network
2.2. Research Interest Themes and Keyword Occurrence
2.3. Fate of Emerging Contaminants in Wastewater
3. Advanced Oxidation Processes (AOPs)
3.1. Hydrogen Peroxide Coupled with UV Radiation
3.2. Photo-Fenton (Fe2+/H2O2/UV)
3.3. Electrochemical Oxidation
4. Photocatalysis Process
4.1. TiO2 Photocatalytic Mechanism
4.2. Factors Affecting Photocatalytic Process
4.2.1. Catalyst Loading
4.2.2. pH
4.2.3. Light Wavelength and Intensity
4.2.4. Initial Concentration Nature of Pollutants
4.2.5. Morphology
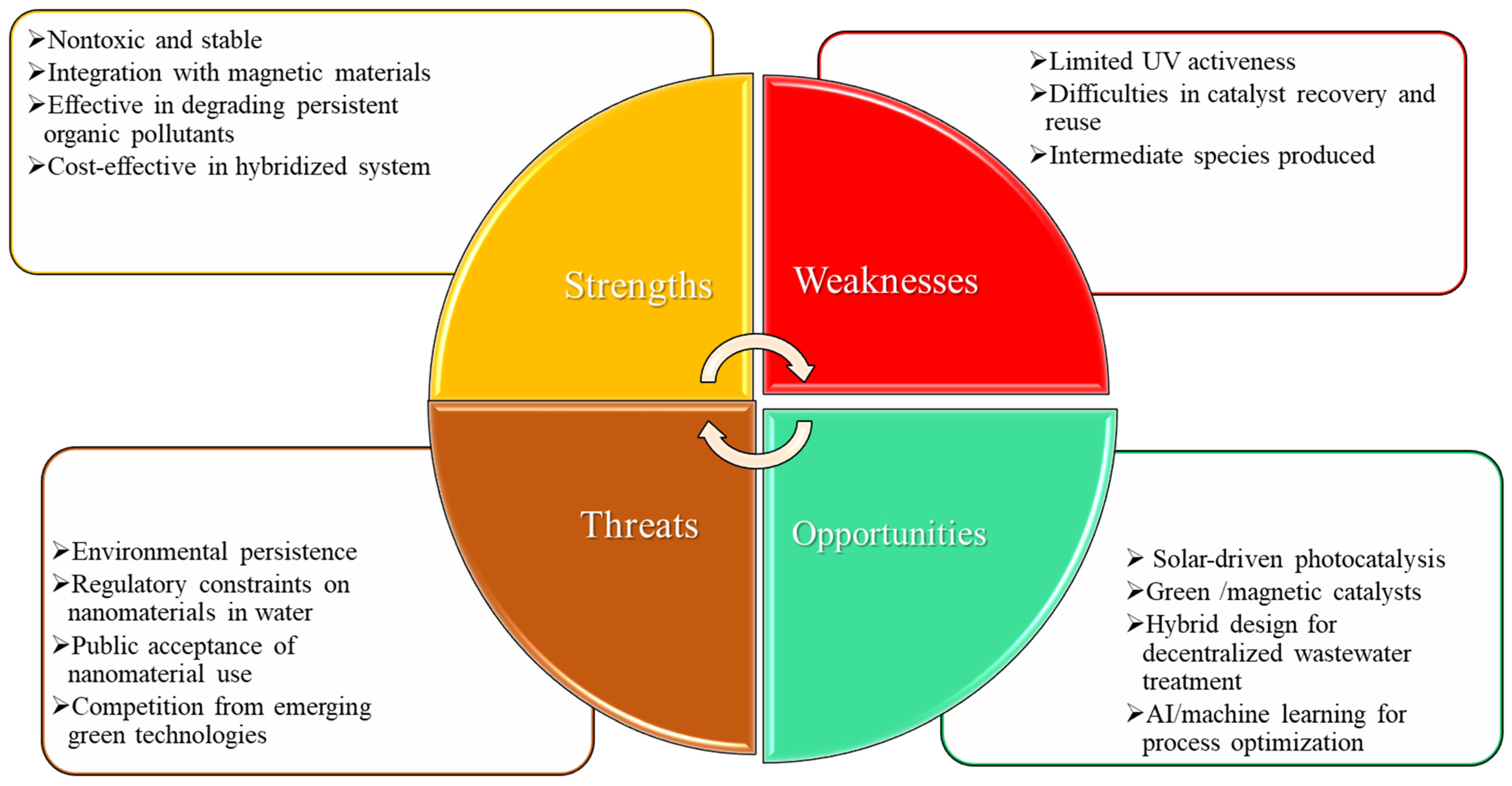
5. Challenges and Prospects of TiO2-Based Photocatalysis
5.1. Challenges of TiO2’s Large Energy Band Gap
5.1.1. Enhancing TiO2 Visible/Sunlight and Near-Infrared (NIR) Light Absorption
Doping with Metal and Non-Metal Elements
Creating Oxygen Vacancies, Defects, and Introducing Dopants
Combining with Conjugated Polymers
Structural and Morphological Modifications
Co-Doping and Synergistic Effects
Hydrogenation and Phase Transformation
Up-Conversion Materials
5.2. Complex Oxidizable Organic Substrates
5.3. Energy-Intensive/Recovery, Recyclability, and Reusability
5.4. Industrial/Large-Scale Application of Semiconductor Photocatalysts
5.5. The Challenges and Prospects of Magnetized TiO2-Based Photocatalysts
6. Conclusions
- Magnetic nanocomposite photocatalysis, which involves coupling TiO2 with magnetic materials (Fe3O4), allows for easy recovery and regeneration of the catalyst with a magnetic field and enhances its practical applicability.
- TiO2 doped with emerging materials such as nitrogen, ionic liquids, sulfur, metal ions, carbon, and hybrid systems with carbon-based materials can increase the photocatalytic activity under visible light response systems.
- The adoption of environmentally friendly synthesis methods of TiO2 nanoparticles with plant or biodegradable-based materials can contribute to the broader goals of sustainable nanotechnology.
- The integration of TiO2 photocatalysis with biological treatment, electrocatalysis, or membrane systems offers multifunctional effects to enhance overall treatment performance.
- The development of standard testing protocols, regulatory frameworks, and environmental risk assessments is critical to support the safe and responsive implementation of TiO2-based photocatalysis.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karri, R.R.; Ravindran, G.; Dehghani, M.H. Wastewater—Sources, toxicity, and their consequences to human health. In Soft Computing Techniques in Solid Waste and Wastewater Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–33. [Google Scholar]
- Bao, H.; Wu, M.; Meng, X.; Han, H.; Zhang, C.; Sun, W. Application of electrochemical oxidation technology in treating high-salinity organic ammonia-nitrogen wastewater. J. Environ. Chem. Eng. 2023, 11, 110608. [Google Scholar] [CrossRef]
- United Nations Sustainable Development. Goal 6: Ensure Access to Water and Sanitation for All. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 10 July 2024).
- Siraj, K.T.; Rao, P. Review on current world water resources scenario and water treatment technologies and techniques. Int. J. Appl. Res. 2016, 2, 262–266. [Google Scholar]
- Bilal, H.; Li, X.; Iqbal, M.S.; Mu, Y.; Tulcan, R.X.S.; Ghufran, M.A. Surface water quality, public health, and ecological risks in Bangladesh—A systematic review and meta-analysis over the last two decades. Environ. Sci. Pollut. Res. 2023, 30, 1–19. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Visible-light-promoted peroxymonosulfate activation for ACE degradation: Overlooked role of photogenerated hole. Appl. Catal. B Environ. Energy 2025, 365, 124881. [Google Scholar]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Network capture effect-driven enhanced activation of peroxymonosulfate by iron-doped carbon quantum dots derived from ferrous gluconate for efficient ciprofloxacin degradation: DFT calculations and mechanism analysis. J. Mater. Chem. A 2025, 26, 1–16. [Google Scholar]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.; Lim, J. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Srivastav, M.; Gupta, M.; Agrahari, S.K.; Detwal, P. Removal of refractory organic compounds from wastewater by various advanced oxidation process-a review. Curr. Environ. Eng. 2019, 6, 8–16. [Google Scholar] [CrossRef]
- Kweinor Tetteh, E.; Opoku Amankwa, M.; Armah, E.K.; Rathilal, S. Fate of COVID-19 occurrences in wastewater systems: Emerging detection and treatment technologies—A review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Abbood, N.S.; Ali, N.S.; Khader, E.H.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Photocatalytic degradation of cefotaxime pharmaceutical compounds onto a modified nanocatalyst. Res. Chem. Intermed. 2023, 49, 43–56. [Google Scholar] [CrossRef]
- López, J.; Rey, A.; Viñuelas-Zahinos, E.; Álvarez, P.M. Preparation of a new green magnetic Fe3O4@TiO2-P25 photocatalyst for solar advanced oxidation processes in water. J. Environ. Chem. Eng. 2023, 11, 109999. [Google Scholar] [CrossRef]
- Mohod, A.V.; Momotko, M.; Shah, N.S.; Marchel, M.; Imran, M.; Kong, L.; Boczkaj, G. Degradation of Rhodamine dyes by Advanced Oxidation Processes (AOPs)–Focus on caviatation and photocatalysis-A critical review. Water Resour. Ind. 2023, 30, 100220. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Jiang, L.; Yu, H.; Zhao, Y.; Chen, H.; Yuan, X.; Liang, J.; Li, H.; Wu, Z. Defective polymeric carbon nitride: Fabrications, photocatalytic applications and perspectives. Chem. Eng. J. 2022, 427, 130991. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Zhou, S.; Yu, H.; Liang, J.; Chu, W.; Li, H.; Wang, H.; Wu, Z.; Yuan, X. Strategies to extend near-infrared light harvest of polymer carbon nitride photocatalysts. Coord. Chem. Rev. 2021, 439, 213947. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.-S.; Jiang, Y.-X.; Sun, L.; Sun, X.-H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahu, J.; Poonia, A.K.; Ghosh, P. Current perspective of nano-engineered metal oxide based photocatalysts in advanced oxidation processes for degradation of organic pollutants in wastewater. Chem. Eng. Res. Des. 2023, 190, 667–686. [Google Scholar] [CrossRef]
- Gelover, S.; Gómez, L.A.; Reyes, K.; Leal, M.T. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006, 40, 3274–3280. [Google Scholar] [CrossRef]
- Kaswan, V.; Kaur, H. A comparative study of advanced oxidation processes for wastewater treatment. Water Pract. Technol. 2023, 18, 1233–1254. [Google Scholar] [CrossRef]
- Zawrah, M.; Snousy, M.G. Environmental Challenges of Nanotechnology in Subsurface. In Environmental Science and Engineering; Studium Press LLC: Houston, TX, USA, 2017; Volume 10, pp. 379–416. [Google Scholar]
- Chakraborty, A.; Ruzimuradov, O.; Gupta, R.K.; Cho, J.; Prakash, J. TiO2 nanoflower photocatalysts: Synthesis, modifications and applications in wastewater treatment for removal of emerging organic pollutants. Environ. Res. 2022, 212, 113550. [Google Scholar] [CrossRef]
- Kaur, K.; Badru, R.; Singh, P.P.; Kaushal, S. Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 2020, 8, 103666. [Google Scholar] [CrossRef]
- Kong, X.; Bai, R.; Wang, S.; Wu, B.; Zhang, R.; Li, H. Recovery of phosphorus from aqueous solution by magnetic TiO2*/Fe3O4 composites. Chem. Phys. Lett. 2022, 787, 139234. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef]
- Boccuni, F.; Rondinone, B.; Petyx, C.; Iavicoli, S. Potential occupational exposure to manufactured nanoparticles in Italy. J. Clean. Prod. 2008, 16, 949–956. [Google Scholar] [CrossRef]
- Lyons, K. Nanotechnology: Transforming food and the environment. Food First Backgrounder 2010, 16, 1–4. [Google Scholar]
- Kerry, R.G.; Rout, J.R.; Das, G.; Fraceto, L.F.; Paramithiotis, S.; Patra, J.K. Applications of nanotechnology in food and agriculture. In Food Molecular Microbiology; CRC Press: Boca Raton, FL, USA, 2019; pp. 223–249. [Google Scholar]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique properties of surface-functionalized nanoparticles for bio-application: Functionalization mechanisms and importance in application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Bukht, R. Responsibility, Regulation and the Construction of Markets of Nanotechnologies in Food and Food Packaging: The Cases of Canada and India. Ph.D. Thesis, The University of Manchester (United Kingdom), Manchester, UK, 2016. [Google Scholar]
- Henshaw, D.L.; O’Carroll, M.J. Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on Possible Effects of Electromagnetic Fields (EMF) on Human Health. 2009. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=31882392b69b95d2a7dfc4c61d83c8862f53b52f (accessed on 15 August 2025).
- Samaras, T.; Leitgeb, N.; Auvinen, A.; Danker-Hopfe, H.; Mild, K.H.; Mattsson, M.-O.; Norppa, H.; Rubin, G.J.; Scarfi, M.R.; Schüz, J. Scientific Committee on Emerging and Newly Identified Health Risks. Opinion on Potential Health Effects of Exposure to Electromagnetic Fields. Bioelectromagnetics 2015, 36, 480–484. [Google Scholar]
- Castellar, J.A.; Torrens, A.; Buttiglieri, G.; Monclus, H.; Arias, C.A.; Carvalho, P.N.; Galvao, A.; Comas, J. Nature-based solutions coupled with advanced technologies: An opportunity for decentralized water reuse in cities. J. Clean. Prod. 2022, 340, 130660. [Google Scholar] [CrossRef]
- Poyatos, J.M.; Muñio, M.M.; Almecija, M.C.; Torres, J.C.; Hontoria, E.; Osorio, F. Advanced Oxidation Processes for Wastewater Treatment: State of the Art. Water Air Soil Pollut. 2010, 205, 187–204. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Amo-Duodu, G.; Rathilal, S. Anaerobic Digested Wastewater CO2 Sequestration Using a Biophotocatalytic System with a Magnetized Photocatalyst (Fe-TiO2). Molecules 2022, 27, 5213. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Biophotocatalytic reduction of CO2 in anaerobic biogas produced from wastewater treatment using an integrated system. Catalysts 2022, 12, 76. [Google Scholar] [CrossRef]
- Dlamini, C.P. Evaluation of Micro-Scaled TiO2 on Degradation and Recovery of mTiO2 from Treated Drinking Water. Ph.D. Thesis, Durban University of Technology, Durban, South Africa, 2016. [Google Scholar]
- Janzeer, Y. Surface Modification of Titanium and Titanium Alloys to Enhance Bone Healing. Ph.D. Thesis, King’s College London, London, UK, 2013. [Google Scholar]
- Umar, M.; Aziz, H.A. Photocatalytic Degradation of Organic Pollutants in Water. Intech 2013, 8, 196–197. [Google Scholar] [CrossRef]
- Laoufi, N.A.; Tassalit, D.; Bentahar, F. The degradation of phenol in water solution by TiO2 photocatalysis in a helical reactor. Glob. NEST J. 2008, 10, 404–418. [Google Scholar]
- Liu, H.; Zhang, Z.-G.; He, H.-W.; Wang, X.-X.; Zhang, J.; Zhang, Q.-Q.; Tong, Y.-F.; Liu, H.-L.; Ramakrishna, S.; Yan, S.-Y. One-step synthesis heterostructured g-C3N4/TiO2 composite for rapid degradation of pollutants in utilizing visible light. Nanomaterials 2018, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Si, X.; He, L.; Zhang, J.; Sun, Y. Strategies for enhancing the photocatalytic activity of semiconductors. Int. J. Hydrogen Energy 2024, 58, 1249–1265. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J. Cu2O: A catalyst for the photochemical decomposition of water? Chem. Commun. 1999, 12, 1069–1070. [Google Scholar] [CrossRef]
- Finlayson, M.F.; Wheeler, B.L.; Kakuta, N.; Park, K.H.; Bard, A.J.; Campion, A.; Fox, M.A.; Webber, S.E.; White, J.M. Determination of flat-band position of cadmium sulfide crystals, films, and powders by photocurrent and impedance techniques, photoredox reaction mediated by intragap states. J. Phys. Chem. 1985, 89, 5676–5681. [Google Scholar] [CrossRef]
- Zheng, N.-C.; Ouyang, T.; Chen, Y.; Wang, Z.; Chen, D.-Y.; Liu, Z.-Q. Ultrathin CdS shell-sensitized hollow S-doped CeO2 spheres for efficient visible-light photocatalysis. Catal. Sci. Technol. 2019, 9, 1357–1364. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 2010, 49, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lv, S.; Li, Z.; Zou, Z. Organic–inorganic composite photocatalyst of gC3N4 and TaON with improved visible light photocatalytic activities. Dalton Trans. 2010, 39, 1488–1491. [Google Scholar] [CrossRef]
- Chun, W.-J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Memar, A.; Phan, C.M.; Tade, M.O. Influence of surfactants on Fe2O3 nanostructure photoanode. Int. J. Hydrogen Energy 2012, 37, 16835–16843. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Chen, X.-T.; Xue, Z.-L. Microwave-assisted synthesis and photocatalytic properties of flower-like Bi2WO6 and Bi2O3–Bi2WO6 composite. J. Colloid Interface Sci. 2013, 394, 69–77. [Google Scholar] [CrossRef]
- Cai, L.; Kisch, H. Visible light induced photoelectrochemical properties of n-BiVO4 and n-BiVO4/p-Co3O4. J. Phys. Chem. C 2008, 112, 548–554. [Google Scholar]
- Hardee, K.L.; Bard, A.J. Semiconductor electrodes: X. Photoelectrochemical behavior of several polycrystalline metal oxide electrodes in aqueous solutions. J. Electrochem. Soc. 1977, 124, 215. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrost. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Ammar, S.H.; Abdulnabi, W.A. Synthesis, characterization and environmental remediation applications of polyoxometalates-based magnetic zinc oxide nanocomposites (Fe3O4@ZnO/PMOs). Environ. Nanotechnol. Monit. Manag. 2020, 13, 100289. [Google Scholar] [CrossRef]
- Huang, M.; Xu, C.; Wu, Z.; Huang, Y.; Lin, J.; Wu, J. Photocatalytic discolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dye. Pigment. 2008, 77, 327–334. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Sun, J.; Sun, R.; Sun, S.; Qiao, L. Photocatalytic degradation and kinetics of Orange G using nano-sized Sn(IV)/TiO2/AC photocatalyst. J. Mol. Catal. A Chem. 2006, 260, 241–246. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Jonidi-Jafari, A.; Farzadkia, M.; Esrafili, A.; Gholami, M. Enhancement of photocatalytic activity of Cu-doped ZnO nanorods for the degradation of an insecticide: Kinetics and reaction pathways. J. Environ. Manag. 2017, 186, 1–11. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Ebrahim, S.E. Highly efficient visible-light-driven photocatalytic degradation of organic pollutants by using magnetically separable supported heterogeneous nanocomposites (SiO2/Fe3O4/Ag2WO4). Environ. Nanotechnol. Monit. Manag. 2021, 16, 100554. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Saffari, R.; Shariatinia, Z.; Jourshabani, M. Synthesis and photocatalytic degradation activities of phosphorus containing ZnO microparticles under visible light irradiation for water treatment applications. Environ. Pollut. 2020, 259, 113902. [Google Scholar] [CrossRef]
- Ammar, S.H.; Salman, M.D.; Shafi, R.F. Keggin-and Dawson-type polyoxotungstates immobilized on poly (3, 4-ethylenedioxythiophene)-coated zerovalent iron nanoparticles: Synthesis, characterization and their catalytic oxidative desulfurization activity. J. Environ. Chem. Eng. 2021, 9, 104904. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C: Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations: A Review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Corrêa, A.X.R.; Tiepo, E.N.; Somensi, C.A.; Sperb, R.M.; Radetski, C.M. Use of Ozone-Photocatalytic Oxidation O3 /UV/TiO2 and Biological Remediation for Treatment of Produced Water from Petroleum Refineries. J. Environ. Eng. 2010, 136, 40–45. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of synthesized magnetic TiO2-based membranes for wastewater treatment: A review. Materials 2021, 14, 3524. [Google Scholar] [CrossRef]
- Diya’uddeen, B.H.; Daud, W.M.A.W.; Aziz, A.A. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Prot. 2011, 89, 95–105. [Google Scholar] [CrossRef]
- Naeem, K.; Feng, O. Effect of calcination on photocatalytic activity of Fe3+-doped TiO2 nanoparticles for degradation of phenol under UV irradiation. In Proceedings of the 2009 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–8 January 2009; pp. 348–352. [Google Scholar]
- Fahad, S.; Sönmez, O.; Saud, S.; Wang, D.; Wu, C.; Adnan, M.; Arif, M. Engineering Tolerance in Crop Plants Against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Begum, S.; Mishra, S.R.; Ahmaruzzaman, M. Fabrication of ZnO–SnO2 nanocomposite and its photocatalytic activity for enhanced degradation of Biebrich scarlet. Environ. Sci. Pollut. Res. 2022, 29, 87347–87360. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.; Martens, W.N.; Brown, R.; Hashib, M. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Das, L.; Dutta, M.; Basu, J.K. Photocatalytic degradation of phenol from industrial effluent using titania-zirconia nanocomposite catalyst. Int. J. Environ. Sci. 2013, 4, 415–431. [Google Scholar]
- Saien, J.; Shahrezaei, F. Organic pollutants removal from petroleum refinery wastewater with nanotitania photocatalyst and UV light emission. Int. J. Photoenergy 2012, 2012, 703074. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-light photocatalysts and their perspectives for building photocatalytic membrane reactors for various liquid phase chemical conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Ameta, S.C.; Ameta, R. Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Boltersdorf, J.; King, N.; Maggard, P.A. Flux-mediated crystal growth of metal oxides: Synthetic tunability of particle morphologies, sizes, and surface features for photocatalysis research. CrystEngComm 2015, 17, 2225–2241. [Google Scholar] [CrossRef]
- Janssens, R.; Cristóvão, B.M.; Bronze, M.R.; Crespo, J.G.; Pereira, V.J.; Luis, P. Photocatalysis using UV-A and UV-C light sources for advanced oxidation of anti-cancer drugs spiked in laboratory-grade water and synthetic urine. Ind. Eng. Chem. Res. 2019, 59, 647–653. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Hou, W.-M.; Ku, Y. Enhanced photocatalytic decomposition of gaseous isopropyl alcohol in a polymer electrolyte cell. Aerosol Air Qual. Res. 2013, 13, 1570–1581. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Fu, Y.; Guan, Y.; Yao, J.; Xiao, L.; Zeng, G. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 2012, 286, 41–48. [Google Scholar] [CrossRef]
- Kertèsz, S.; Cakl, J.; Jiránková, H. Submerged hollow fiber microfiltration as a part of hybrid photocatalytic process for dye wastewater treatment. Desalination 2014, 343, 106–112. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Garcia, H. Solar light photocatalytic CO2 reduction: General considerations and selected bench-mark photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Rani, A.; Reddy, R.; Sharma, U.; Mukherjee, P.; Mishra, P.; Kuila, A.; Sim, L.C.; Saravanan, P. A review on the progress of nanostructure materials for energy harnessing and environmental remediation. J. Nanostructure Chem. 2018, 8, 255–291. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Azmoon, P.; Farhadian, M.; Pendashteh, A.; Tangestaninejad, S. Adsorption and photocatalytic degradation of oilfield produced water by visible-light driven superhydrophobic composite of MIL-101(Cr)/Fe3O4-SiO2: Synthesis, characterization and optimization. Appl. Surf. Sci. 2023, 613, 155972. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Olateju, I.I.; Adesina, O.A. TiO2/anthill clay as a heterogeneous catalyst for solar photocatalytic degradation of textile wastewater: Catalyst characterization and optimization studies. Materialia 2019, 8, 100484. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Ezugbe, E.O.E.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO42− from oil refinery wastewater using a photo-catalytic system—Comparing TiO2 and zeolite efficiencies. Water 2020, 12, 5–14. [Google Scholar] [CrossRef]
- Benhebal, H.; Chaib, M.; Salmon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef]
- Hu, B.; Sun, Q.; Zuo, C.; Pei, Y.; Yang, S.; Zheng, H.; Liu, F. A highly efficient porous rod-like Ce-doped ZnO photocatalyst for the degradation of dye contaminants in water. Beilstein J. Nanotechnol. 2019, 10, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Boutra, B.; Trari, M. Solar photodegradation of a textile azo dye using synthesized ZnO/Bentonite. Water Sci. Technol. 2017, 75, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.M.; Dey, S.C.; Rahman, M.M.; Zakaria, A.M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. A kaolinite/TiO2 TiO2/ZnO-based novel ternary composite for photocatalytic degradation of anionic azo dyes. Bull. Mater. Sci. 2020, 43, 27. [Google Scholar] [CrossRef]
- Ravi, K.; Mohan, B.S.; Sree, G.S.; Raju, I.M.; Basavaiah, K.; Rao, B.V. ZnO/RGO nanocomposite via hydrothermal route for photocatalytic degradation of dyes in presence of visible light. Int. J. Chem. Stud. 2018, 6, 20–26. [Google Scholar]
- Yusuff, A.S.; Taofeek Popoola, L.; Aderibigbe, E.I. Solar photocatalytic degradation of organic pollutants in textile industry wastewater by ZnO/pumice composite photocatalyst. J. Environ. Chem. Eng. 2020, 8, 103907. [Google Scholar] [CrossRef]
- Srinivas, B.; Kumar, B.G.; Muralidharan, K. Stabilizer free copper sulphide nanostructures for rapid photocatalytic decomposition of rhodamine B. J. Mol. Catal. A Chem. 2015, 410, 8–18. [Google Scholar] [CrossRef]
- Meena, K.; Shanthi, M. Eco-Friendly Gelatin–Cerium–Copper Sulphide Nanoparticles for Enhanced Sunlight Photocatalytic Activity. Sustainability 2022, 14, 15325. [Google Scholar] [CrossRef]
- Berkani, M.; Smaali, A.; Kadmi, Y.; Almomani, F.; Vasseghian, Y.; Lakhdari, N.; Alyane, M. Photocatalytic degradation of Penicillin G in aqueous solutions: Kinetic, degradation pathway, and microbioassays assessment. J. Hazard. Mater. 2022, 421, 126719. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Alalm, M.G.; Fujii, M. Modeling and optimization of photocatalytic degradation of methylene blue using lanthanum vanadate. In Materials Science Forum; Trans Tech Publications, Ltd.: Baech, Switzerland, 2020; pp. 97–103. [Google Scholar]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef]
- Nasiri, A.; Tamaddon, F.; Mosslemin, M.H.; Gharaghani, M.A.; Asadipour, A. New magnetic nanobiocomposite CoFe2O4@methycellulose: Facile synthesis, characterization, and photocatalytic degradation of metronidazole. J. Mater. Sci. Mater. Electron. 2019, 30, 8595–8610. [Google Scholar] [CrossRef]
- Sikiru, S.; Abiodun, O.A.; Sanusi, Y.K.; Sikiru, Y.A.; Soleimani, H.; Yekeen, N.; Haslija, A.A. A comprehensive review on nanotechnology application in wastewater treatment a case study of metal-based using green synthesis. J. Environ. Chem. Eng. 2022, 10, 108065. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Angelis-Dimakis, A.; Ramirez Canon, A. Recent advances, influencing factors, and future research prospects using photocatalytic process for produced water treatment. Water Sci. Technol. 2022, 85, 769–788. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- Zia, A.; Akhter, P.; Nazir, A.; Hussain, M.; Park, Y.-K. Synergistic effect of metal-doped TiO2/AC for efficient visible light driven cationic dye degradation. Sep. Purif. Technol. 2025, 361, 131402. [Google Scholar] [CrossRef]
- Qi, Z.; Li, G.; Guo, X. Multi-Element Synergy in Photocatalytic Materials Integrated Mechanism-Design-Preparation Strategies. Mater. Res. Express 2025, 12, 062001. [Google Scholar]
- You, C.-S.; Noh, J.-Y.; Choi, Y.; Jung, S.-C. Degradation of antibiotic oxytetracycline using surface reconstituted TiO2 photocatalyst. Appl. Surf. Sci. 2025, 687, 162244. [Google Scholar] [CrossRef]
- Ayappan, C.; Kannan, S.K.; Ochiai, T.; Zhang, X.; Xing, R.; Liu, S.; Fujishima, A. Commercialization aspects for TiO2-based indoor air purification. Trends Chem. 2025, 7, 134–148. [Google Scholar] [CrossRef]
- Rastinifard, N.; Tota-Maharaj, P. Application of Uv/O3/TiO2/Hydrogen Peroxide-Based Advanced Oxidation Processes for Wastewater Treatment (Focused on Constructed Wetlands): A Review. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5220953 (accessed on 15 August 2025).
- Gopal, S.; Jayabalan, J.; Jayaraman, D.; Perumal, M.; Santhi, V.M. Amalgamation of Advanced Oxidation Process with Biological Techniques for Treatment of Tannery Wastewater. Phys. Chem. Earth Parts A/B/C 2025, 138, 103883. [Google Scholar] [CrossRef]
- Cen, Y.; Zhou, Y.; Zhu, M.; Zheng, K.; Zhou, S. Preparation of the Ti/TiO2-RNTs/SnO2–Sb–Ni-La Electrode and Its Electrochemical Degradation of Oily Wastewater. Langmuir 2025, 41, 8766–8780. [Google Scholar] [CrossRef]
- Aghel, S.; Bahramifar, N.; Younesi, H.; Tanha Ziyarati, M. Evaluation of Photocatalytic Degradation of Bisphenol A by Reusable Fe3O4/SiO2/TiO2 Magnetic Nanocomposite: Optimization by Response Surface Methodology. Int. J. Environ. Res. 2025, 19, 77. [Google Scholar] [CrossRef]
- Li, C.; Ding, G.; Wang, P.; Liu, K.; Yang, B.; Liao, G. Emerging frontiers of nickel–aluminium layered double hydroxide heterojunctions for photocatalysis. Dalton Trans. 2025, 54, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Behera, D.; Priyadarshini, P.; Parida, K. ZIF-8 metal–organic frameworks and their hybrid materials: Emerging photocatalysts for energy and environmental applications. Dalton Trans. 2025, 54, 2681–2708. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhong, S.; Li, K.; Wei, S.; Li, M.; Liu, R. Ionic liquids: The emerging “cardiotonic” for photocatalytic materials. Coord. Chem. Rev. 2025, 529, 216461. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Shinnur, M.V.; Pedeferri, M.; Ferrari, A.M.; Rosa, R.; Meroni, D. Toward Sustainable Photocatalysis: Addressing Deactivation and Environmental Impact of Anodized and Sol–Gel Photocatalysts. Adv. Sustain. Syst. 2025, 9, 2401017. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Shabani, A. Artificial intelligence techniques. Results Chem. 2025, 15, 102276. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation-A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Yang, J.-F.; Mao, L.-F. The impact of the location of Cu substitutions and electric field on visible light absorption in defective titanium dioxide: Ab initio calculations. J. Optoelectron. Adv. Mater. 2018, 20, 56–60. [Google Scholar]
- Xu, Y.-M.; Chen, S.; Chen, S.-L.; Wang, A.-J. Improved N2 photo-fixation performance of nanocrystalline TiO2 films by photon localization effects and Fe doping. New J. Chem. 2024, 48, 6494–6504. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Fu, N.; Li, W.; Zhang, G. Facile synthesis of Mn-doped TiO2 nanotubes with enhanced visible light photocatalytic activity. J. Appl. Electrochem. 2018, 48, 1197–1203. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, Y.; Zhou, J.; Xiao, W.; Feng, Y. Preparation, characterization, and photocatalytic activity of N, S-codoped TiO2 nanoparticles. J. Am. Ceram. Soc. 2009, 92, 938–941. [Google Scholar] [CrossRef]
- Ding, Y.; Nagpal, P. Standalone anion-and co-doped titanium dioxide nanotubes for photocatalytic and photoelectrochemical solar-to-fuel conversion. Nanoscale 2016, 8, 17496–17505. [Google Scholar]
- Chen, X.; Meng, C.; Wang, Y.; Zhao, Q.; Li, Y.; Chen, X.M.; Zhou, Y. Laser-synthesized rutile TiO2 with abundant oxygen vacancies for enhanced solar water evaporation. ACS Sustain. Chem. Eng. 2020, 8, 1095–1101. [Google Scholar] [CrossRef]
- YCao, Y.; Zhou, P.; Tu, Y.; Liu, Z.; Dong, B.-W.; Azad, A.; Ma, D.; Wang, D.; Zhang, X.; Yang, Y.; et al. Modification of TiO2 nanoparticles with organodiboron molecules inducing stable surface Ti3+ complex. IScience 2019, 20, 195–204. [Google Scholar]
- Song, Y.; Zhang, J.; Yang, L.; Cao, S.; Yang, H.; Jiang, L.; Dan, Y.; Le Rendu, P.; Nguyen, T. Photocatalytic activity of TiO2 based composite films by porous conjugated polymer coating of nanoparticles. Mater. Sci. Semicond. Process. 2016, 42, 54–57. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, K.; Schreck, M.; Liu, T.; Tervoort, E.; Niederberger, M. Gas-phase nitrogen doping of monolithic TiO2 nanoparticle-based aerogels for efficient visible light-driven photocatalytic H2 production. ACS Appl. Mater. Interfaces 2021, 13, 53691–53701. [Google Scholar] [CrossRef]
- Su, Y.; Liu, L.; Wen, S. Broadband NaYF4: Yb, Tm@NaYF4: Yb, Nd@TiO2 nanoparticles anchored on SiO2/carbon electrospun fibers for photocatalytic degradation of organic pollutants. ACS Appl. Nano Mater. 2021, 4, 12576–12587. [Google Scholar] [CrossRef]
- Daskalova, D. Combined Structural and Plasmonic Enhancement of Nanometer-Thin Film Photocatalysis for Solar-Driven Wastewater Treatment. ACS Appl. Nano Mater. 2023, 6, 5204–15212. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. Stable and near-infrared absorption enhanced dye-sensitized solar cell based on silver nanoplates@silica nanocrystals. Mater. Res. Bull. 2018, 104, 164–172. [Google Scholar] [CrossRef]
- Wu, L.; Guo, C.; Feng, R.; Zhang, H.; Shi, N.; Li, Y.; Su, H.; Cui, X.; Song, F. Co-doping of P (V) and Ti (III) in leaf-architectured TiO2 for enhanced visible light harvesting and solar photocatalysis. J. Am. Ceram. Soc. 2021, 104, 5719–5732. [Google Scholar] [CrossRef]
- Wang, M.; Deng, K.; Lü, W.; Deng, X.; Li, K.; Shi, Y.; Ding, B.; Cheng, Z.; Xing, B.; Han, G.; et al. Rational design of multifunctional Fe@γ-Fe2O3@H-TiO2 nanocomposites with enhanced magnetic and photoconversion effects for wide applications: From photocatalysis to imaging-guided photothermal cancer therapy. Adv. Mater. 2018, 30, 1706747. [Google Scholar] [CrossRef]
- Nair, P.R.; Ramirez, C.R.S.; Pelaes, R.F.C.; Krishnan, B.; Martínez, J.A.A.; Avellaneda, D.A.; Pinilla, M.A.G.; Shaji, S. IR laser induced defects and morphology in titania nanoparticles as black titania for environmental remediation. Appl. Surf. Sci. 2025, 709, 163827. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Borges, M.; Torres-García, S.; Medina-Alayón, M.; Acosta-Mora, P.; Del-Castillo, J.; Menéndez-Velázquez, A.; García-Delgado, A.; Mullins, C.; Esparza, P. There is plenty of energy at the bottom”: A spectral conversion approach for upconversion-powered water-splitting PEC cell. J. Power Sources 2025, 625, 235668. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-infrared light responsive TiO2 for efficient solar energy utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A review on metal ions modified TiO2 for photocatalytic degradation of organic pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Umar, K.; Ibrahim, M.N.M.; Ahmad, A.; Rafatullah, M. Synthesis of Mn-doped TiO2 by novel route and photocatalytic mineralization/intermediate studies of organic pollutants. Res. Chem. Intermed. 2019, 45, 2927–2945. [Google Scholar] [CrossRef]
- Santos, F.; Azevedo, E.; Dezotti, M. Photocatalysis as a tertiary treatment for petroleum refinery wastewaters. Braz. J. Chem. Eng. 2006, 23, 451–460. [Google Scholar] [CrossRef]
- Li, G.; An, T.; Nie, X.; Sheng, G.; Zeng, X.; Fu, J.; Lin, Z.; Zeng, E.Y. Mutagenicity assessment of produced water during photoelectrocatalytic degradation. Environ. Toxicol. Chem. Int. J. 2007, 26, 416–423. [Google Scholar] [CrossRef]
- Gouma, P.; Lee, J. Photocatalytic nanomats clean up produced water from fracking. Transl. Mater. Res. 2014, 1, 025002. [Google Scholar] [CrossRef]
- Islam, M.T.; Dominguez, A.; Turley, R.S.; Kim, H.; Sultana, K.A.; Shuvo, M.; Alvarado-Tenorio, B.; Montes, M.O.; Lin, Y.; Gardea-Torresdey, J. Development of photocatalytic paint based on TiO2 and photopolymer resin for the degradation of organic pollutants in water. Sci. Total Environ. 2020, 704, 135406. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Tugaoen, H.O.N.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total Environ. 2018, 613, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Lee, C.-G.; Javed, H.; Zhang, D.; Kim, J.-H.; Westerhoff, P.; Li, Q.; Alvarez, P.J. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Shoneye, A. Photocatalytic Mineralisation of Toxic Chlorophenols and Removal of Cr (VI) in Aqueous Solution Using Cocatalyst Decorated TiO2. Ph.D. Thesis, UCL (University College), London, UK, 2021. [Google Scholar]
- Pillai, S.C.; McGuinness, N.B.; Byrne, C.; Han, C.; Lalley, J.; Nadagouda, M.; Falaras, P.; Kontos, A.G.; Gracia-Pinilla, M.A.; OShea, K. Photocatalysis as an effective advanced oxidation process. Adv. Oxid. Process. Water Treat. Fundam. Appl. 2017, 16, 333–381. [Google Scholar] [CrossRef]
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current research trends on emerging contaminants pharmaceutical and personal care products (PPCPs): A comprehensive review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef]
- Sun, Q.; Hou, P.; Wu, S.; Yu, L.; Dong, L. The enhanced photocatalytic activity of Ag-Fe2O3-TiO2 performed in Z-scheme route associated with localized surface plasmon resonance effect. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127304. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Z.; Qi, S.; Yin, Z.; Deng, S.; Zhang, M.; Sun, Z. The quaternary system of Ag2S/ZnS co-modified ZnO/TiO2 nanotree arrays: Excellent photocatalysis and photoelectrochemistry performance. Appl. Surf. Sci. 2021, 538, 148044. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, L.; Yang, H.; Song, K.; Hou, H. Tailored fabrication of TiO2/In2O3 hybrid mesoporous nanofibers towards enhanced photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127455. [Google Scholar] [CrossRef]
- Ali, H.M.; Roghabadi, F.A.; Ahmadi, V. Solid-supported photocatalysts for wastewater treatment: Supports contribution in the photocatalysis process. Sol. Energy 2023, 255, 99–125. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayanandan, A. Review on support materials used for immobilization of nano-photocatalysts for water treatment applications. Inorganica Chim. Acta 2022, 255, 121284. [Google Scholar] [CrossRef]
- Srikanth, B.; Goutham, R.; Narayan, R.B.; Ramprasath, A.; Gopinath, K.; Sankaranarayanan, A. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Mhike, W.; Adalima, J.L.; Tichapondwa, S. Removal of organic dyes from water and wastewater using magnetic ferrite-based titanium oxide and zinc oxide nanocomposites: A review. Catalysts 2021, 11, 1543. [Google Scholar] [CrossRef]
- Gupta, V.K.; Eren, T.; Atar, N.; Yola, M.L.; Parlak, C.; Karimi-Maleh, H. CoFe2O4@TiO2 decorated reduced graphene oxide nanocomposite for photocatalytic degradation of chlorpyrifos. J. Mol. Liq. 2015, 208, 122–129. [Google Scholar] [CrossRef]
- Krishna, S.; Sathishkumar, P.; Pugazhenthiran, N.; Guesh, K.; Mangalaraja, R.; Kumaran, S.; Gracia-Pinilla, M.; Anandan, S. Heterogeneous sonocatalytic activation of peroxomonosulphate in the presence of CoFe2O4/TiO2 nanocatalysts for the degradation of Acid Blue 113 in an aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104024. [Google Scholar]
- Loeb, S.K.; Alvarez, P.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2018, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Piao, L. Considerations for a more accurate evaluation method for photocatalytic water splitting. Angew. Chem. Int. Ed. 2020, 59, 18312–18320. [Google Scholar] [CrossRef] [PubMed]



| Examples of Nanomaterials | Novel Properties of Nanomaterials |
|---|---|
| Carbon nanotubes (CNTs), magnetite, zeolites, TiO2, and nano-Ag | Photocatalytic activity, high selectivity and permeability, hydrophilicity, low toxicity to humans, intense antimicrobial activity, high chemical and mechanical stability, among others. |
| Fullerene derivatives and nano-TiO2 | Low cost, high selectivity and stability, low human toxicity, photocatalytic activity in solar spectrum, among others. |
| CNTs, titanium dioxide (Ag/TiO2), and nano-silver | Ease of use, high chemical stability, low cost and toxicity, potent antimicrobial activity, among others. |
| Nanofibers, metal oxide, and nanoscale or CNTs | Easy reuse, tunable surface chemistry, short intraparticle diffusion distance, more adsorption and selective sites, accessible adsorption sites, and high specific surface area. |
| Semiconductor | Crystal Structure | Band Gap Structure = 7 | Ref. | ||
|---|---|---|---|---|---|
|
Conduction Band (CB) |
Valence Band (VB) | Eg/eV | |||
| TiO2 | Anatase | −0.50 | 2.70 | 3.20 | [46] |
| ZnO | −0.31 | 2.89 | 3.20 | [47] | |
| CuO | −1.16 | 0.85 | 2.00 | [48] | |
| CdS | −0.90 | 1.50 | 2.40 | [49] | |
| ZnS | −1.04 | 2.56 | 3.60 | [50] | |
| g-C3N4 | −1.30 | 1.40 | 2.70 | [51,52] | |
| g-C3N4 | −1.53 | 1.16 | 2.70 | [53] | |
| Ta3N5 | −0.75 | 1.35 | 2.10 | [54] | |
| TaON | −0.75 | 1.75 | 2.50 | [55] | |
| Fe2O3 | 0.28 | 2.48 | 2.20 | [56] | |
| Bi2O3 | 0.33 | 3.13 | 2.80 | [57] | |
| BiVO4 | −0.30 | 2.10 | 2.40 | [58] | |
| WO3 | −0.10 | 2.70 | 2.80 | [59] | |
| Ag3PO4 | Cubic | 0.04 | 2.49 | 2.45 | [60] |
| Photocatalyst | Photocatalyst Load | Irradiation Source | Contaminant | Exposure Time (Minutes) | Photodegradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4@Al2O3-PMo | 0.5–3 g/L | UV (360 nm) | Cibacron brilliant yellow 3G-P | 300 | >90 | [61] |
| Pt-TiO2 | 0.5–6.0 g/L | UV | Methyl orange | Not given | 90.5 | [62] |
| Sn/TiO2/AC | 5.0–15.0 g/L | UV | Orange G | 60 | 99.1 | [63] |
| Cu-doped ZnO | 0.1–1 g/L | 125 W medium-pressure UVC lamp | Diazinon | 120 | 96.97 | [64] |
| Fe3O4@ZnO/PMOs | 0.25–1.5 g/L | 40 W white LED lamps | Methyl orange | 180 | 98.2 | [61] |
| Fe3O4@SiO2@Ag2WO4@Ag2S | 0.5–3 g/L | Xenon + LED lamps (95 W) | Methyl blue | 60 | 99.9 | [65] |
| Bi2O3/SnO2 | (20–60) mg/50 mL | 350 W Xenon lamp | Bisphenol A | 60 | 93.42 | [66] |
| P-ZnO1.8% | 0.5–3 g/L | 300 W halogen lamp | Rhodamine B | 180 | 99 | [67] |
| Fe0@PEDOT\PW12 | 0.5–3 g/L | Visible light | Oxidative desulfurization | 60 | 98.4 | [68] |
| Type of Wastewater | Type of Catalyst | Target Pollutant | Operating Conditions | Light Source | Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Textile industry wastewater | TiO2/anthill | Colour | IC (3.01); CL (2.5 g/L); pH (2); T (67 min) | Sunlight | 70.92 | [99] |
| Petroleum refinery | TiO2 | COD and SO42− | COD: (1226 mg/L); pH (8); CL (1.5 g/L); T (150 min) | UV light | 92 | [100] |
| Industrial wastewater | ZnO | Phenol and benzoic acid | IC (50 mg/L); CL (1.0 g/L); T (120 min) | UV light | 69.75 | [101] |
| Industrial wastewater | Ce-doped ZnO | Rhodamine B (RhB) | IC (10 mg/L); CL (0.7 g/L); pH (9.0); T (120 min) | Sunlight | 97.66 | [102] |
| Textile industry wastewater | ZnO/bentonite | Solophenyl Red 3BL (SR 3BL) | IC (0.75 mg/L); CL (0.75 g/L); pH (6), T (160 min) | Sunlight | 92 | [103] |
| Industrial wastewater | Kaolinite/TiO2/ZnO | Remazol Red (RR), an anionic azo dye | IC (100 mg/L); CL (100 mg/L); pH (2.5); T (120 min) | Sunlight | 98 | [104] |
| Synthetic wastewater | ZnO/RGO | Congo red (CR) and Eosin yellow (EY) contaminate | IC (10 mg/L); CL (0.05 g/L); pH (3.0); T (120 min) | Visible light | 51 | [105] |
| Textile industry wastewater | ZnO/pumice composite | Dye-containing wastewater | IC (3.01 mg/L); CL (3.0 g/L); pH (4.01); T (45.04 min) | Sunlight | 90.17 | [106] |
| Synthetic wastewater | CuS flowers | Rhodamine B (Rh B) | CL (1 g/L); T (12 min) | Visible light | ∼99 | [107] |
| Synthetic wastewater | Gelatin–cerium–copper sulfide Ge-Ce-CuS nanoparticles | Malachite green oxalate dye (MGO dye) | IC (3 × 10−4 M); CL (1.5 g/L); pH (9); T (120 min); | Sunlight | 90.7 | [108] |
| Pharmaceutical industry treatment | Persulfate Sodium (PPS) | Penicillin G (PG) | IC (5 mg/L); CL (0.8 g/L); t pH (6), (22 °C); MS (500 rpm) | UV light | 72.72 | [109] |
| Textile industry wastewater | Lanthanum Vanadate (LaVO4) | Methylene blue (MB) | IC (10 mg/L); CL (0.3 g/L); pH (7) | UV light | 91 | [110] |
| Synthetic wastewater | TiO2/GO graphene oxide composite | Methylene blue (MB) | IC (5 mg/L); CL (0.2 g/L); pH (10); T (4 h). | UV–vis | 99 | [111] |
| Pharmaceutical industry treatment | Nanobiocomposite CoFe2O4@methycellulose (MC) | Metronidazole (MNZ) antibiotic | IC (5 mg/L); CL (0.2 g/L); pH (11); T (120 min) | UV light | 85.3 | [112] |
| Thematic Area | Key Findings | Considerable Remarks | Future Research | Reference |
|---|---|---|---|---|
| TiO2 photocatalysis | Synergistic effect of metal-doped TiO2/AC for efficient visible light-driven cationic dye degradation, Ag-doped TiO2/AC with enhanced surface functionality, reactivity, and cyclic stability showed 87.25% Rh B degradation. | Advances in environmental remediation using metal-doped TiO2/AC heterojunctions (e.g., Ag-TiO2 or TiO2-ZnO, TiO2-graphene) for charge separation. | Doping with metals/non-metals to extend visible light activity. | [116] |
| Multi-element synergy in photocatalytic material-integrated mechanism–design–preparation strategies | Quantitative analysis of the complex interactions between elements and the integrated design of low-cost materials, elemental doping, and surface plasma. | Immobilization of supports to improve recovery potential. Scalability of eco-friendly synthesis routes (bio-mediated TiO2 nanostructures). | [117] | |
| Degradation of antibiotic oxytetracycline using surface-reconstituted TiO2 photocatalyst | The synergistic effect of co-doping compared to single-component doping. | Understanding of charge transfer mechanism and improving band gap energy reduction under responsive light. | [118] | |
| Advanced oxidation process (AOP) | Commercialization aspects for TiO2-based indoor air purification | Designing strategies to improve their photon utilization and deactivation resistance, and regeneration of TiO2-based photocatalysts. | TiO2-based air purification is proposed to demonstrate the innovative commercialization direction. | [119] |
| Application of Uv/O3/Tio2/hydrogen peroxide-based advanced oxidation processes for wastewater treatment | Hybrid system (UV/TiO2/O3) for pollutant removal with pathogen inactivation. Also, ozone-based AOPs are efficient at detoxifying a variety of resistant effluents. | To establish a solid theoretical foundation for the implementation of UV/H2O2- and O3/H2O2-based AOPs in wastewater management. | [120] | |
| Amalgamation of advanced oxidation process with biological techniques for the treatment of tannery wastewater | Pre-treatment of wastewater by AOP converts the recalcitrant organic pollutants to simpler and biodegradable compounds, allowing the wastewater to be treated by subsequent biological treatment. | A cost-effective methodology for the treatment of tannery wastewater to comply with applicable current regulations worldwide must explore the use of AOP coupling with biological treatment technologies. | [121] | |
| Preparation of the Ti/TiO2-RNTs/SnO2–Sb–Ni-La electrode and its electrochemical degradation of oily wastewater | The electrochemical oxidation method is expected to reduce energy loss. | Electrochemical AOPs using renewable energy sources and exploring suitable cost-effective electrode materials. | [122] | |
| Magnetic nanomaterials | Evaluation of photocatalytic degradation of Bisphenol A by reusable Fe3O4/SiO2/TiO2 magnetic nanocomposite: optimization by response surface methodology | The potential of Fe3O4/SiO2/TiO2 using both UVA and solar light. Agglomeration and loss of surface activity over time. | Magnetic TiO2 for easy recovery and reuse with magnetic separation technology. Environmental toxicity and long-term stability have not been fully assessed for scalability applications. | [123] |
| Emerging materials | Emerging frontiers of nickel–aluminum-layered double hydroxide heterojunctions for photocatalysis | Ni–Al LDH-based heterojunctions in photocatalytic applications, such as H2 evolution, CO2 reduction, and pollutant removal. | Exploit new Ni–Al LDH-based heterojunctions for high-performance photocatalytic applications, large surface area, tunable band gap and morphology, abundant reaction sites, and high activity, selectivity, and photostability. | [124] |
| ZIF-8 metal–organic frameworks and their hybrid materials: Emerging photocatalysts for energy and environmental applications | Applications of ZIF-8-based photocatalysts in light-driven H2 evolution, H2O2 evolution, CO2 reduction, and dye and drug degradation. | Developing cost-effective, scalable, and environmentally friendly ZIF-8 composites for industrial applications. | [125] | |
| Ionic liquids: The emerging “cardiotonic” for photocatalytic materials | Ionic liquids (ILs) and photocatalytic materials (PMs) enhance the properties (hydrogen bonds effect, electrostatic effect, polarity effect, and coordination effect). | Research in high-powered PMs using the strategy of IL modification. | [126] | |
| Environmental relevance | Toward sustainable photocatalysis: Addressing deactivation and environmental impact of anodized and sol–gel photocatalysts | Photocatalytic coating preparation, like chemical sol–gel and electrochemical anodic oxidation, generates the oxide directly from a titanium substrate. Life cycle assessment (LCA) was used to quantify and compare the potential environmental impacts associated with the two different TiO2 production processes. | Developing more eco-friendly application strategies, like magnetic photocatalysts and synthesis routes (sol–gel photocatalysts). Future research on iron oxide-based composites for environmental remediation. | [127] |
| AI-integrated process | Artificial intelligence (AI) techniques | Fe3O4/TiO2 nanocomposites were synthesized and optimized for enhanced photocatalytic degradation of organic pollutants. An AI-assisted model was developed to predict removal efficiency based on experimental data. | The potential of integrating AI with advanced photocatalysts for wastewater treatment and suggests a scalable strategy for industrial applications. | [128] |
| Conditions | Current Challenges | Prospective Way Forward |
|---|---|---|
| Cost-effective synthesis route | * Nanomaterials required to improve TiO2 production * Cheap design method * Synthesis route with eco-friendly and non-toxic precursors | * Developing and optimizing cost-effective TiO2 synthesis methods to maximize production yield * Regenerative and reusable photocatalyst approach |
| Morphology control | * Synthesis of nanoparticles with good crystallinity, surface area, and small particle size | * Develop a synthesis method to maximize morphology * Develop a kinetic model for the predictive morphology of photocatalysts |
| Visible light absorption | * Modification with cheap co-catalysts * Concentration of doped materials | * Develop and optimize a cost-effective modification process |
| Product selectivity and optimization | * Sensitivity and selectivity of nanomaterials to improve product yield* Optimizing the selective product | * Tuning the band structure and accumulation of illumination * TiO2 modification and regeneration * Optimizing the synthesis process, multi-factors, or operating conditions |
| Charge separation efficiency | * Difficult to separate photocatalysts after being used | * Develop semiconductors with suitable band positions to couple with TiO2 * Develop magnetized TiO2 to enhance magnetic separation and regeneration usability |
| Cost-effective photocatalytic reactor design | * Design of a suitable photocatalytic reactor | * Establishment of hybridized solar/UV–photocatalytic reactor * Commercialization of reactors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munien, C.; Rathilal, S.; Tetteh, E.K. Challenges and Prospects of TiO2-Based Photocatalysis for Wastewater Treatment: Keyword Analysis. Catalysts 2025, 15, 801. https://doi.org/10.3390/catal15090801
Munien C, Rathilal S, Tetteh EK. Challenges and Prospects of TiO2-Based Photocatalysis for Wastewater Treatment: Keyword Analysis. Catalysts. 2025; 15(9):801. https://doi.org/10.3390/catal15090801
Chicago/Turabian StyleMunien, Caressa, Sudesh Rathilal, and Emmanuel Kweinor Tetteh. 2025. "Challenges and Prospects of TiO2-Based Photocatalysis for Wastewater Treatment: Keyword Analysis" Catalysts 15, no. 9: 801. https://doi.org/10.3390/catal15090801
APA StyleMunien, C., Rathilal, S., & Tetteh, E. K. (2025). Challenges and Prospects of TiO2-Based Photocatalysis for Wastewater Treatment: Keyword Analysis. Catalysts, 15(9), 801. https://doi.org/10.3390/catal15090801









