Tribocatalytic Degradation of Organic Dyes by Disk-Shaped PTFE and Titanium: A Powder-Free Catalytic Technology for Wastewater Treatment
Abstract
1. Introduction
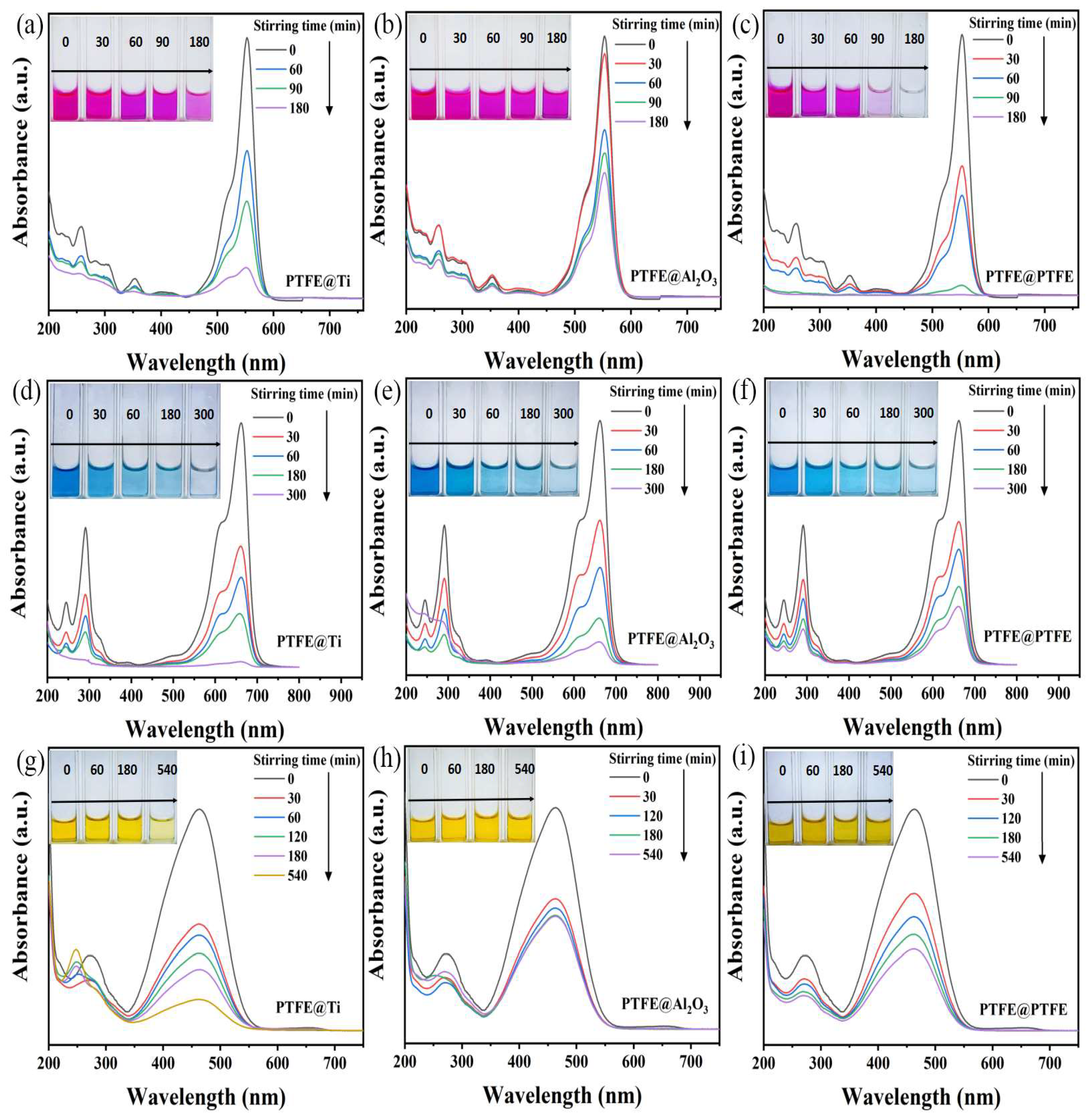
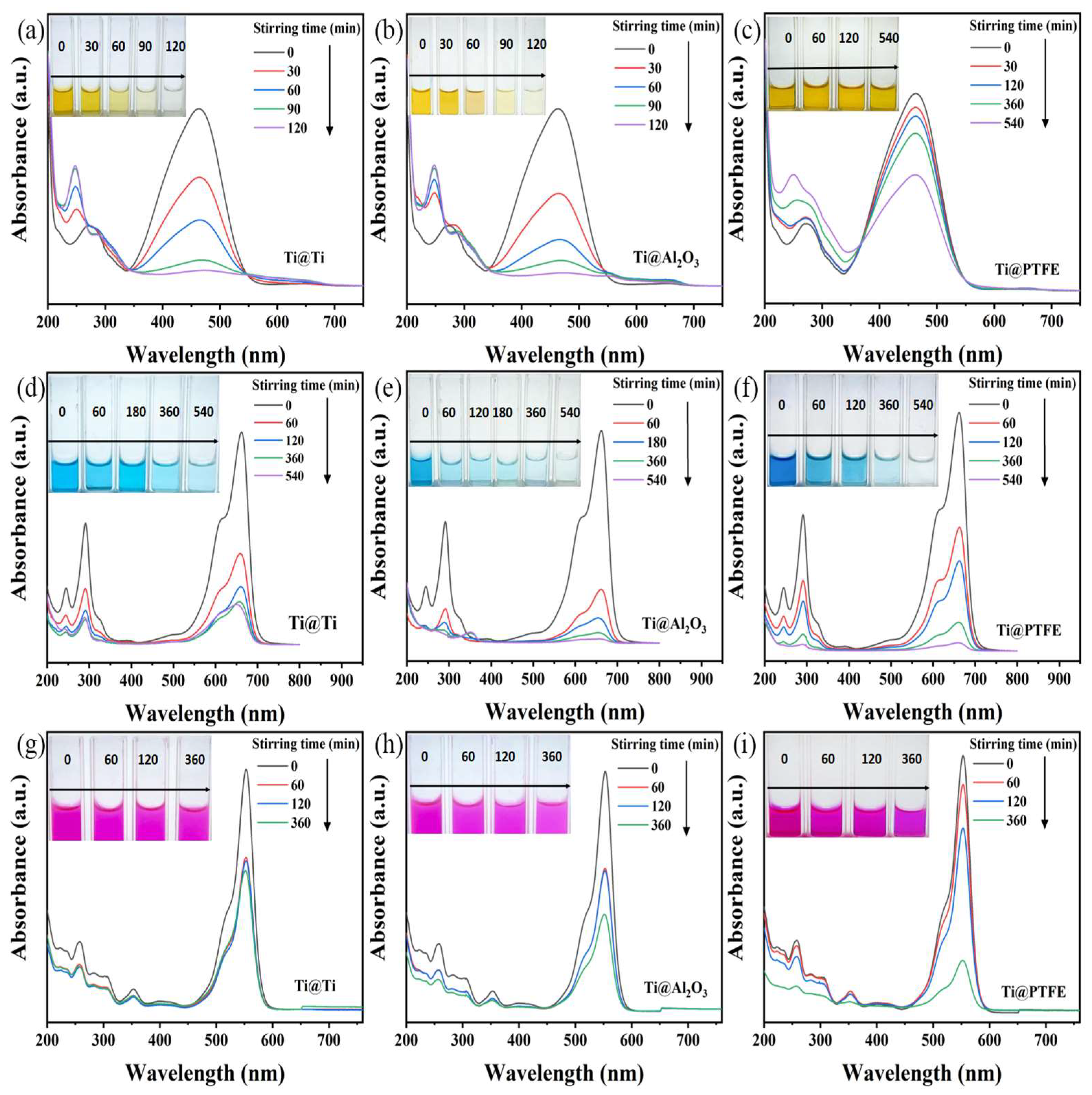
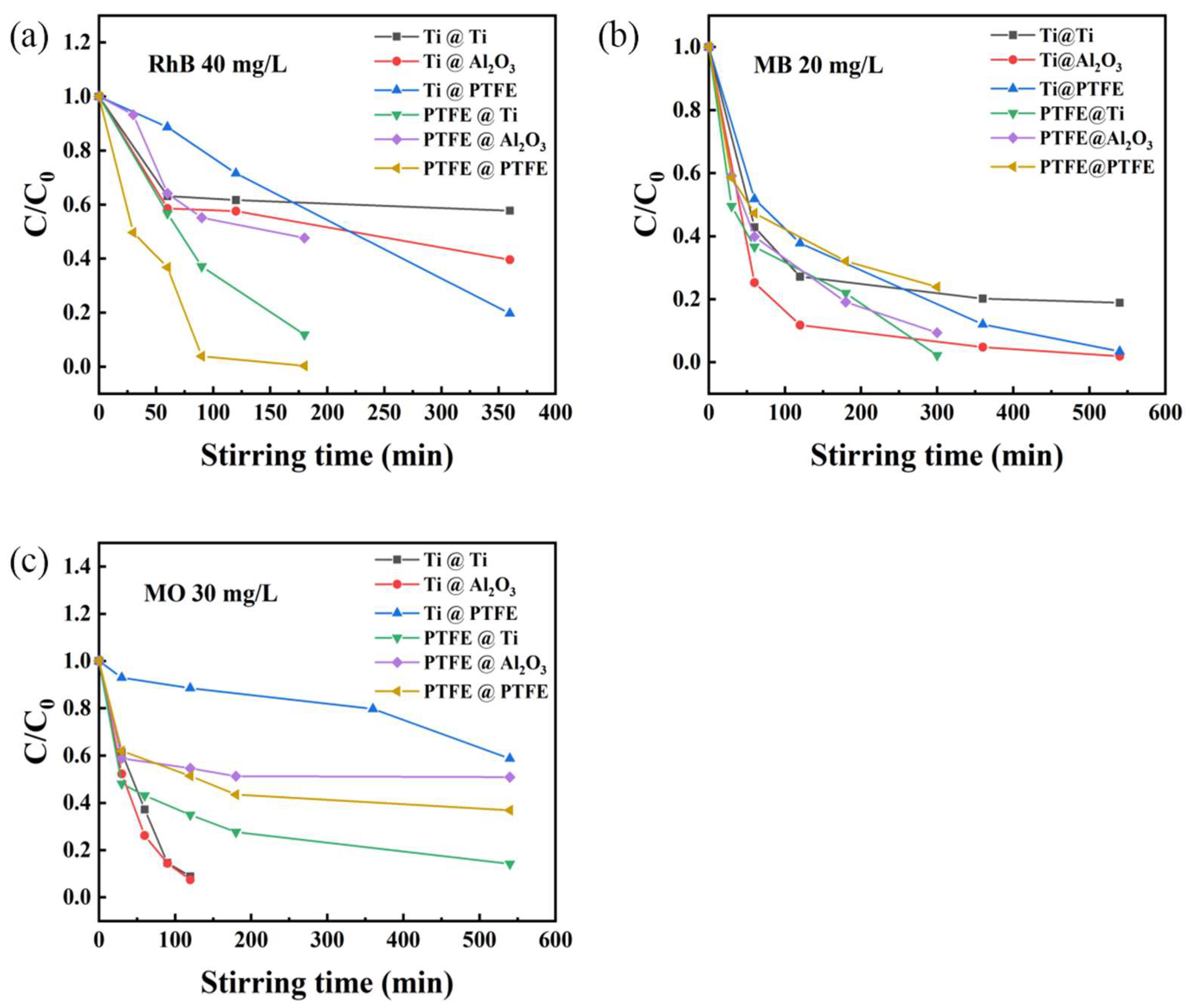
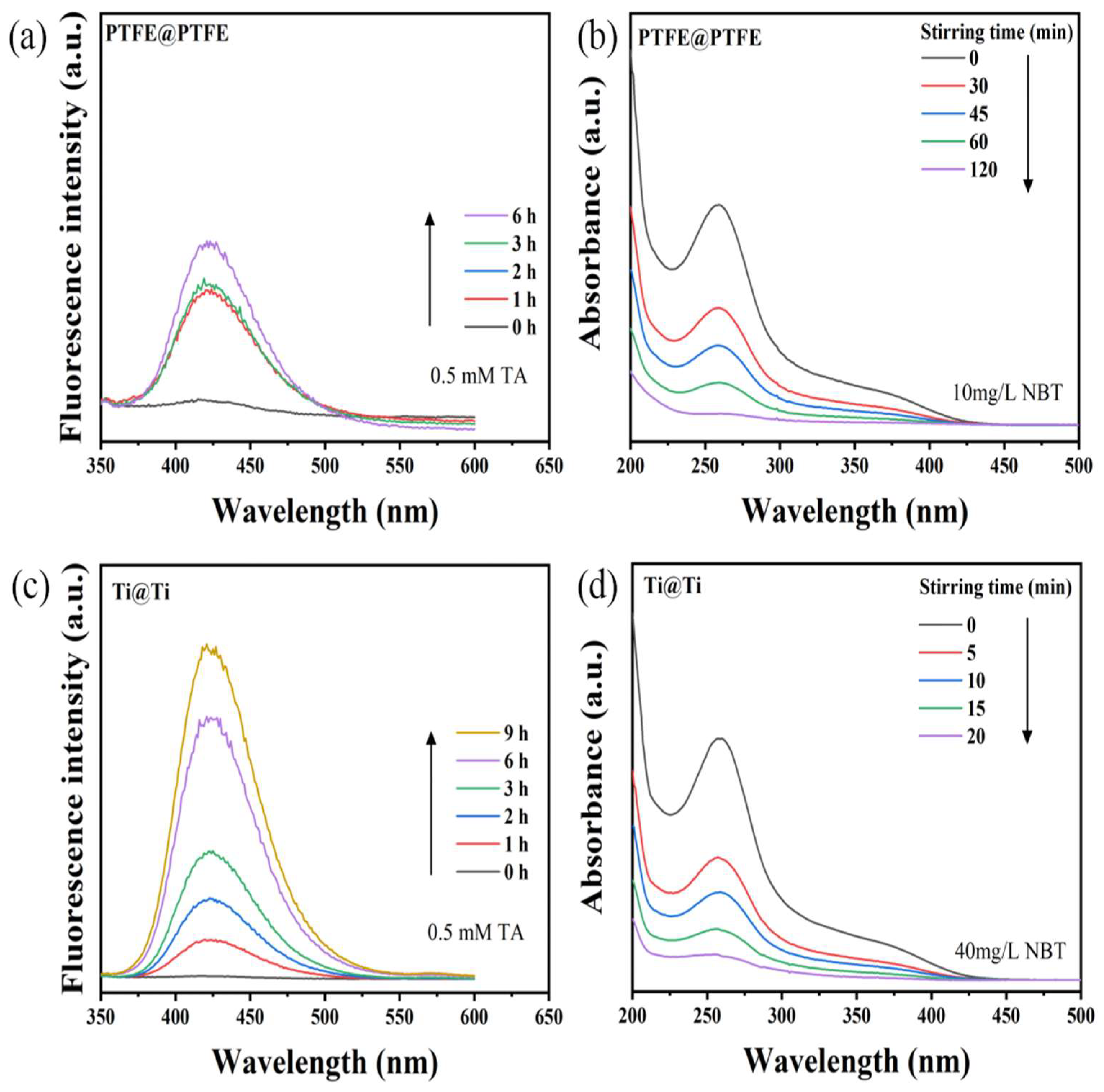
2. Results and Discussion
2.1. Organic Dyes Degradation
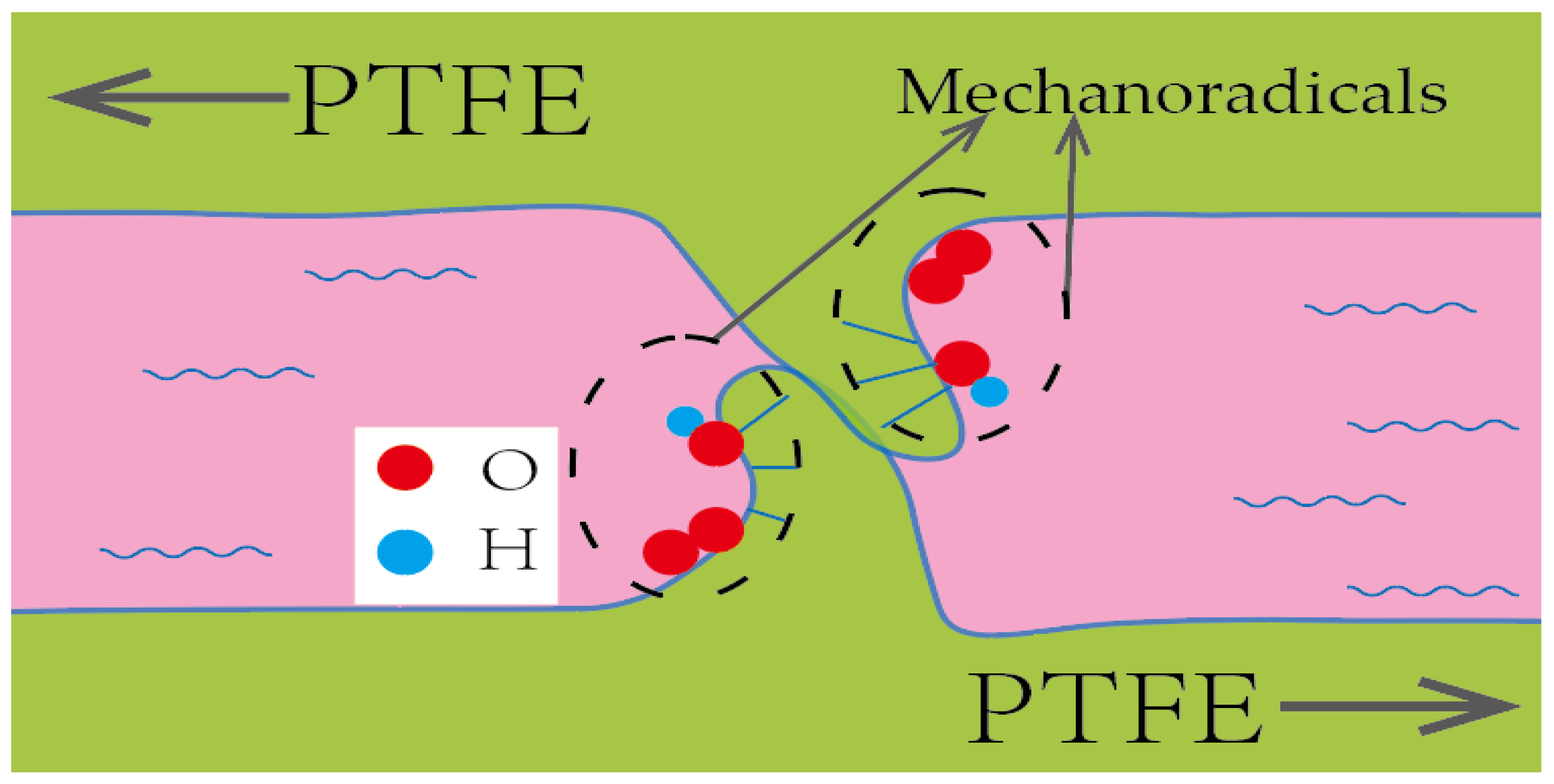
2.2. Mechanism Analysis
3. Materials and Methods
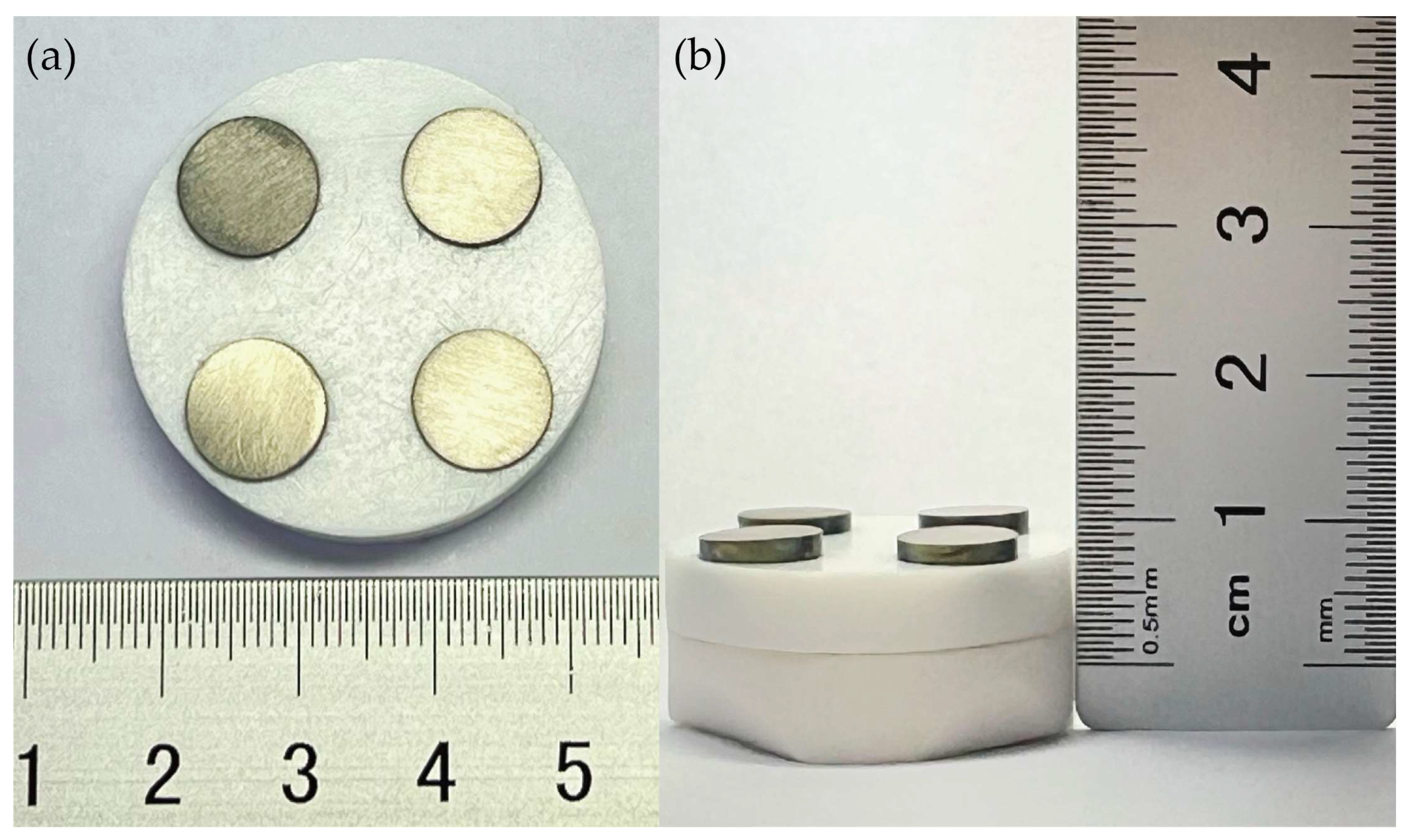
3.1. PTFE and Ti Disks Assembled as Magnetic Rotary Disks
3.2. Modifying Glass Beakers with PTFE, Ti, and Al2O3 Disks on Their Bottoms
3.3. Degradation of Organic Dyes Through Magnetic Stirring
3.4. Detection for Hydroxyl Radicals and Superoxide Radicals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Topinka, J.; Rossner, P.; Milcová, A.; Schmuczerová, J.; Pěnčíková, K.; Rossnerová, A.; Ambrož, A.; Štolcpartová, J.; Bendl, J.; Hovorka, J.; et al. Day-to-day variability of toxic events induced by organic compounds bound to size segregated atmospheric aerosol. Environ. Pollut. 2015, 202, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.C. Persistent Organic Pollutants (POPs) and Related Chemicals in the Global Environment: Some Personal Reflections. Environ. Sci. Technol. 2021, 55, 9400–9412. [Google Scholar] [CrossRef] [PubMed]
- Habesoglu, S.; Atici, A.A. Assessment of pollution indices and human health risk related to 13 heavy metal contents in surface water of Sihke Pond (Van), Turkey. Spectrosc. Lett. 2022, 55, 464–477. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Zhuang, W.; Hachem, K.; Bokov, D.; Javed Ansari, M.; Taghvaie Nakhjiri, A. Ionic liquids in pharmaceutical industry: A systematic review on applications and future perspectives. J. Mol. Liq. 2022, 349, 118145. [Google Scholar] [CrossRef]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. Recent developments in photocatalysis of industrial effluents: A review and example of phenolic compounds degradation. Chemosphere 2022, 296, 133688. [Google Scholar] [CrossRef]
- Yasir, M.W.; Siddique, M.B.A.; Shabbir, Z.; Ullah, H.; Riaz, L.; Nisa, W.U.; Shafeeq, U.R.; Shah, A.A. Biotreatment potential of co-contaminants hexavalent chromium and polychlorinated biphenyls in industrial wastewater: Individual and simultaneous prospects. Sci. Total Environ. 2021, 779, 146345. [Google Scholar] [CrossRef]
- Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules 2022, 27, 618. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling Agricultural Wastes and By-products in Organic Farming: Biofertilizer Production, Yield Performance and Carbon Footprint Analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- Almeida, S.; Ozkan, S.; Gonçalves, D.; Paulo, I.; Queirós, C.S.G.P.; Ferreira, O.; Bordado, J.; Santos, R.G.D. A Brief Evaluation of Antioxidants, Antistatics, and Plasticizers Additives from Natural Sources for Polymers Formulation. Polymers 2022, 15, 6. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Bai, Z.; Ma, L.; Strokal, M.; Kroeze, C.; Chen, X.; Zhang, F.; Shi, X. Mitigating phosphorus pollution from detergents in the surface waters of China. Sci. Total Environ. 2022, 804, 150125. [Google Scholar] [CrossRef] [PubMed]
- Silvello, M.A.C.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef]
- Morra, P.; Lisi, R.; Spadoni, G.; Maschio, G. The assessment of human health impact caused by industrial and civil activities in the Pace Valley of Messina. Sci. Total Environ. 2009, 407, 3712–3720. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and human health impacts of volatile organic compounds: A perspective review. Chemosphere 2022, 313, 137489. [Google Scholar] [CrossRef]
- Bal, G.; Thakur, A. Distinct approaches of removal of dyes from wastewater: A review. Mater. Today Proc. 2022, 50, 1575–1579. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of dye-containing wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Annamalai, S.; Santhanam, M.; Selvaraj, S.; Maruthamuthu, S.; Pandian, K.; Pazos, M. ‘Green technology’: Bio-stimulation by an electric field for textile reactive dye contaminated agricultural soil. Sci. Total Environ. 2018, 624, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Cao, L.; Song, C.; Cao, X.; Zhou, Y. Regulation of the Nutrient Cycle Pathway and the Microbial Loop Structure by Different Types of Dissolved Organic Matter Decomposition in Lakes. Environ. Sci. Technol. 2022, 57, 297–309. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Duan, X.; Sarmah, A.K.; Zhao, X. Remediation of environmentally persistent organic pollutants (POPs) by persulfates oxidation system (PS): A review. Sci. Total Environ. 2022, 863, 160818. [Google Scholar] [CrossRef]
- Dapaah, M.F.; Niu, Q.; Yu, Y.Y.; You, T.; Liu, B.; Cheng, L. Efficient persistent organic pollutant removal in water using MIL-metal–organic framework driven Fenton-like reactions: A critical review. Chem. Eng. J. 2022, 431, 134182. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, H.; Zhou, P.; Lai, L.; Liu, W.; Ao, Z.; Yao, G.; Zhang, H.; Lai, B. Insights into the role of in-situ and ex-situ hydrogen peroxide for enhanced ferrate (VI) towards oxidation of organic contaminants. Water Res. 2021, 203, 117548. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, F.; Fang, M.; Liu, B.; Zhang, B.; Zhong, Z.; Yu, L.; Zhang, Y.; Tan, X.; Wang, X. Catalyst-Free Extraction of U (VI) in Solution by Tribocatalysis. Adv. Sci. 2024, 11, 2404397. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schumuki, P. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Young, J.B.; Lim, H.; Klein, W.M.; Chen, H.; Xi, Y.; Gai, B.; Deutsch, T.G.; Yoon, J. Printed assemblies of GaAs photoelectrodes with decoupled optical and reactive interfaces for unassisted solar water splitting. Nat. Energy 2017, 2, 17043. [Google Scholar] [CrossRef]
- Duan, C.; Qin, X.; Wang, X.; Feng, X.; Yu, H.; Dai, L.; Wang, W.; Zhao, W. Simultaneous mechanical refining and phosphotungstic acid catalysis for improving the reactivity of kraft-based dissolving pulp. Cellulose 2019, 26, 5685–5694. [Google Scholar] [CrossRef]
- Domen, K.; Ikeda, S.; Takata, T.; Tanaka, A.; Hara, M.; Kondo, J.N. Mechano-catalytic overall water-splitting into hydrogen and oxygen on some metal oxides. Appl. Energy 2000, 67, 159–179. [Google Scholar] [CrossRef]
- Hara, M.; Komoda, M.; Hasei, H.; Yashima, M.; Ikeda, S.; Takata, T.; Kondo, J.N.; Domen, K. A Study of Mechano-Catalysts for Overall Water Splitting. J. Phys. Chem. B 2000, 104, 780–785. [Google Scholar] [CrossRef]
- Takata, T.; Ikeda, S.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Mechano-catalytic overall water splitting on some oxides (II). Appl. Catal. A Gen. 2000, 200, 255–262. [Google Scholar] [CrossRef]
- Xu, X.; Mao, C.; Ke, S.; Song, J.; Gu, Y.; Li, N.; Chen, W. Greatly enhanced tribocatalytic degradation of organic dyes by Fe2O3 nanoparticles through Ti and Al2O3 coatings. J. Mater. Sci. 2025, 60, 7333–7342. [Google Scholar] [CrossRef]
- Che, J.; Gao, Y.; Wu, Z.; Ma, J.; Wang, Z.; Liu, C.; Jia, Y.; Wang, X. Review on tribocatalysis through harvesting friction energy for mechanically-driven dye decomposition. J. Alloys Compd. 2024, 1002, 175413. [Google Scholar] [CrossRef]
- Hu, J.; Ma, W.; Pan, Y.; Cheng, Z.; Yu, S.; Gao, J.; Zhang, Z.; Wan, C.; Qiu, C. Insights on the mechanism of Fe doped ZnO for tightly-bound extracellular polymeric substances tribo-catalytic degradation: The role of hydration layers at the interface. Chemosphere 2021, 276, 130170. [Google Scholar] [CrossRef]
- Hu, J.; Ma, W.; Pan, Y.; Chen, Z.; Zhang, Z.; Wan, C.; Sun, Y.; Qiu, C. Resolving the Tribo-catalytic reaction mechanism for biochar regulated Zinc Oxide and its application in protein transformation. J. Colloid Interface Sci. 2021, 607, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Mao, C.; Lei, H.; Guo, Z.; Jia, X.; Cui, X.; Huang, J.; Fei, L.; Jia, Y.; Chen, W. Exceptional tribo-catalytic degradation of concentrated methyl orange and methylene blue solutions by DXN-RT30 TiO2 nanoparticles. Ceram. Int. 2023, 50, 4737–4745. [Google Scholar] [CrossRef]
- Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, Z.; Chen, W. Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity. Nanomaterials 2022, 13, 46. [Google Scholar] [CrossRef]
- Lei, H.; Jia, X.; Wang, H.; Cui, X.; Jia, Y.; Fei, L.; Chen, W. Tribo-Catalytic Conversions of H2O and CO2 by NiO Particles in Reactors with Plastic and Metallic Coatings. Coatings 2023, 13, 396. [Google Scholar] [CrossRef]
- Jia, X.; Wang, H.; Lei, H.; Mao, C.; Cui, X.; Liu, Y.; Jia, Y.; Yao, W.; Chen, W. Boosting tribo-catalytic conversion of H2O and CO2 by Co3O4 nanoparticles through metallic coatings in reactors. J. Adv. Ceram. 2023, 12, 1833–1843. [Google Scholar] [CrossRef]
- Cui, X.; Wang, H.; Lei, H.; Jia, X.; Jiang, Y.; Fei, L.; Jia, Y.; Chen, W. Surprising tribo-catalytic conversion of H2O and CO2 into flammable gases utilizing frictions of copper in water. ChemistrySelect 2023, 8, e202204146. [Google Scholar] [CrossRef]
- Lei, H.; Wu, Z.; Wang, H.; Mao, C.; Guo, Z.; Fei, L.; Chen, W. Converting H2O and CO2 into chemical fuels by nickel via friction. Surf. Interfaces 2024, 46, 104203. [Google Scholar] [CrossRef]
- Barrocas, B.; Monteiro, O.C.; Nunes, M.R.; Silvestre, A.J. Influence of Re and Ru doping on the structural, optical and photocatalytic properties of nanocrystalline TiO2. SN Appl. Sci. 2019, 1, 556. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Hassan, M.A.; Patil, D.R.; Kolekar, S.S.; Ryu, S.-W. One-Pot in Situ Hydrothermal Growth of BiVO4/Ag/rGO Hybrid Architectures for Solar Water Splitting and Environmental Remediation. Sci. Rep. 2017, 7, 8404. [Google Scholar] [CrossRef]
- Vale, M.; Barrocas, B.T.; Serôdio, R.M.; Oliveira, M.C.; Lopes, J.M.; Marques, A.C. Robust Photocatalytic MICROSCAFS® with Interconnected Macropores for Sustainable Solar-Driven Water Purification. Int. J. Mol. Sci. 2024, 25, 5958. [Google Scholar] [CrossRef]
- Cui, X.; Guo, Z.; Lei, H.; Jia, X.; Mao, C.; Ruan, L.; Zhou, X.; Wang, Z.; Chen, F.; Chen, W. Tribo-Catalytic Degradation of Methyl Orange Solutions Enhanced by Silicon Single Crystals. Coatings 2023, 13, 1804. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, R.; Mao, C.; Hu, Y.; Chen, W. Contrasting tribocatalytic degradations of organic dyes by two different commercial silicon powders. J. Adv. Dielectr. 2024, 15, 2450025. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Zhao, M. 3D flower-like ferrous(II) phosphate nanostructures as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose at nanomolar level. Talanta 2018, 182, 230–240. [Google Scholar] [CrossRef]
- Liu, R.; Fu, Y.; Zhan, H. Spectrophotometric Determination of Superoxide Anion Radical with Nitroblue Tetrazolium. J. Instrum. Anal. 2008, 27, 355. [Google Scholar]
- Baytekin, B.; Baytekin, H.T.; Grzybowski, B.A. What Really Drives Chemical Reactions on Contact Charged Surfaces? J. Am. Chem. Soc. 2012, 134, 7223–7226. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Zhou, Z.; Ke, S.; Mao, C.; Song, J.; Chen, W. Tribocatalytic Degradation of Organic Dyes by Disk-Shaped PTFE and Titanium: A Powder-Free Catalytic Technology for Wastewater Treatment. Catalysts 2025, 15, 754. https://doi.org/10.3390/catal15080754
Zhu H, Zhou Z, Ke S, Mao C, Song J, Chen W. Tribocatalytic Degradation of Organic Dyes by Disk-Shaped PTFE and Titanium: A Powder-Free Catalytic Technology for Wastewater Treatment. Catalysts. 2025; 15(8):754. https://doi.org/10.3390/catal15080754
Chicago/Turabian StyleZhu, Hanze, Zeren Zhou, Senhua Ke, Chenyue Mao, Jiannan Song, and Wanping Chen. 2025. "Tribocatalytic Degradation of Organic Dyes by Disk-Shaped PTFE and Titanium: A Powder-Free Catalytic Technology for Wastewater Treatment" Catalysts 15, no. 8: 754. https://doi.org/10.3390/catal15080754
APA StyleZhu, H., Zhou, Z., Ke, S., Mao, C., Song, J., & Chen, W. (2025). Tribocatalytic Degradation of Organic Dyes by Disk-Shaped PTFE and Titanium: A Powder-Free Catalytic Technology for Wastewater Treatment. Catalysts, 15(8), 754. https://doi.org/10.3390/catal15080754





