Controllable Preparation of TiO2/SiO2@Blast Furnace Slag Fiber Composites Based on Solid Waste Carriers and Study on Mechanism of Photocatalytic Degradation of Urban Sewage
Abstract
1. Introduction
2. Results and Discussion
2.1. TG-DTA Analysis
2.2. XRD and BET Analysis
2.3. SEM-EDS Analysis
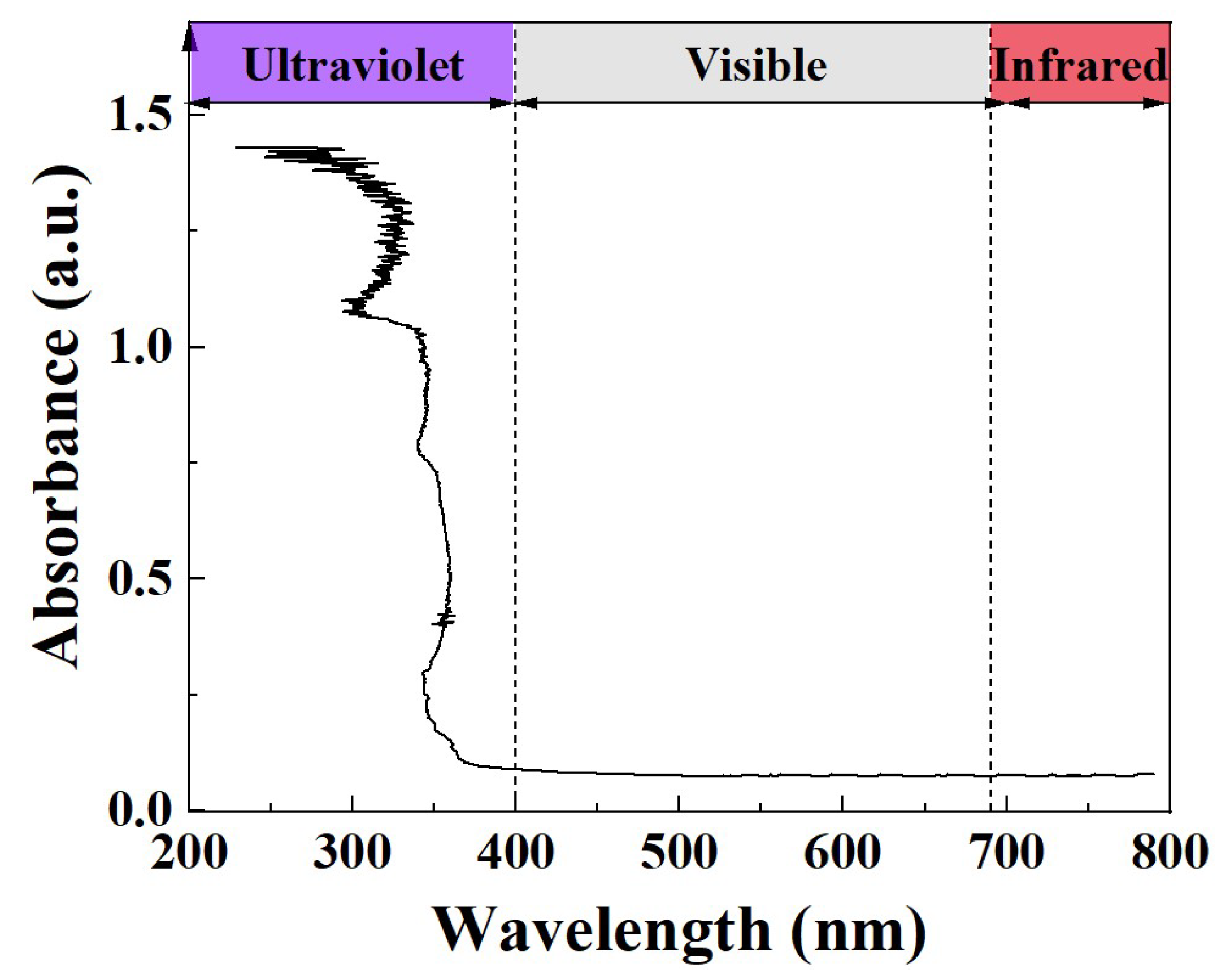
2.4. UV-Vis-NIR Absorption Spectrum
2.5. Photocatalytic Activity Evaluation
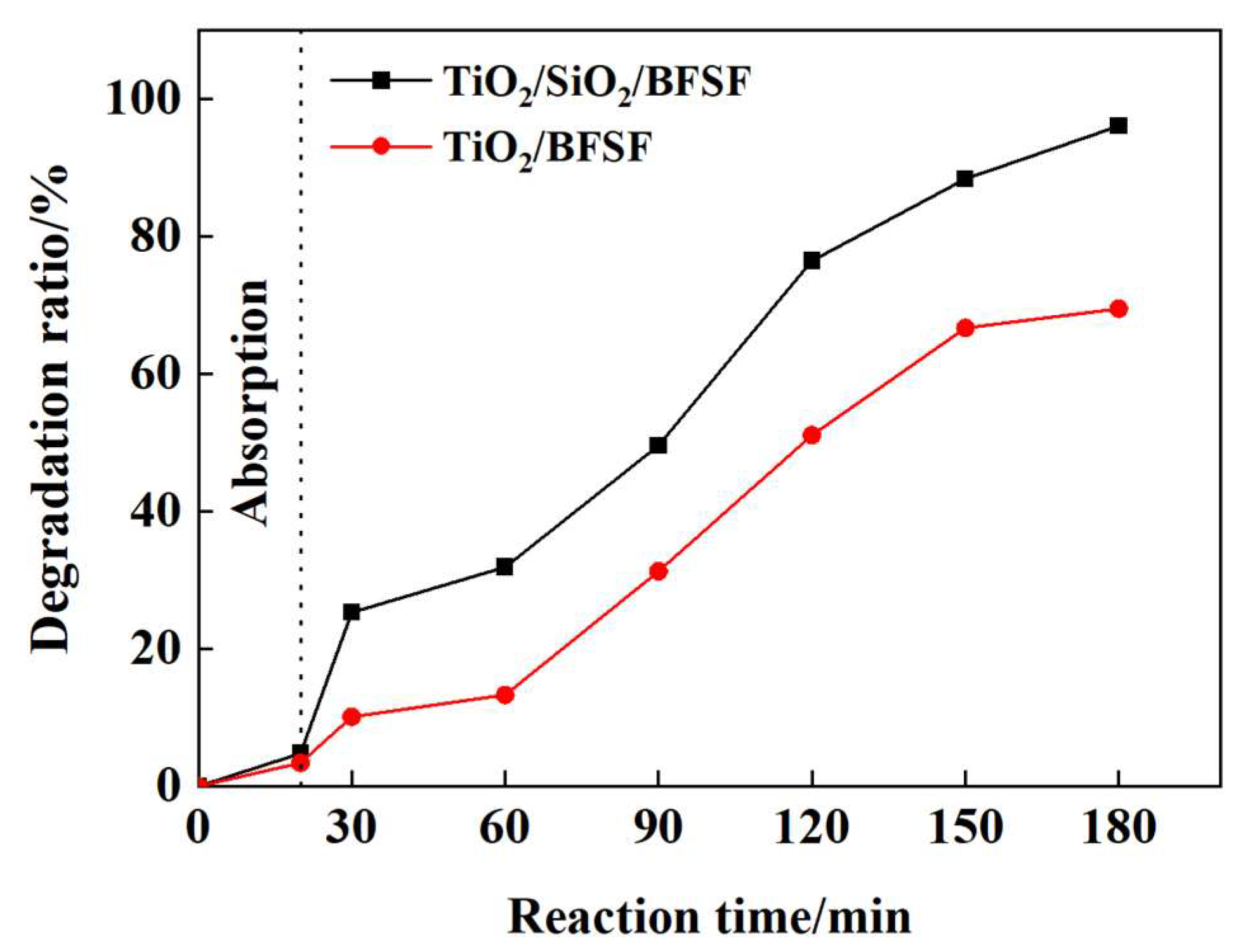
2.5.1. Effect of SiO2 on Photocatalytic Activity
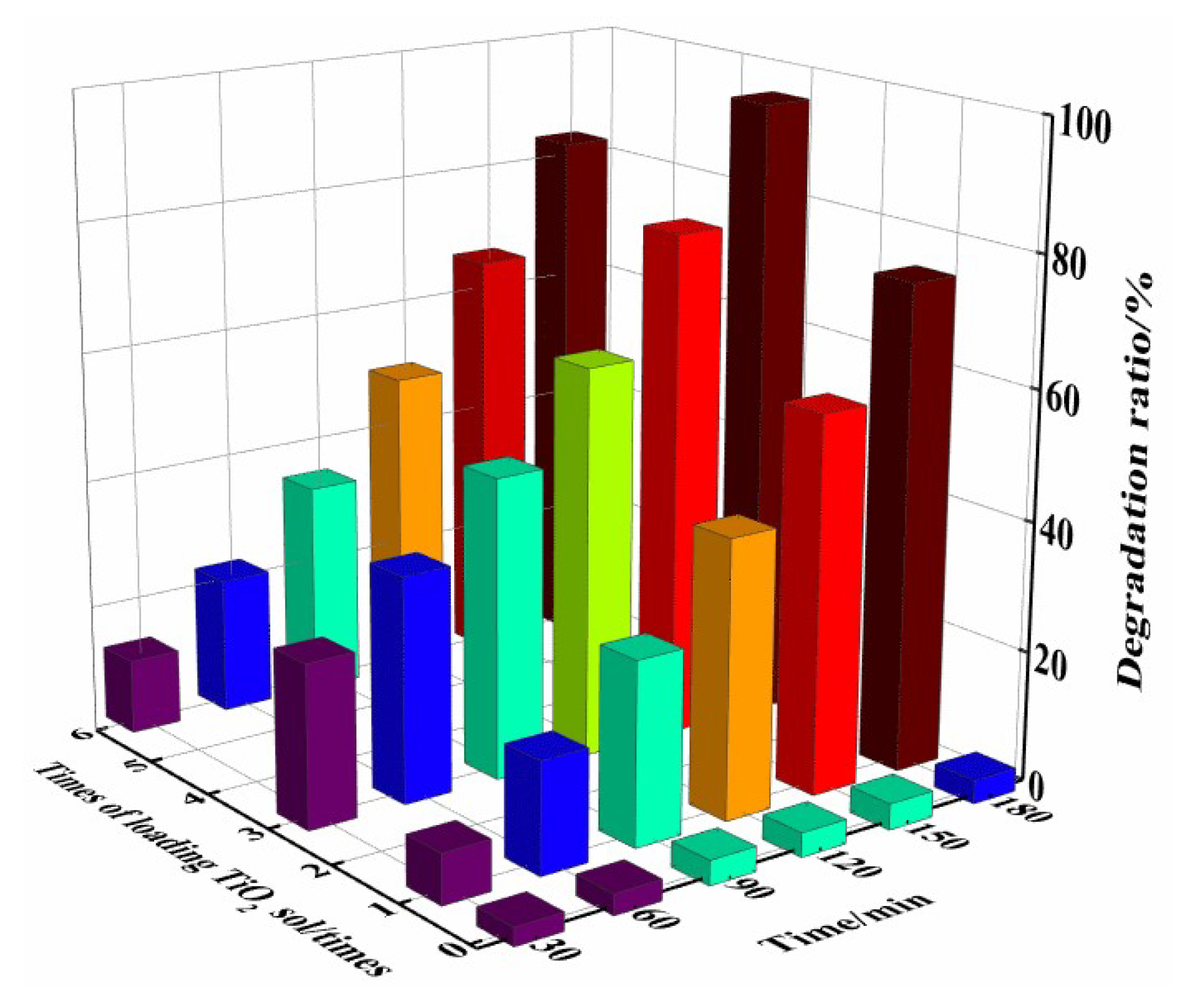
2.5.2. Effect of Loading Times of TiO2 Sol on Photocatalytic Activity
2.5.3. Effect of Calcination Temperature on Photocatalytic Activity
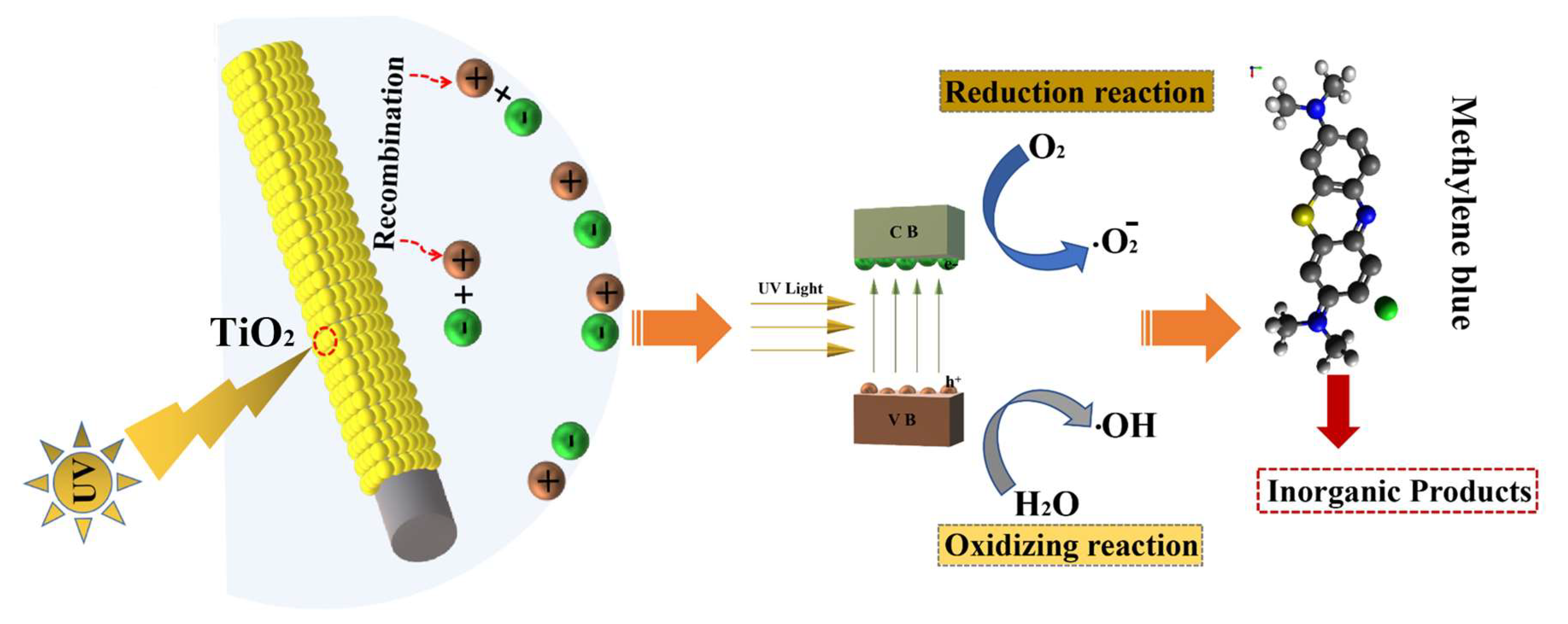
2.5.4. Photocatalysis Mechanism Research
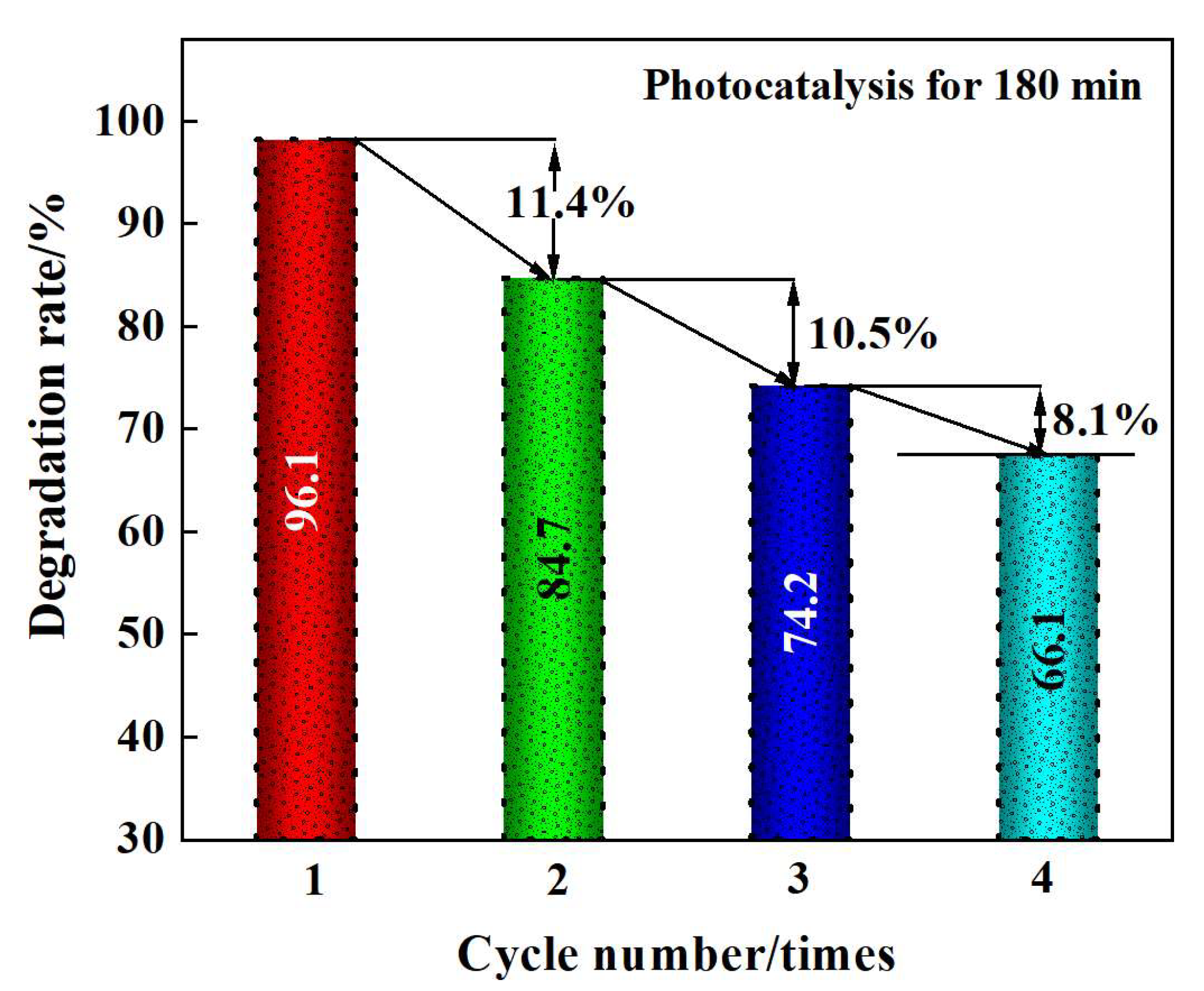
2.5.5. Recycling Property
2.5.6. Evaluation of Photocatalytic Activity of Different Photocatalytic Materials
3. Experimental Section
3.1. Raw Materials and Reagents
3.2. Material Preparation
3.3. Characterization
3.4. Photocatalytic Activity
3.5. Photocatalytic Activity Mechanism Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.J.; Wang, H.; Zhu, X.; Liao, Q.; Ding, B. Centrifugal granulation performance of liquid with various viscosities for heat recovery of blast furnace slag. Appl. Therm. Eng. 2015, 89, 494–504. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Zhang, Y.Z.; Long, Y.; Zou, Z.S.; Pei, J.J. Crystallisation behaviour of blast furnace slag modified by adding fly ash. Ceram. Int. 2018, 44, 11628–11634. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Zhang, Y.; Yang, A.; Zhao, K. Research on modifying blast furnace slag as a raw material of slag fiber. Mater. Manuf. Process. 2015, 30, 374–380. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhang, Y.Z.; Yang, A.M.; Xing, H.W.; Tian, T.L.; Li, Z.H. Preparation and properties of slag wool board using modified polyvinyl alcohol as binder. Mater. Manuf. Process. 2015, 31, 168–172. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhang, H.Y.; Yu, X.L.; Liu, W.; Wang, J.E.; Liu, Q.C.; Chen, L. Preparation, characterization and activity evaluation of heterojunction ZrTi2O6/TiO2 photocatalyst. Mater. Chem. Phys. 2010, 124, 1057–1064. [Google Scholar] [CrossRef]
- Perović, K.; dela Rosa, F.M.; Kovačić, M.; Kušić, H.; Štangar, U.L.; Fresno, F.; Dionysiou, D.D.; Loncaric Bozic, A. Recent achievements in development of tio2-based composite photocatalytic materials for solar driven water purification and water splitting. Materials 2020, 13, 1338. [Google Scholar] [CrossRef]
- Shen, S.; Kronawitter, C.; Kiriakidis, G. An overview of photocatalytic materials. J. Mater. 2017, 3, 1–2. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Rosa, D.; D’Agostino, F.; Bavasso, I.; Palma, L.D. An innovative and easy method for iron-doped titania synthesis. Chem. Eng. Trans. 2023, 101, 13–18. [Google Scholar] [CrossRef]
- Bashiri, R.; Samsudin, M.F.R.; Mohamed, N.M.; Suhaimi, N.A.; Ling, L.Y.; Sufian, S.; Kait, C.F. Influence of growth time on photoelectrical characteristics and photocatalytic hydrogen production of decorated Fe2O3 on TiO2 nanorod in photoelectrochemical cell. Appl. Surf. Sci. 2020, 510, 145482. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zang, S.Q.; Lou, X.W. Hierarchical hollow heterostructures for photocatalytic CO2 reduction and water splitting. Small Methods 2019, 4, 1900586. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Kashi, S.; Sun, L.; Wang, X. Advances in photocatalytic self-cleaning, superhydrophobic and electromagnetic interference shielding textile treatments. Adv. Colloid Interface Sci. 2020, 277, 102116. [Google Scholar] [CrossRef]
- Vélez-Peña, E.; Pérez-Obando, J.; Pais-Ospina, D.; Marín-Silva, D.A.; Pinotti, A.; Cánneva, A.; Donadelli, J.A.; Damonte, L.; Pizzio, L.R.; Osorio-Vargas, P.; et al. Self-cleaning and antimicrobial photo-induced properties under indoor lighting irradiation of chitosan films containing melon/TiO2 composites. Appl. Surf. Sci. 2020, 508, 144895. [Google Scholar] [CrossRef]
- Kosco, J.; Bidwell, M.; Cha, H.; Martin, T.; Howells, C.T.; Sachs, M.; Anjum, D.H.; Lopez, S.G.; Zou, L.; Wadsworth, A.; et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 2020, 19, 559–565. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic degradation of gemifloxacin antibiotic using zn-co-ldh@biochar nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Ya, J.; Yang, N.; Hu, F.; Liu, Z.; Lei, E. Preparation and activity evaluation of TiO2/Cu-TiO2 composite catalysts. J. Sol-Gel Sci. Technol. 2014, 73, 322–331. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef]
- Seo, J.H.; Yoon, H.N.; Kim, S.H.; Bae, S.J.; Jang, D.I.; Kil, T.G.; Park, S.M.; Lee, H.K. An overview on the physicochemical properties and photocatalytic pollutant removal performances of TiO2-incorporated cementitious composites. Korean Soc. Compos. Mater. 2020, 33, 68–75. [Google Scholar] [CrossRef]
- Löffler, F.B.; Altermann, F.J.; Bucharsky, E.C.; Schell, K.G.; Vera, M.L.; Traid, H.; Dwojak, A.; Litter, M.I. Morphological characterization and photocatalytic efficiency measurements of pure silica transparent open-cell sponges coated with TiO2. Int. J. Appl. Ceram. Technol. 2020, 17, 1930–1939. [Google Scholar] [CrossRef]
- Gong, Y.; Song, J.; Bi, H.; Tian, Z. Optimization design of the mix ratio of a nano-TiO2/CaCO3-basalt fiber composite modified asphalt mixture based on response surface methodology. Appl. Sci. 2020, 10, 4596. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Czyzewski, A.; Wanag, A.; Dubicki, M.; Sadlowski, M.; Wrobel, R.J.; Morawski, A.W. Photocatalytic oxidation of nitric oxide over AgNPs/TiO2-loaded carbon fiber cloths. J. Env. Manag. 2020, 262, 110343. [Google Scholar] [CrossRef]
- Zhang, Y.; Duoerkun, G.; Shi, Z.; Cao, W.; Liu, T.; Liu, J.; Zhang, L.; Li, M.; Chen, Z. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020, 571, 213–221. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile construction of all-solid-state z-scheme g-C3N4/TiO2 thin film for the efficient visible-light degradation of organic pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef]
- Ren, J.; Guo, S.Y.; Zhao, T.J.; Chen, J.Z.; Nicolas, R.S.; Zhang, L.H. Constructing a novel nano-TiO2/epoxy resin composite and its application in alkali-activated slag/fly ash pastes. Constr. Build. Mater. 2020, 232, 117218. [Google Scholar] [CrossRef]
- Shao, N.; Li, S.; Yan, F.; Su, Y.; Liu, F.; Zhang, Z. An all-in-one strategy for the adsorption of heavy metal ions and photodegradation of organic pollutants using steel slag-derived calcium silicate hydrate. J. Hazard. Mater. 2020, 382, 121120. [Google Scholar] [CrossRef]
- Malekhosseini, H.; Khosravi, M.; Mahanpoor, K.; Motiee, F. Preparation and characterization of nano ZnFe2O4 supported on copper slag and its effects on the degradation of p-xylene aqueous solution. J. Appl. Chem. Res. 2020, 14, 57–71. [Google Scholar]
- Song, Z.; Gao, H.; Zhang, W.; Wang, D. Reinforce of hydrotalcite-like loaded TiO2 composite material prepared by ti-bearing blast furnace slag for photo-degradation of tetracycline. J. Water Process Eng. 2020, 36, 101399. [Google Scholar] [CrossRef]
- Yu, J.G.; Liu, W.; Yu, H.G. A one-pot approach to hierarchically nanoporous titania hollow microspheres with high photocatalytic activity. Cryst. Growth Des. 2007, 8, 930–934. [Google Scholar] [CrossRef]
- Kwon, C.H.; Kim, J.H.; Jung, I.S.; Shin, H.; Yoon, K.H. Preparation and characterization of TiO2-SiO2 nano-composite thin films. Ceram. Int. 2003, 29, 851–856. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R.S.; Subbarao, P.S. Optical investigation of sol–gel-synthesized and spin-coated SiO2/TiO2 multilayers. Nanomater. Energy 2023, 12, 44–48. [Google Scholar] [CrossRef]
- Kermadi, S.; Agoudjil, N.; Sali, S.; Boumaour, M.; Bourgeois, S.M.C.; de Lucas, M. Sol-gel synthesis of xTiO2 (100-x) SiO2 nanocomposite thin films: Structure, optical and antireflection properties. Thin Solid Films 2014, 564, 170–178. [Google Scholar] [CrossRef]
- Kermadi, S.; Agoudjil, N.; Sali, S.; Zougar, L.; Boumaour, M.; Broch, L.; En Naciri, A.; Placido, F. Microstructure and optical dispersion characterization of nanocomposite sol-gel TiO2-SiO2 thin films with different compositions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 145–154. [Google Scholar] [CrossRef]
- Rasalingam, S.; Peng, R.; Koodali, R.T. Removal of hazardous pollutants from wastewaters: Applications of TiO2-SiO2 mixed oxide materials. J. Nanomater. 2014, 2014, 617405. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Liu, Z.; Liu, X.; Song, Y.; Feng, X.; Zhang, B. Functionalizing slag wool fibers with photocatalytic activity by anatase TiO2 and ctab modification. Ceram. Int. 2018, 44, 5842–5847. [Google Scholar] [CrossRef]
- Ahmed, M.A.; El-Katori, E.E.; Gharni, Z.H. Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol-gel method. J. Alloys Compd. 2013, 553, 19–29. [Google Scholar] [CrossRef]
- Anwar, D.I.; Mulyadi, D. Synthesis of Fe-TiO2 composite as a photocatalyst for degradation of methylene blue. Procedia Chem. 2015, 17, 49–54. [Google Scholar] [CrossRef]
- Schnabel, T.; Jautzus, N.; Mehling, S.; Springer, C.; Londong, J. Photocatalytic degradation of hydrocarbons and methylene blue using floatable titanium dioxide catalysts in contaminated water. Water Reuse 2021, 11, 224–235. [Google Scholar] [CrossRef]
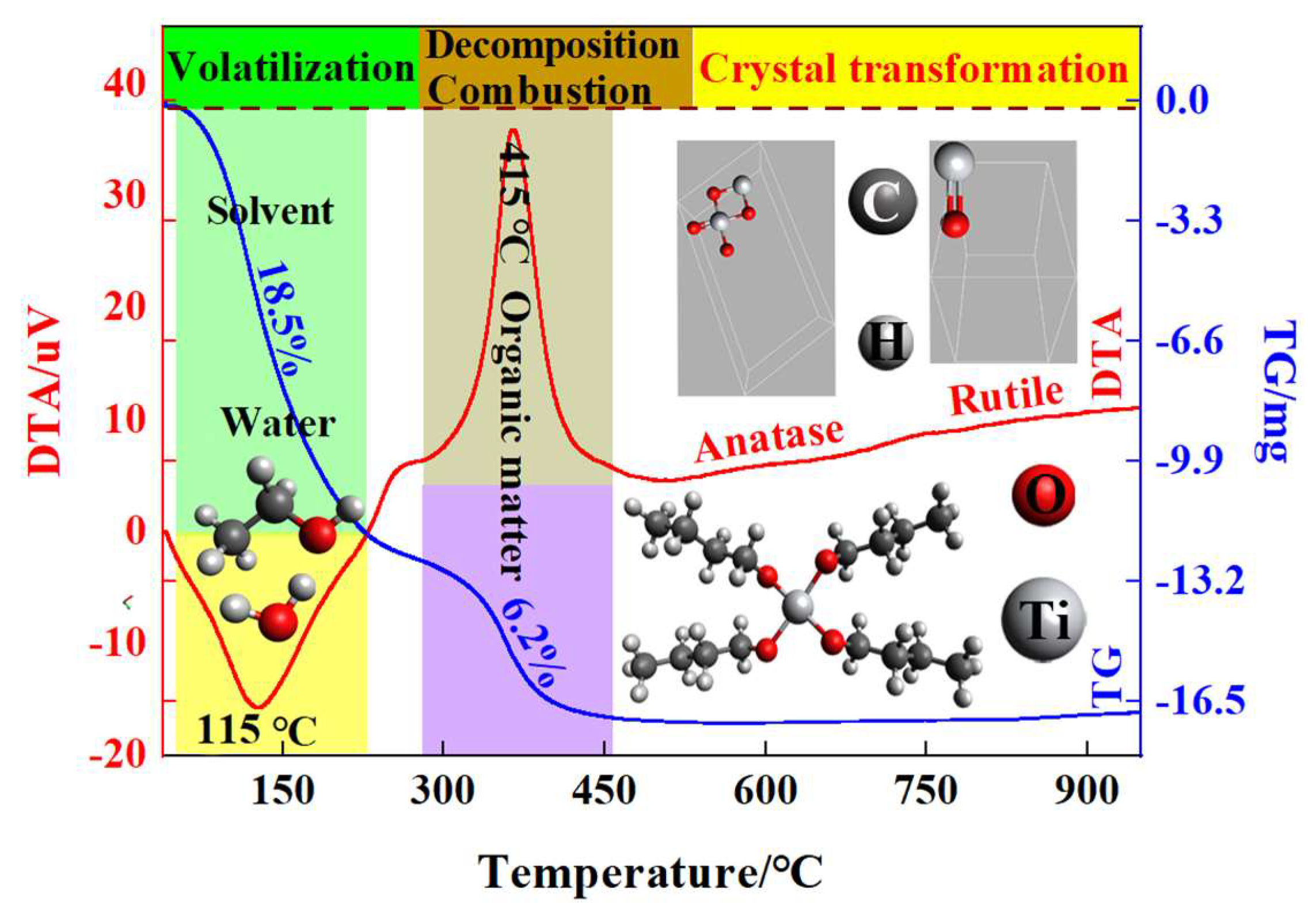
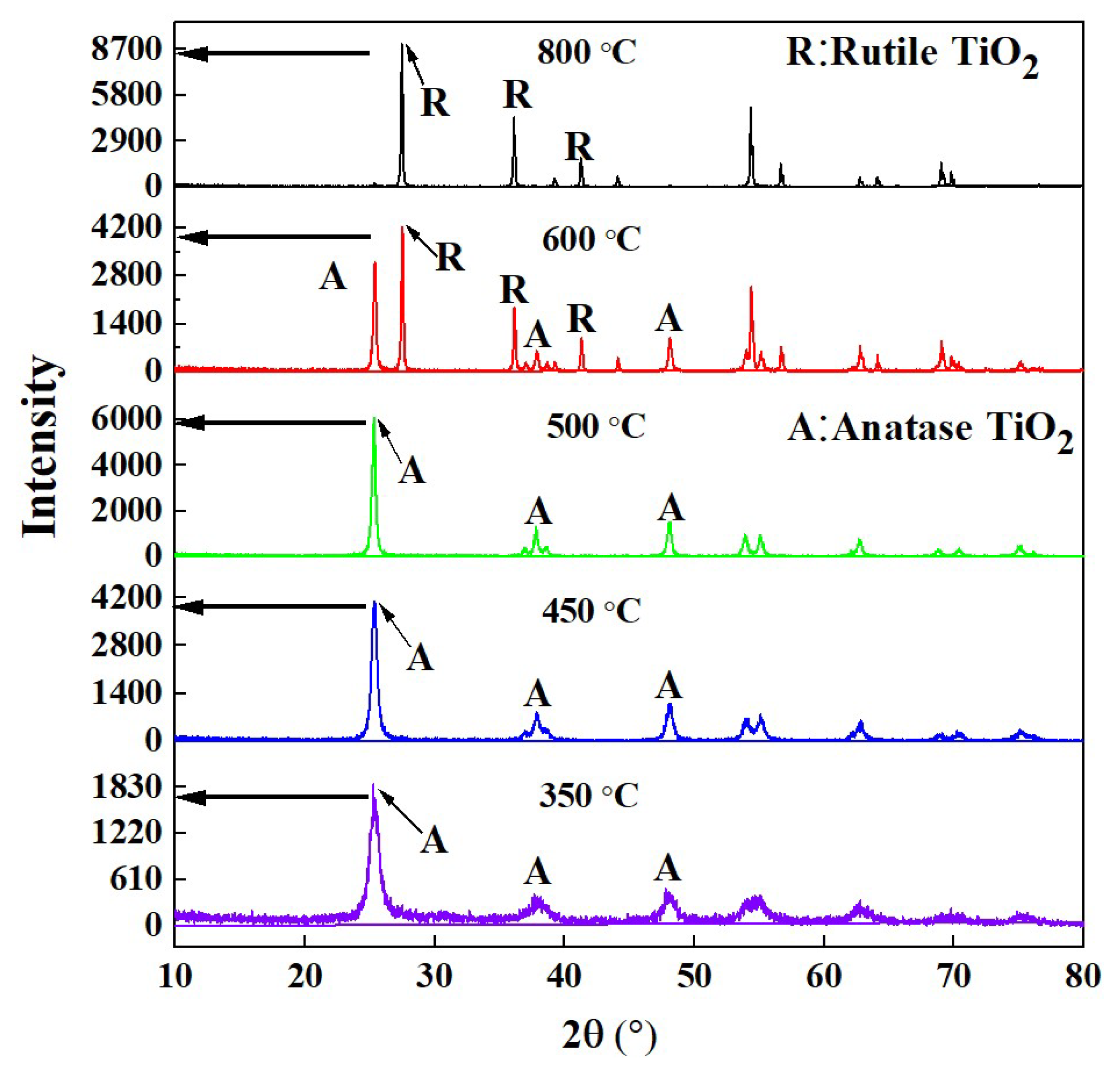
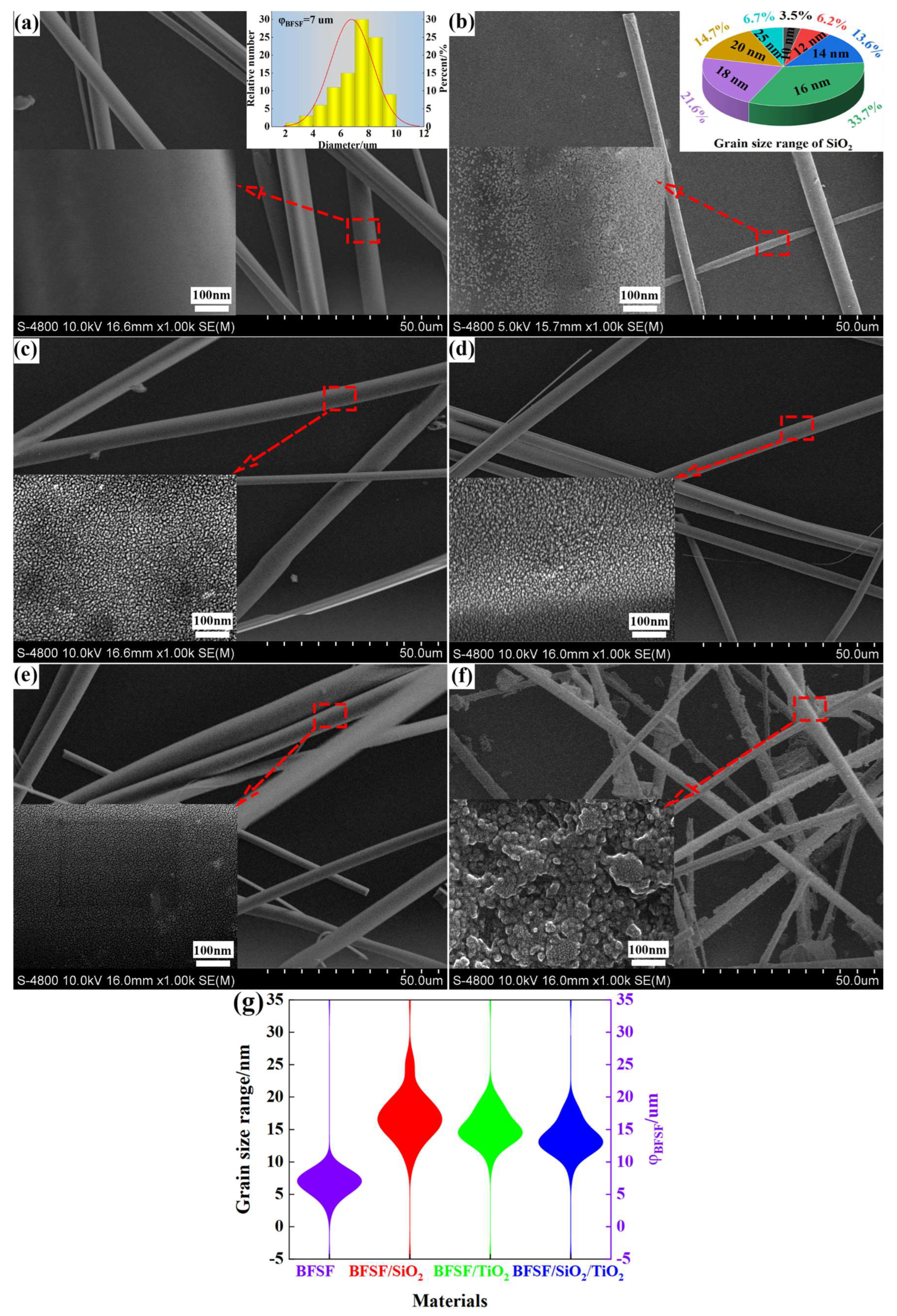
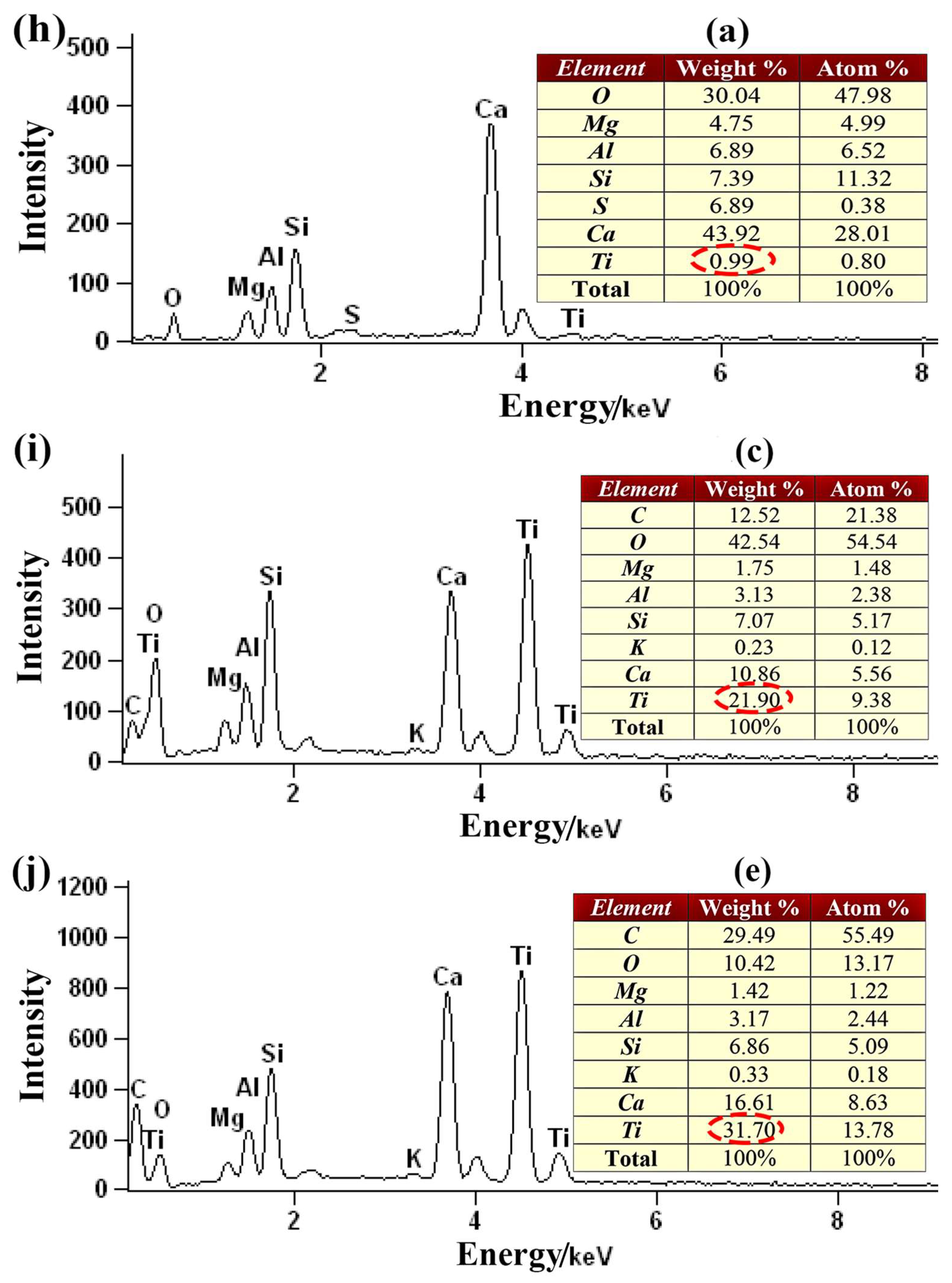


| Calcination Temperature/°C | Average Grain Size of TiO2/nm | Average Specific Surface Area/m2·g−1 | Average Pore Volume/cm3/g | Average Pore Size/nm |
|---|---|---|---|---|
| 350 | 12.4 | 114.16 | 0.34 | 9.5 |
| 450 | 19.6 | 102.34 | 0.62 | 22.3 |
| 500 | 32.4 | 67.13 | 0.53 | 18.2 |
| 600 | 97.3 | 49.24 | 0.41 | 11.1 |
| 800 | 200.4 | 15.67 | 0.38 | 8.4 |
| Photocatalytic Materials | TiO2/SiO2@ BFSF | TiO2/SiO2@ BFSF + BTA | TiO2/SiO2@ BFSF + BQ | TiO2/SiO2@ BFSF + AO |
|---|---|---|---|---|
| 1 h | 32% | 17% | 22% | 27% |
| 2 h | 78% | 36% | 51% | 64% |
| 3 h | 96% | 51% | 70% | 82% |
| Photocatalytic Materials | Fe2O3/TiO2 | BFSF with TiO2 | Fe-TiO2 | Glass Fiber with TiO2 | Steel Tissue with TiO2 |
|---|---|---|---|---|---|
| Degradation time | 70 min | 3 h | 3 h | 4 h | 6 h |
| Degradation ratio | 94.2% | 96% | 98.5% | 96.6% | 98.1% |
| Ref. | [38] | -- | [39] | [40] | [40] |
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | S | Acidity Coefficient | Diameter/um |
|---|---|---|---|---|---|---|---|
| 37.7 | 15.4 | 33.6 | 5.9 | 4.8 | 1.28 | 1.2 | 7–14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wu, J.; Zhu, G.; Han, X.; Zhao, J.; Li, Y.; Li, Y.; Gu, S. Controllable Preparation of TiO2/SiO2@Blast Furnace Slag Fiber Composites Based on Solid Waste Carriers and Study on Mechanism of Photocatalytic Degradation of Urban Sewage. Catalysts 2025, 15, 755. https://doi.org/10.3390/catal15080755
Luo X, Wu J, Zhu G, Han X, Zhao J, Li Y, Li Y, Gu S. Controllable Preparation of TiO2/SiO2@Blast Furnace Slag Fiber Composites Based on Solid Waste Carriers and Study on Mechanism of Photocatalytic Degradation of Urban Sewage. Catalysts. 2025; 15(8):755. https://doi.org/10.3390/catal15080755
Chicago/Turabian StyleLuo, Xinwen, Jinhu Wu, Guangqian Zhu, Xinyu Han, Junjian Zhao, Yaqiang Li, Yingying Li, and Shaopeng Gu. 2025. "Controllable Preparation of TiO2/SiO2@Blast Furnace Slag Fiber Composites Based on Solid Waste Carriers and Study on Mechanism of Photocatalytic Degradation of Urban Sewage" Catalysts 15, no. 8: 755. https://doi.org/10.3390/catal15080755
APA StyleLuo, X., Wu, J., Zhu, G., Han, X., Zhao, J., Li, Y., Li, Y., & Gu, S. (2025). Controllable Preparation of TiO2/SiO2@Blast Furnace Slag Fiber Composites Based on Solid Waste Carriers and Study on Mechanism of Photocatalytic Degradation of Urban Sewage. Catalysts, 15(8), 755. https://doi.org/10.3390/catal15080755




