Alcalase Specificity by Different Substrate Proteins Under Different Conditions: The Enzyme Immobilization on Carrageenan Beads Strongly Affects the pH/Activity Curve Depending on the Substrate Protein
Abstract
1. Introduction
2. Results and Discussion
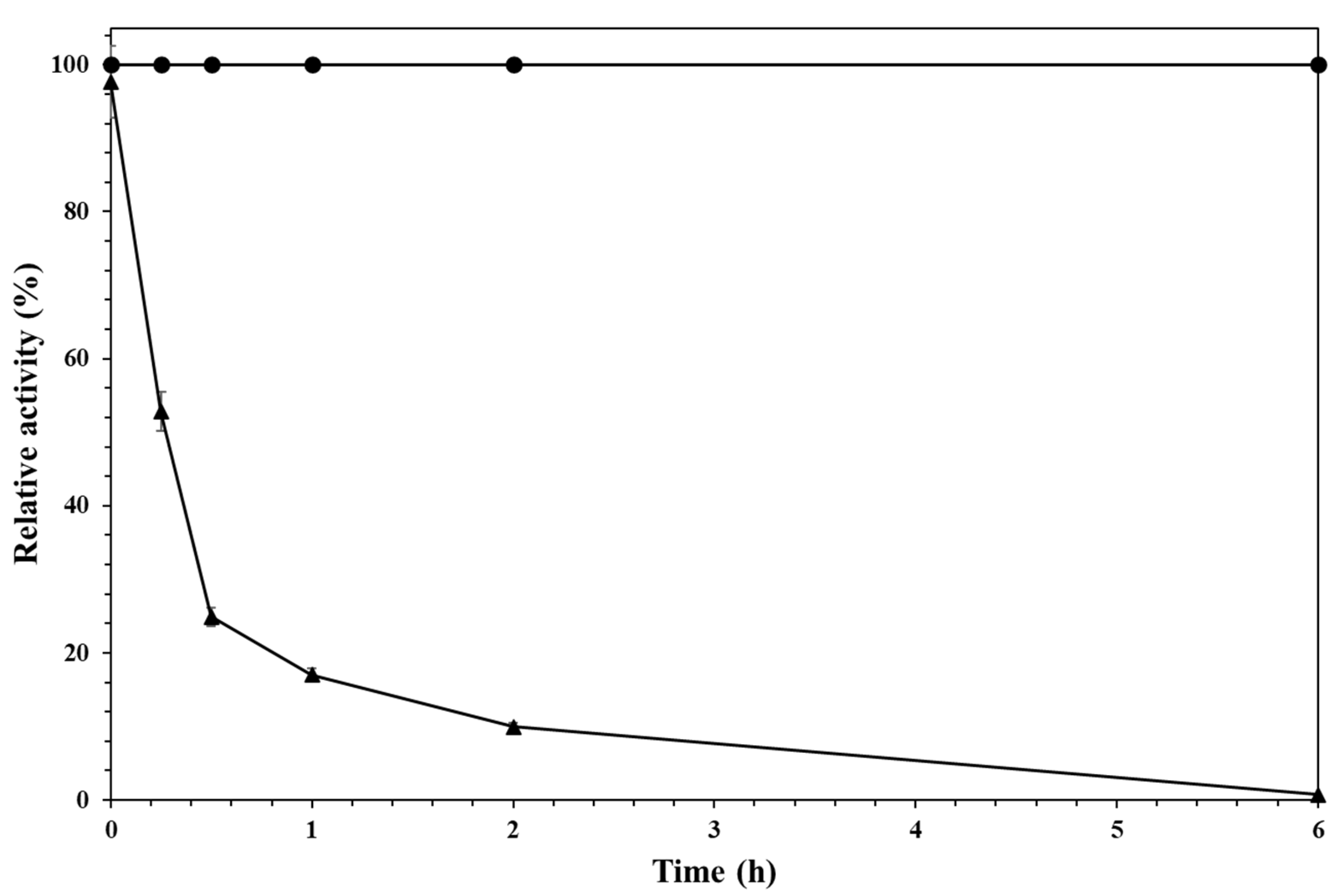
2.1. Immobilization of Alcalase and Preliminary Evaluation of the Enzyme Specificity Versus Different Substrate Proteins
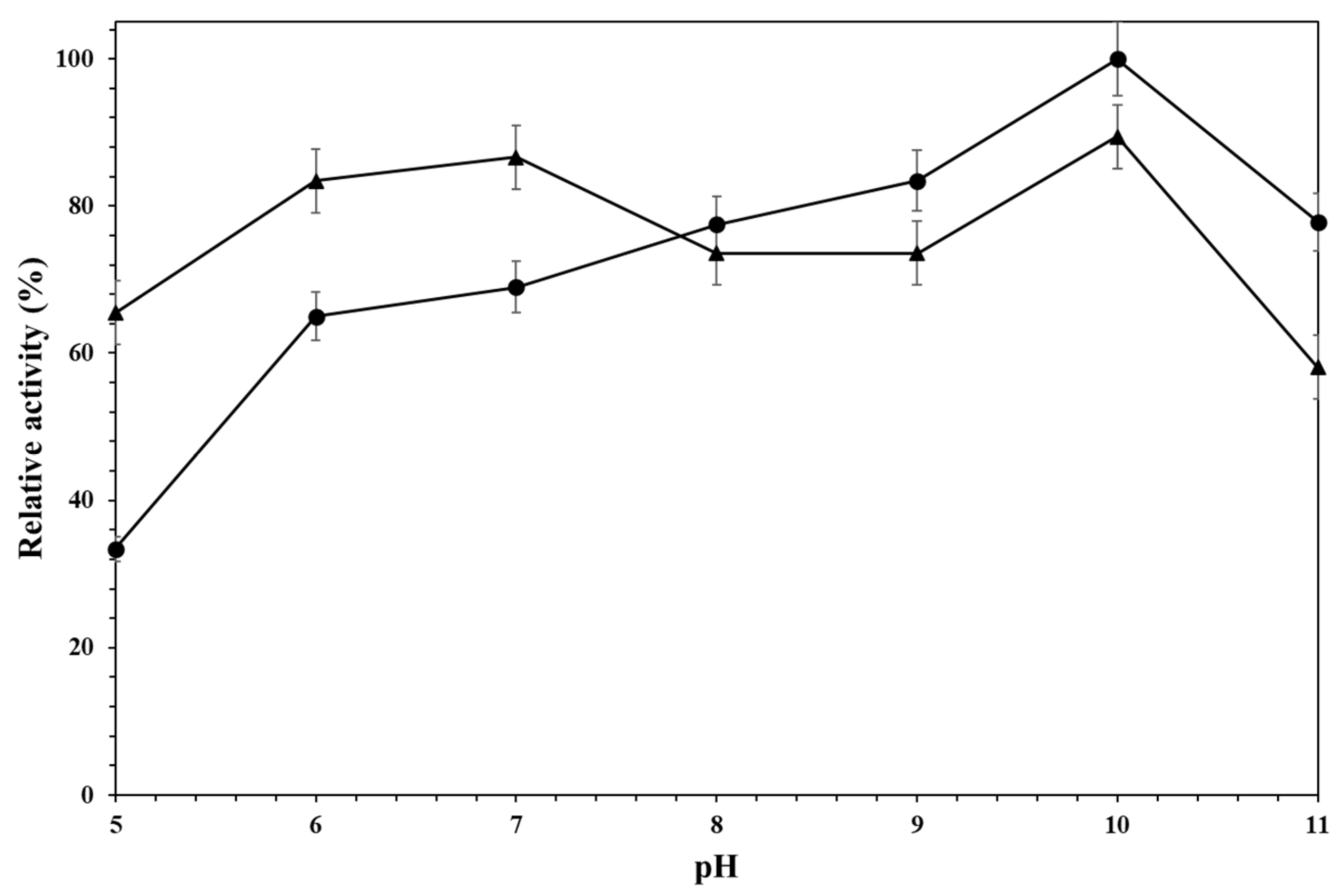
2.2. Effect of the pH Value on the Activity of Different Alcalase Formulations Versus Different Proteins
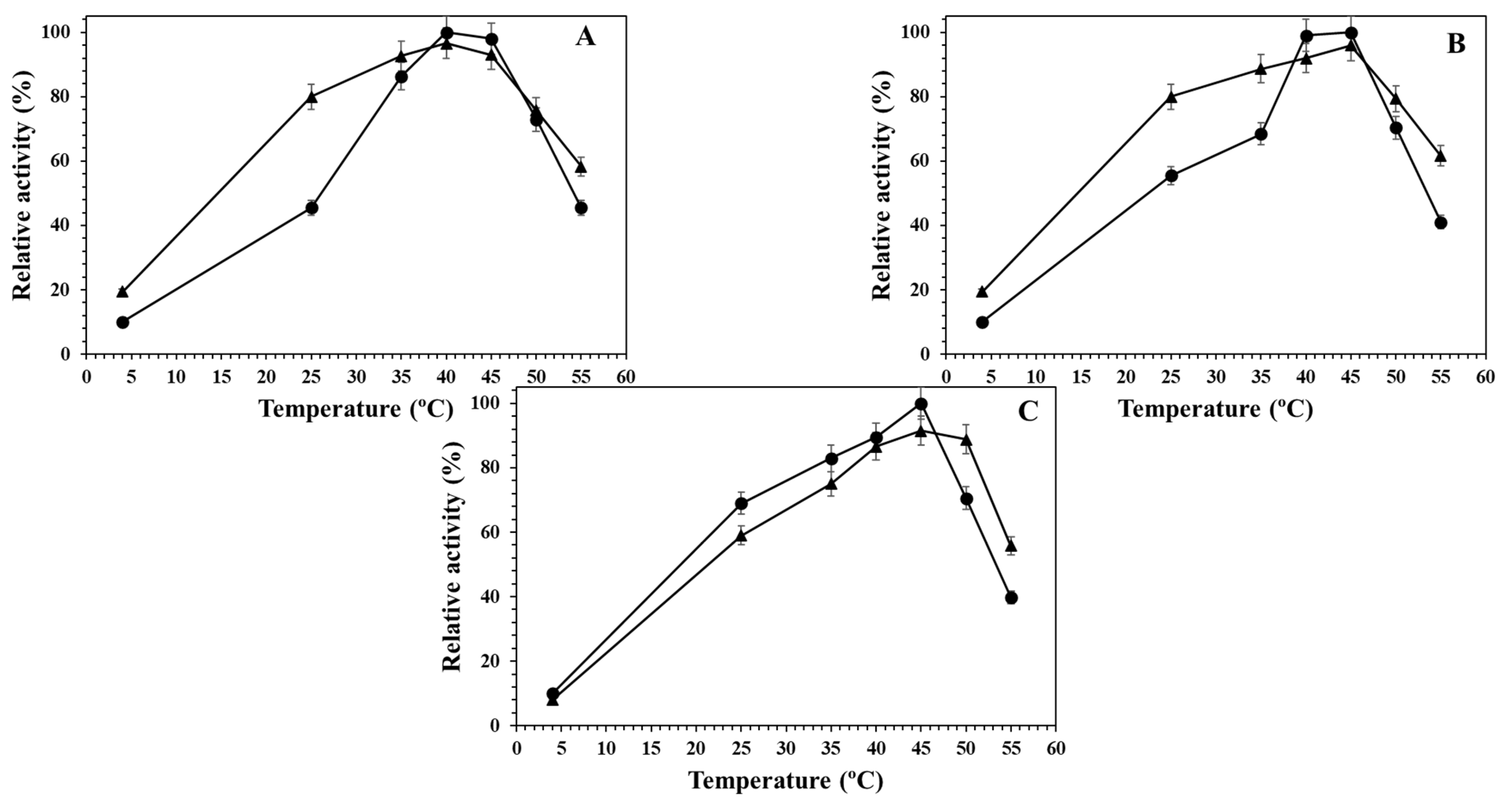
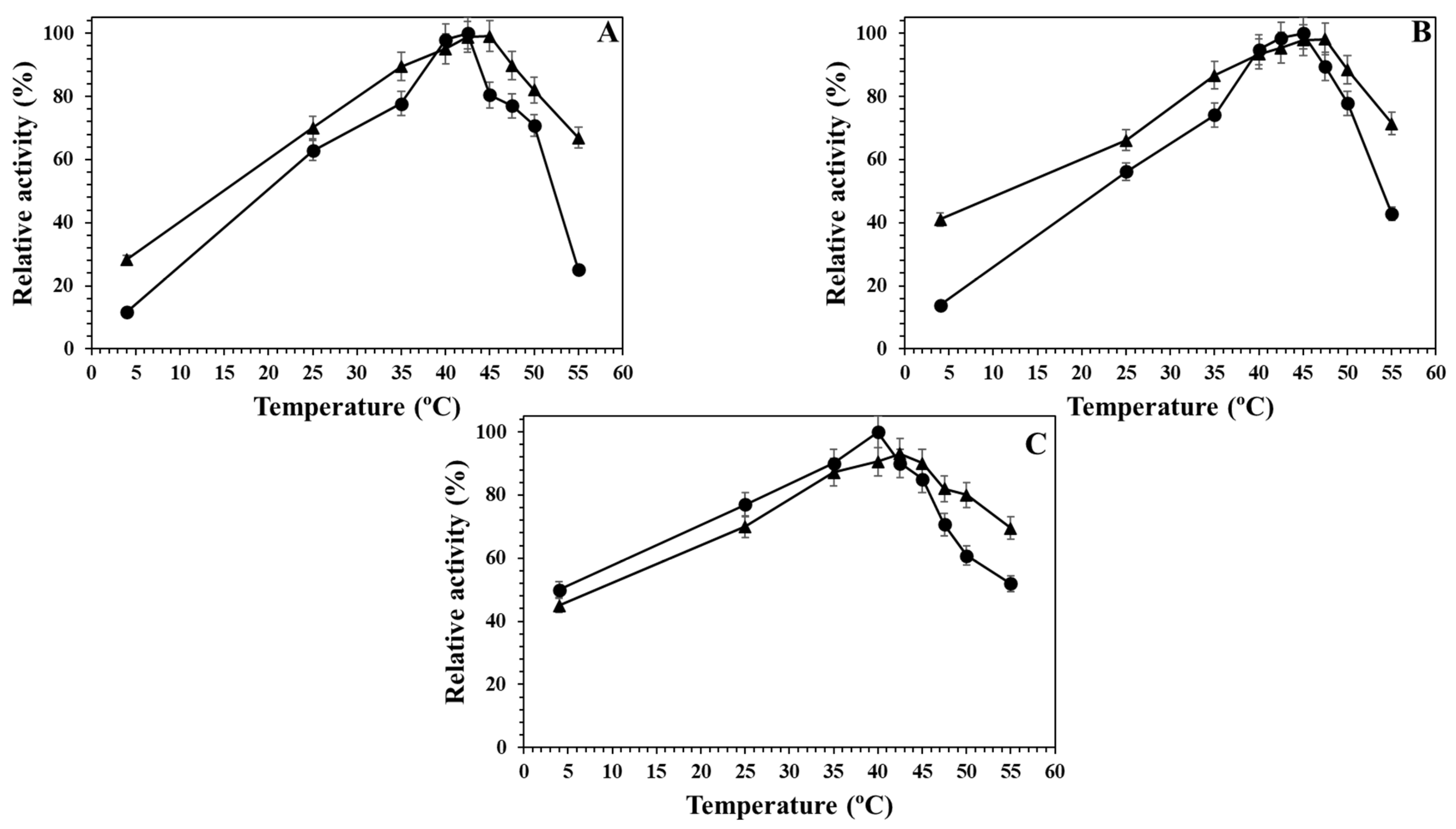
2.3. Effect of the Temperature on the Different Alcalase Formulations at Different pH Values
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Carrageenan Beads
3.2.2. Enzyme Immobilization
3.2.3. Activity of the Different Alcalase Formulations in the Hydrolysis of BSA, CS, and HB
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Nayak, V.; Deepika, D.; Jude Martis, G.; Gaonkar, S.L. Advancements in Biocatalysis: A Comprehensive Review of Applications in Organic Synthesis. Synth. Commun. 2025, 55, 443–464. [Google Scholar] [CrossRef]
- Gu, J.; Mu, W.; Xu, Y.; Nie, Y. From Discovery to Application: Enabling Technology-Based Optimizing Carbonyl Reductases Biocatalysis for Active Pharmaceutical Ingredient Synthesis. Biotechnol. Adv. 2025, 79, 108496. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial Biocatalysis Today and Tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological Applications of Proteases in Food Technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Tavano, O.L. Protein Hydrolysis Using Proteases: An Important Tool for Food Biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Naveed, M.; Nadeem, F.; Mehmood, T.; Bilal, M.; Anwar, Z.; Amjad, F. Protease—A Versatile and Ecofriendly Biocatalyst with Multi-Industrial Applications: An Updated Review. Catal. Lett. 2021, 151, 307–323. [Google Scholar] [CrossRef]
- Naeem, M.; Manzoor, S.; Abid, M.-U.-H.; Tareen, M.B.K.; Asad, M.; Mushtaq, S.; Ehsan, N.; Amna, D.; Xu, B.; Hazafa, A. Fungal Proteases as Emerging Biocatalysts to Meet the Current Challenges and Recent Developments in Biomedical Therapies: An Updated Review. J. Fungi 2022, 8, 109. [Google Scholar] [CrossRef]
- Maurer, K.H. Detergent Proteases. Curr. Opin. Biotechnol. 2004, 15, 330–334. [Google Scholar] [CrossRef]
- Kumar, D.; Savitri, T.N.; Verma, R.; Bhalla, T.C.; Savitri; Thakur, N.; Verma, R.; Bhalla, T.C. Microbial Proteases and Application as Laundry Detergent Additive. Res. J. Microbiol. 2008, 3, 661–672. [Google Scholar] [CrossRef]
- Vojcic, L.; Pitzler, C.; Körfer, G.; Jakob, F.; Martinez, R.; Maurer, K.-H.; Schwaneberg, U. Advances in Protease Engineering for Laundry Detergents. N. Biotechnol. 2015, 32, 629–634. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Kong, D.; Xu, P. Production and Functionality of Food-Derived Bioactive Peptides: A Review. Mini-Rev. Med. Chem. 2018, 18, 1524–1535. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Morellon-Sterling, R.; Tacias-Pascacio, V.G. Biological Activities of Peptides Obtained by Pepsin Hydrolysis of Fishery Products. Process Biochem. 2022, 120, 53–63. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Rather, I.A.; Fernandez-Lafuente, R. Bioactive Peptides from Fisheries Residues: A Review of Use of Papain in Proteolysis Reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Morellon-Sterling, R.; El-Siar, H.; Tavano, O.L.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Ficin: A Protease Extract with Relevance in Biotechnology and Biocatalysis. Int. J. Biol. Macromol. 2020, 162, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Tavano, O.; Murcia, Á.B.; Torrestina-Sánchez, B.; Fernandez-Lafuente, R. Peptides with Biological and Technofunctional Properties Produced by Bromelain Hydrolysis of Proteins from Different Sources: A Review. Int. J. Biol. Macromol. 2023, 253, 127244. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the Production of Bioactive Peptides: A Review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant Proteases for Bioactive Peptides Release: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef]
- Ahangari, H.; Yazdani, P.; Ebrahimi, V.; Soofiyani, S.R.; Azargun, R.; Tarhriz, V.; Eyvazi, S. An Updated Review on Production of Food Derived Bioactive Peptides; Focus on the Psychrotrophic Bacterial Proteases. Biocatal. Agric. Biotechnol. 2021, 35, 102051. [Google Scholar] [CrossRef]
- Montalbetti, C.A.G.N.; Falque, V. Amide Bond Formation and Peptide Coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kasche, V. Mechanism and Yields in Enzyme Catalysed Equilibrium and Kinetically Controlled Synthesis of β-Lactam Antibiotics, Peptides and Other Condensation Products. Enzyme Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Kasche, V.; Haufler, U.; Riechmann, L. Equilibrium and Kinetically Controlled Synthesis with Enzymes: Semisynthesis of Penicillins and Peptides. Methods Enzymol. 1987, 136, 280–292. [Google Scholar] [CrossRef]
- Kumar, D.; Bhalla, T.C. Microbial Proteases in Peptide Synthesis: Approaches and Applications. Appl. Microbiol. Biotechnol. 2005, 68, 726–736. [Google Scholar] [CrossRef]
- Origone, A.; Barberis, S.; Illanes, A.; Guzmán, F.; Camí, G.; Liggieri, C.; Martínez, R.; Bernal, C. Improvement of Enzymatic Performance of Asclepias Curassavica, L. Proteases by Immobilization. Application to the Synthesis of an Antihypertensive Peptide. Process Biochem. 2020, 95, 36–46. [Google Scholar] [CrossRef]
- Di, J.; Li, Y.; Zhang, Y.; Goh, K.-L.; Zheng, M. Enzymatic Synthesis of Antioxidant Peptides with Controllable and Adjustable Molecular Weights Using Magnetically Recyclable Immobilized Alcalase. Int. J. Biol. Macromol. 2025, 306, 141473. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, A.M.; Morawski, K.; Florczak, T. Extremophilic Proteases as Novel and Efficient Tools in Short Peptide Synthesis. J. Ind. Microbiol. Biotechnol. 2017, 44, 1325–1342. [Google Scholar] [CrossRef] [PubMed]
- Mechri, S.; Bouacem, K.; Amziane, M.; Dab, A.; Nateche, F.; Jaouadi, B. Identification of a New Serine Alkaline Peptidase from the Moderately Halophilic Virgibacillus natechei Sp. Nov., Strain FarD T and Its Application as Bioadditive for Peptide Synthesis and Laundry Detergent Formulations. Biomed Res. Int. 2019, 2019, 6470897. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-N.; Zhang, Y.-M.; Wen, Y.; Jiang, Y.; Wang, C.-H. Protease-Catalyzed Rational Synthesis of Uric Acid-Lowering Peptides in Non-Aqueous Medium. Int. J. Pept. Res. Ther. 2022, 28, 61. [Google Scholar] [CrossRef]
- Kawashiro, K.; Sugahara, H.; Tsukioka, T.; Sugiyama, S.; Hayashi, H. Effect of Ester Moiety of Substrates on Enantioselectivity of Protease Catalysis in Organic Media. Biotechnol. Lett. 1996, 18, 1381–1386. [Google Scholar] [CrossRef]
- Zhao, H.; Jackson, L.; Song, Z.; Olubajo, O. Enhancing Protease Enantioselectivity by Ionic Liquids Based on Chiral- or ω-Amino Acids. Tetrahedron Asymmetry 2006, 17, 1549–1553. [Google Scholar] [CrossRef]
- Miyazawa, T.; Minowa, H.; Yamada, T. Enhancement of Enantioselectivity in the Bacillus Subtilis Protease-Catalyzed Hydrolysis of N-Free Amino Acid Esters Using the Ester Grouping-Modification Approach. Biotechnol. Lett. 2006, 28, 295–299. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Qian, Z.; Wei, L.; Xie, J.; Tong, M.; Zhang, Y. Protein Engineering of an Alkaline Protease from Bacillus licheniformis (BLAP) for Efficient and Specific Chiral Resolution of the Racemic Ethyl Tetrahydrofuroate. Enzym. Microb. Technol. 2024, 181, 110523. [Google Scholar] [CrossRef]
- Ruan, L.-T.; Zheng, R.-C.; Zheng, Y.-G.; Shen, Y.-C. Purification and Characterization of R -Stereospecific Amidase from Brevibacterium epidermidis ZJB-07021. Int. J. Biol. Macromol. 2016, 86, 893–900. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Zhang, Y.; Sun, A.; Hu, Y. Utilization of One Novel Deep-Sea Microbial Protease Sin3406-1 in the Preparation of Ethyl (S)-3-Hydroxybutyrate through Kinetic Resolution. World J. Microbiol. Biotechnol. 2018, 34, 124. [Google Scholar] [CrossRef]
- Dong, L.; Qi, S.; Jia, J.; Zhang, Y.; Hu, Y. Enantioselective Resolution of (±)-1-Phenylethyl Acetate Using the Immobilized Extracellular Proteases from Deep-Sea Bacillus Sp. DL-1. Biocatal. Biotransformation 2022, 40, 258–274. [Google Scholar] [CrossRef]
- Gonzalez-Vasquez, A.D.; Hocine, E.S.; Urzúa, M.; Rocha-Martin, J.; Fernandez-Lafuente, R. Changes in Ficin Specificity by Different Substrate Proteins Promoted by Enzyme Immobilization. Enzyme Microb. Technol. 2024, 181, 110517. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, H.E.; Mink, D.L.; WubboLts, M.G. Dispelling the Myths—Biocatalysis in Industrial Synthesis. Science 2003, 299, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Martínez-Martínez, M.; Bargiela, R.; Streit, W.R.; Golyshina, O.V.; Golyshin, P.N. Estimating the Success of Enzyme Bioprospecting through Metagenomics: Current Status and Future Trends. Microb. Biotechnol. 2016, 9, 22–34. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Bargiela, R.; Ferrer, M. Metagenomics and the Search for Industrial Enzymes. In Biotechnology of Microbial Enzymes; Academic Press: Cambridge, MA, USA, 2017; pp. 167–184. [Google Scholar] [CrossRef]
- Ferrer, M.; Martinez-Abarca, F.; Golyshin, P. Mining Genomes and ‘Metagenomes’ for Novel Catalysts. Curr. Opin. Biotechnol. 2005, 16, 588–593. [Google Scholar] [CrossRef]
- Fernández-Arrojo, L.; Guazzaroni, M.-E.; López-Cortés, N.; Beloqui, A.; Ferrer, M. Metagenomic Era for Biocatalyst Identification. Curr. Opin. Biotechnol. 2010, 21, 725–733. [Google Scholar] [CrossRef]
- Renata, H.; Wang, Z.J.; Arnold, F.H. Expanding the Enzyme Universe: Accessing Non-Natural Reactions by Mechanism-Guided Directed Evolution. Angew. Chem. Int. Ed. 2015, 54, 3351–3367. [Google Scholar] [CrossRef]
- Chen, K.; Arnold, F.H. Engineering New Catalytic Activities in Enzymes. Nat. Catal. 2020, 3, 203–213. [Google Scholar] [CrossRef]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef]
- Alonso, S.; Santiago, G.; Cea-Rama, I.; Fernandez-Lopez, L.; Coscolín, C.; Modregger, J.; Ressmann, A.K.; Martínez-Martínez, M.; Marrero, H.; Bargiela, R.; et al. Genetically Engineered Proteins with Two Active Sites for Enhanced Biocatalysis and Synergistic Chemo- and Biocatalysis. Nat. Catal. 2020, 3, 319–328. [Google Scholar] [CrossRef]
- Roda, S.; Fernandez-Lopez, L.; Benedens, M.; Bollinger, A.; Thies, S.; Schumacher, J.; Coscolín, C.; Kazemi, M.; Santiago, G.; Gertzen, C.G.W.; et al. A Plurizyme with Transaminase and Hydrolase Activity Catalyzes Cascade Reactions. Angew. Chem. Int. Ed. 2022, 61, e202207344. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martín, A.; Amigot-Sánchez, R.; Fernandez-Lopez, L.; Gonzalez-Alfonso, J.L.; Roda, S.; Alcolea-Rodriguez, V.; Heras-Márquez, D.; Almendral, D.; Coscolín, C.; Plou, F.J.; et al. Sub-Micro- and Nano-Sized Polyethylene Terephthalate Deconstruction with Engineered Protein Nanopores. Nat. Catal. 2023, 6, 1174–1185. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Roda, S.; Gonzalez-Alfonso, J.L.; Plou, F.J.; Guallar, V.; Ferrer, M. Design and Characterization of In-One Protease-Esterase PluriZyme. Int. J. Mol. Sci. 2022, 23, 13337. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, A.; Davis, B.G. Chemical Modification in the Creation of Novel Biocatalysts. Curr. Opin. Chem. Biol. 2011, 15, 211–219. [Google Scholar] [CrossRef]
- DeSantis, G.; Jones, J.B. Chemical Modification of Enzymes for Enhanced Functionality. Curr. Opin. Biotechnol. 1999, 10, 324–330. [Google Scholar] [CrossRef]
- Inada, Y.; Yoshimoto, T.; Matsushima, A.; Saito, Y. Engineering Physicochemical and Biological Properties of Proteins by Chemical Modification. Trends Biotechnol. 1986, 4, 68–73. [Google Scholar] [CrossRef]
- Davis, B.G. Chemical Modification of Biocatalysts. Curr. Opin. Biotechnol. 2003, 14, 379–386. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the Functional Properties of Thermophilic Enzymes by Chemical Modification and Immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling Chemical Modification and Immobilization to Improve the Catalytic Performance of Enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical Modification in the Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial Use of Immobilized Enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters Necessary to Define an Immobilized Enzyme Preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity via Immobilization Techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Tavano, O.; Bolivar, J.M.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Sabir, J.S.M.; Tacias-Pascacio, V.G.; Fernandez-Lafuente, R. A Review on the Immobilization of Pepsin: A Lys-Poor Enzyme That Is Unstable at Alkaline PH Values. Int. J. Biol. Macromol. 2022, 210, 682–702. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Castañeda-Valbuena, D.; Berenguer-Murcia, Á.; Kamli, M.R.; Tavano, O.; Fernandez-Lafuente, R. Immobilization of Papain: A Review. Int. J. Biol. Macromol. 2021, 188, 94–113. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Tavano, O.; Abellanas-Perez, P.; de Andrades, D.; Santiz-Gómez, J.A.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. A Review on the Immobilization of Bromelain. Int. J. Biol. Macromol. 2024, 273, 133089. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Qamar, S.A.; Carballares, D.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Proteases Immobilized on Nanomaterials for Biocatalytic, Environmental and Biomedical Applications: Advantages and Drawbacks. Biotechnol. Adv. 2024, 70, 108304. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, A.P.; Gonçalves, L.R.B.; de Albuqueque da Silva, T.L.; Fernandez-Lafuente, R.; Silva, I.J. da Alcalase Immobilization in Iota-Carrageenan-Matrix Hydrogel Beads Derived from the Macroalga Solieria filiformis. Enzyme Microb. Technol. 2025, 188, 110636. [Google Scholar] [CrossRef] [PubMed]
- DeLange, R.J.; Smith, E.L. Subtilisin Carlsberg. I. Amino Acid Composition; Isolation and Composition of Peptides from the Tryptic Hydrolysate. J. Biol. Chem. 1968, 243, 2134–2142. [Google Scholar] [CrossRef]
- Singh, S.; Bajaj, B.K. Potential Application Spectrum of Microbial Proteases for Clean and Green Industrial Production. Energy Ecol. Environ. 2017, 2, 370–386. [Google Scholar] [CrossRef]
- Güntelberg, A.V.; Ottesen, M. Preparation of Crystals Containing the Plakalbumin-Forming Enzyme from Bacillus subtilis. Nature 1952, 170, 802. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Takagi, H. Microbial Alkaline Proteases. Biotechnol. Adv. 1999, 17, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Sumantha, A.; Larroche, C.; Pandey, A. Microbiology and Industrial Biotechnology of Food-Grade Proteases: A Perspective. Food Technol. Biotechnol. 2006, 44, 211–220. [Google Scholar]
- Rao, M.B.; Tanksale, A.M.; Ghatge, M.S.; Deshpande, V.V. Molecular and Biotechnological Aspects of Microbial Proteases. Microbiol. Mol. Biol. Rev. 1998, 62, 597–635. [Google Scholar] [CrossRef]
- Adamson, N.J.; Reynolds, E.C. Characterization of Casein Phosphopeptides Prepared Using Alcalase: Determination of Enzyme Specificity. Enzyme Microb. Technol. 1996, 19, 202–207. [Google Scholar] [CrossRef]
- Jonović, M.; Žuža, M.; Đorđević, V.; Šekuljica, N.; Milivojević, M.; Jugović, B.; Bugarski, B.; Knežević-Jugović, Z. Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins. Catalysts 2021, 11, 305. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Abbasi, S.; Tafaghodinia, B. Multi-Immobilization of Viscozyme, Alcalase and Flavourzyme by Nanomagnetic Combi-CLEAs Method for Oil and Protein Hydrolysates Extraction from Rice Bran in Aqueous Phase. Int. J. Biol. Macromol. 2025, 311, 143719. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Chen, X.; Zhu, Z.; Li, S.; Song, J.; Zheng, Z.; Cong, X.; Cheng, S. Co-Immobilization of Alcalase/Dispase for Production of Selenium-Enriched Peptide from Cardamine violifolia. Foods 2024, 13, 1753. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Márquez-López, J.C.; Pedroche, J.; Álvarez-López, A.I.; del Carmen Millán-Linares, M.; Lardone, P.J.; Carrillo-Vico, A. Characterisation and Beneficial Effects of a Lupinus angustifolius Protein Hydrolysate Obtained by Immobilisation of the Enzyme Alcalase®. Food Funct. 2024, 15, 3722–3730. [Google Scholar] [CrossRef]
- Parandi, E.; Mousavi, M.; Kiani, H.; Nasirijoonaghani, S.; Rouhi, M.; Rashidi Nodeh, H.; Assadpour, E.; Zhang, F.; Jafari, S.M. Controlled and Rapid Microreactor-Assisted Hydrolysis of Sesame Protein by a Novel Nanobiocatalyst; Immobilized Alcalase over Activated Sol–Gel Magnetic Nanosupport. Chem. Eng. J. 2024, 496, 153900. [Google Scholar] [CrossRef]
- Wu, S.; Gu, H.; Wang, Y.; Wang, Y.; Raghavan, V.; Wang, J. Alcalase Immobilized on Novel Porous Hydrogel Beads via CO2 Bubble Strategy to Reduce Cow’s Milk Allergenicity. Chem. Eng. J. 2025, 513, 162875. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, H.; Xu, Z.; Wu, H.; Lan, C.Q.; Zhang, J. Immobilization of Alcalase on Polydopamine Modified Magnetic Particles. Biochem. Eng. J. 2024, 207, 109310. [Google Scholar] [CrossRef]
- Ait Braham, S.; Hussain, F.; Morellon-Sterling, R.; Kamal, S.; Kornecki, J.F.; Barbosa, O.; Kati, D.E.; Fernandez-Lafuente, R. Cooperativity of Covalent Attachment and Ion Exchange on Alcalase Immobilization Using Glutaraldehyde Chemistry: Enzyme Stabilization and Improved Proteolytic Activity. Biotechnol. Prog. 2019, 35, e2768. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Pessela, B.C.C.; Mateo, C.; Palomo, J.M.; Batalla, P.; Fernández-Lafuente, R.; Guisán, J.M. Adsorption Behavior of Bovine Serum Albumin on Lowly Activated Anionic Exchangers Suggests a New Strategy for Solid-Phase Proteomics. Biomacromolecules 2006, 7, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Vaiana, S.M.; Emanuele, A.; Palma-Vittorelli, M.B.; Palma, M.U. Irreversible Formation of Intermediate BSA Oligomers Requires and Induces Conformational Changes. Proteins Struct. Funct. Bioinform. 2004, 55, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Militello, V.; Casarino, C.; Emanuele, A.; Giostra, A.; Pullara, F.; Leone, M. Aggregation Kinetics of Bovine Serum Albumin Studied by FTIR Spectroscopy and Light Scattering. Biophys. Chem. 2004, 107, 175–187. [Google Scholar] [CrossRef]
- Bulone, D.; Martorana, V.; San Biagio, P.L. Effects of Intermediates on Aggregation of Native Bovine Serum Albumin. Biophys. Chem. 2001, 91, 61–69. [Google Scholar] [CrossRef]
- Jordan, G.M.; Yoshioka, S.; Terao, T. The Aggregation of Bovine Serum Albumin in Solution and in the Solid State. J. Pharm. Pharmacol. 1994, 46, 182–185. [Google Scholar] [CrossRef]
- Hagiwara, T.; Kumagai, H.; Nakamura, K. Fractal Analysis of Aggregates Formed by Heating Dilute BSA Solutions Using Light Scattering Methods. Biosci. Biotechnol. Biochem. 1996, 60, 1757–1763. [Google Scholar] [CrossRef]
- Arakawa, T.; Kita, Y. Protection of Bovine Serum Albumin from Aggregation by Tween 80. J. Pharm. Sci. 2000, 89, 646–651. [Google Scholar] [CrossRef]
- Maruyama, T.; Katoh, S.; Nakajima, M.; Nabetani, H. Mechanism of Bovine Serum Albumin Aggregation during Ultrafiltration. Biotechnol. Bioeng. 2001, 75, 233–238. [Google Scholar] [CrossRef]
- Edelstein, S.J.; Rehmar, M.J.; Olson, J.S.; Gibson, Q.H. Functional Aspects of the Subunit Association-Dissociation Equilibria of Hemoglobin. J. Biol. Chem. 1970, 245, 4372–4381. [Google Scholar] [CrossRef]
- Rosemeyer, M.A.; Huehns, E.R. On the Mechanism of the Dissociation of Haemoglobin. J. Mol. Biol. 1967, 25, 253–273. [Google Scholar] [CrossRef]
- Manning, L.R.; Jenkins, W.T.; Hess, J.R.; Vandegriff, K.; Winslow, R.M.; Manning, J.M. Subunit Dissociations in Natural and Recombinant Hemoglobins. Protein Sci. 1996, 5, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Bispo, J.A.C.; Santos, J.L.R.; Landini, G.F.; Goncalves, J.M.; Bonafe, C.F.S. PH Dependence of the Dissociation of Multimeric Hemoglobin Probed by High Hydrostatic Pressure. Biophys. Chem. 2007, 125, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kunitz, M. Crystalline Soybean Trypsin Inhibitor II. General Properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef]



| Alcalase Formulation | Substrate Protein | ||
|---|---|---|---|
| CS | BSA | HG | |
| Free | 3.14 ± 0.18 | 4.12 ± 0.11 | 5.26 ± 0.22 |
| Immobilized | 2.98 ± 0.13 | 4.64 ± 0.21 | 4.77 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Almeida, A.P.; Abellanas-Perez, P.; Gonçalves, L.R.B.; de Albuquerque, T.L.; da Silva Junior, I.J.; Fernandez-Lafuente, R. Alcalase Specificity by Different Substrate Proteins Under Different Conditions: The Enzyme Immobilization on Carrageenan Beads Strongly Affects the pH/Activity Curve Depending on the Substrate Protein. Catalysts 2025, 15, 750. https://doi.org/10.3390/catal15080750
D’Almeida AP, Abellanas-Perez P, Gonçalves LRB, de Albuquerque TL, da Silva Junior IJ, Fernandez-Lafuente R. Alcalase Specificity by Different Substrate Proteins Under Different Conditions: The Enzyme Immobilization on Carrageenan Beads Strongly Affects the pH/Activity Curve Depending on the Substrate Protein. Catalysts. 2025; 15(8):750. https://doi.org/10.3390/catal15080750
Chicago/Turabian StyleD’Almeida, Alan Portal, Pedro Abellanas-Perez, Luciana Rocha Barros Gonçalves, Tiago Lima de Albuquerque, Ivanildo José da Silva Junior, and Roberto Fernandez-Lafuente. 2025. "Alcalase Specificity by Different Substrate Proteins Under Different Conditions: The Enzyme Immobilization on Carrageenan Beads Strongly Affects the pH/Activity Curve Depending on the Substrate Protein" Catalysts 15, no. 8: 750. https://doi.org/10.3390/catal15080750
APA StyleD’Almeida, A. P., Abellanas-Perez, P., Gonçalves, L. R. B., de Albuquerque, T. L., da Silva Junior, I. J., & Fernandez-Lafuente, R. (2025). Alcalase Specificity by Different Substrate Proteins Under Different Conditions: The Enzyme Immobilization on Carrageenan Beads Strongly Affects the pH/Activity Curve Depending on the Substrate Protein. Catalysts, 15(8), 750. https://doi.org/10.3390/catal15080750









