Ferrocene-Catalyzed Aromatization and Competitive Oxidative Ring Transformations of 1,2-Dihydro-1-Arylpyridazino[4,5-d]Pyridazines
Abstract
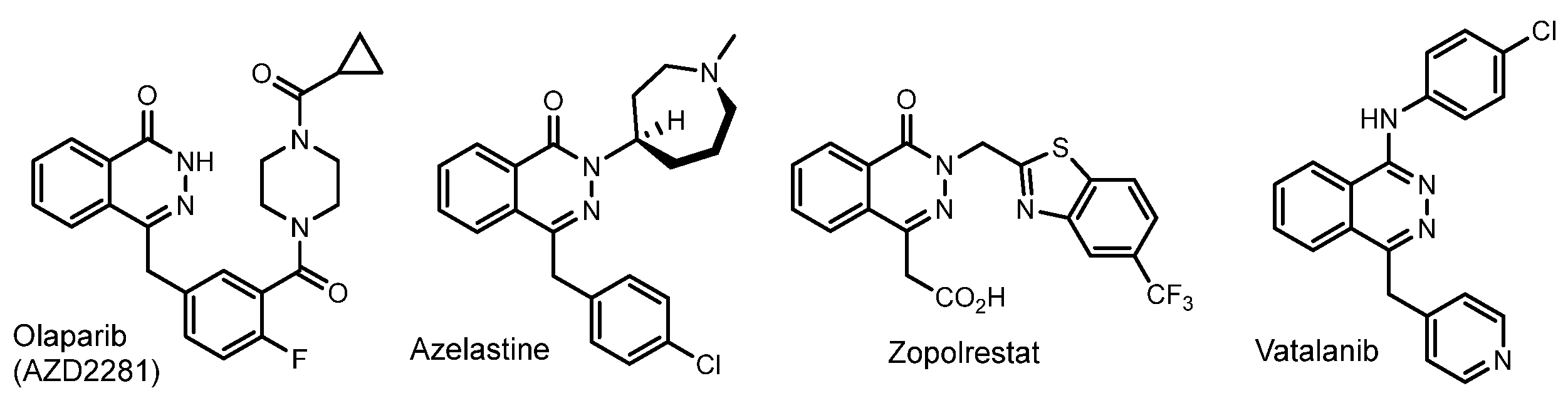
1. Introduction
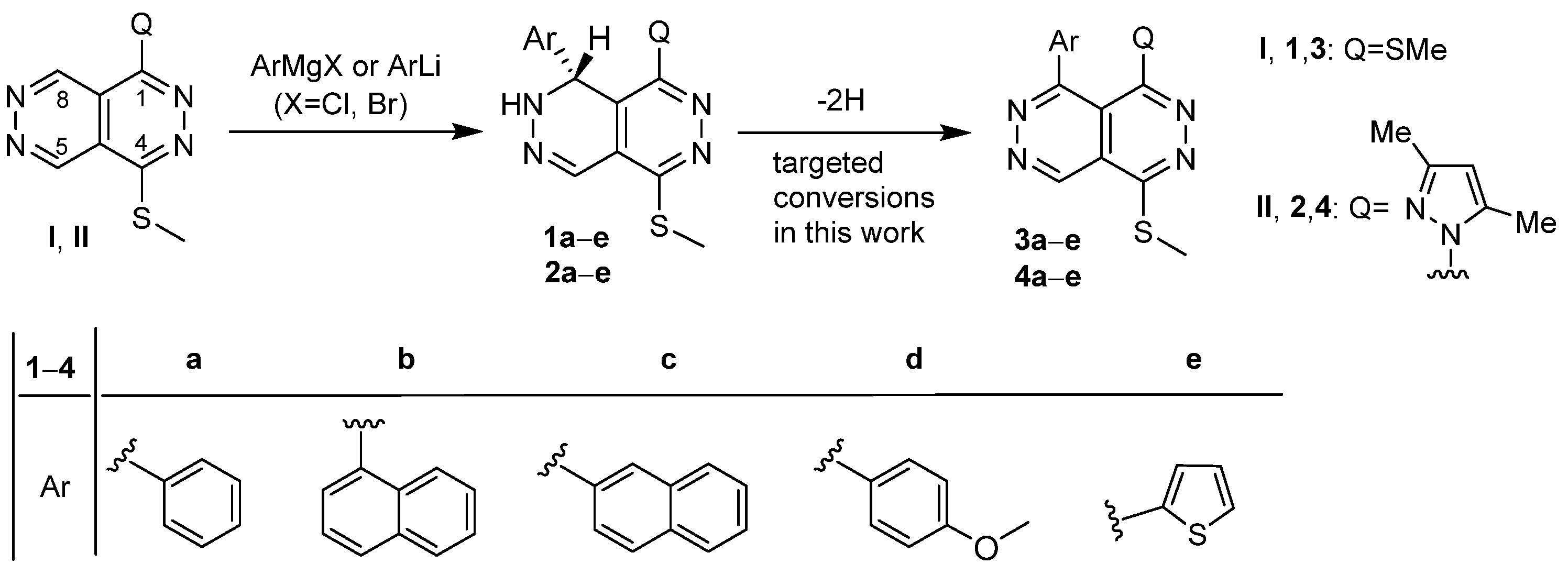
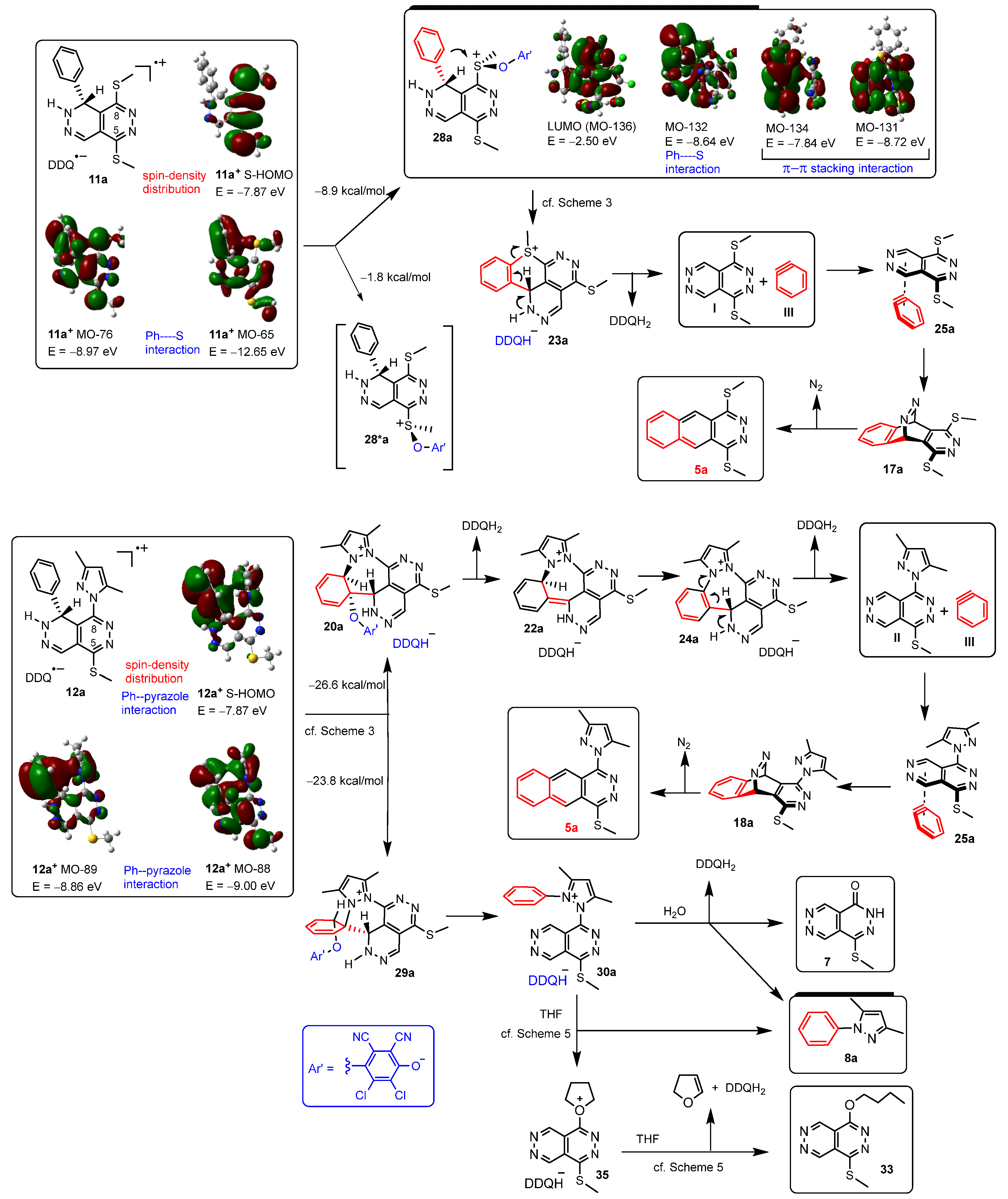
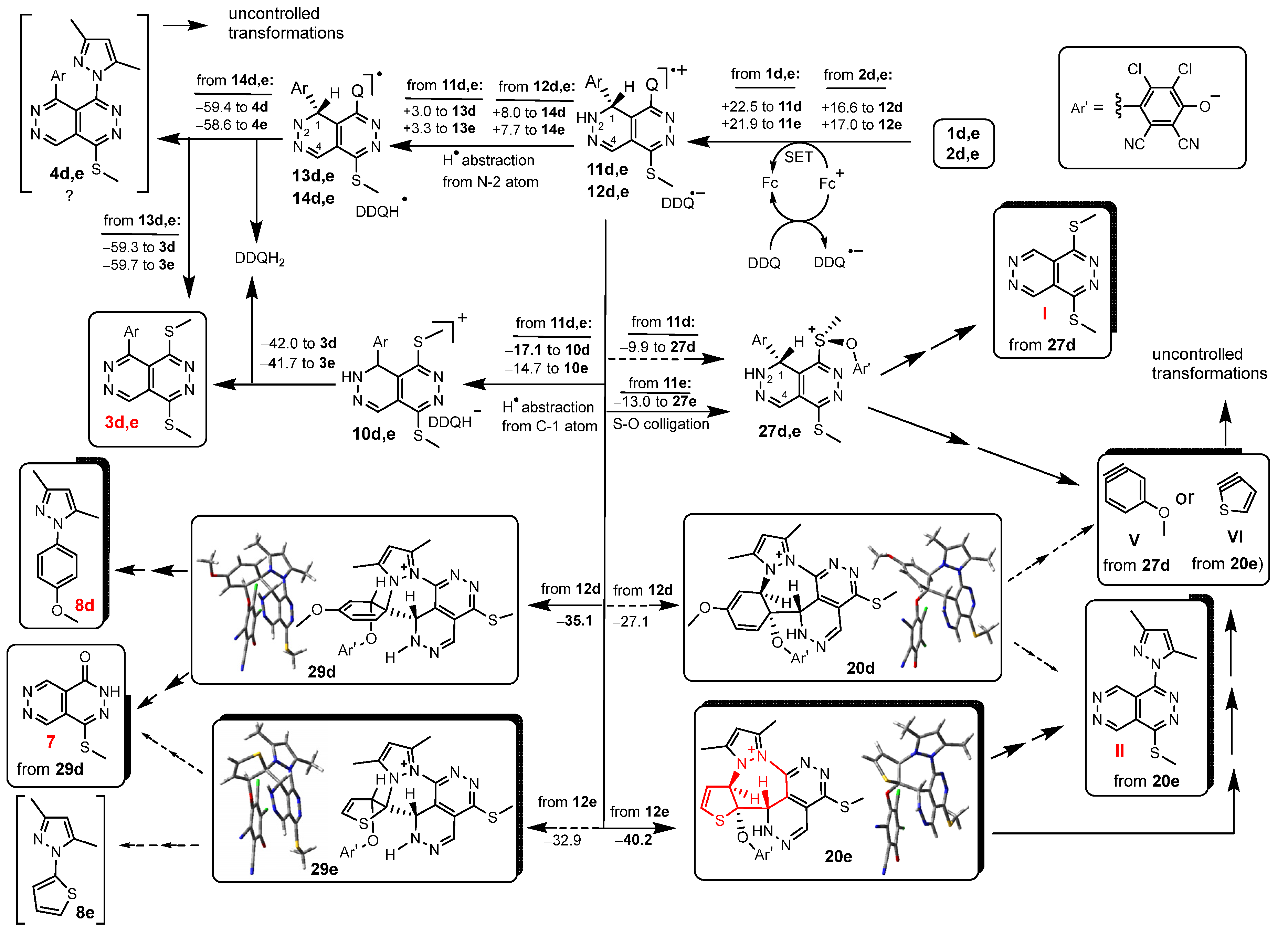
2. Results
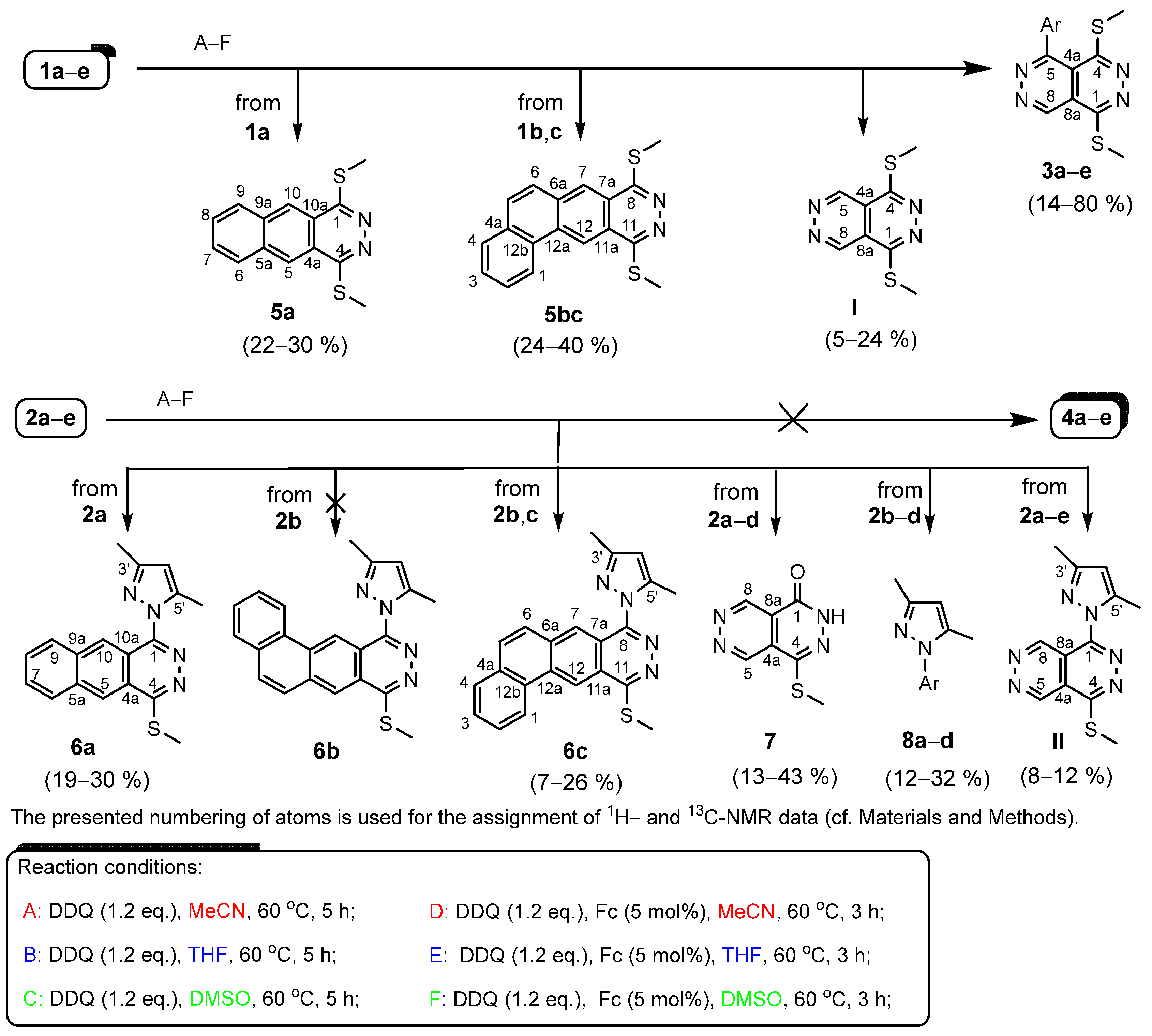
| Entry | Precursor | Product(s) | Yields a by A (%/%) | Yields a by B (%/%) | Yields a by C (%/%) | Yields a by D (%/%) | Yields a by E (%/%) | Yields a by F (%/%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 3a/5a/Ia | 14/–/– | 16/–/– | 18/–/– | 37/24/5 | 45/22/6 | 43/30/6 |
| 2 | 1b | 3b/5bc/I | 17/–/– | 21/–/– | 19/–/– | 35/34/7 | 41/35/6 | 39/32/7 |
| 3 | 1c | 3c/5bc/I | 16/–/– | 16/–/– | 19/–/– | 31/24/5 | 37/34/11 | 32/40/8 |
| 4 | 1d | 3d | 17 | 20 | 24 | 74 | 68 | 80 |
| 5 | 1e | 3e/I | 17/– | 20/– | 22/– | 59/18 | 47/20 | 64/24 |
| 6 | 2a | 6a/II/7/8a | –/–/–/– | –/–/–/– | –/–/–/– | 19/20/7/23 | 26/13/9/19 | 30/19/8/24 |
| 7 | 2b | 6c/II/7/8a | –/–/–/– | –/–/–/– | –/–/–/– | 20/10/17/12 | 20/8/24/18 | 26/9/32/25 |
| 8 | 2c | 6c/II/7/8a | 8/–/–/– | 12/–/–/– | 7/–/–/– | 25/8/36/21 | 22/10/41/27 | 25/9/43/32 |
| 9 | 2d | 7/8d | –/– | –/– | –/– | 44/37 | 37/44 | 46/49 |
| 10 | 2e | II | – | – | – | 52 | 59 | 64 |
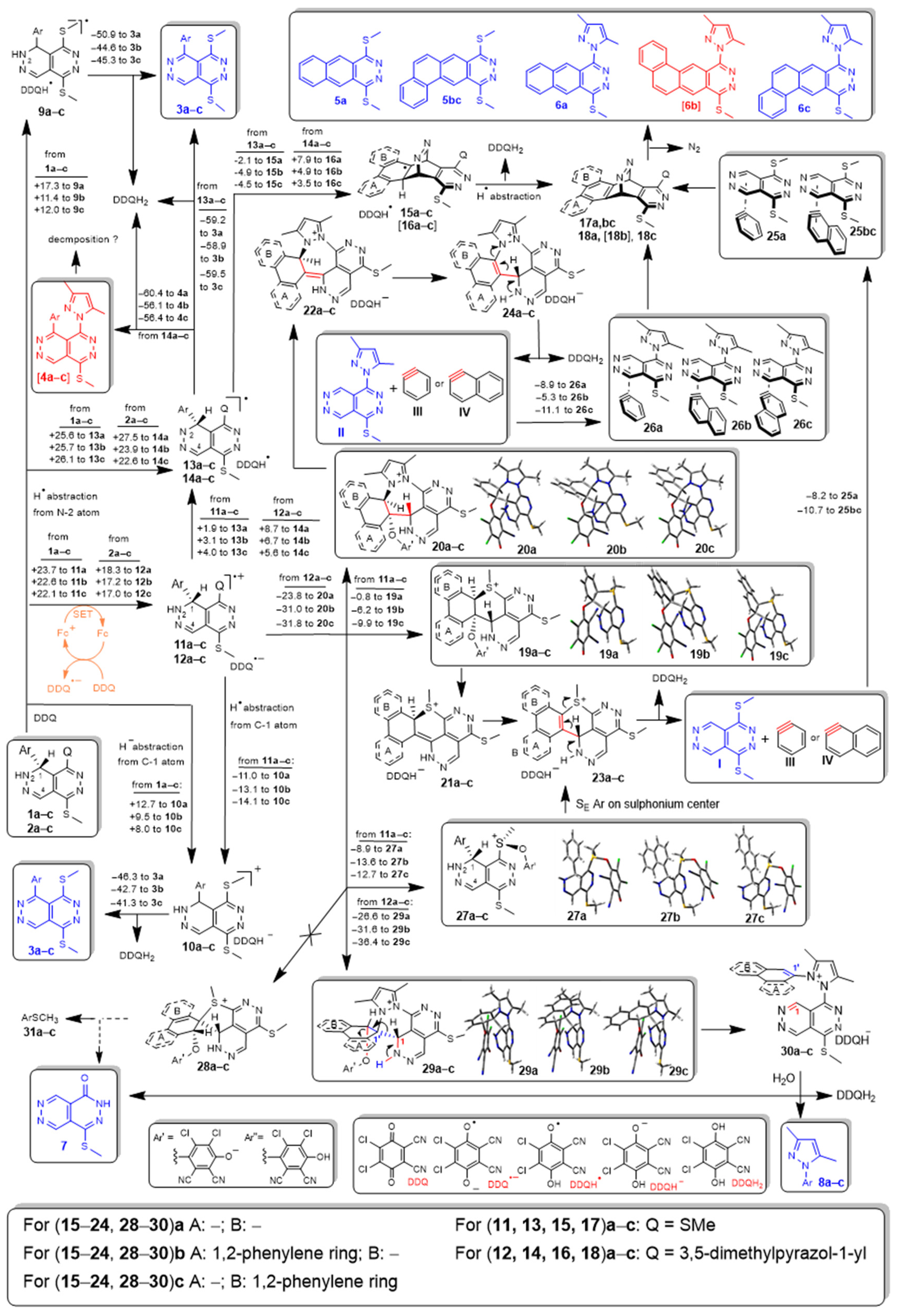
3. Discussion
4. Materials and Methods
4.1. General Procedure for the Dehydroaromatization of 1a–e and 2a–e Effected by DDQ Under the Conditions of Methods A–F
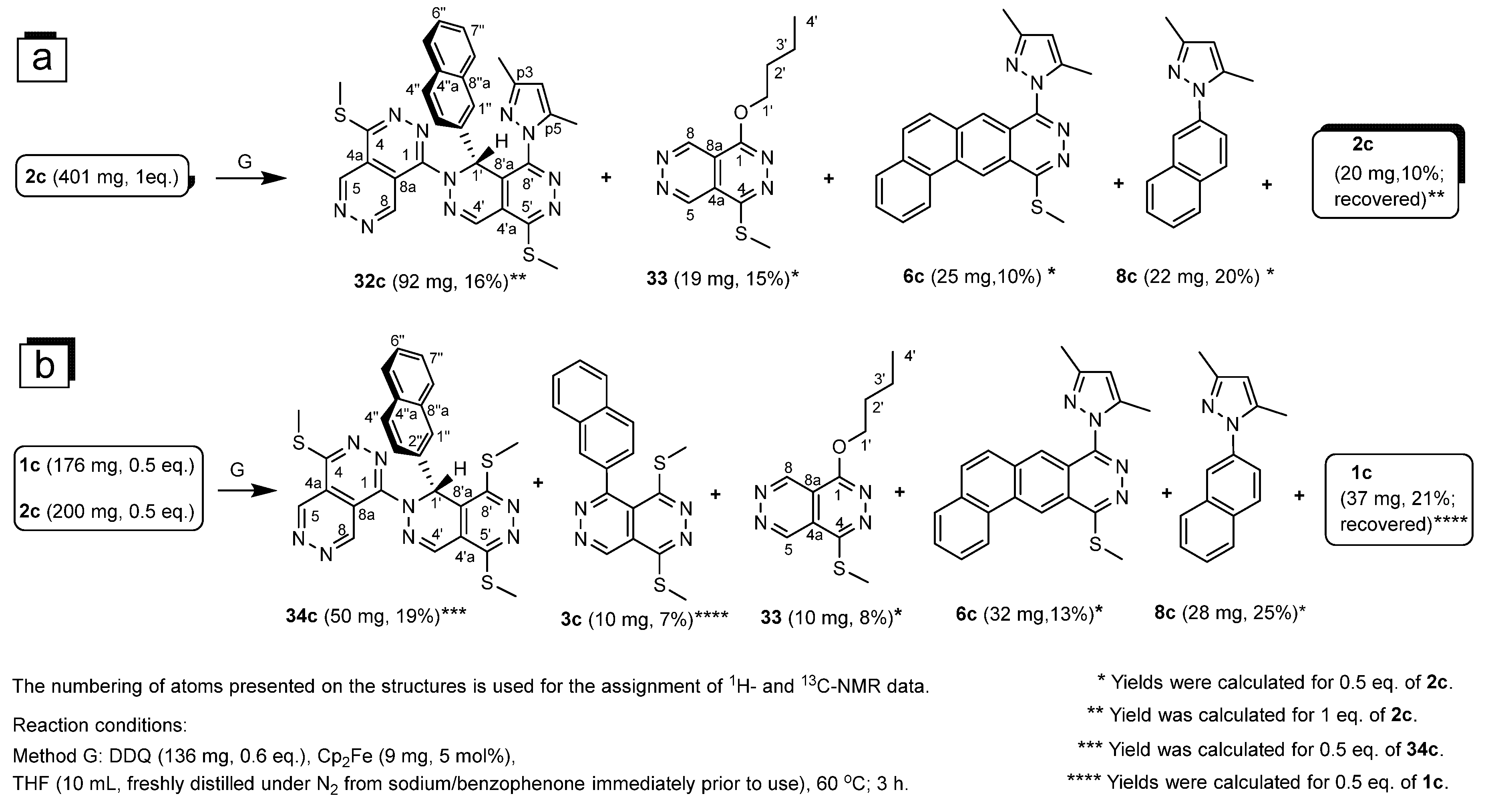
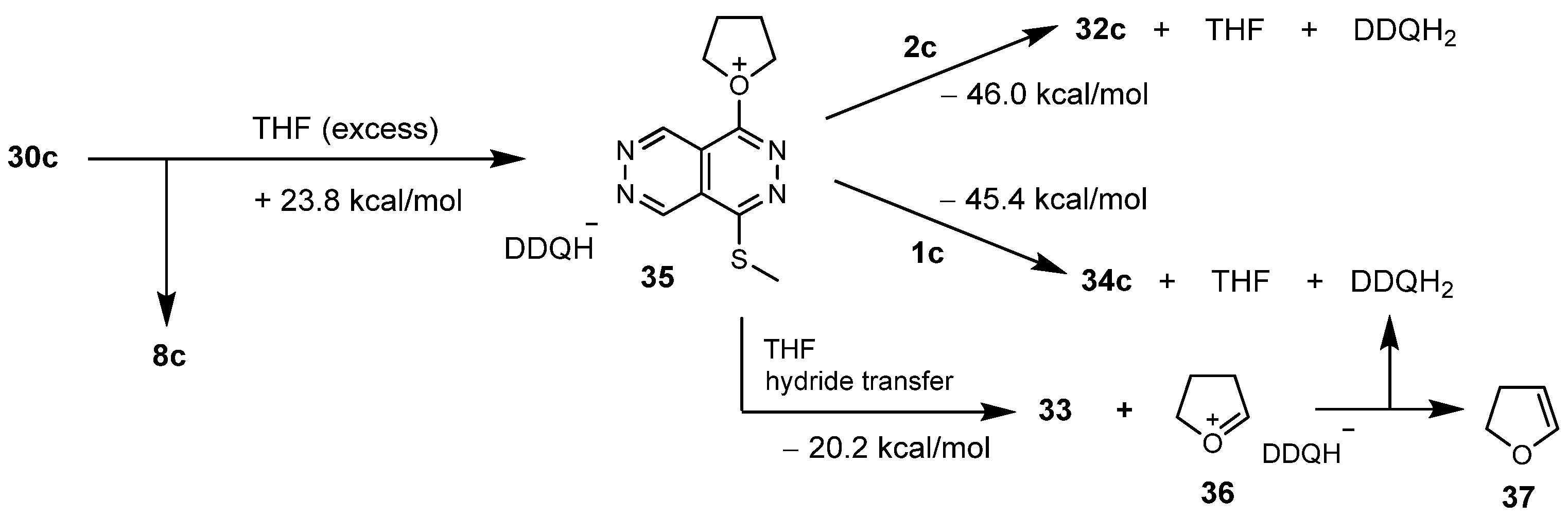
4.2. Procedure for the Reaction of 2c Effected by a Sub-Equimolar Amount of DDQ Under the Conditions of Method G
4.3. Procedure for the Reaction of a 1:1 Mixture of 1c and 2c Effected by a Sub-Equimolar Amount of DDQ Under the Conditions of Method G
4.4. Characterization of the Products
4.4.1. 1,4-Bis(methylthio)-5-phenylpyridazino[4,5-d]pyridazine (3a)
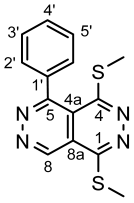
4.4.2. 1,4-Bis(methylthio)-5-(naphthalen-1-yl)pyridazino[4,5-d]pyridazine (3b)
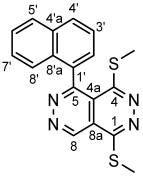
4.4.3. 1,4-Bis(methylthio)-5-(naphthalen-2-yl)pyridazino[4,5-d]pyridazine (3c)
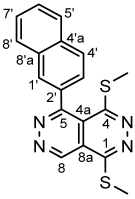
4.4.4. 5-(4-Methoxyphenyl)-1,4-bis(methylthio)pyridazino[4,5-d]pyridazine (5d)
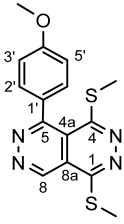
4.4.5. 1,4-Bis(methylthio)-5-(thiophen-2-yl)pyridazino[4,5-d]pyridazine (3e)
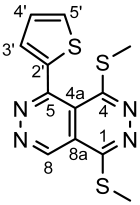
4.4.6. 1,4-Bis(methylthio)benzo[g]phthalazine (5a)
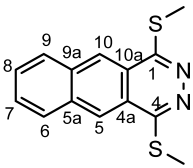
4.4.7. 8,11-Bis(methylthio)naphtho [1,2-g]phthalazine (5bc)
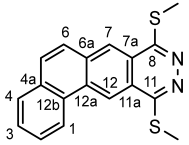
4.4.8. 1-(3,5-Dimethyl-1H-pyrazol-1-yl)-4-(methylthio)benzo[g]phthalazine (6a)
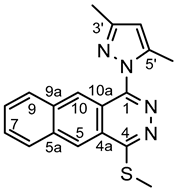
4.4.9. 8-(3,5-Dimethyl-1H-pyrazol-1-yl)-11-(methylthio)naphtho [1,2-g]phthalazine (6c)
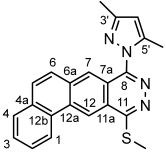
4.4.10. 4-(Methylthio)pyridazino[4,5-d]pyridazin-1(2H)-one (7)
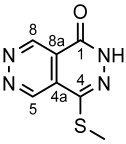
4.4.11. 3,5-Dimethyl-1-phenyl-1H-pyrazole (8a)
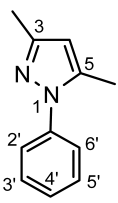
4.4.12. 3,5-Dimethyl-1-(naphthalen-1-yl)-1H-pyrazole (8b)
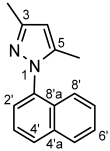
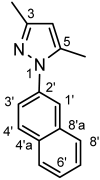
4.4.13. 3,5-Dimethyl-1-(naphthalen-2-yl)-1H-pyrazole (8c)
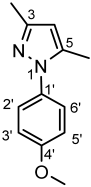
4.4.14. 1-(4-Methoxyphenyl)-3,5-dimethyl-1H-pyrazole (8d)
4.4.15. 8′-(3,5-Dimethyl-1H-pyrazol-1-yl)-4,5′-bis(methylthio)-1′-(naphthalen-2-yl)-1′H- 1,2′-bipyridazino[4,5-d]pyridazine (32c)
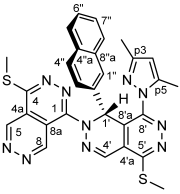
4.4.16. 1-Butoxy-4-(methylthio)pyridazino[4,5-d]pyridazine (33)
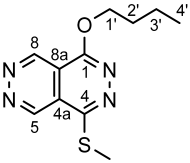
4.4.17. 4,5′,8′-Tris(methylthio)-1′-(naphthalen-2-yl)-1′H-1,2′-bipyridazino[4,5-d]pyridazine (34c)
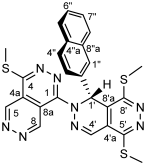
4.4.18. 1,4-Bis(methylthio)pyridazino[4,5-d]pyridazine (I)
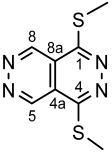
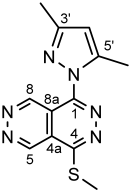
4.4.19. 1-(3,5-Dimethyl-1H-pyrazol-1-yl)-4-(methylthio)pyridazino[4,5-d]pyridazine (II)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akahane, A.; Katayama, H.; Mitsunaga, T.; Kato, T.; Kinoshita, T.; Kita, Y.; Kusunoki, T.; Terai, T.; Yoshida, K.; Shiokawa, Y. Discovery of 6-Oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic Acid (FK 838): A Novel Non-Xanthine Adenosine A1 Receptor Antagonist with Potent Diuretic Activity. J. Med. Chem. 1999, 42, 779–783. [Google Scholar] [CrossRef]
- Meade, E.A.; Wotring, L.L.; Drach, J.C.; Townsend, L.B. Synthesis and Antiproliferative and Antiviral Activity of Carbohydrate Modified Pyrrolo[2,3-d]pyridazin-7-one Nucleosides. J. Med. Chem. 1997, 40, 794–801. [Google Scholar] [CrossRef]
- Kimura, T.; Fujihara, Y.; Shibakawa, N.; Fujiwara, H.; Itoh, E.; Matsunobu, K.; Tabata, K.; Yasuda, H. Pyrrolopyridazine Derivatives. U.S. Patent 6063782 A, 17 July 1996. [Google Scholar]
- Siddiqui, A.A.; Mishra, R.; Shaharyar, M. Synthesis, characterization and antihypertensive activity of pyridazinone derivatives. Eur. J. Med. Chem. 2010, 45, 2283–2290. [Google Scholar] [CrossRef]
- Refaat, H.M.; Khalil, O.M.; Kadry, H.H. Synthesis and anti-inflammatory activity of certain piperazinylthienylpyridazine derivatives. Arch. Pharm. Res. 2007, 30, 803–811. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Lozach, O. New derivatives of pyrrolo [3,4-d]pyridazinone and their anticancer effects. Farmaco 2004, 59, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Boxer, M.B.; van der Heiden, M.G.; Shen, M.; Skoumbourdis, A.P.; Southall, N.; Veith, H.; Leister, W.; Austin, C.P.; Park, H.W.; et al. Evaluation of thieno [3,2-b]pyrrole [3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg. Med. Chem. Lett. 2010, 20, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghaffar, N.F.; Mohamed, M.K.; Kadah, M.S.; Radwan, A.M.; Said, G.H.; Abd Al, S.N. Synthesis and antitumor activities of some new pyridazinones containing the 2-phenyl-1H-indolyl moiety. J. Chem. Pharm. Res. 2011, 3, 248–259. [Google Scholar]
- Rathish, I.; Javed, K.; Ahmad, S.; Bano, S.; Alam, M.; Akhter, M.; Pillai, K.; Ovais, S.; Samim, M. Synthesis and evaluation of anticancer activity of some novel 6-aryl-2-(p-sulfamylphenyl)-pyridazin-3(2H)-ones. Eur. J. Med. Chem. 2012, 49, 304–309. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; DeJesus, R.E.; Contreras, L.; Rodriguez-Palomares, I.A.; Villanueva, P.J.; Balderrama, K.S.; Monterroza, L.; Larragoity, M.; Varela-Ramirez, A.; Aguilera, R.J. A new pyridazinone exhibits potent cytotoxicity on human cancer cells via apoptosis and poly-ubiquitinated protein accumulation. Cell Biol. Toxicol. 2019, 35, 503–519. [Google Scholar] [CrossRef]
- Sabelli, C.; Tangocci, P.; Zanazzi, P.F. The crystal structure of pyridazino[4,5-d]pyridazine. Acta Cryst. 1969, B25, 2231–2236. [Google Scholar] [CrossRef]
- Zhylenko, I.S.; Solntsev, P.V.; Rusanov, E.B.; Chernega, A.N.; Domasevitch, K.V. Hydrate frameworks involving the pyridazino[4,5-d]pyridazine unit as a multiple hydrogen-bond acceptor. Acta Crystallogr. C 2008, 64, 237–241. [Google Scholar] [CrossRef]
- Haider, N. Inverse-electron-demand Diels–Alder reactions of condensed pyridazines, part 1. Synthesis of phthalazine derivatives from pyridazino[4,5-d]pyridazines. Tetrahedron 1991, 47, 3959–3968. [Google Scholar] [CrossRef]
- Heinisch, G.; Kirchner, I. Synthesen und Reaktionen von Pyridazinderivaten, 11. Mitt.: 5-Acyl-4-pyridazincarbonsäurrester als „key intermediates” für 4-Acylpyridazine und 4-Alkyl-bzw. 4-Arylpyridazino[4,5-d]pyridazin-1 (2H) one. Monatsh. Chem. 1979, 110, 365–376. [Google Scholar] [CrossRef]
- Heinisch, G.; Jentzsch, A.; Pailer, M. Darstellung C-4-substituierter Pyridazine durch homolytische Alkylierung bzw. Acylierung. Monatsh. Chem. 1974, 105, 648–652. [Google Scholar] [CrossRef]
- Braun, M.; Hanel, G.; Heinisch, G. 4,5-Diacylpyridazine: Synthese und Umsetzung zu 1,4-Diaryl- bzw. 1,4-Dialkyl-pyridazino [4,5—d]pyridazinen 8. Mitt. über pyridazine. Monatsh. Chem. 1978, 109, 63–71. [Google Scholar] [CrossRef]
- Haider, N.; Heinisch, G.; Kirchner, I. Pyridazin-Analoga biologisch aktiver Verbindungen, 2. Mitt. 4-Aryl-pyridazino[4,5-d]pyridazine mit cyclischem Amin-Substituenten an C-1. Arch. Pharm. 1982, 315, 778–783. [Google Scholar] [CrossRef]
- Nagy, Z.T.; Lőrincz, K.; Csámpai, A.; Kotschy, A. The selective functionalization of pyridazino[4,5-d]pyridazines using polar organometallic reagents. Heterocycles 2007, 71, 141–151. [Google Scholar] [CrossRef]
- Csámpai, A.; Abrán, Á.; Kudar, V.; Túrós, G.; Wamhoff, H.; Sohár, P. Synthesis, NMR, IR spectroscopic and X-ray study of novel [pyridazin-3(2H)-one-6-yl]ferrocenes and related ferrocenophane derivatives. Study on ferrocenes. Part 14. J. Organomet. Chem. 2005, 690, 802–810. [Google Scholar] [CrossRef]
- Csókás, D.; Zupkó, I.; Károlyi, B.I.; Drahos, L.; Holczbauer, T.; Palló, A.; Czugler, M.; Csámpai, A. Synthesis, spectroscopy, X-ray analysis and in vitro antiproliferative effect of ferrocenylmethylene-hydrazinylpyridazin-3(2H)-ones and related ferroceno[d]pyridazin-1(2H)-ones. J. Organomet. Chem. 2013, 743, 130–138. [Google Scholar] [CrossRef]
- Csókás, D.; Károlyi, B.I.; Bősze, S.; Szabó, I.; Báti, G.; Drahos, L.; Csámpai, A. 2, 3-Dihydroimidazo[1,2-b]ferroceno[d]pyridazines and a 3,4-dihydro-2H-pyrimido[1,2-b]ferroceno[d]pyridazine: Synthesis, structure and in vitro antiproliferation activity on selected human cancer cell lines. J. Organomet. Chem. 2014, 750, 41–48. [Google Scholar] [CrossRef]
- Jernei, T.; Bősze, S.; Szabó, R.; Hudecz, F.; Majrik, K.; Csámpai, A. N-ferrocenylpyridazinones and new organic analogues: Synthesis, cyclic voltammetry, DFT analysis and in vitro antiproliferative activity associated with ROS-generation. Tetrahedron 2017, 73, 6181–6192. [Google Scholar] [CrossRef]
- Alaoui, N.-E.E.; Boulhaoua, M.; Hutai, D.; Oláh-Szabó, R.; Bősze, S.; Hudecz, F.; Csámpai, A. Synthetic and DFT Modelling Studies on Suzuki–Miyaura Reactions of 4,5-Dibromo-2-methylpyridazin-3(2H)-one with Ferrocene Boronates, Accompanied by Hydrodebromination and a Novel Bridge-Forming Annulation In Vitro Cytotoxic Activity of the Ferrocenyl–Pyridazinone Products. Catalysts 2022, 12, 578. [Google Scholar] [CrossRef]
- Hati, S.; Holzgrabe, U.; Sen, S. Oxidative dehydrogenation of C–C and C–N bonds: A convenient approach to access diverse (dihydro)heteroaromatic compounds. Beilstein J. Org. Chem. 2017, 13, 1670–1692. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Kovacikova, L.; Pawar, P.; Gaikwad, M.; Bohác, A.; Dawane, B. An updates: Oxidative aromatization of THβC to β-carbolines and their application for the β-carboline alkaloids synthesis. Tetrahedron 2024, 155, 133903. [Google Scholar] [CrossRef]
- Fatykhov, R.F.; Khalymbadzha, I.A.; Sharapov, A.D.; Potapova, A.P.; Mochulskaya, N.N.; Tsmokalyuk, A.N.; Ivoilova, A.V.; Mozharovskaia, P.N.; Santra, S.; Chupakhin, O.N. MnO2-Mediated Oxidative Cyclization of “Formal” Schiff’s Bases: Easy Access to Diverse Naphthofuro-Annulated Triazines. Molecules 2022, 27, 7105. [Google Scholar] [CrossRef]
- Utecht-Jarzyńska, G.; Kowalczyk, A.; Jasiński, M. Fluorinated and Non-Fluorinated 1,4-Diarylpyrazoles via MnO2-Mediated Mechanochemical Deacylative Oxidation of 5-Acylpyrazolines. Molecules 2022, 27, 8446. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Basak, A.K.; Baishya, G.; Narsaiah, A.V. Iodoxybenzoic acid (IBX): An efficient and novel oxidizing agent for the aromatization of 1,4-dihydropyridines. Synthesis 2006, 3, 451–454. [Google Scholar] [CrossRef]
- Nageswar, Y.V.D.; Ramesh, K.; Rakhi, K. IBX-Mediated Organic Transformations in Heterocyclic Chemistry-A Decade Update. Front. Chem. 2022, 10, 841751. [Google Scholar] [CrossRef]
- Varala, R.; Seema, V.; Alam, M.M.; Dubasi, N.; Vummadi, R.D. Iodoxybenzoic Acid (IBX) in Organic Synthesis: A Septennial Review. Curr. Org. Synth. 2024, 21, 607–664. [Google Scholar] [CrossRef]
- Hati, S.; Sen, S. N-Bromo-succinimide promoted synthesis of β-carbolines and 3,4-dihydro-β-carbolines from tetrahydro-β-carbolines. Tetrahedron Lett. 2016, 57, 1040–1043. [Google Scholar] [CrossRef]
- Yang, R.; Xiong, Y.; Deng, S.; Bai, J.; Song, X.-R.; Xiao, Q. NBS-mediated bromination and dehydrogenation of tetrahydro-quinoline in one pot: Scope and mechanistic study. RSC Adv. 2023, 13, 33495–33499. [Google Scholar] [CrossRef]
- Kamal, A.; Sathish, M.; Prasanthi, A.V.G.; Chetna, J.; Tangella, Y.; Srinivasulu, V.; Shankaraiah, N.; Alarifi, A. An efficient one-pot decarboxylative aromatization of tetrahydro-β-carbolines by using N-chlorosuccinimide: Total synthesis of norharmane, harmane and eudistomins. RSC Adv. 2015, 5, 90121–90126. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, T.; Li, J.-X. Metal-free oxidative synthesis of quinazolinones via dual amination of sp3 C–H bonds. Chem. Commun. 2014, 50, 6471–6474. [Google Scholar] [CrossRef] [PubMed]
- Litvić, M.; Cepanec, I.; Filipan, M.; Kos, K.; Bartolinčić, A.; Drušković, V.; Tibi, M.M.; Vinković, V. Mild, selective, and high-yield oxidation of hantzsch 1,4-dihydropyridines with lead(IV) acetate. Heterocycles 2005, 65, 23–35. [Google Scholar] [CrossRef]
- Hussain, K.; Wadhwa, D. Highly Efficient, One Pot Synthesis and Oxidation of Hantsch 1,4-Dihydropyridines Mediated by Iodobenzene Diacetate (III) Using Conventional Heating, Ultrasonic and Microwave Irradiation. Int. J. Org. Chem. 2014, 4, 174–181. [Google Scholar] [CrossRef]
- Varma, R.S.; Kumar, D.J. Solid state oxidation of 1,4-dihydropyridines to pyridines using phenyliodine(III) bis(trifluoroacetate) or elemental sulfur. Chem. Soc. Perkin Trans. 1 1999, 12, 1755–1757. [Google Scholar] [CrossRef]
- Szabó, T.; Hazai, V.; Volk, B.; Milen, M.; Simig, G. First total synthesis of the β-carboline alkaloids trigonostemine A, trigonostemine B and a new synthesis of pityriacitrin and hyrtiosulawesine. Tetrahedron Lett. 2019, 60, 1471–1475. [Google Scholar] [CrossRef]
- Manasa, K.L.; Tangella, Y.; Ramu, G.; Babu, B.N. TCCA. A Mild Reagent for Decarboxylative/Dehydrogenative Aromatization of Tetrahydro-β-carbolines: Utility in the Total Synthesis of Norharmane, Harmane, Eudistomin U, I and N. ChemistrySelect 2017, 2, 9162–9167. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Dilmaghani, K.A.; Roozijoy, A. Aromatization of Hantzsch Ester 1,4-Dihydropyridines with Iodine under Normal Conditions and Ultrasound Irradiation. J. Chin. Chem. Soc. 2005, 52, 1001–1004. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H.; Wang, X.; Zhi, S.; Kan, Y.; Wang, C. Synthesis of Pyrrole via a Silver-Catalyzed 1,3-Dipolar Cycloaddition/Oxidative Dehydrogenative Aromatization Tandem Reaction. J. Org. Chem. 2017, 82, 4194–4202. [Google Scholar] [CrossRef]
- Kumar, P.; Kadyan, K.; Duhan, M.; Sindhu, J.; Hussain, K.; Lal, S. Silica-supported ceric ammonium nitrate (CAN): A simple, mild and solid-supported reagent for quickest oxidative aromatization of Hantzsch 1,4-dihydropyridines. Chem. Pap. 2019, 73, 1153–1162. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Salehi, P.; Ghorbani-Choghamarani, A.; Safaiee, M.; Shahamirian, M. Silica Chromate as a Novel Oxidizing Agent for the Oxidation of 1,4-Dihydropyridines. Synth. Commun. 2007, 37, 1817–1823. [Google Scholar] [CrossRef]
- Cai, X.-H.; Yang, H.-J.; Zhang, G.-L. Aromatization of 1,4-dihydropyridines with selenium dioxide. Can. J. Chem. 2005, 83, 273–275. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Zhao, B.-J.; Cheng, J.-P. Mechanisms of the Oxidations of NAD(P)H Model Hantzsch 1,4-Dihydropyridines by Nitric Oxide and Its Donor N-Methyl-N-nitrosotoluene-p-sulfonamide. J. Org. Chem. 2000, 65, 8158–8163. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Yin, B.; Li, C.; Liu, P.; Li, J.; Shi, Z. DDQ-Induced Dehydrogenation of Heterocycles for CC Double Bond Formation: Synthesis of 2-Thiazoles and 2-Oxazoles. Chem. Asian J. 2013, 8, 1408–14011. [Google Scholar] [CrossRef]
- Patir, S.; Ertürk, E. An Entry to the Azocino[4,3-b]indole Framework through a Dehydrogenative Activation of 1,2,3,4-Tetrahydrocarbazoles Mediated by DDQ: Formal Synthesis of (±)-Uleine. J. Org. Chem. 2011, 76, 335–338. [Google Scholar] [CrossRef]
- Alsharif, M.A.; Raja, Q.A.; Majeed, N.A.; Jassas, R.S.; Alsimaree, A.A.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Alsantali, R.I.; Moussa, Z.; et al. DDQ as a versatile and easily recyclable oxidant: A systematic review. RSC Adv. 2021, 11, 29826–29858. [Google Scholar] [CrossRef]
- Bisek, B.; Chaladaj, W. Access to 2-Alkenyl-furans via a Cascade of Pd-Catalyzed Cyclization/Coupling Followed by Oxidative Aromatization with DDQ. J. Org. Chem. 2024, 89, 7275–7279. [Google Scholar] [CrossRef]
- Knall, A.-C.; Hollauf, M.; Slugovc, C. Kinetic studies of inverse electron demand Diels–Alder reactions (iEDDA) of norbornenes and 3,6-dipyridin-2-yl-1,2,4,5-tetrazine. Tetrahedron Lett. 2014, 55, 4763–4766. [Google Scholar] [CrossRef]
- Zjang, J.; Zhang, H.; Wang, Z.; Yao, W. Concise Synthesis of Tetrasubstituted 1,6-Dihydropyridazine and Pyridazine Derivatives. Synthesis 2024, 56, 3915–3922. [Google Scholar] [CrossRef]
- Litvic′, M.F.; Litvic, M.; Vinkovic, V. An efficient, metal-free, room temperature aromatization of Hantzsch 1,4-dihydropyridines with urea–hydrogen peroxide adduct, catalyzed by molecular iodine. Tetrahedron 2008, 64, 5649–5656. [Google Scholar] [CrossRef]
- Tuo, X.; Chen, S.; Jiang, P.; Ni, P.; Wang, X.; Deng, G.-J. Iodine-catalyzed convergent aerobic dehydroaromatization toward benzazoles and benzazines. RSC Adv. 2020, 10, 8348–8351. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Sen, S. Cerium Chloride Catalyzed, 2-Iodoxybenzoic Acid Mediated Oxidative Dehydrogenation of Multiple Heterocycles at Room Temperature. Eur. J. Org. Chem. 2017, 3, 1277–1280. [Google Scholar] [CrossRef]
- Zhou, W.; Taboonpong, P.; Aboo, A.H.; Zhang, L.; Jiang, J.; Xiao, J. A Convenient Procedure for the Oxidative Dehydrogenation of N-Heterocycles Catalyzed by FeCl2/DMSO. Synlett 2016, 27, 1806–1809. [Google Scholar] [CrossRef][Green Version]
- Jung, D.; Kim, M.H.; Kim, J. Cu-Catalyzed Aerobic Oxidation of Di-tert-butyl Hydrazodicarboxylate to Di-tert-butyl Azodicarboxylate and Its Application on Dehydrogenation of 1,2,3,4-Tetrahydroquinolines under Mild Conditions. Org. Lett. 2016, 18, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Iosub, A.V.; Stahl, S.S. Catalytic Aerobic Dehydrogenation of Nitrogen Heterocycles Using Heterogeneous Cobalt Oxide Supported on Nitrogen-Doped Carbon. Org. Lett. 2015, 17, 4404–4407. [Google Scholar] [CrossRef]
- Fujita, K.; Tanaka, Y.; Kobayashi, M.; Yamaguchi, R. Homogeneous perdehydrogenation and perhydrogenation of fused bicyclic N-heterocycles catalyzed by iridium complexes bearing a functional bipyridonate ligand. J. Am. Chem. Soc. 2014, 136, 4829–4832. [Google Scholar] [CrossRef]
- Wu, J.; Talwar, D.; Johnston, S.; Yan, M.; Xiao, J. Acceptorless dehydrogenation of nitrogen heterocycles with a versatile iridium catalyst. Angew. Chem. Int. Ed. 2013, 52, 6983–6987. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Xia, Y.; Wu, L. Palladium Nanoparticles Stabilized by Metal–Carbon Covalent Bonds as an Expeditious Catalyst for the Oxidative Dehydrogenation of Nitrogen Heterocycles. ChemCatChem 2017, 9, 2463–2466. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Shah, N.; Dauvois, V.; Li, H.; Namboothiri, I.N.; Doris, E. Cooperative dehydrogenation of N-heterocycles using a carbon nanotube-rhodium nanohybrid. Chemistry 2015, 21, 7039–7042. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Cheong, W.C.; Zhang, C.; Zheng, L.; Yan, W.; Yu, R.; Chen, C.; Li, Y. Nitrogen-coordinated cobalt nanocrystals for oxidative dehydrogenation and hydrogenation of N-heterocycles. Chem. Sci. 2019, 10, 5345–5352. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Liu, M.; Bartling, S.; Lund, H.; Rabeah, J.; Dyson, P.J.; Beller, M.; Jagadeesh, R.V. A Cobalt Nanocatalyst for the Hydrogenation and Oxidative Dehydrogenation of N-heterocycles. ChemCatChem 2024, 16, e202301027. [Google Scholar] [CrossRef]
- Sun, K.; Shan, H.; Ma, R.; Wang, P.; Neumann, H.; Lu, G.-P.; Beller, M. Catalytic oxidative dehydrogenation of N-heterocycles with nitrogen/phosphorus co-doped porous carbon materials. Chem. Sci. 2022, 13, 6865–6872. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.A.; Huang, H.; Zhou, F.; Deng, G.-J.; Li, C.-J. Catalytic dehydrogenative aromatization: An alternative route to functionalized arenes. Org. Chem. Front. 2015, 2, 279–287. [Google Scholar] [CrossRef]
- Kirsch, S.F.; Wegener, M. Oxidation by dehydrogenation. Compr. Org. Synth. II 2014, 7, 1–25. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, Y.; Zhang, Z.; Jia, Z.; Loh, T.-P. A Rare Earth Metal Catalyzed Aerobic Dehydrogenation of N-Heterocycles. Org. Lett. 2023, 25, 4468–4472. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kawashita, Y.; Hayashi, M. Oxidative aromatization of 1,3,5-trisubstituted pyrazolines and Hantzsch 1,4-dihydropyridines by Pd/C in acetic acid. Org. Lett. 2002, 4, 3955–3957. [Google Scholar] [CrossRef]
- Bera, S.; Bera, A.; Banerjee, D. Nickel-Catalyzed Dehydrogenation of N-Heterocycles Using Molecular Oxygen. Org. Lett. 2020, 22, 6458–6463. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, Q.; Zhang, L.; Luo, S. Photoredox Mediated Acceptorless Dehydrogenative Coupling of Saturated N-Heterocycles. ACS Catal. 2019, 9, 3589–3594. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, R.; Li, W.; Sun, H.; Chen, G.; Zhan, F.; Zhao, H. Enhanced catalytic performance for aerobic dehydrogenation of N-heterocycles over bimetallic CoOX-CeO2 catalysts derived from Ce-based MOFs. Mol. Catal. 2024, 567, 114450. [Google Scholar] [CrossRef]
- Enders, L.; Casadio, D.S.; Aikonen, S.; Lenarda, A.; Wirtanen, T.; Hu, T.; Hitela, S.; Ribeiro, L.S.; Pereira, M.F.R.; Helaja, J. Air oxidized activated carbon catalyst for aerobic oxidative aromatizations of N-heterocycles. Catal. Sci. Technol. 2021, 11, 5962–5972. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hikawa, H.; Enda, T.; Kikkawa, S.; Azumaya, I. Synthesis of 1-Aryl-β-Carbolines via a Pd-Catalyzed Oxidative Pictet-Spengler Reaction/Aromatization Cascade Using Benzylic Alcohols in Water. Eur. J. Org. Chem. 2025, 28, e202401269. [Google Scholar] [CrossRef]
- Ju, S.; Zhou, X.; Jin, H.; Yang, Y.; Yang, L.; Wu, J. Stereoselective oxidative C3–N bond dehydrogenation and aromatization of 1-carboxyl substituted tetrahydroisoquinolines employing pipecolate oxidase. Green Synth. Catal. 2024, in press. [CrossRef]
- Fabbrizzi, L. The ferrocenium/ferrocene couple: A versatile redox switch. ChemTexts 2020, 6, 22. [Google Scholar] [CrossRef]
- Chen, N.; Wu, Z.-J.; Xu, H.-C. Ferrocene as a Redox Catalyst for Organic Electrosynthesis. Israel. J. Chem. 2023, 64, e202300097. [Google Scholar] [CrossRef]
- Bauer, E.B. Recent Catalytic Applications of Ferrocene and Ferrocenium Cations in the Syntheses of Organic Compounds. Molecules 2024, 29, 5544. [Google Scholar] [CrossRef]
- Astruc, D. The numerous paths of ferrocene. Nat. Chem. 2023, 15, 1650. [Google Scholar] [CrossRef]
- Hernández-Muñoz, L.S.; Galano, A.; Astudillo-Sánchez, P.D.; Abu-Omar, M.M.; González, F.J. The mechanism of mediated oxidation of carboxylates with ferrocene as redox catalyst in absence of grafting effects. An experimental and theoretical approach. Electrochim. Acta 2014, 136, 542–549. [Google Scholar] [CrossRef]
- Sariga, V.A. The Renaissance of Ferrocene-Based Electrocatalysts: Properties, Synthesis Strategies, and Applications. Top. Curr. Chem. 2023, 381, 32. [Google Scholar] [CrossRef]
- Salman, H.M.A.; Mahmoud, M.R.; Abou-El-Wafa, M.H.M.; Rabie, U.M.; Crabtree, R.H. Redox reactions via outer sphere charge transfer complexation: The interaction of ferrocenes with σ- and π-type acceptors. Inorg. Chem. Commun. 2004, 7, 1209–1212. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Hehre, J.W.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Alcarazo, M.; Kozhushkov, S.I. Synthetic Applications of Sulfonium Salts. Eur. J. Inorg. Chem. 2020, 26, 2486–2500. [Google Scholar] [CrossRef] [PubMed]
- Loco, D.; Chataigner, I.; Piquemal, J.-P.; Spezia, R. Efficient and Accurate Description of Diels–Alder Reactions Using Density Functional Theory. ChemPhysChem 2022, 23, e202200349. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.M.; Mackey, J.L.; Garg, N.K.; Houk, K.N. The Role of Aryne Distortions, Steric Effects, and Charges in Regioselectivities of Aryne Reactions. J. Am. Chem. Soc. 2014, 136, 15798–15805. [Google Scholar] [CrossRef]
- Ricca, A.; Bauschlicher, C.W., Jr.; Allamandola, L.J. The infrared spectroscopy of polycyclic aromatic hydrocarbons with five- and seven-membered fused ring defects. Astrophys. J. 2011, 729, 94. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wang, Z.Y.; Wang, S.; Dai, N.N.; Xiao, Y.; Zhou, Y.; Tian, W.C.; Sun, D.; Li, Q.; Wang, Y.; Wei, W.T. Carbon-carbon triple bond cleavage and reconstitution to achieve aryl amidation using nitrous acid esters. Nat. Commun. 2025, 16, 993. [Google Scholar] [CrossRef]
- Sivaguru, P.; Wang, Z.; Zanoni, G.; Bi, X. Cleavage of carbon–carbon bonds by radical reactions. Chem. Soc. Rev. 2019, 48, 2615–2656. [Google Scholar] [CrossRef]
- Liang, Y.F.; Bilal, M.; Tang, L.Y.; Wang, T.Z.; Guan, Y.Q.; Cheng, Z.; Zhu, M.; Wei, J.; Jiao, N. Carbon-Carbon Bond Cleavage for Late-Stage Functionalization. Chem. Rev. 2023, 123, 12313–12370. [Google Scholar] [CrossRef]
- Roque, J.B.; Kuroda, Y.; Göttemann, L.T.; Sarpong, R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science 2018, 361, 171–174. [Google Scholar] [CrossRef]
- Liao, J.; Yang, X.; Ouyang, L.; Huang, J.; Luo, R. Recent advances in cascade radical cyclization of radical acceptors for the synthesis of carbo- and heterocycles. Org. Chem. Front. 2021, 8, 1345–1363. [Google Scholar] [CrossRef]
- Paik, J. Olaparib: A Review as First-Line Maintenance Therapy in Advanced Ovarian Cancer. Target. Oncol. 2021, 16, 847–856. [Google Scholar] [CrossRef]
- Williams, P.B.; Crandall, E.; Sheppard, J.D. Azelastine hydrochloride, a dual-acting anti-inflammatory ophthalmic solution, for treatment of allergic conjunctivitis. Clin. Ophthalmol. 2010, 4, 993–1001. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, H.; Zhang, L.; Zhao, Y.; Chen, S.; Chen, Y.; Peng, X.; Li, Q.; Yuan, M.; Hu, X. Zopolrestat as a human glyoxalase I inhibitor and its structural basis. ChemMedChem 2013, 8, 1462–1464. [Google Scholar] [CrossRef]
- Scott, E.N.; Meinhardt, G.; Jacques, C.; Laurent, D.; Thomas, A.L. Vatalanib: The clinical development of a tyrosine kinase inhibitor of angiogenesis in solid tumours. Expert Opin. Investig. Drugs 2007, 16, 367–379. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutai, D.; Nagy, T.Z.; Emődi, V.; Csámpai, A. Ferrocene-Catalyzed Aromatization and Competitive Oxidative Ring Transformations of 1,2-Dihydro-1-Arylpyridazino[4,5-d]Pyridazines. Catalysts 2025, 15, 742. https://doi.org/10.3390/catal15080742
Hutai D, Nagy TZ, Emődi V, Csámpai A. Ferrocene-Catalyzed Aromatization and Competitive Oxidative Ring Transformations of 1,2-Dihydro-1-Arylpyridazino[4,5-d]Pyridazines. Catalysts. 2025; 15(8):742. https://doi.org/10.3390/catal15080742
Chicago/Turabian StyleHutai, Dániel, Tibor Zs. Nagy, Veronika Emődi, and Antal Csámpai. 2025. "Ferrocene-Catalyzed Aromatization and Competitive Oxidative Ring Transformations of 1,2-Dihydro-1-Arylpyridazino[4,5-d]Pyridazines" Catalysts 15, no. 8: 742. https://doi.org/10.3390/catal15080742
APA StyleHutai, D., Nagy, T. Z., Emődi, V., & Csámpai, A. (2025). Ferrocene-Catalyzed Aromatization and Competitive Oxidative Ring Transformations of 1,2-Dihydro-1-Arylpyridazino[4,5-d]Pyridazines. Catalysts, 15(8), 742. https://doi.org/10.3390/catal15080742








