Highly Effective Asymmetric Henry Reaction Catalyzed by Chiral Complex of Cu (II)-Aziridine-Functionalized Organophosphorus Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Chiral Catalysts 1–12
2.2. Asymmetric Nitroaldol (Henry) Reaction Catalyzed by Complexes of Cu (II) with Chiral Systems 1–12
2.3. Asymmetric Henry Reaction Promoted by Aziridinyl Phosphine 5Cu (II) Complex—Scope of the Starting Materials
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Asymmetric Nitroaldol (Henry) Reaction—General Procedure
1-Phenyl-2-nitroethanol 13
(R)-1-(2-Methoxyphenyl)-2-nitroethanol 14
(R)-1-(2-Nitrophenyl)-2-nitroethanol 15
(R)-1-(4-Chlorophenyl)-2-nitroethanol 16
(R)-1-(4-Methoxyphenyl)-2-nitroethanol 17
(R)-1-(4-Nitrophenyl)-2-nitroethanol 18
(R)-1-(naphthalen-1-yl)-2-nitroethanol 19
(R)-1-(4-bromophenyl)-2-nitroethanol 21
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garg, A.; Rendina, D.; Bendale, H.; Akiyama, T.; Ojima, I. Recent advances in catalytic asymmetric synthesis. Front. Chem. 2024, 12, 1398397. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-M.; Cheng, Y.-Z.; Duan, Y.; Zhang, Y.-D.; Fan, Q.-H.; You, S.-L.; Luo, S.; Zhu, S.-F.; Fu, X.-F.; Zhou, Q.-L. Recent Progress of Asymmetric Catalysis from a Chinese Perspective. CCS Chem. 2023, 5, 2685–2716. [Google Scholar] [CrossRef]
- Tamatam, R.; Shin, D. Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016–2020): A Recapitulation of Chirality. Pharmaceuticals 2023, 16, 339. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Paniraj, A.S.R.; Tambe, Y.B. Developments in the Catalytic Asymmetric Synthesis of Agrochemicals and Their Synthetic Importance. J. Agric. Food Chem. 2021, 69, 14761–14780. [Google Scholar] [CrossRef] [PubMed]
- Ballini, R.; Palmieri, A. Nitroalkanes: Synthesis, Reactivity, and Applications, 1st ed.; Wiley-VCH GmbH: Weinheim, Germany, 2021; pp. 59–105. [Google Scholar]
- Dong, L.; Chen, F.-E. Asymmetric catalysis in direct nitromethane-free Henry reactions. RSC Adv. 2020, 10, 2113–2326. [Google Scholar] [CrossRef]
- Xu, D.; Sun, Q.; Quan, Z.; Sun, W.; Wang, X. The synthesis of chiral tridentate ligands from L-proline and their application in the copper(II)-catalyzed enantioselective Henry reaction. Tetrahedron Asymmetry 2017, 28, 954–963. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Skarżewski, J. New chiral thiols and C2-symmetrical disulfides of Cinchona alkaloids: Ligands for the asymmetric Henry reaction catalyzed by CuII complexes. Tetrahedron Asymmetry 2009, 20, 1992–1998. [Google Scholar] [CrossRef]
- Wang, Z.; He, J.; Mu, Y. Synthesis of chiral salan ligands with bulky substituents and their application in Cu-catalyzed asymmetric Henry reaction. J. Organomet. Chem. 2020, 928, 121546. [Google Scholar] [CrossRef]
- Ishihara, K.; Kato, Y.; Takeuchi, N.; Hayashi, Y.; Hagiwara, Y.; Shibuya, S.; Natsume, T.; Matsugi, M. Asymmetric Henry Reaction Using Cobalt Complexes with Bisoxazoline Ligands Bearing Two Fluorous Tags. Molecules 2023, 28, 7632. [Google Scholar] [CrossRef]
- Rénio, M.R.R.; Sousa, F.J.P.M.; Tavares, N.C.T.; Valente, A.J.M.; da Silva Serra, M.E.; Murtinho, D. (3S,4S)-N-substituted-3,4-dihydroxypyrrolidines as ligands for the enantioselective Henry reaction. Appl. Organomet. Chem. 2021, 35, e6175. [Google Scholar] [CrossRef]
- Alammari, A.S.; Al-Majid, A.M.; Barakat, A.; Alshahrani, S.; Ali, M.; Islam, M.S. Asymmetric Henry Reaction of Nitromethane with Substituted Aldehydes Catalyzed by Novel In Situ Generated Chiral Bis(β-Amino Alcohol-Cu(Oac)2·H2O Complex. Catalysts 2021, 11, 1208. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leśniak, S.; Sznajder, E.; Kiełbasiński, P. Highly enantioselective Henry reaction catalyzed by chiral tridentate heterorganic ligands. Tetrahedron Asymmetry 2009, 20, 1547–1549. [Google Scholar] [CrossRef]
- Rao, D.H.S.; Chatterjee, A.; Padhi, S.K. Biocatalytic approaches for enantio and diastereoselective synthesis of chirl β-nitroalcohols. Org. Biomol. Chem. 2021, 19, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-H.; Wan, N.-W.; Da, X.-Y.; Mou, X.-Q.; Wang, Z.-X.; Chen, Y.-Z.; Liu, Z.-Q.; Zheng, Y.-G. Enantiocomplementary synthesis of β-adrenergic blocker precursors via biocatalytic nitration of phenyl glycidyl ethers. Bioorg. Chem. 2023, 138, 106640. [Google Scholar] [CrossRef]
- Tentori, F.; Brenna, E.; Colombo, D.; Crotti, M.; Gatti, F.G.; Ghezzi, M.C.; Pedrocchi-Fantoni, G. Biocatalytic Approach to Chiral β-Nitroalcohols by Enantioselective Alcohol Dehydrogenase-Mediated Reduction of α-Nitroketones. Catalysts 2018, 8, 308. [Google Scholar] [CrossRef]
- De Jesús Cruz, P.; Johnson, J.S. Crystallization-Enabled Henry Reactions: Stereoconvergent Construction of Fully Substituted [N]-Asymmetric Centers. J. Am. Chem. Soc. 2022, 144, 15803–15811. [Google Scholar] [CrossRef]
- Szymańska, J.; Rachwalski, M.; Pieczonka, A.M. Highly Efficient Asymmetric [3+2]-Cycloaddition Promoted by Chiral Aziridine-Functionalized Organophosphorus Compounds. Molecules 2024, 29, 3283. [Google Scholar] [CrossRef]
- Doğan, Ö.; Çağli, E. PFAM catalyzed enantioselective diethylzinc addition to imines. Turk. J. Chem. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel-Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective Mannich Reaction Promoted by Chiral Phosphinoyl-Aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Adamczyk, J.; Marciniak, L.; Pieczonka, A.M.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines. Catalysts 2021, 11, 968. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Mielgo, A. Unveiling Reliable Catalysts for the Asymmetric Nitroaldol (Henry) Reaction. Angew. Chem. Int. Ed. 2004, 43, 5442–5444. [Google Scholar] [CrossRef]
- Cho, J.; Nayab, S.; Jeong, J.H. An efficient synthetic approach towards a single diastereomer of (2R,3R)-N2,N3-bis((S)-1-phenylethyl)butane-2,3-diamine via metalation and demetalation. Transit. Met. Chem. 2020, 45, 9–17. [Google Scholar] [CrossRef]
- Subba Reddy, B.V.; Madhusudana Reddy, S.; Manisha, S.; Madan, C. Asymmetric Henry reaction catalyzed by a chiral Cu(II) complex: A facile enantioselective synthesis of (S)-2-nitro-1-arylethanols. Tetraheron Asymmetry 2011, 22, 530–535. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Qi, G.; Judeh, Z.M.A. Efficient Asymmetric Copper(I)-Catalyzed Henry Reaction Using Chiral N-Alkyl-C1-tetrahydro-1,1′-bisisoquinolines. Eur. J. Org. Chem. 2011, 2011, 4892–4898. [Google Scholar] [CrossRef]
- Yoshisawa, A.; Feula, A.; Male, L.; Leach, A.G.; Fossey, J.S. Rigid and concave, 2,4-cis-substituted azetidine derivatives: A platform for asymmetric catalysis. Sci. Rep. 2018, 8, 6541. [Google Scholar] [CrossRef]
- Yearick Spangler, K.; Wolf, C. Asymmetric Copper(I)-Catalyzed Henry Reaction with an Aminoindanol-Derived Bisoxazolidine Ligand. Org. Lett. 2009, 11, 4724–4727. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Marciniak, L.; Rachwalski, M.; Leśniak, S. Enantiodivergent aldol condensation in the presence of aziridine/acid/water systems. Symmetry 2020, 12, 930. [Google Scholar] [CrossRef]

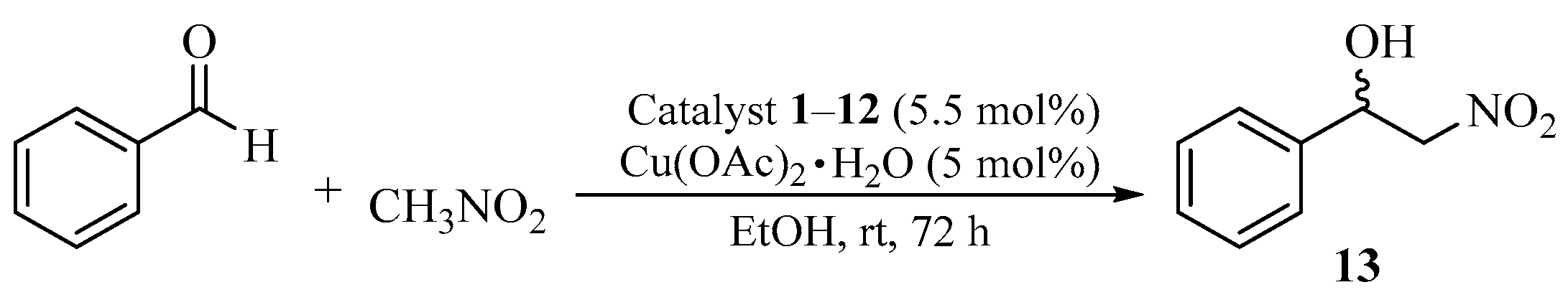
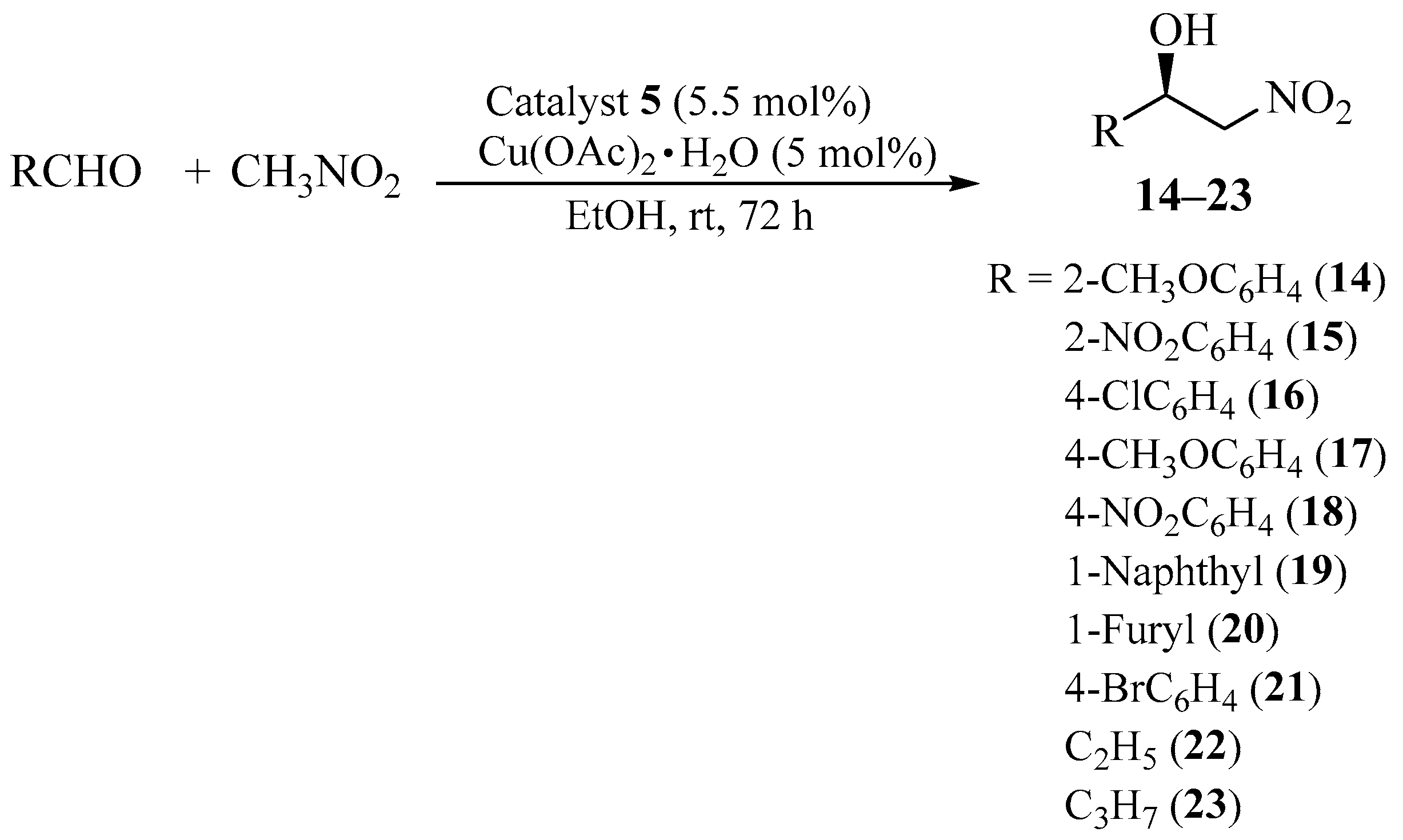

| Entry | Catalyst | Yield (%) | ee (%) a | Abs. Conf. b |
|---|---|---|---|---|
| 1 | 1 | 70 | 40 | (R) |
| 2 | 2 | 68 | 55 | (S) |
| 3 | 3 | 67 | 44 | (S) |
| 4 | 4 | 62 | 34 | (S) |
| 5 | 5 | 90 | 96 | (R) |
| 6 | 6 | 92 | 90 | (S) |
| 7 | 7 | 85 | 56 | (S) |
| 8 | 8 | 82 | 55 | (S) |
| 9 | 9 | 65 | 56 | (S) |
| 10 | 10a | 89 | 80 | (R) |
| 11 | 11a | 70 | 74 | (S) |
| 12 | 11b | 78 | 62 | (S) |
| 13 | 12a | 64 | 50 | (S) |
| Entry | R | Product | Yield (%) | ee (%) a | Abs. Conf. b |
|---|---|---|---|---|---|
| 1 | 2-CH3OC6H4 | 14 | 92 | 90 | (R) |
| 2 | 2-NO2C6H4 | 15 | 90 | 84 | (R) |
| 3 | 4-ClC6H4 | 16 | 88 | 80 | (R) |
| 4 | 4-CH3OC6H4 | 17 | 91 | 84 | (R) |
| 5 | 4-NO2C6H4 | 18 | 78 | 94 | (R) |
| 6 | 1-Naphthyl | 19 | 70 | 64 | (R) |
| 7 | 1-Furyl | 20 | 0 | – | n.d. |
| 8 | 4-BrC6H4 | 21 | 85 | 86 | (R) |
| 9 | C2H5 | 22 | traces | – | n.d. |
| 10 | C3H7 | 23 | traces | – | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachwalski, M.; Wojtaszek, J.; Szymańska, J.; Pieczonka, A.M. Highly Effective Asymmetric Henry Reaction Catalyzed by Chiral Complex of Cu (II)-Aziridine-Functionalized Organophosphorus Compounds. Catalysts 2025, 15, 179. https://doi.org/10.3390/catal15020179
Rachwalski M, Wojtaszek J, Szymańska J, Pieczonka AM. Highly Effective Asymmetric Henry Reaction Catalyzed by Chiral Complex of Cu (II)-Aziridine-Functionalized Organophosphorus Compounds. Catalysts. 2025; 15(2):179. https://doi.org/10.3390/catal15020179
Chicago/Turabian StyleRachwalski, Michał, Julia Wojtaszek, Julia Szymańska, and Adam M. Pieczonka. 2025. "Highly Effective Asymmetric Henry Reaction Catalyzed by Chiral Complex of Cu (II)-Aziridine-Functionalized Organophosphorus Compounds" Catalysts 15, no. 2: 179. https://doi.org/10.3390/catal15020179
APA StyleRachwalski, M., Wojtaszek, J., Szymańska, J., & Pieczonka, A. M. (2025). Highly Effective Asymmetric Henry Reaction Catalyzed by Chiral Complex of Cu (II)-Aziridine-Functionalized Organophosphorus Compounds. Catalysts, 15(2), 179. https://doi.org/10.3390/catal15020179








