SnBi Catalytic Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate
Abstract
1. Introduction
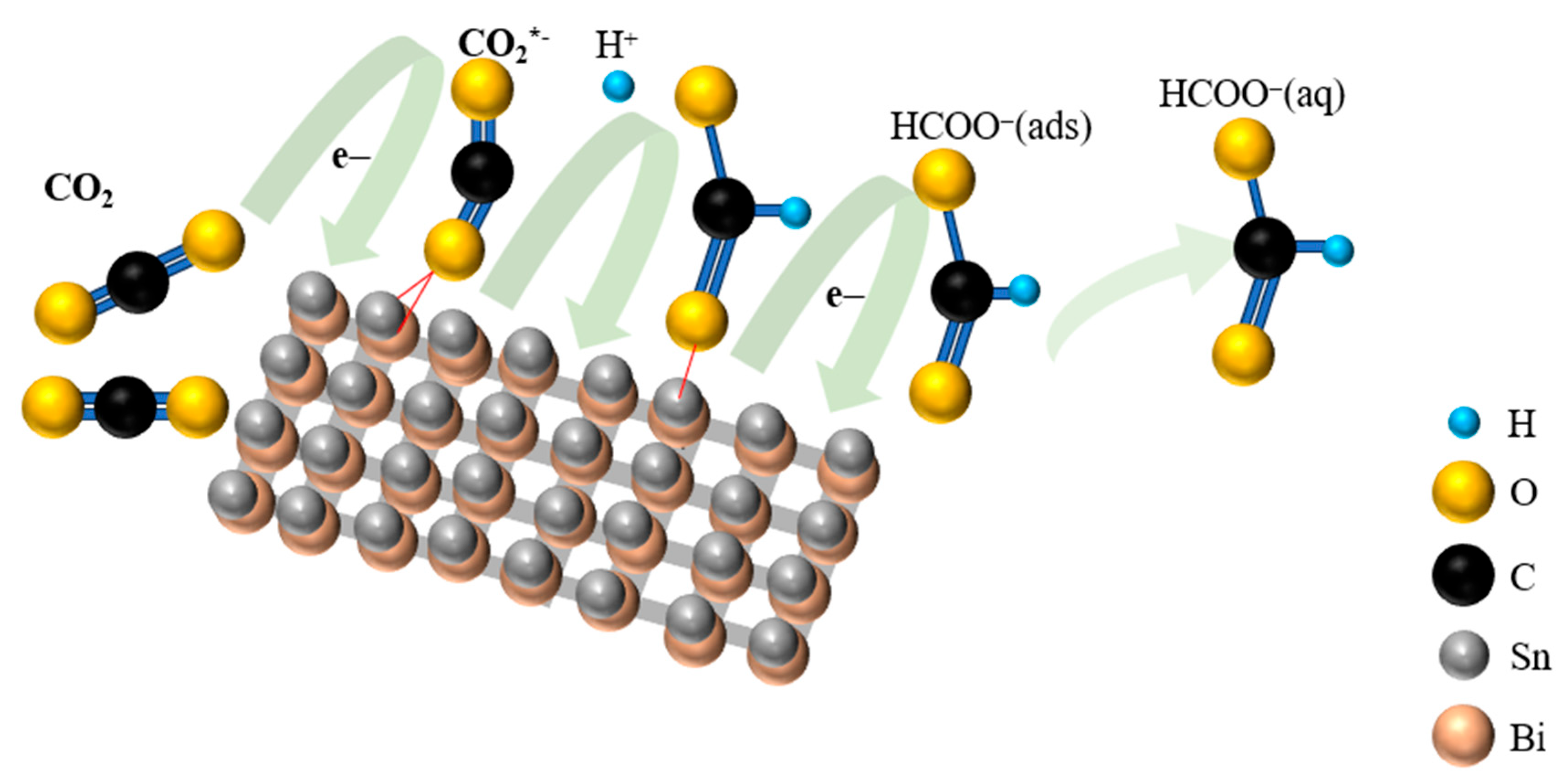
2. Results and Discussion
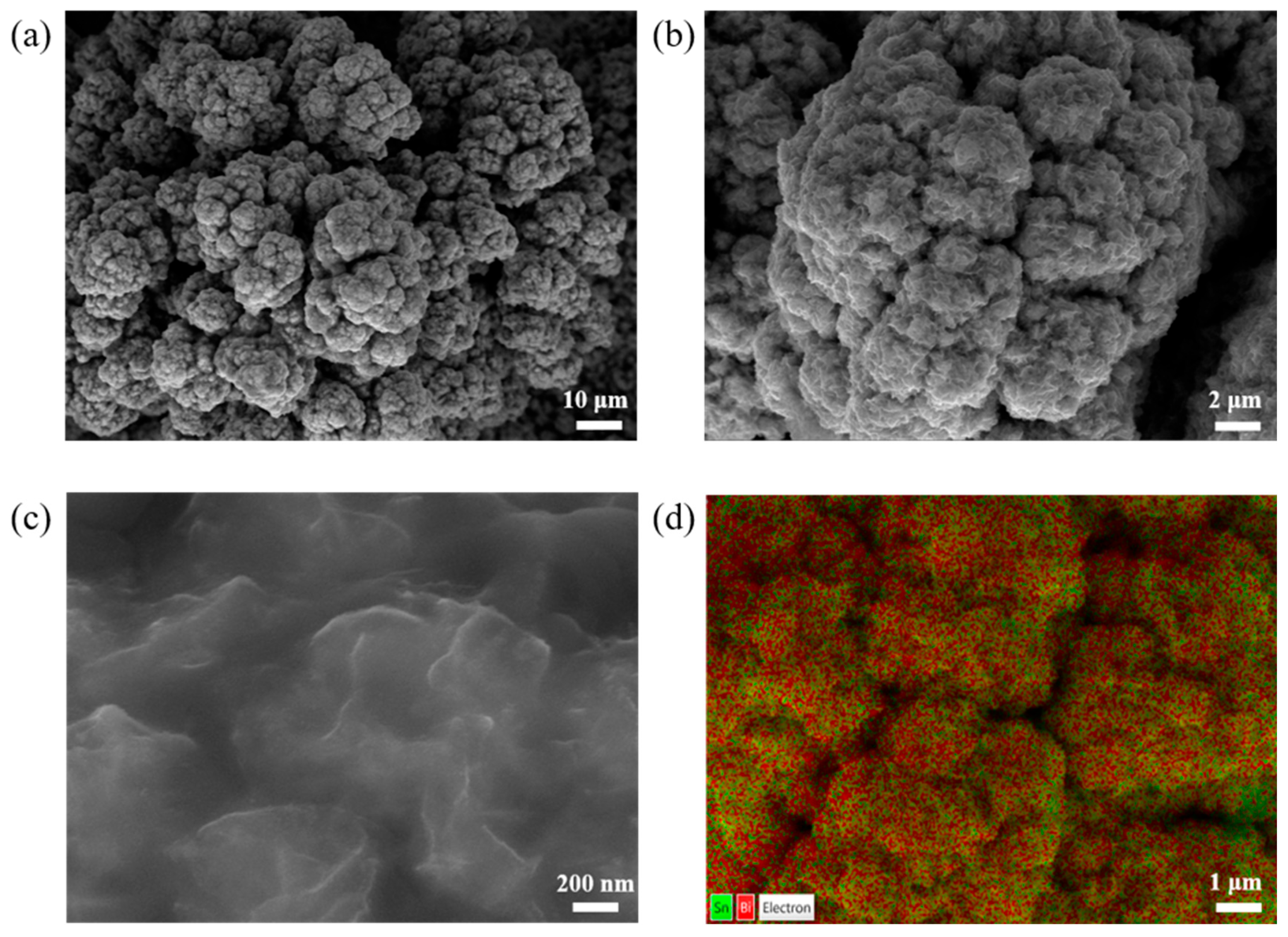
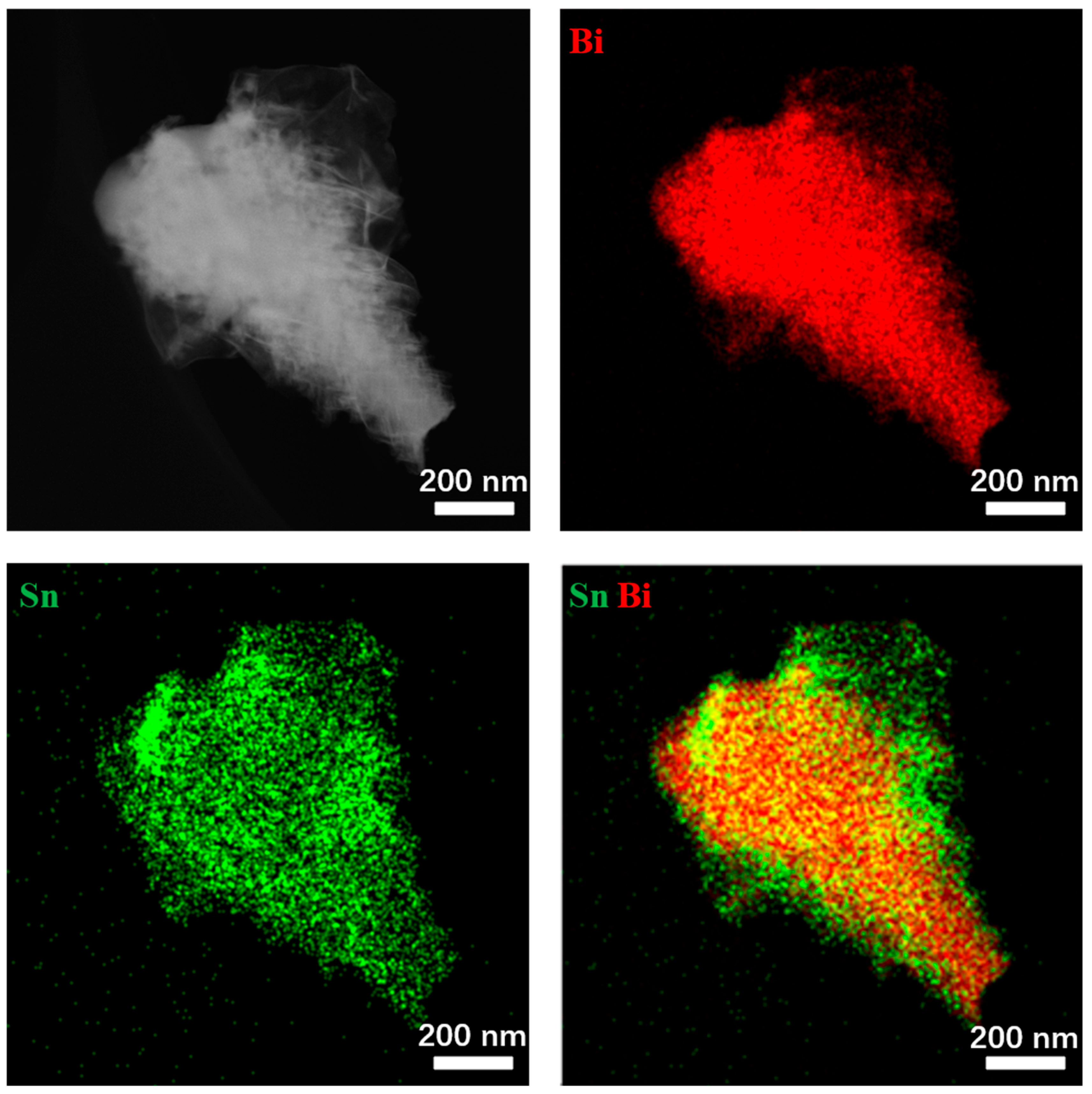
2.1. Morphological and Structural Characterization
2.2. Electrochemical Properties
2.2.1. Effect of Different Sn2+:Bi3+ Molar Ratios on Catalyst Activity
2.2.2. Effect of Deposition Current Density on the Performance of SnBi Catalytic Electrode
2.3. Impedance Characteristics of SnBi Catalytic Electrodes
2.4. Stability of SnBi Catalytic Electrodes
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Pretreated Copper Foam Substrate
3.3. Fabrication of the SnBi Double Electrode
3.4. Material Characterization
3.5. Electrochemical Measurements
3.6. Product Analysis
- F—Faraday constant (96,485 C·mol−1);
- n—amount of formate ions produced (mol);
- Q—total charge passed through the system during the specified reaction time (C).
- N—Number of electrons transferred per mole of CO2 converted to H2, CO, or other gaseous products (mol e−/mol CO2);
- c—Volume percentage of the target gas in the total gas mixture (%);
- υ—CO2 flow rate at the H-cell outlet (measured by gas flow meter, mL·min−1);
- t—Electrolysis duration (min);
- Vm—Molar volume of gas (at standard conditions: 22.4 L·mol−1).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Who Ambient Air Quality Database, 2022 Update: Status Report; World Health Organization: Geneva, Switzerland, 2023; p. 44. [Google Scholar]
- Nwabara, U.O.; Cofell, E.R.; Verma, S.; Negro, E.; Kenis, P.J. Durable Cathodes and Electrolyzers for the Efficient Aqueous Electrochemical Reduction of CO2. ChemSusChem 2020, 13, 855–875. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Duarah, P.; Haldar, D.; Yadav, V.S.K.; Purkait, M.K. Progress in the Electrochemical Reduction of CO2 to Formic Acid: A Review on Current Trends and Future Prospects. J. Environ. Chem. Eng. 2021, 9, 106394. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.N.; Legrand, U.; Pahija, E.; Tavares, J.R.; Boffito, D.C. From CO2 to Formic Acid Fuel Cells. Ind. Eng. Chem. Res. 2021, 60, 803–815. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Avila-Bolívar, B.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Improving Trade-Offs in the Figures of Merit of Gas-Phase Single-Pass Continuous CO2 Electrocatalytic Reduction to Formate. Chem. Eng. J. 2021, 405, 126965. [Google Scholar] [CrossRef]
- Onishi, N.; Iguchi, M.; Yang, X.C.; Kanega, R.; Kawanami, H.; Xu, Q.; Himeda, Y. Development of Effective Catalysts for Hydrogen Storage Technology Using Formic Acid. Adv. Energy Mater. 2019, 9, 1801275. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural Defects on Converted Bismuth Oxide Nanotubes Enable Highly Active Electrocatalysis of Carbon Dioxide Reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef]
- Jin, S.; Hao, Z.M.; Zhang, K.; Yan, Z.H.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem.-Int. Ed. 2021, 60, 20627–20648. [Google Scholar] [CrossRef]
- Perry, S.C.; Leung, P.K.; Wang, L.; de León, C.P. Developments on Carbon Dioxide Reduction: Their Promise, Achievements, and Challenges. Curr. Opin. Electrochem. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- Yang, Z.W.; Chen, J.M.; Qiu, L.Q.; Xie, W.J.; He, L.N. Molecular Engineering of Metal Complexes for Electrocatalytic Carbon Dioxide Reduction: From Adjustment of Intrinsic Activity to Molecular Immobilization. Angew. Chem.-Int. Ed. 2022, 61, e202205301. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Salehi, M.; Le, Q.V.; Seifitokaldani, A.; Dinh, C.T. Fundamentals of Electrochemical CO2 Reduction on Single-Metal-Atom Catalysts. ACS Catal. 2020, 10, 10068–10095. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhang, Z.D.; Zhao, Z.H.; Huang, H.; Anjum, D.H.; Wang, D.S.; He, J.H.; Huang, K.W. Tunable Selectivity for Electrochemical CO2 Reduction by Bimetallic Cu-Sn Catalysts: Elucidating the Roles of Cu and Sn. ACS Catal. 2021, 11, 11103–11108. [Google Scholar] [CrossRef]
- Wen, G.B.; Lee, D.U.; Ren, B.H.; Hassan, F.M.; Jiang, G.P.; Cano, Z.P.; Gostick, J.; Croiset, E.; Bai, Z.Y.; Yang, L.; et al. Orbital Interactions in Bi-Sn Bimetallic Electrocatalysts for Highly Selective Electrochemical CO2 Reduction toward Formate Production. Adv. Energy Mater. 2018, 8, 1802427. [Google Scholar] [CrossRef]
- Chen, F.R.; Yao, Z.C.; Lyu, Z.H.; Fu, J.J.; Zhang, X.L.; Hu, J.S. Recent Advances in P-Block Metal Chalcogenide Electrocatalysts for High-Efficiency CO2 Reduction. Escience 2024, 4, 100172. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Song, Y.; Huang, L.; Guo, W.; Cao, X.; Dou, Y.; Cheng, L.; Li, G.; Hu, Q.J. Recent Development of Nanomaterials for Carbon Dioxide Electroreduction. SmartMat 2022, 3, 35–53. [Google Scholar] [CrossRef]
- Yang, Z.N.; Oropeza, F.E.; Zhang, K.H.L. P-Block Metal-Based (Sn, In, Bi, Pb) Electrocatalysts for Selective Reduction of CO2 to Formate. APL Mater. 2020, 8, 060901. [Google Scholar] [CrossRef]
- Li, P.F.; Yang, F.Q.; Li, J.; Zhu, Q.; Xu, J.W.; Loh, X.J.; Huang, K.W.; Hu, W.P.; Lu, J. Nanoscale Engineering of P-Block Metal-Based Catalysts toward Industrial-Scale Electrochemical Reduction of CO2. Adv. Energy Mater. 2023, 13, 2301597. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Li, Y.; Li, C. Electrocatalytic CO2 Reduction over Bimetallic Bi-Based Catalysts: A Review. CCS Chem. 2022, 5, 544–567. [Google Scholar] [CrossRef]
- Wang, Y.H.; Jiang, W.J.; Yao, W.; Liu, Z.L.; Liu, Z.; Yang, Y.; Gao, L.Z. Advances in Electrochemical Reduction of Carbon Dioxide to Formate over Bismuth-Based Catalysts. RARE Met. 2021, 40, 2327–2353. [Google Scholar] [CrossRef]
- Proietto, F.; Patel, U.; Galia, A.; Scialdone, O. Electrochemical Conversion of CO2 to Formic Acid Using a Sn Based Electrode: A Critical Review on the State-of-the-Art Technologies and Their Potential. Electrochim. Acta 2021, 389, 138753. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Mu, K.; Ma, X.; Jiao, M.; Wang, Z.; Limpachanangkul, P.; Chalermsinsuwan, B.; Gao, Y.; Li, Y.; et al. Recent Progress of Sn-Based Derivative Catalysts for Electrochemical Reduction of CO2. Energy Technol. 2021, 9, 2000799. [Google Scholar] [CrossRef]
- Li, J.; Jiao, J.Q.; Zhang, H.C.; Zhu, P.; Ma, H.F.; Chen, C.; Xiao, H.; Lu, Q. Two-Dimensional SnO2 Nanosheets for Efficient Carbon Dioxide Electroreduction to Formate. ACS Sustain. Chem. Eng. 2020, 8, 4975–4982. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, H.; Won, J.H.; Sim, W.; Kang, S.J.; Choi, H.; Sharma, M.; Oh, H.S.; Ringe, S.; Kwon, Y.; et al. Design of Less Than 1 Nm Scale Spaces on SnO2 Nanoparticles for High-Performance Electrochemical CO2 Reduction. Adv. Funct. Mater. 2022, 32, 2107349. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, T.; Xie, R.; Hou, Z.; Ji, X.; Pang, Y.; Chen, S.; Titirici, M.M.; Weng, H.; Chai, G. Facet Engineering to Regulate Surface States of Topological Crystalline Insulator Bismuth Rhombic Dodecahedrons for Highly Energy Efficient Electrochemical CO2 Reduction. Adv. Mater. 2021, 33, 2008373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Chen, Q.; Zhou, Y.-J.; Li, H.-M.; Fu, J.-W.; Liu, M. Cu-Based Bimetallic Catalysts for CO2 Reduction Reaction. Adv. Sens. Energy Mater. 2022, 1, 100023. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, F.L.; Dong, J.; Jia, K.C.; Sun, T.T.; Xu, L.B. Recent Advances in Designing Efficient Electrocatalysts for Electrochemical Carbon Dioxide Reduction to Formic Acid/Formate. J. Electroanal. Chem. 2023, 928, 117018. [Google Scholar] [CrossRef]
- Avila-Bolívar, B.; Montiel, V.; Solla-Gullón, J. Electrochemical Reduction of CO2 to Formate on Nanoparticulated Bi-Sn-Sb Electrodes. Chemelectrochem 2022, 9, e202200272. [Google Scholar] [CrossRef]
- Yang, S.P.; Sun, Y.; Wang, C.L.; Lv, L.; Hu, M.X.; Jin, J.S.; Xie, H.X. One-Step Co-Electrodeposition of SnBi for Efficient Electrochemical Reduction of Carbon Dioxide to Formic Acid. Catal. Sci. Technol. 2023, 13, 758–766. [Google Scholar] [CrossRef]
- Zheng, W.R.; Liu, M.J.; Lee, L.Y.S. Best Practices in Using Foam-Type Electrodes for Electrocatalytic Performance Benchmark. ACS Energy Lett. 2020, 5, 3260–3264. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Wang, Q.N.; Wu, C. Rapid and Scalable Synthesis of Bismuth Dendrites on Copper Mesh as a High-Performance Cathode for Electroreduction of CO2 to Formate. J. CO2 Util. 2020, 36, 96–104. [Google Scholar] [CrossRef]
- Chen, Z.; Löber, M.; Rokicińska, A.; Ma, Z.; Chen, J.; Kuśtrowski, P.; Meyer, H.-J.; Dronskowski, R.; Slabon, A. Increased Photocurrent of CuWO4 Photoanodes by Modification with The Oxide Carbodiimide Sn2O(NCN). Dalton Trans. 2020, 49, 3450–3456. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Li, R.Z.; Xu, J.J.; Tang, Y.F.; Huang, F.Q. Long-Life and High Volumetric Capacity Bi2Sn2O7 Anode with Interpenetrating Bi-O and Sn-O Networks. Cell Rep. Phys. Sci. 2022, 3, 101109. [Google Scholar] [CrossRef]
- Sheng, B.B.; Cao, D.F.; Liu, C.J.; Chen, S.M.; Song, L. Support Effects in Electrocatalysis and Their Synchrotron Radiation-Based Characterizations. J. Phys. Chem. Lett. 2021, 12, 11543–11554. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Wang, W.H.; Zhang, J.Q.; Wang, H.Z.; Yang, Z.X.; Ning, H.; Zhu, J.X.; Zhang, Y.L.; Guan, L.; Teng, X.L.; et al. Carbon Sustained SnO2-Bi2O3 Hollow Nanofibers as Janus Catalyst for High-Efficiency CO2 Electroreduction. Chem. Eng. J. 2021, 426, 131867. [Google Scholar] [CrossRef]
- Lou, W.S.; Peng, L.W.; He, R.N.; Liu, Y.Y.; Qiao, J.L. CuBi Electrocatalysts Modulated to Grow on Derived Copper Foam for Efficient CO2-to-Formate Conversion. J. Colloid Interface Sci. 2022, 606, 994–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Chen, F.F.; Zhang, F.F.; Liu, Y.D.; Hao, X.Y.; Jin, H.K.; Zhang, X.H.; Lu, Z.M.; Dong, H.; et al. Mass-Transfer-Enhanced Hydrophobic Bi Microsheets for Highly Efficient Electroreduction of CO2 to Pure Formate in a Wide Potential Window. Appl. Catal. B-Environ. Energy 2023, 322, 122127. [Google Scholar] [CrossRef]
- Peng, C.; Yang, S.T.; Luo, G.; Yan, S.; Chen, N.; Zhang, J.B.; Chen, Y.S.; Wang, X.C.; Wang, Z.Q.; Wei, W.; et al. Ampere-Level CO2-to-Formate Electrosynthesis Using Highly Exposed Bismuth(110) Facets Modified with Sulfur-Anchored. Chem 2023, 9, 2830–2840. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S.N.; Mandler, D.; Wang, X.; Qiao, S.Z.; Xu, Z.C.J. Approaches for Measuring the Surface Areas of Metal Oxide Electrocatalysts for Determining their Intrinsic Electrocatalytic Activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef]
- Wang, J.G.; Zou, J.S.; Hu, X.; Ning, S.L.; Wang, X.J.; Kang, X.W.; Chen, S.W. Heterostructured Intermetallic CuSn Catalysts: High Performance Towards the Electrochemical Reduction of CO2 to Formate. J. Mater. Chem. A 2019, 7, 27514–27521. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.J.; Shao, Q.; Yin, R.G.; Guo, J.; Li, Y.Y.; Huang, X.Q. Phase and Structure Modulating of Bimetallic CuSn Nanowires Boosts Electrocatalytic Conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Peng, L.W.; Wang, Y.F.; Wang, Y.X.; Xu, N.N.; Lou, W.S.; Liu, P.X.; Cai, D.Q.; Huang, H.T.; Qiao, J.L. Separated Growth of Bi-Cu Bimetallic Electrocatalysts on Defective Copper Foam for Highly Converting CO2 to Formate with Alkaline Anion-Exchange Membrane Beyond KHCO3 Electrolyte. Appl. Catal. B-Environ. 2021, 288, 120003. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, L. Effects of the Catalyst Dynamic Changes and Influence of the Reaction Environment on the Performance of Electrochemical CO2 Reduction. Adv. Mater. 2022, 34, 2103900. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xie, H.; Lv, L.; Xu, J.; Li, X.; Wang, C.; Ma, A. SnBi Catalytic Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate. Catalysts 2025, 15, 698. https://doi.org/10.3390/catal15080698
Liu Z, Xie H, Lv L, Xu J, Li X, Wang C, Ma A. SnBi Catalytic Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate. Catalysts. 2025; 15(8):698. https://doi.org/10.3390/catal15080698
Chicago/Turabian StyleLiu, Zhuoqi, Hangxin Xie, Li Lv, Jialin Xu, Xinbo Li, Chunlai Wang, and Aijing Ma. 2025. "SnBi Catalytic Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate" Catalysts 15, no. 8: 698. https://doi.org/10.3390/catal15080698
APA StyleLiu, Z., Xie, H., Lv, L., Xu, J., Li, X., Wang, C., & Ma, A. (2025). SnBi Catalytic Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate. Catalysts, 15(8), 698. https://doi.org/10.3390/catal15080698





