Abstract
As a persistent organic pollutant, methoxychlor has drawn considerable environmental attention. Photocatalysis, recognized for its environmentally friendly characteristics, has been widely utilized for the degradation of contaminants. In this study, the photocatalytic material BiVO4@diatomite was successfully synthesized via the liquid-phase precipitation method. The synthesized material was comprehensively characterized using X-ray diffraction (XRD), energy-dispersive spectroscopy (EDS), scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), UV-vis diffuse reflectance spectroscopy (DRS), and a Brunauer–Emmett–Teller (BET) analysis, providing robust evidence for the material’s stability and biocompatibility. The results confirmed the successful deposition of BiVO4 onto the diatomite surface. Furthermore, the effects of various parameters, including the initial methoxychlor concentration, pH, light exposure duration, and illumination intensity, on the photocatalytic degradation efficiency of methoxychlor by BiVO4@diatomite were systematically investigated to optimize degradation performance. The identification of optimal reaction conditions and the proposed degradation mechanism based on experimental findings will be valuable for guiding future studies and practical applications in environmental pollution control. The integration of BiVO4 with diatomite in this study yields a novel composite system with significantly enhanced photocatalytic degradation performance, offering fresh insights into the design of efficient, stable, and eco-friendly materials for pollutant removal.
1. Introduction
Over the past few decades, a wide range of organic compounds have been synthesized in large quantities for industrial, agricultural, and public health purposes [1]. However, some organics exhibit persistent ecotoxicity; one prominent example is methoxychlor [2,3], an organochlorine insecticide that has been used as a substitute for dichlorodiphenyltrichloroethane (DDT). Studies have demonstrated that methoxychlor poses potential risks to human health and exhibits high toxicity toward aquatic organisms, affecting the growth, development, and survival of fish, amphibians, and invertebrates. Methoxychlor can mimic the action of natural hormones, particularly estrogen, leading to disruptions in the reproductive systems of animals. The use of methoxychlor may result in the accumulation of pollutants in water sources and environmental systems. It has been reported that the release of toxic substances has caused significant pollution to crops and marine organisms [2,4], thereby posing substantial threats to human health and exerting profound impacts on individual well-being and security. Developing an efficient cleaning process to degrade methoxychlor in the environment is therefore of great significance.
Photocatalysis has been recognized as a critical approach for degrading organic pollutants such as methoxychlor [5,6], offering substantial benefits to both ecological systems and human well-being. Photocatalytic reactions involve photochemical processes facilitated by catalysts, representing the fundamental interaction between light and material entities [7]. Based on the properties of semiconductor materials that can be excited by light to generate strong oxidative holes and reductive photogenerated electrons, photocatalysts can decompose water into oxygen and hydrogen under visible light and oxidize or degrade environmental pollutants, mineralizing organic contaminants into non-toxic and harmless substances. This catalytic redox technology is characterized by its environmentally friendly nature and low energy consumption [8].
In recent years, bismuth-containing compound photocatalysts with visible-light activity have garnered significant attention. The filled 6 s orbital of Bi3+ overlaps partially with the O 2p orbital, thereby reducing the band gap. Consequently, Bi(III) compounds exhibit excellent photo-response properties under visible-light irradiation [9]. BiVO4 represents a novel type of visible-light-responsive catalyst [7,10,11,12], featuring ferroelectric properties and ion conductivity. Moreover, it can serve as both a cathode material and a non-toxic yellow pigment. Its wavelength response range extends beyond 500 nm, attracting extensive interest due to its high utilization of visible light and catalytic decomposition capabilities for water and organic matter [13]. The photocatalytic performance of BiVO4 is closely associated with its preparation method, crystal structure, particle size, and microscopic morphology [12]. BiVO4 exhibits three crystal structures: monoclinic scheelite structure (ms-BiVO4), tetragonal scheelite structure (ts-BiVO4), and tetragonal zircon structure (tz-BiVO4). Among these crystal forms, ms-BiVO4 exhibits a relatively narrow band gap of approximately 2.4 eV, which enables it to effectively absorb photons within the visible-light spectrum. This characteristic provides ms-BiVO4 with a significant advantage over traditional semiconductors in terms of visible-light utilization [14]. The photocatalytic mechanism of BiVO4 relies on its ability to generate electron–hole pairs upon light irradiation. When exposed to visible light, electrons in the valence band are excited to the conduction band, leaving holes in the valence band. These holes can directly oxidize pollutants, while the electrons can react with oxygen molecules to produce reactive oxygen species, further facilitating the degradation process.
Moreover, significant efforts have been devoted to enhancing the photocatalytic efficiency of BiVO4. Key strategies include doping with various elements, forming heterojunctions by coupling with other semiconductors, and modifying their surface morphology. The formation of heterojunctions through coupling with other semiconductors facilitates improved charge separation and transfer efficiency. Diatomite, primarily composed of biologically derived SiO2, is abundant and cost-effective [15,16]. As a natural material, diatomite exhibits a meso-/macro-porous structure, strong acid resistance, a large specific surface area, excellent physical/chemical stability, low thermal conductivity, and numerous active groups with a high adsorption capacity. Among various support materials, diatomite serves as an effective carrier for dispersing bismuth vanadate. It has demonstrated outstanding performance in certain reactions, enhancing both charge and proton transfer efficiency during photocatalytic processes [16,17].
In this study, a method for preparing BiVO4@diatomite powder via the liquid-phase precipitation approach was designed and implemented. Bi(NO3)3·5H2O and NH4VO3 were employed as the starting materials for the reaction. A comprehensive series of tests were conducted to characterize the phase composition and morphology of the synthesized material. Furthermore, the visible-light-driven degradation of methoxychlor was investigated under varying conditions, including different concentrations, pH levels, and light intensities. The photocatalytic activity and recyclability of the catalyst were also evaluated systematically. Finally, the identification of degradation products was achieved using gas chromatography–mass spectrometry (GC-MS) and other advanced characterization techniques. Additionally, the underlying degradation reaction mechanism was deduced based on the experimental results. Diatomite is a naturally occurring, porous material that is rich in silica, making it an excellent candidate for catalyst support. Its strong adsorption capacity, high surface area, eco-friendly characteristics, and cost-effectiveness have attracted significant attention from researchers. By loading BiVO4 onto diatomite, improved light absorption, enhanced particle dispersion, and more efficient charge separation can be achieved, thereby promoting superior photocatalytic performance [18,19].
2. Results and Discussion
2.1. Phase and Morphology Analysis of BiVO4@Diatomite
2.1.1. XRD Analysis
The crystal structure of BiVO4@diatomite was investigated, and its XRD pattern is presented in Figure 1. According to the standard card (JCPDS 14-0688), as shown in Figure 1, the peaks at 2θ = 18.76°, 28.97°, 30.60°, 34.54°, 35.31°, 40.10°, 42.50°, 46.09°, 46.78°, 47.37°, 49.94°, 50.26°, 53.23°, 58.32°, and 59.68° correspond to the characteristic diffraction peaks of monoclinic BiVO4, attributed to the (110), (121), (040), (200), (002), (141), (211), (150), (132), (240), (042), (202), (222), (161), (251), and (321) crystal planes, respectively. These results confirm that BiVO4 was successfully synthesized without being coated by diatomite and that its crystalline transformation occurred. The XRD pattern of BiVO4@diatomite in Figure 1 shows a trend similar to that of natural diatomite. Based on the standard card (JCPDS 85-0621), the peaks at 2θ = 17.50°, 21.48°, 27.83°, and 30.56° are identified as the characteristic diffraction peaks of SiO2, corresponding to the (110), (111), (012), and (211) crystal planes, respectively. The distinct and intense diffraction peaks with minimal impurity peaks indicate that BiVO4@diatomite possesses good crystallinity and high purity. The XRD pattern of diatomite exhibits a broad and diffuse diffraction peak, indicative of an amorphous structure [20].
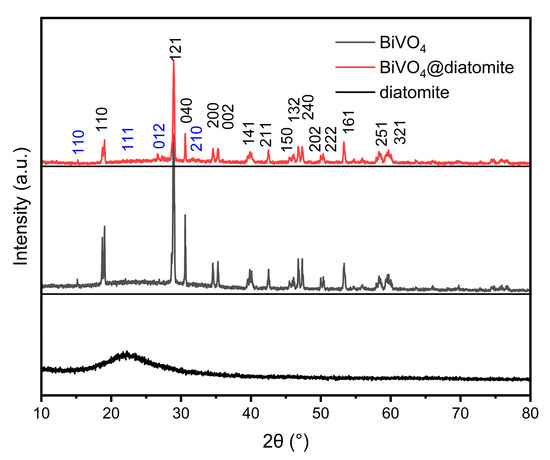
Figure 1.
XRD pattern of BiVO4 and BiVO4@diatomite showing the phase structure and crystallinity of the synthesized materials.
2.1.2. SEM and EDS Analysis
The microstructure of the materials was investigated using scanning electron microscopy (SEM) analysis as shown in (Figure 2a–d). Figure 2a,b presents the SEM images of BiVO4 synthesized via the liquid-phase precipitation method. The particle size distribution of BiVO4 is relatively uniform, exhibiting excellent dispersibility. As shown in Figure 2b, the product consists of mesoporous spherical particles with an average particle size ranging from 1 to 2 μm and a pore diameter of approximately 73.23 nm. Figure 2c displays the SEM image of acid-washed diatomite, revealing a smooth and orderly surface. The primary structure is disc-shaped with a diameter of approximately 21 μm, and the surface exhibits a uniform pore structure with a pore diameter of about 0.5 μm. The SEM image of BiVO4@diatomite shown in Figure 2d indicates that the surface of diatomite is coated with a layer of viscous material while retaining its original pore structure, suggesting that the coating process does not alter the fundamental structure of diatomite. By comparing Figure 2c,d, it can be concluded that BiVO4 has been successfully loaded onto the surface of diatomite.
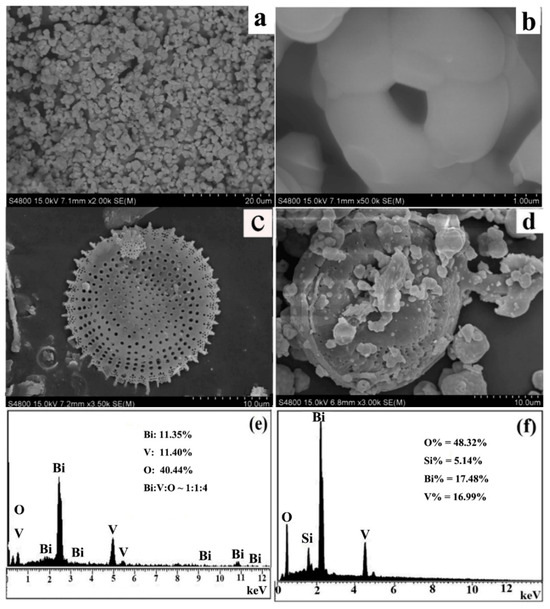
Figure 2.
SEM image of (a,b) BiVO4; (c) acid-washed diatomite; (d) BiVO4@diatomite and EDS analysis of (e) BiVO4; and (f) BiVO4@diatomite.
Figure 2e,f depicts the Energy Dispersive X-ray Spectroscopy (EDS) spectra of BiVO4 and BiVO4@diatomite. As illustrated in Figure 2e, the atomic ratio of Bi, V, and O is approximately 1:1:4, which aligns well with the XRD results. In the EDS spectrum of BiVO4@diatomite (Figure 2f), the atomic ratio of Bi, V, and Si is approximately 1:1:0.30, consistent with the input ratio of BiVO4 and diatomite. Although diatomite is primarily composed of SiO2, the relatively lower atomic ratio of Si (0.30) observed in the EDS spectrum of BiVO4@diatomite can be attributed to the surface coverage of the BiVO4 particles on the diatomite framework. This surface shielding effect may partially suppress Si signals during EDS analysis, particularly in regions with dense BiVO4 deposition. Furthermore, EDS is a surface-sensitive analytical technique and may not accurately represent the bulk composition. To verify the elemental distribution and assess sample homogeneity, EDS mappings were conducted across multiple regions, revealing a consistent elemental presence throughout the sample.
2.1.3. Infrared Spectrum and UV-Vis Diffuse Reflectance (DRS) Analysis and Specific Surface Area (BET) Analysis of Catalysts
Figure 3a presents the infrared spectra of diatomite, BiVO4, and BiVO4@diatomite. For diatomite, the IR peaks observed at 3461.6 cm−1 can be attributed to the OH stretching vibration of the hydroxyl groups present in diatomite. The broad band at approximately 1639.4 cm−1 corresponds to the OH vibration mode of physically adsorbed H2O and the bending vibration of water molecules retained within the silica matrix of diatomite. The peaks at approximately 479.6 cm−1 and 1112.5 cm−1 may be assigned to the asymmetric stretching modes of Si–O–Si bonds, while the peak at 796.4 cm−1 may correspond to the stretching vibration of Al–O–Si bonds [20].
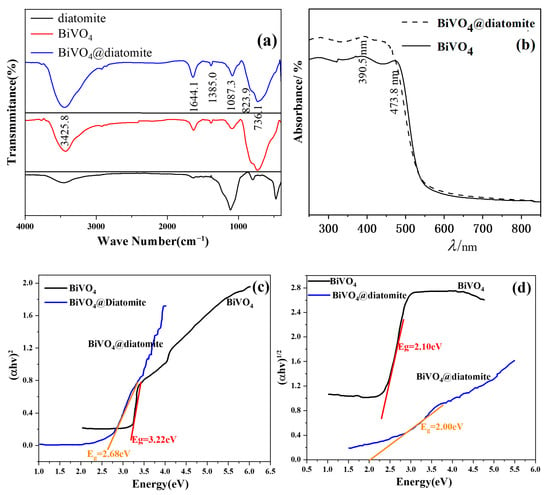
Figure 3.
Infrared spectra (a) and UV-Vis diffuse reflectance (DRS) spectrum (b) of BiVO4 and BiVO4@diatomite; (c) fit curves of (αhν)2~hν plots; and (d) fit curves of (αhν)1/2~hν plots.
After incorporation of BiVO4, the symmetric stretching vibration absorption peak of V-O and the deformation bending vibration absorption peak of VO43− are located at 823.9 cm−1 and 736.1 cm−1, respectively [21]. The peak at 1087.3 cm−1 corresponds to the stretching vibration absorption of the V=O double bond in BiVO4 [22], which overlaps with the antisymmetric stretching vibration absorption peak of the Si-O-Si bond [23]. Consequently, this absorption peak is stronger than that of the uncoated state. The absorption peaks at 1385 cm−1 and 1633 cm−1 represent the symmetric and antisymmetric stretching vibrations of the nitro group, indicating that nitrate ions were not completely removed during the preparation process. The absorption peak at 3425.8 cm−1 arises from the stretching vibration of hydroxyl groups on the surfaces of BiVO4 and SiO2, confirming the successful loading of BiVO4 onto diatomite [24].
Figure 3b illustrates the ultraviolet-visible diffuse reflectance (DRS) spectra of BiVO4@diatomite and BiVO4. Pure BiVO4 exhibits an absorption edge at approximately 473.8 nm, consistent with previous reports [25]. Diatomite shows a steep absorption band at 390.5 nm [26]. After loading BiVO4 onto diatomite, the absorption edge of the composite red-shifts to approximately 455.6 nm as shown in Figure 3. This results in significantly enhanced light absorption intensity for BiVO4@diatomite compared to BiVO4 in both the ultraviolet and visible light regions, demonstrating that diatomite as a carrier improves the light absorption performance and photocatalytic activity of BiVO4.
The band gap between the valence band (VB) and conduction band (CB) of the material can be determined using the Tauc equation: (αhν)n = A (hν − Eg) [27], where A denotes the proportionality constant, α represents the light absorption coefficient, hν signifies the photon energy, Eg corresponds to the band gap width (i.e., the energy value of the band gap) of the semiconductor, and n equals 2 for direct bandgap semiconductors and 1/2 for indirect bandgap semiconductors. Consequently, by extrapolating the (αhν)2 versus hν curve to the x-axis, the direct bandgap energy (Eg) of the material can be estimated. Similarly, by extrapolating the (αhν)1/2 versus hν curve to the x-axis, the indirect bandgap energy (Eg) can be determined.
As shown in Figure 3c, the direct bandgaps of pure BiVO4 and BiVO4@diatomite are calculated to be 3.22 eV and 2.68 eV, respectively. Meanwhile, as depicted in Figure 3d, the indirect bandgaps of pure BiVO4 and BiVO4@diatomite are estimated as 2.10 eV and 2.00 eV, respectively. These results indicate that BiVO4@diatomite demonstrates a stronger response to visible light compared to pure BiVO4.
The reasons can be summarized as follows: diatomite possesses a large specific surface area, high chemical stability, and excellent adsorption capacity, making it an ideal candidate for use as a catalyst carrier. The reduction in Eg is attributed to the formation of heterojunction structures within the composite, which enhances its response to visible light [28]. The presence of heterojunction structures in BiVO4@diatomite results in efficient visible-light-driven degradation performance. Diatomite, as a photocatalyst carrier, can effectively reduce heterojunction agglomeration during the preparation process, ensuring a larger effective surface area and improving both adsorption performance and photocatalytic activity [29].
Moreover, diatomite can suppress electron–hole recombination, thereby enhancing the photocatalytic degradation performance of the material [18]. The coexistence of BiVO4 and diatomite generates synergistic effects: diatomite prevents the aggregation of BiVO4 nanoparticles, ensures the exposure of more active sites, and consequently improves the degradation efficiency of methoxychlor.
Table 1 presents the specific surface area and pore volume data of the catalyst before and after diatomite loading, as measured by a physical adsorption instrument. As shown in Table 1, the specific surface areas of BiVO4 and BiVO4@diatomite are 1.59 m2/g and 4.89 m2/g, respectively, while the corresponding pore volumes are 0.005646 cm3/g and 0.01735 cm3/g. The presence of diatomite significantly enhances the specific surface area and pore volume of BiVO4@diatomite. This increase in specific surface area and pore volume leads to a greater number of surface active sites available for reaction participation [30], thereby providing a mechanistic explanation for the observed enhancement in photodegradation efficiency. For comparison purpose, the surface area and pore volume of diatomite adopted from our reported literature data [31].

Table 1.
Specific surface area and pore volume of catalysts before and after BiVO4 loading on diatomite.
2.2. The Degradation of Methoxychlor on BiVO4@Diatomite
2.2.1. Effect of Carrier Balance on Degradation Efficiency of Methoxychlor
Figure 4a,b compare the degradation efficiencies of methoxychlor by BiVO4@diatomite and BiVO4 under the conditions of Cmethoxychlor = 40 mg/L, pH = 8, illumination intensity: 32.2 × 103 μW/cm2, and dosage of catalyst: 5.28 g/L. With the extension of the illumination time, the degradation efficiency of methoxychlor exhibits a gradually increasing trend. After 6 h of illumination, the removal efficiency of methoxychlor by BiVO4@diatomite reached 95.35%, which is higher than that of BiVO4 (85.82%) under the same conditions. This indicates that BiVO4@diatomite demonstrates superior photocatalytic degradation performance. This enhanced performance may be attributed to the ideal pore channels provided by diatomite, which facilitate the vertical growth of BiVO4 nanosheets on diatomite disks. Additionally, the chemical combination between BiVO4 and diatomite improves the thermal stability of BiVO4, hinders its aggregation, and increases the specific surface area from 1.5 m2/g to 4.89 m2/g (Table 1). These factors enhance the contact area between BiVO4 and methoxychlor, thereby promoting photocatalytic degradation. Furthermore, methoxychlor can be continuously transferred to the surface of BiVO4 through adsorption by diatomite, ensuring sustained photocatalytic degradation until the complete removal of methoxychlor is achieved [32].
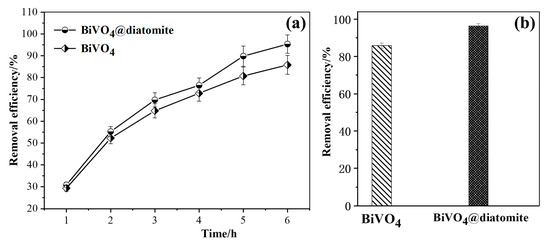
Figure 4.
(a) The removal effects of different carriers over time; (b) Maximum removal efficiency of different carriers. Degradation condition: Cmethoxychlor = 40 mg/L, pH = 8, illumination intensity: 32.2 × 103 μW/cm2, and dosage of catalyst: 5.28 g/L.
On the other hand, the high photocatalytic performance of BiVO4@diatomite can also be ascribed to the heterojunction structures within the composite and the synergistic effect between BiVO4 and diatomite. Diatomite exhibits an intense absorption band with a steep edge near 390 nm, causing the absorption edge of BiVO4@diatomite to shift toward a longer wavelength region of the visible light photo-response [33]. Moreover, BiVO4 is homogeneously dispersed on the surface of diatomite, and is rich in Si–OH groups that act as Lewis bases to trap photogenerated holes, thereby contributing to the formation of hydroxyl radicals (·OH). These radicals effectively oxidize organic pollutants, significantly enhancing the photocatalytic activity of the catalyst.
2.2.2. Effect of Initial Concentration of Methoxychlor on the Degradation of Methoxychlor
As shown in Figure 5a, with the extension of the illumination time, the degradation efficiency of methoxychlor at different concentrations gradually increased. Notably, when the initial concentration of methoxychlor was 40 mg/L, its degradation efficiency reached the highest value under various illumination durations, achieving up to 94.31% efficiency after 6 h of experimental light exposure. Figure 5b illustrates that, after 6 h of photocatalytic degradation, the degradation efficiency of methoxychlor varies depending on its initial concentration. Specifically, when the initial concentration of methoxychlor was 40 mg/L, the degradation efficiency reached its maximum value of 94.31%. In contrast, when the initial concentration was 50 mg/L, the degradation efficiency decreased significantly. The photocatalytic activity of BiVO4@diatomite under different initial concentrations of methoxychlor has the following order: 40 mg/L > 20 mg/L > 50 mg/L > 10 mg/L > 30 mg/L.
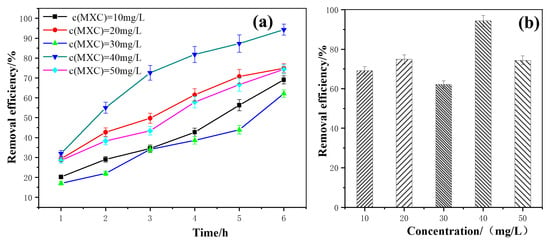
Figure 5.
(a) The removal effects of different initial concentration over time; (b) Maximum removal efficiency of different initial concentration. Degradation condition: pH = 8, illumination intensity: 32.2 × 103 μW/cm2, and dosage of catalyst: 5.28 g/L.
The aforementioned phenomenon can be attributed to the fact that, at low concentrations of methoxychlor, the catalytic activity of BiVO4@diatomite is primarily influenced by the adsorption capacity of diatomite on the catalyst surface. Appropriately increasing the concentration of methoxychlor enhances the adsorption capacity of pollutants per unit area on the catalyst surface, thereby accelerating the reaction rate and improving the degradation efficiency. However, when the concentration of methoxychlor exceeds 40 mg/L, the light penetration ability of the solution weakens, leading to a reduction in the number of photons participating in the photocatalytic reaction and a consequent decrease in photocatalytic activity [34]. Additionally, at higher concentrations, active radicals are limited to reacting with a restricted number of substrates, which prevents timely decomposition. Subsequently, intermediates may become adsorbed on the catalyst surface and re-react to reform the initial structure of methoxychlor, ultimately causing a decline in the degradation efficiency.
2.2.3. Effect of pH on the Degradation of Methoxychlor
As shown in Figure 6a, under different pH conditions, with the extension of the illumination time, the degradation efficiency of methoxychlor exhibited a gradual increasing trend. Notably, at a solution pH of 8, the degradation efficiency was the highest at each time point, reaching a maximum value of 94.31% after 6 h of light exposure. This suggests that the degradation efficiency of methoxychlor initially increases with rising pH, reaching its peak at 94.31% under pH 8 (Figure 6b). However, when the pH of the solution was increased to 9 or 10, the degradation rate decreased significantly. Therefore, the photocatalytic activity of BiVO4@diatomite under different pH conditions has the following order: pH = 8 > pH = 7 > pH = 6 > pH = 9 > pH = 10. These results demonstrate that the degradation of methoxychlor by BiVO4@diatomite is highly sensitive to pH.
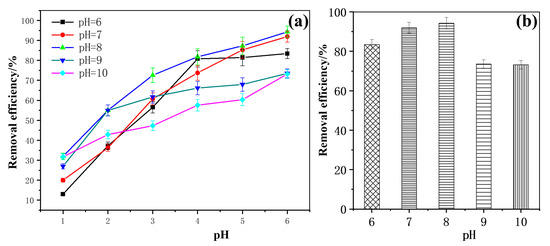
Figure 6.
(a) The removal effects of different pH over time; (b) Maximum removal efficiency of different pH. Degradation condition: Cmethoxychlor = 40 mg/L, illumination intensity: 32.2 × 103 μW/cm2, and dosage of catalyst: 5.28 g/L.
Mechanism analysis of the effect of pH on the degradation efficiency of methoxychlor: Under weakly acidic (pH = 6) and neutral (pH = 7) conditions, adjusting the pH by adding dilute HNO3 introduces a small amount of H+, which alters the form of vanadate. This change affects the stability of bismuth vanadate, inhibits the photocatalytic reaction, and consequently reduces the degradation efficiency. At pH = 9 and pH = 10, the removal efficiency decreased. There are two possible reasons for this decline. First, when the pH of the medium is raised, the addition of dilute NaOH increases the concentration of OH- in the reaction medium, hindering the degradation of methoxychlor in the reaction solution. Second, for the adsorption on the surface of the catalyst BiVO4@diatomite under alkaline conditions, Bi3+ ions are prone to hydrolysis and polymerization, forming highly polymeric species (nBi3+ + 2nOH− → [BinOn]n+ + nH2O). This process affects the activity of the catalyst [35] and leads to a decrease in the removal efficiency of methoxychlor degradation at pH = 9 and pH = 10.
2.2.4. Effect of Illumination Intensity on the Degradation of Methoxychlor
As shown in Figure 7a,b, with the increase in illumination intensity, the degradation efficiency of methoxychlor also increased correspondingly. The illumination intensities of the quartz–tungsten–halogen (QTH) light source at powers of 100 W, 300 W, and 500 W were measured using the FZ-A radiometer and found to be 1.64 × 103 μW/cm2, 24.3 × 103 μW/cm2, and 32.2 × 103 μW/cm2, respectively. When the power of the QTH lamp was increased from 100 W to 300 W, the degradation efficiency of methoxychlor increased by 10.25%. Furthermore, when the power was increased from 300 W to 500 W, the degradation efficiency increased by 22.95%. These results indicate that the photocatalytic activity of BiVO4@diatomite under different illumination intensities has the following order: 500 W > 300 W > 100 W.
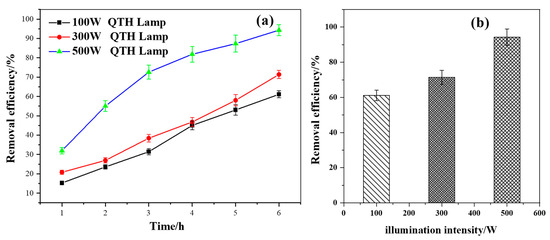
Figure 7.
(a) The removal effects of different carriers over time; (b) Maximum removal efficiency of different carriers. Effect of illumination intensity on the degradation and removal efficiency of methoxychlor.
It can be inferred that the enhancement in degradation efficiency can be attributed to the fact that, as the photocatalytic reaction progresses, a higher-intensity light source generates more photons, thereby exciting more valence-band electrons and increasing the generation rate of photogenerated electron–hole pairs. This enhanced generation rate [36] facilitates the rapid progression of the photocatalytic oxidation reaction.
2.2.5. Reuse Activity of BiVO4@Diatomite Photocatalyst
Figure 8a illustrates the repeated utilization performance of BiVO4@diatomite and BiVO4 under experimental conditions. Under optimal conditions, the degradation efficiency of methoxychlor by BiVO4@diatomite reached a higher value of 94.31%, compared to 85.82% for BiVO4 alone. When the regenerated BiVO4@diatomite and BiVO4 photocatalysts were used for the second cycle, their respective degradation efficiencies decreased to 80.24% and 74.02%. As the number of repeated cycles increased, the degradation efficiency of methoxychlor gradually declined due to the gradual deactivation of active sites on the catalyst surface during repeated use. After four cycles of photodegradation, the degradation efficiency of methoxychlor by BiVO4@diatomite remained at 40.24%, indicating that the BiVO4@diatomite system exhibits good reusability.
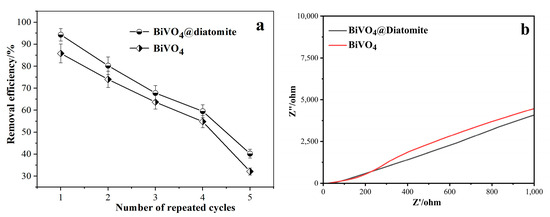
Figure 8.
Reuse activity of BiVO4@diatomite. (a) Electrochemical impedance spectroscopy of BiVO4 and BiVO4@diatomite. (b) Degradation condition: CMETHOXYCHLOR = 40 mg/L, pH = 8, illumination intensity: 32.2 × 103 μW/cm2, and dosage of catalyst: 5.28 g/L.
To further evaluate the electronic transport properties of the material, electrochemical impedance spectroscopy (EIS) analysis was conducted. As shown in Figure 8b, the impedance spectrum demonstrated that BiVO4@Diatomite exhibited slightly lower impedance than pure BiVO4, suggesting enhanced electronic conductivity. The improved charge transport behavior indicates a more efficient utilization of the applied current, which facilitates the effective separation and migration of photogenerated charge carriers.
2.2.6. Possible Mechanism of BiVO4@Diatomite on the Degradation of Methoxychlor
In this study, the degradation products of methoxychlor catalyzed by BiVO4@diatomite were identified using GC-MS analysis. The detected products include 1,1-dimethylcyclopentane (a), 2,2-dimethylpentane (b), cyclohexane (c), 2-methylpentane (d), 3-methylpentane (e), and 2-methyl-1-pentene (f). Owing to the broad distribution of m/z values among the degradation products, the mass spectra of each compound are presented separately, as illustrated in Figure 9.
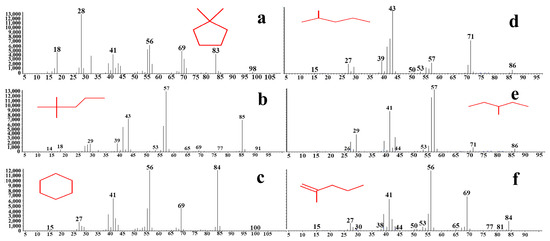
Figure 9.
Mass spectrum of methoxychlor degraded by BiVO4@Diatomite. (a) 1,1-dimethylcyclopentane; (b) 2,2-dimethylpentane; (c) cyclohexane; (d) 2-methylpentane; (e) 3-methylpentane; (f) 2-methyl-1-pentene.
As shown in Figure 9, a prominent peak at m/z = 43 corresponds to the fragment ion peak of 2-methylpentane (d). A significant peak at m/z = 57 represents the fragment ion peaks of 3-methylpentane (e) and 2, 2-dimethylpentane (b). Additionally, a strong peak at m/z = 56 is attributed to the fragment ion peak of 2-methyl-1-pentene (f). Furthermore, a pronounced peak at m/z = 84 corresponds to the molecular ion peak of cyclohexane (c), while a strong peak at m/z = 28 is associated with the fragment ion peak of 1, 1-dimethylcyclopentane (a), an alkane.
As reported by X. Xu, a trapping experiment was carried out using electron spin resonance (ESR) to investigate the degradation mechanisms of free radicals in BiVO4 photocatalysis under visible-light irradiation. The study confirmed that ·OH and h+ are the dominant reactive species, offering detailed insights into the generation pathways of ·OH during the photocatalytic process of BiVO4 [37]. Under visible-light irradiation, electrons in the valence band of BiVO4 can absorb photon energy and transition to the conduction band (e−), leaving behind holes (h+) in the valence band. The h+ can react with H2O adsorbed on the surface of BiVO4 to generate hydroxyl radicals (·OH) (Figure 10), while the e− can react with O2 to form superoxide radicals (·O2−). These reactive oxygen species subsequently attack methoxychlor molecules present in the surrounding environment. The plausible degradation pathway for methoxychlor catalyzed by BiVO4@diatomite proceeds as follows: Methoxychlor first undergoes chlorine removal under the action of ·OH hydroxyl radicals, followed by dehydrogenation mediated by BiVO4@diatomite. Olefin compounds are generated and subsequently oxidized under the action of BiVO4@diatomite to produce p-methoxybenzaldehyde.
The reaction process is as follows:
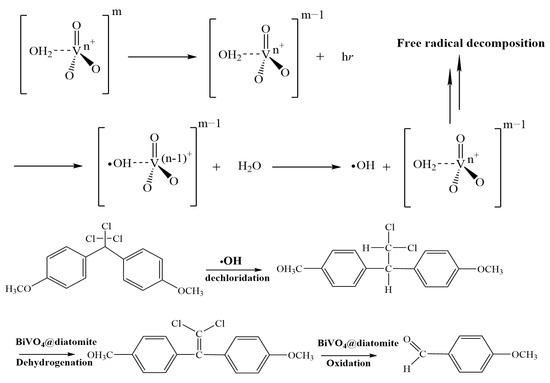

Figure 10.
Possible pathway for BiVO4@diatomite in the degradation of MXC.
The potential pathways for the further catalytic degradation of the intermediate product, p-methoxybenzaldehyde, are illustrated as follows (Figure 11):
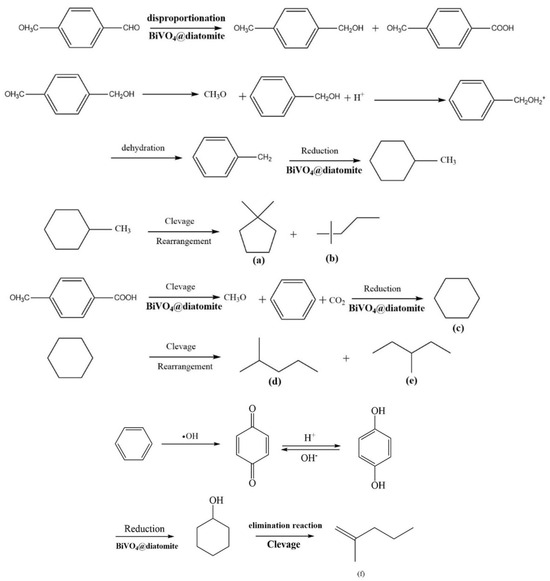
Figure 11.
Possible pathway for BiVO4@diatomite in the degradation of intermediate product p-methoxybenzaldehyde.
P-methoxybenzaldehyde undergoes disproportionation into methoxybenzyl alcohol and p-methoxybenzoic acid under redox conditions mediated by BiVO4@diatomite. Methoxybenzyl alcohol is subsequently demethoxylated, protonated, dehydrated to form methylbenzene, and further reduced to methylcyclohexane via the action of BiVO4@diatomite. Methylcyclohexane is then cleaved and rearranged into 1, 1-dimethylcyclopentane (a) and 2, 2-dimethylpentane (b). Simultaneously, p-methoxybenzoic acid undergoes demethoxylation and decarboxylation to produce a benzene ring, which is subsequently reduced to cyclohexane (c). Cyclohexane further splits into 2-methylpentane (d) and 3-methylpentane (e). The formation of 2-methyl-1-pentene (f) involves a more complex process. Following the demethoxylation and decarboxylation of p-methoxybenzoic acid, the benzene ring is oxidized to benzoquinone by hydroxyl radicals. Benzoquinone undergoes proton exchange to form p-hydroxyphenol, which is subsequently reduced to hydroxycyclohexane [38], Hydroxycyclohexane is then eliminated and cleaved to yield 2-methyl-1-pentene (f).
3. Experimental Section
3.1. Experimental Reagents
All reagents herein were of analytical grade and were not further purified. Bismuth nitrate (Bi(NO3)3·5H2O), ammonium vanadate (NH4VO3), sodium dioctylsulfosuccinate (AOT), HNO3, and HCl were purchased from Sinopharm Reagent Co., Ltd. (Shanghai, China), NaOH and n-hexane were purchased from Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China), and diatomite was obtained from Huali Diatomite Products Co., Ltd. (Shanghai, China). All chemicals were used without further purification and their purity was above 98%.
3.2. Preparation of BiVO4
A total of 4.8510 g of bismuth nitrate pentahydrate (Bi(NO3)3•5H2O, 10.0 mmol) was ultrasonically dissolved in 10 mL of a 2 mol/L nitric acid (HNO3) solution to prepare a 1 mol/L Bi(NO3)3 solution. Subsequently, 1.1698 g of ammonium vanadate (NH4VO3, 10.0 mmol) was dissolved in 10 mL of a 6 mol/L sodium hydroxide (NaOH) solution to prepare a 1 mol/L NH4VO3 solution. The NH4VO3 solution was then added dropwise to the Bi(NO3)3 solution using a peristaltic pump (Baoding Lange Constant Flow Pump Co., Ltd., Baoding, China). The pH of the reaction mixture was adjusted to 6 with 1 mol/L NaOH, followed by stirring in an oil bath at 80 °C for 30 min. The reaction was continued for 1 h and subsequently aged for 24 h. All reactions were carried out under atmospheric pressure, and stirring speed was maintained at 400 rpm throughout. Suction filtration was performed, and the precipitate was thoroughly washed with deionized water and absolute ethanol. Vacuum drying at 80 °C yielded a loose bright yellow powder, which served as the precursor for the catalyst. The precursor was transferred to a muffle furnace and calcined at 600 °C for 1 h at a heating rate of 2 °C/min. After cooling to room temperature, the sample was ground evenly in a mortar to obtain the final catalyst, BiVO4.
3.3. Preparation of BiVO4@Diatomite
Firstly, 100 g of natural diatomite was added to a 500 mL three-necked flask for pickling. Subsequently, 200 mL of deionized water and 100 mL of 37% concentrated hydrochloric acid (HCl) were sequentially introduced into the flask. The resulting solution was then stirred magnetically for 10 h. Following the completion of the pickling process, the solution underwent suction filtration, followed by thorough washing with deionized water and absolute ethanol. Finally, the sample was vacuum-dried at 100 °C to obtain acid-washed diatomite. The diatomite was then stored in an air-tight container prior to further use.
In the subsequent step, 0.10 g of acid-washed diatomite was dispersed in a 1 mol/L bismuth nitrate (Bi(NO3)3) solution. A 1 mol/L ammonium vanadate (NH4VO3) solution was subsequently added dropwise to the mixture of diatomite and Bi(NO3)3 via a peristaltic pump while maintaining the pH value at 6. The subsequent steps were identical to those described in Section 2.2 (Preparation of BiVO4). The final product obtained was BiVO4@diatomite.
3.4. Material Characterization
All instruments were calibrated before use to ensure measurement accuracy. Powder X-ray diffraction (XRD) patterns were recorded at room temperature on a D/max 2550 VB/PC X-ray diffract meter (CuKα radiation, λ = 0.15406 nm). Micro-region components on the catalyst surface were analyzed using JSM-6360LV EDS (JEOL, Tokyo, Japan). Surface microstructures were observed with a JSM-5900LV SEM (JEOL, Japan). Changes in functional groups were determined by an IRPrestige-21 FTIR spectrometer (Shimadzu, Tokyo, Japan). UV-Vis diffuse reflectance spectra were recorded using a UV2600 spectrophotometer (Shimadzu, Japan). Adsorption performance and pore structure were measured with an ASAP2405N instrument (Micromeritics, Norcross, GA, USA), and specific surface areas were calculated using the BET method. The degradation products were determined by GC-MS.
3.5. Photocatalytic Experiment
Each photocatalytic experiment was performed in triplicate to ensure reproducibility, and the average values were reported. The temperature during photocatalytic reactions was maintained at 25 ± 2 °C. A specific amount of methoxychlor was added to n-hexane and dissolved via ultrasonication. Subsequently, 0.20 g of AOT and a suitable volume of deionized water were introduced to prepare 100 mL of methoxychlor solution with an initial concentration ranging from 10 to 50 mg/L. The prepared solution was transferred to a 250 mL three-necked quartz flask, followed by the addition of the BiVO4@diatomite photocatalyst. The mixture was magnetically stirred in a dark environment for 1 h to ensure uniform dispersion of the catalyst and achieve adsorption equilibrium of methoxychlor on the catalyst surface prior to light exposure. Under visible light irradiation (32.2 × 103 μW/cm2, 500 W quartz–tungsten–halogen (QTH) lamp), the reflux reaction was magnetically stirred.
Samples (5 mL) were collected at 0, 1, 2, 3, 4, 5, and 6 h of illumination. The samples were cooled to room temperature and extracted twice with n-hexane (15 mL for the first extraction and 10 mL for the second). The supernatants from both extractions were combined and transferred to a 50 mL spinner bottle, then concentrated to approximately 1 mL using a rotary evaporator set at 35 °C. The extract was subsequently transferred to a 5 mL volumetric flask, and the one-necked flask was rinsed three times with n-hexane to combine all extracts. The final volume was adjusted to the designated level, and the degradation rate was determined using a FULI 9790 gas chromatograph (Osaka, Japan). The standard curve was constructed following the same procedure.
During the photocatalytic degradation of methoxychlor by BiVO4@diatomite powder, the adsorption behavior of methoxychlor onto BiVO4 must be considered. To evaluate this, adsorption experiments were conducted under identical experimental conditions except for the absence of light. These experiments were performed at room temperature for 1 h in the dark to reach equilibrium, allowing the deduction of adsorption effects and other influencing factors. For blank carrier determination, the operating conditions used for standard solution measurements were applied to analyze the samples, with each sample measured in triplicate. The concentration of methoxychlor after adsorption (Cs) and after photocatalytic degradation (C1) were measured relative to the initial concentration (C0, mg/L). The removal efficiency (α) and adsorption efficiency (β) were calculated as follows:
Removal efficiency/% (α) = (1 − C1/C0) × 100%
Adsorption efficiency/% (β) = (1 − Cs/C0) × 100%
The degradation efficiency of methoxychlor was calculated as the difference between the overall removal efficiency and the adsorption efficiency.
An adsorption experiment was conducted as follows: the prepared solution was transferred into a 250 mL three-necked quartz flask, followed by the addition of 0.5 g of BiVO4@diatomite photocatalyst. The mixture was magnetically stirred in the dark for 1 h to ensure uniform dispersion of the catalyst and to establish adsorption equilibrium of methoxychlor on the catalyst surface prior to light exposure. Conducting the experiment in the dark prevents photocatalytic degradation and allows for the accurate evaluation of the catalyst’s adsorption capacity during methoxychlor degradation [3].
Condition Optimization Experiment (Photo-Fenton Reaction)
To evaluate the photo-Fenton degradation efficiency of BiVO4@diatomite under varying operational conditions, 100 mL of methoxychlor solution with an initial concentration ranging from 10 to 50 mg/L was prepared. Methoxychlor was dissolved in n-hexane through ultrasonication, followed by the addition of 0.20 g of AOT and 100 mL of deionized water to form a stable emulsion. This emulsion was then transferred into a 250 mL three-necked quartz flask, where 0.5 g of BiVO4@diatomite photocatalyst was introduced. The suspension was magnetically stirred in the dark for 1 h to achieve adsorption equilibrium. Subsequently, the photo-Fenton reaction was initiated under visible light irradiation (32.2 × 103 μW/cm2) using a 500 W quartz–tungsten–halogen (QTH) lamp, with continuous magnetic stirring maintained at 400 rpm. Aliquots were collected at 60 min intervals for analysis to assess the degradation performance. All experiments were performed in triplicate, and average values were reported to ensure data accuracy and reproducibility.
4. Conclusions
The monoclinic scheelite BiVO4 was successfully immobilized on diatomite through the liquid-phase precipitation method for the photocatalytic degradation of methoxychlor. The characterization results confirmed the successful deposition of BiVO4 onto the diatomite surface. The photocatalytic activity assessments revealed that the optimal reaction conditions are as follows: an initial methoxychlor concentration of 40 mg/L, a solution pH of 8, and a light intensity of 32.2 × 103 μW/cm2, under which the maximum degradation efficiency reaches 94.31%, indicating high catalytic performance. Diatomite, with its high specific surface area and porosity, provides excellent adsorption capabilities and reaction sites. BiVO4, as a well-known photocatalyst with strong redox properties, facilitates light-driven electron transfer processes, making it suitable for driving chemical reactions. Notably, after four consecutive cycles of methoxychlor degradation, the catalyst retains a degradation efficiency of 56.53%, demonstrating its remarkable stability and reusability. The underlying mechanism is attributed to the synergistic effects of diatomite’s high surface area and porosity, which offer optimal reaction sites, in conjunction with BiVO4′s redox properties that facilitate the formation of heterojunctions that are conducive to efficient light-driven chemical reactions. This material demonstrates considerable potential as a stable, efficient, and reusable photocatalytic redox catalyst for environmental remediation and restoration.
Author Contributions
N.I.: Investigation, Data curation, Writing—original draft. X.H.: Methodology, Data curation, Validation, Writing—review and editing. K.M.H. and I.B.: Writing—review and editing, Conceptualization. H.Y.: Methodology, Validation. Y.Y.: Supervision, Writing—review and editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (20577010, 20971043), and the Open Project Program of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, Jilin University.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We extend our heartfelt gratitude to Anbang Dai for his significant contributions to the conceptualization and validation of this work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nikolova, C.; Gutierrez, T. Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yu, L.; Li, J.; Zhu, W.; Li, Y.; Li, Y.; Liu, S. Unveiling the Spatial Patterns and Potential Sources of Black Carbon in Lake Sediments from Diverse Developing Areas in a Representative Mining and Metallurgy Region of Central China. J. Soils Sediments 2024, 24, 970–979. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Yuan, H.; Dai, A.; Ni, C. Photocatalytic Degradation of Methoxychlor by Diatomite@Bi2WO6 under Visible Irradiations. J. Mol. Struct. 2023, 1275, 135545. [Google Scholar] [CrossRef]
- Ullah, S.; Zuberi, A.; Alagawany, M.; Farag, M.R.; Dadar, M.; Karthik, K.; Tiwari, R.; Dhama, K.; Iqbal, H.M. Cypermethrin Induced Toxicities in Fish and Adverse Health Outcomes: Its Prevention and Control Measure Adaptation. J. Environ. Manag. 2018, 206, 863–871. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-industrialized Applications. A Comprehensive Approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Gutierrez-Mata, A.G.; Velazquez-Martínez, S.; Álvarez-Gallegos, A.; Ahmadi, M.; Hernández-Pérez, J.A.; Ghanbari, F.; Silva-Martínez, S. Recent Overview of Solar Photocatalysis and Solar Photo-Fenton Processes for Wastewater Treatment. Int. J. Photoenergy 2017, 2017, 8528063. [Google Scholar] [CrossRef]
- Liu, X.; Lou, Z. Integration of Multi-Strategy Modifications in an Au Cocatalyst-Loaded 2D/2D BiVO4/P-Doped g-C3N4 Z-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2025, 680, 161328. [Google Scholar] [CrossRef]
- Tian, H.; Wang, A.; Pan, H.; Zhang, H.; Yang, S. Advances and Perspectives on Photochemical/Electrochemical Synthesis of Nitrogen, Sulfur, and Phosphorus Heteroatom Containing Compounds from Biomass-Based Feedstocks. Ind. Crops Prod. 2024, 222, 119738. [Google Scholar] [CrossRef]
- Qian, X.; Yue, D.; Tian, Z.; Reng, M.; Zhu, Y.; Kan, M.; Zhang, T.; Zhao, Y. Carbon Quantum Dots Decorated Bi2WO6 Nanocomposite with Enhanced Photocatalytic Oxidation Activity for VOCs. Appl. Catal. B 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Sharma, N.; Pap, Z.; Garg, S.; Hernadi, K. Photocatalyst Composites from Bi-Based and Carbon Materials for Visible Light Photodegradation. In Green Photocatalytic Semiconductors: Recent Advances and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 145–178. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Štrbac, D.; Far, L.Đ.; Dramićanin, M.D. Improved Coloristic Properties and High NIR Reflectance of Environment-Friendly Yellow Pigments Based on Bismuth Vanadate. Ceram. Int. 2018, 44, 22731–22737. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Han, J.; Wang, S.; Zhang, Z.; Wei, Z.; Wu, Z.; Zhu, Y.; Guo, Q. An Investigation of Z-Scheme Homojunction BiVO4 Photocatalysts for Efficient Green Removal of Ammonia Nitrogen. J. Alloys Compd. 2024, 1008, 176640. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Fang, Y.; Li, R.; Huang, Y. Hydrothermal Synthesis of m-BiVO4/t-BiVO4 Heterostructure for Organic Pollutants Degradation: Insight into the Photocatalytic Mechanism of Exposed Facets from Crystalline Phase Controlling. J. Hazard. Mater. 2020, 399, 123159. [Google Scholar] [CrossRef] [PubMed]
- Daye, S.; Vrindaa, S.; Dae, H.; Giulia, G.; Kyoung-Shin, C. p-Type BiVO4 for Solar O2 Reduction to H2O2. J. Am. Chem. Soc. 2025, 147, 3261–3273. [Google Scholar] [CrossRef]
- Wang, S.; Feng, K.; Zhang, D.; Yang, D.; Xiao, M.; Zhang, C.; He, L.; Yan, B.; Ozin, G.A.; Sun, W. Stable Cu Catalysts Supported by Two-Dimensional SiO2 with Strong Metal-Support Interaction. Adv. Sci. 2022, 9, 2104972. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, X.; Zheng, S.; Yin, J.; Shen, X.; Zhang, T. Regulating Pore Structure of Diatomite with Alkali Dissolution and Its Influence on Humidity Control Performance. Ceram. Int. 2024, 50, 42322–42332. [Google Scholar] [CrossRef]
- Ezzatahmadi, N.; Bao, T.; Liu, H.; Millar, G.J.; Ayoko, G.A.; Zhu, J.; Zhu, R.; Liang, X.; He, H.; Xi, Y. Catalytic Degradation of Orange II in Aqueous Solution Using Diatomite-Supported Bimetallic Fe/Ni Nanoparticles. RSC Adv. 2018, 8, 7687–7696. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Q.; Xu, B.i.n.; Chen, S.; Ding, Y.; Jin, Z.; Guo, W.; Jia, X. Efficient degradation of imidacloprid in wastewater by a novel p-n heterogeneous Ag2O/BiVO4/diatomite composite under hydrogen peroxide. J. Ind. Eng. Chem. 2023, 123, 187–200. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, L.; Shen, M.; Guo, M.; Zhu, P.; Jin, W. Novel Preparation of Bi/BiVO4 Catalyst Supported by Alkali-Modified Diatomite and Its Visible Light-Driven Degradation Performance. Nano 2021, 16, 2150034. [Google Scholar] [CrossRef]
- Yusan, S.; Korzhynbayeva, K.; Aytas, S.; Tazhibayeva, S.; Musabekov, K. Preparation and Investigation of Structural Properties of Magnetic Diatomite Nanocomposites Formed with Different Iron Content. J. Alloy. Compd. 2014, 608, 8–13. [Google Scholar] [CrossRef]
- Khan, I.; Ali, S.; Mansha, M.; Qurashi, A. Sonochemical Assisted Hydrothermal Synthesis of Pseudo-Flower Shaped Bismuth Vanadate (BiVO4) and Their Solar-Driven Water Splitting Application. Ultrason. Sonochem. 2017, 36, 386–392. [Google Scholar] [CrossRef]
- Xu, D.; Xia, T.; Fan, W.; Bai, H.; Ding, J.; Mao, B.; Shi, W. MOF-Derived Co3O4 Thin Film Decorated BiVO4 for Enhancement of Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2019, 491, 497–504. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Li, J.; Tian, Z.; Yu, F. Highly-Dispersed Ni–NiO Nanoparticles Anchored on an SiO2 Support for an Enhanced CO Methanation Performance. Catalysts 2019, 9, 506. [Google Scholar] [CrossRef]
- Yun, G.; Arunachalam, M.; Ahn, K.S.; Nam, K.M.; Ha, J.S.; Kang, S.H. Multilayered Fluorine Doped SnO2 Inverse Opal/WO3/BiVO4 Film for Solar Water Oxidation: Systematic Development and Defined Role of Each Layer. J. Electrochem. Soc. 2019, 166, H750–H763. [Google Scholar] [CrossRef]
- Liaqat, M.; Riaz, K.N.; Iqbal, T.; Nabi, G.; Rizwan, M.; Shakil, M. Fabrication of Novel BiVO4/Bi2O3 Heterostructure with Superior Visible Light Induced Photocatalytic Properties. Nanotechnology 2022, 34, 015711. [Google Scholar] [CrossRef]
- Cai, L.; Gong, J.; Liu, J.; Zhang, H.; Song, W.; Ji, L. Facile Preparation of Nano-Bi2MoO6/Diatomite Composite for Enhancing Photocatalytic Performance under Visible Light Irradiation. Materials 2018, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, Y.; Lei, T.; Yang, Z.; Yan, J.; Jiang, D. Preparation of Ternary BiVO4/g-C3N4/Diatomite Composites for Enhanced Photodegradation of Rhodamine B and Formaldehyde. J. Photochem. Photobiol. A Chem. 2024, 457, 115906. [Google Scholar] [CrossRef]
- Ao, M.; Liu, K.; Tang, X.; Li, Z.; Peng, Q.; Huang, J. BiOCl/TiO2/Diatomite Composites with Enhanced Visible-Light Photocatalytic Activity for the Degradation of Rhodamine B. Beilstein J. Nanotechnol. 2019, 10, 1412–1422. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Oh, W.C. Synthesis of BiVO4-GO-PVDF nanocomposite: An excellent, newly designed material for high photocatalytic activity towards organic dye degradation by tuning band gap energies. Solid State Sci. 2018, 80, 22–30. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Tan, B.; Zhang, N.; Bao, J.; He, G. Ce–Co Interaction Effects on the Catalytic Performance of Uniform Mesoporous Cex–Coγ Catalysts in Hg0 Oxidation Process. Fuel 2018, 226, 18–26. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, L.P.; Yang, Y.X. Structural Investigation of Some Important Chinese Diatomites. Glass Phys. Chem. 2009, 35, 673–679. [Google Scholar] [CrossRef]
- Hua, C.; Liu, X.; Ren, S.; Zhang, C.; Liu, W. Preparation of Visible Light-Responsive Photocatalytic Paper Containing BiVO4@Diatomite/MCC/PVBCFs for Degradation of Organic Pollutants. Ecotoxicol. Environ. Saf. 2020, 202, 110897. [Google Scholar] [CrossRef]
- Dai, N.; Yi, S.; Zhang, X.; Feng, L.; Ding, H.; Song, D.; Liu, X.; Rao, J.; Zhang, Y. Typical Synthesis of an Iron-Modified Laponite@Diatomite Composite for Photo-Fenton Degradation of Methyl Orange Dyes. Appl. Surf. Sci. 2023, 607, 155214. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Sheng, Y.; Chen, F. Inhibited Photocorrosion and Improved Photocatalytic H2-Evolution Activity of CdS Photocatalyst by Molybdate Ions. Appl. Surf. Sci. 2018, 463, 27–33. [Google Scholar] [CrossRef]
- Kaur, K.; Gautam, U.K. Influence of support textural property on CO2 to methane activity of Ni/SiO2 catalysts. Appl. Catal. B Environ. 2022, 317, 121692. [Google Scholar] [CrossRef]
- He, W.; Liu, L.; Ma, T.; Han, H.; Zhu, J.; Liu, Y.; Guo, K. Controllable Morphology CoFe2O4/g-C3N4 p–n Heterojunction Photocatalysts with Built-in Electric Field Enhance Photocatalytic Performance. Appl. Catal. B 2022, 306, 121107. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Fan, Z.; Zhao, D.; Xiong, S.; Zhang, B.; Zhou, S.; Liu, G. Mechanisms for·O2- and·OH Production on Flowerlike BiVO4 Photocatalysis Based on Electron Spin Resonance. Front. Chem. 2018, 6, 64. [Google Scholar] [CrossRef]
- Ren, D.; Jiang, S.; Fu, L.; Wang, Z.; Zhang, S.; Zhang, X.; Chen, W. Laccase Immobilized on Amino-Functionalized Magnetic Fe3O4–SiO2 Core–Shell Material for 2,4-Dichlorophenol Removal. Environ. Technol. 2022, 43, 2697–2711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).