Influence of Metal Compounds on Structural and Electrochemical Characteristics of Chars from PVC Pyrolysis
Abstract
1. Introduction
2. Results
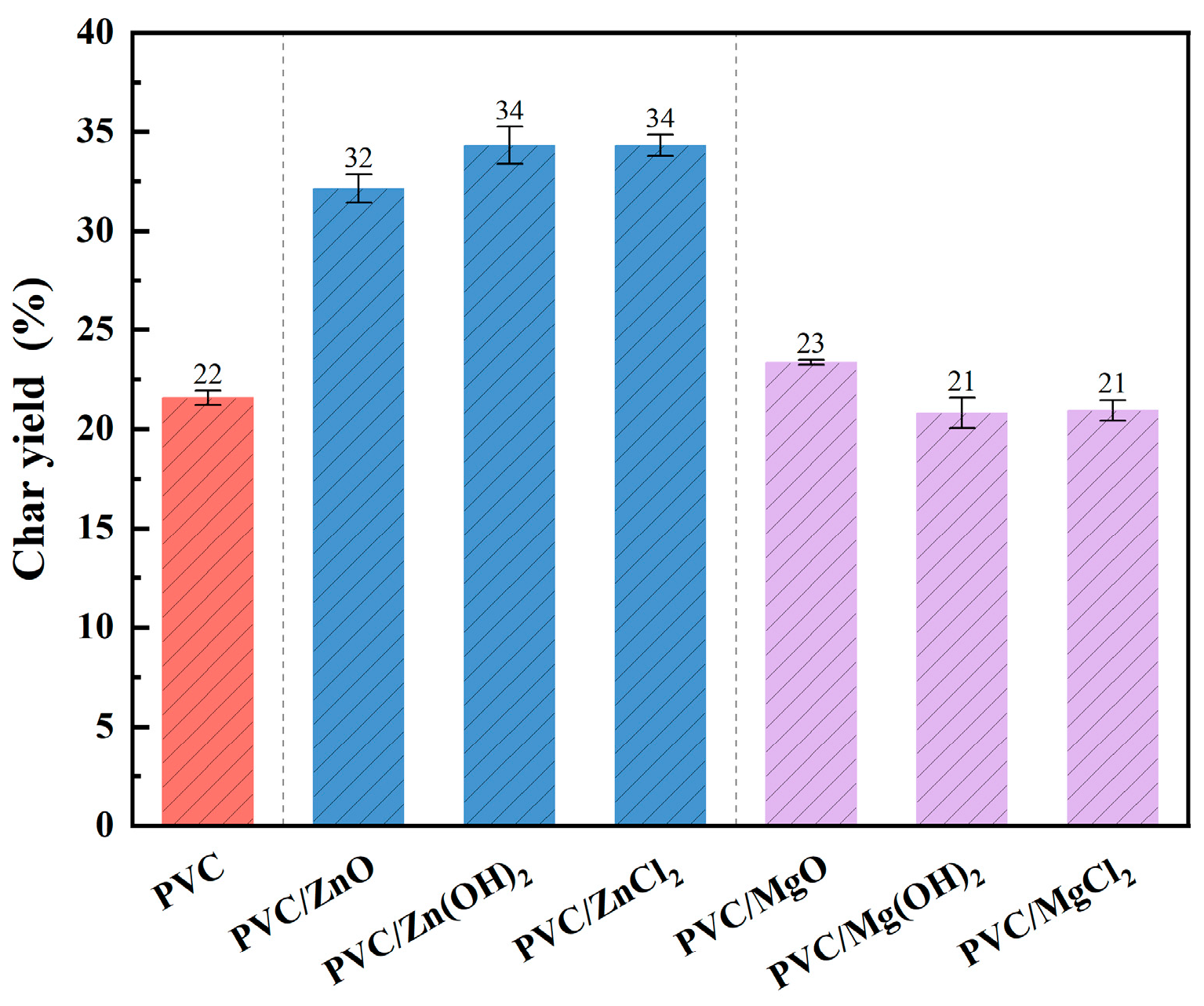
2.1. Char Yield
2.2. Analysis of Char
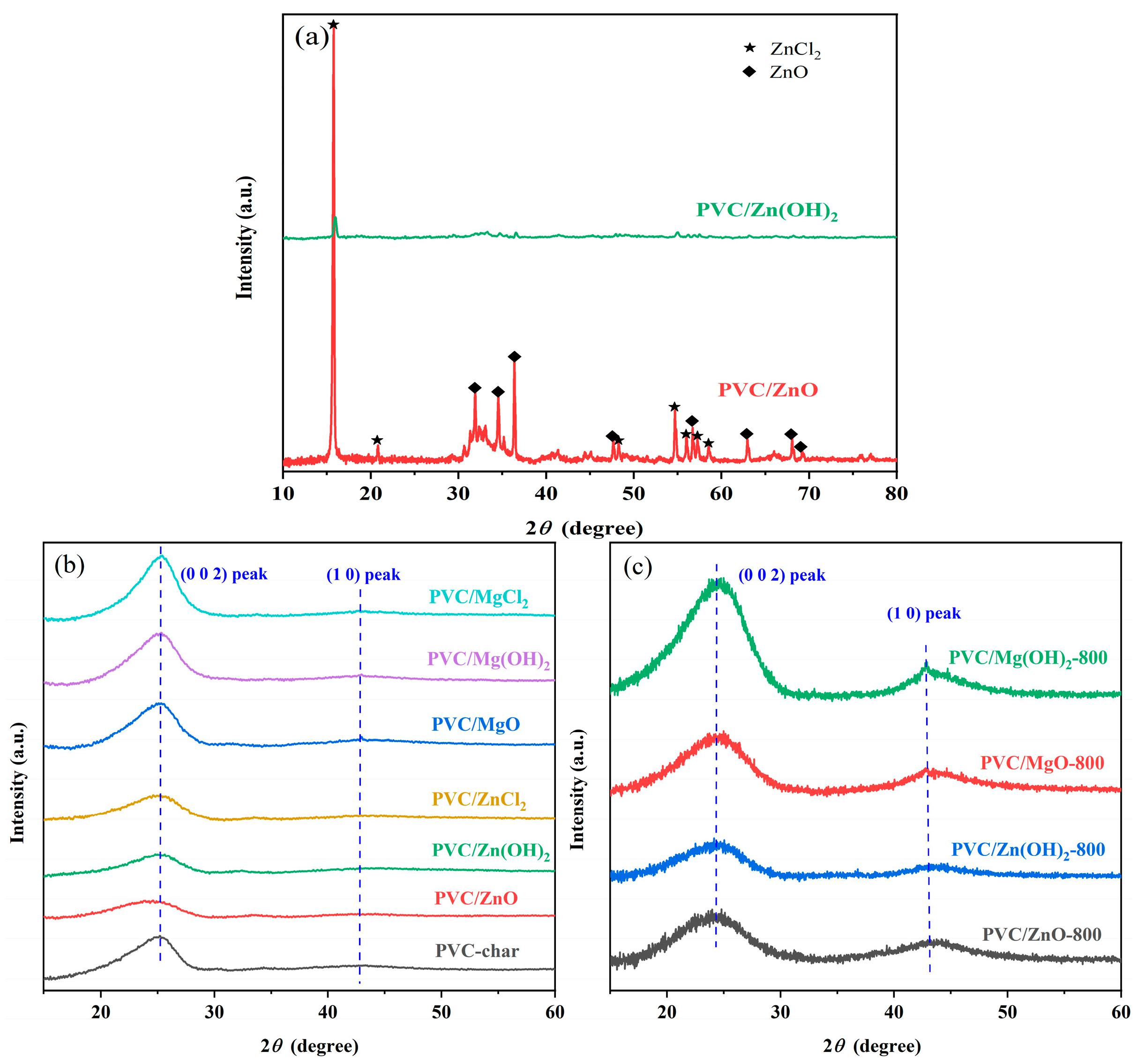
2.2.1. X-Ray Diffraction Analysis
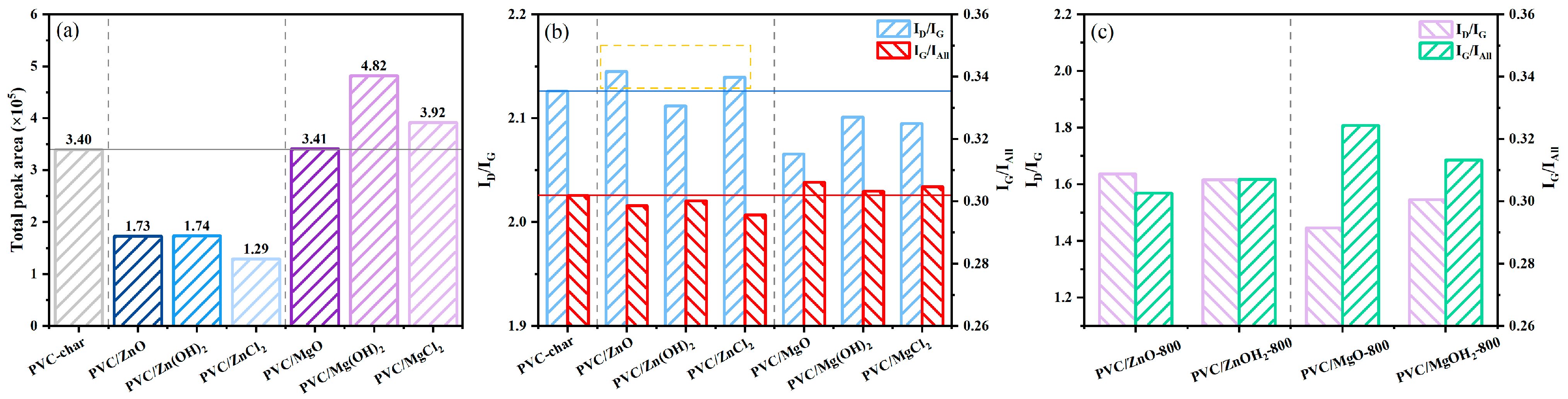
2.2.2. Raman Spectroscopy
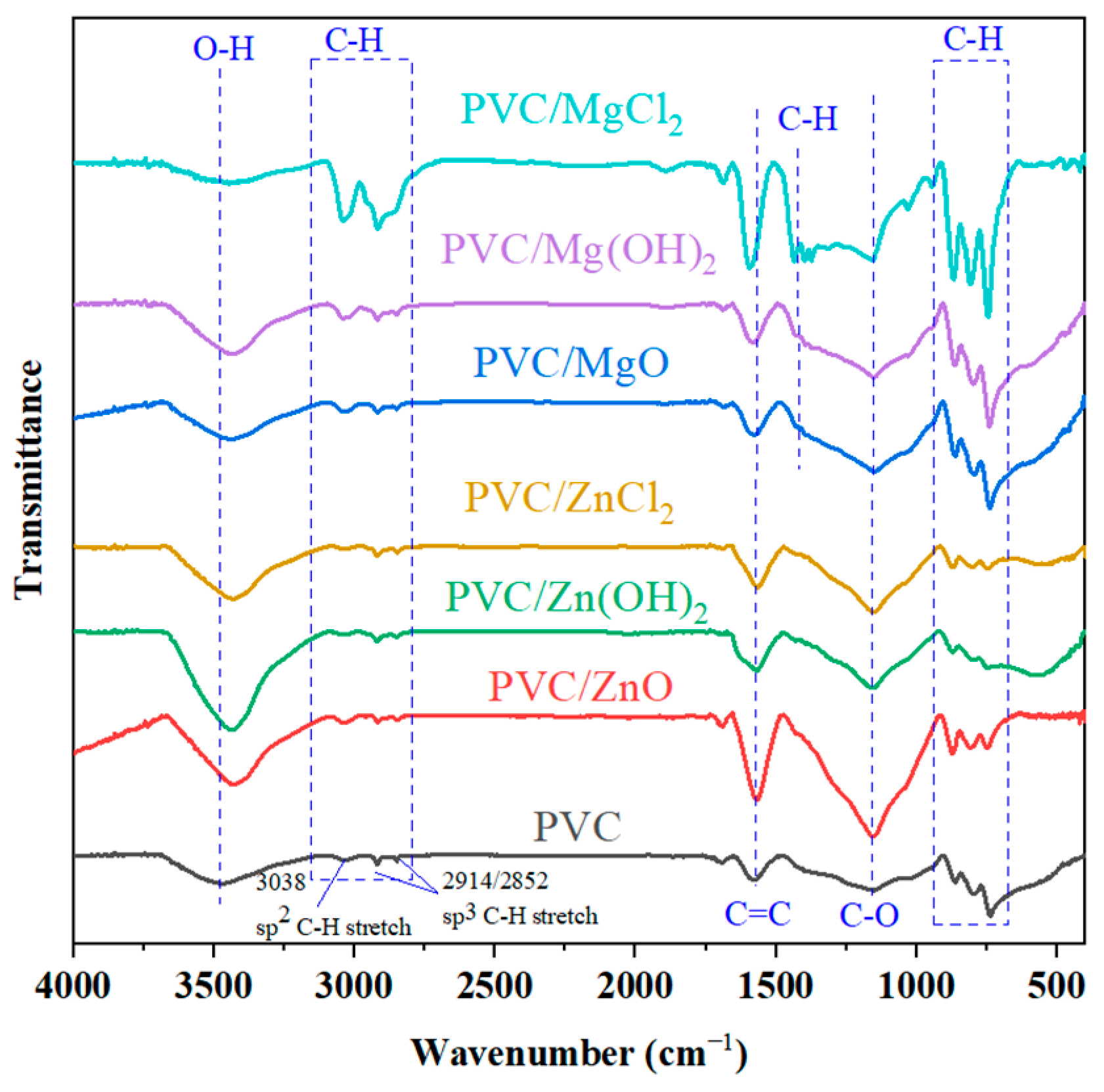
2.2.3. FTIR Analysis
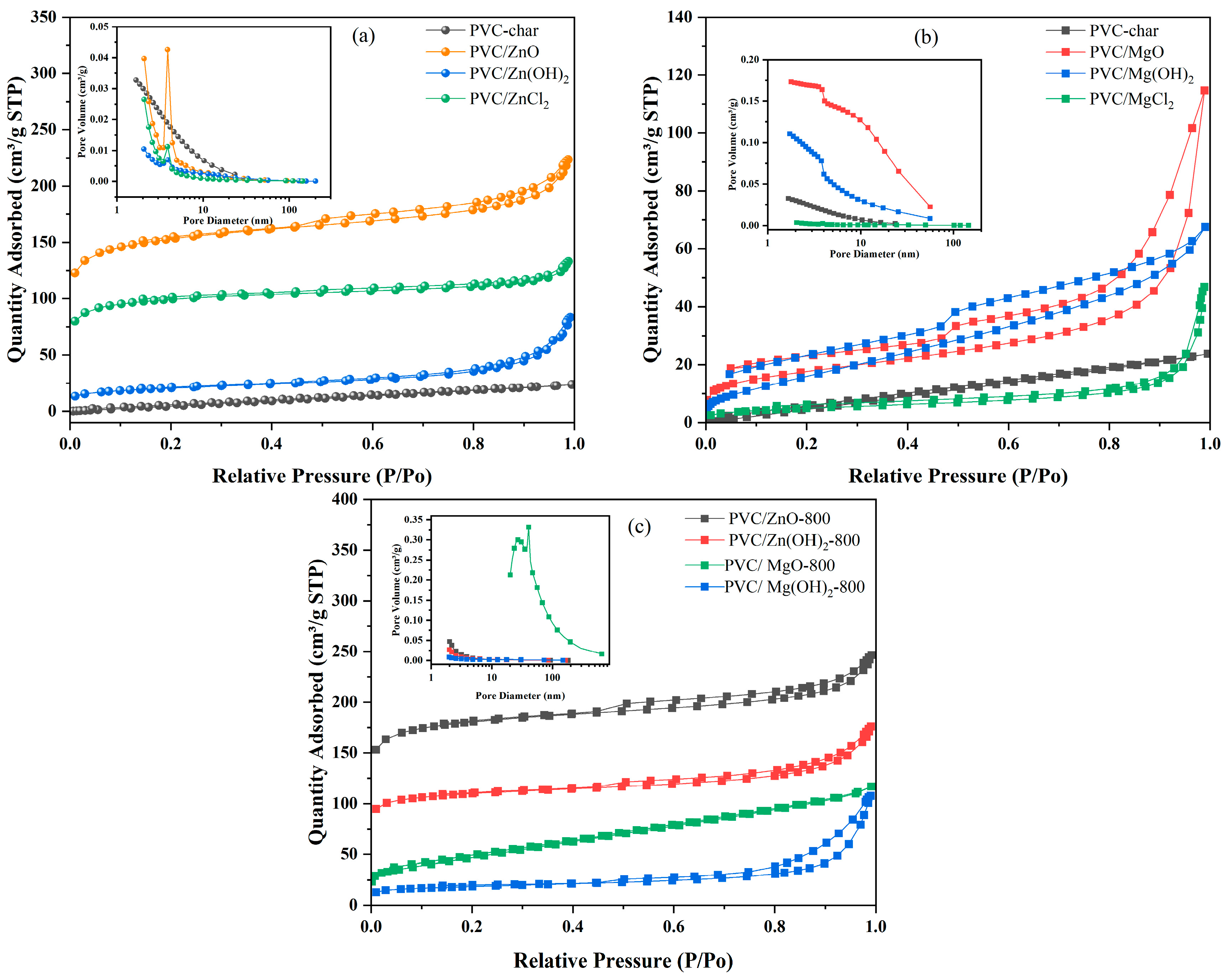
2.2.4. BET Analysis
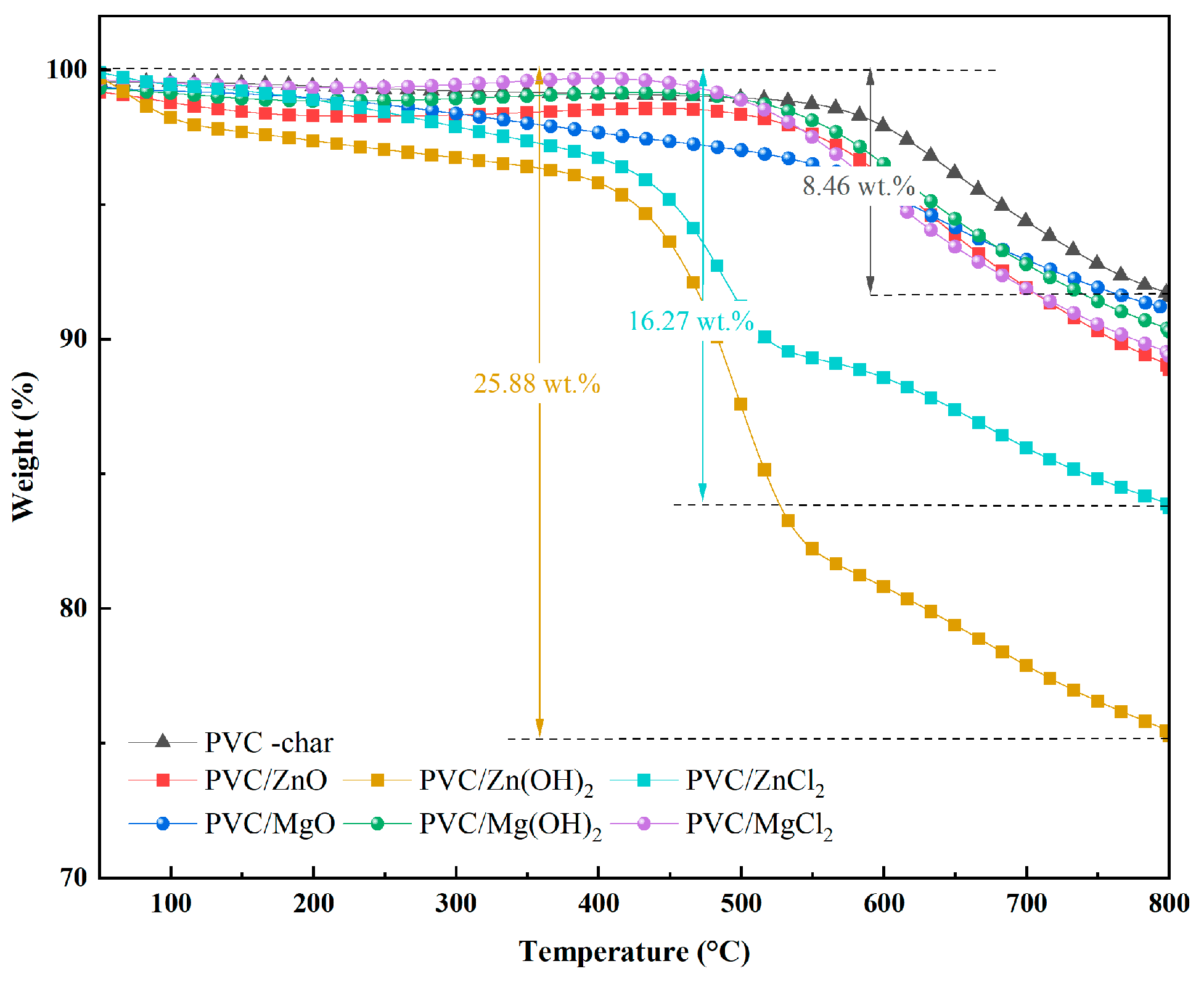
2.2.5. Thermogravimetric Characteristics
2.3. Electrochemical Analysis of Chars
3. Discussion
4. Materials and Methods
4.1. Sample Preparation and Pyrolysis Procedure
4.2. Char Characterization
4.3. Electrochemical Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.M.; Gull, N.; Khan, R.U.; Butt, M.T.Z. Polyvinylchloride (PVC): Structure and Properties Relationship. In Polyvinylchloride-Based Blends; Visakh, P.M., Darie-Nita, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 19–47. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Manag. 2023, 166, 245–258. [Google Scholar] [CrossRef]
- Zhang, L. Insights into the evolution of chemical structure and pyrolysis reactivity of PVC-derived hydrochar during hydrothermal carbonization. RSC Adv. 2023, 13, 27212–27224. [Google Scholar] [CrossRef] [PubMed]
- Lorang, S.; Yang, Z.; Zhang, H.; Lü, F.; He, P. Achievements and policy trends of extended producer responsibility for plastic packaging waste in Europe. Waste Dispos. Sustain. Energy 2022, 4, 91–103. [Google Scholar] [CrossRef]
- Havaei, M.; Akin, O.; Locaspi, A.; John Varghese, R.; Minette, F.; Romers, E.; De Meester, S.; Van Geem, K.M. Beyond the Landfill: A critical review of techniques for End-of-Life Polyvinyl chloride (PVC) valorization. Waste Manag. 2025, 193, 105–134. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, C.; Li, F.; Liu, H.; Yang, J. Stocks and flows of polyvinyl chloride (PVC) in China: 1980–2050. Resour. Conserv. Recycl. 2020, 154, 104584. [Google Scholar] [CrossRef]
- Tang, Y.; Song, M.; Dong, J.; Li, G.; Ye, C.; Hu, Y.; Fang, Y.; Wang, L.; Zheng, Y. Synergetic effect of in-situ CaO on PVC plastic pyrolysis characteristics: TG and Py GC/MS analysis. Polym. Degrad. Stab. 2025, 234, 111205. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, A.; Beltrán, M. Thermogravimetric kinetic study of poly(vinyl chloride) pyrolysis. Polym. Degrad. Stab. 1995, 48, 219–229. [Google Scholar] [CrossRef]
- Lian, X.; Wang, S.; Shan, R.; Gu, J.; Zhao, C.; Zhao, W.; Yuan, H.; Chen, Y. Pyrolysis characterization and mechanism studies of different structural plastics: A comparative study at optimal temperatures. Energy 2024, 313, 133986. [Google Scholar] [CrossRef]
- Wu, J.; Papanikolaou, K.G.; Cheng, F.; Addison, B.; Cuthbertson, A.A.; Mavrikakis, M.; Huber, G.W. Kinetic Study of Polyvinyl Chloride Pyrolysis with Characterization of Dehydrochlorinated PVC. ACS Sustain. Chem. Eng. 2024, 12, 7402–7413. [Google Scholar] [CrossRef]
- Al-Yaari, M.; Dubdub, I. Pyrolytic Behavior of Polyvinyl Chloride: Kinetics, Mechanisms, Thermodynamics, and Artificial Neural Network Application. Polymers 2021, 13, 4359. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, H.; Qing, T.; Zhang, J.; Tian, K. Transformation and kinetics of chlorine-containing products during pyrolysis of plastic wastes. Chemosphere 2021, 284, 131348. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Zeng, G.; Cheng, X.; Tong, H.; Wang, D. Thermal decomposition mechanisms of poly(vinyl chloride): A computational study. Waste Manag. 2018, 76, 483–496. [Google Scholar] [CrossRef]
- Meng, H.; Liu, J.; Xia, Y.; Hu, B.; Sun, H.; Li, J.; Lu, Q. Migration and transformation mechanism of Cl during polyvinyl chloride pyrolysis: The role of structural defects. Polym. Degrad. Stab. 2024, 224, 110750. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.H. Chemical recycling of plastic wastes with alkaline earth metal oxides: A review. Sci. Total Environ. 2023, 905, 167251. [Google Scholar] [CrossRef]
- Torres, D. Hydrochloric acid removal from the thermogravimetric pyrolysis of PVC. J. Anal. Appl. Pyrolysis 2020, 149, 104831. [Google Scholar] [CrossRef]
- Kots, P.A.; Vance, B.C.; Quinn, C.M.; Wang, C.; Vlachos, D.G. A two-stage strategy for upcycling chlorine-contaminated plastic waste. Nat. Sustain. 2023, 6, 1258–1267. [Google Scholar] [CrossRef]
- Meng, T.-T.; Zhang, H.; Lü, F.; Shao, L.-M.; He, P.-J. Comparing the effects of different metal oxides on low temperature decomposition of PVC. J. Anal. Appl. Pyrolysis 2021, 159, 105312. [Google Scholar] [CrossRef]
- Masuda, Y.; Uda, T.; Terakado, O.; Hirasawa, M. Pyrolysis study of poly(vinyl chloride)–metal oxide mixtures: Quantitative product analysis and the chlorine fixing ability of metal oxides. J. Anal. Appl. Pyrolysis 2006, 77, 159–168. [Google Scholar] [CrossRef]
- Zhou, X.-L. Upcycling waste polyvinyl chloride: One-pot synthesis of valuable carbon materials and pipeline-quality syngas via pyrolysis in a closed reactor. J. Hazard. Mater. 2022, 427, 128210. [Google Scholar] [CrossRef]
- Tao, R.; Li, B.; Wu, Y.; Zhang, W.; Zhao, L.; Yuan, H.; Gu, J.; Chen, Y. Based on experiment and quantum chemical calculations: A study of the co-pyrolysis mechanism of polyesterimide enameled wires with polyvinyl chloride and the catalytic effect of endogenous metal Cu. Resour. Environ. Sustain. 2024, 17, 100167. [Google Scholar] [CrossRef]
- Shibata, E.; Yamamoto, S.; Kasai, E.; Nakamura, T. Formation behavior of PCDD/Fs in PVC pyrolysis with copper oxide. Chemosphere 2003, 50, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Zhou, N.; Yuan, H.; Guo, Q.; Jiao, L.; Ma, Z. Catalytic stepwise pyrolysis for dechlorination and chemical recycling of PVC-containing mixed plastic wastes: Influence of temperature, heating rate, and catalyst. Sci. Total Environ. 2024, 908, 168344. [Google Scholar] [CrossRef]
- Karayildirim, T.; Yanik, J.; Yuksel, M.; Saglam, M.; Vasile, C.; Bockhorn, H. The effect of some fillers on PVC degradation. J. Anal. Appl. Pyrolysis 2006, 75, 112–119. [Google Scholar] [CrossRef]
- Ji, M.; Chen, L.; Que, J.; Zheng, L.; Chen, Z.; Wu, Z. Effects of transition metal oxides on pyrolysis properties of PVC. Process Saf. Environ. Prot. 2020, 140, 211–220. [Google Scholar] [CrossRef]
- Müller, J.; Dongmann, G. Formation of aromatics during pyrolysis of PVC in the presence of metal chlorides. J. Anal. Appl. Pyrolysis 1998, 45, 59–74. [Google Scholar] [CrossRef]
- Blazsó, M.; Jakab, E. Effect of metals, metal oxides, and carboxylates on the thermal decomposition processes of poly (vinyl chloride). J. Anal. Appl. Pyrolysis 1999, 49, 125–143. [Google Scholar] [CrossRef]
- Peña, G.D.J.G.; Rahman, R.K.; Raj, A.; Stephen, S.; Anjana, T.; Brito, J.L. Effect of fuel flow rate on the characteristics of soot generated from unsubstituted and disubstituted aromatic hydrocarbon flames: Experimental and numerical study. Combust. Flame 2018, 190, 224–239. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Millan, M. A study on pyrolysis of wood of different sizes at various temperatures and pressures. Fuel 2023, 342, 127846. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.; Liu, X.; Millan, M. On the effect of pellet density on biomass pyrolysis in a pressurized fixed bed reactor. Fuel 2023, 354, 129191. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, B.; Qiao, Y.; Zhang, J.; Wang, W.; Yao, H.; Yu, Y.; Xu, M. Understanding the pyrolysis mechanism of polyvinylchloride (PVC) by characterizing the chars produced in a wire-mesh reactor. Fuel 2016, 166, 526–532. [Google Scholar] [CrossRef]
- Zou, J.; Fan, C.; Jiang, Y.; Liu, X.; Zhou, W.; Xu, H.; Huang, L. A preliminary study on assessing the Brunauer-Emmett-Teller analysis for disordered carbonaceous materials. Microporous Mesoporous Mater. 2021, 327, 111411. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Sonibare, O.O.; Haeger, T.; Foley, S.F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Berrueco, C.; Fidalgo, B.; Paterson, N.; Millan, M. Influence of temperature and particle size on structural characteristics of chars from Beechwood pyrolysis. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]

| Sample | Structure Parameters | ||||
|---|---|---|---|---|---|
| d002 (nm) | La (nm) | Lc (nm) | N | fa | |
| PVC char | 0.362 | 3.419 | 1.654 | 4.564 | 0.888 |
| PVC/ZnO | 0.366 | 3.150 | 1.418 | 3.869 | 0.917 |
| PVC/Zn(OH)2 | 0.358 | 2.468 | 1.650 | 4.615 | 0.951 |
| PVC/ZnCl2 | 0.359 | 3.156 | 1.614 | 4.492 | 0.894 |
| PVC/MgO | 0.360 | 3.315 | 1.808 | 5.023 | 0.866 |
| PVC/Mg(OH)2 | 0.358 | 3.626 | 1.818 | 5.077 | 0.888 |
| PVC/MgCl2 | 0.355 | 3.448 | 1.972 | 5.553 | 0.869 |
| Sample | SBET (a) (m2/g) | Daver (b) (nm) | Vtotal (c) (cm3/g) |
|---|---|---|---|
| PVC char | 38.31 | 3.81 | 0.03 |
| PVC/ZnO | 518.11 | 2.66 | 0.34 |
| PVC/Zn(OH)2 | 72.95 | 7.08 | 0.12 |
| PVC/ZnCl2 | 337.49 | 2.43 | 0.20 |
| PVC/MgO | 62.15 | 11.44 | 0.17 |
| PVC/Mg(OH)2 | 66.70 | 6.27 | 0.10 |
| PVC/MgCl2 | 18.11 | 16.01 | 0.07 |
| PVC/ZnO-800 | 609.38 | 2.50 | 0.38 |
| PVC/Zn(OH)2-800 | 371.69 | 2.93 | 0.27 |
| PVC/MgO-800 | 171.40 | 4.22 | 0.18 |
| PVC/Mg(OH)2-800 | 64.41 | 10.35 | 0.16 |
| Band Type | Raman Shift (cm−1) | Description |
|---|---|---|
| D1 | 1350 | Aromatic structure of more than six rings; disordered graphitic lattice |
| D2 | 1620 | Defect carbon crystal structure |
| D3 | 1500 | Amorphous sp2-carbon, organic molecules, fragments, functional groups |
| D4 | 1200 | Disordered graphitic lattice, polyene, ionic impurities |
| G | 1580 | Aromatic benzene ring C=C vibration; ideal graphitic lattice |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Ding, T.; Zhao, X.; Xu, G.; Wen, C.; Yu, J. Influence of Metal Compounds on Structural and Electrochemical Characteristics of Chars from PVC Pyrolysis. Catalysts 2025, 15, 660. https://doi.org/10.3390/catal15070660
Sun J, Ding T, Zhao X, Xu G, Wen C, Yu J. Influence of Metal Compounds on Structural and Electrochemical Characteristics of Chars from PVC Pyrolysis. Catalysts. 2025; 15(7):660. https://doi.org/10.3390/catal15070660
Chicago/Turabian StyleSun, Jiayou, Tianyang Ding, Xue Zhao, Guorong Xu, Chang Wen, and Jie Yu. 2025. "Influence of Metal Compounds on Structural and Electrochemical Characteristics of Chars from PVC Pyrolysis" Catalysts 15, no. 7: 660. https://doi.org/10.3390/catal15070660
APA StyleSun, J., Ding, T., Zhao, X., Xu, G., Wen, C., & Yu, J. (2025). Influence of Metal Compounds on Structural and Electrochemical Characteristics of Chars from PVC Pyrolysis. Catalysts, 15(7), 660. https://doi.org/10.3390/catal15070660








