Comparative Study on Ni/MgO-Al2O3 Catalysts for Dry and Combined Steam–CO2 Reforming of Methane
Abstract
1. Introduction
2. Results and Discussion
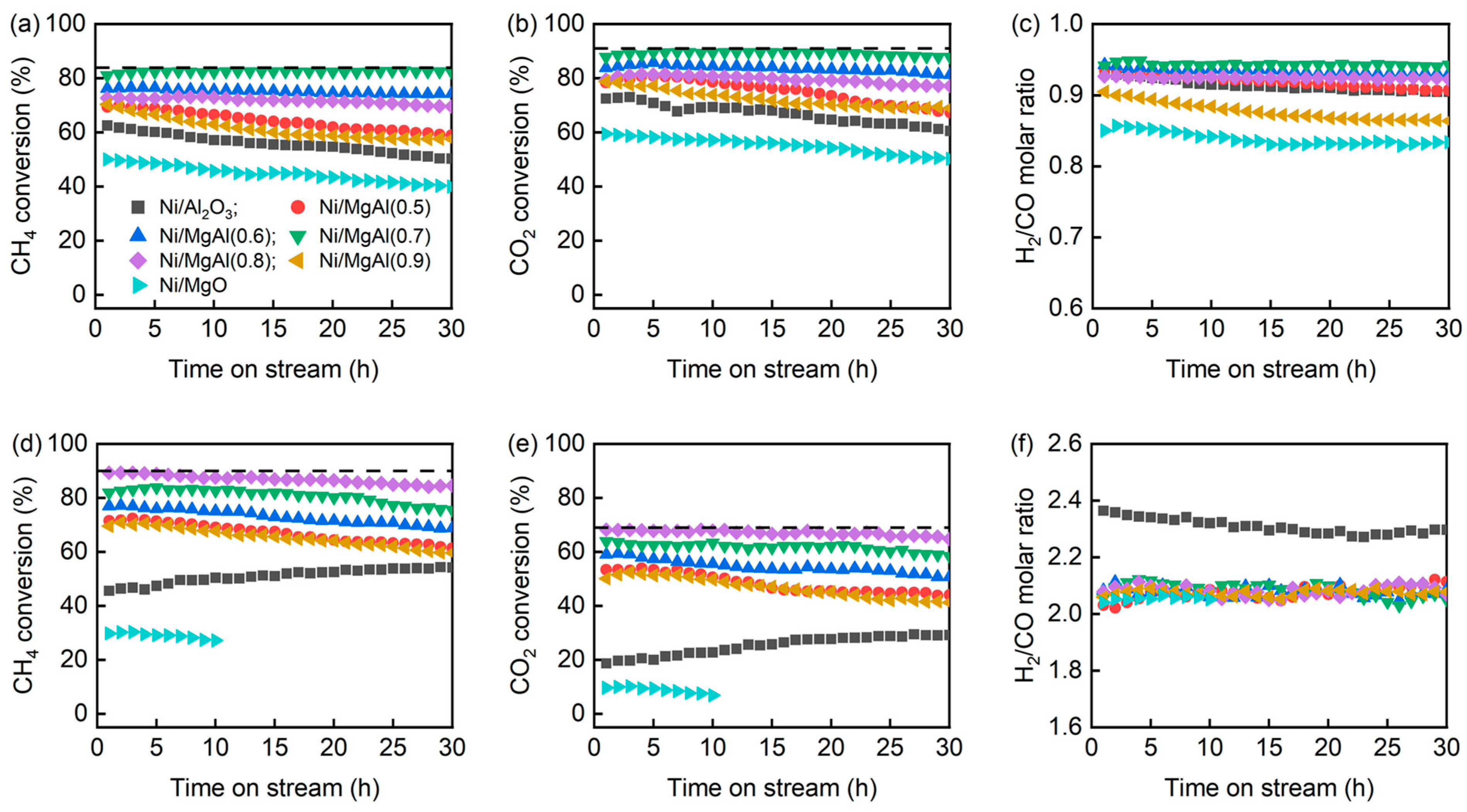
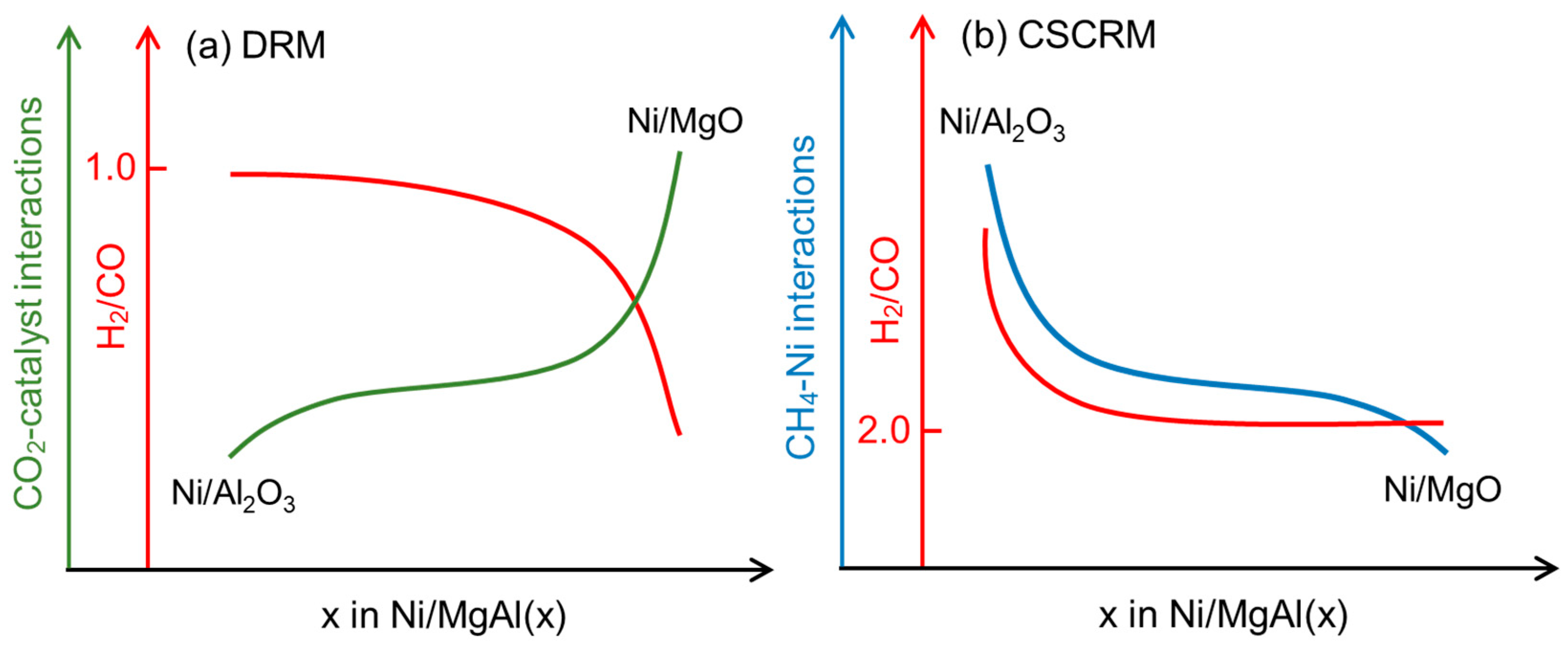
2.1. Catalytic Performance
2.2. Physicochemical Properties of Catalysts
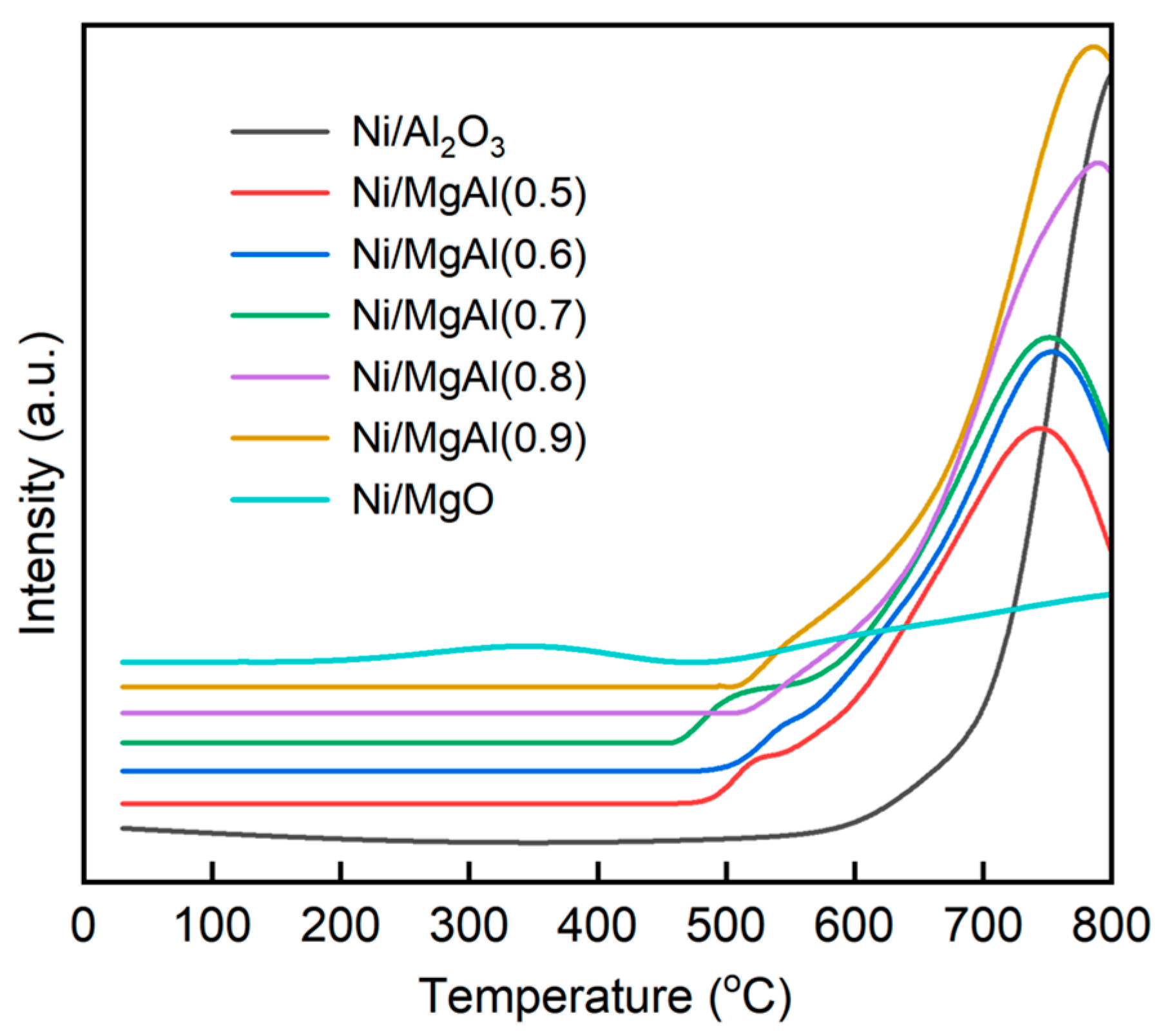
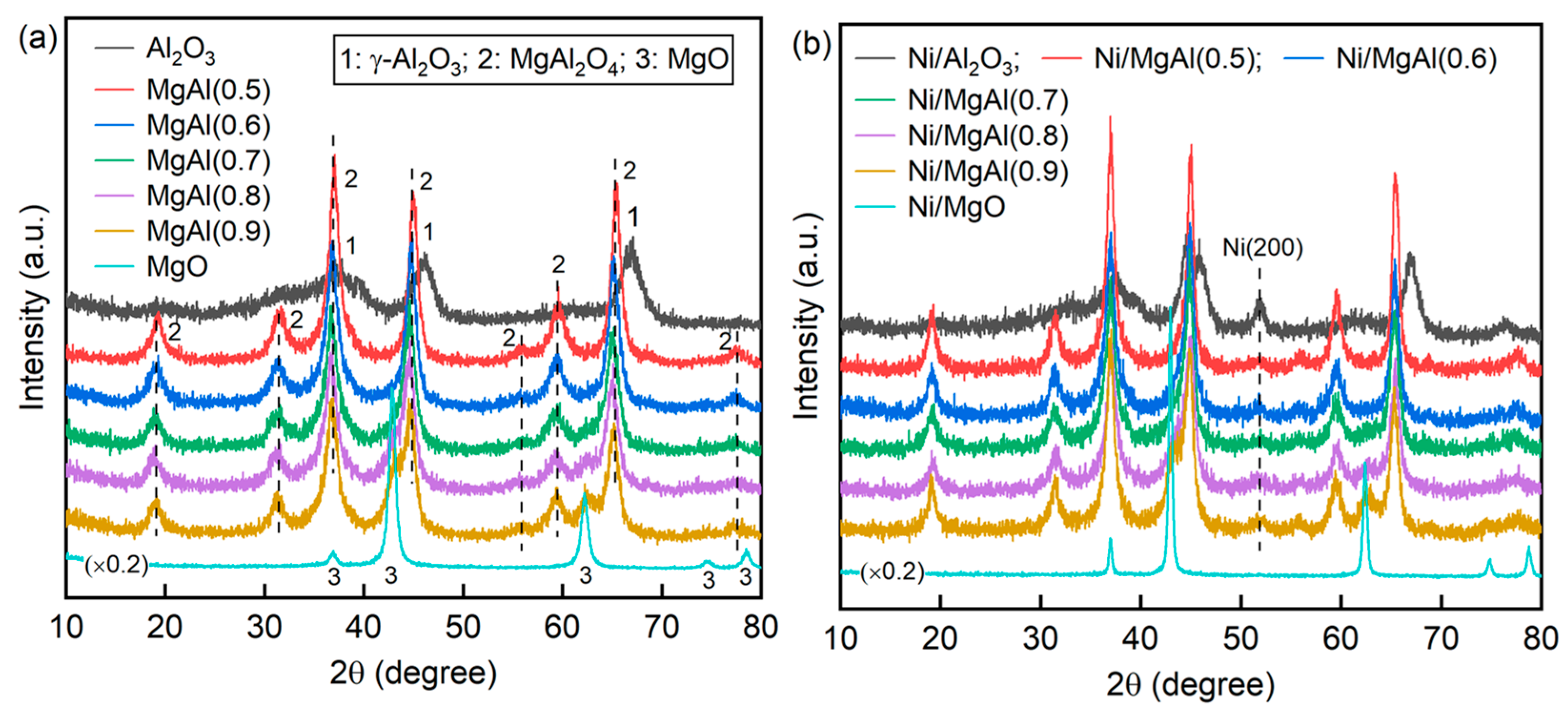
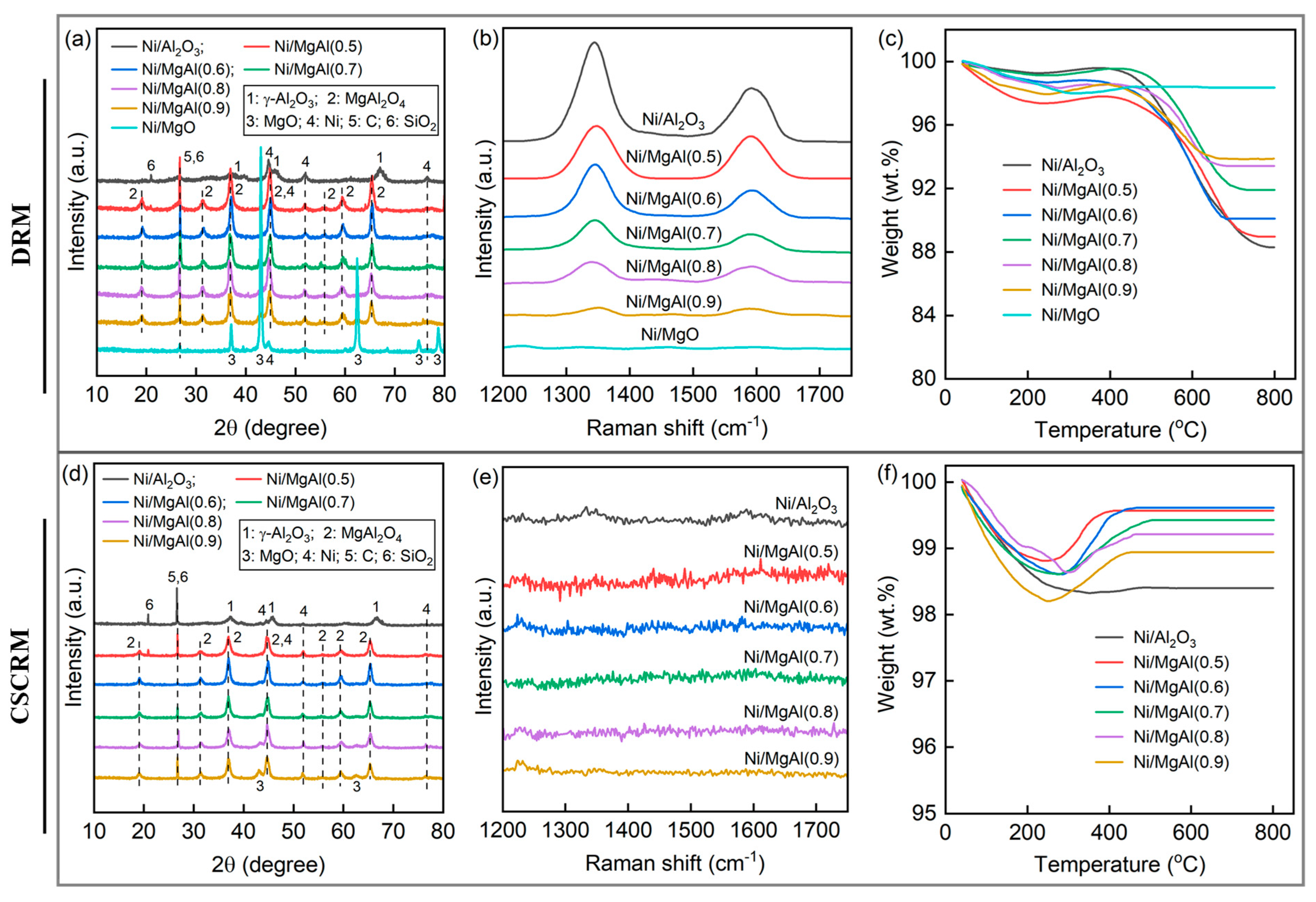
2.2.1. Fresh Catalysts
2.2.2. Spent Catalysts
2.3. Long-Term Test of Screened Catalysts
3. Experimental Section
3.1. Materials
3.2. Support Preparation
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.5. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [PubMed]
- Radlik, M.; Adamowska-Teyssier, M.; Krzton, A.; Koziel, K.; Krajewski, W.; Turek, W.; Da Costa, P. Dry reforming of methane over Ni/Ce0.62Zr0.38O2 catalysts: Effect of Ni loading on the catalytic activity and on H2/CO production. C. R. Chim. 2015, 18, 1242–1249. [Google Scholar]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheedu, R.; Bagabas, A.; Chaudhary, M.L.; Frusteri, F.; et al. The effect of modifier identity on the performance of Ni-based catalyst supported on γ-Al2O3 in dry reforming of methane. Catal. Today 2020, 348, 236–242. [Google Scholar]
- Dan, M.; Mihet, M.; Lazar, M.D. Hydrogen and/or syngas production by combined steam and dry reforming of methane on nickel catalysts. Int. J. Hydrogen Energy 2020, 45, 26254–26264. [Google Scholar]
- Zhao, Y.; Qi, L.; Cheng, Z.; Zhou, Z. Syngas production via combined steam and carbon dioxide reforming of methane over Ni-CexM1-xO2 (M = Ti or Zr) catalysts. Ind. Eng. Chem. Res. 2022, 61, 12978–12988. [Google Scholar]
- Wu, X.; Xu, L.; Chen, M.; Lv, C.; Wen, X.; Cui, Y.; Wu, C.; Yang, B.; Miao, Z.; Hu, X. Recent progresses in the design and fabrication of highly efficient Ni-based catalysts with advanced catalytic activity and enhanced anti-coke performance toward CO2 reforming of methane. Front. Chem. 2020, 8, 581923. [Google Scholar]
- Mohanty, U.S.; Ali, M.; Azhar, M.R.; Al-Yaseri, A.; Keshavarz, A.; Iglauer, S. Current advances in syngas (CO + H2) production through bi-reforming of methane using various catalysts: A review. Int. J. Hydrogen Energy 2021, 46, 32809–32845. [Google Scholar]
- Shi, Y.; Tian, X.; Deng, Z.; Shi, W.; Fan, W.; Wang, F. Review and outlook of confined Ni catalysts for the dry reforming of methane reaction. Energy Fuels 2024, 38, 1633–1656. [Google Scholar]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A 2004, 273, 75–82. [Google Scholar]
- Hadian, N.; Rezaei, M.; Mosayebi, Z.; Meshkani, F. CO2 reforming of methane over nickel catalysts supported on nanocrystalline MgAl2O4 with high surface area. J. Nat. Gas Chem. 2012, 21, 200–206. [Google Scholar]
- Hadian, N.; Rezaei, M. Combination of dry reforming and partial oxidation of methane over Ni catalysts supported on nanocrystalline MgAl2O4. Fuel 2013, 113, 571–579. [Google Scholar]
- Kim, H.; Eissa, A.A.; Kim, S.; Lee, H.; Kim, W.; Seo, D.; Lee, K.; Yoon, W. One-pot synthesis of a highly mesoporous Ni/MgAl2O4 spinel catalyst for efficient steam methane reforming: Influence of inert annealing. Catal. Sci. Technol. 2021, 11, 4447–4458. [Google Scholar]
- Yu, S.; Hu, Y.; Cui, H.; Cheng, Z.; Zhou, Z. Ni-based catalysts supported on MgAl2O4 with different properties for combined steam and CO2 reforming of methane. Chem. Eng. Sci. 2021, 232, 116379. [Google Scholar]
- Cosimo, J.I.D.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar]
- Li, X.; Huang, Y.; Zhang, Q.; Luan, C.; Vinokurov, V.A.; Huang, W. Highly stable and anti-coking Ni/MoCeZr/MgAl2O4-MgO complex support catalysts for CO2 reforming of CH4: Effect of the calcination temperature. Energy Convers. Manag. 2019, 179, 166–177. [Google Scholar]
- Bach, V.R.; de Camargo, A.C.; de Souza, T.L.; Cardozo, L.; Alves, H.J. Dry reforming of methane over Ni/MgO-Al2O3 catalysts: Thermodynamic equilibrium analysis and experimental application. Int. J. Hydrogen Energy 2020, 45, 5252–5263. [Google Scholar]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2021, 284, 119082. [Google Scholar]
- Fang, X.; Zhang, J.; Liu, J.; Wang, C.; Huang, Q.; Xu, X.; Peng, H.; Liu, W.; Wang, X.; Zhou, W. Methane dry reforming over Ni/Mg-Al-O: On the significant promotional effects of rare earth Ce and Nd metal oxides. J. CO2 Util. 2018, 25, 242–253. [Google Scholar]
- Park, H.; Kim, B.; Lee, Y.; Ahn, S.; Kim, K.; Hong, G.; Yun, S.; Jeon, B.; Bae, J.; Roh, H. CO2 reforming of CH4 using coke oven gas over Ni/MgO-Al2O3 catalysts: Effect of the MgO:Al2O3 ratio. Catalysts 2021, 11, 1468. [Google Scholar]
- Tao, X.; Yu, F.; Yu, P.; Yu, H.; Zhao, T.; Li, M.; Wang, H. Local coordination configuration of Ni and Co in MgAl2O4 spinel structure and the performance of NiCo/MgO-Al2O3 catalyst for dry reforming of methane. Chem. Eng. J. 2025, 507, 160708. [Google Scholar]
- Schiaroli, N.; Lucarelli, C.; de Luna, G.S.; Fornasari, G.; Vaccari, A. Ni-based catalysts to produce synthesis gas by combined reforming of clean biogas. Appl. Catal. A 2019, 582, 117087. [Google Scholar]
- Koo, K.; Roh, H.; Seo, Y.; Seo, D.; Yoon, W.; Park, S. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Appl. Catal. A 2008, 340, 183–190. [Google Scholar]
- Koo, K.; Roh, H.; Jung, U.; Seo, D.; Seo, Y.; Yoon, W. Combined H2O and CO2 reforming of CH4 over nano-sized Ni/MgO-Al2O3 catalysts for synthesis gas production for gas to liquid (GTL): Effect of Mg/Al mixed ratio on coke formation. Catal. Today 2009, 146, 166–171. [Google Scholar]
- Xin, J.; Cui, H.; Cheng, Z.; Zhou, Z. Bimetallic Ni-Co/SBA-15 catalysts prepared by urea co-precipitation for dry reforming of methane. Appl. Catal. A 2018, 554, 95–104. [Google Scholar]
- Zhao, Y.; Geng, J.; Zhu, H.; Zhang, Q.; Wang, H.; Feng, X. Ordered mesoporous Ni–Mg–Al2O3 as an effective catalyst for CO2 reforming of CH4. Int. J. Hydrogen Energy 2023, 48, 7192–7201. [Google Scholar]
- Zhang, C.; Gao, Y.; Wang, H.; Jiang, L.; Zhang, D.; Ming, S.; Fu, G.; Wang, C.; Lv, Z. Hydrophobic driving fabrication of highly dispersed PtNi in Zr-doped 3D hollow flower-like MgAl2O4 spheres with abundant O vacancies for enhanced dry reforming of methane. J. Colloid Interface Sci. 2025, 685, 244–254. [Google Scholar]
- Koo, K.; Lee, S.; Jung, U.; Roh, H.; Yoon, W. Syngas production via combined steam and carbon dioxide reforming of methane over Ni-Ce/MgAl2O4 catalysts with enhanced coke resistance. Fuel Process. Technol. 2014, 119, 151–157. [Google Scholar]
- Dan, M.; Mihet, M.; Barbu-Tudoran, L.; Lazar, M. Biogas upgrading to syngas by combined reforming using Ni/CeO2-Al2O3 with bimodal pore structure. Microporous Mesoporous Mater. 2022, 341, 112015. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Song, D.; Jung, U.; Kim, Y.; Im, H.; Lee, T.; Lee, K.; Koo, K. Influence of supports on the catalytic activity and coke resistance of Ni catalyst in dry reforming of methane. Catalysts 2022, 12, 216. [Google Scholar] [CrossRef]
- Hu, Y. Solid-solution catalysts for CO2 reforming of methane. Catal. Today 2009, 148, 206–211. [Google Scholar]
- Serrano-Lotina, A.; Martin, A.J.; Folgado, M.A.; Daza, L. Dry reforming of methane to syngas over La-promoted hydrotalcite clay-derived catalysts. Int. J. Hydrogen Energy 2012, 37, 12342–12350. [Google Scholar]
- Nguyen-Phu, H.; Kim, T.; Kim, Y.; Kang, K.; Cho, H.; Kim, J.; Ro, I. Role of phase in NiMgAl mixed oxide catalysts for CO2 dry methane reforming (DRM). Catal. Today 2023, 411, 113894. [Google Scholar]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J. Effect of NiAl2O4 formation on Ni/Al2O3 stability during dry reforming of methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar]
- Qiu, H.; Ran, J.; Huang, X.; Ou, Z.; Niu, J. Unrevealing the influence that preparation and reaction parameters have on Ni/Al2O3 catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2022, 47, 34066–34074. [Google Scholar]
- Huang, C.; Tai, C.; Huang, C.; Chien, Y. Low-loss microwave dielectrics in the spinel-structured (Mg1-xNix)Al2O4 solid solutions. J. Am. Ceram. Soc. 2010, 93, 1999–2003. [Google Scholar]
- Han, R.; Xing, S.; Wang, Y.; Wei, L.; Li, Z.; Yang, C.; Song, C.; Liu, Q. Two birds with one stone: MgO promoted Ni-CaO as stable and coke-resistant bifunctional materials for integrated CO2 capture and conversion. Sep. Purif. Technol. 2023, 307, 122808. [Google Scholar]
- Boukha, Z.; Jiménez-González, C.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B 2014, 158, 190–201. [Google Scholar]
- Bae, Y.; Hong, J. Enhancement of surface morphology and catalytic kinetics of NiAl2O4 spinel-derived Ni catalyst to promote dry reforming of methane at low temperature for the direct application to a solid oxide fuel cell. Chem. Eng. J. 2022, 446, 136978. [Google Scholar]
- Charisiou, N.D.; Papageridis, K.N.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKetbi, M.; Polychronopoulou, K.; Goula, M.A. Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2019, 44, 256–273. [Google Scholar]
- Liu, Q.; Sun, J.; Feng, Q.; Ji, S.; Wang, Z. A La-promoted Ni/MgAl2O4 catalyst with superior methanation performance for the production of synthetic natural gas. Catal. Today 2020, 339, 127–134. [Google Scholar]
- Fakeeha, A.; Kurdi, A.; Al, B.; Ibrahim, A.A.; Abasaeed, A.E.; Al-Fatesh, A.S. Performance study of methane dry reforming on Ni/ZrO2 catalyst. Energies 2022, 15, 3841. [Google Scholar]
- Zhao, R.; Cao, K.; Ye, R.; Tang, Y.; Du, C.; Liu, F.; Zhao, Y.; Chen, R.; Shan, B. Deciphering the stability mechanism of Pt-Ni/Al2O3 catalysts in syngas production via DRM. Chem. Eng. J. 2024, 491, 151966. [Google Scholar]
- Guilhaume, N.; Bianchi, D.; Wandawa, R.A.; Yin, W.; Schuurman, Y. Study of CO2 and H2O adsorption competition in the combined dry/steam reforming of biogas. Catal. Today 2021, 375, 282–289. [Google Scholar]
- Ponugoti, P.V.; Pathmanathan, P.; Rapolu, J.; Gomathi, A.; Janardhanan, V.M. On the stability of Ni/γ-Al2O3 catalyst and the effect of H2O and O2 during biogas reforming. Appl. Catal. A 2023, 651, 119033. [Google Scholar]
- Qin, Z.; Wang, X.; Dong, L.; Su, T.; Li, B.; Zhou, Y.; Jiang, Y.; Luo, X.; Ji, H. CO2 methanation on Co/TiO2 catalyst: Effects of Y on the support. Chem. Eng. Sci. 2019, 210, 115245. [Google Scholar]
- Shi, H.; Tian, C.; Liu, X.; Sun, N.; Song, C.; Zheng, H.; Gao, K.; Wang, X.; Jiang, Z.; Xuan, Y.; et al. Ni-phyllosilicate nanotubes coated by CeO2 for ultra-efficiency of 36.9% and near-limit CO2 conversion in solar-driven conversion of CO2-to-fuel. Chem. Eng. J. 2023, 454, 140063. [Google Scholar]
- Serrano-Lotina, A.; Daza, L. Influence of the operating parameters over dry reforming of methane to syngas. Int. J. Hydrogen Energy 2014, 39, 4089–4094. [Google Scholar]
- Wysocka, I.; Mielewczyk-Gryn, A.; Lapinski, M.; Cieslik, B.; Rogala, A. Effect of small quantities of potassium promoter and steam on the catalytic properties of nickel catalysts in dry/combined methane reforming. Int. J. Hydrogen Energy 2021, 46, 3847–3864. [Google Scholar]
- Bae, D.; Kim, Y.; Ko, E.; Han, S.; Lee, J.; Kim, M.; Kang, D. Methane pyrolysis and carbon formation mechanisms in molten manganese chloride mixtures. Appl. Energy 2023, 336, 120810. [Google Scholar]
- Lee, H.; Kim, A.; Park, M.; Jo, J.; Lee, D.; Bae, J. Combined steam and CO2 reforming of CH4 using coke oven gas on nickel-based catalyst: Effects of organic acids to nickel dispersion and activity. Chem. Eng. J. 2015, 280, 771–781. [Google Scholar]
- Wang, C.; Jie, X.; Qiu, Y.; Zhao, Y.; Al-Megren, H.A.; Alshihri, S.; Edwards, P.P.; Xiao, T. The importance of inner cavity space within Ni@SiO2 nanocapsule catalysts for excellent coking resistance in the high-space-velocity dry reforming of methane. Appl. Catal. B 2019, 259, 118019. [Google Scholar]
- Su, T.; Gong, B.; Xie, X.; Luo, X.; Qin, Z.; Ji, H. Effect of cobalt on the activity of nickel-based/magnesium-substituted hydroxyapatite catalysts for dry reforming of methane. Chin. J. Chem. Eng. 2024, 76, 281–291. [Google Scholar]
- Koo, K.; Lee, J.; Jung, U.; Kim, S.; Yoon, W. Combined H2O and CO2 reforming of coke oven gas over Ca-promoted Ni/MgAl2O4 catalyst for direct reduced iron production. Fuel 2015, 153, 303–309. [Google Scholar]
- Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Al-Fatesh, A.S. Dry reforming of methane using Ni catalyst supported on ZrO2: The effect of different sources of zirconia. Catalysts 2021, 11, 827. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Biogas reforming of carbon dioxide to syngas production over Ni-Mg-Al catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar]
- Guo, J.; Lou, H.; Zheng, X. The deposition of coke from methane on a Ni/MgAl2O4 catalyst. Carbon 2007, 45, 1314–1321. [Google Scholar]
- Han, J.; Zhan, Y.; Street, J.; To, F.; Yu, F. Natural gas reforming of carbon dioxide for syngas over Ni-Ce-Al catalysts. Int. J. Hydrogen Energy 2017, 42, 18364–18374. [Google Scholar]
- Sehested, J.; Gelten, J.; Remediakis, I.; Bengaard, H.; Norskov, J. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar]



| Sample a | SBET (m2/g) | Vp b (cm3/g) | dp b (nm) | Ni Content c (wt.%) | H2 Uptake (μmol/g) | dNi-XRD d (nm) | dNi-HRTEM e (nm) |
|---|---|---|---|---|---|---|---|
| MgO | 21.9 | 0.12 | 32.7 | ||||
| Al2O3 | 197.0 | 0.53 | 10.6 | ||||
| MgAl(0.5) | 92.1 | 0.40 | 17.3 | ||||
| MgAl(0.6) | 133.1 | 0.54 | 19.4 | ||||
| MgAl(0.7) | 141.7 | 0.71 | 20.5 | ||||
| MgAl(0.8) | 155.0 | 0.92 | 24.6 | ||||
| MgAl(0.9) | 110.8 | 0.49 | 17.9 | ||||
| Ni/MgO | 41.8 | 0.15 | 28.0 | 10.3 | 3.4 | - | 4.9 |
| Ni/Al2O3 | 135.1 | 0.50 | 14.0 | 10.2 | 14.4 | 6.9 | 6.4 |
| Ni/MgAl(0.5) | 50.3 | 0.40 | 31.0 | 9.6 | 17.8 | 10.3 | 8.8 |
| Ni/MgAl(0.6) | 100.4 | 0.56 | 22.0 | 9.5 | 19.5 | 8.9 | 8.3 |
| Ni/MgAl(0.7) | 107.8 | 0.53 | 18.1 | 9.5 | 22.1 | 8.5 | 7.6 |
| Ni/MgAl(0.8) | 108.5 | 0.41 | 13.2 | 9.8 | 24.8 | 7.9 | 7.1 |
| Ni/MgAl(0.9) | 81.0 | 0.37 | 16.9 | 9.7 | 16.9 | 11.4 | 10.5 |
| Mg/Al Molar Ratio (x) | Lattice Parameter of MgAl2O4 in MgAl(x) and Ni/MgAl(x) (Å) | |||
|---|---|---|---|---|
| MgAl(x) | Fresh Ni/MgAl(x) | Spent Ni/MgAl(x) in DRM | Spent Ni/MgAl(x) in CSCRM | |
| 0.5 | 8.0636 | 8.0515 | 8.0622 | 8.0593 |
| 0.6 | 8.0728 | 8.0588 | 8.0678 | 8.0687 |
| 0.7 | 8.1025 | 8.0611 | 8.0652 | 8.0746 |
| 0.8 | 8.0975 | 8.0791 | 8.0921 | 8.0817 |
| 0.9 | 8.0801 | 8.0615 | 8.0816 | 8.0765 |
| Catalyst | DRM | CSCRM | |||||
|---|---|---|---|---|---|---|---|
| dNi-XRD a (nm) | dNi-TEM b (nm) | Coke c (wt.%) | Coking Rate (wt.%/h) | dNi-XRD a (nm) | dNi-TEM b (nm) | Coke c (wt.%) | |
| Ni/γ-Al2O3 | 8.7 | 8.4 | 11.3 | 0.38 | 8.8 | 9.0 | n.d. |
| Ni/MgAl(0.5) | 15.5 | 13.7 | 9.0 | 0.30 | 16.3 | 15.9 | n.d. |
| Ni/MgAl(0.6) | 11.7 | 12.8 | 8.6 | 0.29 | 13.6 | 14.3 | n.d. |
| Ni/MgAl(0.7) | 10.8 | 11.5 | 7.7 | 0.26 | 14.0 | 12.7 | n.d. |
| Ni/MgAl(0.8) | 11.7 | 10.9 | 5.2 | 0.17 | 13.4 | 11.8 | n.d. |
| Ni/MgAl(0.9) | 13.1 | 13.0 | 4.7 | 0.16 | 14.8 | 15.4 | n.d. |
| Ni/MgO | 8.2 | 8.6 | n.d. | - | - | - | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Zhou, Y.; Cui, H.; Zhou, Z. Comparative Study on Ni/MgO-Al2O3 Catalysts for Dry and Combined Steam–CO2 Reforming of Methane. Catalysts 2025, 15, 659. https://doi.org/10.3390/catal15070659
Zheng T, Zhou Y, Cui H, Zhou Z. Comparative Study on Ni/MgO-Al2O3 Catalysts for Dry and Combined Steam–CO2 Reforming of Methane. Catalysts. 2025; 15(7):659. https://doi.org/10.3390/catal15070659
Chicago/Turabian StyleZheng, Tingting, Yuqi Zhou, Hongjie Cui, and Zhiming Zhou. 2025. "Comparative Study on Ni/MgO-Al2O3 Catalysts for Dry and Combined Steam–CO2 Reforming of Methane" Catalysts 15, no. 7: 659. https://doi.org/10.3390/catal15070659
APA StyleZheng, T., Zhou, Y., Cui, H., & Zhou, Z. (2025). Comparative Study on Ni/MgO-Al2O3 Catalysts for Dry and Combined Steam–CO2 Reforming of Methane. Catalysts, 15(7), 659. https://doi.org/10.3390/catal15070659





