A Simple Fabrication of Tourmaline-Supported Ni-NiAl2O4 Nanocomposites for Enhanced Methane Dry Reforming Activity
Abstract
1. Introduction
2. Results and Discussion
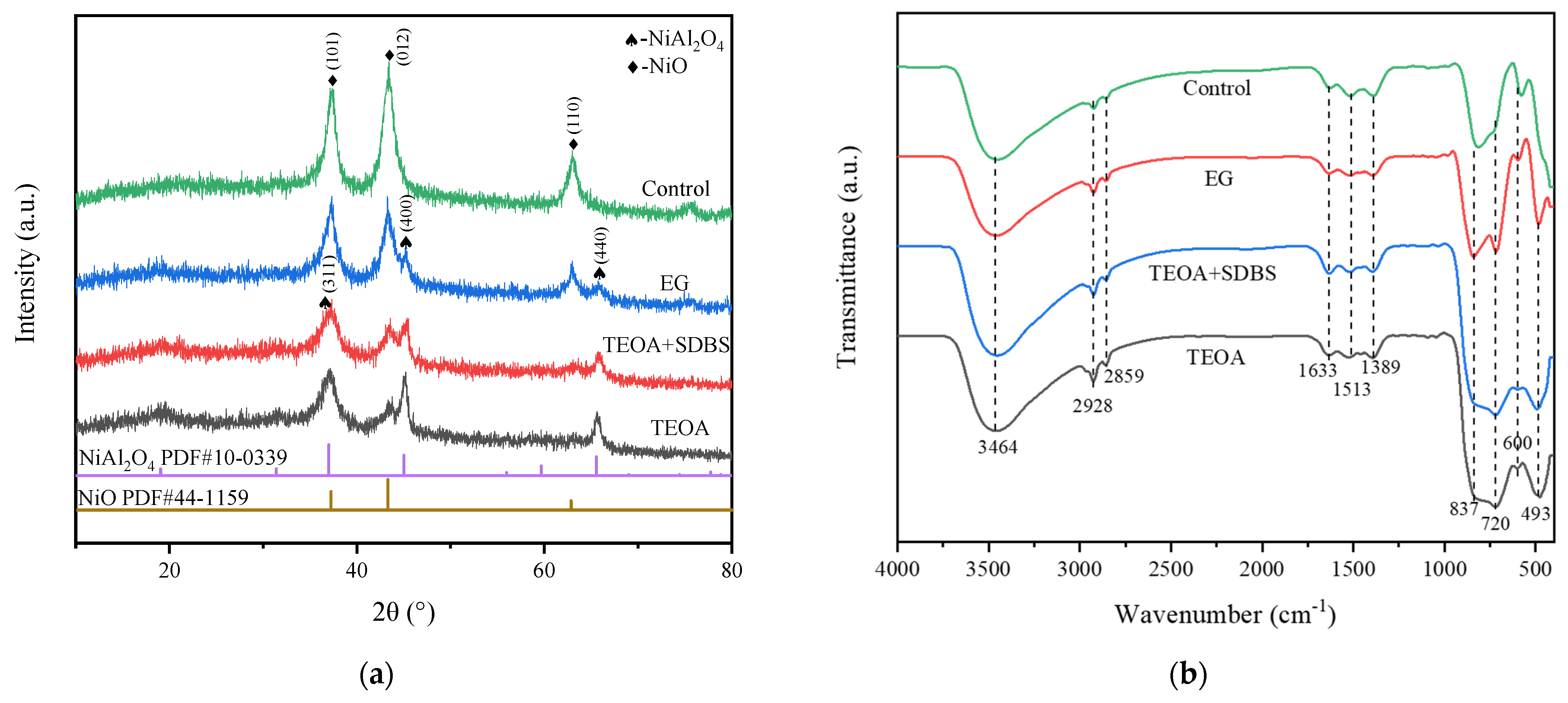
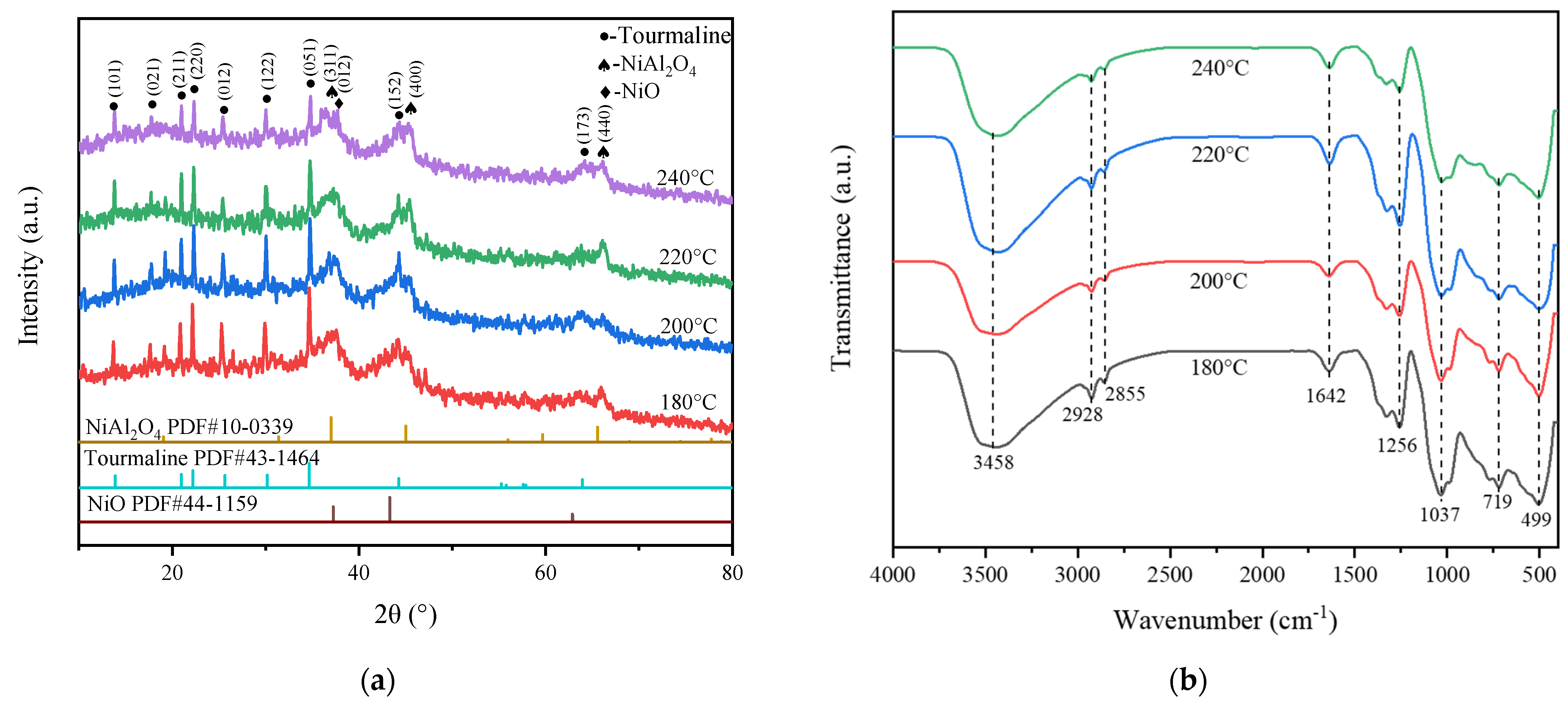
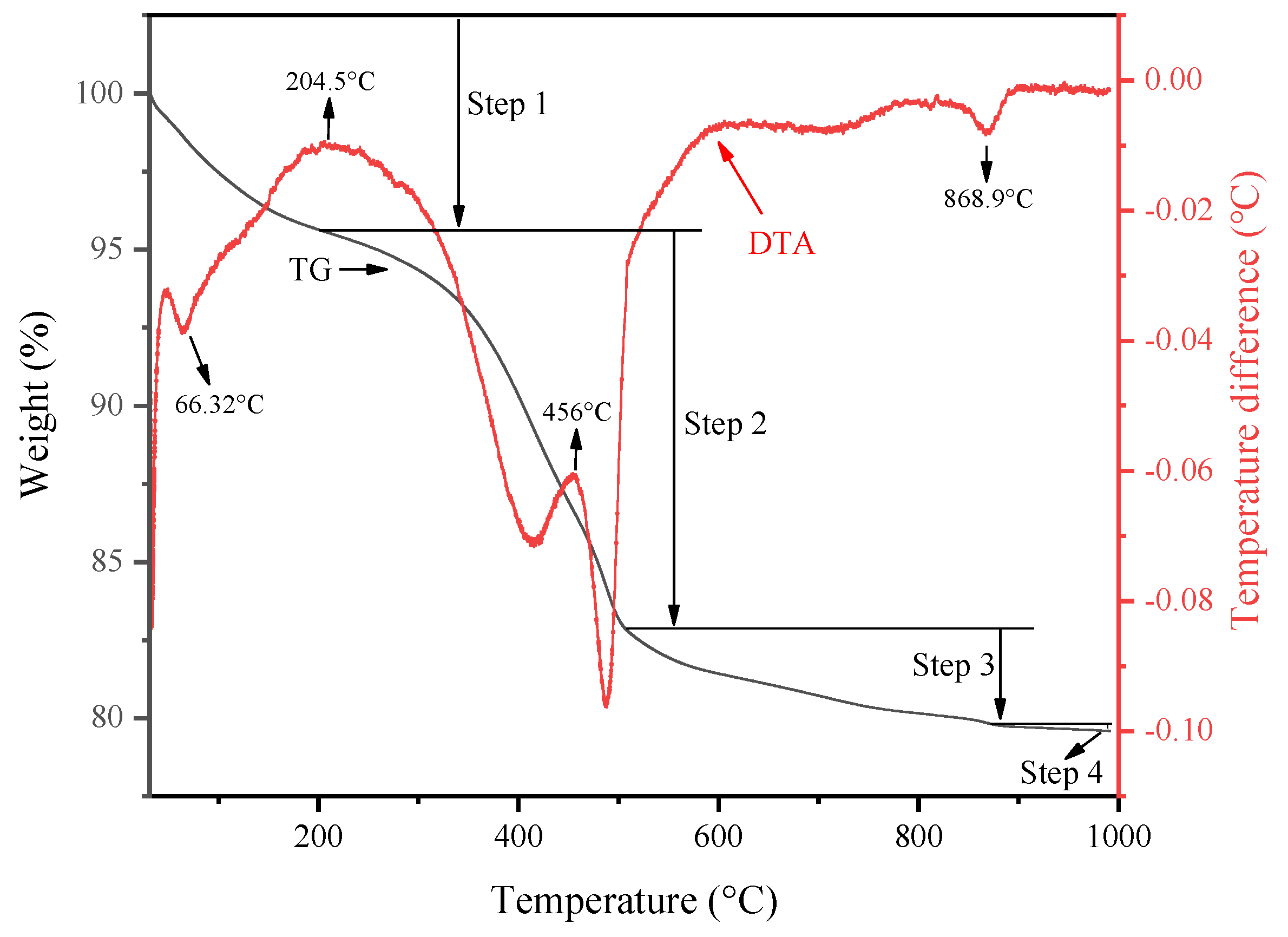
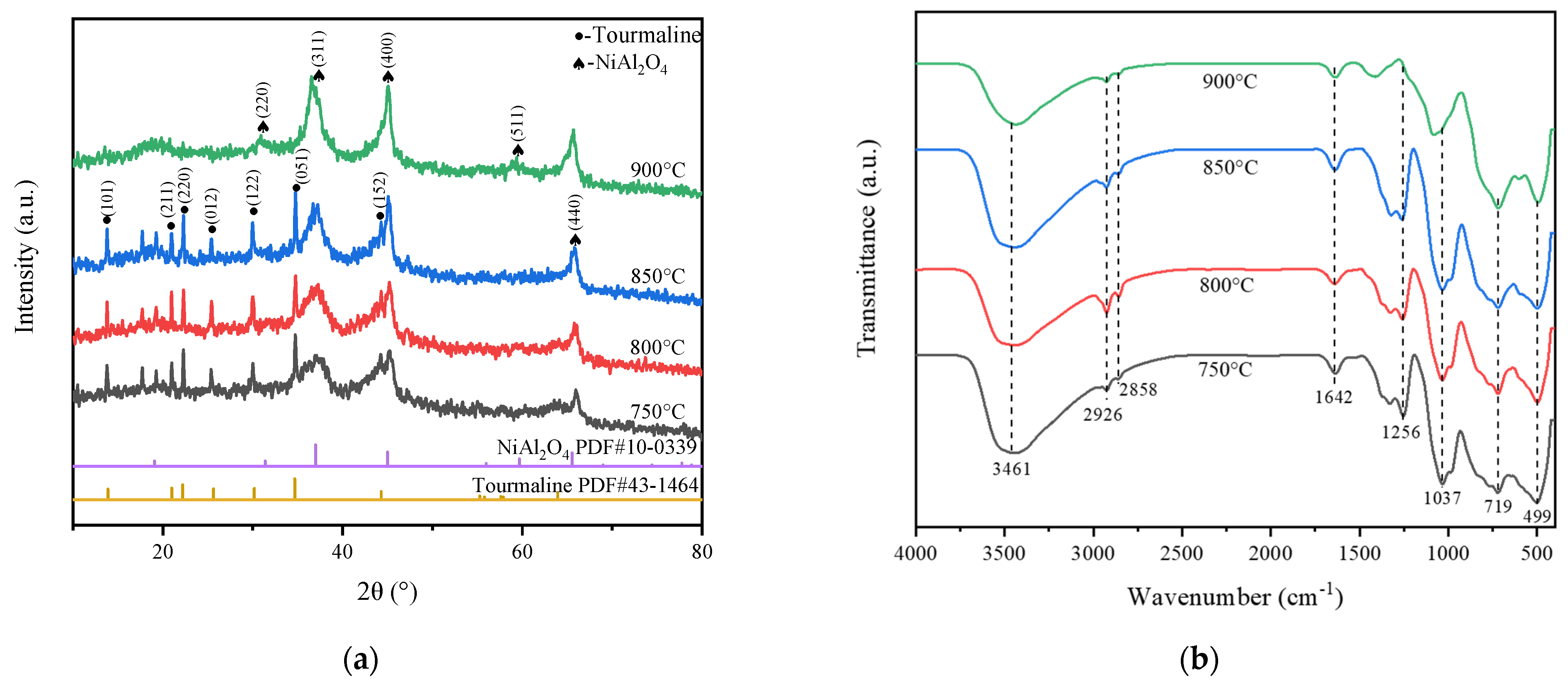
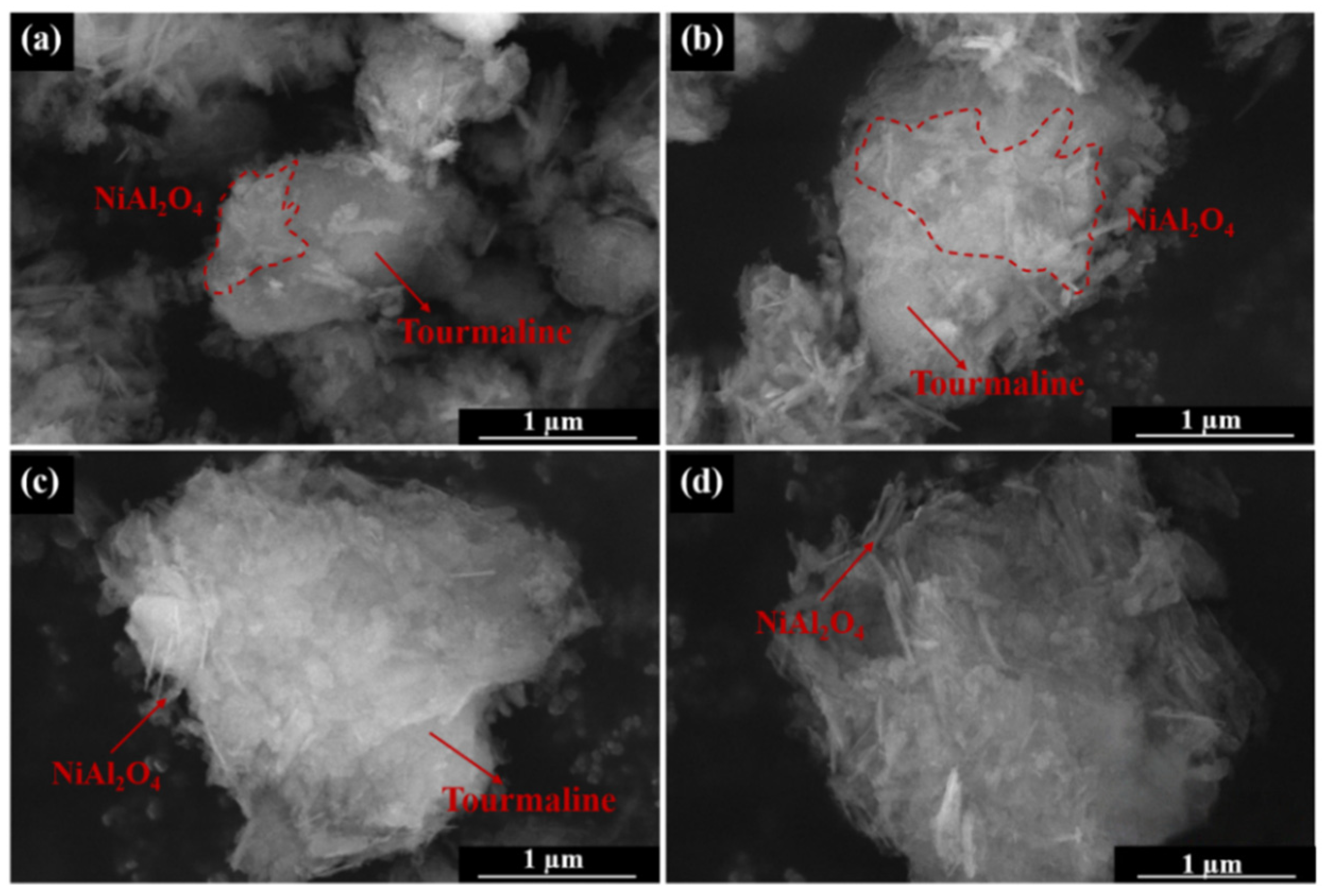
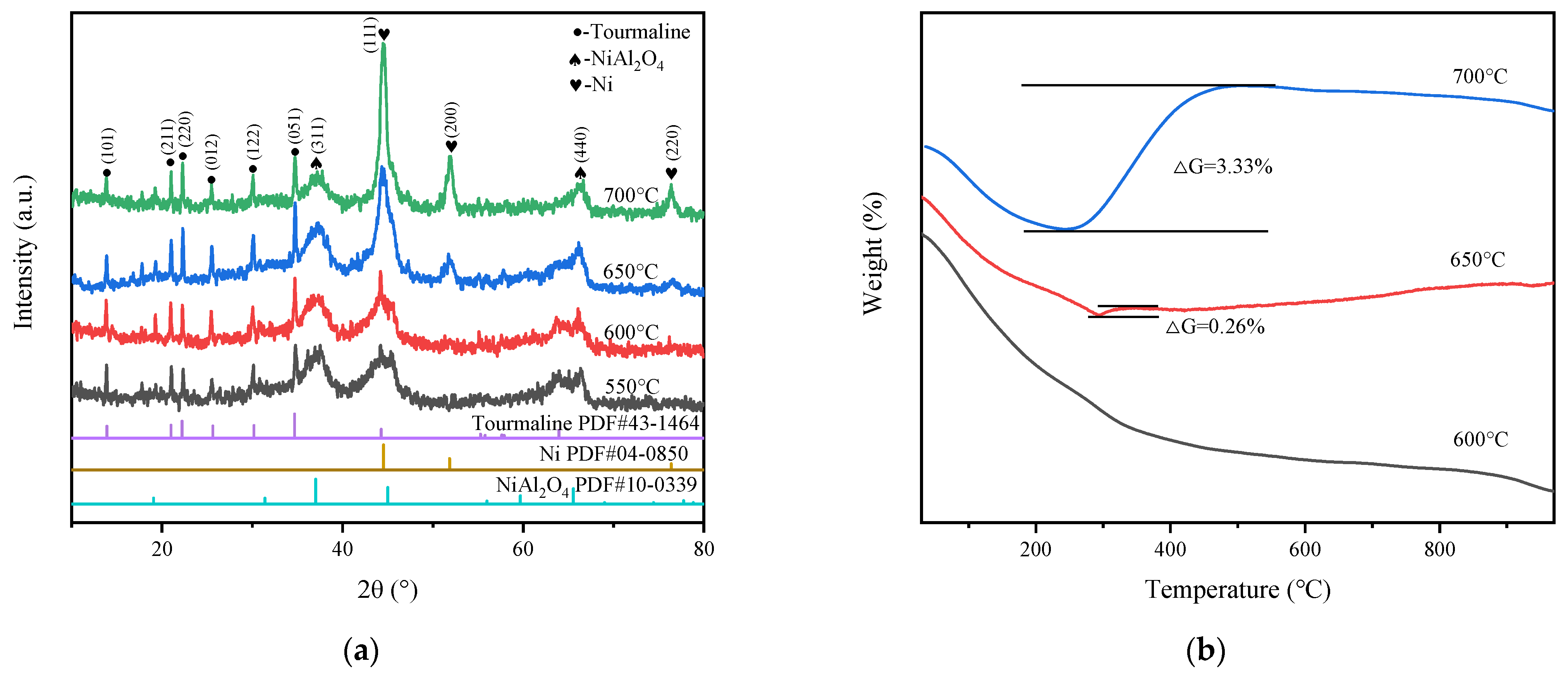
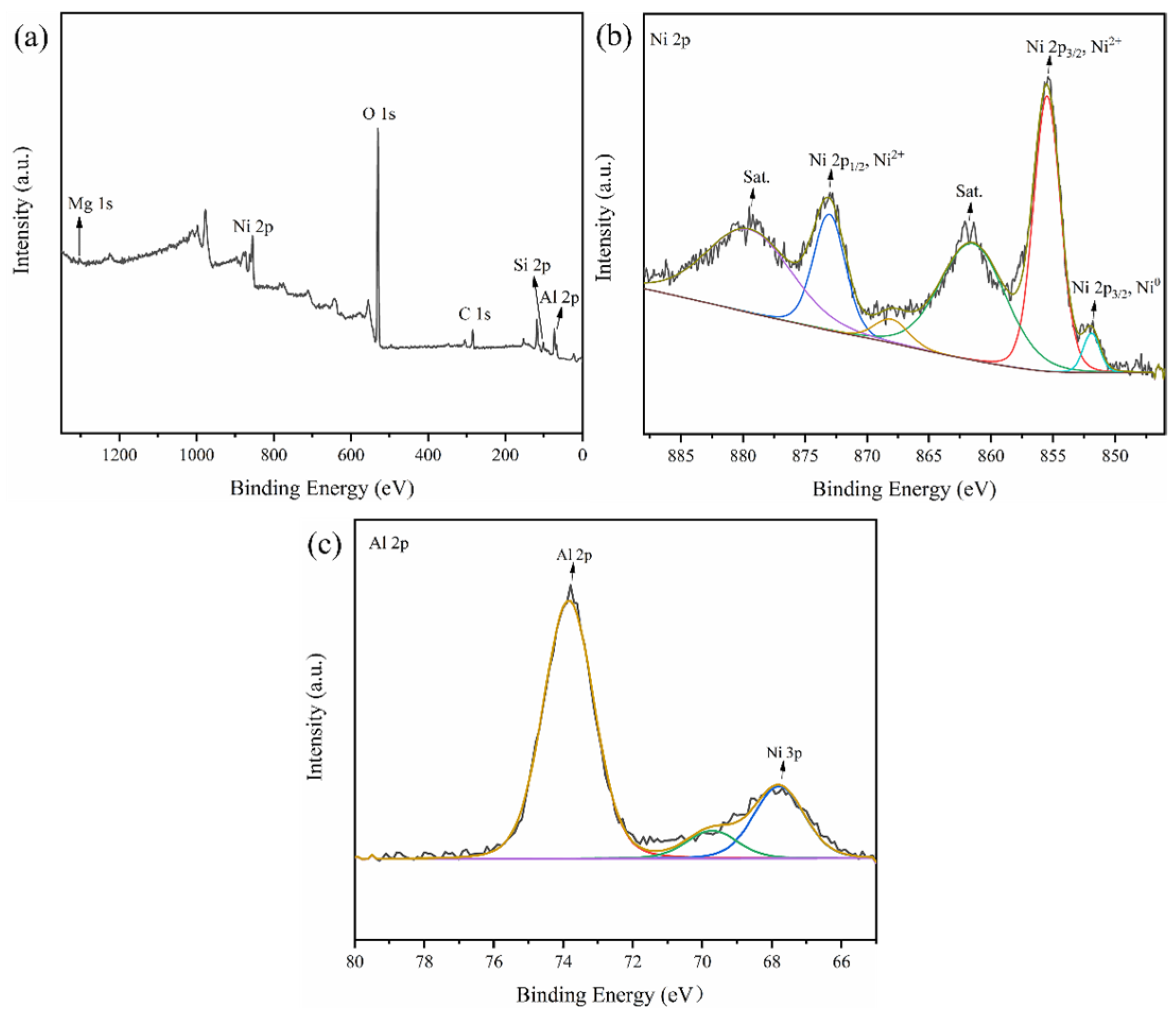
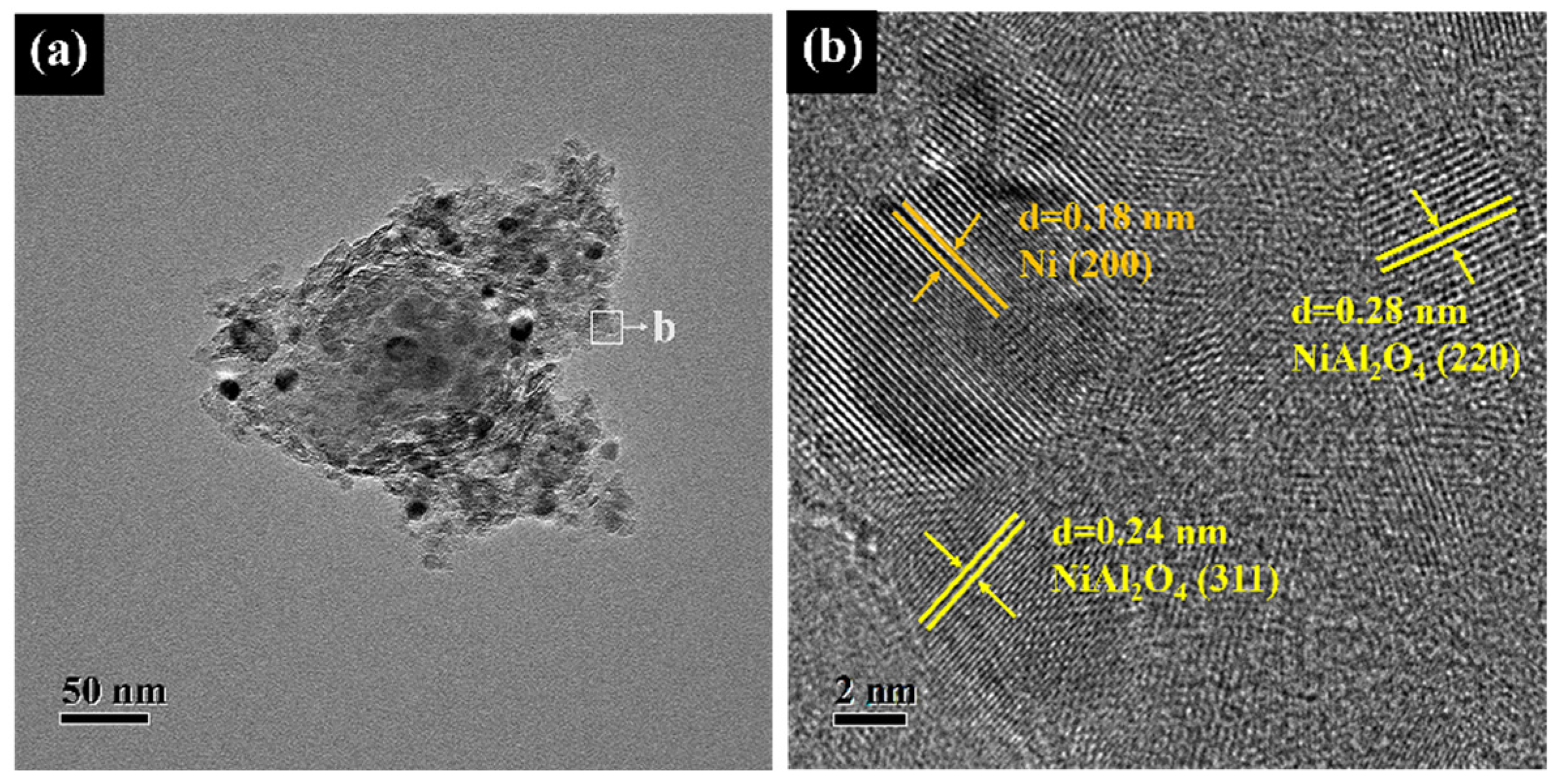
2.1. Catalysts Characterization
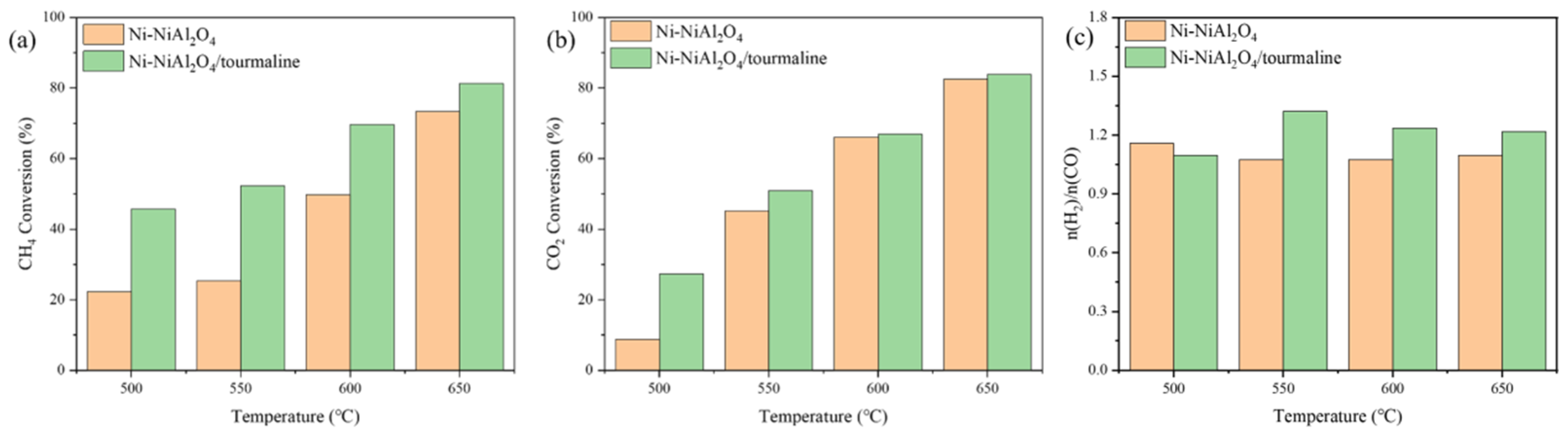
2.2. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ni-NiAl2O4/Tourmaline Composite
3.3. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Xie, X.; Ren, H.; Gao, X. A short review on nickel-based catalysts in dry reforming of methane: Influences of oxygen defects on anti-coking property. Mater. Today Proc. 2021, 42, 153–160. [Google Scholar] [CrossRef]
- Xie, T.; Zheng, H.-Y.; Xu, K.-D.; Zhang, Z.-Y.; Yang, B.-L.; Yu, B. High performance Ni-based porous catalytically activated absorbers and establishment of kinetic model for complex solar methane dry reforming reaction system. Chem. Eng. Sci. 2021, 239, 116625. [Google Scholar] [CrossRef]
- Grabchenko, M.; Pantaleo, G.; Puleo, F.; Kharlamova, T.S.; Zaikovskii, V.I.; Vodyankina, O.; Liotta, L.F. Design of Ni-based catalysts supported over binary La-Ce oxides: Influence of La/Ce ratio on the catalytic performances in DRM. Catal. Today 2021, 382, 71–81. [Google Scholar] [CrossRef]
- Plou, J.; Durán, P.; Herguido, J.; Peña, J.A. Hydrogen from synthetic biogas by catalyzed MDR and SIP: Screening of catalyst and iron oxide mixtures. Fuel 2015, 140, 470–476. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, L.; Chen, Y.; Shen, G.; Wang, L.; Zhang, X.; Zou, J.-J. Mo-doped Ni-based catalyst for remarkably enhancing catalytic hydrogen evolution of hydrogen-storage materials. Int. J. Hydrogen Energy 2020, 45, 15560–15570. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Ashok, J.; Kawi, S. A comprehensive review of anti-coking, anti-poisoning and anti-sintering catalysts for biomass tar reforming reaction. Chem. Eng. Sci. X 2020, 7, 100065. [Google Scholar] [CrossRef]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based nano-catalysts for the dry reforming of methane. Catal. Today 2020, 343, 26–37. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, M.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y. NixAl1O2-δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism. Appl. Catal. B Environ. 2021, 291, 120074. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional roles of second metals in catalyzing methane decomposition over the Ni-based catalysts for hydrogen production: A critical review. Int. J. Hydrogen Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Pan, C.; Guo, Z.; Dai, H.; Ren, R.; Chu, W. Anti-sintering mesoporous Ni-Pd bimetallic catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 16133–16143. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Wang, Z.; Jiang, B.; Kawi, S. Dry reforming of methane on Ni/mesoporous-Al2O3 catalysts: Effect of calcination temperature. Int. J. Hydrogen Energy 2021, 46, 31041–31053. [Google Scholar] [CrossRef]
- Martín-Jimeno, F.J.; Suárez-García, F.; Paredes, J.I.; Martínez-Alonso, A.; Tascón, J.M.D. Nickel nanoparticle/carbon catalysts derived from a novel aqueous-synthesized metal-organic framework for nitroarene reduction. J. Alloys Compd. 2021, 853, 157348. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Ticali, P.; Summa, P.; Mai, S.; Skorupska, K.; Behrens, M. Support effect on Ni-based mono- and bimetallic catalysts in CO2 hydrogenation. Nanoscale 2024, 16, 17378–17392. [Google Scholar] [CrossRef]
- Deng, G.; Nie, G.; He, X.; Li, L.; Sun, Z.; Duan, L. La2O3-Modified Ni-Based Catalysts Supported on Ordered Mesoporous Silicas for CO2 Methanation. ACS Appl. Nano Mater. 2024, 7, 28436–28447. [Google Scholar] [CrossRef]
- González-Ingelmo, M.; García, M.; Oropeza, F.; Alvarez, P.; Blanco, C.; Santamaría, R.; García Rocha, V. Ultra-high dispersion of Ni-based OER catalysts on graphene 3D networks enhances the in-situ Fe3+ catalytic activation. J. Mater. Chem. A 2023, 11, 24248–24260. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, P.; Cao, X.; Chen, J.; Pu, T.; Shi, B.; Xu, J.; Moon, J.; Wu, Z.; Han, Y.-F. Vacancy engineering of the nickel-based catalysts for enhanced CO2 methanation. Appl. Catal. B Environ. 2021, 282, 119561. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Volodin, A.A.; Fursikov, P.V.; Mozhzhuhin, S.A.; Lototskyy, M.V.; Yartys, V.A. Metal hydride-Graphene composites for hydrogen based energy storage. J. Alloys Compd. 2022, 896, 162881. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.H.; Choi, I.-G.; Choi, J.W. Enhancement of bio-oil hydrodeoxygenation activity over Ni-based bimetallic catalysts supported on SBA-15. Renew. Energy 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Liu, J.; Xiong, B. Mechanistic understanding of CO2 hydrogenation to methane over Ni/CeO2 catalyst. Appl. Surf. Sci. 2021, 558, 149866. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Jiang, S.; Yu, Q.; Xie, X.; Zhuang, R. Mesoporous molecular sieve-based materials for catalytic oxidation of VOC: A review. J. Environ. Sci. 2023, 125, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, D.F.P.; Thyssen, V.V.; De Almeida, P.M.M.; Gomes, R.S.; Borges, M.C.; Duarte De Farias, A.M.; Assaf, E.M.; Fraga, M.A.; Brandão, S.T. Renewable hydrogen from glycerol reforming over nickel aluminate-based catalysts. Catal. Today 2017, 289, 96–104. [Google Scholar] [CrossRef]
- Salhi, N.; Boulahouache, A.; Petit, C.; Kiennemann, A.; Rabia, C. Steam reforming of methane to syngas over NiAl2O4 spinel catalysts. Int. J. Hydrogen Energy 2011, 36, 11433–11439. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; De Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158–159, 190–201. [Google Scholar] [CrossRef]
- Menon, S.G.; Swart, H.C. Microwave-assisted synthesis of blue-green NiAl2O4 nanoparticle pigments with high near-infrared reflectance for indoor cooling. J. Alloys Compd. 2020, 819, 152991. [Google Scholar] [CrossRef]
- Srifa, A.; Kaewmeesri, R.; Fang, C.; Itthibenchapong, V.; Faungnawakij, K. NiAl2O4 spinel-type catalysts for deoxygenation of palm oil to green diesel. Chem. Eng. J. 2018, 345, 107–113. [Google Scholar] [CrossRef]
- Gayathri, R.C.; Elakkiya, V.; Sumathi, S. Effect of method of preparation on the photocatalytic activity of NiAl2O4. Inorg. Chem. Commun. 2021, 129, 108634. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X.; Liu, H.; Chen, Y.; Dong, S.; Ji, M.; Chen, D.; Xu, C.; Xie, H.; Zhu, C.; et al. Comparative study of mineral with different structures supported Fe-Ni catalysts for steam reforming of toluene. Fuel 2022, 315, 123253. [Google Scholar] [CrossRef]
- Han, F.; Liu, Q.; Li, D.; Ouyang, J. An emerging and high-performance sepiolite-supported Ni catalyst for low-temperature CO2 methanation: The critical role of hydroxyl groups. J. Environ. Chem. Eng. 2023, 11, 110331. [Google Scholar] [CrossRef]
- Daza-Gómez, L.-C.; Ruiz-Ruiz, V.-F.; Mendoza-Nieto, J.A.; Pfeiffer, H.; Díaz, D. Co3O4 nanostructures and Co3O4 supported on halloysite nanotubes: New highly active and thermally stable feasible catalysts for CO oxidation. Appl. Clay Sci. 2020, 190, 105590. [Google Scholar] [CrossRef]
- Zhou, P.; Han, X.; Wu, L.; Qi, M.; Shen, Y.; Nie, J.; Liu, X.; Wang, F.; Bian, L.; Liang, J. Facile construction of novel BiOBr/black-TiO2/tourmaline composites for the synergistic degradation of tetracycline in aqueous. Sep. Purif. Technol. 2025, 362, 131976. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Qiao, G.; Wang, X.; Lu, G.; Cui, H.; Wang, X. Sepiolite/amorphous nickel hydroxide hierarchical structure for high capacitive supercapacitor. J. Alloys Compd. 2021, 881, 160519. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Li, T.; Zhang, C.; Zhu, L.; Wang, S. Selective hydrodeoxygenation of lignin-derived phenolics to cycloalkanes over highly stable NiAl2O4 spinel-supported bifunctional catalysts. Chem. Eng. J. 2022, 429, 132181. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, X.; Ullah, M.; He, L.; Ikram, M.; Khan, M.; Ma, L.; Li, L.; Zhang, G.; Shi, K. Three-dimensional flower-like Ni9S8/NiAl2O4 nanocomposites composed of ultra-thin porous nanosheets: Fabricated, characterized and ultra-fast NOx gas sensors at room temperature. J. Alloys Compd. 2020, 825, 154151. [Google Scholar] [CrossRef]
- Roniboss, A.; Subramani, A.; Ramamoorthy, R.; Yuvaraj, S.; Sundararajan, M.; Dash, C.S. Investigation of structural, optical and magnetic behavior of MAl2O4 (M = Zn and Co) nanoparticles via microwave combustion technique. Mater. Sci. Semicond. Process. 2021, 123, 105507. [Google Scholar] [CrossRef]
- Tangcharoen, T.; T-Thienprasert, J.; Kongmark, C. Effect of calcination temperature on structural and optical properties of MAl2O4 (M = Ni, Cu, Zn) aluminate spinel nanoparticles. J. Adv. Ceram. 2019, 8, 352–366. [Google Scholar] [CrossRef]
- Yang, H.; Mu, B.; Wang, Q.; Xu, J.; Wang, A. Resource and sustainable utilization of quartz sand waste by turning into cobalt blue composite pigments. Ceram. Int. 2021, 47 Pt A, 13806–13813. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Li, G.; Cui, B.; Wei, D.; Shen, Y. Synthesis of clinoptilolite-supported BiOCl/TiO2 heterojunction nanocomposites with highly-enhanced photocatalytic activity for the complete degradation of xanthates under visible light. Chem. Eng. J. 2021, 407, 126697. [Google Scholar] [CrossRef]
- Babińska, J.; Dyrek, K.; Pieczka, A.; Sojka, Z.J.E.J.O.M. X and Q band EPR studies of paramagnetic centres in natural and heated tourmaline. Eur. J. Mineral. 2008, 20, 233–240. [Google Scholar] [CrossRef]
- Benrabaa, R.; Barama, A.; Boukhlouf, H.; Guerrero-Caballero, J.; Rubbens, A.; Bordes-Richard, E.; Löfberg, A.; Vannier, R.-N. Physico-chemical properties and syngas production via dry reforming of methane over NiAl2O4 catalyst. Int. J. Hydrogen Energy 2017, 42, 12989–12996. [Google Scholar] [CrossRef]
- Yancheshmeh, M.S.; Sahraei, O.A.; Aissaoui, M.; Iliuta, M.C. A novel synthesis of NiAl2O4 spinel from a Ni-Al mixed-metal alkoxide as a highly efficient catalyst for hydrogen production by glycerol steam reforming. Appl. Catal. B Environ. 2020, 265, 118535. [Google Scholar] [CrossRef]
- Yang, L.; Bukhovko, M.P.; Malek, A.; Li, L.; Jones, C.W.; Agrawal, P.K.; Davis, R.J. Steam reforming of ethylene over nickel-based spinel oxides. Appl. Catal. A Gen. 2020, 603, 117739. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Liang, J.; Fang, B.; Piao, Y.A.; Hao, M.; Wang, Z. Tourmaline-Modified FeMnTiOx Catalysts for Improved Low-Temperature NH3-SCR Performance. Environ. Sci. Technol. 2019, 53, 6989–6996. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, X.; Zhou, P.; Bian, L.; Wang, F. A Simple Fabrication of Tourmaline-Supported Ni-NiAl2O4 Nanocomposites for Enhanced Methane Dry Reforming Activity. Catalysts 2025, 15, 658. https://doi.org/10.3390/catal15070658
Wang J, Wang X, Zhou P, Bian L, Wang F. A Simple Fabrication of Tourmaline-Supported Ni-NiAl2O4 Nanocomposites for Enhanced Methane Dry Reforming Activity. Catalysts. 2025; 15(7):658. https://doi.org/10.3390/catal15070658
Chicago/Turabian StyleWang, Jin, Xianku Wang, Pengfei Zhou, Liang Bian, and Fei Wang. 2025. "A Simple Fabrication of Tourmaline-Supported Ni-NiAl2O4 Nanocomposites for Enhanced Methane Dry Reforming Activity" Catalysts 15, no. 7: 658. https://doi.org/10.3390/catal15070658
APA StyleWang, J., Wang, X., Zhou, P., Bian, L., & Wang, F. (2025). A Simple Fabrication of Tourmaline-Supported Ni-NiAl2O4 Nanocomposites for Enhanced Methane Dry Reforming Activity. Catalysts, 15(7), 658. https://doi.org/10.3390/catal15070658







