Mechanistic Behavior of Basicity of Bimetallic Ni/ZrO2 Mixed Oxides for Stable Oxythermal Reforming of CH4 with CO2
Abstract
1. Introduction
2. Result and Discussion
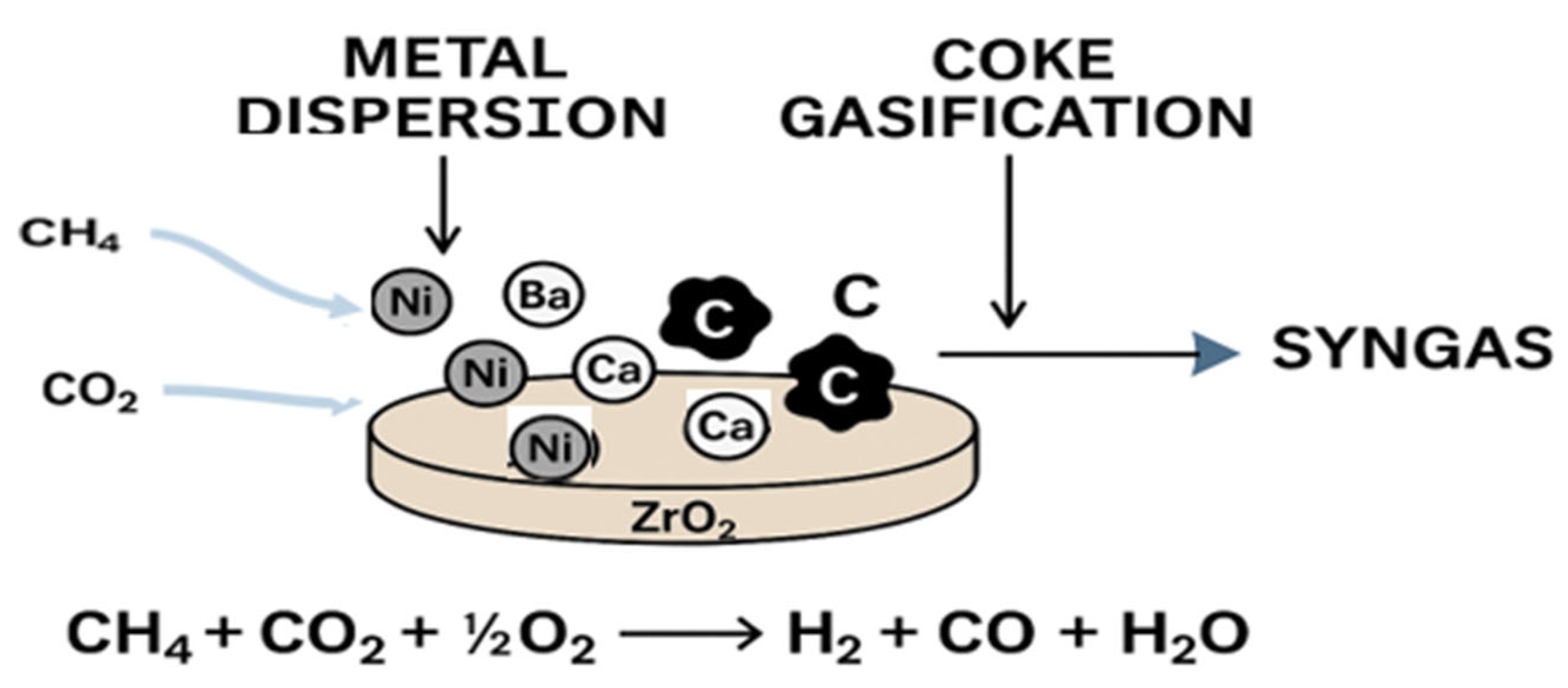
2.1. Catalyst Surface Properties
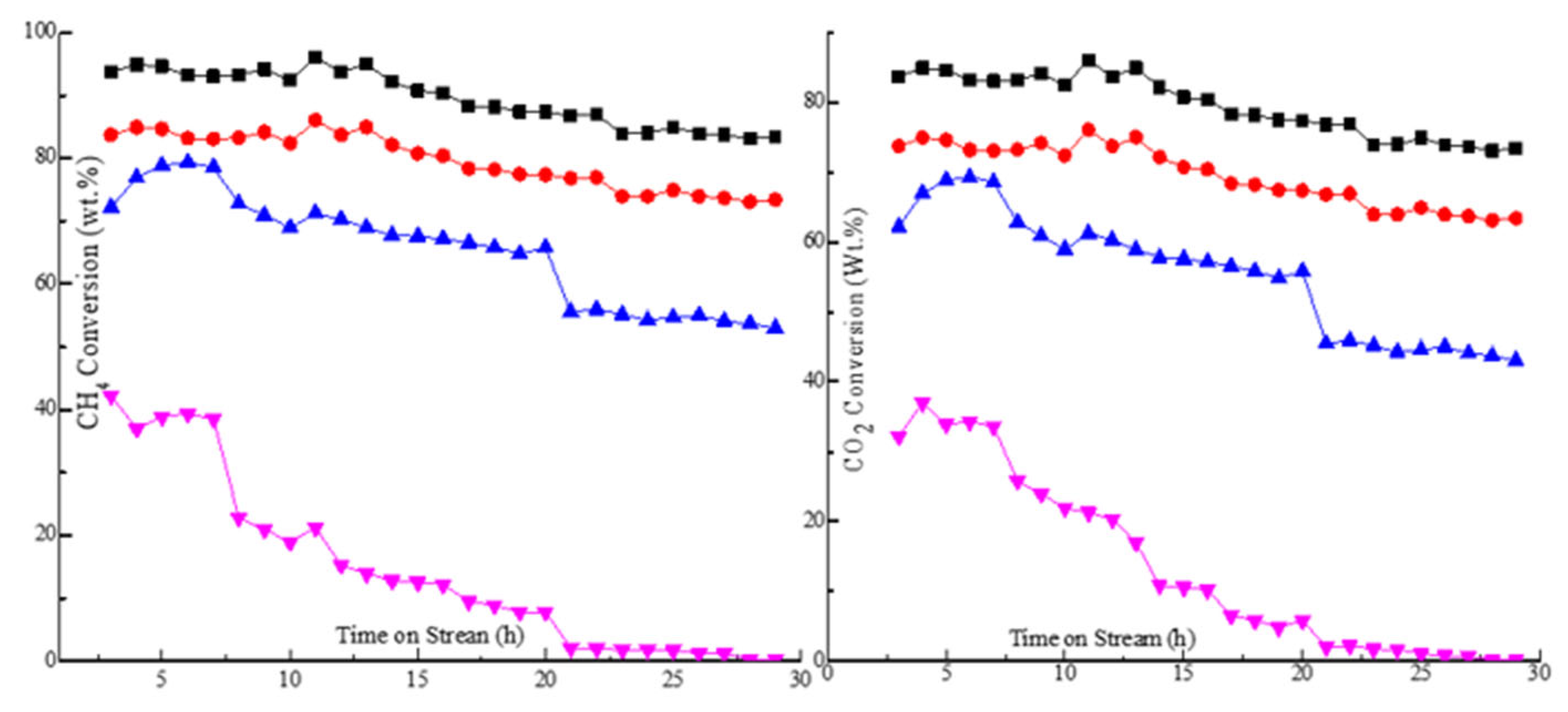
2.2. Effect of Ba and Ca Metal Loading on Oxythermal Reforming
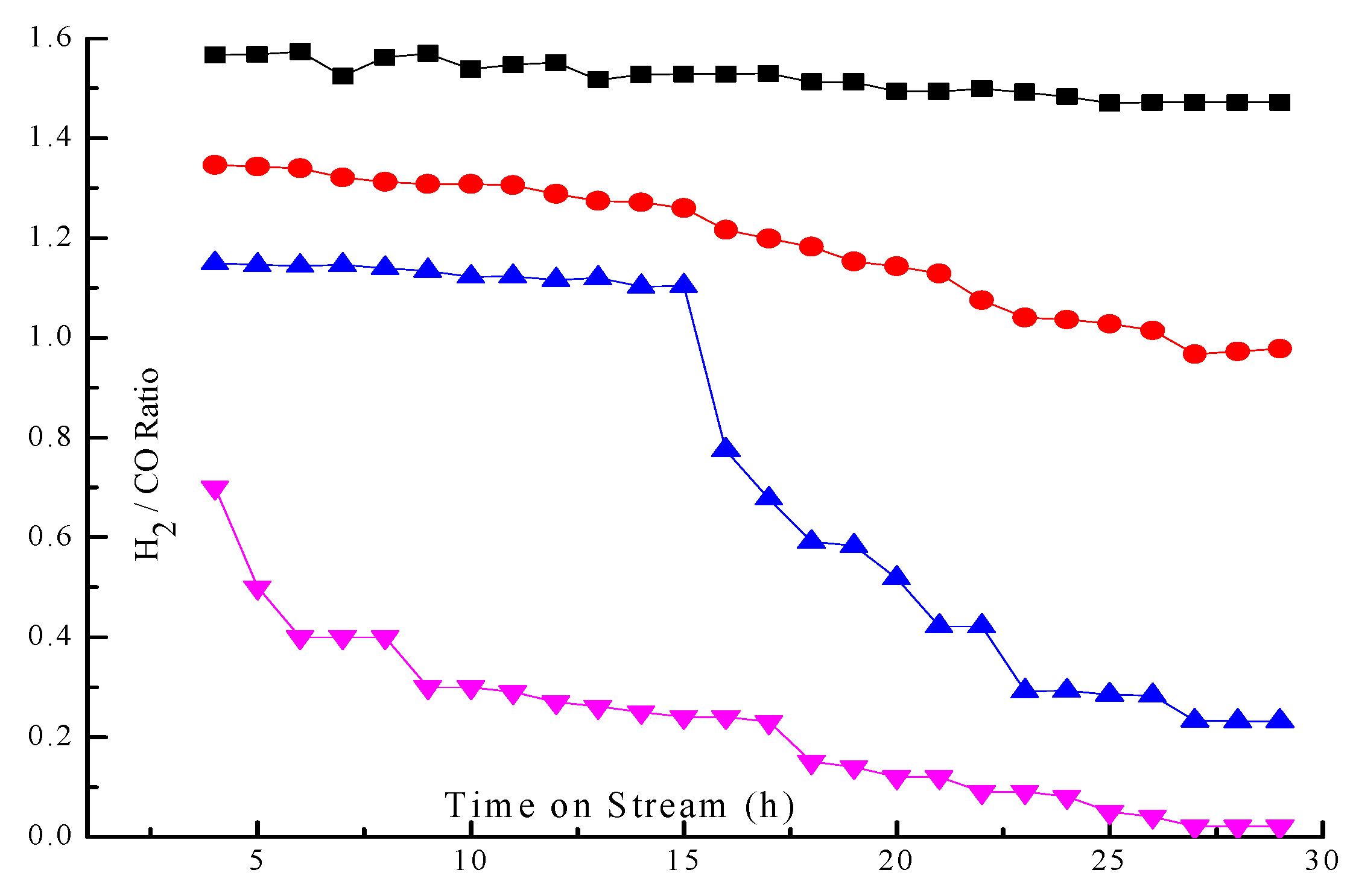
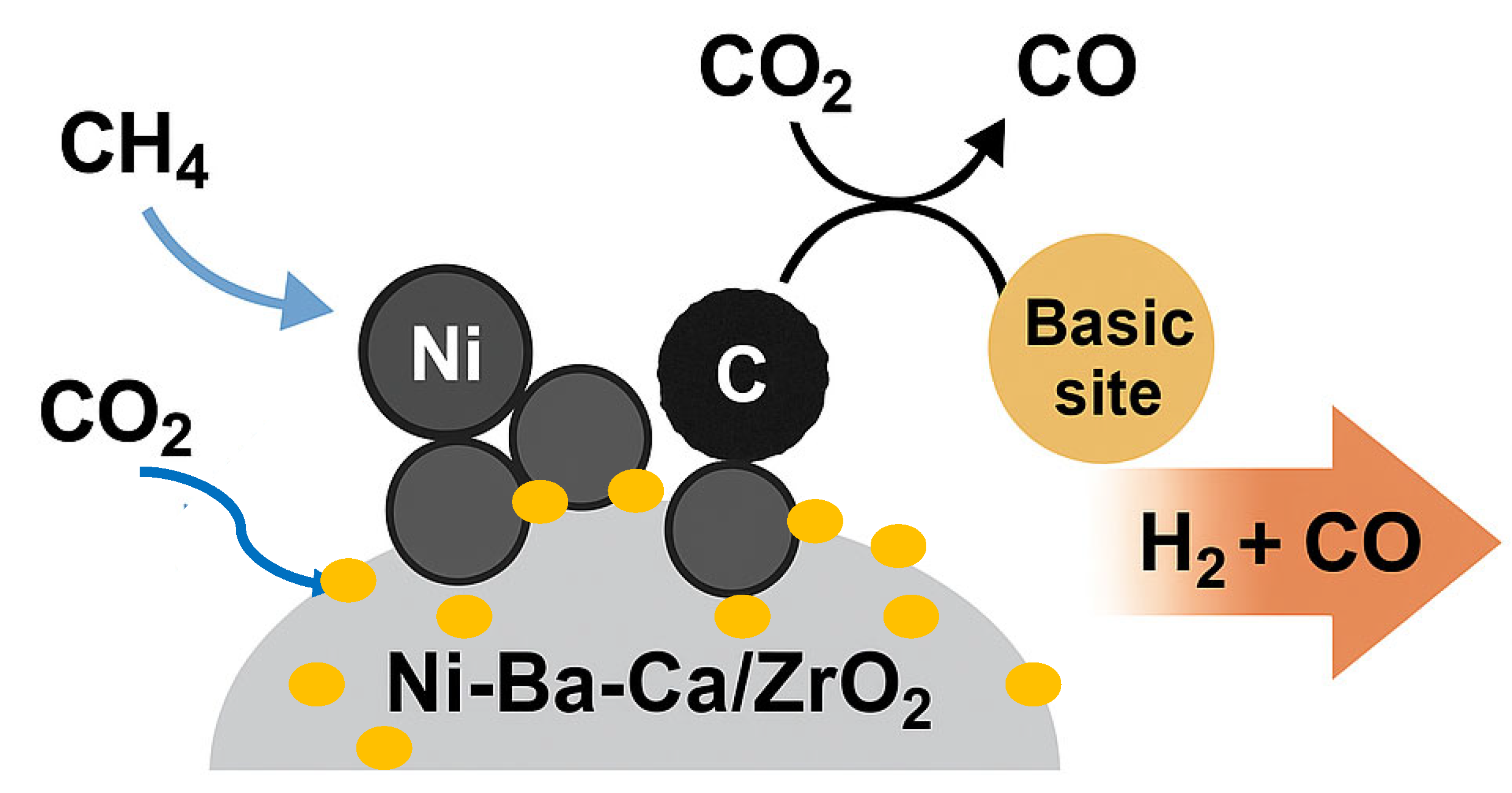
2.3. Effect of Basicity on Conversion and Selectivity
3. Experimental Methods
3.1. Synthesis of Mixed Oxide Catalytic Materials
3.2. Characterization and Catalytic Activity
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Matteo, D.; Joshua, M. Quantifying the flexibility of hydrogen production systems to support large-scale renewable energy integration. J. Power Sources 2018, 399, 383–391. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L. Modeling and optimization of an energy generation island based on renewable technologies and hydrogen storage systems. Int. J. Hydrogen Energy 2012, 37, 2081–2093. [Google Scholar] [CrossRef]
- Avril, S.; Arnaud, S.; Florentin, G.; Vinard, A. Multi-objective optimization of batteries and hydrogen storage technologies for remote photovoltaic systems. Energy 2010, 35, 5300–5308. [Google Scholar] [CrossRef]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Current advances in syngas (CO + H2) production through bi-reforming of methane using various catalysts: A review. Int. J. Hydrogen Energy 2021, 46, 32809–32845. [Google Scholar] [CrossRef]
- Pereñguez, R.; González-DelaCruz, V.; Holgado, J.; Caballero, A. Modifying the Size of Nickel Metallic Particles H2/CO Treatment in Ni/ZrO2 Methane Dry Reforming Catalysts. Appl. Catal. B 2011, 1, 82–88. [Google Scholar] [CrossRef]
- Moradi, A.; Rahmanzadeh, G.R.; Sharifnia, M.S. Kinetic investigation of CO2 reforming of CH4 over La-Ni based perovskite. Chem. Eng. J. 2010, 162, 787–791. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Ni Loadings on the Activity and Coke Formation of MgO-Modified Ni/Al2O3Nanocatalyst in Dry Reforming of Methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2020, 284, 119082. [Google Scholar] [CrossRef]
- Nahar, G. Hydrogen production from reforming of biogas: Review of technological advances and an Indian perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Bhavani, A.G.; Kim, W.Y.; Kim, J.Y.; Lee, J.S. Improved activity and coke resistance by promoters of nanosized trimetallic catalysts for autothermal carbon dioxide reforming of methane. Appl. Catal. A Gen. 2013, 450, 63–72. [Google Scholar] [CrossRef]
- Pal, D.B. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, E.S.; Regli, K.S.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling Enhanced Activity, Selectivity, and Coke Resistance of Pt–Ni Bimetallic Clusters in Dry Reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Gu, Y.; Yang, S.; Tang, Z.; Sun, Y.; Wu, Q. Techno-economic evaluation of CO2-rich natural gas dry reforming for linear alpha olefins production. Energy Convers. Manag. 2020, 205, 112348. [Google Scholar] [CrossRef]
- Tatsuya, T.; Shin-Nosuke, F.; Masashi, I.; Koichi, E. Autothermal reforming of methane over Ni catalysts supported over CaO–CeO2–ZrO2 solid solution. Appl. Catal. A Gen. 2003, 240, 223–233. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, Q.; Zhang, J.; Sun, Y.; Zhu, Y. NiO-MgO nanoparticles confined inside SiO2 frameworks to achieve highly catalytic performance for CO2 reforming of methane. Mole. Catal. 2017, 432, 31–36. [Google Scholar] [CrossRef]
- Barzegari, F.; Rezaei, M.; Kazemeini, M.; Farhadi, F.; Keshavarz, A. The Influence of Lanthanide on NiO-MgO-SiO2 Catalysts for Syngas Production via propane steam reforming. Mole. Catal. 2020, 499, 111281. [Google Scholar] [CrossRef]
- Yahia Ahmad, H.; Assem, T.; Mohamed, T.; Hany, A.; El-Sayed, A.K.; Al-Qaradawi, S.Y. Design of Ni/La2O3 catalysts for dry reforming of methane: Understanding the impact of synthesis methods. Int. J. Hydrogen Energy 2022, 47, 41294–41309. [Google Scholar] [CrossRef]
- Yasotha, K.; Warintorn, T.; Kesada, S.; Sibudjing, K. Inverse NiAl2O4 on LaAlO3–Al2O3: Unique Catalytic Structure for Stable CO2 Reforming of Methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Pant, K.K.; Choudary, N.V. Tuning the Metal-Support Interaction of Methane Tri-Reforming Catalysts for Industrial Flue Gas Utilization. Int. J. Hydrogen Energy 2020, 45, 1911–1929. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef]
- Min, J.-E.; Lee, Y.-J.; Park, H.-G.; Zhang, C.; Jun, K.-W. Carbon Dioxide Reforming of Methane on Ni–MgO–Al2O3 Catalysts Prepared by Sol–Gel Method: Effects of Mg/Al Ratios. J. Ind. Eng. Chem. 2015, 26, 375–383. [Google Scholar] [CrossRef]
- Fei, J.; Hou, Z.; Zheng, X.; Yashima, T. Doped Ni Catalysts for Methane Reforming with CO2. Catal. Lett. 2004, 98, 241–246. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Bobadilla, L.F.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. CO2 Reforming of Methane over Ni-Ru Supported Catalysts: On the Nature of Active Sites by Operando DRIFTS Study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar] [CrossRef]
- Katheria, S.; Kunzru, D.; Deo, G. Kinetics of Steam Reforming of Methane on Rh–Ni/MgAl2O4 Catalyst. React. Kinet. Mech. Catal. 2020, 130, 91–101. [Google Scholar] [CrossRef]
- Álvarez Moreno, A.; Ramirez-Reina, T.; Ivanova, S.; Roger, A.-C.; Centeno, M.Á.; Odriozola, J.A. Bimetallic Ni–Ru and Ni–Re Catalysts for Dry Reforming of Methane: Understanding the Synergies of the Selected Promoters. Front. Chem. 2021, 9, 694976. [Google Scholar] [CrossRef] [PubMed]
- Torimoto, M.; Sekine, Y. Effects of Alloying for Steam or Dry Reforming of Methane: A Review of Recent. Studies. Catal. Sci. Technol. 2022, 12, 3387–3411. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Chatterjee, S.; Huang, K.-W. The Insignificant Role of Dry Reforming of Methane in CO2 Emission Relief. ACS Energy Lett. 2020, 5, 2881–2885. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Batchu, R.; Buelens, L.C.; Detavernier, C.; Marin, G.B. Mechanism of Carbon Deposits Removal from Supported Ni Catalysts. Appl. Catal. B Environ. 2018, 239, 502–512. [Google Scholar] [CrossRef]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry Reforming of Methane with Carbon Dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Kwon, D.; Kang, J.Y.; An, S.; Yang, I.; Jung, J.C. Tuning the Base Properties of Mg–Al Hydrotalcite Catalysts Using Their Memory Effect. J. Energy Chem. 2020, 46, 229–236. [Google Scholar] [CrossRef]
- Adamu, S.; Bawah, A.-R.; Muraza, O.; Malaibari, Z.; Hossain, M.M. Effects of Metal Support Interaction on Dry Reforming of Methane over Ni/Ce-Al2O3 Catalysts. Can. J. Chem. Eng. 2020, 98, 2425–2434. [Google Scholar] [CrossRef]
- Hong Phuong, P.; Cam Anh, H.; Tri, N.; Phung Anh, N.; Cam Loc, L. Effect of Support on Stability and Coke Resistance of Ni-Based Catalyst in Combined Steam and CO2 Reforming of CH4. ACS Omega 2022, 7, 20092–20103. [Google Scholar] [CrossRef]
- De Coster, V.; Srinath, N.V.; Theofanidis, S.A.; Pirro, L.; Van Alboom, A.; Poelman, H.; Sabbe, M.K.; Marin, G.B.; Galvita, V.V. Looking inside a Ni-Fe/MgAl2O4 Catalyst for Methane Dry Reforming via Mössbauer Spectroscopy and in Situ QXAS. Appl. Catal. B Environ. 2022, 300, 120720. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Xuan, T.N.; Filippo, B.; Claudio, E.; Vladimiro, D.S.; Vladimiro, D.S. Methane Reforming Processes: Advances on Mono- and Bimetallic Ni-Based Catalysts Supported on Mg-Al Mixed Oxides. Catalysts 2023, 13, 379. [Google Scholar] [CrossRef]

| Catalyst Code a | Metal Content wt.% | Elemental Analysis% | ||||||
|---|---|---|---|---|---|---|---|---|
| Ni | Ba | Ca | Zr | Ni | Ba | Ca | Zr | |
| Ni/ZrO2 | 20 | 0 | 0 | 80 | 19.97 | 0 | 0 | 78.98 |
| Ni–Ba/ZrO2 | 20 | 10 | 0 | 70 | 19.91 | 9.89 | 0 | 69.91 |
| Ni–Ca/ZrO2 | 20 | 0 | 10 | 70 | 19.97 | 0 | 9.91 | 69.89 |
| Ni–Ba–Ca/ZrO2 | 20 | 5 | 5 | 70 | 19.98 | 4.82 | 4.81 | 69.87 |
| Catalyst Code | Surface Area m2/g a | Pore Diameter (nm) a | Pore Volume (cm3/g) a | Metal Dispersion (%) b | Metallic Surface Area m2/g b | Coke Content wt.% c |
|---|---|---|---|---|---|---|
| Ni/ZrO2 | 58.71 | 2.09 | 0.02 | 0.1 | 0.01 | 19.46 |
| Ni–Ba/ZrO2 | 131.41 | 7.1 | 0.57 | 4.8 | 2.1 | 3.57 |
| Ni–Ca/ZrO2 | 145.18 | 7.6 | 0.61 | 5.2 | 2.6 | 1.68 |
| Ni–Ba–Ca/ZrO2 | 157.15 | 8.3 | 0.67 | 7.4 | 3.1 | 0.90 |
| Catalyst Code | Basic Sites µmol/g | |||
|---|---|---|---|---|
| Weak (<200 °C) | Medium (200–350 °C) | Strong (350–600 °C) | Very Strong (>600 °C) | |
| Ni/ZrO2 | 12.41 | 5.1 | 0.31 | 2.6 |
| Ni–Ba/ZrO2 | 5.39 | 0.1 | 0.41 | 19.6 |
| Ni–Ca/ZrO2 | 11.24 | 0.8 | 1.97 | 39.8 |
| Ni–Ba–Ca/ZrO2 | 15.91 | 1.3 | 2.7 | 51.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bong, H.J.; Subba Reddy, N.G.; Bhavani, A.G. Mechanistic Behavior of Basicity of Bimetallic Ni/ZrO2 Mixed Oxides for Stable Oxythermal Reforming of CH4 with CO2. Catalysts 2025, 15, 700. https://doi.org/10.3390/catal15080700
Bong HJ, Subba Reddy NG, Bhavani AG. Mechanistic Behavior of Basicity of Bimetallic Ni/ZrO2 Mixed Oxides for Stable Oxythermal Reforming of CH4 with CO2. Catalysts. 2025; 15(8):700. https://doi.org/10.3390/catal15080700
Chicago/Turabian StyleBong, Hyuk Jong, Nagireddy Gari Subba Reddy, and A. Geetha Bhavani. 2025. "Mechanistic Behavior of Basicity of Bimetallic Ni/ZrO2 Mixed Oxides for Stable Oxythermal Reforming of CH4 with CO2" Catalysts 15, no. 8: 700. https://doi.org/10.3390/catal15080700
APA StyleBong, H. J., Subba Reddy, N. G., & Bhavani, A. G. (2025). Mechanistic Behavior of Basicity of Bimetallic Ni/ZrO2 Mixed Oxides for Stable Oxythermal Reforming of CH4 with CO2. Catalysts, 15(8), 700. https://doi.org/10.3390/catal15080700









