Recent Progress in Photocatalytic Hydrogen Production Using 2D MoS2 Based Materials
Abstract
1. Introduction
2. Synthetic Methods for the Preparation of MoS2
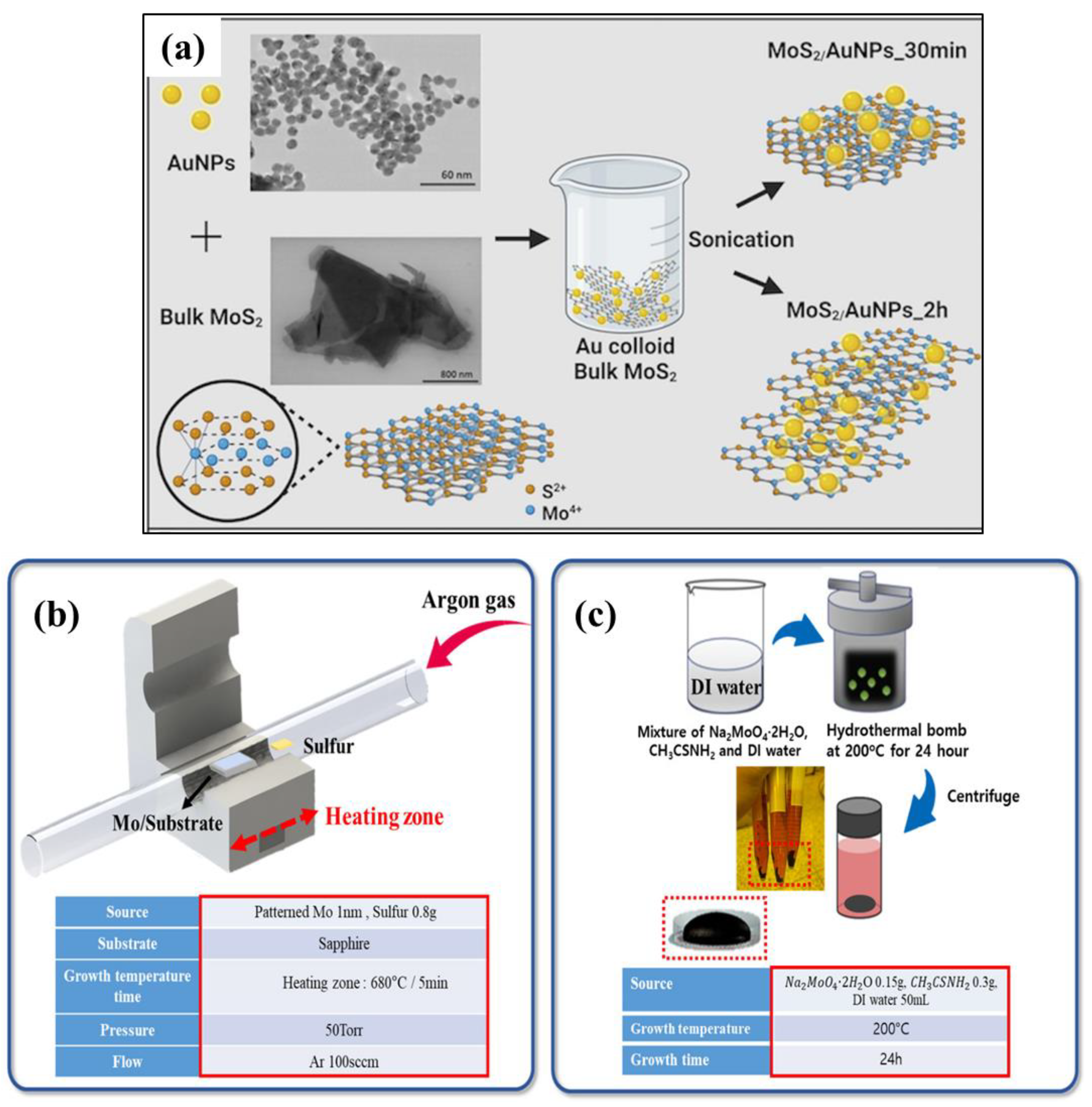
2.1. Exfoliation Approach
2.2. Physical Vapor Deposition (PVD) Approach
2.3. Chemical Vapor Deposition (CVD) Approach
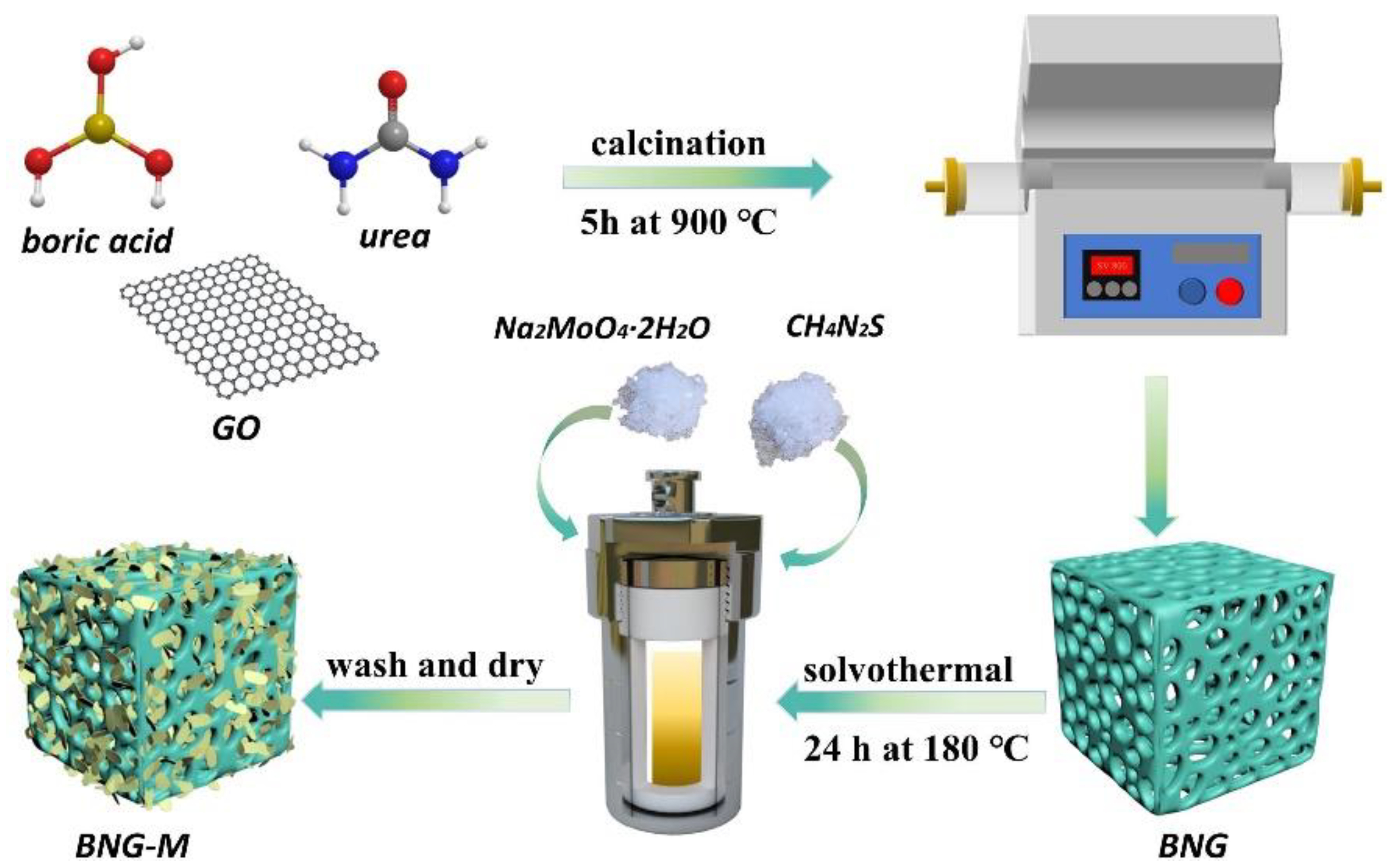
2.4. Hydrothermal/Solvothermal Approach
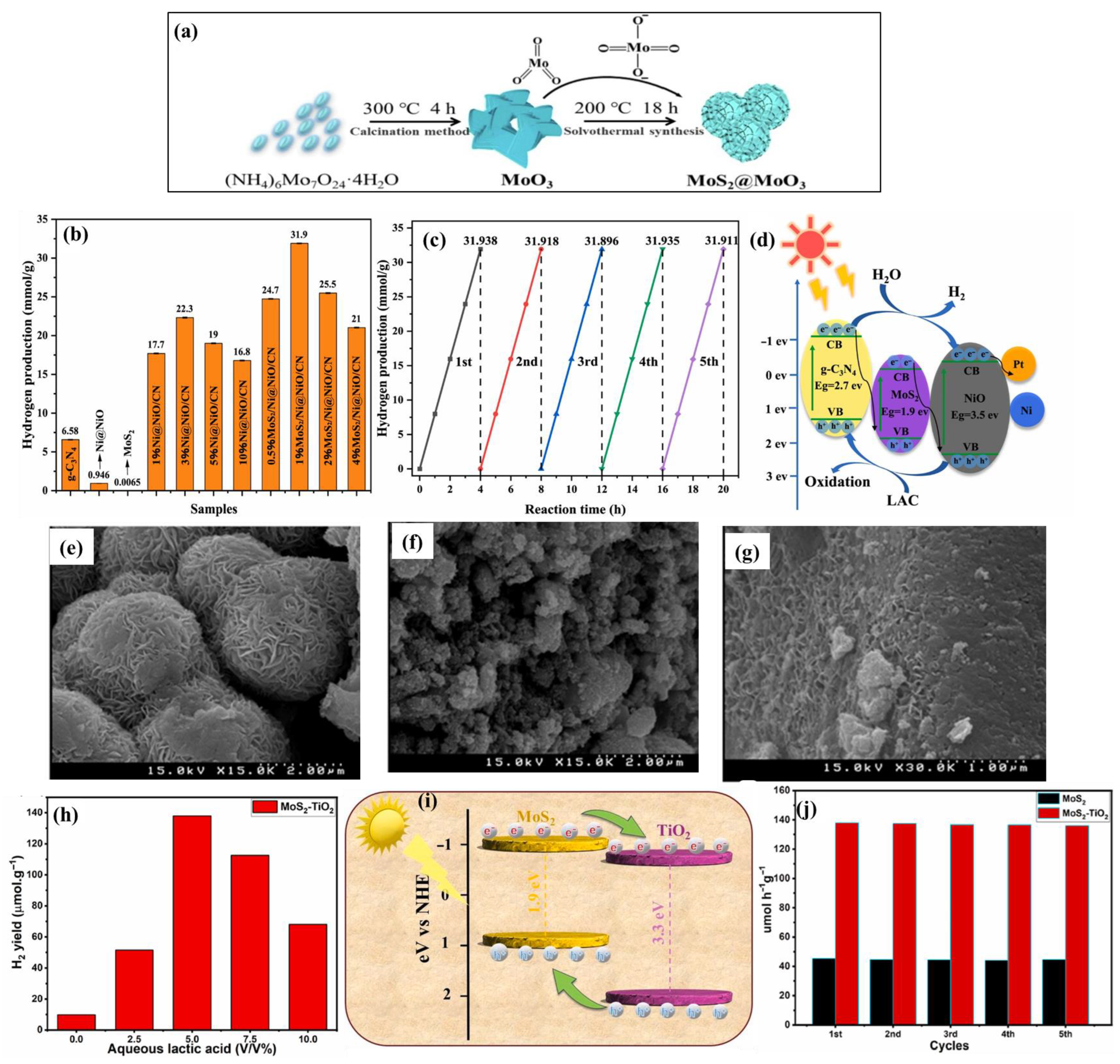
2.5. Calcination Method
2.6. Sol-Gel Method
2.7. Electrochemical Method
3. Progress in H2 Evolution
3.1. MoS2/Metal Oxides
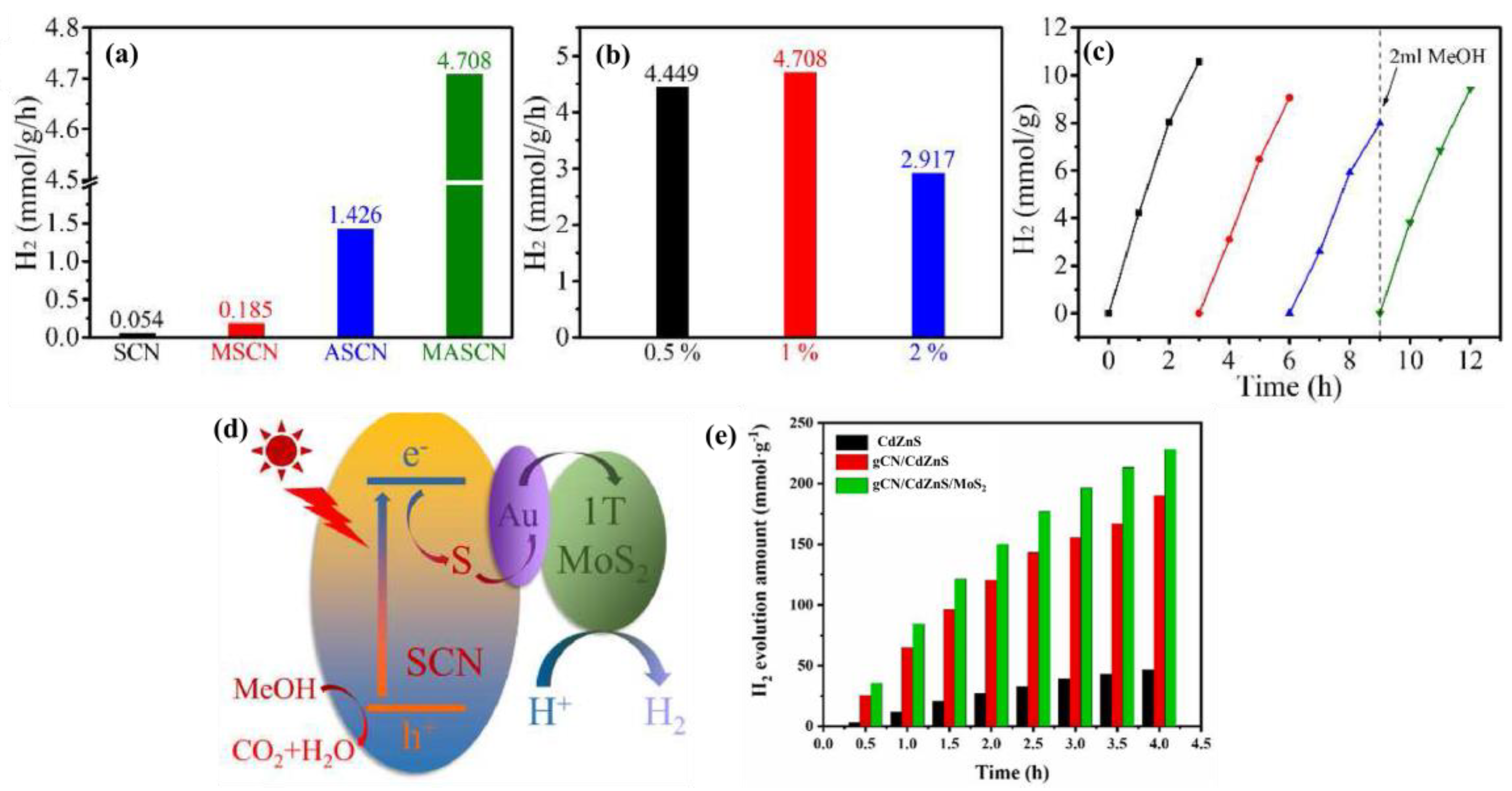
3.2. MoS2/Carbon, Graphene, Graphitic Carbon Nitride, and CNTs
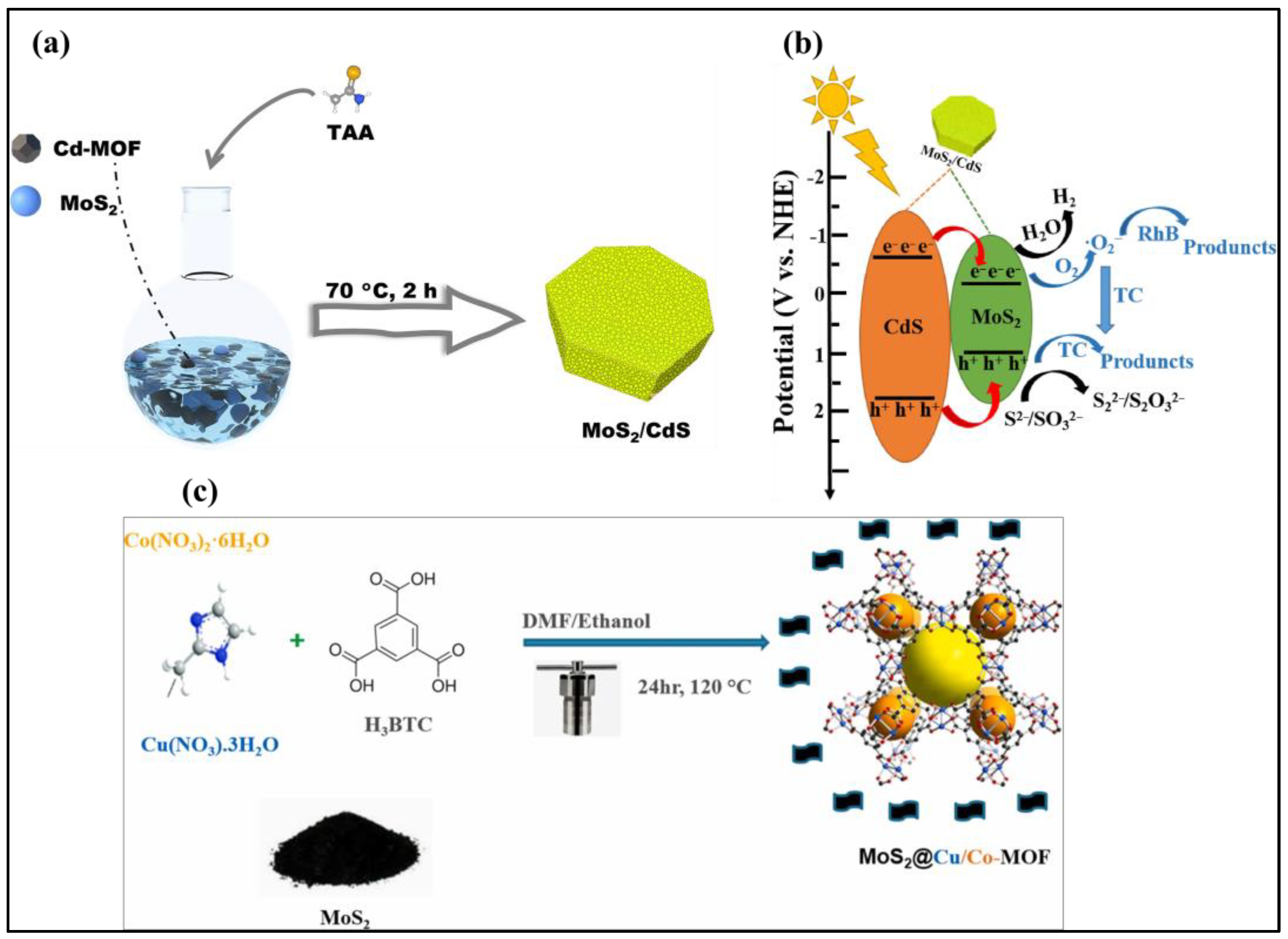
3.3. MoS2/Carbon/MOF/ZIF
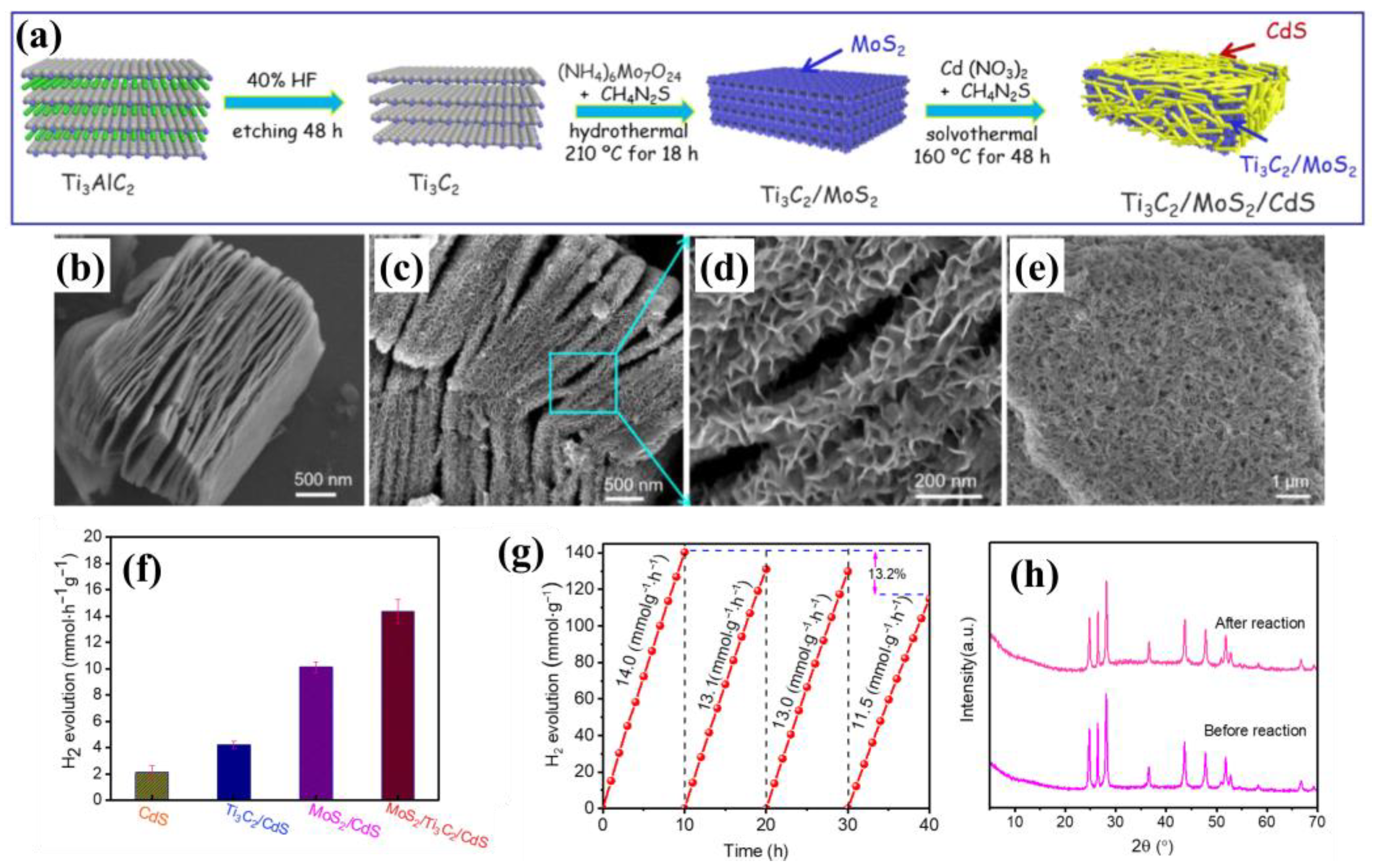
3.4. MoS2/Polymers/MXenes
3.5. MoS2/Sulfides
3.6. Other MoS2-Based PC Materials for H2 Evolution
4. Conclusions, Limitations, and Future Perspectives
- i.
- The stability of MoS2 for long-term application should be improved.
- ii.
- The mechanism for improved H2 evolution is still not clear. A depth study and more clarifications are required to understand the role of cocatalysts in photocatalytic hydrogen production.
- iii.
- The design and development of cocatalysts at the molecular and atomic levels should be considered for future research.
- iv.
- Previous studies show that Ni, Co, or P doping to MoS2 NSs enhances the hydrogen production. Thus, it is expected to explore single-atom-doped MoS2 as a cocatalyst for hydrogen production.
- v.
- We believe that future research may also focus on density functional theory (DFT) and experimental investigations to analyze the electron transfer route for the enhanced hydrogen production.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gałecka, A.; Pyra, M. Changes in the Global Structure of Energy Consumption and the Energy Transition Process. Energies 2024, 17, 5644. [Google Scholar] [CrossRef]
- Khan, I.; Altaf, A.; Sadiq, S.; Khan, S.; Khan, A.; Khan, S.; Humayun, M.; Khan, A.; Abumousa, R.A.; Bououdina, M. Towards sustainable solutions: Comprehensive review of advanced porous materials for CO2 capture, hydrogen generation, pollutant degradation, and energy application. Chem. Eng. J. Adv. 2025, 21, 100691. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, S.; Wang, T.; Yu, Y.; Wang, Y.; Li, Y.; Gao, W. The Influencing Factors and Future Development of Energy Consumption and Carbon Emissions in Urban Households: A Review of China’s Experience. Appl. Sci. 2025, 15, 2961. [Google Scholar] [CrossRef]
- Azni, M.A.; Md Khalid, R.; Hasran, U.A.; Kamarudin, S.K. Review of the Effects of Fossil Fuels and the Need for a Hydrogen Fuel Cell Policy in Malaysia. Sustainability 2023, 15, 4033. [Google Scholar] [CrossRef]
- Pustějovská, K.; Janovská, K.; Jursová, S. Alternative Sources of Energy in Transport: A Review. Processes 2023, 11, 1517. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chang, X.; Wang, W.; Fan, H. Graphitic Carbon Nitride for Photocatalytic Hydrogen Production from Water Splitting: Nano-Morphological Control and Electronic Band Tailoring. Nanomaterials 2025, 15, 45. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism. Catalysts 2021, 11, 405. [Google Scholar] [CrossRef]
- Ahasan, T.; Edirisooriya, E.M.N.T.; Senanayake, P.S.; Xu, P.; Wang, H. Advanced TiO2-Based Photocatalytic Systems for Water Splitting: Comprehensive Review from Fundamentals to Manufacturing. Molecules 2025, 30, 1127. [Google Scholar] [CrossRef]
- Li, X.; Zang, Y.; Zhang, J.; Zhang, L.; Zhang, J.; Huang, M.; Wang, J. Orange Peel Biochar–CdS Composites for Photocatalytic Hydrogen Production. Inorganics 2024, 12, 156. [Google Scholar] [CrossRef]
- Cai, M.; Zha, X.; Zhuo, Z.; Bai, J.; Wang, Q.; Cheng, Q.; Wei, Y.; Sun, S. Enhanced Photocatalytic Hydrogen Production of ZnIn2S4 by Using Surface-Engineered Ti3C2Tx MXene as a Cocatalyst. Materials 2023, 16, 2168. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, H.; Yin, H.; Yuan, C.; Lv, H.; Fei, Q.; Zhang, Y.; Zhao, Q.; Zheng, M.; Zhang, Y. Facile Synthesis of P-Doped ZnIn2S4 with Enhanced Visible-Light-Driven Photocatalytic Hydrogen Production. Molecules 2023, 28, 4520. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Sadiq, S.; Wu, P.; Humayun, M.; Ullah, S.; Yaseen, W.; Khan, S.; Khan, A.; Abumousa, R.A.; Bououdina, M. Synergizing black gold and light: A comprehensive analysis of biochar-photocatalysis integration for green remediation. Carbon Capture Sci. Technol. 2024, 13, 100315. [Google Scholar] [CrossRef]
- Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Comprehensive Review on g-C3N4-Based Photocatalysts for the Photocatalytic Hydrogen Production under Visible Light. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tang, X.; Liu, P.; Huang, Q.; Li, T.; Ju, L. Recent Progress of Ion-Modified TiO2 for Enhanced Photocatalytic Hydrogen Production. Molecules 2024, 29, 2347. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Wu, H.; Peng, Y.; Li, Y.; Deng, P.; Zhang, L.; Hou, Y. Efficient separation of charge carriers in the C–S bonded COF@Co9S8 S-scheme heterostructure for enhancing the photocatalytic H2 and H2O2 production. Int. J. Hydrogen Energy 2025, 130, 33–44. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem. Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Wang, W.; Wang, L. Montmorillonite-hybridized g-C3N4 composite modified by NiCoP cocatalyst for efficient visible-light-driven photocatalytic hydrogen evolution by dye-sensitization. Int. J. Hydrogen Energy 2019, 44, 4114–4122. [Google Scholar] [CrossRef]
- Aleithan, S.H.; Laradhi, S.S.; Al-Amer, K.; El-Lateef, H.M.A. Synergistic MoS2–Gold Nanohybrids for Sustainable Hydrogen Production. Catalysts 2025, 15, 550. [Google Scholar] [CrossRef]
- Machín, A.; Soto-Vázquez, L.; Colón-Cruz, C.; Valentín-Cruz, C.A.; Claudio-Serrano, G.J.; Fontánez, K.; Resto, E.; Petrescu, F.I.; Morant, C.; Márquez, F. Photocatalytic Activity of Silver-Based Biomimetics Composites. Biomimetics 2021, 6, 4. [Google Scholar] [CrossRef]
- Alshammari, H.M.; Alotaibi, M.H.; Aldosari, O.F.; Alsolami, A.S.; Alotaibi, N.A.; Alzahrani, Y.A.; Alhumaimess, M.S.; Alotaibi, R.L.; El-Hiti, G.A. A Process for Hydrogen Production from the Catalytic Decomposition of Formic Acid over Iridium-Palladium Nanoparticles. Materials 2021, 14, 3258. [Google Scholar] [CrossRef]
- Xiao, Z.; Lin, X.; Feng, W.; Chen, B.; Meng, Q.; Wang, T. Efficient Hydrogen Production from the Aqueous-Phase Reforming of Biomass-Derived Oxygenated Hydrocarbons over an Ultrafine Pt Nanocatalyst. Catalysts 2023, 13, 1428. [Google Scholar] [CrossRef]
- Kumar, R.R.; Gupta, S.; Anbalagan, A.K.; Khan, A.; Tai, N.-H.; Lee, C.-H.; Lin, H.-N. Time-Dependent Growth of Sputtered MoS2 Films on ZnO Nanorods for Enhanced NO2 Sensing Performance. Micromachines 2025, 16, 659. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, A.k.; Hu, F.-C.; Chan, W.K.; Gandhi, A.C.; Gupta, S.; Chaudhary, M.; Chuang, K.-W.; Ramesh, A.K.; Billo, T.; Sabbah, A.; et al. Gamma-Ray Irradiation Induced Ultrahigh Room-Temperature Ferromagnetism in MoS2 Sputtered Few-Layered Thin Films. ACS Nano 2023, 17, 6555–6564. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Liao, K.-W.; Fahmi, F.R.Z.; Modak, V.A.; Tsai, S.-H.; Ke, S.-W.; Wang, C.-H.; Chen, L.-C.; Chen, K.-H. Thickness-Dependent Photocatalysis of Ultra-Thin MoS2 Film for Visible-Light-Driven CO2 Reduction. Catalysts 2021, 11, 1295. [Google Scholar] [CrossRef]
- Peng, C.; Ling, Z.; Qu, M.; Cao, C.; Chen, G.; Shi, W.; Wei, B. Enhanced Performance of Flexible Organic Photovoltaics Based on MoS2 Micro-Nano Array. Molecules 2023, 28, 813. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Samy, O.; El Moutaouakil, A. A Review on MoS2 Energy Applications: Recent Developments and Challenges. Energies 2021, 14, 4586. [Google Scholar] [CrossRef]
- Du, X.; Xu, Y.; Shan, A.; Wang, R. Recent Progress in the Synthesis and Engineering of High-Performance MoS2 Electrocatalysts for the Hydrogen Evolution Reaction. Catalysts 2025, 15, 626. [Google Scholar] [CrossRef]
- de Sousa, B.P.; Estrada, A.C.; Fateixa, T.T.S. One-step synthesis of few-layer MoS2/Au nanostructures mediated by colloidal gold exfoliation with potential for surface-enhanced Raman scattering applications. Appl. Surf. Sci. 2025, 689, 162492. [Google Scholar] [CrossRef]
- Muratore, C.; Hu, J.J.; Wang, B.; Haque, M.A.; Bultman, J.E.; Jespersen, M.L.; Shamberger, P.J.; McConney, M.E.; Naguy, R.D.; Voevodin, A.A. Continuous ultra-thin MoS2 films grown by low-temperature physical vapor deposition. Appl. Phys. Lett. 2014, 104, 261604. [Google Scholar] [CrossRef]
- Qin, X.; Ke, P.; Wang, A.; Kim, K. Microstructure, mechanical and tribological behaviors of MoS2-Ti composite coatings deposited by a hybrid HIPIMS method. Surf. Coat. Technol. 2013, 228, 275–281. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Guo, W.; Zhao, M.; Fan, X.; Dong, Y.; Xu, C.; Deng, J.; Fu, Y. Synthesis Methods of Two-Dimensional MoS2: A Brief Review. Crystals 2017, 7, 198. [Google Scholar] [CrossRef]
- Kim, E.; Kim, J.-Y.; Kwon, M.-K. Synthesis of MoS2 Using Chemical Vapor Deposition and Conventional Hydrothermal Methods: Applications to Respiration Sensing. Appl. Sci. 2023, 13, 6084. [Google Scholar] [CrossRef]
- Yan, Z.S.; Long, J.Y.; Zhou, Q.F.; Gong, Y.; Lin, J.H. One-step synthesis of MnS/MoS2/C through the calcination and sulfurization of a bi-metal–organic framework for a high-performance supercapacitor and its photocurrent investigation. Dalton Trans. 2018, 47, 5390–5405. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, N.; Liu, Z.; Du, C. Two-step calcination synthesis of 1T/2H mixed-phase MoS2/g-C3N4 with sulfur defects for efficient removal of Cr(VI) from water. J. Mol. Struct. 2024, 1300, 137262. [Google Scholar] [CrossRef]
- Taffelli, A.; Ligorio, G.; Pancheri, L.; Quaranta, A.; Ceccato, R.; Chiappini, A.; Nardi, M.V.; List-Kratochvil, E.J.; Dirè, S. Large area MoS2 films fabricated via sol-gel used for photodetectors. Opt. Mater. 2023, 135, 113257. [Google Scholar] [CrossRef]
- Vizza, M.; Giurlani, W.; Cerri, L.; Calisi, N.; Leonardi, A.A.; Faro, M.J.L.; Irrera, A.; Berretti, E.; Perales-Rondón, J.V.; Colina, A.; et al. Electrodeposition of Molybdenum Disulfide (MoS2) Nanoparticles on Monocrystalline Silicon. Molecules 2022, 27, 5416. [Google Scholar] [CrossRef]
- Ponomarev, E.A.; Neumann-Spallart, M.; Hodes, G.; Lévy-Clément, C. Electrochemical Deposition of MoS2 Thin Films by Reduction of Tetrathiomolybdate. Thin Solid Film. 1996, 280, 86–89. [Google Scholar] [CrossRef]
- Giurlani, W.; Vizza, M.; Leonardi, A.A.; Lo Faro, M.J.; Irrera, A.; Innocenti, M. Optimization and Characterization of Electrodeposited Cadmium Selenide on Monocrystalline Silicon. Nanomaterials 2022, 12, 610. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Li, C.; Niu, Z.; Du, X.; Liu, Z.; Yue, X. Rational regulation of vacancy species to manage migration paths of carriers in MoS2/TiO2 heterojunctions for efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2022, 47, 28845–28858. [Google Scholar] [CrossRef]
- Di, T.; Deng, Q.; Wang, G.; Wang, S.; Wang, L.; Ma, Y. Photodeposition of CoOx and MoS2 on CdS as dual cocatalysts for photocatalytic H2 production. J. Mater. Sci. Technol. 2022, 124, 209–216. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Tsubaki, N. Activating and optimizing the MoS2@MoO3 S-scheme heterojunction catalyst through interface engineering to form a sulfur-rich surface for photocatalyst hydrogen evolution. Chem. Eng. J. 2022, 438, 135238. [Google Scholar] [CrossRef]
- Priya, B.A.; Sivakumar, T.; Venkateswari, P. Construction of MoS2 nanoparticles incorporated TiO2 nanosheets heterojunction photocatalyst for enhanced visible light driven hydrogen production. Inorg. Chem. Commun. 2022, 136, 109118. [Google Scholar] [CrossRef]
- Lee, G.-C.; Lyu, L.-M.; Hsiao, K.-Y.; Huang, Y.-S.; Perng, T.-P.; Lu, M.-Y.; Chen, L.-J. Induction of a piezo-potential improves photocatalytic hydrogen production over ZnO/ZnS/MoS2 Heterostructures. Nano Energy 2022, 93, 106867. [Google Scholar] [CrossRef]
- Tien, T.-M.; Chuang, Y.; Chen, E.L. Z-scheme driven of MoS2/Co3O4 nano-heterojunction for efficient photocatalysis hydrogen evolution and Rhodamine B degradation. J. Photochem. Photobiol. A Chem. 2023, 444, 114986. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, C.; Zhao, Y.; Fang, F.; Yin, Y.; Ye, Y.; Yang, K.; Yang, Q.; Wang, K. Photocatalytic pollutant elimination and hydrogen production over TiO2 NTs/Bi2S3-MoS2 with Z-scheme configuration: Kinetics and mechanism. Mater. Res. Bull. 2023, 167, 112430. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Lim, S.J.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250. [Google Scholar] [CrossRef]
- Ma, D.; Yin, M.; Liang, K.; Xue, M.; Fan, Y.; Li, Z. Simple synthesis and efficient photocatalytic hydrogen production of WO3-WS2 and WO3–WS2–MoS2. Mater. Sci. Semicond. Process. 2023, 167, 107788. [Google Scholar] [CrossRef]
- Govinda raj, M.; Mahalingam, S.; Gnanarani, S.V.; Jayashree, C.; Ganeshraja, A.S.; Pugazhenthiran, N.; Rahaman, M.; Abinaya, S.; Senthil, B.; Kim, J. TiO2 nanorod decorated with MoS2 nanospheres: An efficient dual-functional photocatalyst for antibiotic degradation and hydrogen production. Chemosphere 2024, 357, 142033. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, C.; Cao, X. Preparation of MoS2/Ni@NiO/g-C3N4 composite catalyst and its photocatalytic performance for hydrogen production. Int. J. Hydrogen Energy 2024, 51, 1078–1086. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, J.; Peng, R.; Cao, M.; Peng, G.; Yuan, W.; Luo, X. Mo–P sites boosting interfacial charge transfer of 2D/3D MoS2/TiO2 heterostructure for efficient photocatalytic hydrogen production and chromium(vi) reduction. Catal. Sci. Technol. 2024, 14, 1579–1587. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Z.; Yang, T.; Feng, H.; Li, L.; Ullah, I.; Xue, S. Photocatalytic hydrogen production and simultaneous tetracycline degradation by selectively depositing growth of MoS2 on the PbTiO3 (00) surface. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133096. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Reddy, B.P.; Kuchi, C.; Basha, C.K.; Al-Zahrani, F.A.M.; Mangiri, R. Enhanced solar-driven photocatalytic hydrogen production, dye degradation, and supercapacitor functionality using MoS2–TiO2 nanocomposite. Ceram. Int. 2024, 50, 38679–38687. [Google Scholar] [CrossRef]
- Lin, K.; Liu, Q.; Yue, X.; Shi, Y.; Song, W.; Wang, D.; Tian, C.; Wu, A.; Fu, H. Rutile TiO2–MoS2–CdS ternary heterojunctions with enhanced charge transfer for photocatalytic pure water splitting. Int. J. Hydrogen Energy 2025, 126, 251–260. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Yan, Z.; Gao, A.; Li, Y.; Luo, Y.; Zhao, N.; Lin, J. A honeycomb-rod-like hierarchical MoO3@MoS2@ZnIn2S4 p-n heterojunction composite photocatalyst for efficient solar hydrogen production. Fuel 2025, 399, 135674. [Google Scholar] [CrossRef]
- Nawaz, R.; Saad, M.; Bahadur, A.; Iqbal, S.; Mahmood, S.; Zidan, A.; Khan, M.S.; Liaquat, R.; Sohail, M.; Alotaibi, M.T. Designing an innovative 2D/2D step scheme α-Fe2O3/BiOBr/MoS2 ternary integrated heterojunction with unparalleled visible-light-induced remarkable photocatalytic H2 evolution. Int. J. Hydrogen Energy 2025, 99, 112–122. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhao, J.; Weon, S.; Li, L.; Zhang, P. Photocatalytic hydrogen-recovering wastewater treatment through dual-functional hierarchical heterostructure of MoS2/SrWO4. Next Res. 2025, 2, 100162. [Google Scholar] [CrossRef]
- Oskoei, A.; Khaleghi, M.; Sheibani, S. Modification of MoS2/ZnO nanocomposite for efficient photocatalytic degradation of water pollutants and hydrogen evolution. J. Water Process Eng. 2025, 71, 107404. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.; Chen, H.; Kang, X.; Wang, P.; Xian, Z.; Zhang, Z.; Cai, Y.; Lu, G. Encapsulation of Pt/TiO2@MoS2+x with polymethyl methacrylate for enhancement of photocatalytic hydrogen evolution in seawater. Chem. Eng. J. 2025, 514, 163378. [Google Scholar] [CrossRef]
- Wan, A.; Ren, Z.; He, Y.; Zhang, Y.; Zhang, S.; Tu, M.; Li, J.; Qiu, M. A promising type-II/Z-scheme ternary MoS2/Bi2S3/Bi2WO6 heterostructure photoanode for photocatalytic fuel cell effective hydrogen production and electricity generation in organic wastewater. Appl. Surf. Sci. 2025, 690, 162607. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Chu, X.-S.; Dang, Y.-Y.; Liu, X.-Y.; Ma, T.; Li, J.-Y.; Wang, C.-Y. 3D porous BN/rGO skeleton embedded by MoS2 nanostructures for simulated-solar-light induced hydrogen production. Chem. Eng. J. 2022, 435, 132441. [Google Scholar] [CrossRef]
- Jiao, Y.; Qin, J.; Li, Y.; Wang, J.; He, Z.; Li, Z. Hollow carbon spheres coated with layered MoS2 decorated carbon nitride for enhanced photocatalytic hydrogen performance. Mater. Chem. Phys. 2022, 288, 126321. [Google Scholar] [CrossRef]
- Yuan, H.; Fang, F.; Dong, J.; Xia, W.; Zeng, X.; Shangguan, W. Enhanced photocatalytic hydrogen production based on laminated MoS2/g-C3N4 photocatalysts. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128575. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. MoS2/CdS rod-like nanocomposites as high-performance visible light photocatalyst for water splitting photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 8247–8260. [Google Scholar] [CrossRef]
- Xue, K.; Yu, L.; Luo, H.; Ji, X.; Li, X.; Zhu, H.; Zhang, Y. Surface engineering of phase controlled defective 1 T-MoS2 QDs@g-C3Nx material for significantly enhanced hydrogen evolution under visible-light irradiation. Sep. Purif. Technol. 2023, 308, 122920. [Google Scholar] [CrossRef]
- Pan, M.; Gao, L.; Wang, P.; Wang, X.; Yu, H. Dual-heterophase MoS2-MoC@rGO cocatalyst with rapid electron transfer and interfacial H2-evolution reaction toward efficient photocatalytic activity of TiO2. J. Alloys Compd. 2023, 939, 168721. [Google Scholar] [CrossRef]
- Masimukku, S.; Tsai, D.-L.; Lin, Y.-T.; Chang, I.-L.; Wu, J.J. Low-frequency vibration induced piezoelectric boost to photocatalytic hydrogen evolution through 2D-2D-stacked MoS2-carbon nitride. Appl. Surf. Sci. 2023, 614, 156147. [Google Scholar] [CrossRef]
- Xu, Y.; Ouyang, J.; Zhang, L.; Long, H.; Song, Y.; Cui, Y. Embedded 1T-rich MoS2 into C3N4 hollow microspheres for effective photocatalytic hydrogen production. Chem. Phys. Lett. 2023, 814, 140331. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, T.; Liu, W.; Wang, X.; Xue, Y.; Tian, J. 1T-phase MoS2/holey ultrathin g-C3N4 nanosheets based 2D/2D heterostructure for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2023, 48, 7284–7293. [Google Scholar] [CrossRef]
- Li, D.; Zeng, G.; Wu, Y.; Zhou, Z.; Guo, W.; Li, C. Au induced in-situ formation of ultra-stable 1T-MoS2 on polymeric carbon nitride toward promoted photocatalytic hydrogen production. Int. J. Hydrogen Energy 2023, 48, 34363–34369. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Q.; Bai, J.; Xu, T.; Li, C. One-pot fabrication CoS2 modified MoS2-g-C3N4 ternary heterostructure composites with enhanced photocatalytic hydrogen production from water. Diam. Relat. Mater. 2023, 134, 109764. [Google Scholar] [CrossRef]
- Wei, X.; Wang, M.; Ali, S.; Wang, J.; Zhou, Y.; Zuo, R.; Zhong, Q.; Zhan, C. Enhanced photocatalytic H2 evolution on g-C3N4 nanosheets loaded with nitrogen-doped MoS2 as cocatalysts. Int. J. Hydrogen Energy 2024, 89, 691–702. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Li, Y.; Huang, H.; Li, M.; Long, P.; Cao, X.; Zhang, J.; Zhou, M.; Zhou, Z.; et al. One-step synthesis of 2D/2D gC3N4/MoS2 composites for effective photocatalytic hydrogen evolution. Mater. Sci. Eng. B 2024, 303, 117265. [Google Scholar] [CrossRef]
- Ning, Y.; Lv, D.; Tang, Q.; Wang, H.; Hu, X.; Cao, Y.; Yu, S.; Tian, H. Novel 2D/2D/2D heterojunction of ZnIn2S4/g-C3N4/MoS2 for enhanced photocatalytic hydrogen evolution reaction. Ceram. Int. 2024, 50, 48692–48699. [Google Scholar] [CrossRef]
- Su, L.-X.; Lou, Q.; Shan, C.-X.; Du, D.-J. A novel MoS2-modified hybrid nanodiamond/g-C3N4 photocatalyst for photocatalytic hydrogen evolution. Chem. Phys. 2024, 577, 112135. [Google Scholar] [CrossRef]
- Li, N.; Ma, J.; Wang, W.; Chang, Q.; Liu, L.; Hao, C.; Zhang, H.; Zhang, H.; Hu, S.; Wang, S. Dual S-scheme MoS2/ZnIn2S4/Graphene quantum dots ternary heterojunctions for highly efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2024, 676, 496–505. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, H.; Zhao, L.; Dou, J.; Yin, X. Constructing GO films/CdS nanoparticles/MoS2 nanosheets ternary nanojunction with enhanced photocatalytic hydrogen evolution activity. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134900. [Google Scholar] [CrossRef]
- Imam, M.D.; Badreldin, A.; Kakosimos, K.E.; Al-Hashimi, M.; Abdel-Wahab, A. One-pot synthesis of a CdS–MoS2/CNTs nano-composite for photocatalytic hydrogen production under visible light. Int. J. Hydrogen Energy 2024, 51, 1267–1278. [Google Scholar] [CrossRef]
- Mou, Z.; Meng, T.; Li, J.; Wang, Y.; Wang, X.; Guo, H.; Meng, W.; Zhang, K. Significantly enhanced photocatalytic hydrogen production performance of MoS2/CNTs/CdS with carbon nanotubes as the charge mediators. Int. J. Hydrogen Energy 2024, 51, 748–757. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, H.; Li, Z.; Chu, M.; Xie, G.; Xie, T.; Jiang, L. High photocatalytic activity of g-C3N4/CdZnS/MoS2 heterojunction for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 776–785. [Google Scholar] [CrossRef]
- Muthukumar, R.; Balaji, G. Noble metal-free ternary MoS2/g-C3N4/ZnIn2S4 heterojunction with efficient charge transfer for highly efficient photocatalytic H2 production. Diam. Relat. Mater. 2025, 152, 111941. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, W.; Liu, N.; Huang, H.-T.; Karuppasamy, L.; Yang, J.-J.; Liu, C.-H.; Wu, J.J. Synthesis of MOF/MoS2 composite photocatalysts with enhanced photocatalytic performance for hydrogen evolution from water splitting. Int. J. Hydrogen Energy 2022, 47, 40755–40767. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Wang, X.; Zhang, X.; Tian, J. Structure engineering of 1T/2H multiphase MoS2 via oxygen incorporation over 2D layered porous g-C3N4 for remarkably enhanced photocatalytic hydrogen evolution. Mater. Today Nano 2022, 18, 100204. [Google Scholar] [CrossRef]
- Sima, L.; Li, D.; Dong, L.; Zhang, F. Facile preparation of porous g-C3N4/MoS2 heterojunction for hydrogen production under simulated sunlight. Mater. Today Sustain. 2022, 20, 100217. [Google Scholar] [CrossRef]
- Liu, S.; Chi, D.; Zou, Q.; Ma, Y.; Chen, R.; Zhang, K. MOFs-derived MoS2/C3N4 composites with highly efficient charge separation for photocatalytic H2 evolution. Inorg. Chim. Acta 2022, 533, 120787. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Xiao, W.; Xia, S.; Jin, C.; Wang, W.; Li, S.; Zhong, M.; Wang, S.; Chen, C. Metal-organic frameworks derived porous MoS2/CdS heterostructure for efficient photocatalytic performance towards hydrogen evolution and organic pollutants. Int. J. Hydrogen Energy 2023, 48, 32729–32738. [Google Scholar] [CrossRef]
- Rehan, M.A.; Liang, H.; Li, G. Synergistic role of plasmonic Au-doped MOF with ZnIn2S4/MoS2 nanosheets for boosted photocatalytic hydrogen evolution. Nano Mater. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Tab, A.; Belabed, C.; Mahieddine, A.; Kaci, M.M.; Derkaoui, K.; Chetoui, A.; Al-Saleem, M.S.; Khen, A.; Adnane-Amara, L.; Özacar, M.; et al. 1T/2H-MoS2@Zn-Ni MOF composite: A novel catalyst for efficient hydrogen production and antimicrobial activity. J. Water Process Eng. 2025, 75, 108047. [Google Scholar] [CrossRef]
- Masekela, D.; Yusuf, T.L.; Balogun, S.A.; Makhado, E.; Adeniran, O.I.; Modibane, K.D. Interfacial engineering of MoS2 and bimetallic MOF hybrid for superior piezo-photocatalytic hydrogen production and wastewater treatment. J. Alloys Compd. 2025, 1020, 179304. [Google Scholar] [CrossRef]
- Kshirsagar, S.D.; Shelake, S.P.; Biswas, B.; Singh, A.; Pakhira, S.; Sainath, A.V.S.; Pal, U. In situ decoration of 2D-MoS2/ZIF-67 type II heterojunction for enhanced hydrogen production under simulated sunlight. Catal. Today 2025, 445, 115056. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, A.; Zhang, L.; Dong, P. Construction of S-scheme heterojunction from protonated D-A typed polymer and MoS2 for efficient photocatalytic H2 production. Chin. J. Catal. 2024, 58, 180–193. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Heng, Q.; Chen, W.; Li, X.; Mao, L.; Shangguan, W. Promoted charge separation on 3D interconnected Ti3C2/MoS2/CdS composite for enhanced photocatalytic H2 production. Int. J. Hydrogen Energy 2022, 47, 8284–8293. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Kong, C.; Zhang, F. Defect MoS2 and Ti3C2 nanosheets co-assisted CdS to enhance visible-light driven photocatalytic hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129746. [Google Scholar] [CrossRef]
- Wu, C.; Huang, W.; Liu, H.; Lv, K.; Li, Q. Insight into synergistic effect of Ti3C2 MXene and MoS2 on anti-photocorrosion and photocatalytic of CdS for hydrogen production. Appl. Catal. B Environ. 2023, 330, 122653. [Google Scholar] [CrossRef]
- Ma, L.; Xu, L.; Ding, Y.; Lin, C.; Yang, Y.; Ai, X. Enhancing photocatalytic hydrogen evolution via efficient carrier separation in interpenetrating layered Ti3C2-MoS2-ZnIn2S4 heterojunctions. Int. J. Hydrogen Energy 2024, 82, 713–723. [Google Scholar] [CrossRef]
- Mansoor, S.; Hu, Z.; Zhang, Y.; Tayyab, M.; Khan, M.; Akmal, Z.; Zhou, L.; Lei, J.; Nasir, M.; Zhang, J. Simultaneous hydrogen production with photo reforming of lactic acid over MXene derived MoS2/TiO2/Ti3C2 nanowires. Chin. J. Catal. 2025, 71, 234–245. [Google Scholar] [CrossRef]
- Liu, H.; Tan, P.; Liu, Y.; Zhai, H.; Du, W.; Liu, X.; Pan, J. Ultrafast interfacial charge evolution of the Type-II cadmium Sulfide/Molybdenum disulfide heterostructure for photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 619, 246–256. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, X.; Xu, S.; Guo, Y.; Zhang, D.; Wang, M.; Wei, G.; Yang, X.; Li, Z.; Zhang, Y.; et al. Surface modulation of MoS2/O-ZnIn2S4 to boost photocatalytic H2 evolution. J. Energy Chem. 2022, 75, 276–284. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Zhang, F.-J.; Wang, Y.-R.; Kong, C. Facile formation of Mo-vacancy defective MoS2/CdS nanoparticles enhanced efficient hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128743. [Google Scholar] [CrossRef]
- Chand, M.; Rawat, A.S.; Khanuja, M.; Rawat, S. Hydrogen production activity of MoS2-ZnIn2S4 nanocomposite under visible light irradiation. Mater. Today Proc. 2022, 66, 1951–1954. [Google Scholar] [CrossRef]
- Zhang, K.; Mou, Z.; Cao, S.; Meng, T.; Li, J.; Ling, G.; Zhang, X.; Zhou, Z.; Meng, W. MoS2 grown in situ on CdS nanosheets for boosted photocatalytic hydrogen evolution under visible light. Int. J. Hydrogen Energy 2022, 47, 2967–2975. [Google Scholar] [CrossRef]
- Kumar, D.P.; Seo, S.; Rangappa, A.P.; Kim, S.; Reddy, K.A.J.; Gopannagari, M.; Bhavani, P.; Reddy, D.A.; Kim, T.K. Ultrathin layered Zn-doped MoS2 nanosheets deposited onto CdS nanorods for spectacular photocatalytic hydrogen evolution. J. Alloys Compd. 2022, 905, 164193. [Google Scholar] [CrossRef]
- Kumar, D.P.; Rangappa, A.P.; Kim, S.; Kim, E.; Reddy, K.A.J.; Gopannagari, M.; Bhavani, P.; Reddy, D.A.; Kim, T.K. Boosting charge transfers in cadmium sulfide nanorods with a few layered Ni-doped MoS2 nanosheets for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 40218–40226. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, D.; Li, H.; Kondamareddy, K.K.; Wang, H.; Zhang, B.; Wang, J.; Wu, Q.; Zeng, Y.; Zhang, X.; et al. Enhanced visible Light-Driven photocatalytic hydrogen evolution and stability for noble Metal-Free MoS2/Zn0.5Cd0.5S heterostructures with W/Z phase junctions. Appl. Surf. Sci. 2022, 586, 152770. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, A.; Jiang, L.; Huang, F.; Hu, D.; Duan, G.; Zheng, F. MoS2/HCSs/ZnIn2S4 nanocomposites with enhanced charge transport and photocatalytic hydrogen evolution performance. J. Alloys Compd. 2022, 895, 162504. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, H.; Yang, X.; Xiong, S.; An, X. High-performance MoS2/CdS nanodiamonds for photocatalytic hydrogen evolution under visible light irradiation. Powder Technol. 2022, 406, 117596. [Google Scholar] [CrossRef]
- Chang, C.-J.; Tsai, Z.-T.; Lin, K.-S.; Nian, Y.-H. Enhanced photocatalytic H2 production of flower-like MoS2@Ag2S photocatalysts with matched band structures. J. Photochem. Photobiol. A Chem. 2023, 445, 115027. [Google Scholar] [CrossRef]
- Peng, K.; Zuo, L.; Wang, Y.; Ye, J.; Wang, H.; Jia, Y.; Niu, M.; Su, L.; Zhuang, L.; Li, X. Boosting photocatalytic hydrogen evolution over CdS/MoS2 on the graphene/montmorillonite composites. Appl. Clay Sci. 2023, 236, 106855. [Google Scholar] [CrossRef]
- Liu, N.; Yu, H.; Liu, Y.; Lin, M.; Lei, Z.; Huang, C.; Qi, F.; Zhou, Y.; Ouyang, X. A novel hierarchical S-scheme heterojunction of 0D/3D Zn0.5Cd0.5S nanoparticles/hollow micro-flower MoS2 for improved photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 632, 157579. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Bai, J.; Li, C.; Liang, H. One-dimensional nanowire CdS/MoS2/CNFs with compact heterostructure for photocatalytic evolution of hydrogen. Vacuum 2023, 216, 112469. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Guo, L.; Yang, Z.; Jin, B.; Du, R.; Fu, F.; Wang, D. Plate-on-plate structured MoS2/Cd0.6Zn0.4S Z-scheme heterostructure with enhanced photocatalytic hydrogen production activity via hole sacrificial agent synchronously strengthen half-reactions. J. Colloid Interface Sci. 2023, 630, 341–351. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, H.; Li, X.; Chen, F.; Yang, X.; Xiong, S.; Li, S.; An, X. High-performance visible-light-driven MoS2/CdZnS nanorods for photocatalytic hydrogen production by water splitting. Powder Technol. 2023, 429, 118889. [Google Scholar] [CrossRef]
- Xing, J.; Wang, Y.; Shi, G.; Li, L.; Wu, Y. Defective Cd0.3Zn0.7S/1 T-2 H MoS2 Z-scheme heterojunctions: Rational design with efficient charge transfer for enhanced photocatalytic H2 generation. J. Alloys Compd. 2024, 988, 174302. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, T.; Li, R.; Chen, Y.; Luo, W.; Wu, Y.; Xie, Y.; Wang, Y.; Zhang, Y. Layered deposited MoS2 nanosheets on acorn leaf like CdS as an efficient anti-photocorrosion photocatalyst for hydrogen production. Fuel 2024, 368, 131621. [Google Scholar] [CrossRef]
- Sridevi, R.; Prakasam, A.; Anbarasan, P.M.; Kumar, R.; Karthik, M.; Deepakvijay, K. Facile construction of MoS2 decorated CdS hybrid heterojunction with enhanced hydrogen generation performance. Chem. Phys. Impact 2024, 8, 100584. [Google Scholar] [CrossRef]
- Liu, Q.; You, J.; Xiong, Y.; Liu, W.; Song, M.; Ren, J.; Xue, Q.; Tian, J.; Zhang, H.; Wang, X. Synergistic effect of interstitial phosphorus doping and MoS2 modification over Zn0.3Cd0.7S for efficient photocatalytic H2 production. J. Colloid Interface Sci. 2024, 675, 772–782. [Google Scholar] [CrossRef]
- Chen, C.; Li, Q.; Wang, F.; Hu, C.; Ma, J. Dual-vacancies modulation of 1T/2H heterostructured MoS2-CdS nanoflowers via radiolytic radical chemistry for efficient photocatalytic H2 evolution. J. Colloid Interface Sci. 2024, 661, 345–357. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B.; Sun, H.; Ma, G.; Wang, S.; Li, Y.; Zhang, H.; Xie, H.; Quan, H.; Zhang, H. Stepped fluorinated CdS/MoS2/ZnS nanoparticles constructed on a multifunctional platform with Zn(OH)F nanoflowers for highly active photocatalytic H2 production. Sep. Purif. Technol. 2024, 347, 127461. [Google Scholar] [CrossRef]
- Xi, T.-L.; Liu, L.-j.; Liu, Q.; Wang, H.-W.; Zuo, L.-Y.; Fan, H.-T.; Li, B.; Wang, L.-Y. Hollow MoS2@ZnIn2S4 nanoboxes for improving photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 62, 62–70. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, H.; Zhou, X.; Su, Y.; Du, C. Sulfur vacancy-induced 1T-MoS2 co-catalyst loaded on Zn3In2S6 for simultaneous promoting photocatalytic hydrogen evolution and pollutant degradation. Fuel 2024, 371, 131939. [Google Scholar]
- Hu, G.; Guo, T.; Wang, C.; Liu, J.; Liu, Y.; Guo, Q. High-performance photocatalytic hydrogen evolution in a Zn0.5Cd0.5S/MoS2 p–n heterojunction. Vacuum 2024, 227, 113451. [Google Scholar] [CrossRef]
- Wang, W.; Shan, W.; Hu, Y.; Jiang, H.; Wang, L.; Chen, J.; Liu, Q.; Tang, H. Fast carrier separation induced by the metal-like O-doped MoS2/CoS cocatalyst for achieving photocatalytic and photothermal hydrogen production. Chem. Eng. J. 2024, 493, 152516. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Wang, L.; Geng, Y.; Zhang, M. Preparation of CdIn2S4 nanoparticles@MoS2 microrods heterojunctions for boosted photocatalytic hydrogen production. J. Alloys Compd. 2024, 982, 173750. [Google Scholar] [CrossRef]
- Naikoo, G.A. Boosting green hydrogen production with ZnS@MoS2 2D materials: A structural, electrochemical and photocatalytic analysis. Mater. Today Commun. 2024, 41, 110175. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Bano, M.; Ayyub, M.M.; Hassan, I.U.; Saleh, T.A. Exploring the catalytic capabilities of NiS@MoS2 2D for the production of Green Hydrogen. Nano Trends 2025, 9, 100089. [Google Scholar] [CrossRef]
- Ruan, C.; Gao, K.; Wang, Q.; Luo, S.; Zhang, Y.; Yang, M.; Zhong, S.; Wu, Q.; Tian, Y.; Zhang, Z. Charge carriers oriented accumulation in MoS2/Cd0.5Mn0.5S Schottky junctions for highly efficient photocatalytic H2 evolution. Sep. Purif. Technol. 2025, 362, 131723. [Google Scholar] [CrossRef]
- Tayyab, M.; Xie, Y.; Tan, X.; Usman, M.; Tang, M.-C.; Chen, S.S. A ternary dumbbell MoS2 tipped Zn0.1Cd0.9S nanorods visible light driven photocatalyst for simultaneous hydrogen production with organics degradation in wastewater. Chem. Eng. J. 2025, 505, 159064. [Google Scholar] [CrossRef]
- Men, X.; Liang, H.; Fan, X.; Bai, J. Synergistic incorporation of MoS2 cocatalyst into ZnIn2S4 nanoflower architectures for efficient photocatalytic H2 production and selective benzyl alcohol oxidation. Opt. Mater. 2025, 166, 117208. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Ma, H.; Ye, B.; Zhuang, T.; Lv, Z. Rh single atoms anchored in hollow microflower MoS2/sulfur-vacancy rich CdZnS with dual proton reduction sites for enhanced photocatalytic hydrogen generation. J. Colloid Interface Sci. 2025, 684, 207–214. [Google Scholar] [CrossRef]
- Yan, A.; Zhang, T.; Huang, F.; Wang, Q.; Lu, S.; Zhao, W.; Gao, Y.; Su, Z.; Yuan, H. In situ preparation of MoS2/Sv-ZnIn2S4/ZnS composites with directional charge transfer pathway and boosted photocatalytic hydrogen evolution activity. Int. J. Hydrogen Energy 2025, 128, 319–328. [Google Scholar] [CrossRef]
- Dang, S.; Wang, Y.; Shi, G.; Wu, Y.; Li, L.; Liu, F.; Xing, J.; Wen, A. Construction of 1T-2H mixed-phase MoS2-coated CdS hollow sphere core-shell structure and study on its efficient photocatalytic hydrogen evolution performance. Vacuum 2025, 239, 114427. [Google Scholar] [CrossRef]
- Zhang, N.; Zhai, Z.; Yan, Y.; Feng, X.; Chen, S.; Zhao, Y.; Zhang, B. Constructing 2D/2D heterostructure of ZnIn2S4 nanosheets on cobalt doped MoS2 for enhanced photocatalytic hydrogen production performance. Sep. Purif. Technol. 2025, 362, 131694. [Google Scholar] [CrossRef]
- Deng, S.; Ge, W.; Song, J.; Zhou, Q.; Jiang, Y.; Liu, K.; Deng, S.; Yang, P. Boosted photocatalytic hydrogen production of CdS@1T/2H MoS2 nanorod clusters heterostructure photocatalyst with rich sulfur vacancies. Colloids Surf. A Physicochem. Eng. Asp. 2025, 722, 137317. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Wang, Z.; Zang, Y.; Tao, X. Efficient and long-term photocatalytic H2 evolution stability enabled by Cs2AgBiBr6/MoS2 in aqueous HBr solution. Int. J. Hydrogen Energy 2022, 47, 8829–8840. [Google Scholar] [CrossRef]
- Deng, L.; Li, M.; Luo, L.; Chen, T.; Shan, T.; Yang, X.; Shen, L.; Yang, M.-Q. In-situ construction of FAPbBr3-wrapped MoS2 heterostructure for photocatalytic H2 evolution from dehydrogenation of aromatic alcohols. Appl. Surf. Sci. 2023, 638, 158004. [Google Scholar] [CrossRef]
- Zhao, H.; Greco, R.; Komsa, H.-P.; Sliz, R.; Pitkänen, O.; Kordas, K.; Ojala, S. 1 T′/2 H MoS2 nanoflowers integrated with bismuth halide perovskite for improved photocatalytic hydrogen evolution. Appl. Catal. B Environ. Energy 2024, 357, 124318. [Google Scholar] [CrossRef]
- Jia, J.; Zheng, L.; Li, K.; Zhang, Y.; Xie, H. Two-electron transfer mechanism from 3D/3D nickel selenide/MoS2 heterostructure accelerates photocatalytic hydrogen evolution and tetracycline hydrochloride removal. Chem. Eng. J. 2022, 429, 132432. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Q.; Jia, Y.; Liu, C. Cu2(OH)2CO3 clusters modified MoS2@Ag2S heterojunctions for efficient photocatalytic pollutant degradation and hydrogen evolution. Mater. Lett. 2022, 328, 133140. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. High-yield exfoliation of MoS2 (WS2) monolayers towards efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 431, 133286. [Google Scholar] [CrossRef]
- Zhao, C.-H.; Luo, K.-L.; Li, W. Z-scheme electron transfer mechanism of MoS2/CoP heterostructure for simulated solar light induced hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131652. [Google Scholar] [CrossRef]
- Mandari, K.K.; Kang, M. Highly active Co2P/2H-1T MoS2 cocatalyst with fast charge transfer and H2 production reaction toward effective photocatalytic activity of P-TiO2. Mater. Today Sustain. 2023, 23, 100444. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Xu, J.; Huang, Z.; Lao, H.; Xu, X.; Xu, J.; Tang, H.; Yuan, R.; Wang, Z.; et al. Dual non-metal atom doping enabled 2D 1T-MoS2 cocatalyst with abundant edge-S active sites for efficient photocatalytic H2 evolution. Int. J. Hydrogen Energy 2023, 48, 16987–16999. [Google Scholar] [CrossRef]
- Yanalak, G.; Eroglu, Z.; Yılmaz, S.; Bas, S.Z.; Metin, O.; Patir, I.H. Metal doped black phosphorus/molybdenum disulfide (BP/MoS2–Y (Y: Ni, Co)) heterojunctions for the photocatalytic hydrogen evolution and electrochemical nitrite sensing applications. Int. J. Hydrogen Energy 2023, 48, 14238–14254. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, G.; Chen, M.; Li, B.; Dai, W.; Deng, F.; Yang, L.; Zou, J.; Luo, S. Interfacial oxygen vacancy modulated Ag3PO4 @MoS2 Z-scheme system for efficient photocatalytic hydrogen recovery from antibiotic wastewater. Appl. Catal. B Environ. 2023, 330, 122584. [Google Scholar] [CrossRef]
- Xie, H.; Wang, K.; Li, S.; Jin, Z. Construction of Co9S8/MoS2/Ni2P double S-scheme heterojunction for enhanced photocatalytic hydrogen evolution. Surf. Interfaces 2023, 42, 103353. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Liu, Y.-T.; Perng, T.-P.; Lu, M.-Y.; Chueh, Y.-L.; Chen, L.-J. Enhancing photocatalytic properties of continuous few-layer MoS2 thin films for hydrogen production by water splitting through defect engineering with Ar plasma treatment. Nano Energy 2023, 109, 108295. [Google Scholar] [CrossRef]
- Pundi, A.; Tsai, Z.-T.; Chen, J.; Yu, Y.-H.; Chang, C.-J. Enhanced photocatalytic H2 production activity by loading Ni complex on flower-like MoS2 nanomaterials. J. Taiwan Inst. Chem. Eng. 2024, 161, 105530. [Google Scholar] [CrossRef]
- Chen, Y.H.; Cheng, W.H.; Ruan, J. Uniform and delicate adjustment of plasmonic coupling upon evolved MoS2 crystalline shields on individual gold nanoparticles for photocatalytic water splitting. J. Alloys Compd. 2024, 1009, 176604. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Huang, Y.-F.; Wu, X.-Y.; Ling, M.; Cheng, Y.; Wu, F.-H.; Xu, Q.; Wei, X.-W. Construction of synergistic active sites on MoS2 basal plane by interfacing with C60 derivative for enhanced photocatalytic hydrogen evolution coupled with biomass alcohol conversion. Appl. Surf. Sci. 2024, 649, 159166. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.; Li, T.; Xu, S.; Chen, S.; Yin, H. Enhanced photocatalytic hydrogen evolution through MoS2 quantum dots modification of bismuth-based perovskites. Chem. Commun. 2024, 60, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Miao, H.; Wu, J.; Zhu, X.; Yi, J.; Zhu, X.; Qi, H.; Jiang, Z.; Mo, Z.; Liu, J.; et al. Ingenious regulation and activation of sites in the 2H-MoS2 basal planes by oxygen incorporation for enhanced photocatalytic hydrogen evolution of CdS. Chem. Eng. J. 2024, 499, 156367. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, L.; Xie, X.; Qin, Z.; Ji, H.; Su, T. Construction of Electron Bridge and Activation of MoS2 Inert Basal Planes by Ni Doping for Enhancing Photocatalytic Hydrogen Evolution. Acta Phys.-Chim. Sin. 2024, 40, 2406024. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Cui, R.; Ling, W.; Hong, M.; Sun, R. Flower-structured CoP@MoS2−x S-scheme heterojunction photocatalysts for enhanced hydrogen evolution and selective biomass oxidation. Chem. Eng. J. 2025, 503, 158427. [Google Scholar] [CrossRef]
- Xi, F.; Zhang, L.; Cheng, A.; Sun, H.; Qin, Y.; Yang, B.; Zhang, S.; Ma, J.; Du, X.; Meng, X. Molten salt synthesis of 1T phase dominated O-MoS2 for enhancing photocatalytic hydrogen production performance of CdS via Ohmic junction. J. Colloid Interface Sci. 2025, 686, 1230–1240. [Google Scholar] [CrossRef]
- Kaba, I.; Kerkez-Kuyumcu, Ö. Highly efficient photocatalytic hydrogen production: Cd0.7Zn0.3S nanorod decorated by MoS2/MoC-Mo2C hybrid cocatalyst. J. Water Process Eng. 2025, 74, 107818. [Google Scholar] [CrossRef]
- Bu, S.H.; Cho, W.; Lee, C.; Ham, G.; Yang, B.; Jung, J.; Cha, H.; Park, C. Mechanochemical engineering and supramolecular reconstruction of MoS2 nanosheets with C60-γCD complexes for enhanced photocatalytic and piezoelectric performances. Chem. Eng. J. 2024, 502, 157688. [Google Scholar] [CrossRef]
- Fraga, A.L.S.; Kohlrausch, E.C.; Gulgielmin, L.; Fernandes, J.A.; Santos, J.F.L.; Benvenutti, E.V.; Weibel, D.E.; Khan, S.; Santos, M.J.L. Heterogeneous nucleation on defects in MoS2 nanostructures as a proxy for assessing active sites for hydrogen production. Mater. Lett. 2024, 362, 136175. [Google Scholar] [CrossRef]


| Method | Type | Advantages | Disadvantages | Expected Applications |
|---|---|---|---|---|
| Liquid phase exfoliation | Top-down | Simplicity, High yield | Poor efficiency and toxic nature of solvent | Suitable for large-scale production of few-layer MoS2 for basic research or coatings |
| PVD method | Bottom-up | Strong adhesion, low temperature deposition, scalability | High cost vacuum equipment, slo production speeds | Suitable for thin film based nano devices and microchips |
| CVD method | Bottom-up | Controlable layer numbers and size, large scale | High temperature | Suitable for high-performance electronic or sensor applications |
| Hydrothermal/solvothermal | Bottom-up | High yield and low temperature | Time consuming and limited control over phase purity | Photocatalysis, H2 evolution, and energy storage |
| Calcination method | Bottom-up | Thermal decomposition, volatile removal | High temperature, limited morphology control, Environemntal concerns | Photocatalysis, sensors |
| Sol-gel method | Bottom-up | Low processing temperature, cost-effective | Limited control over particle size and morphology, volume shrinkage | Thin film deposition for optoelectronic devices |
| Electrochemical deposition | Bottom-up | Uniform coating of films on substartes, precision, low temperature, control over reaction conditions, cost effective | Needs conductive substrate-Limited scalability-Requires controlled electrolyte chemistry | Electrochemical applications |
| PC/Cocatalyst | H2 Evolution | Light Source | Sacrificial Agent | References |
|---|---|---|---|---|
| MoS2/TiO2 | 1.41 mmol·g−1·h−1 | 300 W Xe lamp (320 < λ < 780 nm) | TEOA | [41] |
| MoS2@MoO3 | 12,416.8 µmol·h−1·g−1 | 5 W LED lamp (λ = 400–800 nm) | TEOA | [43] |
| ZnO/ZnS/MoS2 | 4.45 mmol·g−1 h−1 | 100 W Xe lamp (AM 1.5 G) | Na2S/Na2SO3 | [45] |
| MoS2/ZnO | 235 µmol·h−1·g−1 | 300 W Xe lamp | Na2S/Na2SO3 | [48] |
| TiO2(Rod)/MoS2 | 7415 μmol·g−1 | 300 W Xe lamp | glycerol/water | [50] |
| α-Fe2O3/BiOBr/MoS2 | 57 mmol·g−1·h−1 | 300 W Xe lamp (λ > 400 nm) | Ethanol | [57] |
| MoS2 nanosheets/CdS nanorods | 71.24 mmol·g−1·h−1 | AM 1.5 G | 10% Lactic acid | [65] |
| MoS2/SgCN | 4708.3 µmol·h−1·g−1 | 300 W Xe lamp | Methanol | [71] |
| MoS2/ZIS/GQDs | 21.63 mmol·g−1·h−1 | 300 W Xenon lamp | - | [77] |
| MOF/MoS2 | 626.3 μmol·h−1·g−1 | 350 W Xe lamp | Formic acid | [83] |
| MoS2/ZIF-67 | 8.13 mmol·g−1·h−1 | - | Na2S/Na2SO3 | [91] |
| MoS2/O-ZnIn2S4 | 4.002 mmol·g−1·h−1 | 300 W Xe lamp | Na2S/Na2SO3 | [99] |
| Ni–MoS2/CdS | 249 mmol·h−1·g−1 | Simulated solar light | Lactic acid | [104] |
| CdS/MoS2/CNFs | 3195.52 μmol·g−1·h−1 | 300 W Xe lamp (λ > 420 nm) | Na2S/Na2SO3 | [111] |
| 1% MoS2/P-Zn0.3Cd0.7S | 30.65 mmol·g−1·h−1 | 300 W Xe lamp | Lactic acid | [117] |
| O-doped MoS2/CoS/Zn0.1Cd0.9S | 95.5 mmol·g−1·h−1 | Xe lamp AM 1.5 G | Lactic acid | [123] |
| Rh1@MoS2/CZS-SVs | 39,827 μmol·h−1·g−1 | 300 W Xe lamp (λ > 420 nm) | Lactic acid | [130] |
| MoS2/Sv-ZnIn2S4/ZnS | 9.5 mmol·g−1·h−1 | 300 W Xe lamp (λ > 420 nm) | TEOA | [131] |
| MoS2/CABB | 87.5 μmol·h−1·g−1 | 300 W Xe lamp (λ > 420 nm) | - | [135] |
| Co9S8/MoS2/Ni2P | 5.69 mmol·g−1·h−1 | - | TEOA | [146] |
| MoS2/38AuNPs−2.75 | 35.08 mmol·g−1·h−1 | 300 W Xe lamp (λ > 400 nm) | 15 vol % TEOA and 0.5 M Na2SO4 | [149] |
| 5% indene-C60 bisadduct (ICBA)/MoS2/CdS | 978 μmol·h−1·g−1 | 300 W Xe lamp (λ > 400 nm) | - | [150] |
| MoS2 QDs/Cs3Bi2I9 | 6.09 mmol·h−1·g−1 | 300 W Xe lamp (λ > 400 nm) | - | [151] |
| O-MoS2/CdS | 58.47 mmol·g−1·h−1 | 300 W Xe lamp (λ > 400 nm) | - | [152] |
| Ni0.08-MoS2/ZnIn2S4 | 7.13 mmol·h−1·g−1 | 300 W Xe lamp (λ > 400 nm) | TEOA | [153] |
| CoP@MoS2-2 | 4339.39 μmol·g−1·h−1 | - | - | [154] |
| O-MoS2/CdS | 532.8 μmol·h−1 | LED light (420 nm) | lactic acid | [155] |
| N-Cd0.7Zn0.3S/1%MoS2 | 132 mmol·g−1·h−1 | - | Na2S/Na2SO3 | [156] |
| N-Cd0.7Zn0.3S/1%MoS2/1%MoC-Mo2C | 168 mmol·g−1·h−1 | - | Na2S/Na2SO3 | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, K.; Oh, T.H. Recent Progress in Photocatalytic Hydrogen Production Using 2D MoS2 Based Materials. Catalysts 2025, 15, 648. https://doi.org/10.3390/catal15070648
Ahmad K, Oh TH. Recent Progress in Photocatalytic Hydrogen Production Using 2D MoS2 Based Materials. Catalysts. 2025; 15(7):648. https://doi.org/10.3390/catal15070648
Chicago/Turabian StyleAhmad, Khursheed, and Tae Hwan Oh. 2025. "Recent Progress in Photocatalytic Hydrogen Production Using 2D MoS2 Based Materials" Catalysts 15, no. 7: 648. https://doi.org/10.3390/catal15070648
APA StyleAhmad, K., & Oh, T. H. (2025). Recent Progress in Photocatalytic Hydrogen Production Using 2D MoS2 Based Materials. Catalysts, 15(7), 648. https://doi.org/10.3390/catal15070648








