Ceria Promoted Ni/SiO2 as an Efficient Catalyst for Carbon Dioxide Reforming of Methane
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Textural Properties of Ni-Based Catalysts
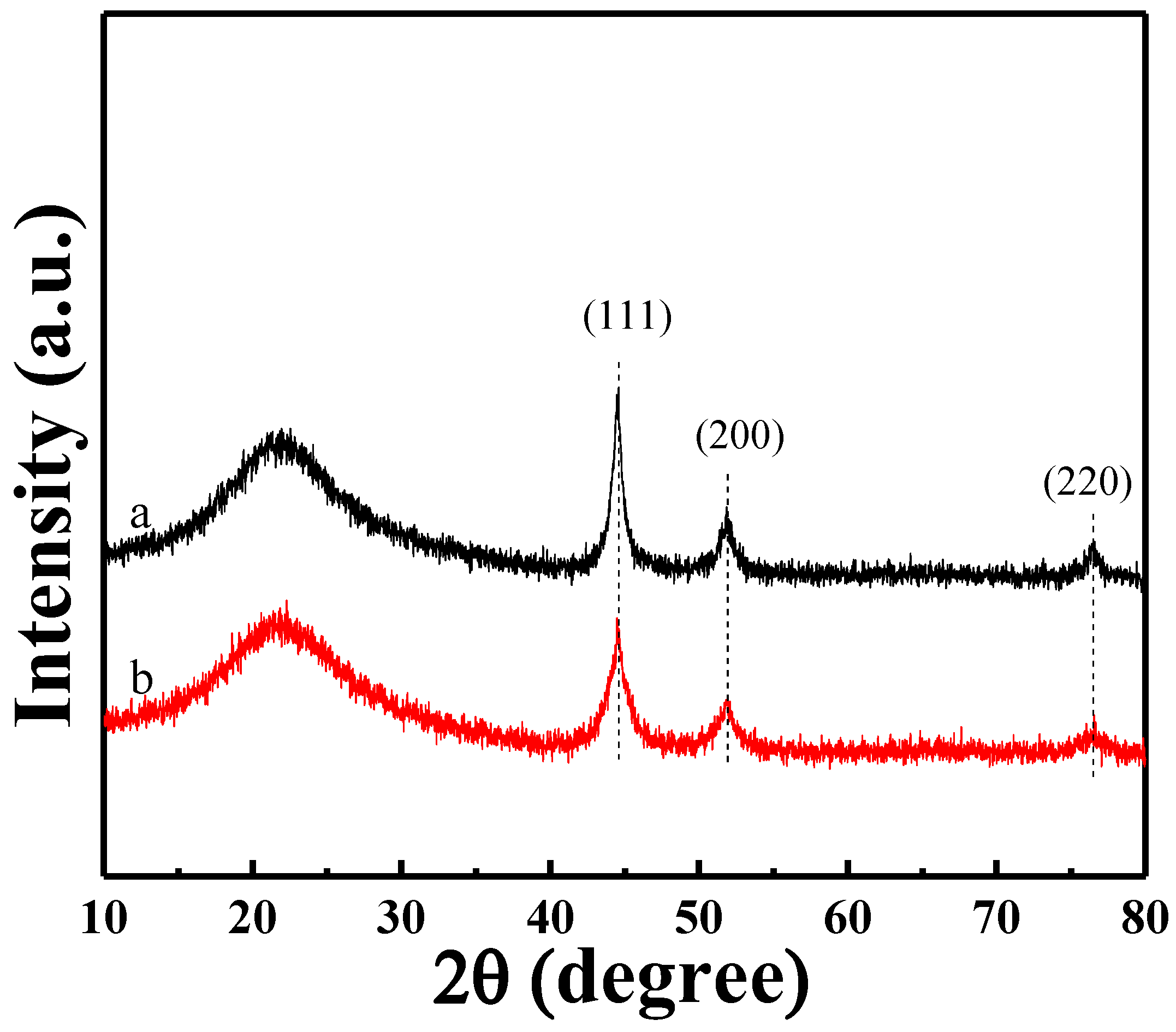
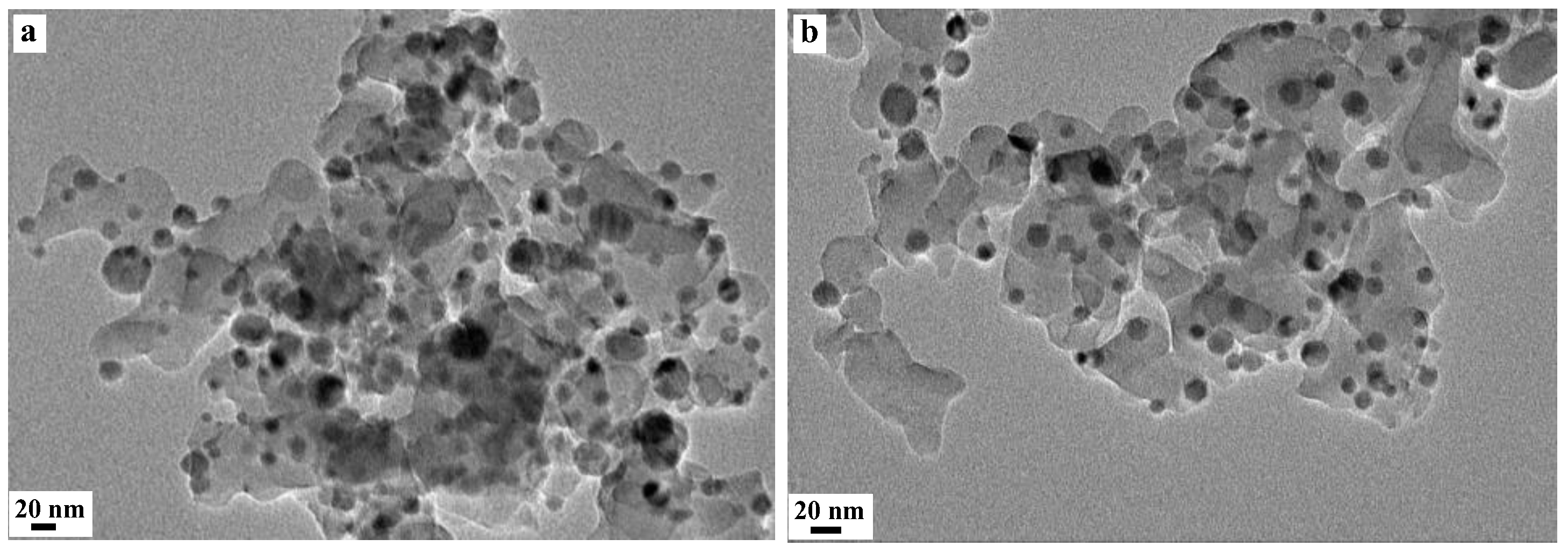
2.1.1. Structural Properties
2.1.2. Textural Properties of Ni-Based Catalysts
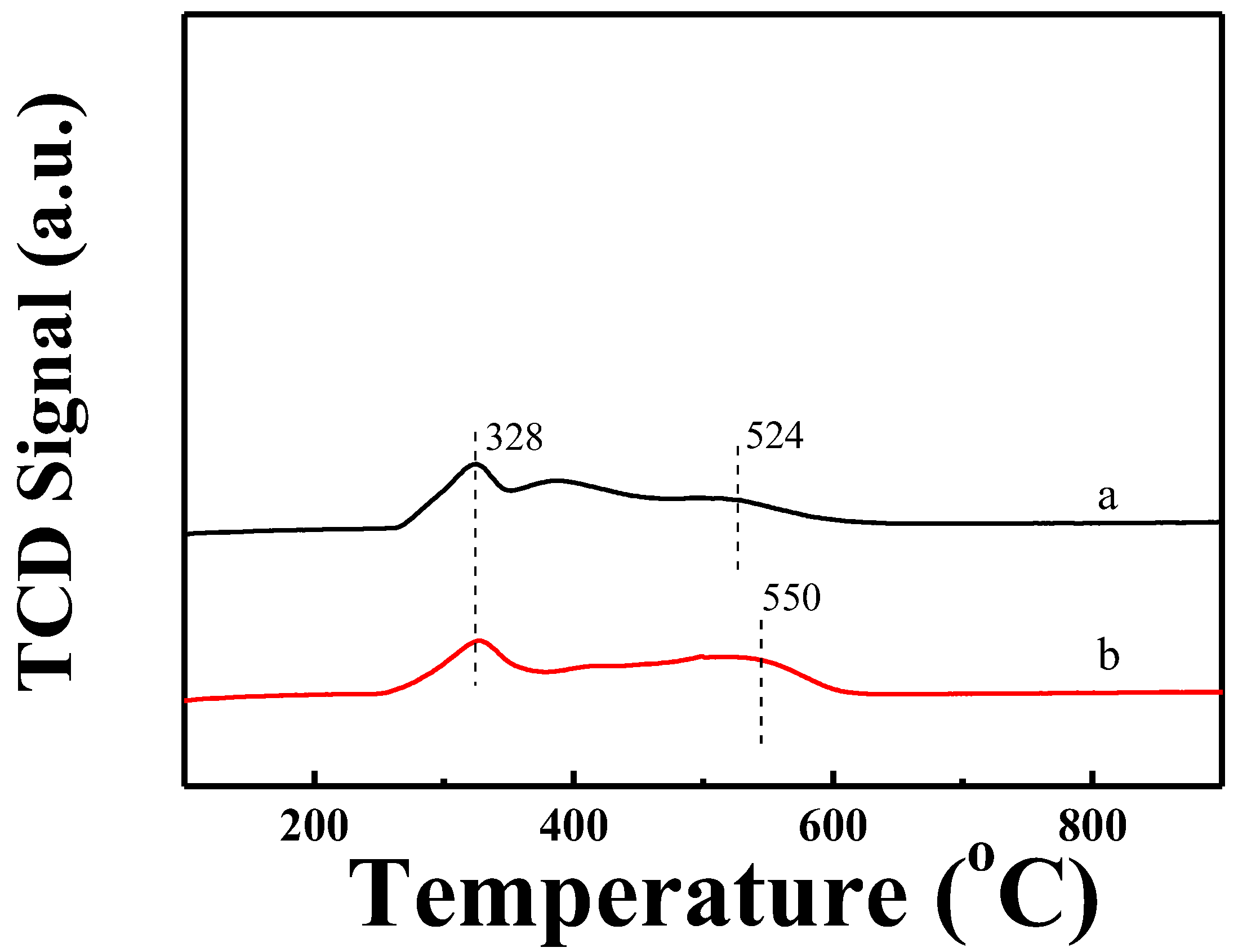
2.2. Reduction Behavior of Ni-Based Catalysts
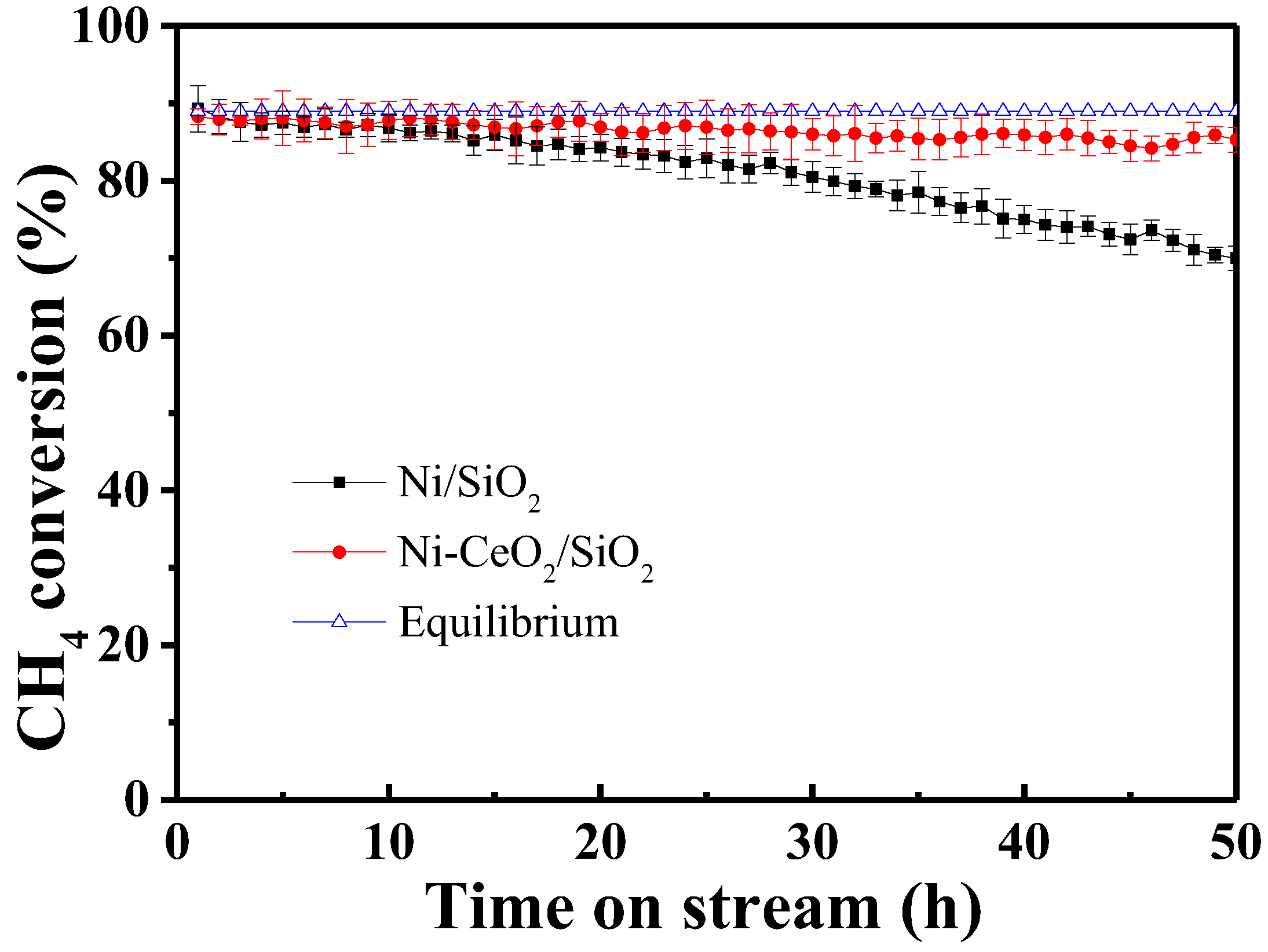
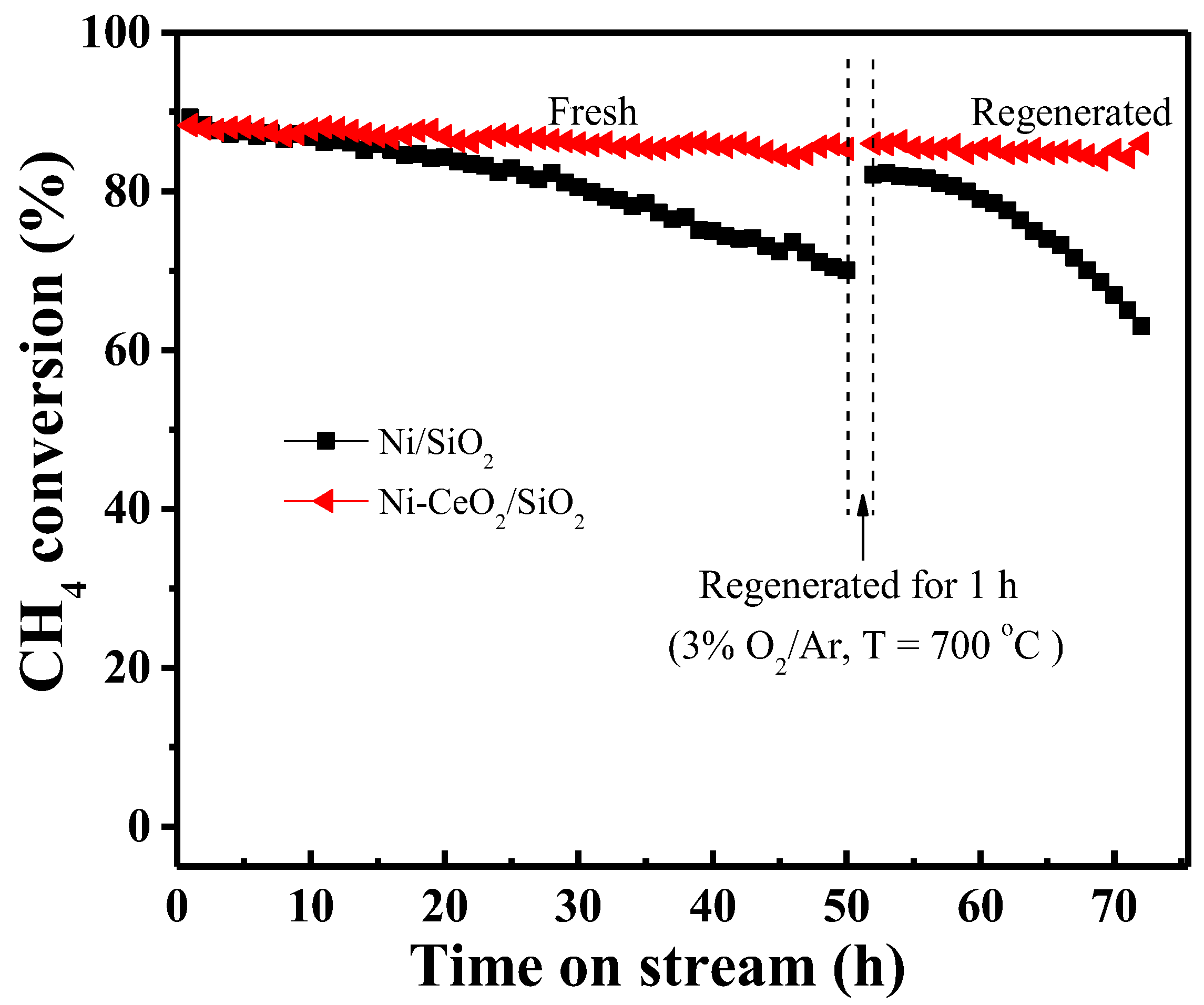
2.3. Catalytic Performance
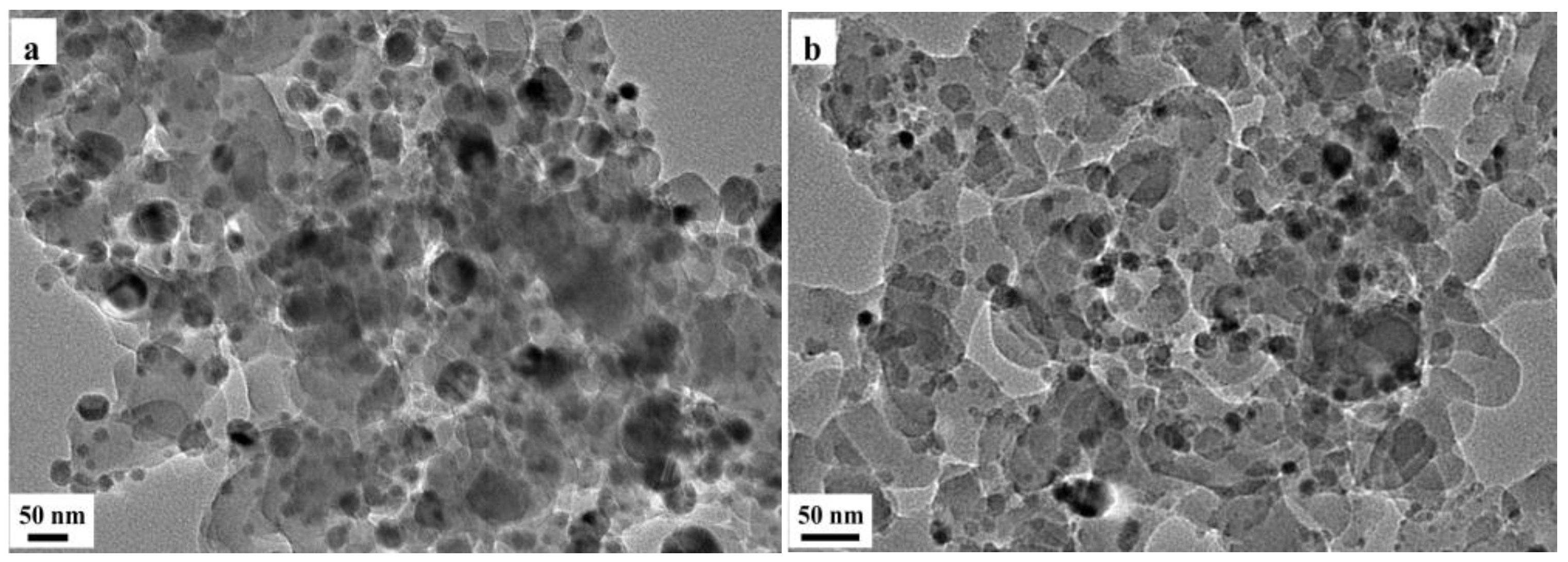
2.4. Characterization of Used Catalysts
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Evaluation of the Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, I.; Jalil, A.A.; Hassan, N.S.; Hamid, M.Y.S. Recent advances in catalytic systems for CO2 conversion to substitute natural gas (SNG): Perspective and challenges. J. Energy. Chem. 2021, 62, 377–407. [Google Scholar] [CrossRef]
- Sreedhar, I.; Varun, Y.; Singh, S.A.; Venugopal, A.; Reddy, B.M. Developmental trends in CO2 methanation using various catalysts. Catal. Sci. Technol. 2019, 9, 4478–4504. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Yan, T.; Deng, J.; Fang, J.; Zhang, D. Emergent Ni catalysts induced by nitride-to-oxide transformation for coking and sintering resistant dry reforming of methane. New J. Chem. 2023, 47, 10604–10612. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Yang, J.; Zhu, K.-K.; Sui, Z.-J.; Chen, D.; Zhu, Y.-A.; Zhou, X.-G. High-throughput screening of alloy catalysts for dry methane reforming. ACS Catal. 2021, 11, 8881–8894. [Google Scholar] [CrossRef]
- Silva, C.G.; Passos, F.B.; Silva, V.T. Influence of the support on the activity of a supported nickel-promoted molybdenum carbide catalyst for dry reforming of methane. J. Catal. 2019, 375, 507–518. [Google Scholar] [CrossRef]
- Moura, I.P.; Reis, A.C.; Bresciani, A.E.; Alves, R.M.B. Carbon dioxide abatement by integration of methane bi-reforming process with ammonia and urea synthesis. Renew. Sustain. Energy Rev. 2021, 151, 111619. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, T.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y.; Guo, Y. Robust nanosheet-assembled Al2O3-supported Ni catalysts for the dry reforming of methane: The effect of nickel content on the catalytic performance and carbon formation. New J. Chem. 2021, 45, 21750–21762. [Google Scholar] [CrossRef]
- Pentyala, P.; Biswas, P.; Jha, P.K. A density functional theory study of Nix (x = 4–16) cluster impregnation effects in multi-metal (Ce, Ti) UiO-66 metal-organic frameworks. New J. Chem. 2023, 47, 8549–8557. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.; Saw, E.T. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef]
- Mahfouz, R.; Estephane, J.; Gennequin, C.; Tidahy, L.; Aouadb, S.; Abi-Aad, E. CO2 reforming of methane over Ni and/or Ru catalysts supported on mesoporous KIT-6: Effect of promotion with Ce. J. Environ. Chem. Eng. 2021, 9, 104662. [Google Scholar] [CrossRef]
- Niu, J.; Ran, J.; Chen, D. Understanding the mechanism of CO2 reforming of methane to syngas on Ni@Pt surface compared with Ni(111) and Pt(111). Appl. Surf. Sci. 2020, 513, 145840. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Tran, T.Q.; Minh, D.P.; Phan, T.S.; Pham, Q.N.; Xuan, H.N. Dry reforming of methane over calcium-deficient hydroxyapatite supported cobalt and nickel catalysts. Chem. Eng. Sci. 2020, 228, 115975. [Google Scholar] [CrossRef]
- Abdulghani, A.J.A.; Park, J.-H.; Kozlov, S.M.; Kang, D.-C.; AlSabban, B.; Pedireddy, S.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-L.; Basset, J.-M.; et al. Methane dry reforming on supported cobalt nanoparticles promoted by boron. J. Catal. 2020, 392, 126–134. [Google Scholar] [CrossRef]
- Azancot, L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Effect of potassium loading on basic properties of Ni/MgAl2O4 catalyst for CO2 reforming of methane. J. CO2 Util. 2021, 52, 101681. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Zhu, J.; Han, B.; Fan, W.; Zhao, L.; Cai, W.; Li, Z.; Xu, L.; Yu, H.; et al. CO2 reforming with methane reaction over Ni@SiO2 catalysts coupled by size effect and metal-support interaction. Fuel 2019, 256, 115954. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Wang, F.; Han, B.; Zhang, L.; Xu, L.; Yu, H.; Shi, W. CO2 reforming with methane over small-sized Ni@SiO2 catalysts with unique features of sintering-free and low carbon. Appl. Catal. B Environ. 2018, 235, 26–35. [Google Scholar] [CrossRef]
- Benrabaa, R.; Barama, A.; Boukhlouf, H.; Jesús, G.-C.; Rubbens, A.; Elisabeth, B.-R.; Axel, L.; Vannier, R.-N. Physico-chemical properties and syngas production via dry reforming of methane over NiAl2O4 catalyst. Int. J Hydrogen Energy 2017, 42, 12989–12996. [Google Scholar] [CrossRef]
- Daoura, O.; Fornasieri, G.; Boutros, M.; Hassan, N.E.; Beaunier, P.; Thomas, C.; Selmane, M.; Miche, A.; Sassoye, C.; Ersen, O.; et al. One-pot prepared mesoporous silica SBA-15-like monoliths with embedded Ni particles as selective and stable catalysts for methane dry reforming. Appl. Catal. B Environ. 2021, 280, 119417. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wu, H.; Yang, W.; He, D. Promoting effect of glucose and β-cyclodextrin on Ni dispersion of Ni/MCM-41 catalysts for carbon dioxide reforming of methane to syngas. Fuel 2014, 136, 19–24. [Google Scholar] [CrossRef]
- Danghyan, V.; Kumar, A.; Mukasyan, A.; Wolf, E.E. An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane. Appl. Catal. B Environ. 2020, 273, 119056. [Google Scholar] [CrossRef]
- Scapinello, M.; Delikonstantis, E.; Stefanidis, G.D. The panorama of plasma-assisted non-oxidative methane reforming. Chem. Eng. Process. 2017, 117, 120–140. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metalorganic frameworks (MOFs)-based catalysts. Appl. Catal. B Environ. 2020, 260, 118195. [Google Scholar] [CrossRef]
- Fang, X.; Peng, C.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W. Methane dry reforming over coke-resistant mesoporous Ni-Al2O3 catalysts prepared by evaporation-induced self-assembly method. ChemCatChem 2015, 7, 3753–3762. [Google Scholar] [CrossRef]
- Costa, K.Ś.D.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Costa, P.D. Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Toniolo, F.S.; Noronha, F.B.; Epron, F.; Duprez, D.; Bion, N. Highly active and stable Ni dispersed on mesoporous CeO2-Al2O3 catalysts for production of syngas by dry reforming of methane. Appl. Catal. B Environ. 2021, 281, 119459. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Liu, J.; Wang, T.; Shen, Y.; Zheng, K.; Jiang, F.; Xu, Y.; Liu, X. Tuning the Lewis acidity of ZrO2 for efficient conversion of CH4 and CO2 into acetic acid. New J. Chem. 2021, 45, 8978–8985. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jiang, Q.; Gao, Y.; Liu, J.; Gu, H.; Neal, L.; Li, F. LaNixFe1-xO3-δ as a robust redox catalyst for CO2 splitting and methane partial oxidation. Energy Fuels 2021, 35, 13921–13929. [Google Scholar] [CrossRef]
- Bakhtiari, K.; Kootenaei, A.S.; Maghsoodi, S.; Azizi, S.; Ghomsheh, S.M.T. Synthesis of high sintering-resistant Ni-modified halloysite based catalysts containing La, Ce, and Co for dry reforming of methane. Ceram. Int. 2022, 48, 37394–37402. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Z.; Osatiashtiani, A.; Nabavi, S.A.; Clough, P.T. Ni-based bimetallic catalysts for hydrogen production via (sorption-enhanced) steam methane reforming. Chem. Eng. J. 2024, 486, 150170. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Fung, W.-Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int. J. Hydrogen Energy 2019, 44, 14303–14315. [Google Scholar] [CrossRef]
- Babakouhi, R.; Alavi, S.M.; Rezaei, M.; Jokar, F.; Varbar, M.; Akbari, E. Hydrogen production through combined dry reforming and partial oxidation of methane over the Ni/Al2O3-CeO2 catalysts . Int. J. Hydrogen Energy 2024, 60, 503–514. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Zhang, W.; Hou, B.; Deng, Y.; Ma, X.; Xu, L. Solution combustion synthesis of Ce-based composite oxygen carriers for chemical looping reforming of methane. ChenCatChem 2024, 16, e202301768. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, H.; Huang, L.; Liu, Y.; Cui, S.; Liu, H.; Zeng, S.; Wang, L. Anti-coking Ni-La2O3/SiO2 catalyst prepared by using a glycine-assisted impregnation method for low-temperature dry reforming of methane. Chem. Lett. 2024, 53, 4. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Z.; Zhang, Y.; Li, G.; Xu, W.; Zheng, Y.; Song, W. Insight into exhaust gas-methane reforming over La2O3-modified Ni/Ca-Al for enhanced hydrogen-rich production. Fuel 2024, 363, 130912. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Contemporary trends in composite Ni-based catalysts for CO2 reforming of methane. Chem. Eng. Sci. 2021, 229, 116072. [Google Scholar] [CrossRef]
- Ren, H.-P.; Song, Y.-H.; Wang, W.; Chen, J.-G.; Cheng, J.; Jiang, J.; Liu, Z.-T.; Liu, Z.-W.; Hao, Z.; Lu, J. Insights into CeO2-modified Ni-Mg-Al oxides for pressurized carbon dioxide reforming of methane. Chem. Eng. J. 2015, 259, 581–593. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S. Methane reforming with CO2 using nickel catalysts supported on yttria-doped SBA-15 mesoporous materials via sol-gel process. Int. J. Hydrogen Energy 2013, 38, 14250–14260. [Google Scholar] [CrossRef]
- Ren, H.-P.; Hao, Q.-Q.; Ding, S.-Y.; Zhao, Y.-Z.; Zhu, M.; Tian, S.-P.; Ma, Q.; Song, W.-Q.; Miao, Z.; Liu, Z.-T. A high-performance Ni/SiO2 prepared by the complexed impregnation method with citric acid for carbon dioxide reforming of methane. Ind. Eng. Chem. Res. 2018, 57, 16257–16263. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Mastro, S.L.; Sodo, A. Ni/CeO2-Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Coelho, L.R.F.; Silva, A.A.A.; Xing, Y.; Rabelo-Neto, R.; Noronha, F.B.; Mattos, L.V. Ni supported on desilicated HZSM-5 as catalyst for biogas reforming. Fuel 2024, 363, 130914. [Google Scholar] [CrossRef]



| Samples | SBET (m2·g−1) a | Vp (cm3·g−1) b | Dp (nm) c | dNiO (nm) d | dNi (nm) e | Dispersion (D%) f |
|---|---|---|---|---|---|---|
| SiO2 | 572 | 0.45 | 3.0 | |||
| Ni/SiO2 | 352 | 0.36 | 3.5 | 13.4 | 13.7 | 7.1 |
| Ni-CeO2/SiO2 | 382 | 0.38 | 3.5 | 12.8 | 10.3 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-P.; Zhang, L.-F.; Hui, Y.-X.; Wu, X.-Z.; Tian, S.-P.; Ding, S.-Y.; Ma, Q.; Zhao, Y.-Z. Ceria Promoted Ni/SiO2 as an Efficient Catalyst for Carbon Dioxide Reforming of Methane. Catalysts 2025, 15, 649. https://doi.org/10.3390/catal15070649
Ren H-P, Zhang L-F, Hui Y-X, Wu X-Z, Tian S-P, Ding S-Y, Ma Q, Zhao Y-Z. Ceria Promoted Ni/SiO2 as an Efficient Catalyst for Carbon Dioxide Reforming of Methane. Catalysts. 2025; 15(7):649. https://doi.org/10.3390/catal15070649
Chicago/Turabian StyleRen, Hua-Ping, Lin-Feng Zhang, Yu-Xuan Hui, Xin-Ze Wu, Shao-Peng Tian, Si-Yi Ding, Qiang Ma, and Yu-Zhen Zhao. 2025. "Ceria Promoted Ni/SiO2 as an Efficient Catalyst for Carbon Dioxide Reforming of Methane" Catalysts 15, no. 7: 649. https://doi.org/10.3390/catal15070649
APA StyleRen, H.-P., Zhang, L.-F., Hui, Y.-X., Wu, X.-Z., Tian, S.-P., Ding, S.-Y., Ma, Q., & Zhao, Y.-Z. (2025). Ceria Promoted Ni/SiO2 as an Efficient Catalyst for Carbon Dioxide Reforming of Methane. Catalysts, 15(7), 649. https://doi.org/10.3390/catal15070649






