Electrocatalytic Investigation of the SOFC Internal CH4 Dry Reforming with Modified Ni/GDC: Effect of Au Content on the Performance Enhancement by Fe-Au Doping
Abstract
1. Introduction
2. Results
2.1. Effect of Au Content on the Performance Enhancement by Fe-Au Doping
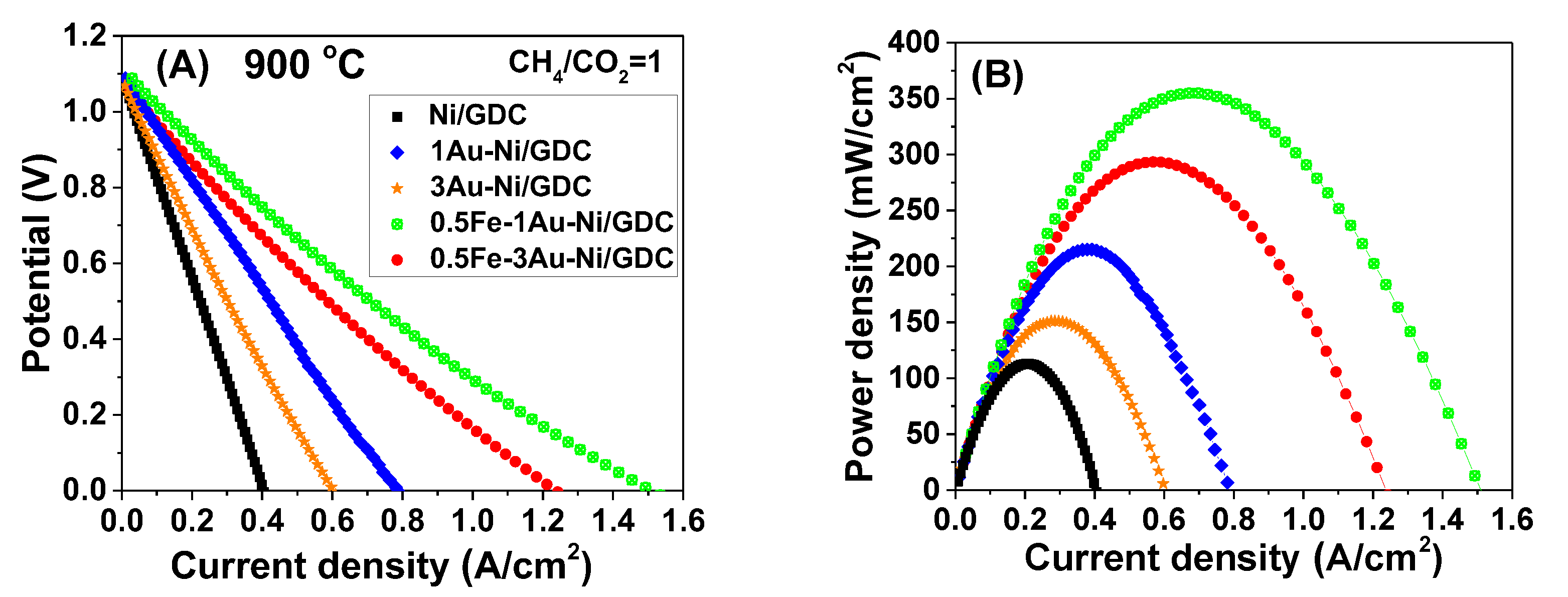

2.1.1. Effect of Fe/Au Modification at Lower Operating Temperature
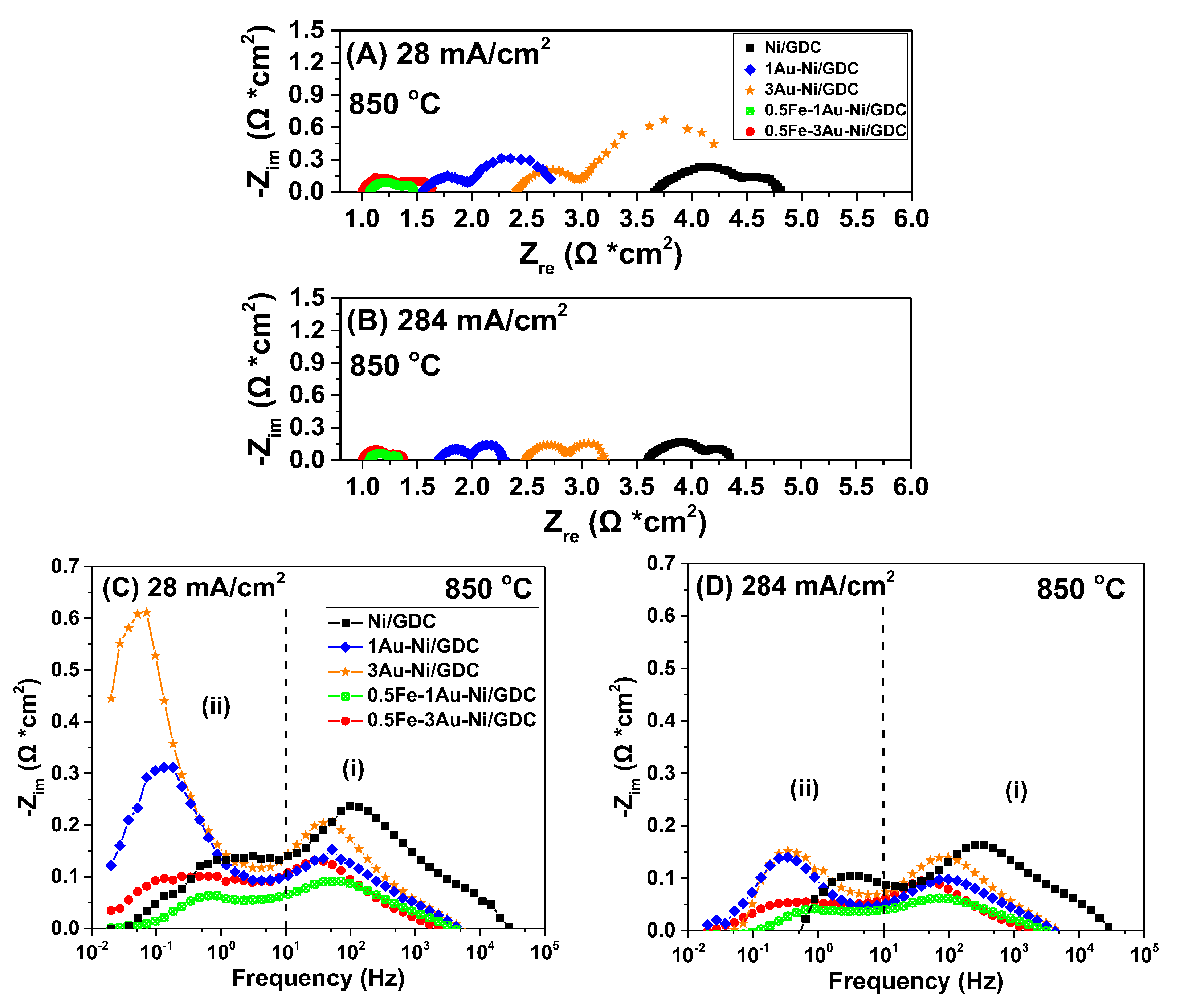
2.1.2. Effect of Fe/Au Modification at Different Applied Polarization
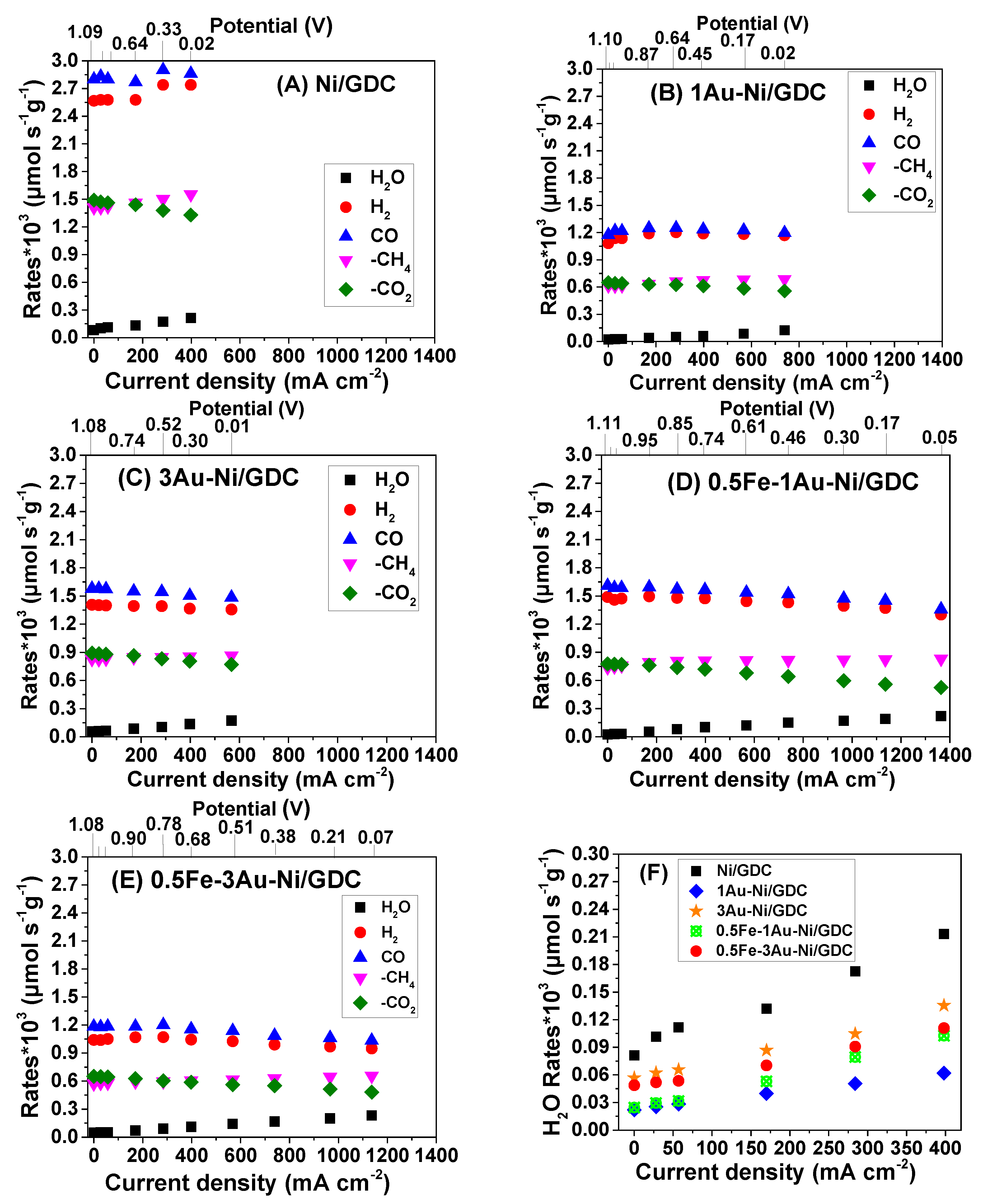
2.2. Effect of Fe/Au Modification on the SOFC IDRM Electrocatalytic Activity—Quantitative Analysis
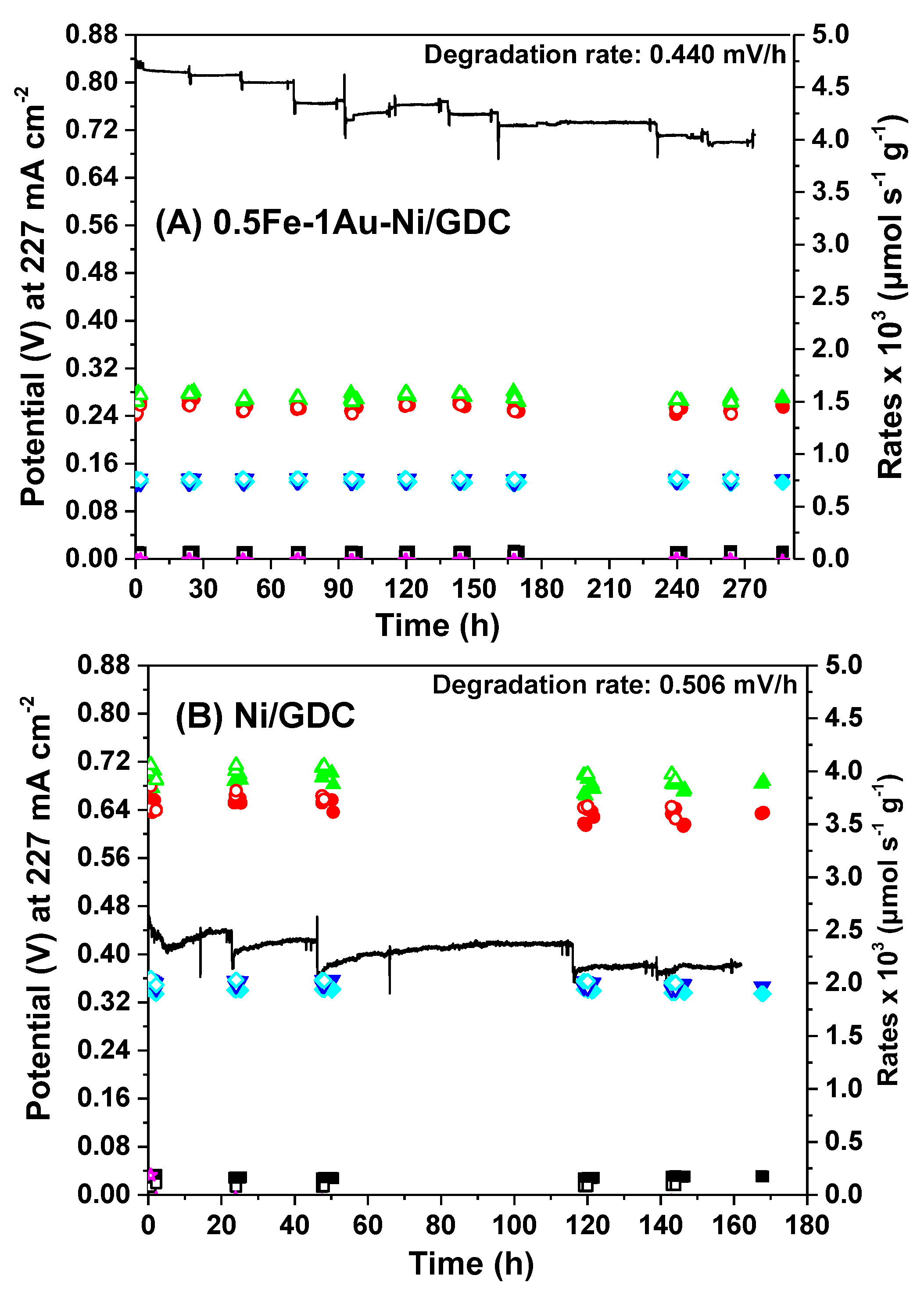
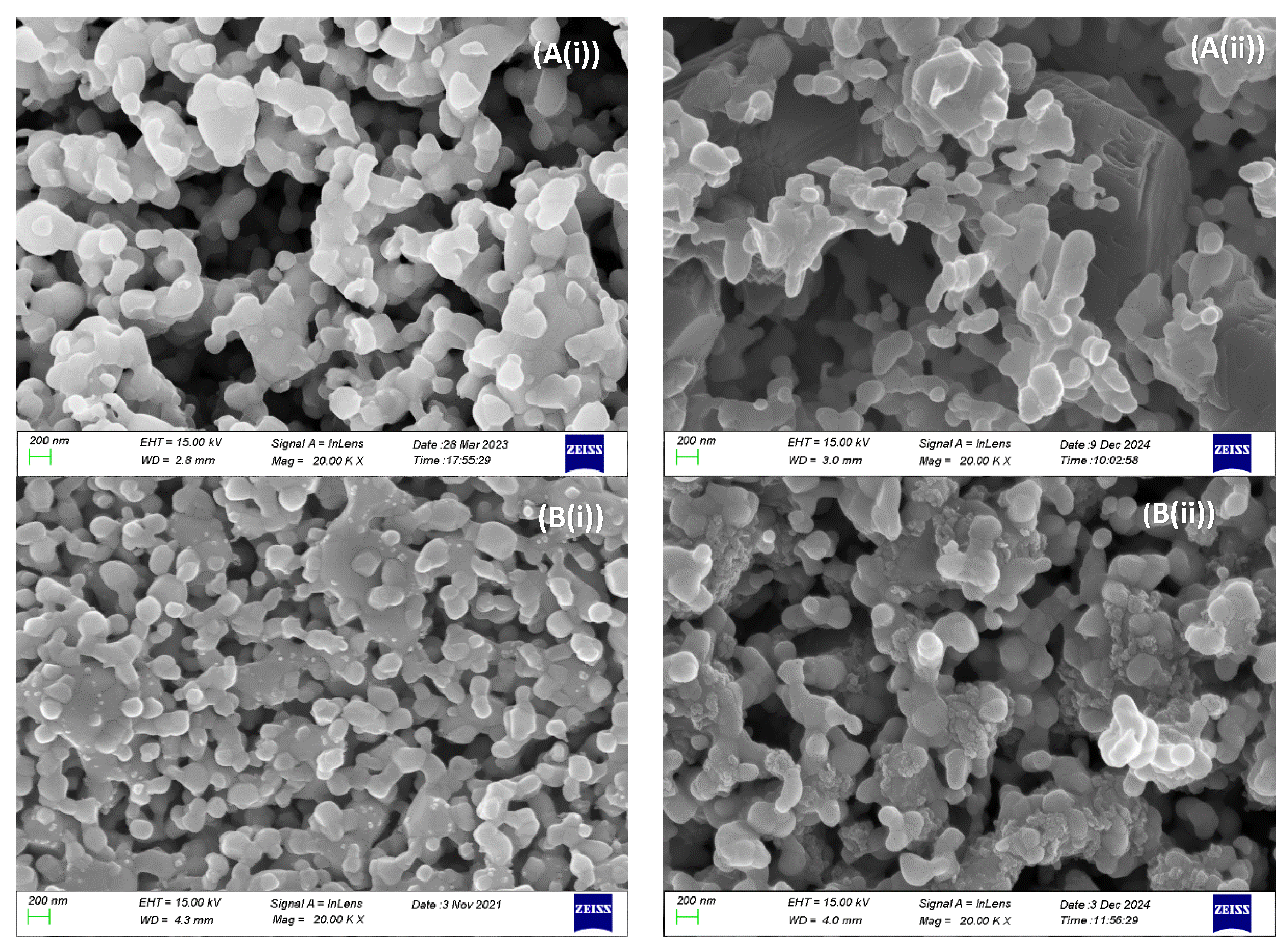
2.3. Electrocatalytic IDRM Stability Test of 0.5Fe-1Au-Ni/GDC vs Ni/GDC
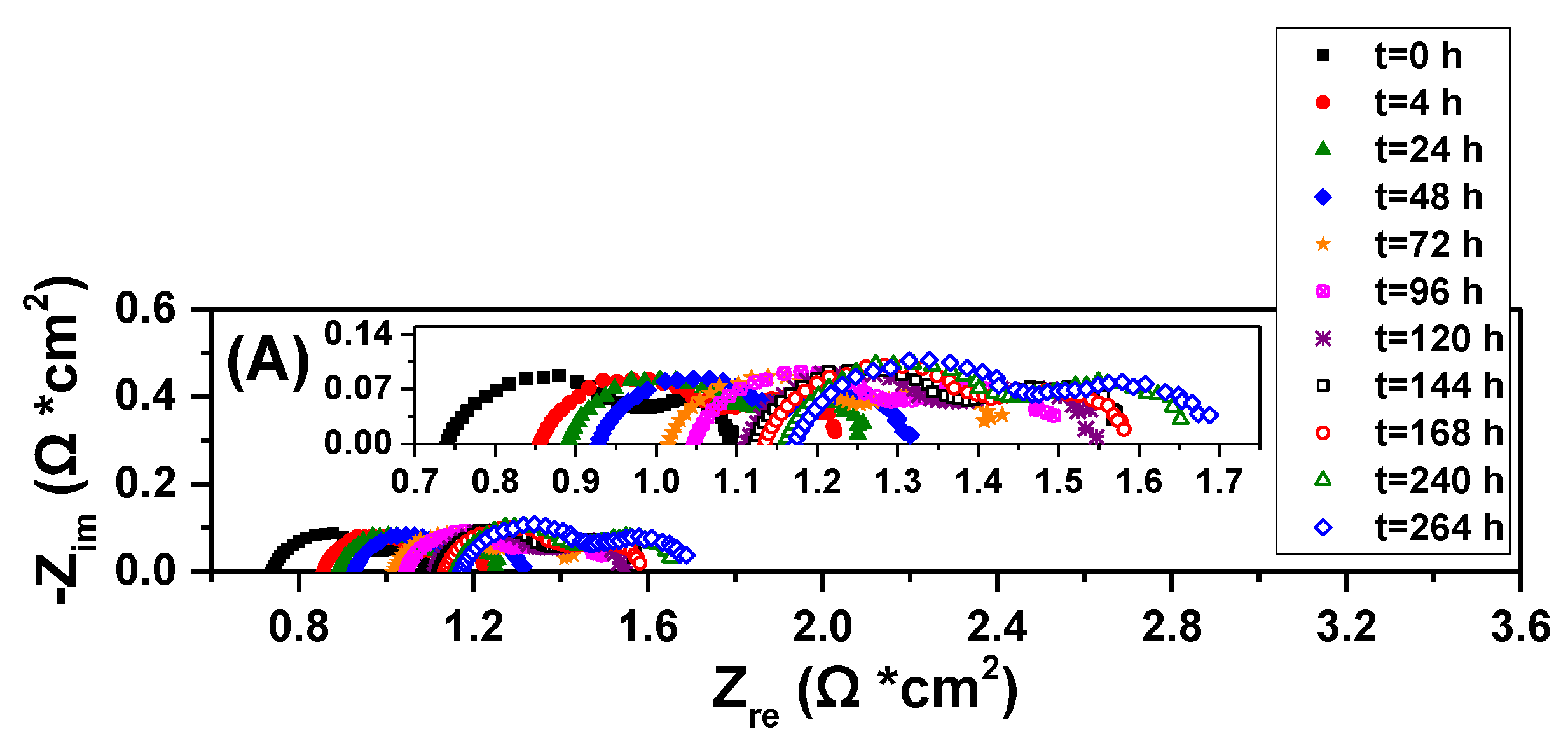

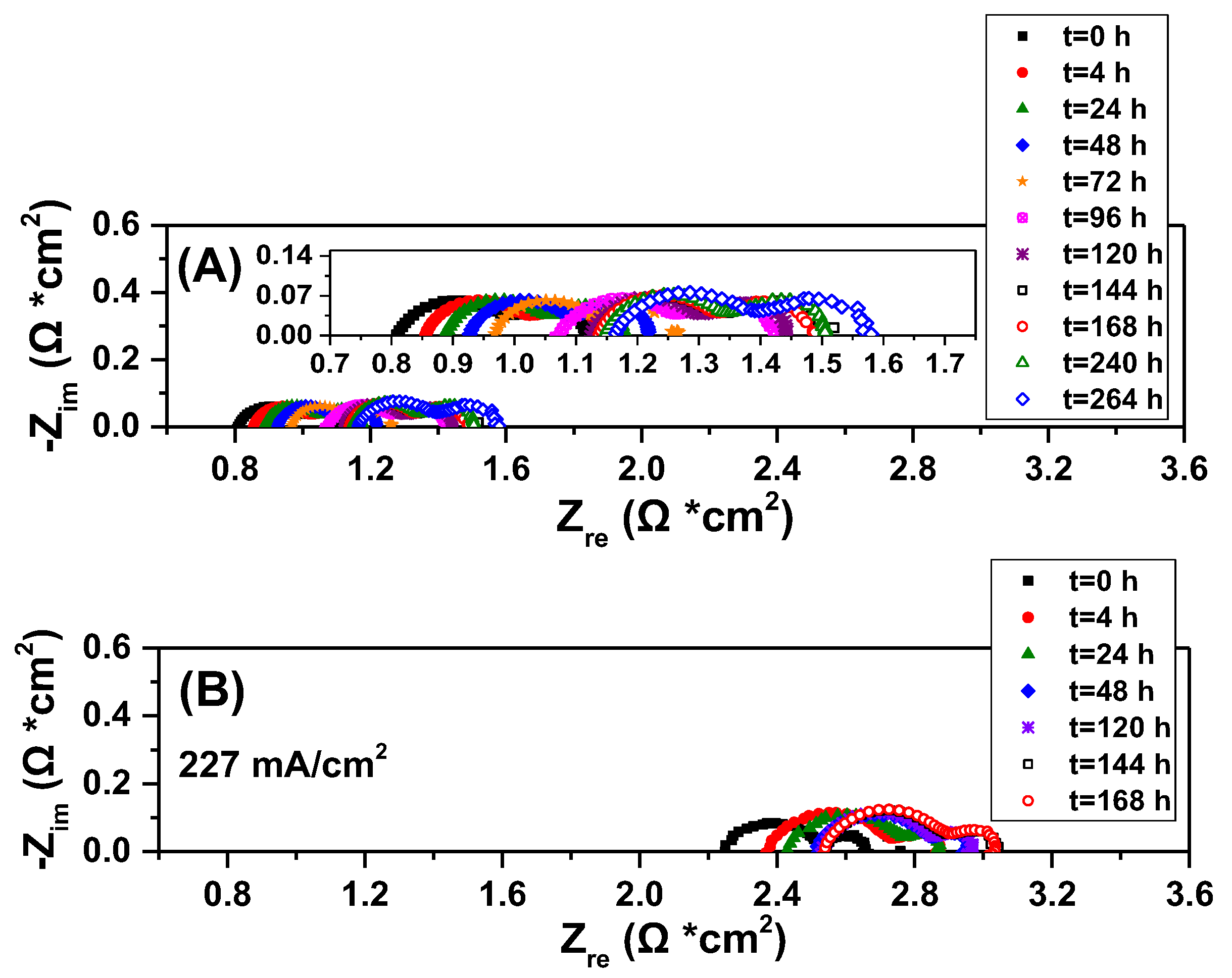

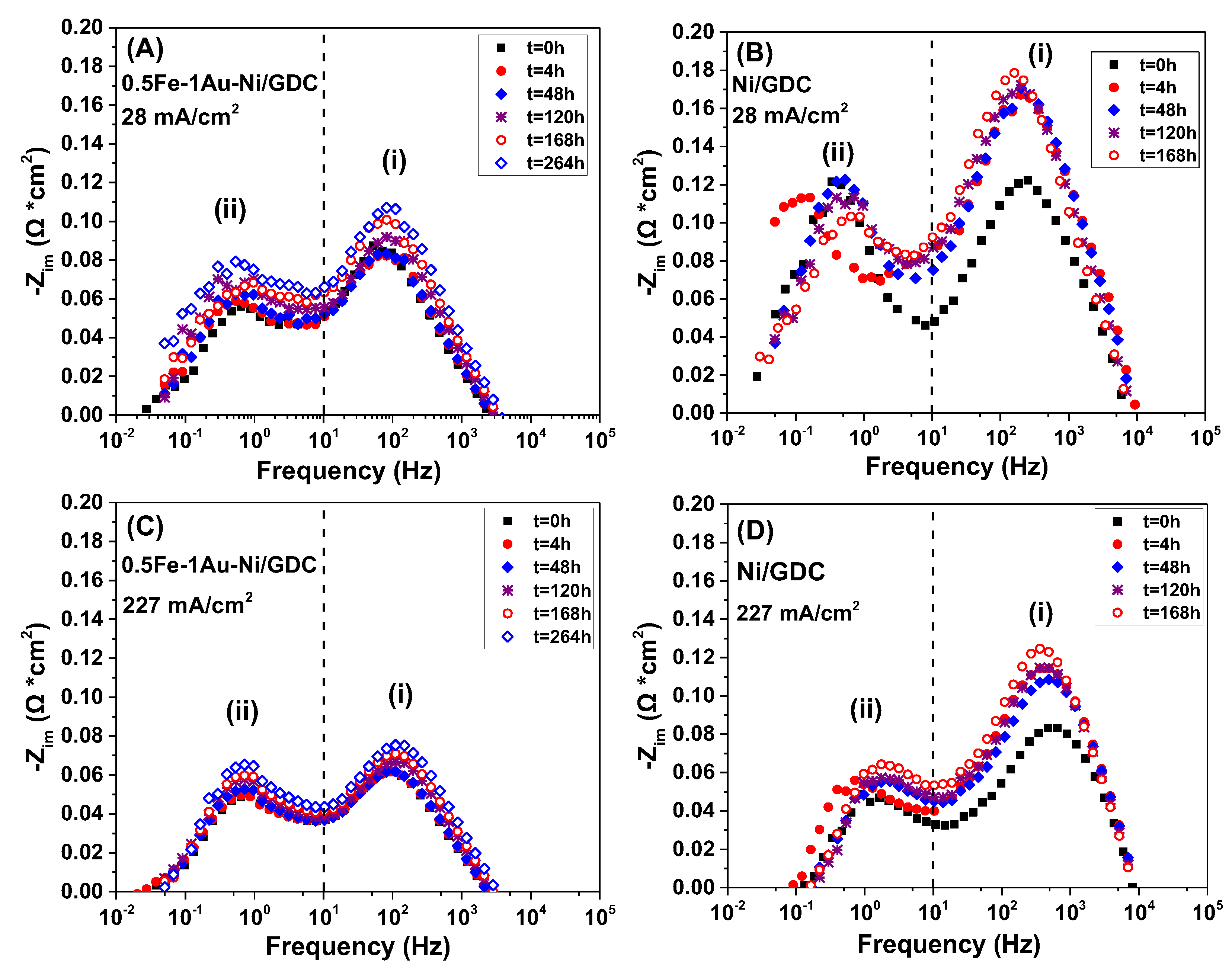
3. Experimental Procedure
3.1. Preparation of Electrocatalysts
3.2. Preparation of Solid Oxide Fuel Cells (SOFCs)
3.3. Electrocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenton, T.M.; Xu, C.; Abrams, J.F.; Ghadiali, A.; Loriani, S.; Sakschewski, B.; Zimm, C.; Ebi, K.L.; Dunn, R.R.; Svenning, J.C.; et al. Quantifying the human cost of global warming. Nat. Sustain. 2023, 6, 1237–1247. [Google Scholar] [CrossRef]
- Spring, C.R.; Cirella, G.T. Fostering Sustainable Development: Green Energy Policy in the European Union and the United States. In Advances in 21st Century Human Settlements; Springer: Berlin/Heidelberg, Germany, 2022; pp. 101–137. [Google Scholar]
- Scarlat, N.; Prussi, M.; Padella, M. Quantification of the carbon intensity of electricity produced and used in Europe. Appl. Energy 2022, 305, 117901. [Google Scholar] [CrossRef]
- Escudero, M.J.; Maffiotte, C.A.; Serrano, J.L. Impedance analysis of electrolyte-supported solid oxide fuel cell with nickel-tungsten-cerium anode fed with CH4-CO2 mixtures and siloxanes. Electrochim. Acta 2022, 427, 140852. [Google Scholar] [CrossRef]
- Trendewicz, A.A.; Braun, R.J. Techno-economic analysis of solid oxide fuel cell-based combined heat and power systems for biogas utilization at wastewater treatment facilities. J. Power Sources 2013, 233, 380–393. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Wang, J.; Yang, J.; Chen, Y.; Guan, W.; Singhal, S.C. Power generation from a symmetric flat-tube solid oxide fuel cell using direct internal dry-reforming of methane. J. Power Sources 2021, 516, 230662. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Santoro, M.; Di Bartolomeo, E.; Luisetto, I.; Aricò, A.S.; Squadrito, G.; Zignani, S.C.; Lo Faro, M. Insights on the electrochemical performance of indirect internal reforming of biogas into a solid oxide fuel cell. Electrochim. Acta 2022, 409, 139940. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Tanveer, W.H.; Sayed, E.T.; Assad, M.E.H.; Allagui, A.; Cha, S.W. On the technical challenges affecting the performance of direct internal reforming biogas solid oxide fuel cells. Renew. Sustain. Energy Rev. 2019, 101, 361–375. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Illathukandy, B.; Aravind, P.V. Direct internal methane reforming in biogas fuelled solid oxide fuel cell; the influence of operating parameters. Energy Sci. Eng. 2021, 9, 1232–1248. [Google Scholar] [CrossRef]
- Lyu, Z.; Wang, Y.; Zhang, Y.; Han, M. Solid oxide fuel cells fueled by simulated biogas: Comparison of anode modification by infiltration and reforming catalytic layer. Chem. Eng. J. 2020, 393, 124755. [Google Scholar] [CrossRef]
- Neofytidis, C.; Dracopoulos, V.; Neophytides, S.G.; Niakolas, D.K. Electrocatalytic performance and carbon tolerance of ternary Au-Mo-Ni/GDC SOFC anodes under CH4-rich Internal Steam Reforming conditions. Catal. Today 2018, 310, 157–165. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Escudero, M.J.; Maffiotte, C.A.; Serrano, J.L. Long-term operation of a solid oxide fuel cell with MoNi–CeO2 as anode directly fed by biogas containing simultaneously sulphur and siloxane. J. Power Sources 2021, 481, 229048. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Souentie, S.; Athanasiou, M.; Niakolas, D.K.; Katsaounis, A.; Neophytides, S.G.; Vayenas, C.G. Mathematical modeling of Ni/GDC and Au–Ni/GDC SOFC anodes performance under internal methane steam reforming conditions. J. Catal. 2013, 306, 116–128. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shiratori, Y.; Furuta, S.; Sasaki, K. Thermo-mechanical reliability and catalytic activity of Ni-Zirconia anode supports in internal reforming SOFC running on biogas. Solid State Ionics 2012, 225, 113–117. [Google Scholar] [CrossRef]
- Shiratori, Y.; Tuyen, T.; Takahashi, Y.; Taniguchi, S.; Sasaki, K. Highly Efficient Biomass Utilization with Solid Oxide Fuel Cell Technology. In Renewable Energy-Trends and Applications; InTech: Houston, TX, USA, 2011. [Google Scholar]
- Pillai, M.; Lin, Y.; Zhu, H.; Kee, R.J.; Barnett, S.A. Stability and coking of direct-methane solid oxide fuel cells: Effect of CO2 and air additions. J. Power Sources 2010, 195, 271–279. [Google Scholar] [CrossRef]
- Jones, C.M.; Persky, J.; Datta, R. Exploring Conditions That Enhance Durability and Performance of a Tubular Solid Oxide Fuel Cell Fed with Simulated Biogas. Energy Fuels 2017, 31, 12875–12892. [Google Scholar] [CrossRef]
- Qiu, P.; Sun, S.; Yang, X.; Chen, F.; Xiong, C.; Jia, L.; Li, J. A review on anode on-cell catalyst reforming layer for direct methane solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 25208–25224. [Google Scholar] [CrossRef]
- Lyu, Z.; Liu, Y.; Sciazko, A.; Komatsu, Y.; Tao, J.; Nakamura, A.; Hara, T.; Sun, K.; Shikazono, N.; Han, M. Co-Generation of Electricity and Chemicals From Methane Using Direct Internal Reforming Solid Oxide Fuel Cells. Adv. Energy Mater. 2024, 15, 2403869. [Google Scholar] [CrossRef]
- Lanzini, A.; Leone, P.; Guerra, C.; Smeacetto, F.; Brandon, N.P.; Santarelli, M. Durability of anode supported Solid Oxides Fuel Cells (SOFC) under direct dry-reforming of methane. Chem. Eng. J. 2013, 220, 254–263. [Google Scholar] [CrossRef]
- Papadam, T.; Goula, G.; Yentekakis, I.V. Long-term operation stability tests of intermediate and high temperature Ni-based anodes’ SOFCs directly fueled with simulated biogas mixtures. Int. J. Hydrogen Energy 2012, 37, 16680–16685. [Google Scholar] [CrossRef]
- Jiang, S.P.; Zhang, S.; Da Zhen, Y.; Wang, W. Fabrication and Performance of Impregnated Ni Anodes of Solid Oxide Fuel Cells. In Progress in Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 257–263. [Google Scholar] [CrossRef]
- Wang, W.; Qu, J.; Julião, P.S.B.; Shao, Z. Recent Advances in the Development of Anode Materials for Solid Oxide Fuel Cells Utilizing Liquid Oxygenated Hydrocarbon Fuels: A Mini Review. Energy Technol. 2019, 7, 33–44. [Google Scholar] [CrossRef]
- Mogensen, M.B.; Chen, M.; Frandsen, H.L.; Graves, C.; Hauch, A.; Hendriksen, P.V.; Jacobsen, T.; Jensen, S.H.; Skafte, T.L.; Sun, X. Ni migration in solid oxide cell electrodes: Review and revised hypothesis. Fuel Cells 2021, 21, 415–429. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, S.P.; Tok, A.I.Y.; Luo, L. GDC-impregnated Ni anodes for direct utilization of methane in solid oxide fuel cells. J. Power Sources 2006, 159, 68–72. [Google Scholar] [CrossRef]
- Livermore, S.J.A.; Cotton, J.W.; Ormerod, R.M. Fuel reforming and electrical performance studies in intermediate temperature ceria-gadolinia-based SOFCs. J. Power Sources 2000, 86, 411–416. [Google Scholar] [CrossRef]
- Jiang, S.P.; Zhang, S.; Zhen, Y.D.; Koh, A.P. Performance of GDC-impregnated Ni anodes of SOFCs. Electrochem. Solid-State Lett. 2004, 7, A282. [Google Scholar] [CrossRef]
- Sun, C.; Stimming, U. Recent anode advances in solid oxide fuel cells. J. Power Sources 2007, 171, 247–260. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Ge, X.; Deng, S.; Chen, B.; Shen, J. A review of different catalytic systems for dry reforming of methane: Conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 2021, 46, 101462. [Google Scholar] [CrossRef]
- Niu, J.; Guo, F.; Ran, J.; Qi, W.; Yang, Z. Methane dry (CO2) reforming to syngas (H2/CO) in catalytic process: From experimental study and DFT calculations. Int. J. Hydrogen Energy 2020, 45, 30267–30287. [Google Scholar] [CrossRef]
- Kan, H.; Lee, H. Enhanced stability of Ni-Fe/GDC solid oxide fuel cell anodes for dry methane fuel. Catal. Commun. 2010, 12, 36–39. [Google Scholar] [CrossRef]
- Cesario, M.R.; Souza, G.S.; Loureiro, F.J.A.; Araújo, A.J.M.; Grilo, J.P.F.; Aouad, S.; Tidahy, H.L.; Macedo, D.A.; Fagg, D.P.; Gennequin, C.; et al. Synthesis of Co–Ni and Cu–Ni based-catalysts for dry reforming of methane as potential components for SOFC anodes. Ceram. Int. 2021, 47, 33191–33201. [Google Scholar] [CrossRef]
- Yentekakis, I.V. Open- and closed-circuit study of an intermediate temperature SOFC directly fueled with simulated biogas mixtures. J. Power Sources 2006, 160, 422–425. [Google Scholar] [CrossRef]
- Papaefthimiou, V.; Shishkin, M.; Niakolas, D.K.; Athanasiou, M.; Law, Y.T.; Arrigo, R.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; et al. On the Active Surface State of Nickel-Ceria Solid Oxide Fuel Cell Anodes During Methane Electrooxidation. Adv. Energy Mater. 2013, 3, 762–769. [Google Scholar] [CrossRef]
- Niakolas, D.K.; Neofytidis, C.S.; Neophytides, S.G. Effect of Au and/or Mo doping on the development of carbon and sulfur tolerant anodes for SOFCs-A short review. Front. Environ. Sci. 2017, 5, 78. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Zhao, C.; Boréave, A.; Retailleau-Mevel, L.; Niakolas, D.; Neofytidis, C.; Vernoux, P. Sulphur tolerance of Au-modified Ni/GDC during catalytic methane steam reforming. Catal. Sci. Technol. 2018, 8, 1578–1588. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neofytidis, C.; Sygellou, L.; Niakolas, D.K. Au-doped Ni/GDC as an Improved Cathode Electrocatalyst for H2O Electrolysis in SOECs. Appl. Catal. B Environ. 2018, 236, 253–264. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Sygellou, L.; Kollia, M.; Niakolas, D.K. Affecting the H2O electrolysis process in SOECs through modification of NiO/GDC; experimental case of Au-Mo-Ni synergy. J. Catal. 2019, 373, 260–275. [Google Scholar] [CrossRef]
- Vibhu, V.; Vinke, I.C.; Zaravelis, F.; Neophytides, S.G.; Niakolas, D.K.; Eichel, R.-A.; de Haart, L.G.J. Performance and Degradation of Electrolyte-Supported Single Cell Composed of Mo-Au-Ni/GDC Fuel Electrode and LSCF Oxygen Electrode during High Temperature Steam Electrolysis. Energies 2022, 15, 2726. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neophytides, S.; Niakolas, D.K. Experimental clarification of the RWGS reaction effect in H2O/CO2 SOEC co-electrolysis conditions. Catalysts 2019, 9, 151. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Kollia, M.; Neophytides, S.; Niakolas, D. The promoting effect of Fe on Ni/GDC for the Solid Oxide H2O electrolysis. Int. J. Energy Res. 2020, 44, 10982–10995. [Google Scholar] [CrossRef]
- Zaravelis, F.; Sygellou, L.; Souvalioti, A.; Niakolas, D.K. Transition metals in Ni/GDC for the reversible solid oxide cell operation: Optimization of the Mo-Au-Ni synergy and further enhancement via substitution of Mo with Fe. Electrochim. Acta 2023, 453, 142343. [Google Scholar] [CrossRef]
- Zaravelis, F.; Niakolas, D.K. Enhancement of the intrinsic Ni/GDC activity under rSOC operation by means of Fe–Au doping: An electro-kinetic study. Int. J. Hydrogen Energy 2023, 48, 36663–36677. [Google Scholar] [CrossRef]
- Ioannidou, E.T.; Neophytides, S.G.; Niakolas, D.K. Ternary Fe- or Mo-Au-Ni/GDC as Candidate Fuel Electrodes for the Internal Dry Reforming of CH4: Physicochemical and Kinetic Investigation. Energies 2024, 17, 184. [Google Scholar] [CrossRef]
- Ioannidou, E.; Neophytides, S.G.; Niakolas, D.K. Au-Mo-Fe-Ni/CeO2 (Gd2O3) As Potential Fuel Electrodes for Internal CO2 Reforming of CH4 in Single SOFCs. ECS Trans. 2023, 111, 2473–2485. [Google Scholar] [CrossRef]
- Escudero, M.J.; Serrano, J.L. Individual impact of several impurities on the performance of direct internal reforming biogas solid oxide fuel cell using W-Ni-CeO2 as anode. Int. J. Hydrogen Energy 2019, 44, 20616–20631. [Google Scholar] [CrossRef]
- Schefold, J.; Brisse, A.; Poepke, H. Long-term Steam Electrolysis with Electrolyte-Supported Solid Oxide Cells. Electrochim. Acta 2015, 179, 161–168. [Google Scholar] [CrossRef]
- Schefold, J.; Brisse, A.; Poepke, H. 23,000 h steam electrolysis with an electrolyte supported solid oxide cell. Int. J. Hydrogen Energy 2017, 42, 13415–13426. [Google Scholar] [CrossRef]
- Jensen, S.H.; Hauch, A.; Hendriksen, P.V.; Mogensen, M.; Bonanos, N.; Jacobsen, T. A Method to Separate Process Contributions in Impedance Spectra by Variation of Test Conditions. J. Electrochem. Soc. 2007, 154, B1325–B1330. [Google Scholar] [CrossRef]
- Giotakos, P.I.; Neophytides, S.G. Physical modeling of the electrochemical impedance spectra for the O2 reduction reaction in HTPEM fuel cells’ cathodic electrochemical interface. Electrochim. Acta 2018, 292, 285–291. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Kane, N.; Luo, Z.; Pei, K.; Sasaki, K.; Choi, Y.; Chen, Y.; Ding, D.; Liu, M. An Efficient Bifunctional Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Adv. Funct. Mater. 2021, 31, 2105386. [Google Scholar] [CrossRef]
- Nuggehalli Sampathkumar, S.; Aubin, P.; Couturier, K.; Sun, X.; Sudireddy, B.R.; Diethelm, S.; Pérez-Fortes, M.; Van Herle, J. Degradation study of a reversible solid oxide cell (rSOC) short stack using distribution of relaxation times (DRT) analysis. Int. J. Hydrogen Energy 2022, 47, 10175–10193. [Google Scholar] [CrossRef]
- Nechache, A.; Mansuy, A.; Petitjean, M.; Mougin, J.; Mauvy, F.; Boukamp, B.A.; Cassir, M.; Ringuedé, A. Diagnosis of a cathode-supported solid oxide electrolysis cell by electrochemical impedance spectroscopy. Electrochim. Acta 2016, 210, 596–605. [Google Scholar] [CrossRef]
- Knibbe, R.; Traulsen, M.L.; Hauch, A.; Ebbesen, S.D.; Mogensen, M. Solid Oxide Electrolysis Cells: Degradation at High Current Densities. J. Electrochem. Soc. 2010, 157, B1209–B1217. [Google Scholar] [CrossRef]
- Hauch, A.; Jensen, S.H.; Bilde-So̸rensen, J.B.; Mogensen, M. Silica Segregation in the Ni∕YSZ Electrode. J. Electrochem. Soc. 2007, 154, A619–A626. [Google Scholar] [CrossRef]
- Moon, D.J.; Ryu, J.W. Electrocatalytic reforming of carbon dioxide by methane in SOFC system. Catal. Today 2003, 87, 255–264. [Google Scholar] [CrossRef]
- Mogensen, D.; Grunwaldt, J.D.; Hendriksen, P.V.; Dam-Johansen, K.; Nielsen, J.U. Internal steam reforming in solid oxide fuel cells: Status and opportunities of kinetic studies and their impact on modelling. J. Power Sources 2011, 196, 25–38. [Google Scholar] [CrossRef]
- Koh, J.H.; Yoo, Y.S.; Park, J.W.; Lim, H.C. Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ionics 2002, 149, 157–166. [Google Scholar] [CrossRef]
- Matsui, T.; Kishida, R.; Kim, J.-Y.; Muroyama, H.; Eguchi, K. Performance Deterioration of Ni–YSZ Anode Induced by Electrochemically Generated Steam in Solid Oxide Fuel Cells. J. Electrochem. Soc. 2010, 157, B776–B781. [Google Scholar] [CrossRef]

| Temperature: 900 °C | ||||
|---|---|---|---|---|
| Applied Polarization | 28 mA cm−2 | 284 mA cm−2 | ||
| Resistance | Rohm (Ω cm2) | Rpol (Ω cm2) | Rohm (Ω cm2) | Rpol (Ω cm2) |
| Ni/GDC | 2.07 | 0.57 | 2.33 | 0.46 |
| 1Au-Ni/GDC | 1.07 | 0.44 | 1.14 | 0.31 |
| 3Au-Ni/GDC | 1.44 | 0.58 | 1.48 | 0.44 |
| 0.5Fe-1Au-Ni/GDC | 0.69 | 0.27 | 0.69 | 0.21 |
| 0.5Fe-3Au-Ni/GDC | 0.71 | 0.35 | 0.71 | 0.28 |
| Element | wt.% Concentration | |
|---|---|---|
| 0.5Fe-1Au-Ni/GDC | Ni/GDC | |
| C | 0.9 | 1.6 |
| Pt | 9.4 | 14.3 |
| Ce | 34.7 | 36.1 |
| Gd | 5.2 | 5.3 |
| Ni | 43.1 | 33.7 |
| O | 6.7 | 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidou, E.; Neophytides, S.G.; Niakolas, D.K. Electrocatalytic Investigation of the SOFC Internal CH4 Dry Reforming with Modified Ni/GDC: Effect of Au Content on the Performance Enhancement by Fe-Au Doping. Catalysts 2025, 15, 618. https://doi.org/10.3390/catal15070618
Ioannidou E, Neophytides SG, Niakolas DK. Electrocatalytic Investigation of the SOFC Internal CH4 Dry Reforming with Modified Ni/GDC: Effect of Au Content on the Performance Enhancement by Fe-Au Doping. Catalysts. 2025; 15(7):618. https://doi.org/10.3390/catal15070618
Chicago/Turabian StyleIoannidou, Evangelia, Stylianos G. Neophytides, and Dimitrios K. Niakolas. 2025. "Electrocatalytic Investigation of the SOFC Internal CH4 Dry Reforming with Modified Ni/GDC: Effect of Au Content on the Performance Enhancement by Fe-Au Doping" Catalysts 15, no. 7: 618. https://doi.org/10.3390/catal15070618
APA StyleIoannidou, E., Neophytides, S. G., & Niakolas, D. K. (2025). Electrocatalytic Investigation of the SOFC Internal CH4 Dry Reforming with Modified Ni/GDC: Effect of Au Content on the Performance Enhancement by Fe-Au Doping. Catalysts, 15(7), 618. https://doi.org/10.3390/catal15070618










