H2 Production from Pyrolysis-Steam Reforming of Municipal Solid Waste and Biomass: A Comparative Study When Using the Self-Derived Char-Based Catalysts
Abstract
1. Introduction
2. Results and Discussions
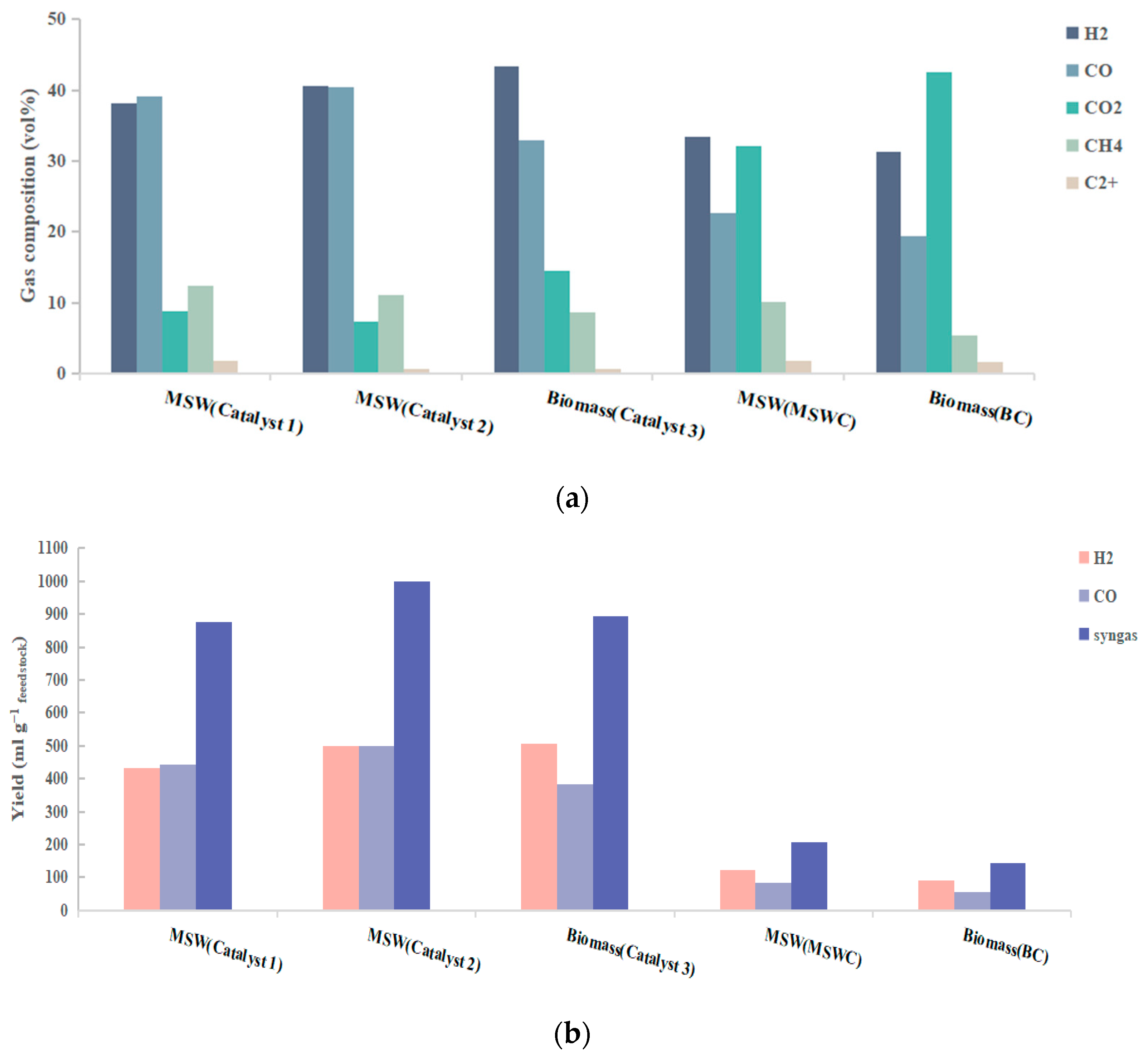
2.1. Catalyst Performance in the Absence of Steam
2.2. H2 Production Performance and Quality of Syngas in the Presence of Steam
2.2.1. Influence of Feedstock Type
- 1.
- MSW contains both biomass and waste plastics, and there exists a synergistic effect during their co-pyrolysis, which is manifested in improved reaction kinetics and reduced energy barriers. Multiple studies have analyzed the synergistic effects of biomass-plastic co-processing, finding that co-processing yields higher H2 production compared to individual processing of either biomass or plastics alone, while also increasing the calorific value of the produced gas and the CGE of the reaction [30,31,32]. Kiran et al. discovered that secondary reactions between plastic volatiles and biomass volatiles are the primary contributors to the co-pyrolysis synergy; their interaction promotes the transfer of H from plastics and O from biomass, thereby enhancing volatile reforming reactions and improving reaction kinetics [31]. Rahul et al. also observed that co-pyrolysis of plastics and biomass improves kinetics and reduces activation energy due to this synergy [30]. Therefore, MSW containing both plastics and biomass demonstrates superior H2 production performance compared to biomass alone.
- 2.
- The pyrolysis volatiles from biomass contain significantly more oxygenated compounds than those from MSW, making biomass-derived volatiles more challenging to reform [33]. And the inherent AAEM species in biochar demonstrate weaker activity in breaking π-bonds within aromatic rings [33], which explains why MSW exhibits greater hydrogen production potential than biomass.
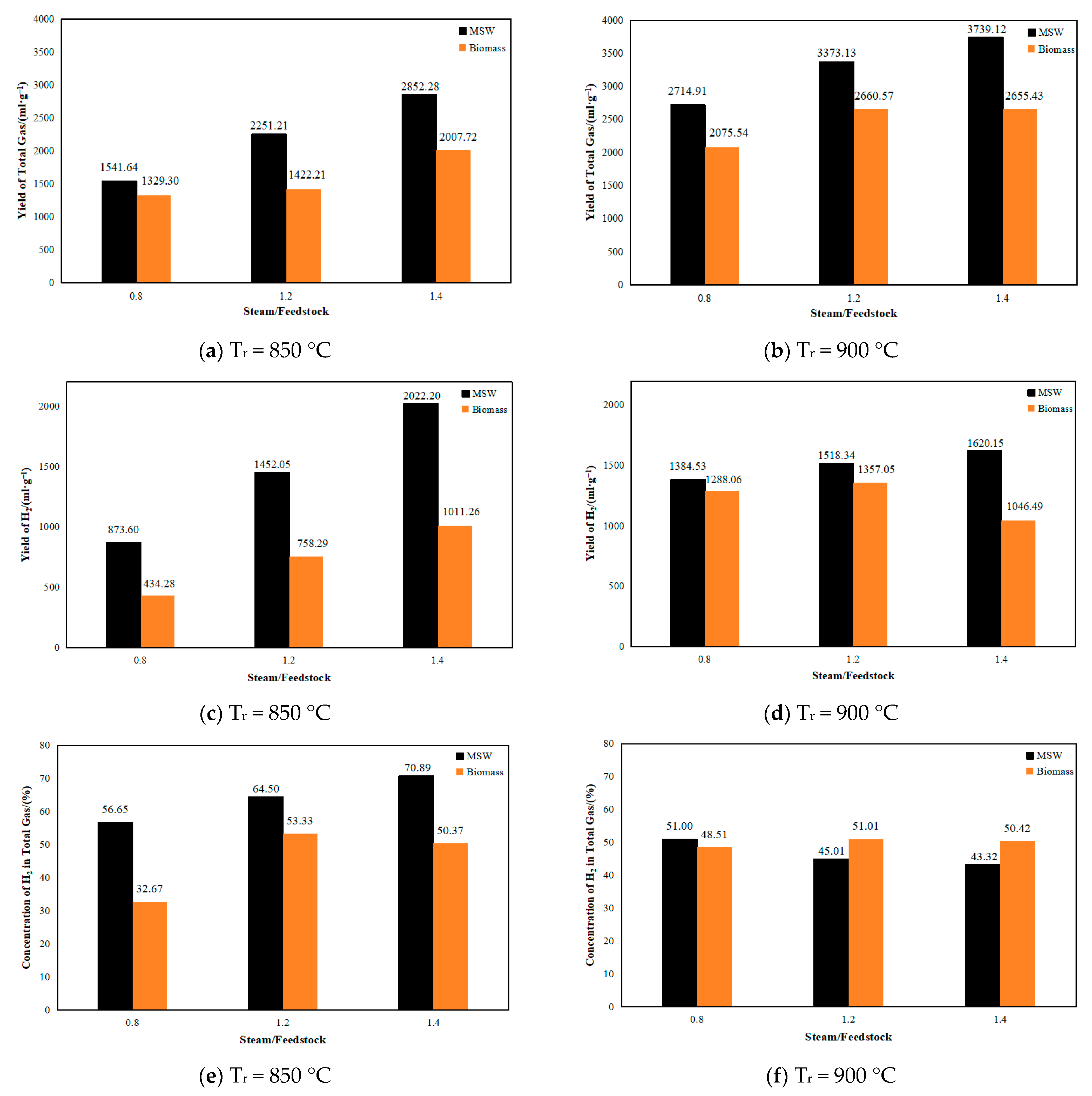
2.2.2. Influence of the Operating Parameters
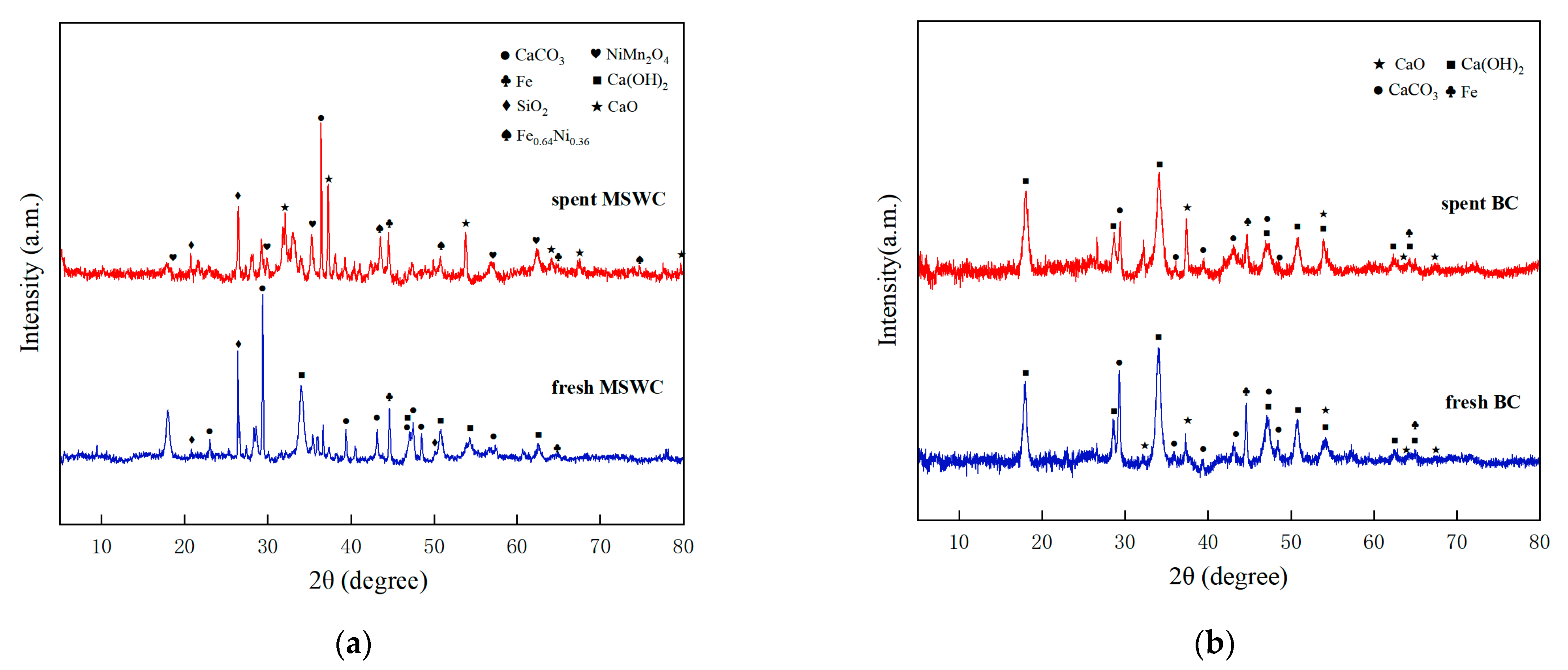
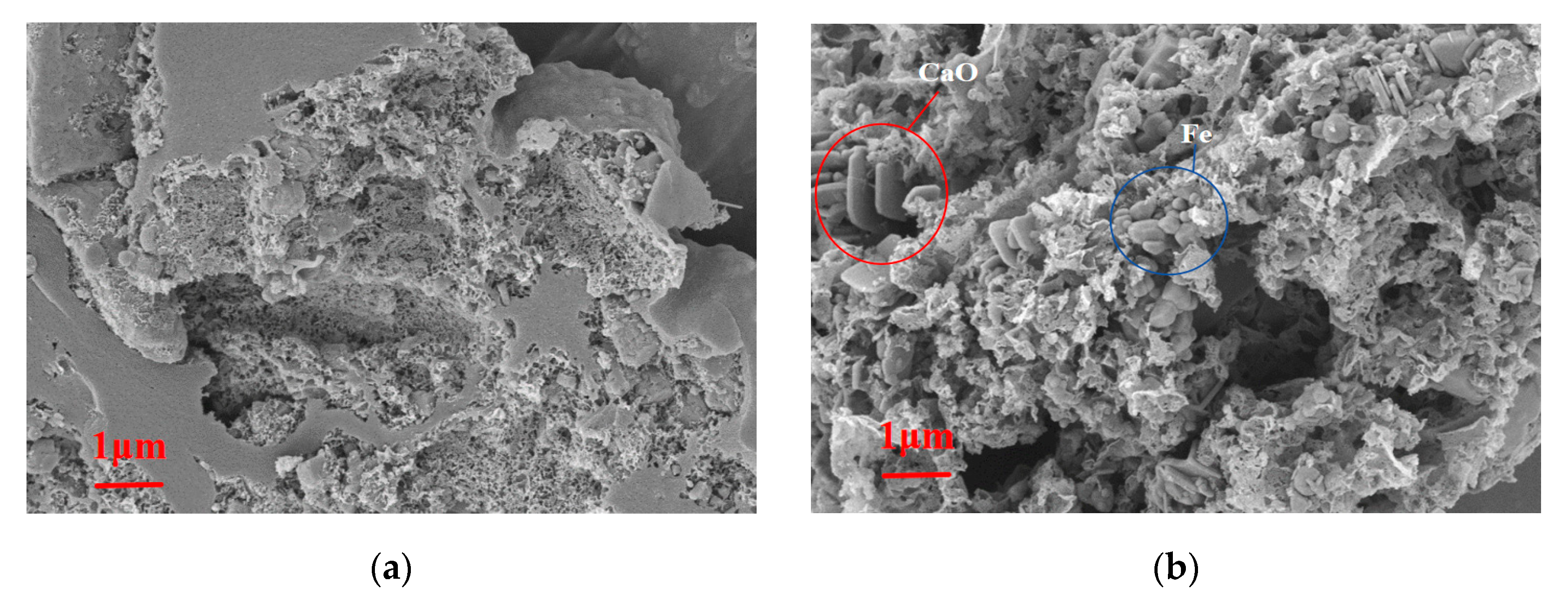
2.3. Catalyst Changes and Recovery
3. Materials and Methods
3.1. Experimental Materials
3.1.1. Characterization of Feedstock
3.1.2. Characterization of Fresh Catalysts
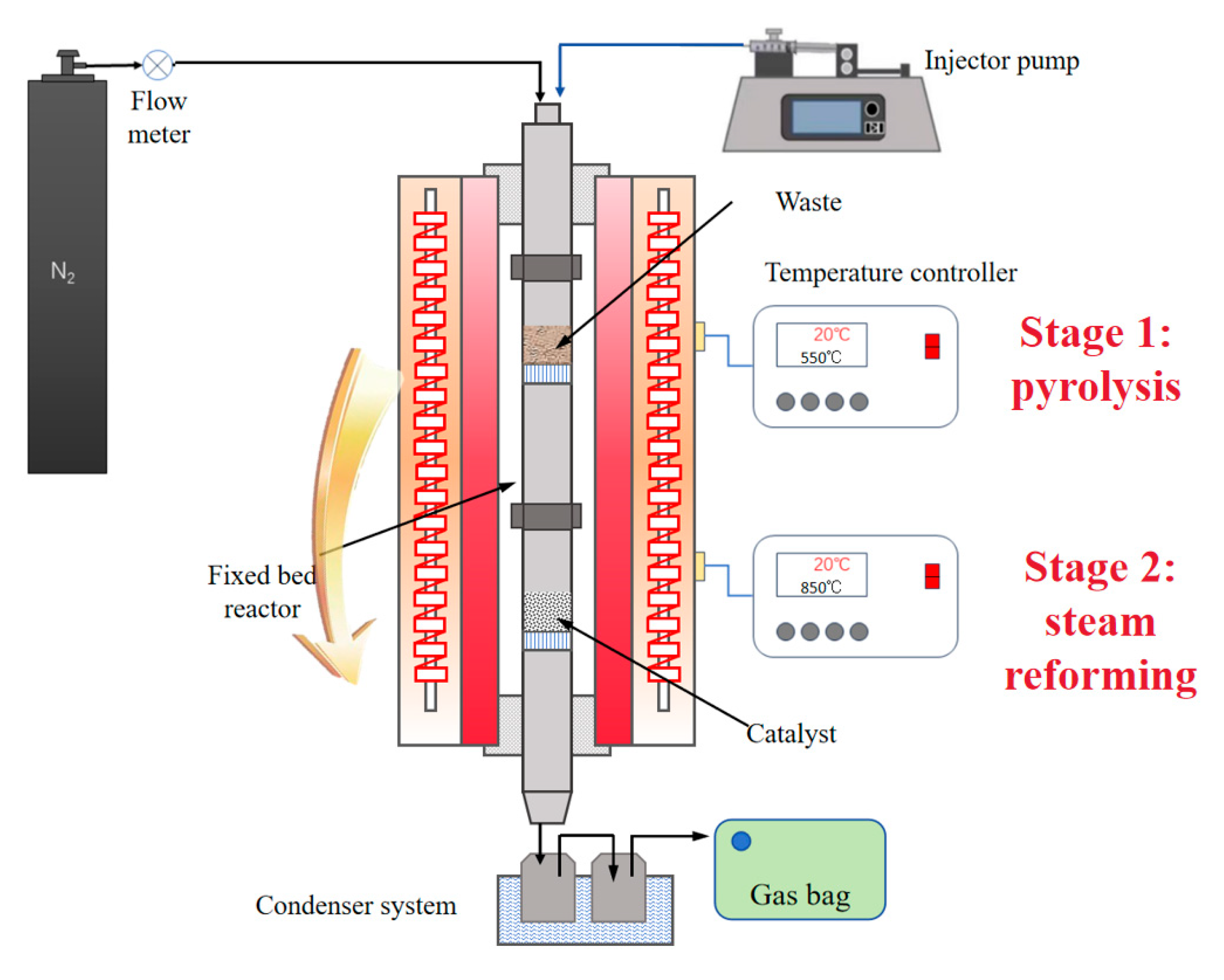
3.2. Experimental Setup
3.3. Characterization and Measurement Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Wu, C.; Dong, L.; Jin, F.; Williams, P.T.; Huang, J. Catalytic steam reforming of volatiles released via pyrolysis of wood sawdust for hydrogen-rich gas production on Fe–Zn/Al2O3 nanocatalysts. Fuel 2015, 158, 999–1005. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Chen, M.; Xin, W.; Yang, Z.; Kong, L. Hydrogen production via catalytic pyrolysis of biomass in a two-staged fixed bed reactor system. Int. J. Hydrogen Energy 2014, 39, 13128–13135. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Barbarias, I.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H2 production. JAAP 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Williams, P.T. Fe−Ni−MCM-41 Catalysts for Hydrogen-Rich Syngas Production from Waste Plastics by Pyrolysis−Catalytic Steam Reforming. Energy Fuel 2017, 31, 8497–8504. [Google Scholar] [CrossRef]
- Li, Y.; Nahil, M.A.; Williams, P.T. Hydrogen/Syngas Production from Different Types of Waste Plastics Using aSacrificial Tire Char Catalyst via Pyrolysis−Catalytic Steam Reforming. Energy Fuel 2023, 37, 6661–6673. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Zhang, S.; Xu, Q.; Yan, Y. Steam Reforming of Bio-Oil for Hydrogen Production: Effect of Ni-Co Bimetallic Catalysts. Chem. Eng. Technol. 2011, 35, 302–308. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Benito, P.L.; Bilbao, J.; Gaoyubo, A.G. Biomass to hydrogen-rich gas via steam reforming of raw bio-oil over Ni/La2O3-αAl2O3 catalyst: Effect of space-time and steam-to-carbon ratio. Fuel 2018, 216, 445–455. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Fierro, J.L.G.; Efstathiou, A.M. The phenol steam reforming reaction over MgO-based supported Rh catalysis. J. Catal. 2004, 228, 417–432. [Google Scholar] [CrossRef]
- Rioche, C.; Kulkarni, S.; Meunier, F.C.; Breen, J.P.; Burch, R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl. Catal. B-Environ. 2005, 61, 130–139. [Google Scholar] [CrossRef]
- Alshareef, R.; Nahil, M.A.; Williams, P.T. Hydrogen Production by Three-Stage (i) Pyrolysis, (ii) Catalytic Steam Reforming, and (iii) Water Gas Shift Processing of Waste Plastic. Energy Fuel 2023, 37, 3894–3907. [Google Scholar] [CrossRef]
- Nabgan, W.; Adbullah, T.A.T.; Mat, R.; Nabgan, B.; Gambo, Y.; Ibrahim, M.; Ahmad, A.; Jalil, A.A.; Triwahyono, S.; Saeh, I. Renewable hydrogen production from bio-oil derivative via catalytic steam reforming: An overview. Renew. Sust. Energy Rev. 2017, 79, 347–357. [Google Scholar] [CrossRef]
- Nabgan, W.; Abdullah, T.A.T.; Mat, R.; Nabgan, B.; Jalil, A.A.; Firmansyah, L.; Triwahyono, S. Production of hydrogen via steam reforming of acetic acid over Ni and Co supported on La2O3 catalyst. Int. J. Hydrogen Energy 2017, 42, 8975–8985. [Google Scholar] [CrossRef]
- Setibudi, H.D.; Aziz, M.A.A.; Abdullah, S.; The, L.P.; Jusoh, R. Hydrogen production from catalytic steam reforming of biomass pyrolysis oil or bio-oil derivatives: A review. Int. J. Hydrogen Energy 2020, 45, 18376–18397. [Google Scholar] [CrossRef]
- Hosokai, S.; Kumabe, K.; Ohshita, M.; Norinaga, K.; Li, C.; Hayashi, J. Mechanism of decompositionof aromatics over charcoal and necessary condition for maintaining its activity. Fuel 2008, 87, 2914–2922. [Google Scholar] [CrossRef]
- Mei, Z.; He, X.; Chen, D.; Wang, N.; Yin, L.; Qian, K.; Feng, Y. Comparison of chars from municipal solid waste and wheat straw for understanding the role of inorganics in char-based catalysts during volatile reforming process. Energy 2021, 229, 120619. [Google Scholar] [CrossRef]
- Mei, Z.; Zhou, H.; Ge, S.; Feng, Y.; Chen, D. Waste-derived char-supported Ni catalyst for syngas methanation: Ni recovery, performance evolution and its implications for assessing char support. Appl. Catal. B-Environ. 2024, 365, 124234. [Google Scholar] [CrossRef]
- Bizkarra, K.; Bermudez, J.M.; Arcelus-Arrillaga, P.; Barrio, V.L.; Cambra, J.F.; Millan, M. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming. Int. J. Hydrogen Energy 2018, 43, 11706–11718. [Google Scholar] [CrossRef]
- Valle, B.; Remiro, A.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Catalysts of Ni/α-Al2O3 and Ni/La2O3-α-Al2O3 for hydrogen production by steam reforming of bio-oil aqueous fraction with pyrolytic lignin retention. Int. J. Hydrogen Energy 2013, 38, 1307–1318. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Arregi, A.; Amutio, M.; Artetxe, M.; Olazar, M. Effect of calcination conditions on the performance of Ni/MgO-Al2O3 catalysts in the system reforming of biomass fast pyrolysis volatiles. Catal. Sci. Technol. 2019, 9, 3947–3963. [Google Scholar] [CrossRef]
- Qin, T.; Yuan, S. Research progress of catalysts for catalytic steam reforming of high temperature tar: A review. Fuel 2023, 331, 125790. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Liang, T.; Yang, Z. Hydrogen production by steam reforming of bio-oil aqueous fraction over Co-Fe/ZSM-5. IOP Conf. Ser. : Earth Environ. Sci. 2018, 113, 012081. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Shan, R.; Gu, J.; Huhe, T.; Ling, X.; Yuan, H.; Chen, Y. Polypropylene pyrolysis and steam reforming over Fe-based catalyst supported on activated carbon for the production of hydrogen-rich syngas. Carbon Resour. Convers. 2023, 6, 173–182. [Google Scholar] [CrossRef]
- An, Y.; Tahmasebi, A.; Zhao, X.; Matamba, T.; Yu, J. Catalytic reforming of palm kernel shell microwave pyrolysis vapors over iron-loaded activated carbon: Enhanced production of phenol and hydrogen. Bioresour. Technol. 2020, 306, 123111. [Google Scholar] [CrossRef]
- Chanburanasiri, N.; Ribeiro, A.M.; Rodrigues, A.E.; Arporwichanop, A.; Laosiripojana, N.; Praserthdam, P.; Assabumrungrat, S. Hydrogen Production via Sorption Enhanced Steam Methane Reforming Process Using Ni/CaO Multifunctional Catalyst. Ind. Eng. Chem. Res. 2011, 50, 13662–13671. [Google Scholar] [CrossRef]
- Zamboni, I.; Courson, C.; Kiennemann, A. Fe-Ca interactions in Fe-based/CaO catalyst/sorbent for CO2 sorption and hydrogen production from toluene steam reforming. Appl. Catal. B Environ. 2017, 203, 154–165. [Google Scholar] [CrossRef]
- Prestipino, M.; Corigliano, O.; Galvagno, A.; Piccolo, A.; Fragiacomo, P. Exploringthe potential of wet biomass gasification with SOFC and ICE cogeneration technologies: Process design, simulation and comparative thermodynamic analysis. Appl. Energy 2025, 392, 125998. [Google Scholar] [CrossRef]
- Chen, D.; Holmen, A.; Sui, Z.; Zhou, X. Carbon mediated catalysis: A review on oxidative dehydrogenation. Chin. J. Catal. 2014, 35, 824–841. [Google Scholar] [CrossRef]
- Vassilev, S.; Braekman-Danheux, C.; Laurent, P. Characterization of refuse-derived char from municipal solid waste: 1. Phase-mineral and chemical composition. Fuel Process. Technol. 1999, 59, 95–134. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Mishra, R.; Chyuan, H.; Lin, C. Progress on co-processing of biomass and plastic waste for hydrogen production. Energy Convers. Manage. 2023, 284, 116983. [Google Scholar] [CrossRef]
- Burra, K.R.G.; Liu, X.; Wang, Z.; Li, J.; Che, D.; Gupta, A.K. Quantifying the sources of synergistic effects in co-pyrolysis of pinewood and polystyrene. Appl. Energy 2021, 302, 117562. [Google Scholar] [CrossRef]
- Fazil, A.; Kumar, S.; Mahajani, S.M. Downdraft co-gasification of high ash biomass and plastics. Energy 2022, 243, 123055. [Google Scholar] [CrossRef]
- Sun, H.; Feng, D.; Sun, S.; Wei, Q.; Zhao, Y.; Zhang, Y.; Xie, M.; Qin, Y. Effect of steam on coke deposition during the tar reforming from corn straw pyrolysis over biochar. Fuel Process. Technol. 2021, 224, 107007. [Google Scholar] [CrossRef]
- Li, C. Importance of volatile-char interactions during the pyrolysis and gasification of low-rank fuels—A review. Fuel 2013, 112, 609–623. [Google Scholar] [CrossRef]
- Mondal, P. From municipal solid waste (MSW) to hydrogen: Performance optimization of a fixed bed gasifier using Box-Benkhen method. Int. J. Hydrogen Energy 2022, 47, 20064–20075. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Cai, Q.; Ding, D. The integrated process for hydrogen production from biomass: Study on the catalytic conversion behavior of pyrolytic vapor in gas-solid simultaneous gasification process. Int. J. Hydrogen Energy 2016, 41, 6653–6661. [Google Scholar] [CrossRef]
- Jia, S.; Ying, H.; Sun, Y.; Sun, N.; Xu, W.; Xu, Y.; Ning, S. Research progress of biomass steam gasification to produce hydrogen-rich syngas and its application. Chem. Ind. Eng. Prog. 2018, 37, 497–504. [Google Scholar] [CrossRef]
- Chai, Y.; Gao, N.; Wang, M.; Wu, C. H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem. Eng. J. 2020, 382, 122947. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, S.; Zhang, S.; Xu, Q.; Yan, Y. Low-temperature biomass gasification and catalytic reforming for hydrogen production. Petrchem. Technol. 2010, 39, 1198–1203. [Google Scholar]


| Feedstock | Catalyst and Operation Parameters | Catalytic Performance | Ref. |
|---|---|---|---|
| Water hyacinth | 9 wt. % Ni/sepiolite + SiC Tp 1 = 650 °C; Tr 1 = 800 °C; Steam/Feedstock = 0. | H2:CO:CO2:CH4 = 77.2:7.8:10.7:4.3 YH2 = 1225 L kg−1feedstock Yliquid = 16 wt. % | [1] |
| HDPE | 14 wt. %NiO/CaAl2O3 Tp = 500 °C; Tr = 700 °C; Steam/Feedstock = 4. | H2:CO:CO2:CH4:C2+ = 71.5:10.5:16.9:0.7:0.4 YH2 = 4231 L kg−1feedstock Yliquid = 0.06 wt. % | [2] |
| Wood sawdust | Fe-ZnO/Al2O3 = 1:1 Tp = 500 °C; Tr = 800 °C; Steam/Feedstock = 0. | H2:CO:CO2:CH4:C2+ = 40.0:24.8:24.7:8.1:2.4 YH2 = 236 L kg−1feedstock | [3] |
| SMWP (Stimulated mixed waste plastics) | Fe-Ni/MCM-41 (Fe/Ni = 10:10) Tp = 500 °C; Tr = 800 °C. mfeedstock = 3.4 g Steam flow rate = 2 mL h−1 | H2:CO:CO2:CH4:C2+ = 46.7:32.2:1.9:6.2:12.9 YH2 = 1129 L kg−1feedstock | [4] |
| LDPE | Tire char Tp = 500 °C; Tr = 1000 °C. mfeedstock = 9.2 g Steam flow rate = 8 mL h−1 | H2:CO:CO2:CH4:C2+ = 49.7:30.4:13.0:5.1:1.8 YH2 = 3261 L kg−1feedstock | [5] |
| Bio-oil | 3Ni9Co/Ce-Zr-O Tr = 850 °C; Moisture content of bio-oil = 57.52 wt. %. | H2:CO:CO2:CH4 = 57.65:28.32:11.01:3.02 YH2 = 72.15 wt. % (in relation to mass of feedstock) | [6] |
| Raw bio-oil | Ni/La2O3-αAl2O3 Tr = 700 °C; S/C = 6. | H2 Concentration = 66 vol% YH2 = 83 wt. % (in relation to mass of feedstock) | [7] |
| Phenol (bio-oil model compound) | Rh/MgCeZrO Tr = 700 °C; Steam/Phenol = 80:1 | H2:CO:CO2 = 9.4:0.4:4.3 Phenol conversion = 70 wt. % | [8] |
| Acetic acid | Rh/CeZrO2 Tr = 761 °C; Steam/acetic acid = 2:1 | H2 selectivity = 70% Acetic acid conversion = 100% | [9] |
| Polypropylene | Co/Al2O3 Tp = 500 °C; Tr = 850 °C; Steam/Feedstock = 1.33. | H2/CO = 3:1 YH2 = 2940 L kg−1feedstock (pyrolysis stage: YH2 = 1781 L kg−1feedstock) | [10] |
| Feedstock | Operating Parameters | EH2/kJ | EH2O 1/kJ | η1 | Egas 2/EH2O |
|---|---|---|---|---|---|
| MSW | Tp = 550 °C, Tr = 850 °C, S/F = 0.8 | 11.15 | 3.32 | 3.36 | 5.341 |
| Tp = 550 °C, Tr = 850 °C, S/F = 1.2 | 18.54 | 4.98 | 3.72 | 5.317 | |
| Tp = 550 °C, Tr = 850 °C, S/F = 1.4 | 25.82 | 5.81 | 4.44 | 5.332 | |
| Tp = 550 °C, Tr = 900 °C, S/F = 0.8 | 17.68 | 3.40 | 5.20 | 9.242 | |
| Tp = 550 °C, Tr = 900 °C, S/F = 1.2 | 19.38 | 5.10 | 3.80 | 7.313 | |
| Tp = 550 °C, Tr = 900 °C, S/F = 1.4 | 20.68 | 5.95 | 3.47 | 6.752 | |
| Biomass | Tp = 550 °C, Tr = 850 °C, S/F = 0.8 | 5.54 | 3.32 | 1.67 | 3.347 |
| Tp = 550 °C, Tr = 850 °C, S/F = 1.2 | 9.68 | 4.98 | 1.95 | 2.790 | |
| Tp = 550 °C, Tr = 850 °C, S/F = 1.4 | 12.91 | 5.81 | 2.22 | 3.224 | |
| Tp = 550 °C, Tr = 900 °C, S/F = 0.8 | 12.86 | 3.40 | 3.78 | 7.592 | |
| Tp = 550 °C, Tr = 900 °C, S/F = 1.2 | 17.33 | 5.10 | 3.40 | 5.198 | |
| Tp = 550 °C, Tr = 900 °C, S/F = 1.4 | 17.09 | 5.95 | 2.87 | 3.389 |
| Catalyst | Percentage by Mass | ||
|---|---|---|---|
| C | Fe | Ca | |
| Fresh Catalyst 2 | 78.32 wt. % 1 | 0.45 wt. % | 6.47 wt. % |
| Spent Catalyst 2 | 23.75 wt. % | 7.05 wt. % | 34.51 wt. % |
| Fresh Catalyst 3 | 85.27 wt. % | 0.08 wt. % | 0.35 wt. % |
| Spent Catalyst 3 | 48.81 wt. % | 0.63 wt. % | 6.43 wt. % |
| Catalyst | Percentage by Original Mass | |
|---|---|---|
| Operating Conditions | Recovered Fe | |
| Spent Catalyst 2 | Tr = 850 °C, S/F = 0 | 250 wt. % |
| Spent Catalyst 3 | Tr = 850 °C, S/F = 0 | 98 wt. % |
| Spent Catalyst 2 | Tr = 850 °C, S/F = 1.4 | 219 wt. % |
| Spent Catalyst 3 | Tr = 850 °C, S/F = 1.4 | 75 wt. % |
| Properties | Items | MSW | Biomass |
|---|---|---|---|
| Proximate analysis/(wt. %, ad. 1) | Moisture | 3.80 | 6.40 |
| Ash | 14.00 | 1.41 | |
| Volatile | 72.73 | 81.70 | |
| Fixed carbon | 9.47 | 10.49 | |
| Ultimate analysis/(wt. %, ad. 1) | C | 53.94 | 47.01 |
| H | 8.12 | 5.60 | |
| N | 0.26 | 0.90 | |
| S | 0.10 | 0.00 | |
| O (diff.) 2 | 19.78 | 38.68 | |
| Physical composition/(wt. %, ad. 1) | Kitchen waste | 12.90 | |
| Paper | 22.90 | ||
| Fiber | 9.20 | ||
| Plastics | 42.40 | ||
| Wood | 2.50 | ||
| Residue | 10.20 |
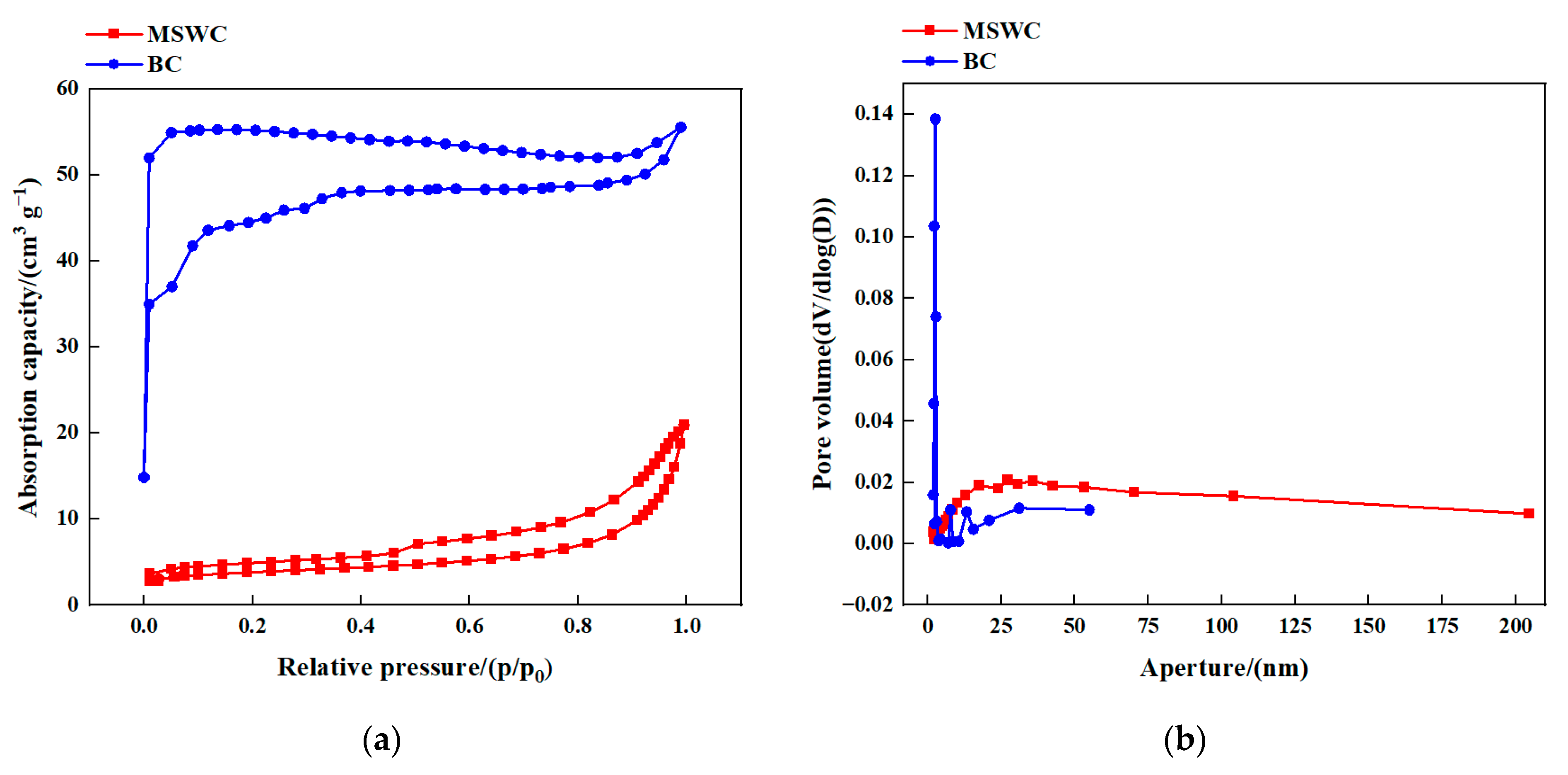
| Char | SBET 1 (m2 g−1) | Sp, micro 2 (m2 g−1) | Vp, micro 3 (cm3 g−1) | Vp 4 (cm3 g−1) | AD 5 (nm) |
|---|---|---|---|---|---|
| MSWC | 13.85 | 7.22 | 0.0030 | 0.0324 | 9.36 |
| BC | 166.66 | 154.76 | 0.0636 | 0.0860 | 2.06 |
| Catalyst | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Fe2O3 | NiO | ZnO |
|---|---|---|---|---|---|---|---|---|---|---|
| MSWC | 0.962 | 3.012 | 3.727 | 18.299 | 0.967 | 18.224 | 2.715 | 5.504 | 0.150 | 0.025 |
| BC | 0.086 | 0.758 | 0.233 | 0.997 | 1.813 | 6.160 | 0.049 | 0.528 | 0.000 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Xiang, C.; Wen, Y.; Hong, W.; Liu, R.; Chen, D.; Chen, D. H2 Production from Pyrolysis-Steam Reforming of Municipal Solid Waste and Biomass: A Comparative Study When Using the Self-Derived Char-Based Catalysts. Catalysts 2025, 15, 531. https://doi.org/10.3390/catal15060531
Qiu M, Xiang C, Wen Y, Hong W, Liu R, Chen D, Chen D. H2 Production from Pyrolysis-Steam Reforming of Municipal Solid Waste and Biomass: A Comparative Study When Using the Self-Derived Char-Based Catalysts. Catalysts. 2025; 15(6):531. https://doi.org/10.3390/catal15060531
Chicago/Turabian StyleQiu, Maijia, Chenhao Xiang, Yitao Wen, Weichen Hong, Renkai Liu, Dehong Chen, and Dezhen Chen. 2025. "H2 Production from Pyrolysis-Steam Reforming of Municipal Solid Waste and Biomass: A Comparative Study When Using the Self-Derived Char-Based Catalysts" Catalysts 15, no. 6: 531. https://doi.org/10.3390/catal15060531
APA StyleQiu, M., Xiang, C., Wen, Y., Hong, W., Liu, R., Chen, D., & Chen, D. (2025). H2 Production from Pyrolysis-Steam Reforming of Municipal Solid Waste and Biomass: A Comparative Study When Using the Self-Derived Char-Based Catalysts. Catalysts, 15(6), 531. https://doi.org/10.3390/catal15060531










