Polyurethane@CeO2 Nanozyme Core–Shell Fibrous Membranes for Enhanced Wound Healing via Balanced Redox Modulation
Abstract
1. Introduction
2. Results
2.1. Optimized CeO2 Nanoparticles
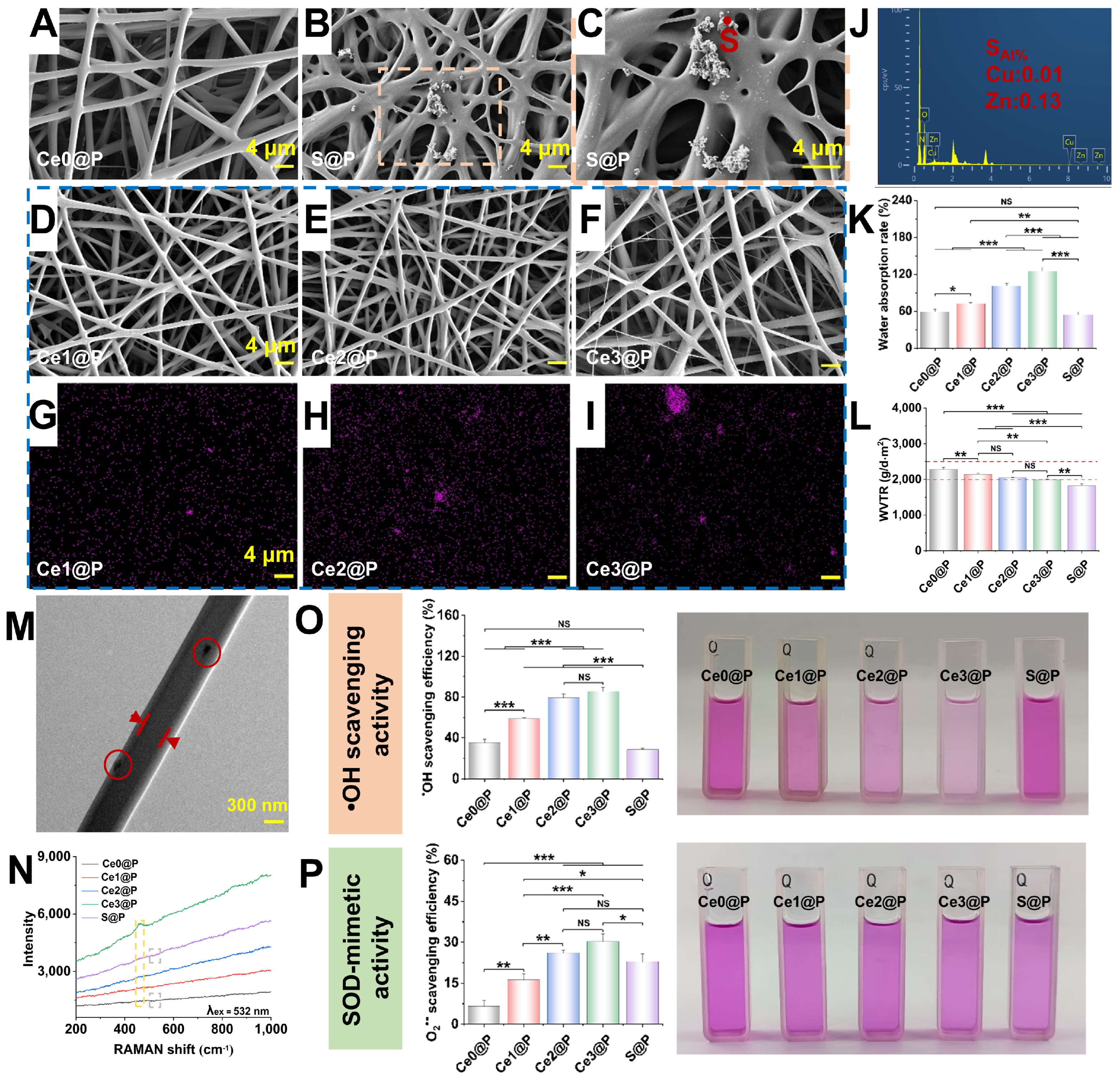
2.2. Characterization of Engineered PU CSFs
2.3. In Vitro Experiments Regulated by Engineered PU CSFs
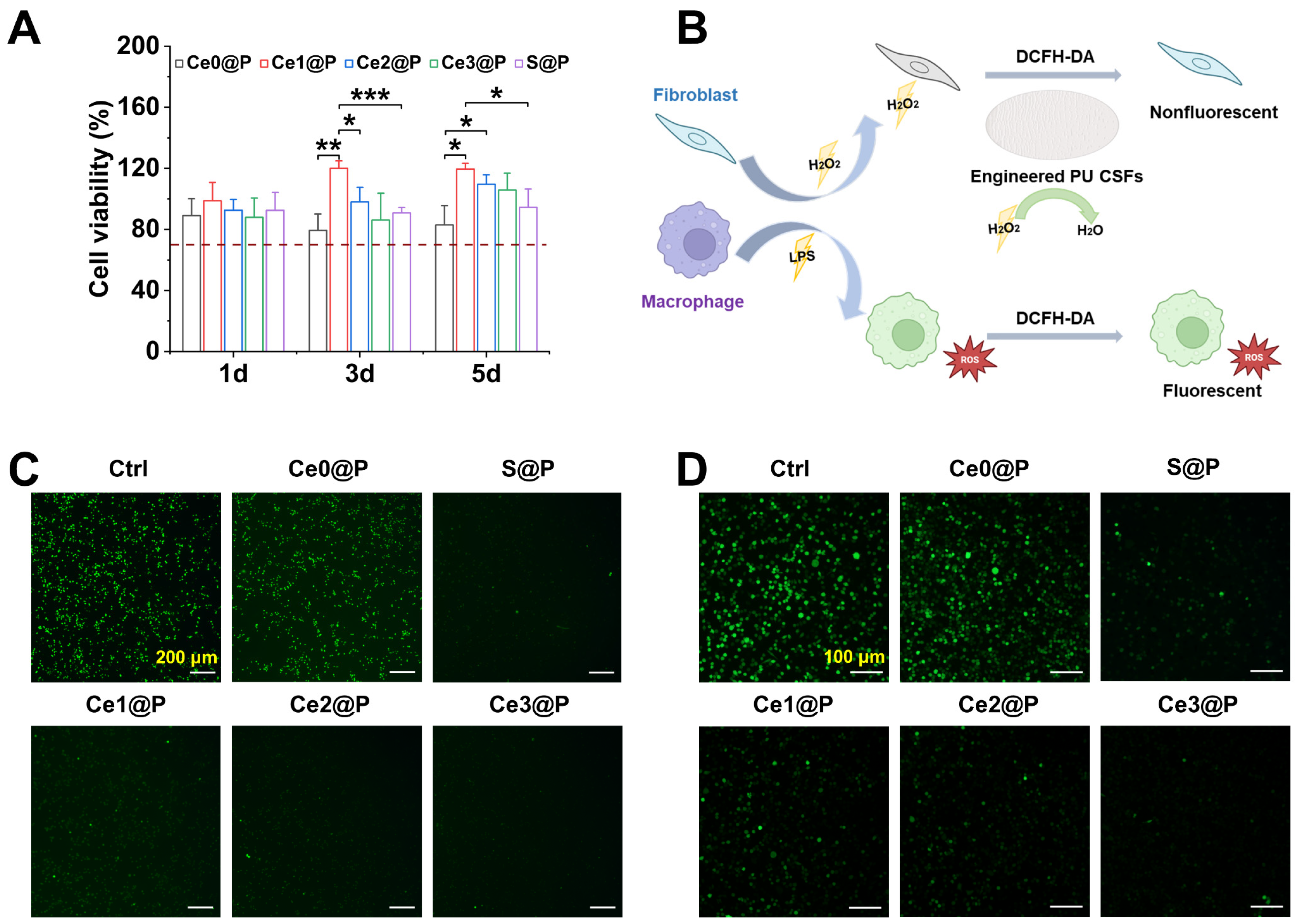
2.3.1. Cellular Compatibility
2.3.2. Cellular ROS Scavenging Activity
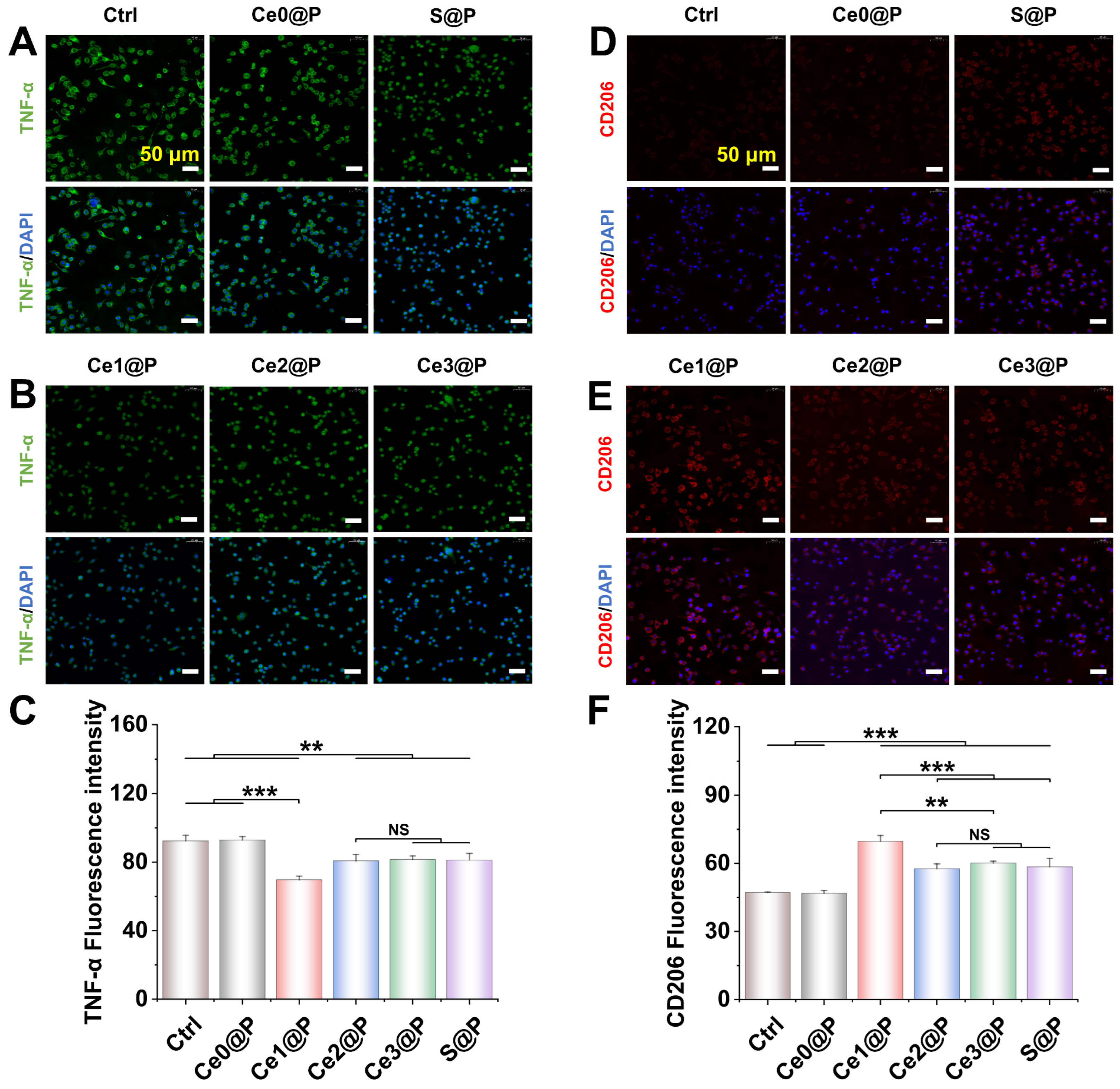
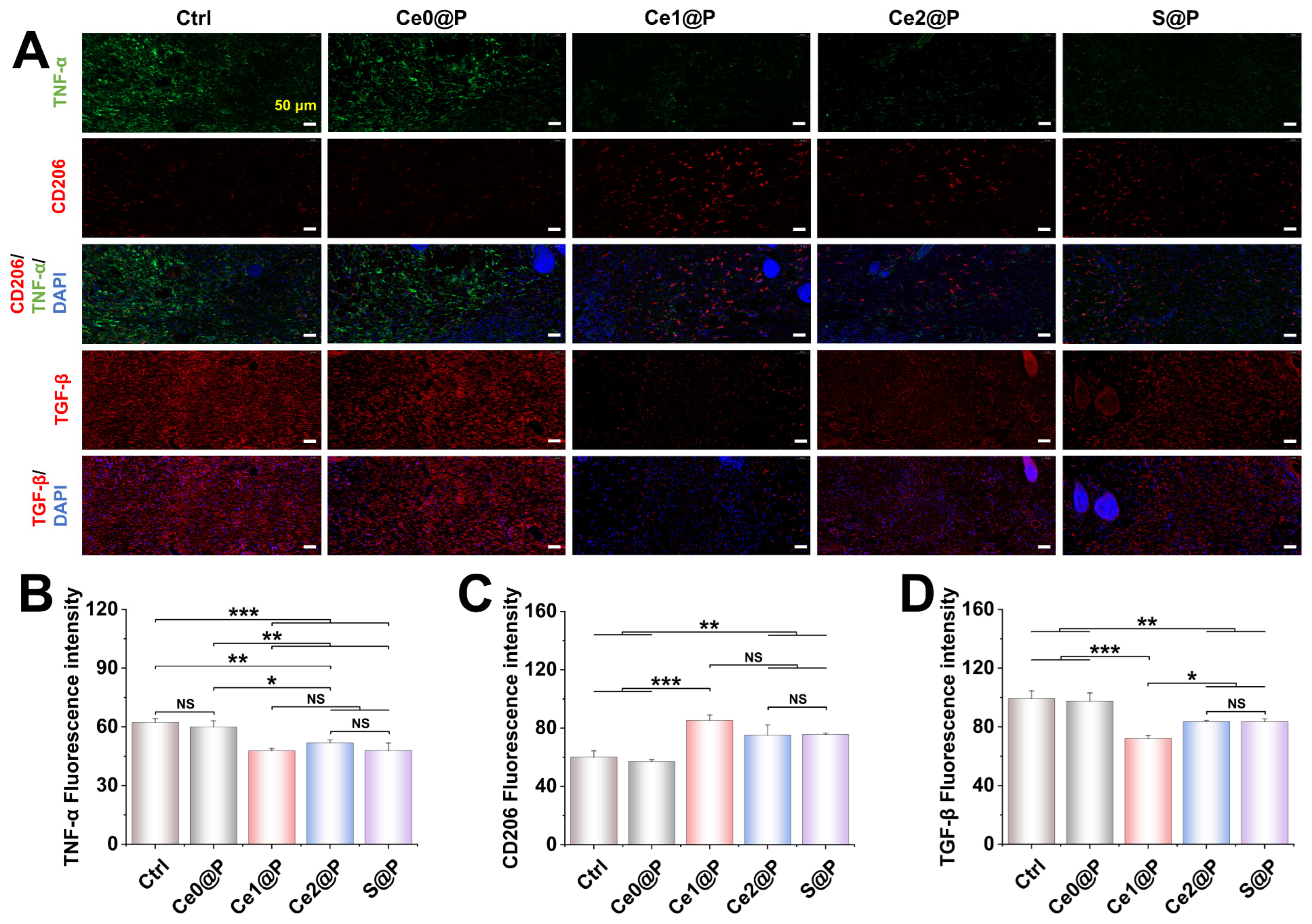
2.3.3. Engineered Membranes Regulating Macrophage Polarization
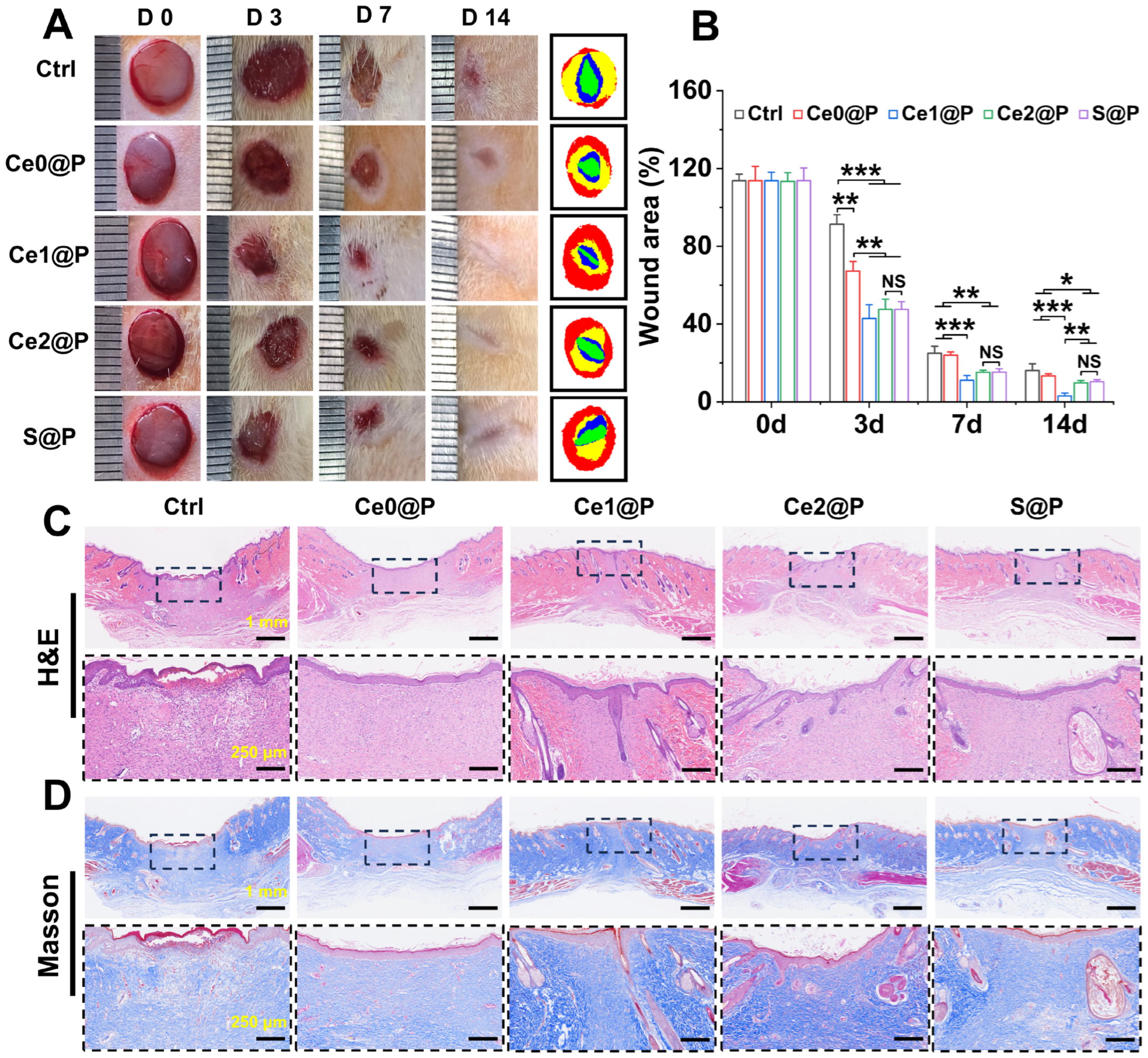
2.4. Wound Healing with Engineered PU CSFs
2.4.1. Wound Healing
2.4.2. Histological Analysis
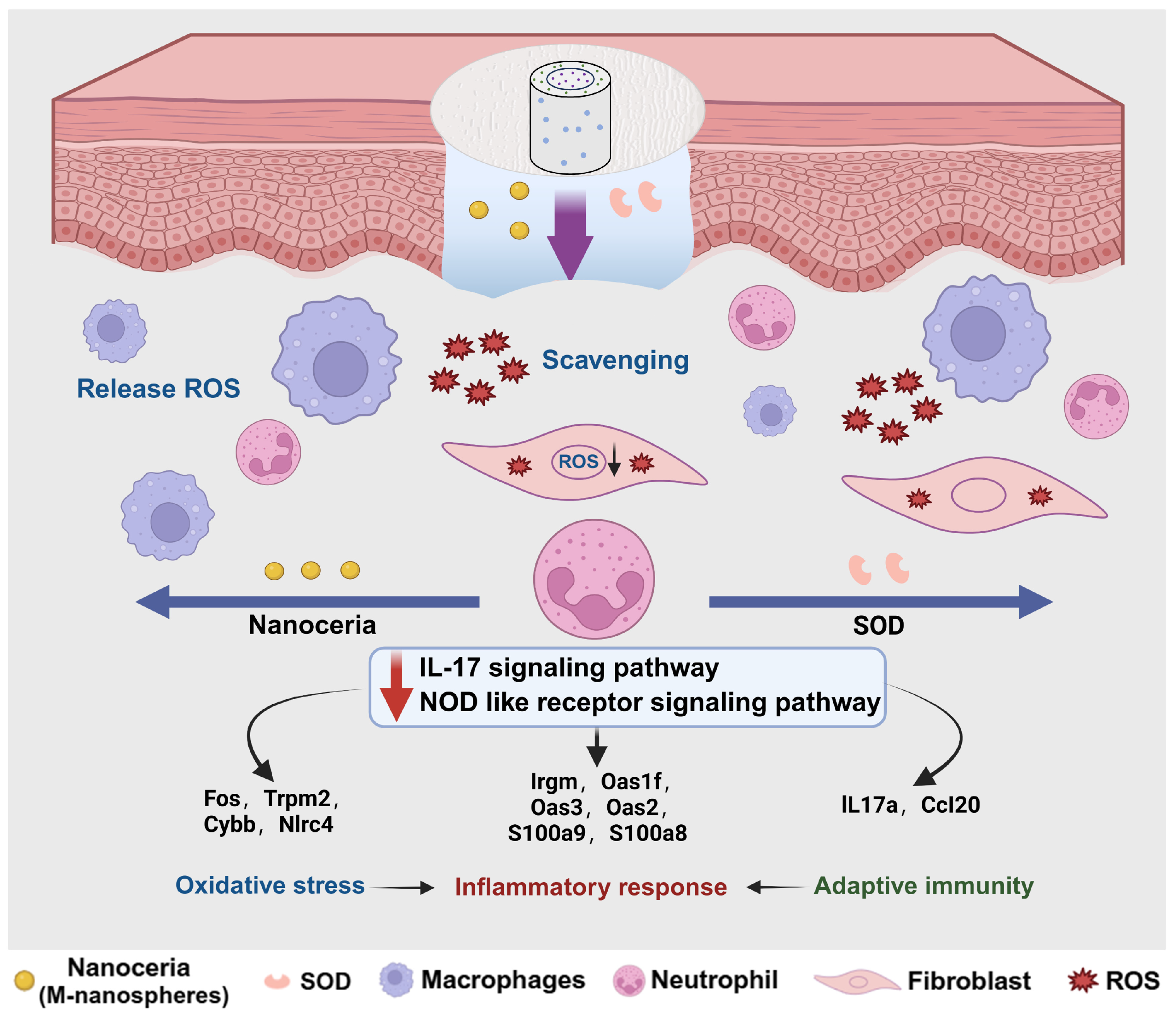
2.5. Regulatory Mechanisms of PU CSFs Embedded with CeO2 vs. SOD
3. Discussion
3.1. Optimal Redox Catalytic Performance of CeO2 Nanoparticles
3.2. Enzyme-Mimetic Activity Modulated by Engineered PU CSFs
3.3. Wound Healing Modulated by Engineered PU CSFs
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of Nanoceria
4.2.1. Synthesis of Ceria Nanoparticles
4.2.2. Characterization and Optimization of Ceria Nanoparticles
4.3. Fabrication of PU Core–Shell Fibers
4.4. Characterization of PU CSFs Membranes
4.4.1. Physicochemical Properties
4.4.2. Water Absorption
4.4.3. Water Vapor Transmission Rate
4.4.4. Redox Catalytic Activity
4.5. Biocompatibility and Environment Regulation Through PU CSFs
4.5.1. Cell Proliferation and Cytocompatibility Test
4.5.2. Cellular Reactive Oxygen Species (ROS) Scavenging Activity
4.5.3. In Vitro Polarization of RAW 264.7 Macrophages
4.6. Skin Wound Treatment
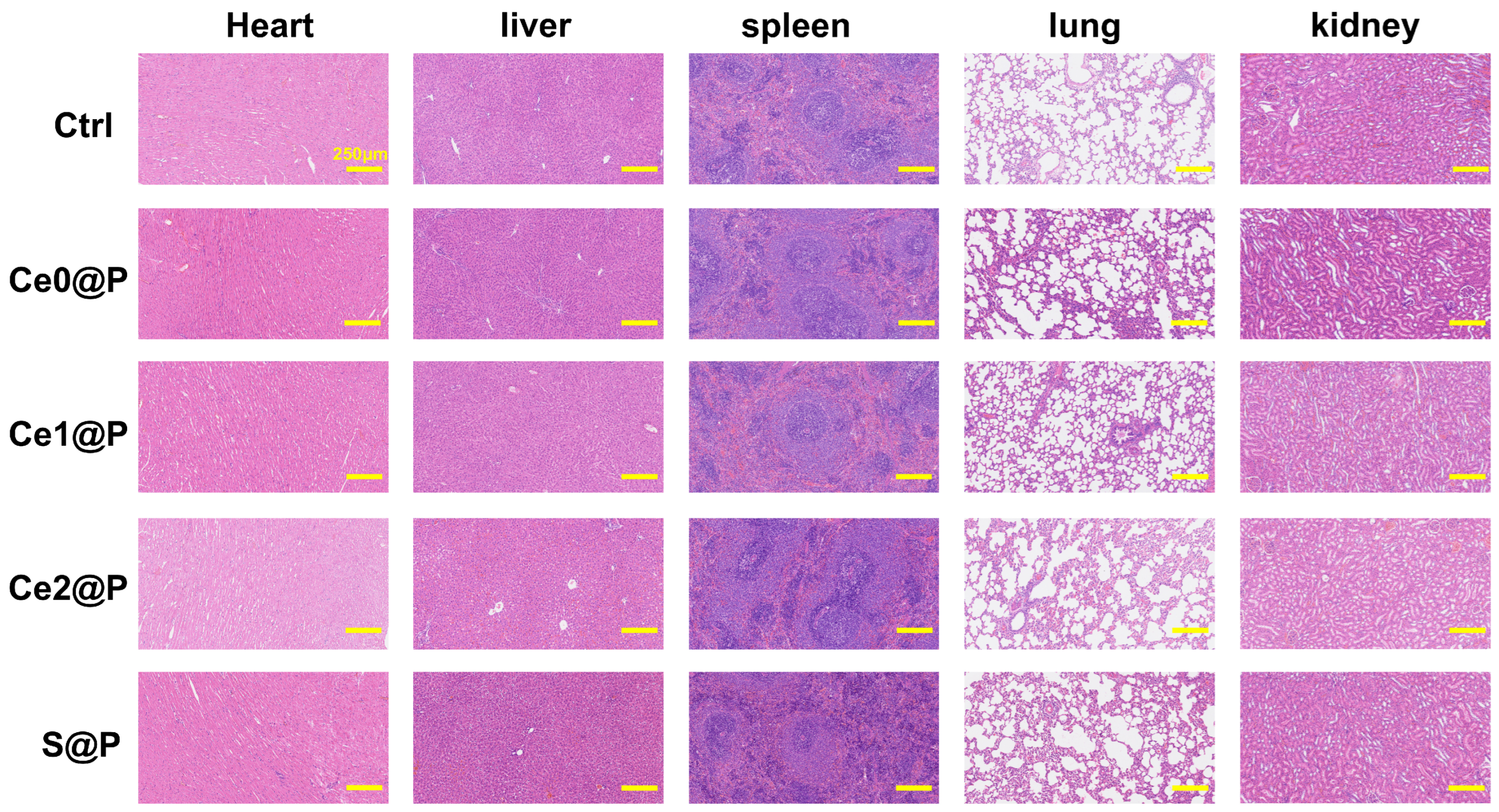
4.6.1. In Vivo Animal Experiments
4.6.2. Histological Staining
4.6.3. Transcriptomic Analysis of Composite Fiber Membrane
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Autologous liquid platelet rich fibrin: A novel drug delivery system. Acta Biomater. 2018, 75, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Nqoro, X.; Taziwa, R. Polymer-Based Functional Materials Loaded with Metal-Based Nanoparticles as Potential Scaffolds for the Management of Infected Wounds. Pharmaceutics 2024, 16, 155. [Google Scholar] [CrossRef]
- Shimada, K.; Ojima, Y.; Ida, Y.; Komiya, T.; Matsumura, H. Negative-pressure wound therapy for donor-site closure in radial forearm free flap: A systematic review and meta-analysis. Int. Wound J. 2022, 19, 316–325. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Wei, S.; Wang, W.; Li, L.; Meng, H.Y.; Feng, C.Z.; Dong, Y.Y.; Fang, X.C.; Dong, Q.Q.; Jiang, W.; Xin, H.L.; et al. Recombinant human epidermal growth factor combined with vacuum sealing drainage for wound healing in Bama pigs. Mil. Med. Res. 2021, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Opdenakker, G.; Van Damme, J.; Vranckx, J.J. Immunomodulation as Rescue for Chronic Atonic Skin Wounds. Trends Immunol. 2018, 39, 341–354. [Google Scholar] [CrossRef]
- Xue, M.L.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Wang, K.; Jia, X.Y.; Fu, C.L.; Yu, H.N.; Wang, Y.P. Antioxidant peptides, the guardian of life from oxidative stress. Med. Res. Rev. 2024, 44, 275–364. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Angkeow, P.; Kuang, J.P.; Ozaki, M.; Irani, K. Rac1 inhibits TNF-α-induced endothelial cell apoptosis: Dual regulation by reactive oxygen species. FASEB J. 2000, 14, 1705–1714. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.X.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.J.; Zheng, X.F.S. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.Q.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Wu, H.B.; Li, F.Y.; Wang, S.F.; Lu, J.X.; Li, J.Q.; Du, Y.; Sun, X.L.; Chen, X.Y.; Gao, J.Q.; Ling, D.S. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2047. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovic, I. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Hemalatha, J.; Senthamil, C.; Sakthivel, C.; Nivetha, A.; Umashankar, J.J.; Prabha, I. Efficient transition metal nanozymes as the alternate for natural enzymes in food analysis and environmental remediation. J. Environ. Chem. Eng. 2024, 12, 112575. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 Nanoparticle-Containing Polymers for Biomedical Applications: A Review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef]
- Cao, X.F.; Zhao, S.; Yan, S.J.; Hu, J.; Dan, Y. Fabrication and Application of CeO2 Nanostructure with Different Morphologies: A Review. J. Renew. Mater. 2020, 8, 1443–1472. [Google Scholar] [CrossRef]
- Abe, H.; Mizoguchi, H.; Eguchi, R.; Hosono, H. Exploration of heterogeneous catalyst for molecular hydrogen ortho-para conversion. Exploration 2024, 4, 20232858. [Google Scholar] [CrossRef]
- Xue, Y.J.; Yang, F.; Wu, L.K.; Xia, D.D.; Liu, Y.S. CeO2 Nanoparticles to Promote Wound Healing: A Systematic Review. Adv. Healthc. Mater. 2024, 13, 2302858. [Google Scholar] [CrossRef]
- Fu, X.X.; Li, P.; Chen, X.; Ma, Y.Y.; Wang, R.; Ji, W.X.; Gu, J.K.; Sheng, B.W.; Wang, Y.Z.; Zhang, Z.H. Ceria nanoparticles: Biomedical applications and toxicity. J. Zhejiang Univ. Sci. B 2024, 25, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Mao, Z.; Huang, W.J.; Liu, L.H.; Li, J.L.; Li, J.L.; Wu, Q.Z. Redox enzyme-mimicking activities of CeO2 nanostructures: Intrinsic influence of exposed facets. Sci. Rep. 2016, 6, 35344. [Google Scholar] [CrossRef]
- Manto, M.J.; Xie, P.F.; Wang, C. Catalytic Dephosphorylation Using Ceria Nanocrystals. ACS Catal. 2017, 7, 1931–1938. [Google Scholar] [CrossRef]
- Venkataronappa, A.; Bankaitis, J.; Seo, J. Enhancing dispersion stability and recyclability of ceria slurry with polyacrylic acid for improved glass polishing performance. J. Ind. Eng. Chem. 2024, 138, 623–631. [Google Scholar] [CrossRef]
- Wu, Z.L.; Li, M.J.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B-Environ. 2015, 174, 403–412. [Google Scholar] [CrossRef]
- Hancock, M.L.; Yokel, R.A.; Beck, M.J.; Calahan, J.L.; Jarrells, T.W.; Munson, E.J.; Olaniyan, G.A.; Grulke, E.A. The characterization of purified citrate-coated cerium oxide nanoparticles prepared via hydrothermal synthesis. Appl. Surf. Sci. 2021, 535, 147681. [Google Scholar] [CrossRef]
- Buckley, C.D.; Ospelt, C.; Gay, S.; Midwood, K.S. Location, location, location: How the tissue microenvironment affects inflammation in RA. Nat. Rev. Rheumatol. 2021, 17, 195–212. [Google Scholar] [CrossRef]
- Machi, K.; Ono, Y.; Iwahashi, H. Citrate, a TCA cycle metabolite, plays a role as a hydroxyl radical scavenger in vitro. J. Photochem. Photobiol. A-Chem. 2024, 454, 115691. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Kamalipooya, S.; Fahimirad, S.; Abtahi, H.; Golmohammadi, M.; Satari, M.; Dadashpour, M.; Nasrabadi, D. Diabetic wound healing function of PCL/cellulose acetate nanofiber engineered with chitosan/cerium oxide nanoparticles. Int. J. Pharm. 2024, 653, 123880. [Google Scholar] [CrossRef]
- Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Ivanova, O.S.; Popov, A.L.; Mysina, E.A.; Artyushkova, E.B.; Kryukov, A.A.; Dodonova, S.A.; Kruglova, M.P.; et al. Influence of the Synthesis Scheme of Nanocrystalline Cerium Oxide and Its Concentration on the Biological Activity of Cells Providing Wound Regeneration. Int. J. Mol. Sci. 2023, 24, 14501. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Walters, G.; Pountos, I.; Giannoudis, P.V. The cytokines and micro-environment of fracture haematoma: Current evidence. J. Tissue Eng. Regen. Med. 2018, 12, E1662–E1677. [Google Scholar] [CrossRef]
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen. 2016, 24, 613–629. [Google Scholar] [CrossRef]
- Axelsson, J.; Xu, D.; Kang, B.N.; Nussbacher, J.K.; Handel, T.M.; Ley, K.; Sriramarao, P.; Esko, J.D. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood 2012, 120, 1742–1751. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.L.; Lu, S.T.; Zhang, N.Y.; Zhang, H.J.; Zhang, J.; Zhang, J. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res. Ther. 2022, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Cuttle, L.; Nataatmadja, M.; Fraser, J.F.; Kempf, M.; Kimble, R.M.; Hayes, M.T. Collagen in the scarless fetal skin wound: Detection with Picrosirius-polarization. Wound Repair Regen. 2005, 13, 198–204. [Google Scholar] [CrossRef]
- Zielinska, W.; Zabrzynski, J.; Gagat, M.; Grzanka, A. The Role of TRPM2 in Endothelial Function and Dysfunction. Int. J. Mol. Sci. 2021, 22, 7635. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.Y.C.; Conquer, J.A.; Grinstein, S.; Cruz, T.F. Interleukin-1β induction of c-fos and collagenase expression in articular chondrocytes: Involvement of reactive oxygen species. J. Cell. Biochem. 1998, 69, 19–29. [Google Scholar] [CrossRef]
- Sundaram, B.; Kanneganti, T.D. Advances in Understanding Activation and Function of the NLRC4 Inflammasome. Int. J. Mol. Sci. 2021, 22, 1048. [Google Scholar] [CrossRef]
- Whitehead, N.P. Enhanced autophagy as a potential mechanism for the improved physiological function by simvastatin in muscular dystrophy. Autophagy 2016, 12, 705–706, Editorial Material. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, S.C.; Choi, E.Y.; Lee, M.H.; Seo, G.S.; Kim, E.C.; Yang, B.J.; Lee, M.S.; Shine, Y.I.; Park, K.I.; et al. Iron chelator induces MIP-3α/CCL20 in human intestinal epithelial cells: Implication for triggering mucosal adaptive immunity. Exp. Mol. Med. 2005, 37, 297–310. [Google Scholar] [CrossRef]
- Mishra, P.; Lee, J.; Kumar, D.; Lauro, R.O.; Costa, N.; Pathania, D.; Kumar, S.; Lee, J.; Singh, L. Engineered Nanoenzymes with Multifunctional Properties for Next-Generation Biological and Environmental Applications. Adv. Funct. Mater. 2022, 32, 21. [Google Scholar] [CrossRef]
- Karmakar, A.; Zhang, Q.L.; Zhang, Y.B. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Leiss-Maier, F.; Mühlhofer, R.; Boesen, B.; Mustafa, G.; Kugler, H.; Zeymer, C. A De Novo Metalloenzyme for Cerium Photoredox Catalysis. J. Am. Chem. Soc. 2024, 146, 25976–25985. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mahmoud, G.A.E.; Sharmoukh, W. A cerium-based MOFzyme with multi-enzyme-like activity for the disruption and inhibition of fungal recolonization. J. Mat. Chem. B 2020, 8, 7548–7556. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, S. Polyoxometalate-Mediated Vacancy-Engineered Cerium Oxide Nanoparticles Exhibiting Controlled Biological Enzyme-Mimicking Activities. Inorg. Chem. 2021, 60, 7475–7489. [Google Scholar] [CrossRef]
- Li, J.H.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.H.; Jiang, X.Q.; Yeung, K.W.K.; Chu, P.K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci. 2018, 5, 1700678. [Google Scholar] [CrossRef]
- Mehta, A.; Scammon, B.; Shrake, K.; Bredikhin, M.; Gil, D.; Shekunova, T.; Baranchikov, A.; Ivanov, V.; Reukov, V. Nanoceria: Metabolic interactions and delivery through PLGA-encapsulation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 114, 111003. [Google Scholar] [CrossRef]
- My Kieu, H.; Yoo Jin, S.; Tae Hyun, Y. Effects of agglomeration on in vitro dosimetry and cellular association of silver nanoparticles. Environ. Sci. Nano 2018, 5, 446–455. [Google Scholar] [CrossRef]
- Masui, T.; Hirai, H.; Imanaka, N.; Adachi, G.; Sakata, T.; Mori, H. Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. J. Mater. Sci. Lett. 2002, 21, 489–491. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lee, Y.H.; Chou, C.L.; Chang, Y.S.; Liu, W.C.; Chiu, H.W. Oxidative stress and potential effects of metal nanoparticles: A review of biocompatibility and toxicity concerns. Environ. Pollut. 2024, 346, 123617. [Google Scholar] [CrossRef]
- Selvaraj, S.; Chauhan, A.; Radhakrishnan, A.; Rana, G.; Dutta, V.; Batoo, K.M.; Ghotakar, S. Cerium Oxide Nanoparticles and Their Polymeric Composites: Advancements in Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2024, 34, 5691–5717. [Google Scholar] [CrossRef]
- Huangfu, Y.N.; Li, S.Y.; Deng, L.D.; Zhang, J.H.; Huang, P.S.; Feng, Z.J.; Kong, D.L.; Wang, W.W.; Dong, A.J. Skin-Adaptable, Long-Lasting Moisture, and Temperature-Tolerant Hydrogel Dressings for Accelerating Burn Wound Healing without Secondary Damage. ACS Appl. Mater. Interfaces 2021, 13, 59695–59707. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 81, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Zheng, Y.X.; Zhou, Y.; Xu, F.; Cui, Y.Z.; Chen, X.Y.; Wang, Z.Y.; Yan, B.X.; Zheng, M.; Man, X.Y. OAS1, OAS2, and OAS3 Contribute to Epidermal Keratinocyte Proliferation by Regulating Cell Cycle and Augmenting IFN-1-Induced Jak1-Signal Transducer and Activator of Transcription 1 Phosphorylation in Psoriasis. J. Investig. Dermatol. 2022, 142, 2635–2645. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Liu, H.; Chen, F.; Wang, H.; Chen, S.; Xie, J.; Zheng, Z.; Li, Z. Alarmins S100A8/A9 promote intervertebral disc degeneration and inflammation-related pain in a rat model through toll-like receptor-4 and activation of the NF-κB signaling pathway. Osteoarthr. Cartil. 2022, 30, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.B.; Karadarevic, D.; Castaño-Rodríguez, N. Immunity-related GTPase IRGM at the intersection of autophagy, inflammation, and tumorigenesis. Inflamm. Res. 2022, 71, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Ren, S.S.; Zhou, Y.; Zheng, K.; Xu, X.W.; Yang, J.; Wang, X.Y.; Miao, L.Y.; Wei, H.; Xu, Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, J.Q.; Tang, J.J.; Lei, X.Y.; Zhang, J.Z.; Zhang, Y.L.; Li, J.D.; Zuo, Y.; Li, Y.B. Self-Activating Phosphorus-Rich Substrate Import Enzyme Catalytic Domains for Bone Regeneration. Adv. Funct. Mater. 2024, 34, 2316428. [Google Scholar] [CrossRef]
- ISO 11137-2; Sterilization of Health Care Products–Radiation–Part 2: Establishing the Sterilization Dose. International Organization for Standardization: Geneva, Switzerland, 2006.
- Davoudabadi, M.; Fahimirad, S.; Ganji, A.; Abtahi, H. Wound healing and antibacterial capability of electrospun polyurethane nanofibers incorporating Calendula officinalis and Propolis extracts. J. Biomater. Sci.-Polym. Ed. 2023, 34, 1491–1516. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Farzamfar, S.; Bastami, F.; Tajerian, R. Fabrication and characterization of electrospun PLLA/collagen nanofibrous scaffold coated with chitosan to sustain release of aloe vera gel for skin tissue engineering. Biomed. Eng. Appl. Basis Commun. 2016, 28, 1650035. [Google Scholar] [CrossRef]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef]
- Taciak, B.; Bialasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, L.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, 13. [Google Scholar] [CrossRef] [PubMed]

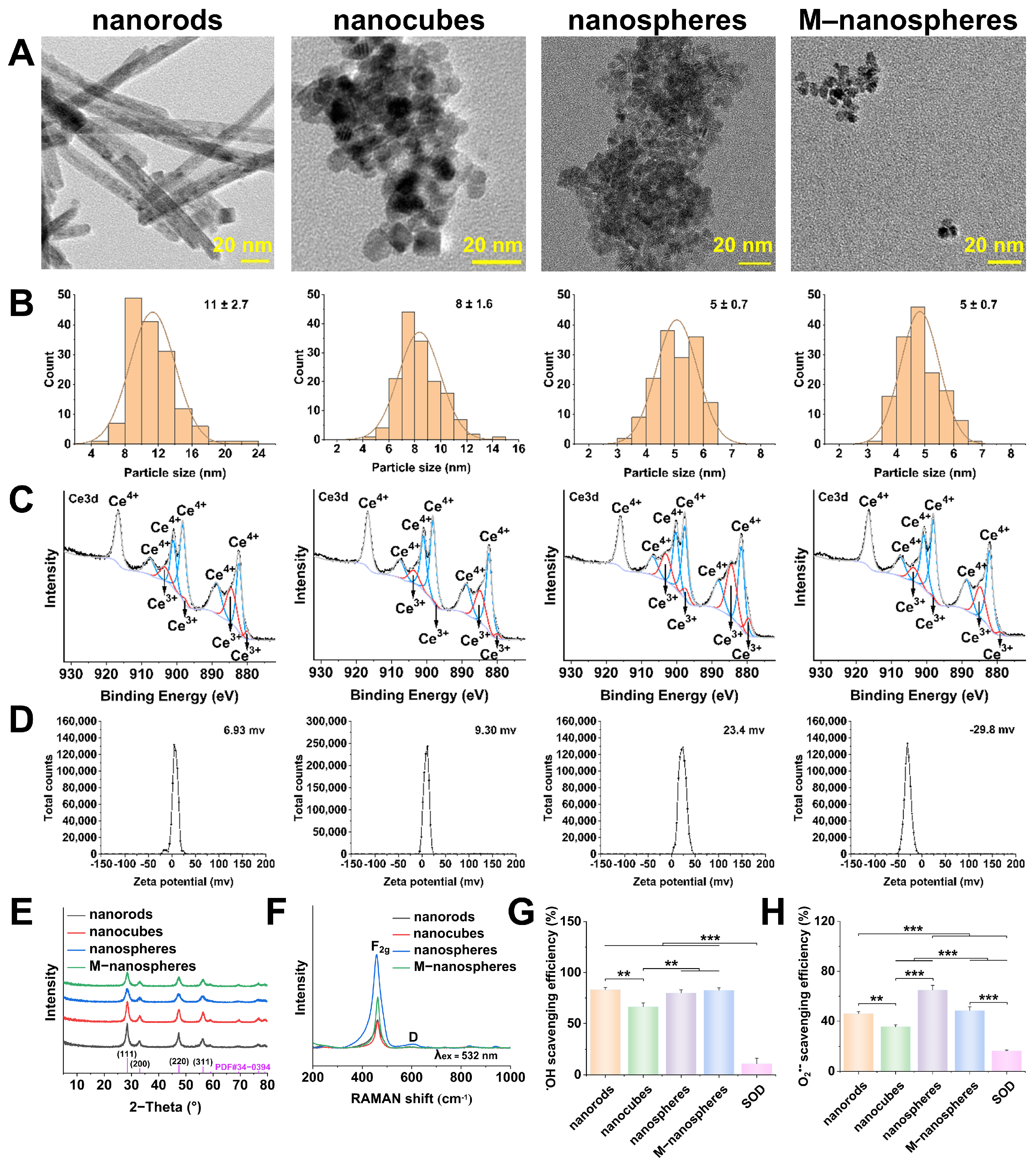

| CeO2 Nanocrystals | Size Distribution from TEM (nm, n = 150) | BET Surface Area (m2/g) | Ce3+ (%) | I600/I460 |
|---|---|---|---|---|
| Nanorods | 11 ± 2.7 (width) | 57.2 | 24.15 | 0.039 |
| Nanocubes | 8 ± 1.6 | 96.0 | 20.51 | 0.023 |
| Nanospheres | 5 ± 0.7 | 123.8 | 33.13 | 0.043 |
| M-nanospheres | 5 ± 0.7 | 77.3 | 19.66 | 0.020 |
| Types | Stability | Biocompatibility | Cost |
|---|---|---|---|
| Cerium-based | Excellent | Excellent | Low |
| Manganese-based | Stable in acidic environments | Medium | Low |
| Pt/Pd NPs | Excellent | Poor | High |
| Groups | Abb. | PU/HFIP (wt/vol%) | Core: PCL/TFE (wt/vol%) | Shell: CeO2/PU (wt%) | SOD (0.001 mg/mL) |
|---|---|---|---|---|---|
| CeO2-0@PU CSFs | Ce0@P | 3.5 | 12 | 0 | / |
| CeO2-1@PU CSFs | Ce1@P | 3.5 | 12 | 5 | / |
| CeO2-2@PU CSFs | Ce2@P | 3.5 | 12 | 10 | / |
| CeO2-3@PU CSFs | Ce3@P | 3.5 | 12 | 15 | / |
| SOD@PU CSFs | S@P | 3.5 | 12 | / | 50 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, J.; Lei, X.; Li, L.; Mu, B.; Du, Q.; Li, Y.; Zuo, Y. Polyurethane@CeO2 Nanozyme Core–Shell Fibrous Membranes for Enhanced Wound Healing via Balanced Redox Modulation. Catalysts 2025, 15, 617. https://doi.org/10.3390/catal15070617
Li Y, Zhang J, Lei X, Li L, Mu B, Du Q, Li Y, Zuo Y. Polyurethane@CeO2 Nanozyme Core–Shell Fibrous Membranes for Enhanced Wound Healing via Balanced Redox Modulation. Catalysts. 2025; 15(7):617. https://doi.org/10.3390/catal15070617
Chicago/Turabian StyleLi, Yuping, Jinzheng Zhang, Xiaoyu Lei, Li Li, Bo Mu, Qingda Du, Yubao Li, and Yi Zuo. 2025. "Polyurethane@CeO2 Nanozyme Core–Shell Fibrous Membranes for Enhanced Wound Healing via Balanced Redox Modulation" Catalysts 15, no. 7: 617. https://doi.org/10.3390/catal15070617
APA StyleLi, Y., Zhang, J., Lei, X., Li, L., Mu, B., Du, Q., Li, Y., & Zuo, Y. (2025). Polyurethane@CeO2 Nanozyme Core–Shell Fibrous Membranes for Enhanced Wound Healing via Balanced Redox Modulation. Catalysts, 15(7), 617. https://doi.org/10.3390/catal15070617







