Construction of A NiS/g-C3N4 Co-Catalyst-Based S-Scheme Heterojunction and Its Performance in Photocatalytic CO2 Reduction
Abstract
1. Introduction
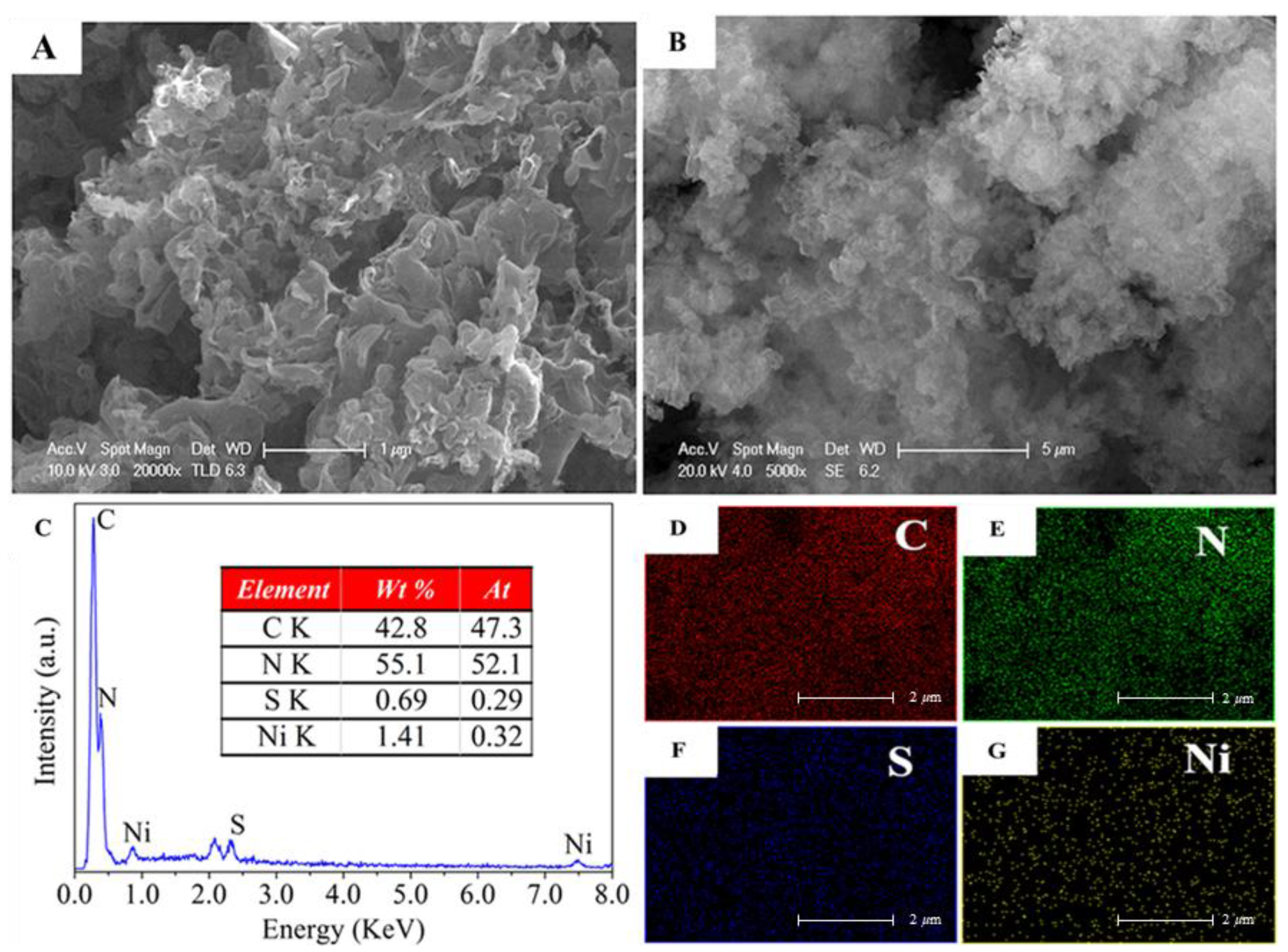
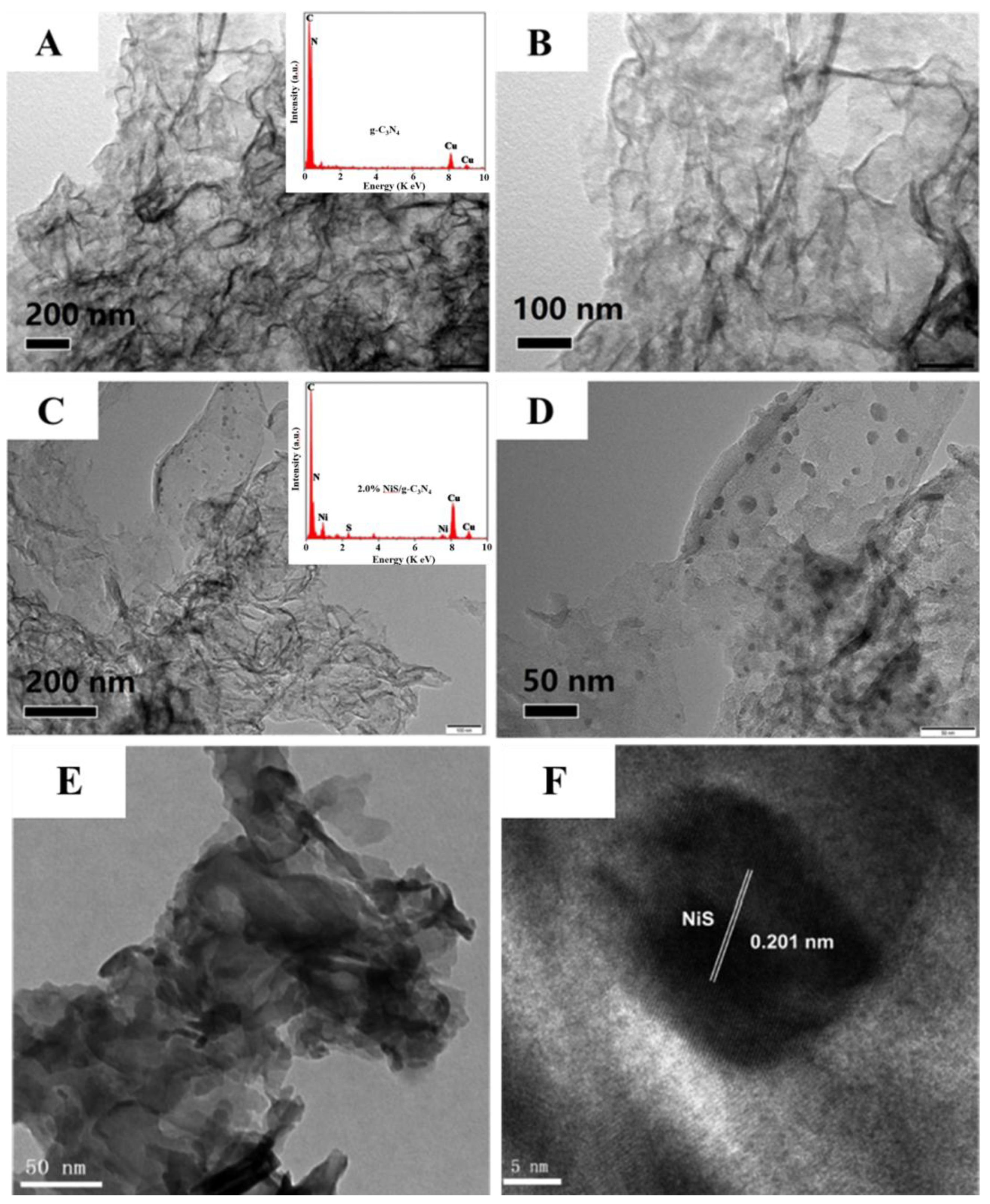
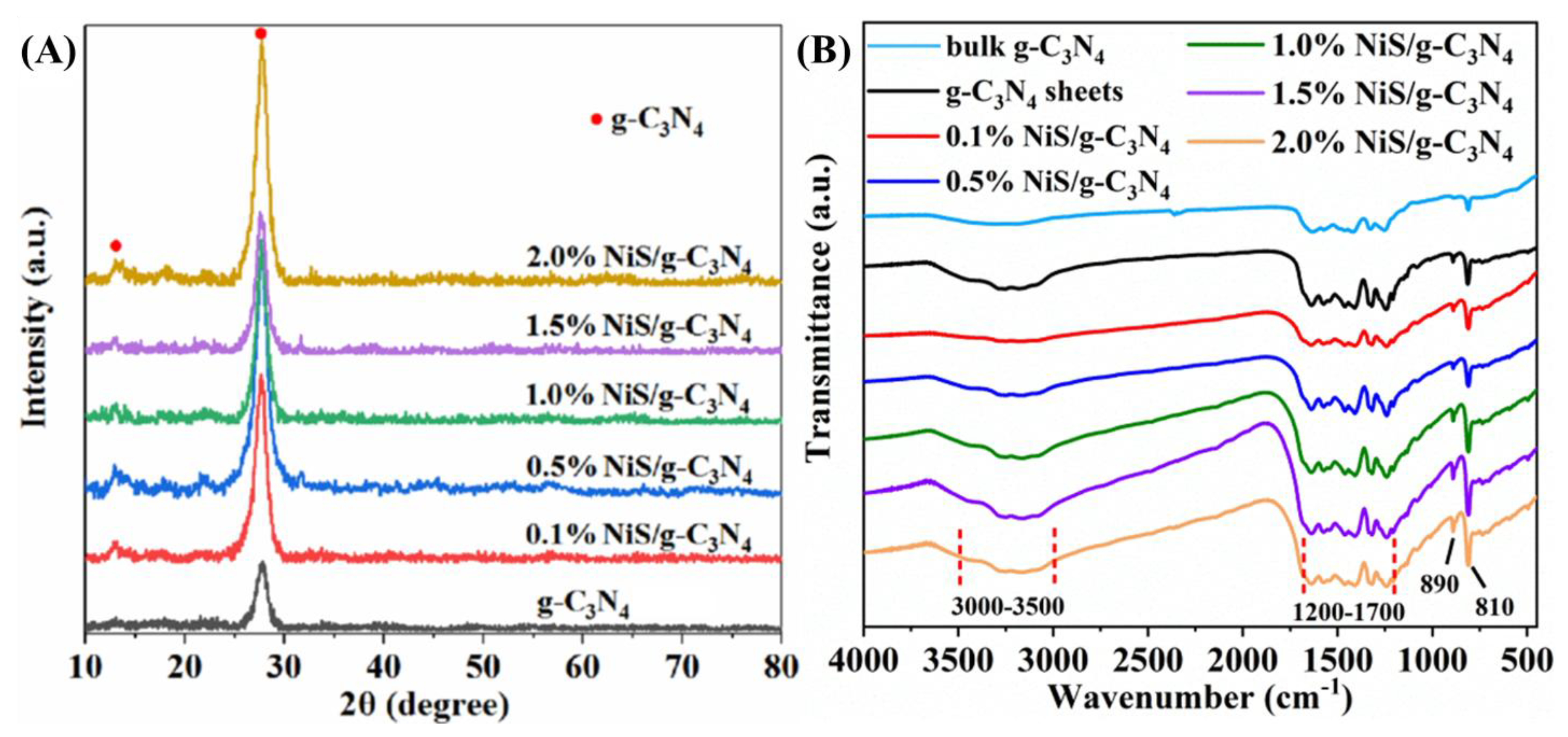
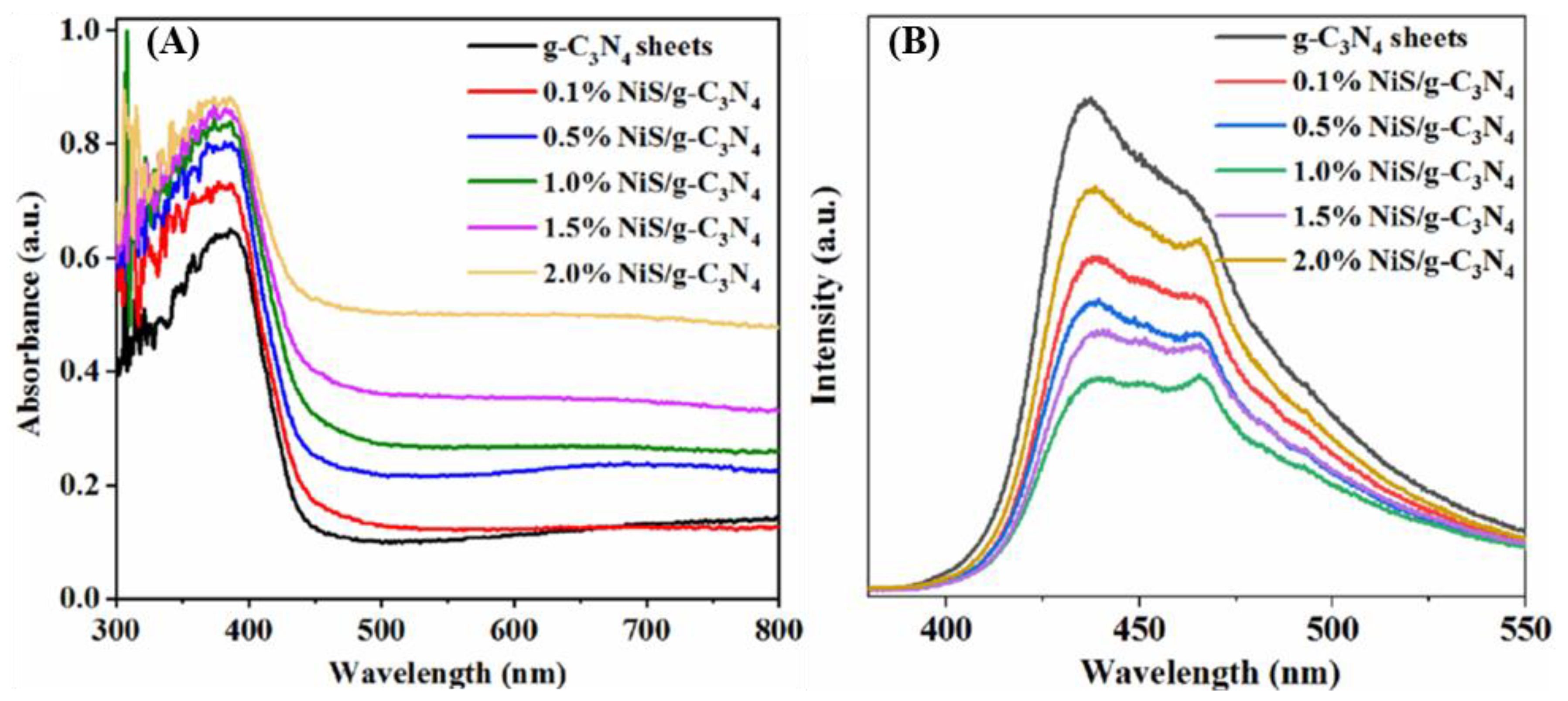
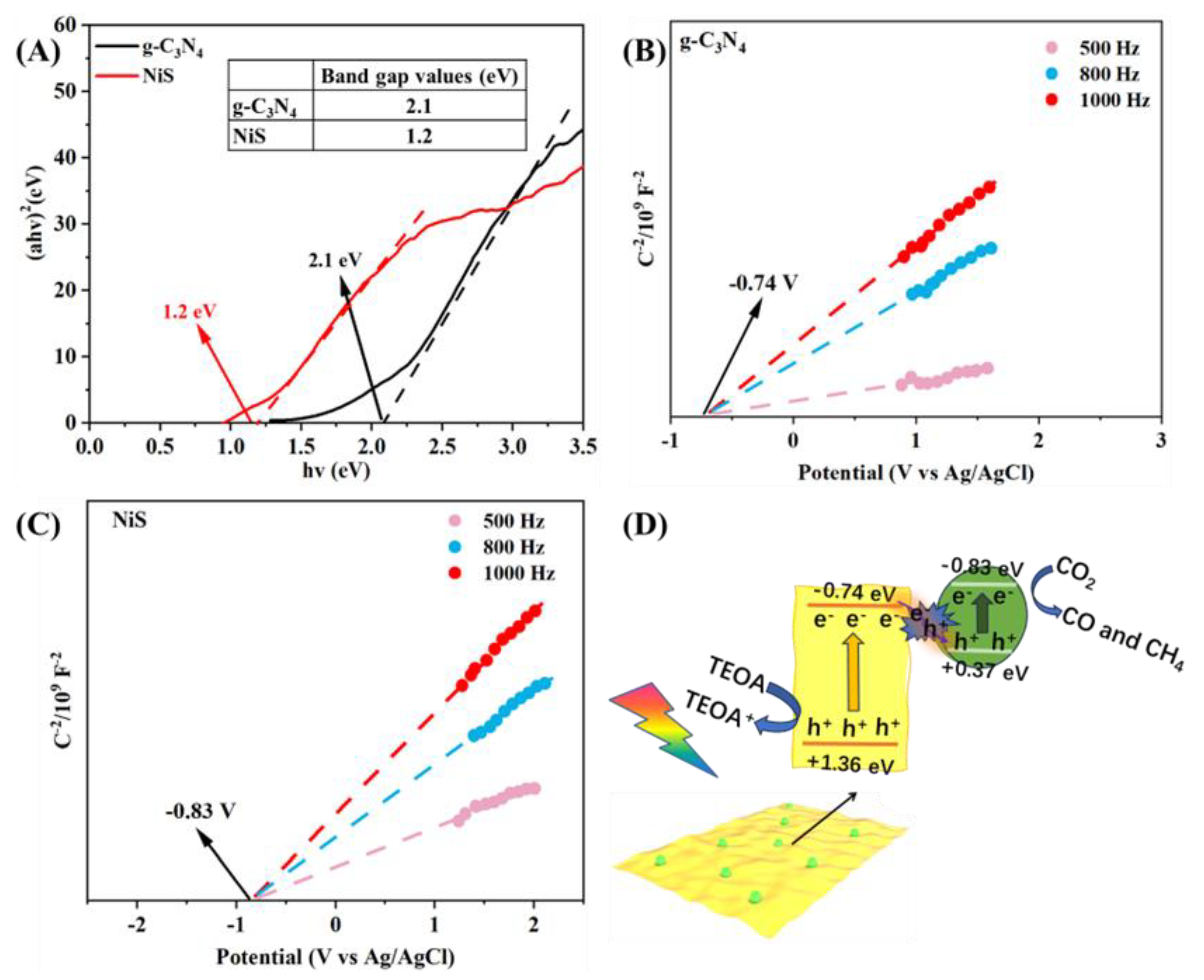
2. Results and Discussion
3. Experimental Section
3.1. Preparation of g-C3N4
3.2. Preparation of NiS/g-C3N4
4. Materials and Methods
4.1. Materials
4.2. Material Characterization
4.3. Photocatalytic Activity Measurement
4.4. Photocatalytic Stability Test
4.5. Electrochemical Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumari, P.; Bahadur, N.; Conlan, X.A.; Laleh, M.; Kong, L.; O’Dell, L.A.; Dumée, L.F.; Merenda, A. Atomically-thin Schottky-like photo-electrocatalytic cross-flow membrane reactors for ultrafast remediation of persistent organic pollutants. Water Res. 2022, 218, 118519. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Sun, Y.; Li, N.; Gao, Y.; Li, H.; Ge, L.; Ma, T. Photocatalytic CO2-to-CH4 Conversion with Ultrahigh Selectivity of 95.93% on S-Vacancy Modulated Spatial In2S3/In2O3 Heterojunction. Adv. Funct. Mater. 2024, 34, 2409031. [Google Scholar] [CrossRef]
- Le Huec, T.; Lopez-Frances, A.; Lazaro, I.A.; Navalon, S.; Baldovi, H.G.; Gimenez-Marques, M. Heteroepitaxial MOF-on-MOF Photocatalyst for Solar-Driven Water Splitting. ACS Nano 2024, 18, 20201–20212. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Salavati-fard, T.; Wang, B. Plasmonic Energetic Electrons Drive CO2 Reduction on Defective Cu2O. ACS Catal. 2023, 13, 6328–6337. [Google Scholar] [CrossRef]
- Le, V.N.; Nguyen, V.C.; Nguyen, H.T.; Tran, H.D.; Tu, T.N.; Kim, W.-S.; Kim, J. Facile synthesis of bimetallic MIL-100(Fe, Al) for enhancing CO2 Adsorption performance. Microporous Mesoporous Mater. 2023, 360, 112716. [Google Scholar] [CrossRef]
- Lee, A.; Wu, S.; Yim, J.E.; Zhao, B.; Sheldon, M.T. Hot Electrons in a Steady State: Interband vs Intraband Excitation of Plasmonic Gold. ACS Nano 2024, 18, 19077–19085. [Google Scholar] [CrossRef]
- Lee, H.; Ferguson, P.W.; Rosen, J. Lower Limb Exoskeleton Systems—Overview. In Wearable Robotics: Systems and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 207–229. [Google Scholar]
- Lee, J.; Jeon, D.J.; Yeo, J.S. Quantum Plasmonics: Energy Transport Through Plasmonic Gap. Adv. Mater. 2021, 33, e2006606. [Google Scholar] [CrossRef]
- Li, A.; Zhu, W.; Li, C.; Wang, T.; Gong, J. Rational design of yolk–shell nanostructures for photocatalysis. Chem. Soc. Rev. 2019, 48, 1874–1907. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; An, W.; Wang, H.; Guo, H.; Liang, Y.; Cui, W. Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO to ethanol. Appl. Catal. B Environ. 2020, 266, 118618. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, L.; Chen, D.; Hao, Z.; Deng, B.; Sun, Y.; Liu, X.; Jia, B.; Chen, L.; Liu, H. Twin S-Scheme g-C3N4/CuFe2O4/ZnIn2S4 Heterojunction with a Self-Supporting Three-Phase System for Photocatalytic CO2 Reduction: Mechanism Insight and DFT Calculations. ACS Catal. 2024, 14, 5326–5343. [Google Scholar] [CrossRef]
- Liu, D.; Ma, H.; Zhu, C.; Qiu, F.; Yu, W.; Ma, L.-L.; Wei, X.-W.; Han, Y.-F.; Yuan, G. Molecular Co-Catalyst Confined within a Metallacage for Enhanced Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2024, 146, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xue, C. Plasmonic Coupling Architectures for Enhanced Photocatalysis. Adv. Mater. 2021, 33, 2005738. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, Z.; Han, H.; Wu, D.; Xu, F.; Wang, H.; Jiang, K.; Cu, M. facile synthesis and the selective adsorption properties. Chem. Eng. J. 2012, 185–186, 151–159. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Xiao, R.; Tan, R.; Qin, Y.; Li, J.; Bai, Y.; Xi, M.; Yang, W.; Fang, Q.; et al. Improving Interface Matching in MOF-on-MOF S-Scheme Heterojunction through π–π Conjugation for Boosting Photoelectric Response. Nano Lett. 2023, 23, 5358–5366. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Zhao, K.; Liu, P.; Sun, Z.; Lu, J. Photocatalytic conversion of CH4 and CO2 to acetic acid over Cu/ZnO catalysts under mild conditions. Chem. Eng. J. 2024, 487, 150690. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Song, X.; Liu, X.; Zhou, W.; Wang, H.; Huo, P. Pd Nanosheet-Decorated 2D/2D g-C3N4/WO3·H2O S-Scheme Photocatalyst for High Selective Photoreduction of CO2 to CO. Inorg. Chem. 2022, 61, 4171–4183. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Z.; Zhang, R.; Xiong, J.; Qiao, Y.; Liu, X.; Lu, X. Hollow Nanoreactors for Controlled Photocatalytic Behaviors: Fundamental Theory, Structure–Performance Relationship, and Catalytic Advantages. Small 2023, 20, 2308142. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Q.; Xu, M.; Yan, C.; Song, X.; Liu, X.; Wang, H.; Zhou, W.; Huo, P. Dual-plasma enhanced 2D/2D/2D g-C3N4/Pd/MoO3−x S-scheme heterojunction for high-selectivity photocatalytic CO2 reduction. Appl. Surf. Sci. 2023, 640, 158420. [Google Scholar] [CrossRef]
- Wang, H.; Yan, C.; Xu, M.; Li, J.; Zhang, Z.; Song, X.; Liu, X.; Huo, P. Pd Nanoparticle-Modified BiOBr/CdS S-Scheme Photocatalyst for Enhanced Conversion of CO2. Inorg. Chem. 2024, 63, 17274–17286. [Google Scholar] [CrossRef]
- Wang, K.; Sun, T.; Ma, H.; Wang, Y.; He, Z.-H.; Wang, H.; Wang, W.; Yang, Y.; Wang, L.; Liu, Z.-T. Energy band modulating of NiO/BiOCl heterojunction with transition from type-II to S-scheme for enhancing photocatalytic CO2 reduction. Chem. Eng. J. 2024, 497, 154711. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Ni–Co Bimetallic Hydroxide Nanosheet Arrays Anchored on Graphene for Adsorption-Induced Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2022, 34, e2202960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Deng, F.; Liu, X.; Li, X.; Luo, X.; Peng, Y.; Zhang, J.; Zou, J.; Ding, L.; et al. Ag quantum dots decorated ultrathin g-C3N4 nanosheets for boosting degradation of pharmaceutical contaminants: Insight from interfacial electric field induced by local surface plasma resonance. Chem. Eng. J. 2023, 463, e2202960. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, Y.; Yan, Y.; Huo, P.; Wang, H. G-C3N4 quantum dots and Au nano particles co-modified CeO2/Fe3O4 micro-flowers photocatalyst for enhanced CO2 photoreduction. Renew. Energy 2021, 179, 756–765. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Zhu, J.; Bi, Q.; Tao, Y.; Guo, W.; Fan, J.; Min, Y.; Li, G. Mo-Modified ZnIn2S4@NiTiO3 S-Scheme Heterojunction with Enhanced Interfacial Electric Field for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2213131. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, S.; Zhao, Z.; Shi, Z.; Yan, S.; Zou, Z. A Facet-Dependent Schottky-Junction Electron Shuttle in a BiVO4{010}–Au–Cu2O Z-Scheme Photocatalyst for Efficient Charge Separation. Adv. Funct. Mater. 2018, 28, 1801214. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Liu, X.; Zhou, W.; Wang, H.; Huo, P. Tailored Linker Defects in UiO-67 with High Ligand-to-Metal Charge Transfer toward Efficient Photoreduction of CO2. Inorg. Chem. 2022, 61, 1765–1777. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Wu, D.; Li, J.; Huo, P.; Wang, H. Improved charge transfer by size-dependent plasmonic Au on C3N4 for efficient photocatalytic oxidation of RhB and CO2 reduction. Chin. J. Catal. 2019, 40, 928–939. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Lv, Y.; An, S.; Wang, R. ZIF-8/g-C3N4 photocatalysts: Enhancing CO2 reduction through improved adsorption and photocatalytic performance. RSC Adv. 2024, 14, 17498–17506. [Google Scholar] [CrossRef]
- Sun, Q.; Cortie, D.; Zhang, S.; Frankcombe, T.J.; She, G.; Gao, J.; Sheppard, L.R.; Hu, W.; Chen, H.; Zhuo, S.; et al. The Formation of Defect-Pairs for Highly Efficient Visible-Light Catalysts. Adv. Mater. 2017, 29, 1605123. [Google Scholar] [CrossRef]
- Sun, K.; Qian, Y.; Jiang, H.L. Metal-Organic Frameworks for Photocatalytic Water Splitting and CO2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202217565. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Meng, F.; Chen, S.; Wang, J.; Liu, C.; Wei, Y.; He, C.; Fan, L.; Zhang, Q.; Ye, W.; et al. Triazine-COF@Silicon nanowire mimicking plant leaf to enhance photoelectrocatalytic CO2 reduction to C2+ chemicals. Green Energy Environ. 2025, 10, 422–432. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Q.; Xu, Z.; Li, J.; Wang, H.; Xu, M.; Yan, C.; Song, X.; Liu, X.; Wang, H.; et al. Interface engineering enhanced g-C3N4/rGO/Pd composites synergetic localized surface plasmon resonance effect for boosting photocatalytic CO2 reduction. Carbon Lett. 2024, 34, 1143–1154. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; Wang, Y.; Ding, Y.; Leung, M.K.H.; Ng, Y.H. Defects of Metal Halide Perovskites in Photocatalytic Energy Conversion: Friend or Foe? Adv. Sci. 2024, 11, e2402471. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Y.; Li, Z.; Lou, Z.; Chen, Q.; Li, Y.; Wang, D.; Mao, J. Engineering a Copper Single-Atom Electron Bridge to Achieve Efficient Photocatalytic CO2 Conversion. Angew. Chem. Int. Ed. 2023, 62, e202218460. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wan, Y.; Nazir, A.; Song, X.; Huo, P.; Wang, H. Fabrication of Zn vacancies-tunable ultrathin-g-C3N4@ZnIn2S4/SWNTs composites for enhancing photocatalytic CO2 reduction. Appl. Surf. Sci. 2023, 613, 155989. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wan, Y.; Nazir, A.; Song, X.; Huo, P.; Wang, H. Synthesis of AgInS2 QDs-MoS2/GO composite with enhanced interfacial charge separation for efficient photocatalytic degradation of tetracycline and CO2 reduction. J. Alloys Compd. 2023, 954, 170159. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Wang, J.; Sun, K.; Wang, D.; Niu, X.; Lin, Z.; Wang, S.; Yang, W.; Huang, J.; Jiang, H.-L. Precise Regulation of the Coordination Environment of Single Co(II) Sites in a Metal–Organic Framework for Boosting CO2 Photoreduction. ACS Catal. 2023, 13, 8760–8769. [Google Scholar] [CrossRef]
- Wang, J.; Tan, H.Y.; Zhu, Y.; Chu, H.; Chen, H.M. Linking the Dynamic Chemical State of Catalysts with the Product Profile of Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17254–17267. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Meng, F.; Bai, G.; Zhang, Q.; Lan, X. Integrating Dual-Metal Sites into Covalent Organic Frameworks for Enhanced Photocatalytic CO2 Reduction. ACS Catal. 2023, 13, 4316–4329. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Y.; Qu, B.; Bai, L.; Liu, Y.; Yang, Z.D.; Zhang, W.; Jing, L.; Fu, H. Construction of Six-Oxygen-Coordinated Single Ni Sites on g-C3N4 with Boron-Oxo Species for Photocatalytic Water-Activation-Induced CO2 Reduction. Adv. Mater. 2021, 33, e2105482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, M.; Jiang, H.; He, H.; Qi, J.; Ma, J. Photocatalytic MOF membranes with two-dimensional heterostructure for the enhanced removal of agricultural pollutants in water. Chem. Eng. J. 2022, 435, 133870. [Google Scholar] [CrossRef]
- Xin, S.; Ma, X.; Lu, J.; Zhang, G.; Huo, S.; Gao, M.; Xu, P.; Liu, W.; Fu, W. Enhanced visible light photoelectrocatalytic degradation of o-chloronitrobenzene through surface plasmonic Au nanoparticles and g-C3N4 co-modified TiO2 nanotube arrays photoanode. Appl. Catal. B Environ. 2023, 323, 122174. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, X.; Jiang, H.; Chen, S.; Huo, P. MOFs-derived C-In2O3/g-C3N4 heterojunction for enhanced photoreduction CO2. J. Environ. Chem. Eng. 2021, 9, 106469. [Google Scholar] [CrossRef]
- Yao, L.; Sheng, X.; Mrachacz-Kersting, N.; Zhu, X.; Farina, D.; Jiang, N. Sensory Stimulation Training for BCI System Based on Somatosensory Attentional Orientation. IEEE Trans. Biomed. Eng. 2019, 66, 640–646. [Google Scholar] [CrossRef]
- Yin, S.; Liu, Y.; Zhou, W.; Wang, H.; Song, X.; Wang, H.; Huo, P. Nanocluster-mediated electron–hole separation for efficient CO2photoreduction. Chem. Eng. J. 2023, 477, 147292. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, G.; Chen, G.; Wang, H.; Zhao, Q.; Yin, W.; Yi, J.; Zhu, X.; Wang, X.; Ning, X. Bimetallic NiCu catalyst derived from spent MOF adsorbent for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2024, 497, 154701. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Ma, S.; Zhang, Y.; Wang, L.; Zhu, G.; Huang, W.; Wang, S. Induced dipole moments in amorphous ZnCdS catalysts facilitate photocatalytic H2 evolution. Nat. Commun. 2024, 15, 2600. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J.; Yu, J.C. A Hollow Porous CdS Photocatalyst. Adv. Mater. 2018, 30, e1804368. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.M.; Wang, L. Hollow Nanostructures for Photocatalysis: Advantages and Challenges. Adv. Mater. 2018, 31, e1801369. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, J.; Leng, J.; Yin, Z.; Yin, Y.; Zhang, F.; Sun, C.; Jin, S. Long-Lived Internal Charge-Separated State in Two-Dimensional Metal–Organic Frameworks Improving Photocatalytic Performance. ACS Energy Lett. 2022, 7, 2323–2330. [Google Scholar] [CrossRef]
- Zhan, T.; Zou, Y.; Yang, Y.; Ma, X.; Zhang, Z.; Xiang, S. Two-dimensional Metal-organic Frameworks for Electrochemical CO2Reduction Reaction. ChemCatChem 2021, 14, e202101453. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Shi, S.; Hao, W.; Yuan, C.; Lu, Z.; Teng, F. Synthesis and photocatalytic activity of Cu2O hollow nanospheres/TiO2 nanosheets by an in-situ water-bath method. J. Alloys Compd. 2022, 899, 163252. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Zhou, T.; Zhang, T. Controllable construction of multishelled p-type cuprous oxide with enhanced formaldehyde sensing. J. Colloid. Interface Sci. 2019, 535, 58–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Chen, S.; Wang, H.; Huo, P. Fabricating Ag/CN/ZnIn2S4 S-Scheme Heterojunctions with Plasmonic Effect for Enhanced Light-Driven Photocatalytic CO2 Reduction. Acta Phys. Chim. Sin. 2023, 39, 2211051. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Liu, G.; Liu, Y.; Zhang, S.; Wang, L.; Zheng, X.; Zhou, L.; Gao, J.; Shi, J.; et al. Hollow Rh-COF@COF S-Scheme Heterojunction for Photocatalytic Nicotinamide Cofactor Regeneration. ACS Catal. 2023, 13, 6619–6629. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Dai, M.-D.; Yang, X.-F.; Yin, H.-J.; Zhang, Y.-W. Copper(II)-Doped Two-Dimensional Titanium-Based Metal–Organic Frameworks toward Light-Driven CO2Reduction to Value-Added Products. Inorg. Chem. 2022, 61, 13981–13991. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Yan, Y.; Huo, P. Charge separation and transfer activated by covalent bond in UiO-66-NH2/RGO heterostructure for CO2 photoreduction. Chem. Eng. J. 2022, 437, 135210. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Yin, H. Construction of A NiS/g-C3N4 Co-Catalyst-Based S-Scheme Heterojunction and Its Performance in Photocatalytic CO2 Reduction. Catalysts 2025, 15, 599. https://doi.org/10.3390/catal15060599
Zhao Q, Yin H. Construction of A NiS/g-C3N4 Co-Catalyst-Based S-Scheme Heterojunction and Its Performance in Photocatalytic CO2 Reduction. Catalysts. 2025; 15(6):599. https://doi.org/10.3390/catal15060599
Chicago/Turabian StyleZhao, Qianyu, and Hengbo Yin. 2025. "Construction of A NiS/g-C3N4 Co-Catalyst-Based S-Scheme Heterojunction and Its Performance in Photocatalytic CO2 Reduction" Catalysts 15, no. 6: 599. https://doi.org/10.3390/catal15060599
APA StyleZhao, Q., & Yin, H. (2025). Construction of A NiS/g-C3N4 Co-Catalyst-Based S-Scheme Heterojunction and Its Performance in Photocatalytic CO2 Reduction. Catalysts, 15(6), 599. https://doi.org/10.3390/catal15060599




