Advanced Biocatalytic Processes for the Conversion of Renewable Feedstocks into High-Value Oleochemicals
Abstract
1. Introduction
2. Biocatalysis in Oleochemistry: Current Status and Progress
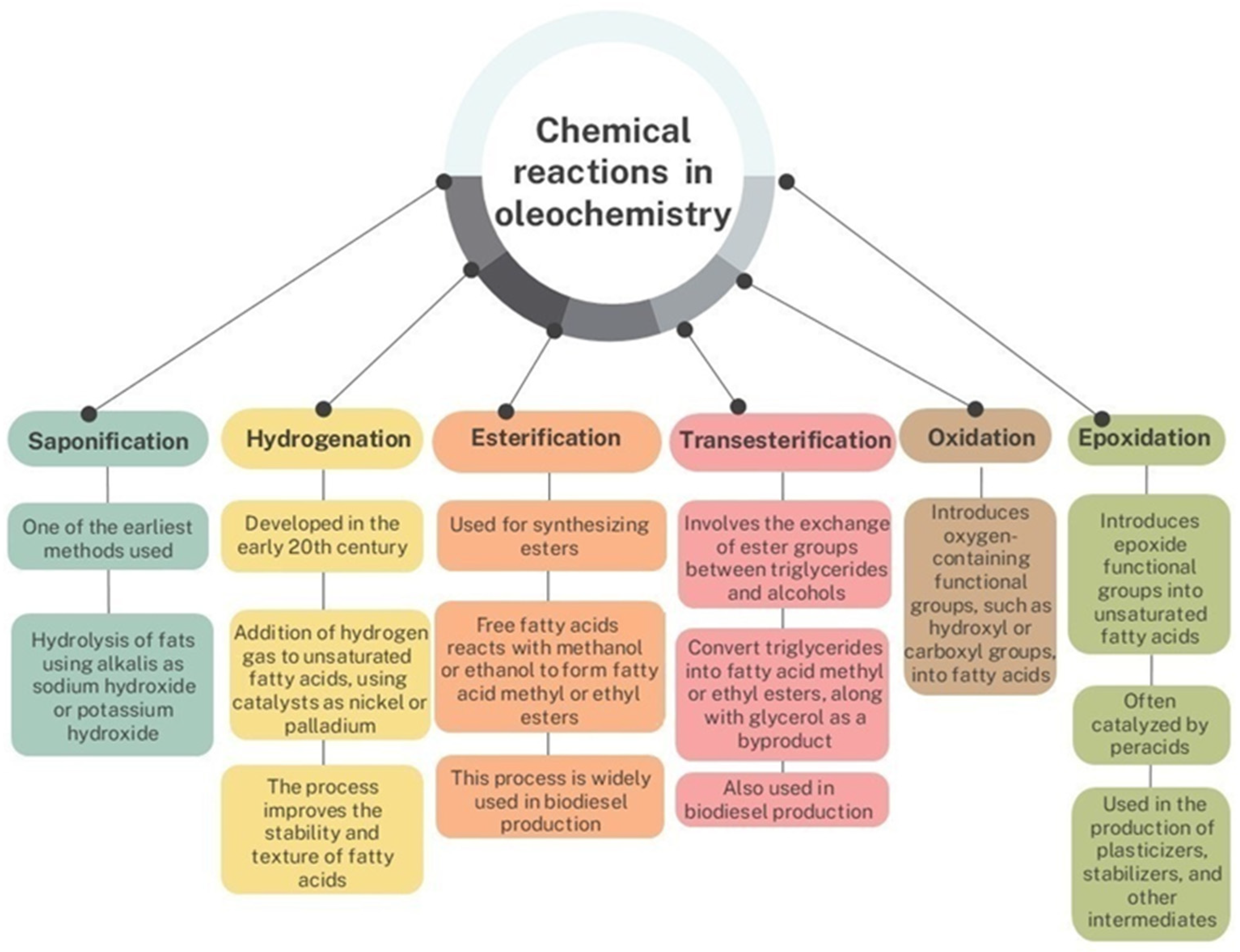
2.1. Oleochemical Synthesis and Chemical Modification of Fatty Acids: An Overview
2.2. Recent Innovations and Chemical Mechanisms of Biocatalysis in Oleochemistry
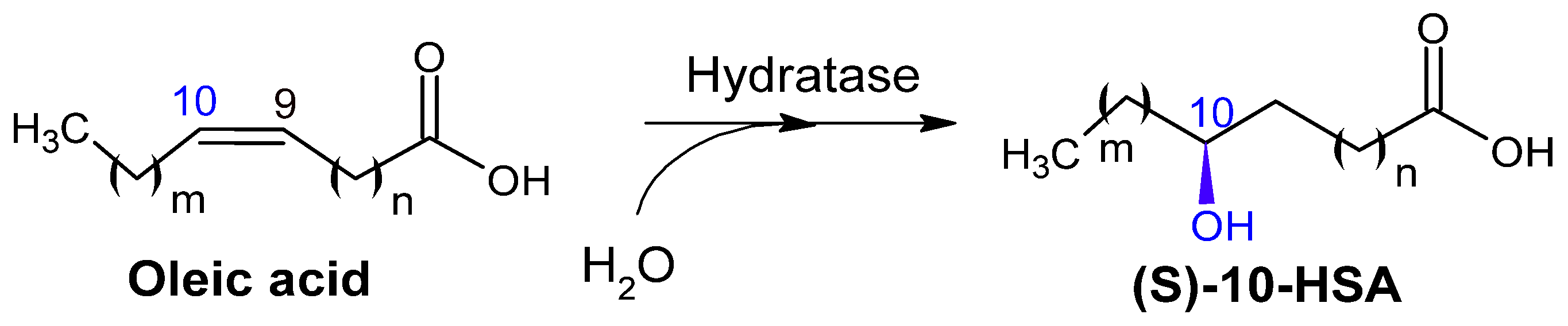
2.2.1. Hydratases
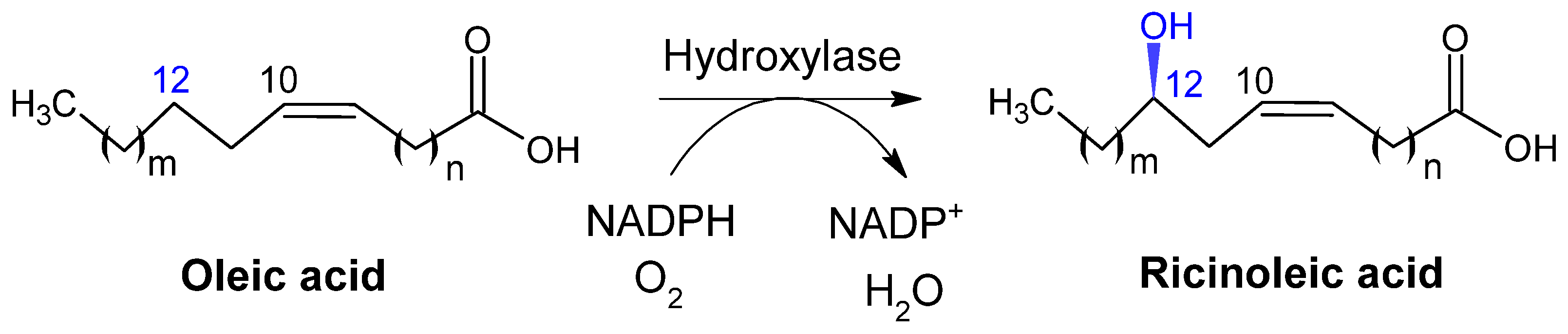
2.2.2. Hydroxylases
2.2.3. Hydroperoxidases
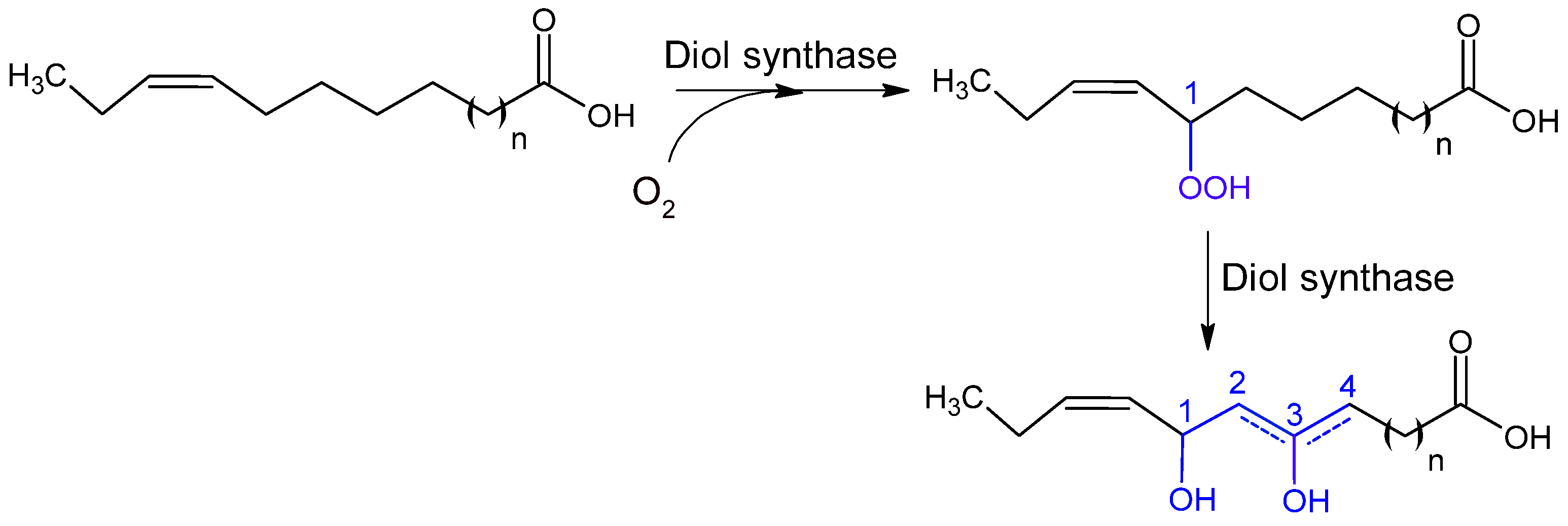
2.2.4. Lipoxygenases
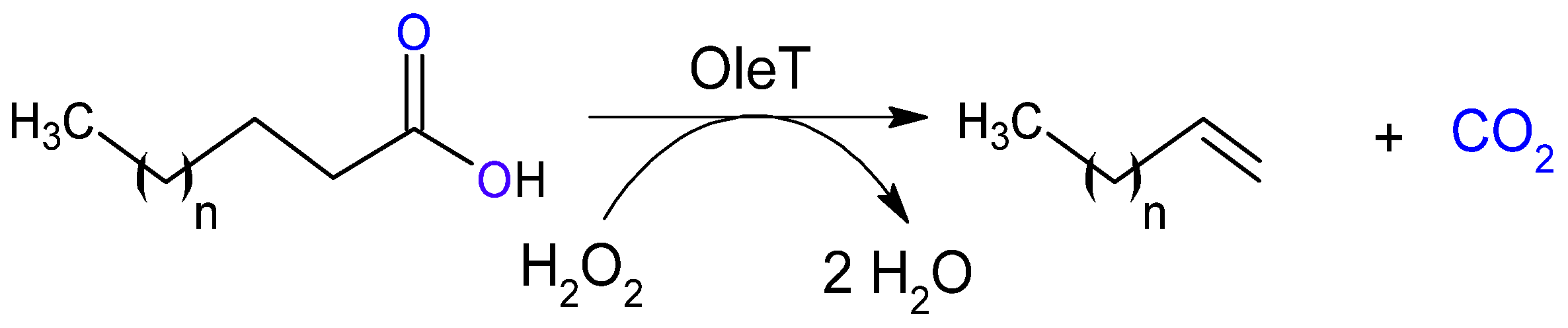
2.2.5. Decarboxylases
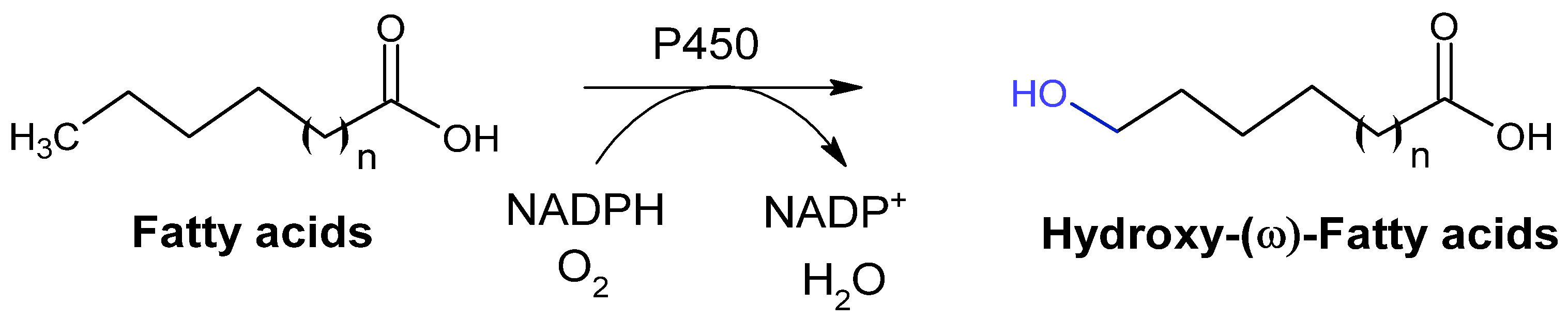
2.2.6. P450 Monooxygenases
3. Environmental Benefits of Biocatalysts in Oleochemistry Compared to Chemical Catalysts
3.1. Reduction of Carbon Dioxide Emissions
3.2. Minimization of Toxic By-Products
3.3. Improved Biodegradability and Compatibility with Green Solvents
4. Market and Economic Considerations
4.1. General Arguments
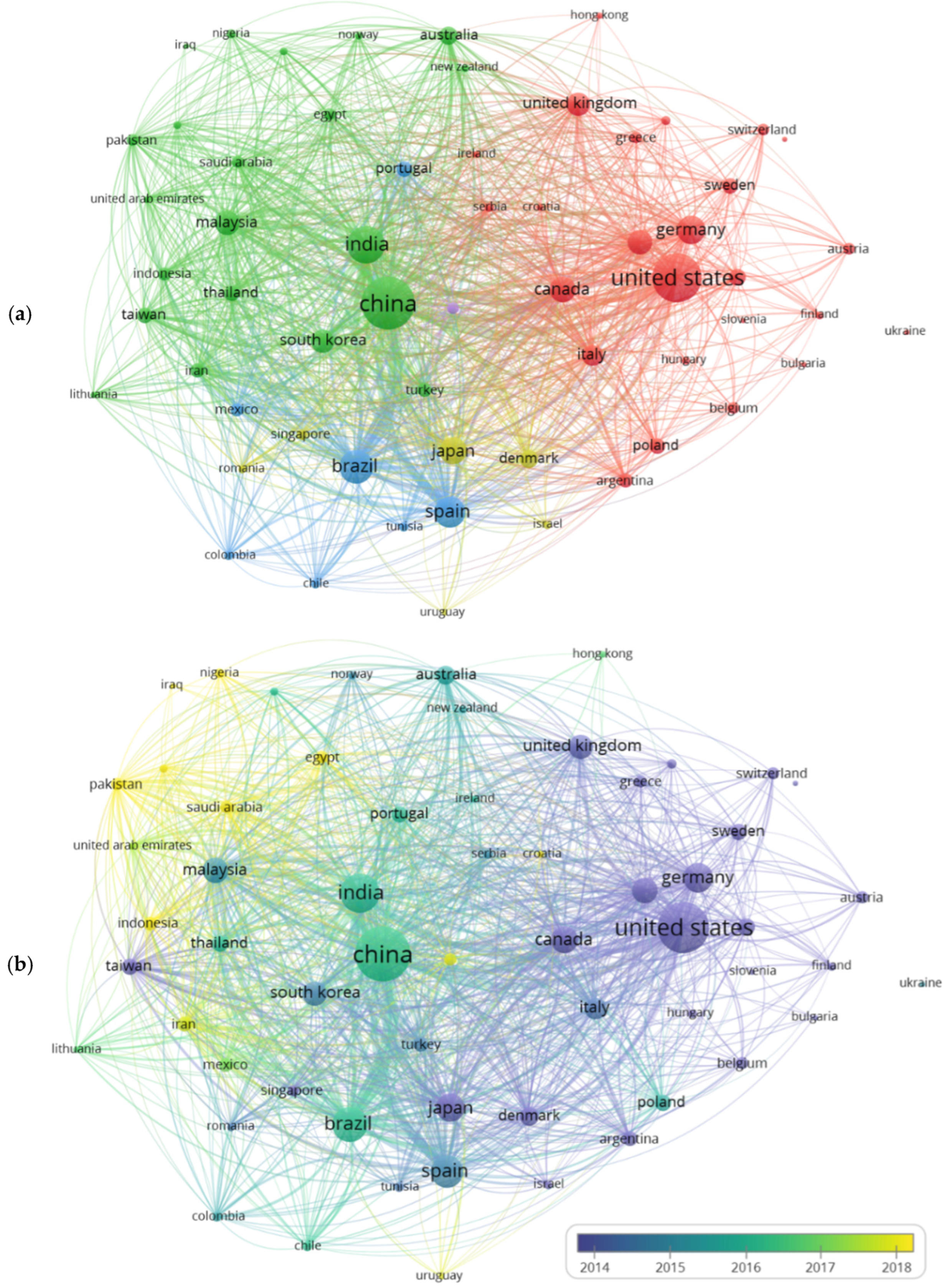
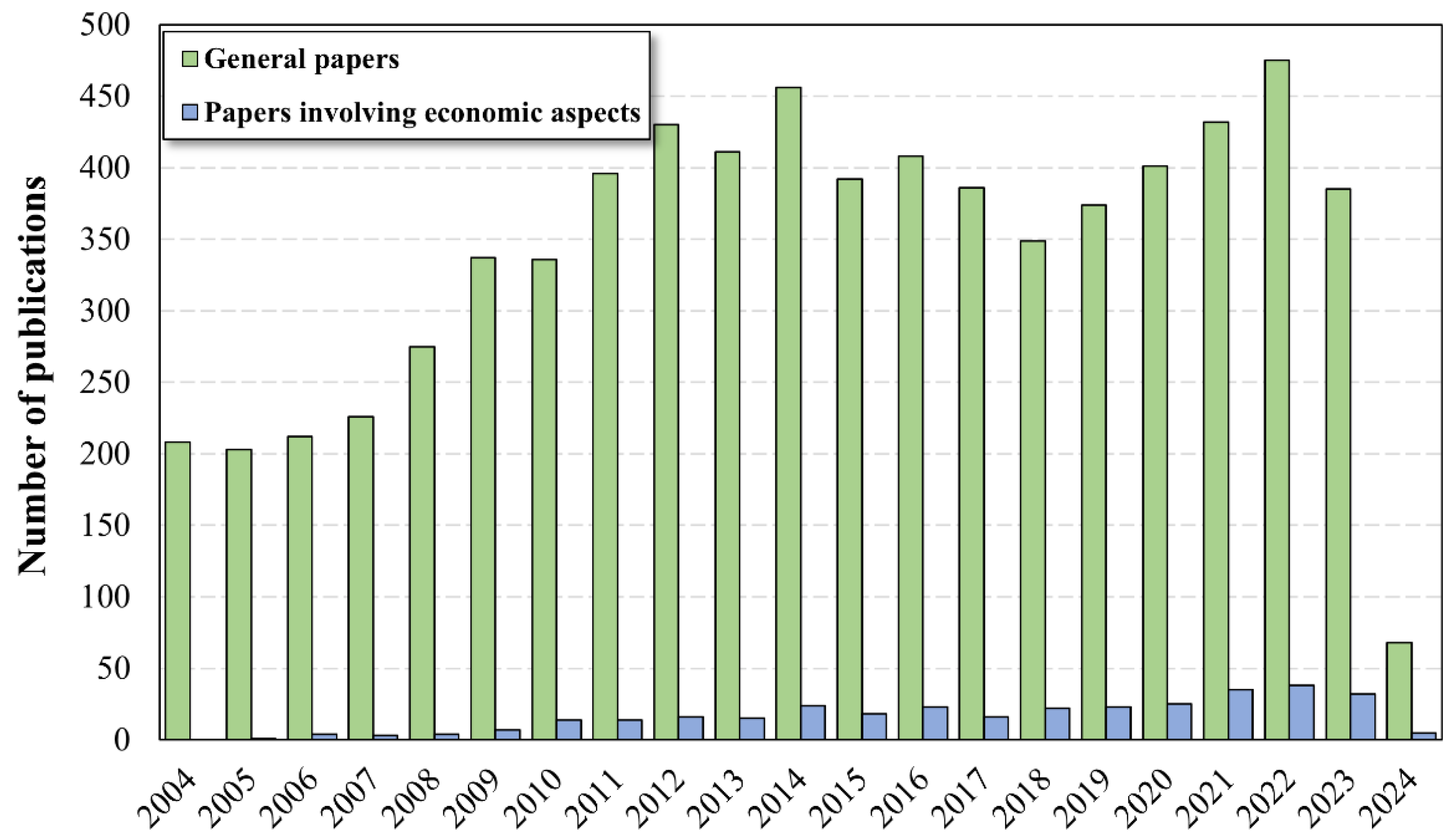
4.2. Bibliometric Evaluation
5. Limitations, Challenges and Final Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marella, E.R.; Holkenbrink, C.; Siewers, V.; Borodina, I. Engineering Microbial Fatty Acid Metabolism for Biofuels and Biochemicals. Curr. Opin. Biotechnol. 2018, 50, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nielsen, J.; Liu, Z. Yeast Based Biorefineries for Oleochemical Production. Curr. Opin. Biotechnol. 2021, 67, 26–34. [Google Scholar] [CrossRef]
- Yan, Q.; Pfleger, B.F. Revisiting Metabolic Engineering Strategies for Microbial Synthesis of Oleochemicals. Metab. Eng. 2020, 58, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; van Dyk, S.; Saddler, J. Repurposing Oil Refineries to “Stand-Alone Units” That Refine Lipids/Oleochemicals to Produce Low-Carbon Intensive, Drop-in Biofuels. J. Clean. Prod. 2022, 376, 134335. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Brondani, M.; dos Santos, M.S.N.; Oro, C.E.D.; Wancura, G.C.; Tres, M.V.; Oliveira, J.V. Demystifying the Enzymatic Biodiesel: How Lipases Are Contributing to Its Technological Advances. Renew. Energy 2023, 216, 119085. [Google Scholar] [CrossRef]
- Awogbemi, O.; Desai, D.A. Application of computational technologies for transesterification of waste cooking oil into biodiesel. Biomass Bioenergy 2025, 194, 107620. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Tres, M.V.; Jahn, S.L.; Oliveira, J.V. Lipases in Liquid Formulation for Biodiesel Production: Current Status and Challenges. Biotechnol. Appl. Biochem. 2020, 67, 648–667. [Google Scholar] [CrossRef]
- Steinke, G.; Cavali, M.; Wancura, J.H.C.; Magro, J.D.; Priamo, W.L.; Mibielli, G.M.; Bender, J.P.; de Oliveira, J.V. Lipase and Phospholipase Combination for Biodiesel Production from Crude Soybean Oil. BioEnergy Res. 2022, 15, 1555–1567. [Google Scholar] [CrossRef]
- Santolin, L.; Fiametti, K.G.; da Silva Lobo, V.; Wancura, J.H.C.; Oliveira, J.V. Enzymatic Synthesis of Eugenyl Acetate from Essential Oil of Clove Using Lipases in Liquid Formulation as Biocatalyst. Appl. Biochem. Biotechnol. 2021, 193, 3512–3527. [Google Scholar] [CrossRef]
- Finco, G.F.; Fiametti, K.G.; Lobo, V.d.S.; da Silva, E.A.; Palú, F.; Wancura, J.H.C.; Rodrigues, M.L.F.; Valério, A.; de Oliveira, J.V. Kinetic Study of Liquid Lipase-catalyzed Glycerolysis of Olive Oil Using Lipozyme. J. Am. Oil Chem. Soc. 2022, 99, 559–568. [Google Scholar] [CrossRef]
- Hosney, H.; Al-Sakkari, E.G.; Mustafa, A.; Ashour, I.; Mustafa, I.; El-Shibiny, A. A Cleaner Enzymatic Approach for Producing Non-Phthalate Plasticiser to Replace Toxic-Based Phthalates. Clean Technol. Environ. Policy 2020, 22, 73–89. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Rosset, D.V.; Tres, M.V.; Oliveira, J.V.; Mazutti, M.A.; Jahn, S.L. Production of Biodiesel Catalyzed by Lipase from Thermomyces lanuginosus in Its Soluble Form. Can. J. Chem. Eng. 2018, 96, 2361–2368. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Fantinel, A.L.; Ugalde, G.A.; Donato, F.F.; de Oliveira, J.V.; Tres, M.V.; Jahn, S.L. Semi-Continuous Production of Biodiesel on Pilot Scale via Enzymatic Hydroesterification of Waste Material: Process and Economics Considerations. J. Clean. Prod. 2021, 285, 124838. [Google Scholar] [CrossRef]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the Time Finally Come for Green Oleochemicals and Biodiesel Production Using Large-Scale Enzyme Technologies? Current Status and New Developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Seidensticker, T. The Basics of Oleochemistry—Basic Oleochemicals. In Chemistry of Renewables; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–60. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.D. FATS and OILS: Formulating and Processing for Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780203483664. [Google Scholar]
- Richardson, A.S.; Conley, C. Process of Decomposing Fats or Oils into Fatty Acids and Glycerin. U.S. Patent 601603A, 29 March 1898. [Google Scholar]
- Lascaray, L. Industrial Fat Splitting. J. Am. Oil Chem. Soc. 1952, 29, 362–366. [Google Scholar] [CrossRef]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Cerqueira Pinto, M.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A. Development of Microbial Oil Wax-Based Oleogel with Potential Application in Food Formulations. Food Bioprocess Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Schmid, U.; Bornscheuer, U.T.; Soumanou, M.M.; McNeill, G.P.; Schmid, R.D. Highly Selective Synthesis of 1,3-Oleoyl-2-Palmitoylglycerol by Lipase Catalysis. Biotechnol. Bioeng. 1999, 64, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, A.; Haraldsson, G.G. Fatty Acid Selectivity of Microbial Lipase and Lipolytic Enzymes from Salmonid Fish Intestines toward Astaxanthin Diesters. J. Am. Oil Chem. Soc. 2004, 81, 347–353. [Google Scholar] [CrossRef]
- Röttig, A.; Wenning, L.; Bröker, D.; Steinbüchel, A. Fatty Acid Alkyl Esters: Perspectives for Production of Alternative Biofuels. Appl. Microbiol. Biotechnol. 2010, 85, 1713–1733. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Bornscheuer, U.T.; Bednarski, W. The Application of Biotechnological Methods for the Synthesis of Biodiesel. Eur. J. Lipid Sci. Technol. 2009, 111, 800–813. [Google Scholar] [CrossRef]
- Jackisch, B.O.; Simmler-Hübenthal, H.; Zschau, W.; Bornscheuer, U.; Durban, M.; Riemer, C. Use of a Thermostable Phospholipase in the Degumming of an Oil or Fat, and a Method for Obtaining a Thermostable Phospholipase. European Patent Application No. EP1788080A, 23 May 2007. pp. 1–56. [Google Scholar]
- Sein, A.; Hitchman, T.; Dayton, L.G. Enzymes in vegetable oil degumming processes. In Industrial Enzyme Applications; Wiley-VHC: Weinheim, Germany, 2019; Chapter 4.2; pp. 323–350. [Google Scholar] [CrossRef]
- Daniel, H.; Otto, R.T.; Binder, M.; Syldatk, C. Production of Sophorolipids from Whey-Development of a Two-Stage Process with Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214. Appl. Microbiol. Biotechnol. 1999, 51, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Fatty Acid Biosynthesis in Microorganisms Being Used for Single Cell Oil Production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Sakuradani, E.; Ando, A.; Ogawa, J.; Shimizu, S. Improved Production of Various Polyunsaturated Fatty Acids through Filamentous Fungus Mortierella alpina Breeding. Appl. Microbiol. Biotechnol. 2009, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Felse, P.A.; Shah, V.; Chan, J.; Rao, K.J.; Gross, R.A. Sophorolipid Biosynthesis by Candida bombicola from Industrial Fatty Acid Residues. Enzym. Microb. Technol. 2007, 40, 316–323. [Google Scholar] [CrossRef]
- Ramani, K.; Boopathy, R.; Vidya, C.; Kennedy, L.J.; Velan, M.; Sekaran, G. Immobilisation of Pseudomonas gessardii Acidic Lipase Derived from Beef Tallow onto Mesoporous Activated Carbon and Its Application on Hydrolysis of Olive Oil. Process Biochem. 2010, 45, 986–992. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Enzymes in Lipid Modification: An Overview. In Lipid Modification by Enzymes and Engineered Microbes; Academic Press: Cambridge, MA, USA, 2018; pp. 1–9. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; van Heuvel, M.; Misset, O. Bacterial Lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [CrossRef]
- Javed, S.; Azeem, F.; Hussain, S.; Rasul, I.; Siddique, M.H.; Riaz, M.; Afzal, M.; Kouser, A.; Nadeem, H. Bacterial Lipases: A Review on Purification and Characterization. Prog. Biophys. Mol. Biol. 2018, 132, 23–34. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial Enzymes with Attractive Applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Caicedo-Paz, A.V.; Mussagy, C.U.; Mesa, V.; Junior, R.H.M.; Valenzuela, R.; de Paula, A.V.; Martinez-Galan, J.P. Enzymatic synthesis of structured lipids from sacha inchi (Plukenetia volubilis) oil with capric acid via acidolysis reaction in stirred tank and packed bed mini reactors. Food Biosci. 2024, 58, 103769. [Google Scholar] [CrossRef]
- Caicedo-Paz, A.V.; Mediavilla, M.; Farías, C.; Valenzuela, R.; Mussagy, C.U.; Martinez-Galan, J.P. Sustainable one-pot solvent-free enzymatic synthesis of capric acid-rich structured lipids to enhance the nutritional value of grape seed oil. Process Biochem. 2024, 144, 160–167. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Hamilton, R.J. Oleochemical Manufacture and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2001; Volume 325. [Google Scholar]
- Gamayurova, V.S.; Zinov’Eva, M.E.; Tran, H.T.T. Features of the Enzymatic Hydrolysis of Castor Oil. Catal. Ind. 2013, 5, 269–273. [Google Scholar] [CrossRef]
- Mensah, M.B.; Awudza, J.A.M.; O’Brien, P. Castor Oil: A Suitable Green Source of Capping Agent for Nanoparticle Syntheses and Facile Surface Functionalization. R. Soc. Open Sci. 2018, 5, 180824. [Google Scholar] [CrossRef] [PubMed]
- Nitbani, F.O.; Tjitda, P.J.P.; Wogo, H.E.; Detha, A.I.R. Preparation of Ricinoleic Acid from Castor Oil: A Review. J. Oleo Sci. 2022, 71, 781–793. [Google Scholar] [CrossRef]
- Rathod, V.K.; Pandit, A.B. Effect of Various Additives on Enzymatic Hydrolysis of Castor Oil. Biochem. Eng. J. 2009, 47, 93–99. [Google Scholar] [CrossRef]
- Broun, P.; Shanklin, J.; Whittle, E.; Somerville, C. Catalytic Plasticity of Fatty Acid Modification Enzymes Underlying Chemical Diversity of Plant Lipids. Science 1998, 282, 1315–1317. [Google Scholar] [CrossRef]
- Thakkar, K.; Kachhwaha, S.S.; Kodgire, P. Multi-Response Optimization of Transesterification Reaction for Biodiesel Production from Castor Oil Assisted by Hydrodynamic Cavitation. Fuel 2022, 308, 121907. [Google Scholar] [CrossRef]
- Metzger, J.O.; Bornscheuer, U. Lipids as Renewable Resources: Current State of Chemical and Biotechnological Conversion and Diversification. Appl. Microbiol. Biotechnol. 2006, 71, 13–22. [Google Scholar] [CrossRef]
- Goh, E.B.; Baidoo, E.E.K.; Keasling, J.D.; Beller, H.R. Engineering of Bacterial Methyl Ketone Synthesis for Biofuels. Appl. Environ. Microbiol. 2012, 78, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Heshof, R.; de Graaff, L.H.; Villaverde, J.J.; Silvestre, A.J.D.; Haarmann, T.; Dalsgaard, T.K.; Buchert, J. Industrial Potential of Lipoxygenases. Crit. Rev. Biotechnol. 2016, 36, 665–674. [Google Scholar] [CrossRef]
- Rincón, L.A.; Ramírez, J.C.; Orjuela, A. Assessment of Degumming and Bleaching Processes for Used Cooking Oils Upgrading into Oleochemical Feedstocks. J. Environ. Chem. Eng. 2021, 9, 21–23. [Google Scholar] [CrossRef]
- Le Borgne, S.; Quintero, R. Biotechnological Processes for the Refining of Petroleum. Fuel Process. Technol. 2003, 81, 155–169. [Google Scholar] [CrossRef]
- Robles-Medina, A.; Gonzalez-Moreno, P.A.; Esteban-Cerdan, L.; Molina-Grima, E. Biocatalysis: Towards Ever Greener Biodiesel Production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef]
- Royce, L.A.; Yoon, J.M.; Chen, Y.; Rickenbach, E.; Shanks, J.V.; Jarboe, L.R. Evolution for Exogenous Octanoic Acid Tolerance Improves Carboxylic Acid Production and Membrane Integrity. Metab. Eng. 2015, 29, 180–188. [Google Scholar] [CrossRef] [PubMed]
- di Bitonto, L.; Todisco, S.; Gallo, V.; Pastore, C. Urban sewage scum and primary sludge as profitable sources of biodiesel and biolubricants of new generation. Bioresour. Technol. Rep. 2020, 9, 100382. [Google Scholar] [CrossRef]
- di Bitonto, L.; Angelini, A.; Pastore, C. Energy Recovery from Municipal Sewage Sludge: An Environmentally Friendly Source for the Production of Biochemicals. Appl. Sci. 2024, 14, 4974. [Google Scholar] [CrossRef]
- Jo, Y.S.; An, J.U.; Oh, D.K. γ-Dodecelactone production from safflower oil via 10-hydroxy-12(Z)-octadecenoic acid intermediate by whole cells of Candida boidinii and Stenotrophomonas nitritireducens. J. Agric. Food Chem. 2014, 62, 6736–6745. [Google Scholar] [CrossRef]
- An, J.U.; Joo, Y.C.; Oh, D.K. New biotransformation process for production of the fragrant compound γ-dodecalactone from 10-hydroxystearate by permeabilized Waltomyces lipofer cells. Appl. Environ. Microbiol. 2013, 79, 2636–2641. [Google Scholar] [CrossRef]
- Wallen, L.; Benedict, R.; Jackson, R. The microbiological production of 10-hydroxystearic acid from oleic acid. Arch. Biochem. Biophys. 1962, 99, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bevers, L.E.; Pinkse, M.W.; Verhaert, P.D.; Hagen, W.R. Oleate hydratase catalyzes the hydration of a nonactivated carbon-carbon bond. J. Bacteriol. 2009, 191, 5010–5012. [Google Scholar] [CrossRef] [PubMed]
- Rosberg-Cody, E.; Liavonchanka, A.; Gobel, C.; Ross, R.P.; O’Sullivan, O.; Fitzgerald, G.F.; Stanton, C. Myosin-cross-reactive antigen (MCRA) protein from Bifidobacterium breve is a FAD-dependent fatty acid hydratase which has a function in stress protection. BMC Biochem. 2011, 12, 9. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Song, Y.; Chen, Y.Q.; Zhang, H.; Chen, W. Myosin-cross-reactive antigens from four different lactic acid bacteria are fatty acid hydratases. Biotechnol. Lett. 2013, 35, 75–81. [Google Scholar] [CrossRef]
- Volkov, A.; Liavonchanka, A.; Kamneva, O.; Fiedler, T.; Goebel, C.; Kreikemeryer, B.; Feussner, I. Myosin cross-reactive antigen of Streptococcus pyogenes M49 encodes a fatty acid double bond hydratase that plays a role in oleic acid detoxification and bacterial virulence. J. Biol. Chem. 2010, 285, 10353–10361. [Google Scholar] [CrossRef]
- Engleder, M.; Pavkov-Keller, T.; Emmerstorfer, A.; Hromic, A.; Schrempf, S.; Steinkellner, G.; Wriessnegger, T.; Leitner, E.; Strohmeier, G.A.; Kaluzna, I.; et al. Structure-based mechanism of oleate hydratase from Elizabethkingia meningoseptica. ChemBioChem 2015, 16, 1730–1734. [Google Scholar] [CrossRef]
- Kang, W.R.; Seo, M.J.; Shin, K.C.; Park, J.B.; Oh, D.K. Comparison of biochemical properties of the original and newly identified oleate hydratases from Stenotrophomonas maltophilia. Appl. Environ. Microbiol. 2017, 83, e03351-16. [Google Scholar] [CrossRef]
- Kim, B.N.; Joo, Y.C.; Kim, Y.S.; Kim, K.R.; Oh, D.K. Production of 10-hydroxystearic acid from oleic acid and olive oil hydrolyzate by an oleate hydratase from Lysinibacillus fusiformis. Appl. Microbiol. Biotechnol. 2012, 95, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.Y.; Lee, J.H.; Yang, K.M.; Joo, Y.C.; Oh, D.K.; Park, J.B. Bioprocess engineering to produce 10-hydroxystearic acid from oleic acid by recombinant Escherichia coli expressing the oleate hydratase gene of Stenotrophomonas maltophilia. Proc. Biochem. 2012, 47, 941–947. [Google Scholar] [CrossRef]
- Kim, K.R.; Oh, H.J.; Park, C.S.; Hong, S.H.; Park, J.Y.; Oh, D.K. Unveiling of novel regio-selective fatty acid double bond hydratases from Lactobacillus acidophilus involved in the selective oxyfunctionalization of mono- and di-hydroxy fatty acids. Biotechnol. Bioeng. 2015, 112, 2206–2213. [Google Scholar] [CrossRef]
- Song, J.W.; Lee, J.H.; Bornscheuer, U.T.; Park, J.B. Microbial synthesis of medium chain α,ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long chain fatty acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Hirata, A.; Kishino, S.; Park, S.B.; Takeuchi, M.; Kitamura, N.; Ogawa, J. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J. Lipid Res. 2015, 56, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Kishino, S.; Park, S.B.; Hirata, A.; Kitamura, N.; Saika, A.; Ogawa, J. Efficient enzymatic production of hydroxy fatty acids by linoleic acid 9 hydratase from Lactobacillus plantarum AKU 1009a. J. Appl. Microbiol. 2016, 120, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Broadwater, J.A.; Whittle, E.; Shanklin, J. Desaturation and hydroxylation. J. Biol. Chem. 2002, 227, 15613–15620. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Bayer, T.; Terholsen, H.; Bornscheuer, U. α-Dioxygenases (α-DOXs): Promising Biocatalysts for the Environmentally Friendly Production of Aroma Compounds. ChemBioChem 2022, 23, 202100693. [Google Scholar] [CrossRef]
- Kaehne, F.; Buchhaupt, M.; Schrader, J. A Recombinant α-Dioxygenase from Rice to Produce Fatty Aldehydes Using E. coli. Appl. Microbiol. Biotechnol. 2011, 90, 989–995. [Google Scholar] [CrossRef]
- Maurer, S.; Schewe, H.; Schrader, J.; Buchhaupt, M. Investigation of Fatty Aldehyde and Alcohol Synthesis from Fatty Acids by αDox- or CAR-Expressing Escherichia coli. J. Biotechnol. 2019, 305, 11–17. [Google Scholar] [CrossRef]
- Otte, K.B.; Kittelberger, J.; Kirtz, M.; Nestl, B.M.; Hauer, B. Whole-Cell One-Pot Biosynthesis of Azelaic Acid. ChemCatChem 2014, 6, 1003–1009. [Google Scholar] [CrossRef]
- Coenen, A.; Ferrer, M.; Jaeger, K.E.; Schörken, U. Synthesis of 12-Aminododecenoic Acid by Coupling Transaminase to Oxylipin Pathway Enzymes. Appl. Microbiol. Biotechnol. 2023, 107, 2209–2221. [Google Scholar] [CrossRef]
- Qi, Y.K.; Pan, J.; Zhang, Z.J.; Xu, J.H. Whole-Cell One-Pot Biosynthesis of Dodecanedioic Acid from Renewable Linoleic Acid. Bioresour. Bioprocess. 2023, 11, 55. [Google Scholar] [CrossRef]
- Seo, M.J.; Shin, K.C.; An, J.U.; Kang, W.R.; Ko, Y.J.; Oh, D.K. Characterization of a Recombinant 7,8-Linoleate Diol Synthase from Glomerella cingulate. Appl. Microbiol. Biotechnol. 2016, 100, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Seo, M.J.; Kang, S.H.; Oh, D.K. Production of 8,11-Dihydroxy Fatty Acids from Oleic and Palmitoleic Acids by Escherichia Coli Cells Expressing Variant 6,8-Linoleate Diol Synthases from Penicillium oxalicum. Biotechnol. Prog. 2022, 38, e3267. [Google Scholar] [CrossRef]
- Seo, M.J.; Shin, K.C.; Oh, D.K. Production of 5,8-Dihydroxy-9,12(Z,Z)-Octadecadienoic Acid From Linoleic Acid by Whole Recombinant Escherichia coli Cells Expressing Diol Synthase From Aspergillus nidulans. Appl. Microbiol. Biotechnol. 2014, 98, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Feussner, I. Lipoxygenases: Structure and reaction mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef]
- de Roos, A.L.; van Dijk, A.A.; Folkertsma, B. Bleaching of Dairy Products. U.S. Patent 20060127533 A1, 15 May 2006. [Google Scholar]
- Palmieri-Thiers, C.; Alberti, J.C.; Canaan, S.; Brunini, V.; Gambotti, C.; Tomi, F.; Oliw, E.H.; Berti, L.; Maury, J. Identification of putative residues involved in the accessibility of the substrate-binding site of lipoxygenase by site-directed mutagenesis studies. Arch. Biochem. Biophys. 2011, 509, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zhang, C.; Bie, X.; Zhao, H.; Diao, H.; Lu, F.; Lu, Z. Improving the thermostability and activity of lipoxygenase from Anabaena sp. PCC7120 by directed evolution and site-directed mutagenesis. J. Mol. Catal. B 2014, 107, 23–30. [Google Scholar] [CrossRef]
- Coffa, G.; Schneider, C.; Brash, A.R. A comprehensive model of positional and stereo control in lipoxygenases. Biochem. Biophys. Res. Commun. 2005, 338, 87–92. [Google Scholar] [CrossRef]
- Coffa, G.; Brash, A.R. A single active site residue directs oxygenation stereospecificity in lipoxygenases: Stereocontrol is linked to the position of oxygenation. Proc. Natl. Acad. Sci. USA 2004, 101, 15579–15584. [Google Scholar] [CrossRef]
- Villaverde, J.J.; van der Vlist, V.; Santos, S.A.O.; Haarmann, T.; Langfelder, K.; Pirttimaa, M.; Nyyssölä, A.; Jylhä, S.; Tamminen, T.; Kruus, K.; et al. Hydroperoxide production from linoleic acid by heterologous Gaeumannomyces graminis tritici lipoxygenase: Optimization and scaleup. Chem. Eng. J. 2013, 217, 82–90. [Google Scholar] [CrossRef]
- Bruhlmann, F.; Bosijokovic, B.; Ullmann, C.; Auffray, P.; Fourage, L.; Wahler, D. Directed evolution of a 13-hydroperoxide lyase (CYP74B) for improved process performance. J. Biotechnol. 2013, 163, 339–345. [Google Scholar] [CrossRef]
- Bruhlmann, F.; Bosijokovic, B. Efficient biochemical cascade for accessing green leaf alcohols. Org. Process Res. Dev. 2016, 20, 1974–1978. [Google Scholar] [CrossRef]
- Rude, M.A.; Baron, T.S.; Brubaker, S.; Alibhai, M.; Del Cardayre, S.B.; Schirmer, A. Terminal olefin (1-alkene) biosynthesis by a novel P450 fatty acid decarboxylase from Jeotgalicoccus species. Appl. Environ. Microbiol. 2011, 77, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, M.; Yan, W.; Bai, W.J.; Wang, X. Enzymatic regio-and enantioselective C–H oxyfunctionalization of fatty acids. ACS Catal. 2021, 11, 10625–10630. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Wang, C.; Zhou, Y.J.; Xu, H.; Li, S. Biochemical characterization of three new α-olefin-producing P450 fatty acid decarboxylases with a halophilic property. Biotechnol. Biofuels 2019, 12, 79. [Google Scholar] [CrossRef]
- Lee, J.W.; Niraula, N.P.; Trinh, C.T. Harnessing a P450 fatty acid decarboxylase from Macrococcus caseolyticus for microbial biosynthesis of odd chain terminal alkenes. Metab. Eng. Commun. 2018, 7, e00076. [Google Scholar] [CrossRef]
- Xu, H.; Ning, L.; Yang, W.; Fang, B.; Wang, C.; Wang, Y.; Xu, J.; Collin, S.; Laurent, F.; Li, S. In vitro oxidative decarboxylation of free fatty acids to terminal alkenes by two new P450 peroxygenases. Biotechnol. Biofuels 2017, 10, 208. [Google Scholar] [CrossRef]
- Iqbal, T.; Murugan, S.; Das, D. A chimeric membrane enzyme and an engineered whole-cell biocatalyst for efficient 1-alkene production. Sci. Adv. 2024, 10, eadl2492. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Murugan, S.; Rajendran, K.; Sidhu, J.S.; Das, D. Unraveling the conversion of fatty acids into terminal alkenes by an integral membrane enzyme, UndB. ACS Catal. 2023, 13, 15516–15525. [Google Scholar] [CrossRef]
- Manley, O.M.; Fan, R.; Guo, Y.; Makris, T.M. Oxidative decarboxylase UndA utilizes a dinuclear iron cofactor. J. Am. Chem. Soc. 2019, 141, 8684–8688. [Google Scholar] [CrossRef]
- Rui, Z.; Harris, N.C.; Zhu, X.; Huang, W.; Zhang, W. Discovery of a family of desaturase-like enzymes for 1-alkene biosynthesis. ACS Catal. 2015, 5, 7091–7094. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Zheng, S.; Xu, H.; Zhou, Y.J.; Gao, Z.; Meng, C.; Li, S. Establishing an enzyme cascade for one-pot production of α-olefins from low-cost triglycerides and oils without exogenous H2O2 addition. Biotechnol. Biofuels 2020, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.; Tee, K.L.; Rattray, N.J.; McLean, K.J.; Leys, D.; Parker, D.A.; Blankley, R.T.; Munro, A.W. Production of alkenes and novel secondary products by P450 Ole TJE using novel H2O2-generating fusion protein systems. FEBS Lett. 2017, 591, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Sorigué, D.; Légeret, B.; Cuiné, S.; Blangy, S.; Moulin, S.; Billon, E.; Beisson, F. An algal photoenzyme converts fatty acids to hydrocarbons. Science 2017, 357, 903–907. [Google Scholar] [CrossRef]
- Sorigué, D.; Légeret, B.; Cuiné, S.; Morales, P.; Mirabella, B.; Guédeney, G.; Beisson, F. Microalgae synthesize hydrocarbons from long-chain fatty acids via a light-dependent pathway. Plant Physiol. 2016, 171, 2393–2405. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Wu, B.; Zhou, J.; Yang, S.; Zhang, F.; Luo, K.; Wang, Y.; Hollmann, F. Photobiocatalysis: More than just an interesting lab curiosity? Chem. Catal. 2024, 4, 101077. [Google Scholar] [CrossRef]
- Li, D.; Han, T.; Xue, J.; Xu, W.; Xu, J.; Wu, Q. Engineering fatty acid photodecarboxylase to enable highly selective decarboxylation of trans fatty acids. Angew. Chem. 2021, 133, 20863–20867. [Google Scholar] [CrossRef]
- Bernhardt, R.; Urlacher, V.B. Cytochrome P450s as promising catalysts for biotechnological application: Chances and limitations. Appl. Microbiol. Biotechnol. 2014, 98, 6185–6203. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases: An update on perspectives for synthetic application. Trends Biotechnol. 2012, 30, 26–36. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.; Groeneboer, S.; Saerens, K.; Soetaert, W. The role of cytochrome P450 monooxygenases in microbial fatty acid metabolism. FEBS J. 2011, 278, 206–221. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schafer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef]
- Munro, A.W.; Leys, D.G.; McLean, K.J.; Marshall, K.R.; Ost, T.; Daff, S.; Dutton, P.L. P450 BM3: The very model of a modern flavocytochrome. Trends Biochem. Sci. 2002, 27, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.A.; Yoshikuni, Y.; Fisher, K.J.; Woolard, F.X.; Ockey, D.; McPhee, D.J.; Keasling, J.D. A novel semi-biosynthetic route for artemisinin production using engineered substrate-promiscuous P450BM3. ACS Chem. Biol. 2009, 4, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Vomund, S.; Grohmann, K.; Kriening, S.; Urlacher, V.B. Rational design of a minimal and highly enriched CYP102A1 mutant library with improved regio-, stereo- and chemoselectivity. ChemBioChem 2009, 10, 853–861. [Google Scholar] [CrossRef]
- Bruhlmann, F.; Fourage, L.; Ullmann, C.; Haefliger, O.P.; Jeckelmann, N. Engineering cytochrome P450 BM3 of Bacillus megaterium for terminal oxidation of palmitic acid. J. Biotechnol. 2014, 184, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Khatri, Y.; Hannemann, F.; Girhard, M.; Kappl, R.; Hutter, M.; Jeckelmann, N.; Dubois, C.; Wahler, D. A natural heme-signature variant of CYP267A1 from Sorangium cellulosum So ce56 executes diverse ω-hydroxylation. FEBS J. 2015, 282, 74–88. [Google Scholar] [CrossRef]
- Durairaj, P.; Malla, S.; Nadarajan, S.P.; Lee, P.G.; Jung, E. Fungal cytochrome P450 monooxygenases of Fusarium oxysporum for the synthesis of ω-hydroxy fatty acids in engineered Saccharomyces cerevisiae. Microb. Cell Fact. 2015, 14, 45. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Transesterification of vegetable oil to biodiesel fuel using alkaline catalyst. Fuel 2011, 90, 42–47. [Google Scholar] [CrossRef]
- Tubino, M.; Junior, J.G.R.; Bauerfeldt, G.F. Biodiesel synthesis: A study of the triglyceride methanolysis reaction with alkaline catalysts. Catal. Comm. 2016, 75, 6–12. [Google Scholar] [CrossRef]
- Canakci, M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef]
- Nurhayati, N.; Anita, S.; Amri, T.A.; Linggawati, A. Esterification of Crude Palm Oil Using H2SO4 and Transesterification Using CaO Catalyst Derived from Anadara granosa. Indones. J. Chem. 2017, 17, 309–315. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. Intensified one-step biodiesel production from high water and free fatty acid waste cooking oils. Fuel 2018, 220, 567–574. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Lopez, A.T.; Rodriguez, J.A. State of art of alkaline earth metal oxides catalysts used in the transesterification of oils for biodiesel production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Haigh, K.F.; Saha, B. Esterification of free fatty acids in used cooking oil using ion-exchange resins as catalysts: An efficient pretreatment method for biodiesel feedstock. Ind. Eng. Chem. Res. 2012, 51, 14653–14664. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, S62–S65. [Google Scholar] [CrossRef]
- Özbay, N.; Oktar, N.; Tapan, N. Esterification of free fatty acids in waste cooking oils (WCO): Role of ion-exchange resins. Fuel 2008, 87, 1789–1798. [Google Scholar] [CrossRef]
- D’Souza, R.; Vats, T.; Chattree, A.; Siril, P.F. Graphene supported magnetically separable solid acid catalyst for the single step conversion of waste cooking oil to biodiesel. Renew. Energy 2018, 126, 1064–1073. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Aziz, A.A. Activity of solid acid catalysts for biodiesel production: A critical review. Appl. Catal. A Gen. 2014, 470, 140–161. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Wang, A.; Quan, W.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-Containing Catalysts for Efficient Biodiesel Production. RSC Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef]
- Nie, Z.; Jiang, P.; Zhang, P.; Liu, D.; Haryono, A.; Zhao, M.; Zhao, H. Zirconia-Coated Magnetic Fe2O3 Nanoparticles Supported 12-Tungstophosphoric Acid: A Novel Environmentally Friendly Catalyst for Biodiesel Production. J. Chin. Chem. Soc. 2021, 68, 837–848. [Google Scholar] [CrossRef]
- Okechukwu, O.D.; Joseph, E.; Nonso, U.C.; Kenechi, N.-O. Improving Heterogeneous Catalysis for Biodiesel Production Process. Clean. Chem. Eng. 2022, 3, 100038. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.M. Lipase and Its Unique Selectivity: A Mini-Review. J. Chem. 2022, 1, 7609019. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and Prospects as An Industrial Biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
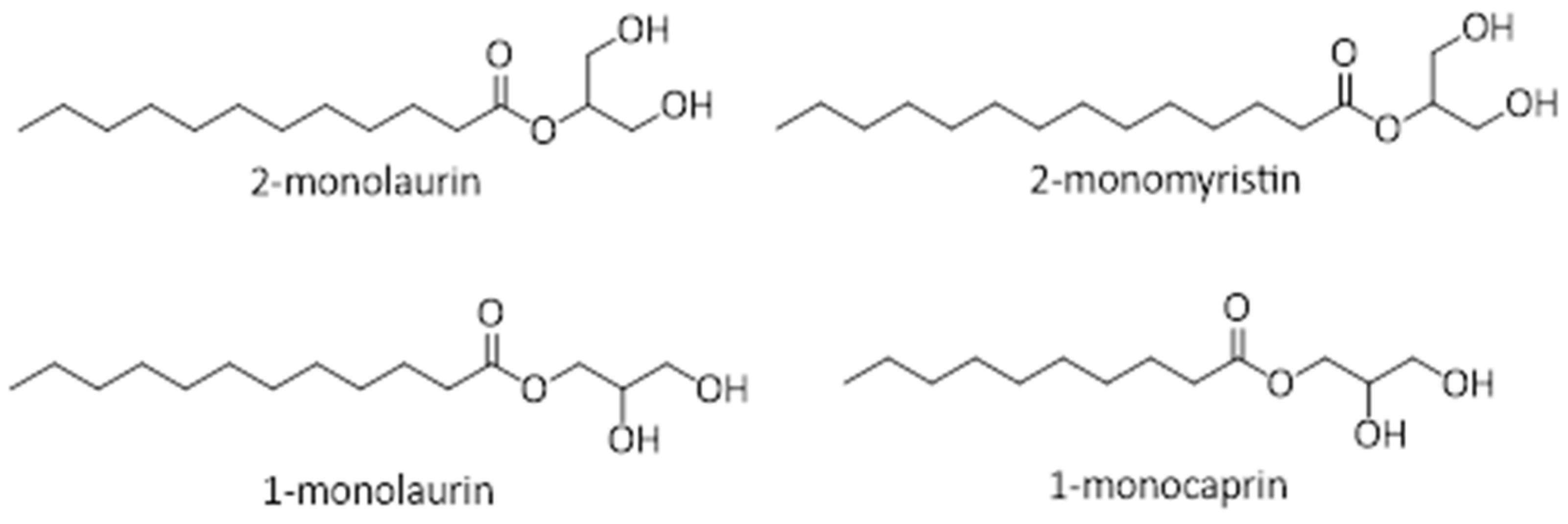
- Nitbani, F.O.; Jumina, J.; Tjitda, P.J.P.; Wogo, H.E.; Detha, A.I.; Nurohmah, B.A. Synthesis of 2-Monolaurin from Pure Lauric Acid. In Proceedings of the AIP Conference Proceedings, Yoyakarta, Indonesia, 30 September 2020. [Google Scholar]
- Jumina; Nurmala, A.; Fitria, A.; Pranowo, D.; Sholikhah, E.N.; Kurniawan, Y.S.; Kuswandi, B. Monomyristin and Monopalmitin Derivatives: Synthesis and Evaluation as Potential Antibacterial and Antifungal Agents. Molecules 2018, 23, 3141. [Google Scholar] [CrossRef]
- Nitbani, F.; Angwarmasse, L.; Bessy, E.; Wogo, H.E.; Detha, A.I.; Tjitda, P.J. Improved Synthesis of α-Glycerol Monolaurate Using Lipozyme TL IM. J. Oleo Sci. 2022, 71, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Nitbani, F.O.; Tjitda, P.J.P.; Detha, A.I.R.; Nitti, F. Modification of A-Glycerol Mono Caprate Synthesis from Capric Acid. Rasayan J. Chem. 2023, 16, 549–556. [Google Scholar] [CrossRef]
- Karmee, S.K. Moving towards the Application of Biocatalysis in Food Waste Biorefinery. Fermentation 2023, 9, 73. [Google Scholar] [CrossRef]
- Lee, S.Y.; Khoiroh, I.; Vo, D.V.N.; Senthil Kumar, P.; Show, P.L. Techniques of Lipid Extraction from Microalgae for Biofuel Production: A Review. Environ. Chem. Lett. 2020, 19, 231–251. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. Lipase as a Green and Sustainable Material for Production of Levulinate Compounds: State of the Art. Mater. Sci. Energy Technol. 2022, 5, 232–242. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase Catalysis in Organic Solvents: Advantages and Applications. Biol. Proced. Online 2016, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Domínguez de María, P. Biocatalysis, Sustainability, and Industrial Applications: Show Me the Metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Satyawali, Y.; Cauwenberghs, L.; Maesen, M.; Dejonghe, W. Lipase Catalyzed Solvent Free Synthesis of Monoacylglycerols in Various Reaction Systems and Coupling Reaction with Pervaporation for in situ Water Removal. Chem. Eng. Process. Intensif. 2021, 166, 108475. [Google Scholar] [CrossRef]
- Mustafa, A.; Niikura, F. Green Synthesis of Isopropyl Palmitate Using Immobilized Candida antarctica Lipase: Process Optimization Using Response Surface Methodology. Clean. Eng. Technol. 2022, 8, 100516. [Google Scholar] [CrossRef]
- Pasha, M.K.; Rahim, M.; Dai, L.; Liu, D.; Du, W.; Guo, M. Comparative Study of a Two-Step Enzymatic Process and Conventional Chemical Methods for Biodiesel Production: Economic and Environmental Perspectives. Chem. Eng. J. 2024, 489, 151254. [Google Scholar] [CrossRef]
- Hosney, H.; Mustafa, A. Semi-Continuous Production of 2-Ethyl Hexyl Ester in a Packed Bed Reactor: Optimization and Economic Evaluation. J. Oleo Sci. 2020, 69, 31–41. [Google Scholar] [CrossRef]
- Holm, H.C.; Nielsen, P.M.; Longin, F. Chapter 15—Upscaling of Enzymatic Processes. In Lipid Modification by Enzymes and Engineered Microbes; AOCS Press: Urbana, IL, USA, 2018; pp. 343–373. ISBN 9780128131671. [Google Scholar]
- Mustafa, A.; Niikura, F.; Pastore, C.; Allam, H.A.; Hassan, O.B.; Mustafa, M.; Inayat, A.; Salah, S.A.; Salam, A.A.; Mohsen, R. Selective Synthesis of Alpha Monoglycerides by a Clean Method: Techno-Economic and Environmental Assessment. Sustain. Chem. Pharm. 2022, 27, 100690. [Google Scholar] [CrossRef]
- Andrade, T.A.; Martín, M.; Errico, M.; Christensen, K.V. Biodiesel Production Catalyzed by Liquid and Immobilized Enzymes: Optimization and Economic Analysis. Chem. Eng. Res. Des. 2019, 141, 1–14. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Tišma, M.; Sundaram, S.; Hessel, V. Is There a Future for Enzymatic Biodiesel Industrial Production in Microreactors? Appl. Energy 2017, 201, 124–134. [Google Scholar] [CrossRef]
- Razzaghi, M.; Homaei, A.; Vianello, F.; Azad, T.; Sharma, T.; Nadda, A.K.; Stevanato, R.; Bilal, M.; Iqbal, H.M.N. Industrial Applications of Immobilized Nano-Biocatalysts. Bioprocess Biosyst. Eng. 2022, 45, 237–256. [Google Scholar] [CrossRef]
- Melo, R.L.F.; Sales, M.B.; de Castro Bizerra, V.; de Sousa Junior, P.G.; Cavalcante, A.L.G.; Freire, T.M.; Neto, F.S.; Bilal, M.; Jesionowski, T.; Soares, J.M.; et al. Recent Applications and Future Prospects of Magnetic Biocatalysts. Int. J. Biol. Macromol. 2023, 253, 126709. [Google Scholar] [CrossRef]
- Pereira, A.S.; de Souza, A.H.; Fraga, J.L.; Villeneuve, P.; Torres, A.G.; Amaral, P.F.F. Lipases as Effective Green Biocatalysts for Phytosterol Esters’ Production: A Review. Catalysts 2022, 12, 88. [Google Scholar] [CrossRef]
- Lisboa, M.C.; Wiltshire, F.M.S.; Fricks, A.T.; Dariva, C.; Carrière, F.; Lima, Á.S.; Soares, C.M.F. Oleochemistry Potential from Brazil Northeastern Exotic Plants. Biochimie 2020, 178, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, G.d.M.; Carvalho, E.E.N.; Lago, R.C.d.; Silva, L.G.M.d.; Souza, L.R.d.; Costa, C.A.R.d.; Boas, E.V.d.B.V. Compositional Analysis of Baru (Dipteryx alata Vogel) Pulp Highlighting Its Industrial Potential. Food Res. Int. 2025, 201, 115548. [Google Scholar] [CrossRef] [PubMed]
- Sorita, G.D.; Favaro, S.P.; Rodrigues, D.d.S.; Silva, W.P.d., Jr.; Leal, W.G.d.O.; Ambrosi, A.; Di Luccio, M. Aqueous Enzymatic Extraction of Macauba (Acrocomia aculeata) Pulp Oil: A Green and Sustainable Approach for High-Quality Oil Production. Food Res. Int. 2024, 182, 114160. [Google Scholar] [CrossRef] [PubMed]
- Borin, G.P.; Alves, R.F.; Júnior, A.D.N.F. Current Status of Biotechnological Processes in the Biofuel Industries. In Bioprocessing for Biomolecules Production; Wiley: Hoboken, NJ, USA, 2019; pp. 47–69. [Google Scholar]
- Gonçalves, T.d.S.; Oro, C.E.D.; Wancura, J.H.C.; dos Santos, M.S.N.; Junges, A.; Dallago, R.M.; Tres, M.V. Challenges for Energy Guidelines in Crop-Based Liquid Biofuels Development in Brazil. Next Sustain. 2023, 2, 100002. [Google Scholar] [CrossRef]
- Wassell, C.S.; Dittmer, T.P. Are Subsidies for Biodiesel Economically Efficient? Energy Policy 2006, 34, 3993–4001. [Google Scholar] [CrossRef]
- Morone, P.; Cottoni, L.; Giudice, F. Biofuels: Technology, Economics, and Policy Issues. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–92. [Google Scholar]

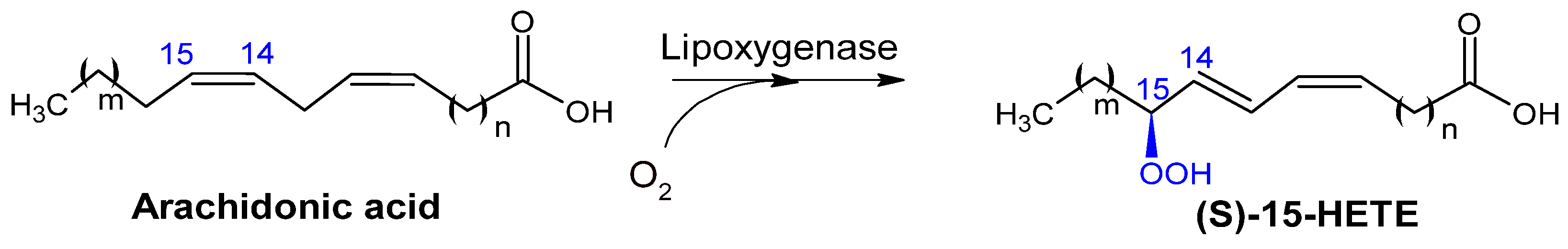




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wancura, J.H.C.; Cipolatti, E.P.; Manoel, E.A.; Nitbani, F.O.; Caicedo-Paz, A.V.; Mussagy, C.U.; Abdellatief, T.M.M.; Mustafa, A.; di Bitonto, L. Advanced Biocatalytic Processes for the Conversion of Renewable Feedstocks into High-Value Oleochemicals. Catalysts 2025, 15, 600. https://doi.org/10.3390/catal15060600
Wancura JHC, Cipolatti EP, Manoel EA, Nitbani FO, Caicedo-Paz AV, Mussagy CU, Abdellatief TMM, Mustafa A, di Bitonto L. Advanced Biocatalytic Processes for the Conversion of Renewable Feedstocks into High-Value Oleochemicals. Catalysts. 2025; 15(6):600. https://doi.org/10.3390/catal15060600
Chicago/Turabian StyleWancura, João H. C., Eliane Pereira Cipolatti, Evelin Andrade Manoel, Febri Odel Nitbani, Angie Vanessa Caicedo-Paz, Cassamo Ussemane Mussagy, Tamer M. M. Abdellatief, Ahmad Mustafa, and Luigi di Bitonto. 2025. "Advanced Biocatalytic Processes for the Conversion of Renewable Feedstocks into High-Value Oleochemicals" Catalysts 15, no. 6: 600. https://doi.org/10.3390/catal15060600
APA StyleWancura, J. H. C., Cipolatti, E. P., Manoel, E. A., Nitbani, F. O., Caicedo-Paz, A. V., Mussagy, C. U., Abdellatief, T. M. M., Mustafa, A., & di Bitonto, L. (2025). Advanced Biocatalytic Processes for the Conversion of Renewable Feedstocks into High-Value Oleochemicals. Catalysts, 15(6), 600. https://doi.org/10.3390/catal15060600










