Enhanced Peroxydisulfate Activation via Fe-Doped BiOBr for Visible-Light Photocatalytic Degradation of Paracetamol
Abstract
1. Introduction
2. Results and Discussion
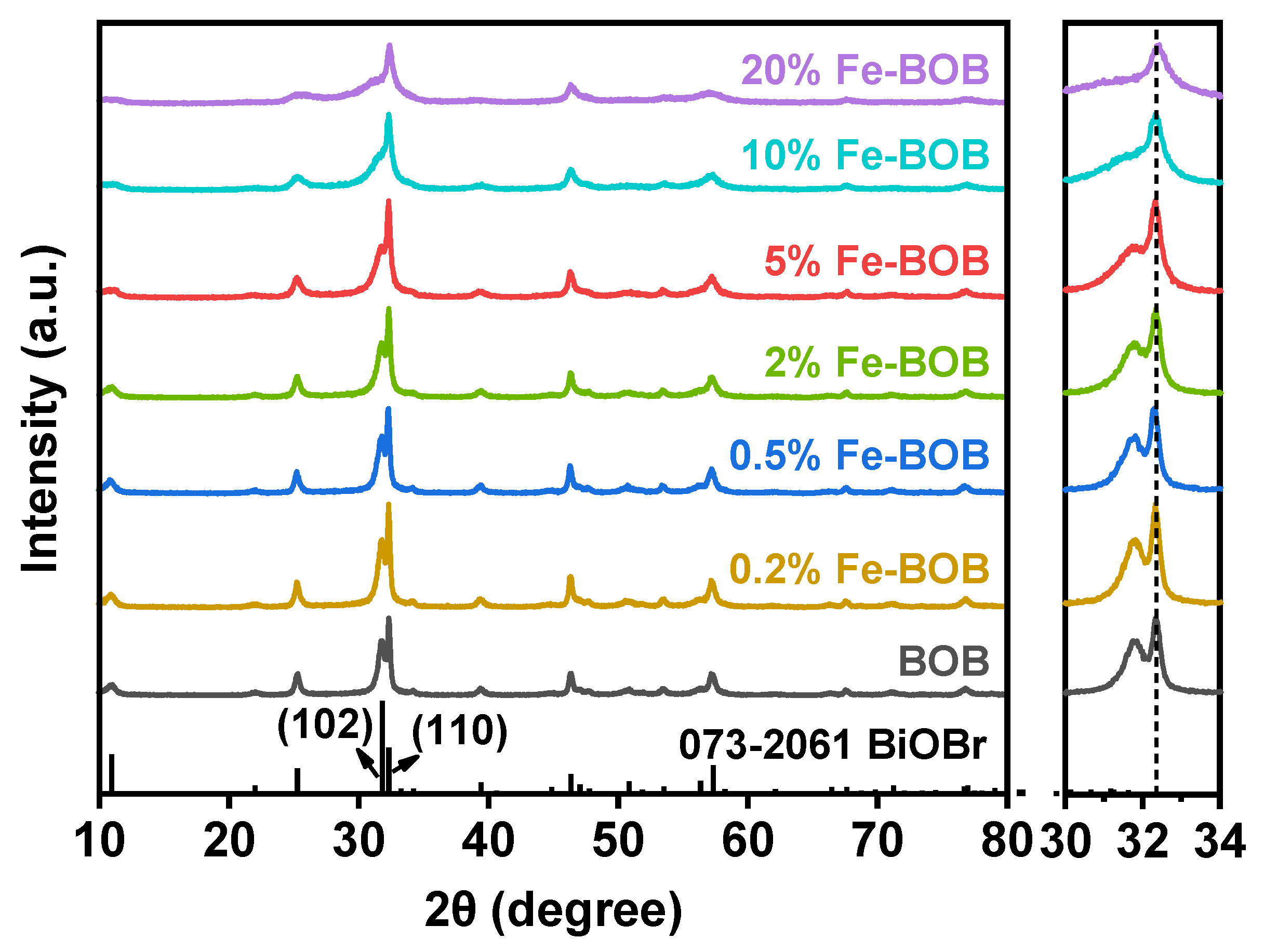
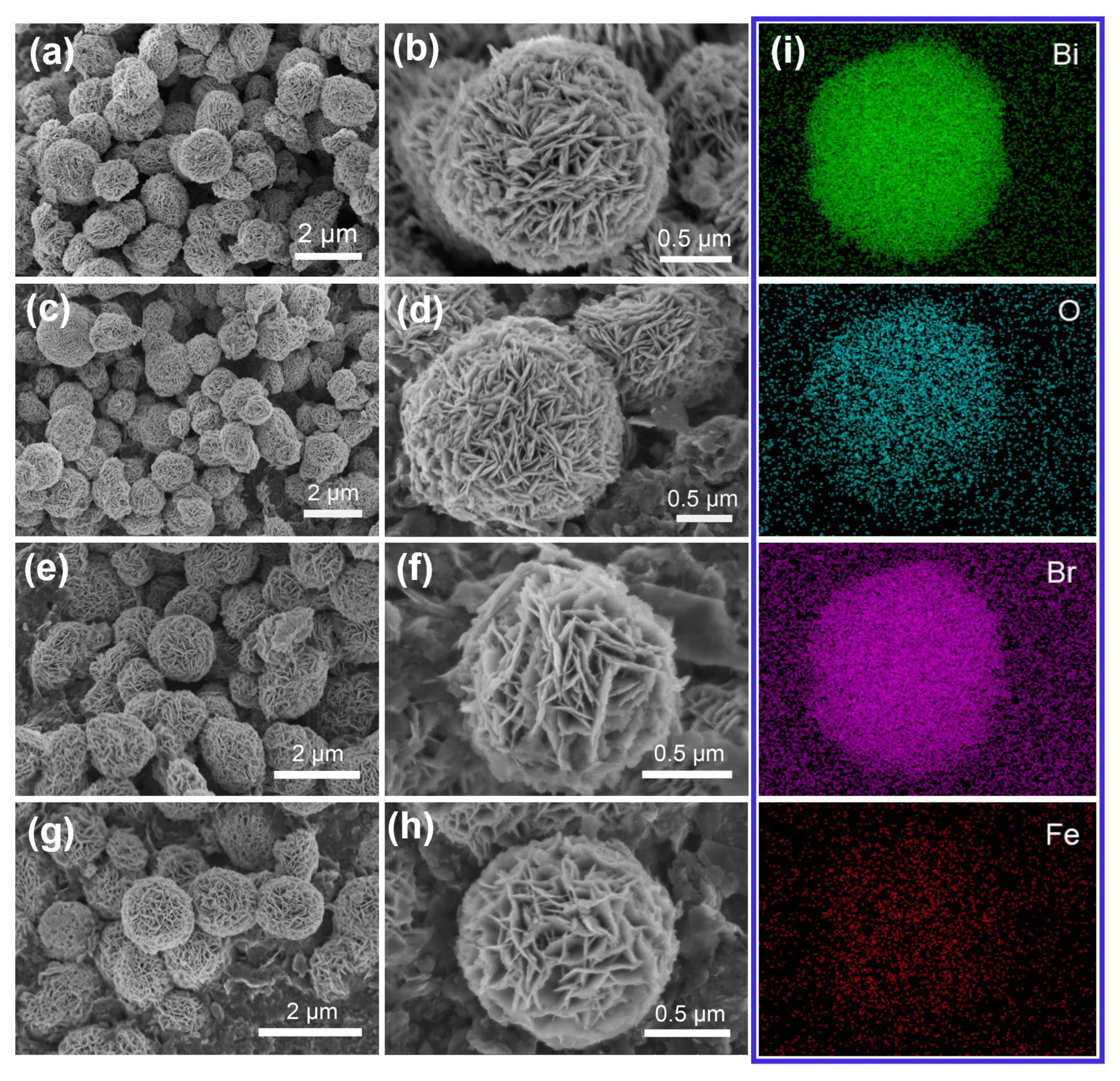
2.1. Characterization of the Fe-BOB Catalysts
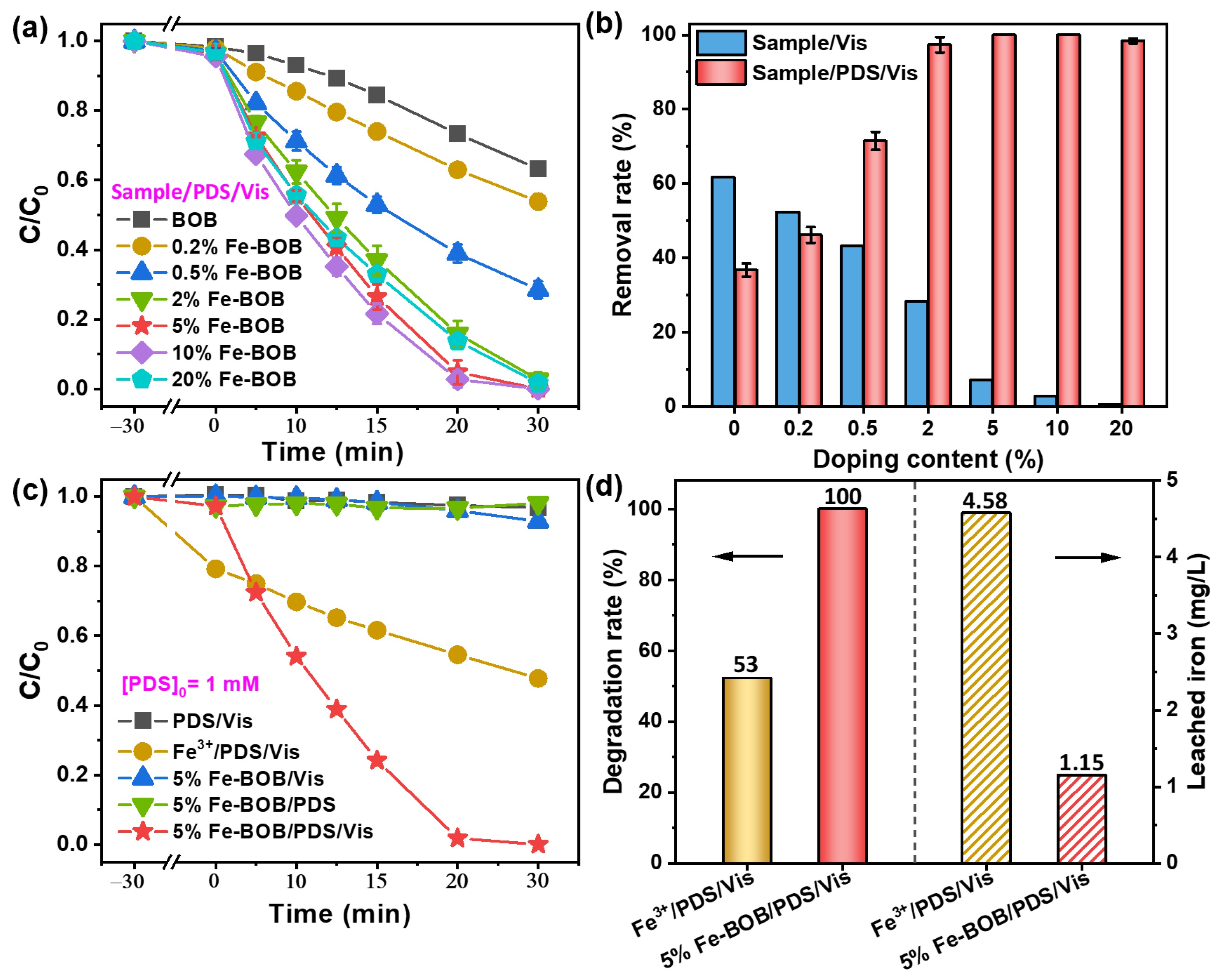
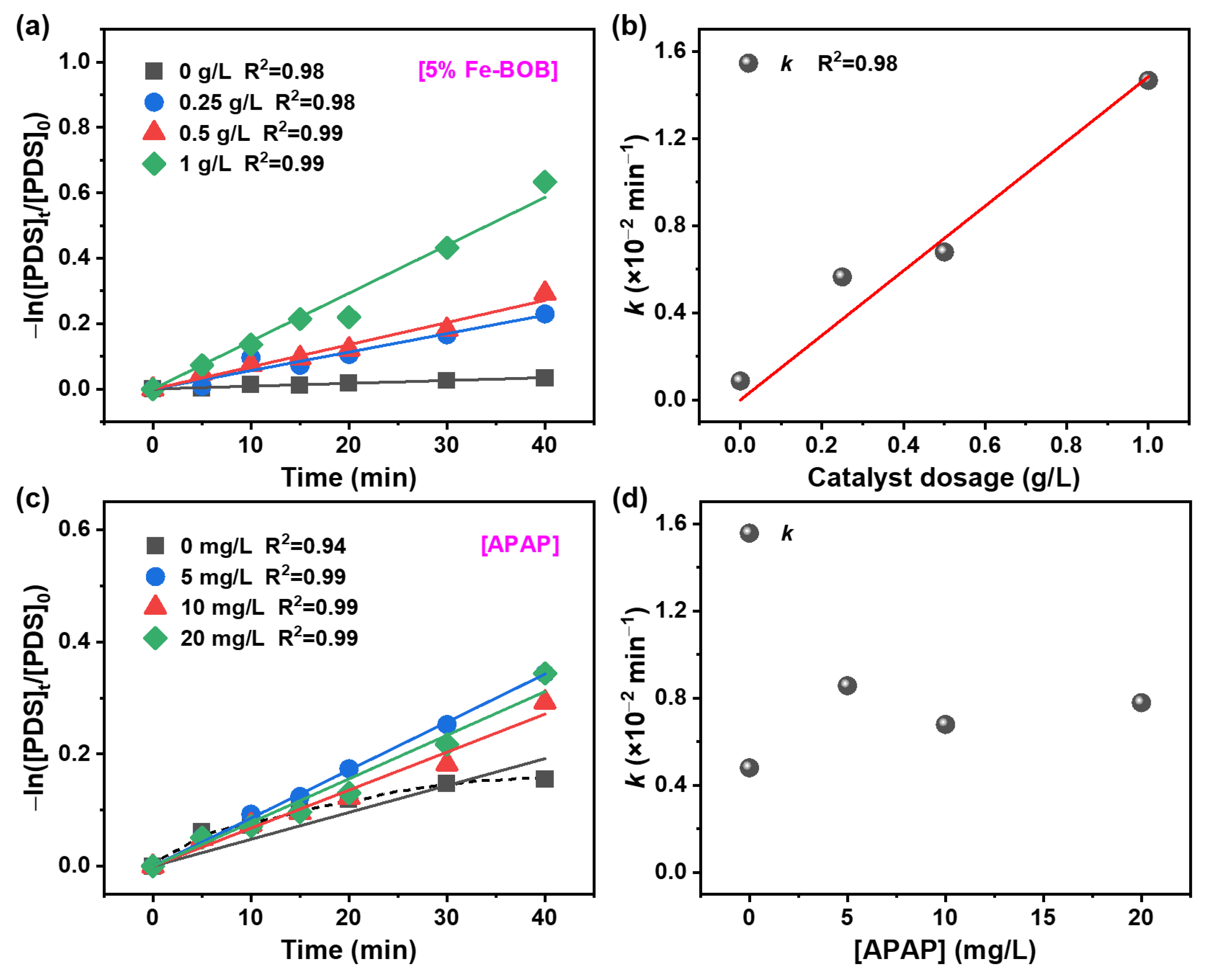
2.2. Evaluation of PDS Activation Performance of Fe-BOB Under Visible Light
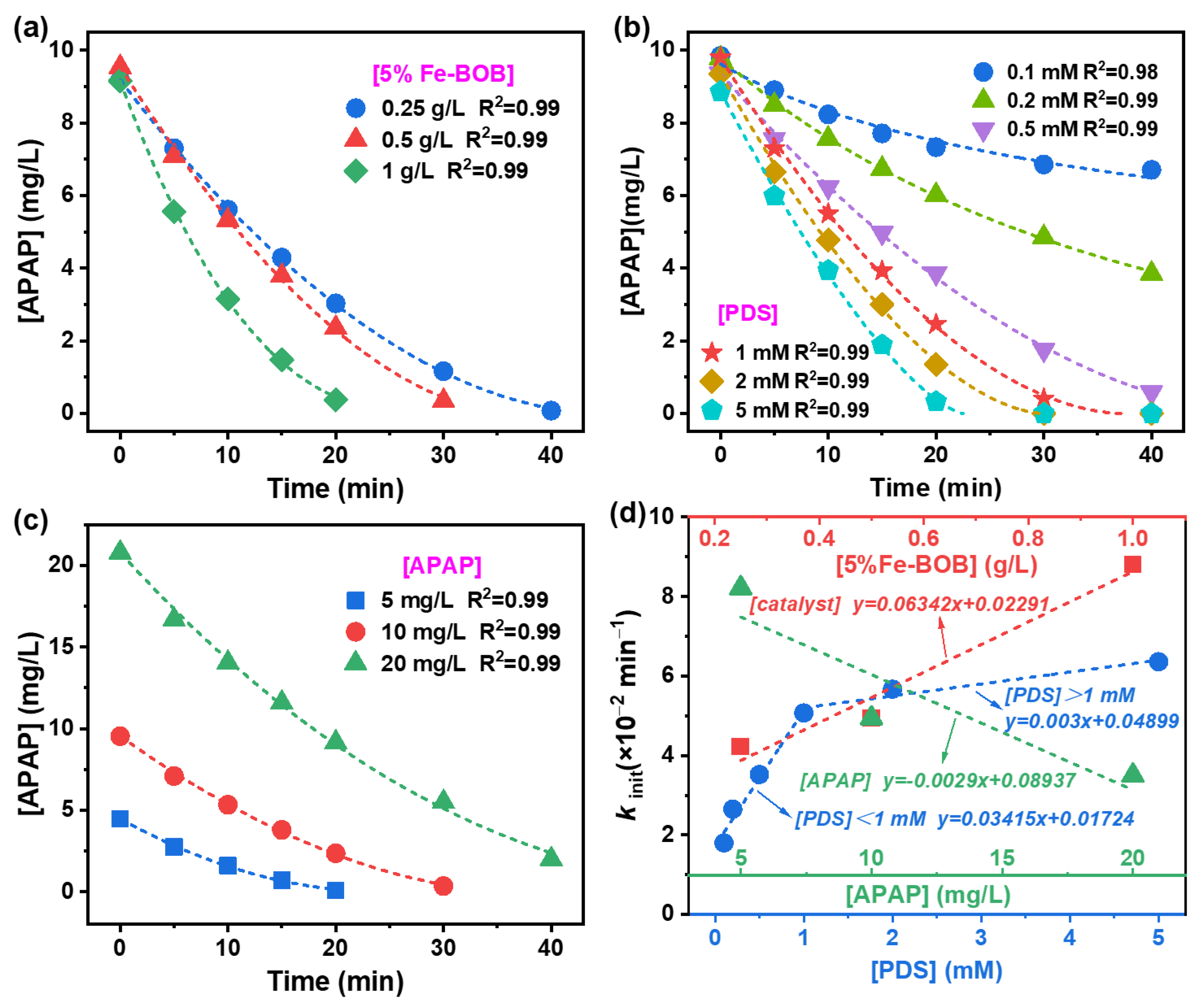
2.3. Degradation Kinetics of APAP over Fe-BOB/PDS
2.4. Identifying and Distributions of Reactive Oxygen Species
2.5. Stability Analysis and Effects of Solution pH on APAP Photodegradation over Fe-BOB/PDS System
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Z.X.; Cheng, X.R.; Kyzas, G.Z.; Fu, J. Pharmaceuticals pollution of aquaculture and its management in China. J. Mol. Liq. 2016, 223, 781–789. [Google Scholar] [CrossRef]
- Peng, Q.C.; Song, J.M.; Li, X.G.; Yuan, H.M.; Liu, M.T.; Duan, L.Q.; Zuo, J.L. Pharmaceutically active compounds (PhACs) in surface sediments of the Jiaozhou Bay, north China. Environ. Pollut. 2020, 266, 115245–115257. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, M. Were persulfate-based advanced oxidation processes really understood? Basic concepts, cognitive biases, and experimental details. Environ. Sci. Technol. 2024, 58, 10415–10444. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.Y.; Wang, J.L.; Cai, B.H.; Lai, B.; Wang, S.B.; Ao, Z.M. Multifunctional roles of MoS2 in persulfate-based advanced oxidation processes for eliminating aqueous organic pollutants: A review. Appl. Catal. B Environ. Energy 2024, 340, 123173. [Google Scholar] [CrossRef]
- Ming, H.B.; Bian, X.Q.; Cheng, J.J.; Yang, C.; Hou, Y.D.; Ding, K.N.; Zhang, J.S.; Anpo, M.; Wang, X.C. Carbon nitride with a tailored electronic structure toward peroxymonosulfate activation: A direct electron transfer mechanism for organic pollutant degradation. Appl. Catal. B Environ. Energy 2024, 341, 123314. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, D.B.; Yang, G.J.; Yuan, X.Z.; Xu, Q.X.; Liu, X.R.; Wang, Y.L.; Guan, R.P.; Fu, Q.Z.; Chen, F. The novel pretreatment of Co2+ activating peroxymonosulfate under acidic condition for dewatering waste activated sludge. J. Taiwan Inst. Chem. E 2019, 102, 259–267. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hasaer, B.; Yang, M.; Liu, R.; Hu, C.Z.; Liu, H.J.; Qu, J.H. Anaerobically-digested sludge disintegration by transition metal ions-activated peroxymonosulfate (PMS): Comparison between Co2+, Cu2+, Fe2+and Mn2+. Sci. Total Environ. 2020, 713, 136530–136540. [Google Scholar] [CrossRef]
- Tang, W.J.; Wang, Z.J.; Guo, S.; Chen, R.; Chen, F.X. Efficient generation of 1O2 by activating peroxymonosulfate on graphitic carbon nanoribbons for water remediation. npj Clean Water 2024, 7, 92. [Google Scholar] [CrossRef]
- Tang, H.; Dai, Z.; Xie, X.D.; Wen, Z.P.; Chen, R. Promotion of peroxydisulfate activation over Cu0.84Bi2.08O4 for visible light induced photodegradation of ciprofloxacin in water matrix. Chem. Eng. J. 2019, 356, 472–482. [Google Scholar] [CrossRef]
- Liu, Y.P.; Gong, Y.H.; Cui, X.; Lu, Y.; Yu, H.B.; Qin, W.C.; Huo, M.X. Visible-light activation of persulfate by a Z-scheme photocatalyst Fe-C3N4/Bi2Sn2O7 for tetracycline degradation. Appl. Catal. A Gen. 2023, 666, 119422. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, Y.J.; Jiang, Z.Q.; Deng, B.L.; Jiang, Z.J. Advances in the removal of organic pollutants from water by photocatalytic activation of persulfate: Photocatalyst modification strategy and reaction mechanism. Chemsuschem 2024, 17, e202400254. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, G.H.; Meng, Y.; Pan, G.X.; Ni, Z.M.; Xia, S.J. Direct Z-scheme CeO2/@LDH core-shell heterostructure for photodegradation of Rhodamine B by synergistic persulfate activation. J. Hazard. Mater. 2021, 408, 124908. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.; Wang, Y.; Li, S.; Cui, B.; Du, Y. Persulfate activation of photocatalysts based on S-scheme Bi3NbO7/BiOBr0.75I0.25 nanosheet heterojunctions for degradation of organic pollutants. ACS Appl. Nano Mater. 2023, 6, 10768–10778. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y.; Li, J. Nanocatalysts for the degradation of refractory pollutants. Catalysts 2024, 14, 444. [Google Scholar] [CrossRef]
- Du, X.; Bai, X.; Xu, L.; Yang, L.; Jin, P.K. Visible-light activation of persulfate by TiO2/g-C3N4 photocatalyst toward efficient degradation of micropollutants. Chem. Eng. J. 2020, 384, 123245–123258. [Google Scholar] [CrossRef]
- Zhang, M.X.; He, J.; Chen, Y.B.; Liao, P.Y.; Liu, Z.Q.; Zhu, M.S. Visible light-assisted peroxydisulfate activation via hollow copper tungstate spheres for removal of antibiotic sulfamethoxazole. Chinese Chem. Lett. 2020, 31, 2721–2724. [Google Scholar] [CrossRef]
- Zhong, X.; Ye, X.Y.; Wu, D.; Zhang, K.X.; Huang, W. A facile heterogeneous system for persulfate activation by CuFe2O4 under LED light irradiation. RSC Adv. 2019, 9, 32328–32337. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Z.; Liu, H.J.; Ji, Q.H.; Qu, J.H.; Li, J.H. Photoactuation healing of α-FeOOH@g-C3N4 catalyst for efficient and stable activation of persulfate. Small 2017, 13, 1702225–1702232. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.X.; Chen, Y.; Chen, Y.J.; Zhuang, L.; Ou, H.S. Enhanced photocatalysis degradation of organophosphorus flame retardant using MIL-101(Fe)/persulfate: Effect of irradiation wavelength and real water matrixes. Chem. Eng. J. 2019, 368, 273–284. [Google Scholar] [CrossRef]
- Wang, M.H.; Yang, L.Y.; Guo, C.P.; Liu, X.F.; He, L.H.; Song, Y.P.; Zhang, Q.X.; Qu, X.W.; Zhang, H.Z.; Zhang, Z.H.; et al. Bimetallic Fe/Ti-based metal-organic framework for persulfate-assisted visible light photocatalytic degradation of orange II. Chemistryselect 2018, 3, 3664–3674. [Google Scholar] [CrossRef]
- Shen, Z.R.; Luo, Z.H.; Chen, J.Y.; Li, Y.W. Oxygen vacancy-mediated exciton effect in hierarchical BiOBr enables dichotomy of energy transfer and electron transfer in photocatalysis. Adv. Funct. Mater. 2023, 33, 2213935. [Google Scholar] [CrossRef]
- Wang, X.S.; Gao, R.; Fan, G.L.; Guo, Y.; Han, C.H.; Gao, Y.L.; Shen, A.; Wu, L.M.; Gu, X.J. Dual defects-induced iron single atoms immobilized in metal-organic framework-derived hollow BiOBr microtubes for low-barrier photocatalytic nitrogen reduction. Angew. Chem. Int. Ed. 2025, 64, e202501297. [Google Scholar] [CrossRef]
- Cui, D.D.; Wang, L.; Du, Y.; Hao, W.C.; Chen, J. Photocatalytic reduction on bismuth-based p-block semiconductors. ACS Sustain. Chem. Eng. 2018, 6, 15936–15953. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Chu, S.P.; Li, J.; Wang, L.L.; Chen, R.; Shao, Y.; Liu, X.W.; Ye, M.M. Efficient degradation of aqueous carbamazepine by bismuth oxybromide-activated peroxide oxidation. Catalysts 2017, 7, 315. [Google Scholar] [CrossRef]
- Bao, Y.P.; Lee, W.J.; Guan, C.T.; Liang, Y.N.; Lim, T.T.; Hu, X. Highly efficient activation of peroxymonosulfate by bismuth oxybromide for sulfamethoxazole degradation under ambient conditions: Synthesis, performance, kinetics and mechanisms. Sep. Purif. Technol. 2021, 276, 119203–119210. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Dou, X.; Chen, Y.; Shi, H. CuBi2O4/BiOBr composites promoted PMS activation for the degradation of tetracycline: S-scheme mechanism boosted Cu2+/Cu+ cycle. Chem. Eng. J. 2022, 431, 134054. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, Y.; Qu, X.; Xiang, Y.; Shen, S.; Zhu, X. Degradation of bisphenol A by Fe-doped BiOBr enhanced UV/persulfate system: Significant role of superoxide radicals. Process Saf. Environ. 2023, 180, 148–159. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.X.; Wang, F.Y.; Xia, M.Z.; Chen, Q.; Ju, X.H. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613–126626. [Google Scholar] [CrossRef]
- Liu, Q.B.; Bai, C.; He, D.; Wu, X.T.; Wu, Y.P.; Chen, R. Oxygen vacancy mediation of BiOX (X=Cl, Br) towards enhanced photocatalytic CO2 reduction activity. J. Environ. Chem. Eng. 2024, 12, 112449. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Z.Q.; Liu, D.M.; Liu, G.S.; Yang, M.; Cui, F.Y.; Wang, W. Ultrathin two-dimensional BiOBrxI1-x solid solution with rich oxygen vacancies for enhanced visible-light-driven photoactivity in environmental remediation. Appl. Catal. B Environ. 2018, 236, 222–232. [Google Scholar] [CrossRef]
- Li, X.B.; Liu, Q.; Deng, F.; Huang, J.T.; Han, L.; He, C.Z.; Chen, Z.; Luo, Y.D.; Zhu, Y.F. Double-defect-induced polarization enhanced OV-BiOBr/Cu2-xS high-low junction for boosted photoelectrochemical hydrogen evolution. Appl. Catal. B Environ. Energy 2022, 314, 121502–121512. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.F.; Yu, J.C. Fe enhanced visible-light-driven nitrogen fixation on BiOBr nanosheets. Chem. Mater. 2020, 32, 1488–1494. [Google Scholar] [CrossRef]
- Bai, C.B.; Zhang, Y.H.; Liu, Q.; Zhu, C.X.; Li, J.; Chen, R. Interfacial complexation between Fe3+ and Bi2MoO6 promote efficient persulfate activation via Fe3+/Fe2+ cycle for organic contaminates degradation upon visible light irradiation. J. Colloid Interface Sci. 2024, 664, 238–250. [Google Scholar] [CrossRef]
- Wang, L.L.; Cheng, H.F. Birnessite (δ-MnO2) mediated degradation of organoarsenic feed additive p-arsanilic acid. Environ. Sci. Technol. 2015, 49, 3473–3481. [Google Scholar] [CrossRef]
- Yu, L.; Wang, C.P.; Ren, X.H.; Sun, H.W. Catalytic oxidative degradation of bisphenol A using an ultrasonic-assisted tourmaline-based system: Influence factors and mechanism study. Chem. Eng. J. 2014, 252, 346–354. [Google Scholar] [CrossRef]
- Huang, S.N.; Tian, F.; Dai, J.W.; Tian, X.S.; Li, G.F.; Liu, Y.L.; Chen, Z.Q.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and 1O2 involved process. Appl. Catal. B Environ. 2021, 298, 120576. [Google Scholar] [CrossRef]
- Jawad, A.; Zhan, K.; Wang, H.; Shahzad, A.; Zeng, Z.; Wang, J.; Zhou, X.; Ullah, H.; Chen, Z.; Chen, Z. Tuning of persulfate activation from a free radical to a nonradical pathway through the incorporation of non-redox magnesium oxide. Environ. Sci. Technol. 2020, 54, 2476–2488. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, H.; Lee, D.; Ko, Y.-J.; Woo, H.; Lee, J.; Lee, C.; Pham, A.L.-T. Activation of hydrogen peroxide by a titanium oxide-supported iron catalyst: Evidence for surface Fe(IV) and its selectivity. Environ. Sci. Technol. 2020, 54, 15424–15432. [Google Scholar] [CrossRef]
- Bai, C.; Guo, W.; Liu, Q.; Li, G.; Guo, S.; Chen, R. Cu2O/BiVO4 heterostructure controllably triggers radical and non-radical persulfate activation via light “on-off” for efficient organic contaminants degradation. Appl. Catal. B Environ. Energy 2024, 344, 123606. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Z.; Tian, F.; Zhou, B.; Zhao, B.; Zhao, H.; Chen, Z.; Liu, Y.; Chen, R. Generation of defect clusters for 1O2 production for molecular oxygen activation in photocatalysis. J. Mater. Chem. A 2017, 5, 23453–23459. [Google Scholar] [CrossRef]
- Tan, C.; Xu, Q.; Sheng, T.; Cui, X.; Wu, Z.; Gao, H.; Li, H. Reactive oxygen species generation in FeOCl nanosheets activated peroxymonosulfate system: Radicals and non-radical pathways. J. Hazard. Mater. 2020, 398, 123084. [Google Scholar] [CrossRef] [PubMed]
- Sin, A.; Machala, L.; Kim, M.; Baďura, Z.; Petr, M.; Polaskova, M.; Novak, P.; Nadagouda, M.N.; Dionysiou, D.D.; Han, C. Development of tungsten-modified iron oxides to decompose an over-the-counter painkiller, Acetaminophen by activating peroxymonosulfate. Sci. Total. Environ. 2024, 951, 175472. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Maqbool, T.; Fu, W.; Yang, Y.; Hou, C.; Guo, J.; Zhang, X. Highly efficient manganese (III) oxide submerged catalytic ceramic membrane for nonradical degradation of emerging organic compounds. Sep. Purif. Technol. 2022, 295, 121272. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, R.; Lin, S.; Zhong, Q.; Zhang, Q.; Hong, J. Regulating the interface electron distribution of iron-based MOFs through ligand functionalization enables efficient peroxymonosulfate utilization and catalytic performance. J. Colloid Interface Sci. 2024, 663, 358–368. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Cheng, M.; Liu, Q.; Chen, R. Enhanced Peroxydisulfate Activation via Fe-Doped BiOBr for Visible-Light Photocatalytic Degradation of Paracetamol. Catalysts 2025, 15, 594. https://doi.org/10.3390/catal15060594
Wang Z, Cheng M, Liu Q, Chen R. Enhanced Peroxydisulfate Activation via Fe-Doped BiOBr for Visible-Light Photocatalytic Degradation of Paracetamol. Catalysts. 2025; 15(6):594. https://doi.org/10.3390/catal15060594
Chicago/Turabian StyleWang, Zhigang, Mengxi Cheng, Qiong Liu, and Rong Chen. 2025. "Enhanced Peroxydisulfate Activation via Fe-Doped BiOBr for Visible-Light Photocatalytic Degradation of Paracetamol" Catalysts 15, no. 6: 594. https://doi.org/10.3390/catal15060594
APA StyleWang, Z., Cheng, M., Liu, Q., & Chen, R. (2025). Enhanced Peroxydisulfate Activation via Fe-Doped BiOBr for Visible-Light Photocatalytic Degradation of Paracetamol. Catalysts, 15(6), 594. https://doi.org/10.3390/catal15060594





