Ce/Mn Co-Doping Induces Synergistic Effects for Low-Temperature NH3-SCR over Ba2Ti5O12 Catalysts
Abstract
1. Introduction
2. Results and Discussion
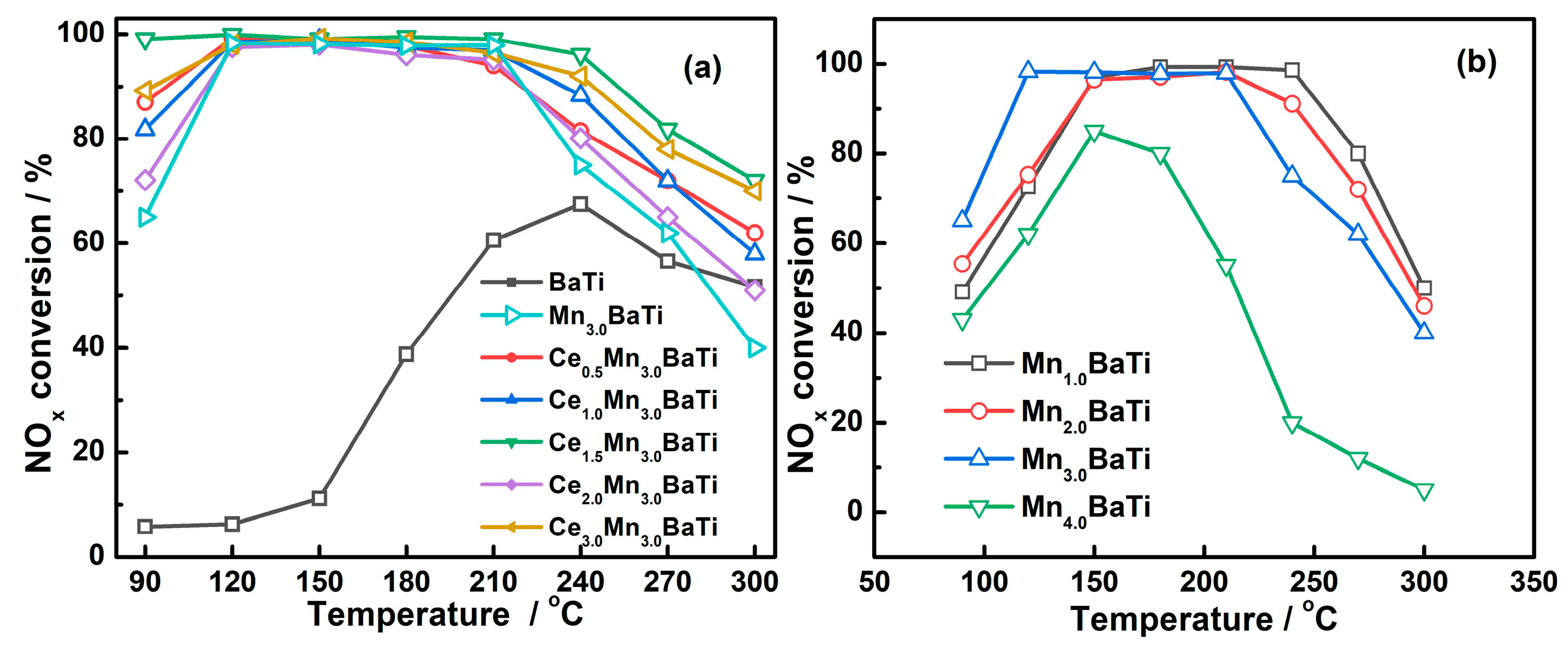
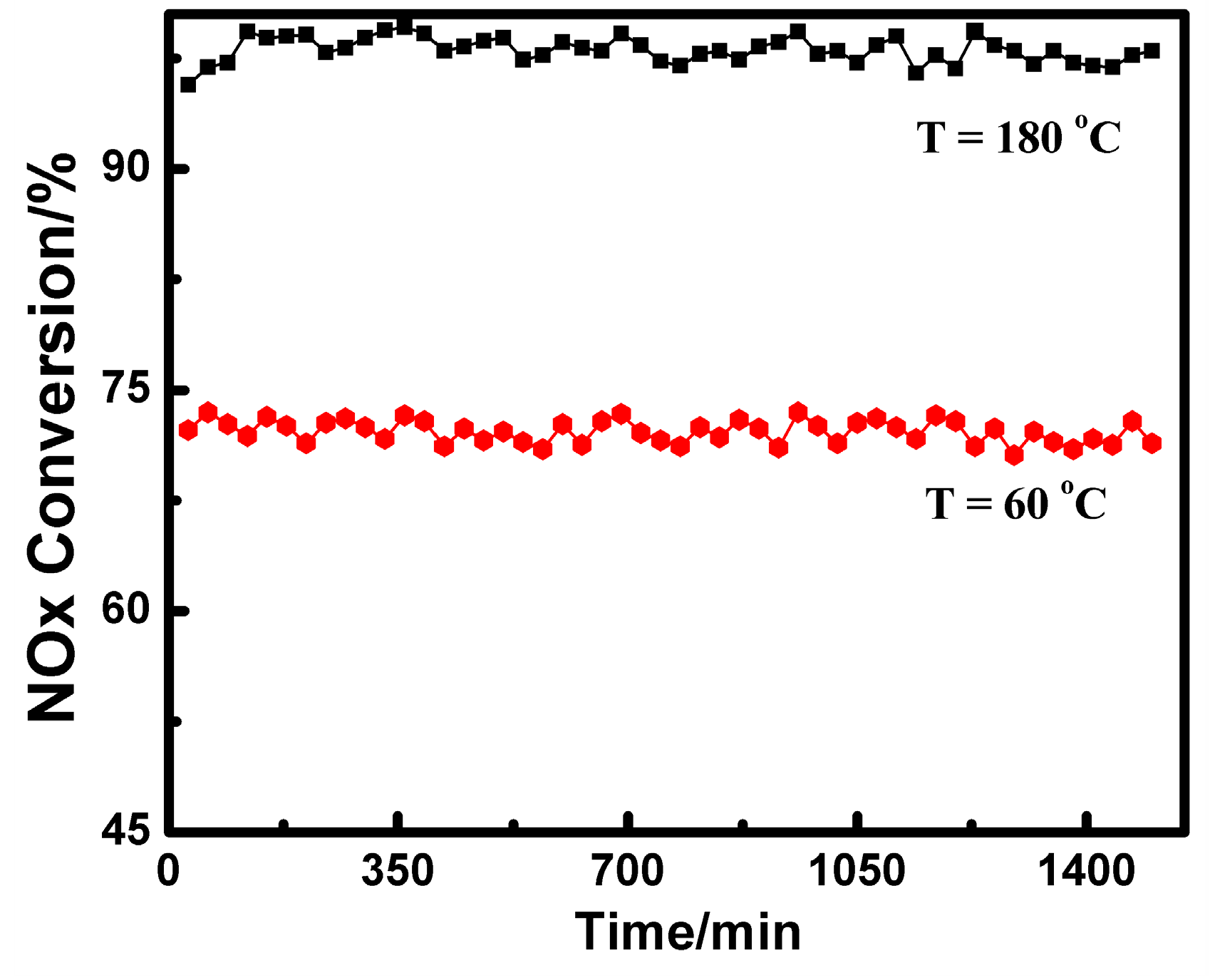
2.1. Catalytic Performance
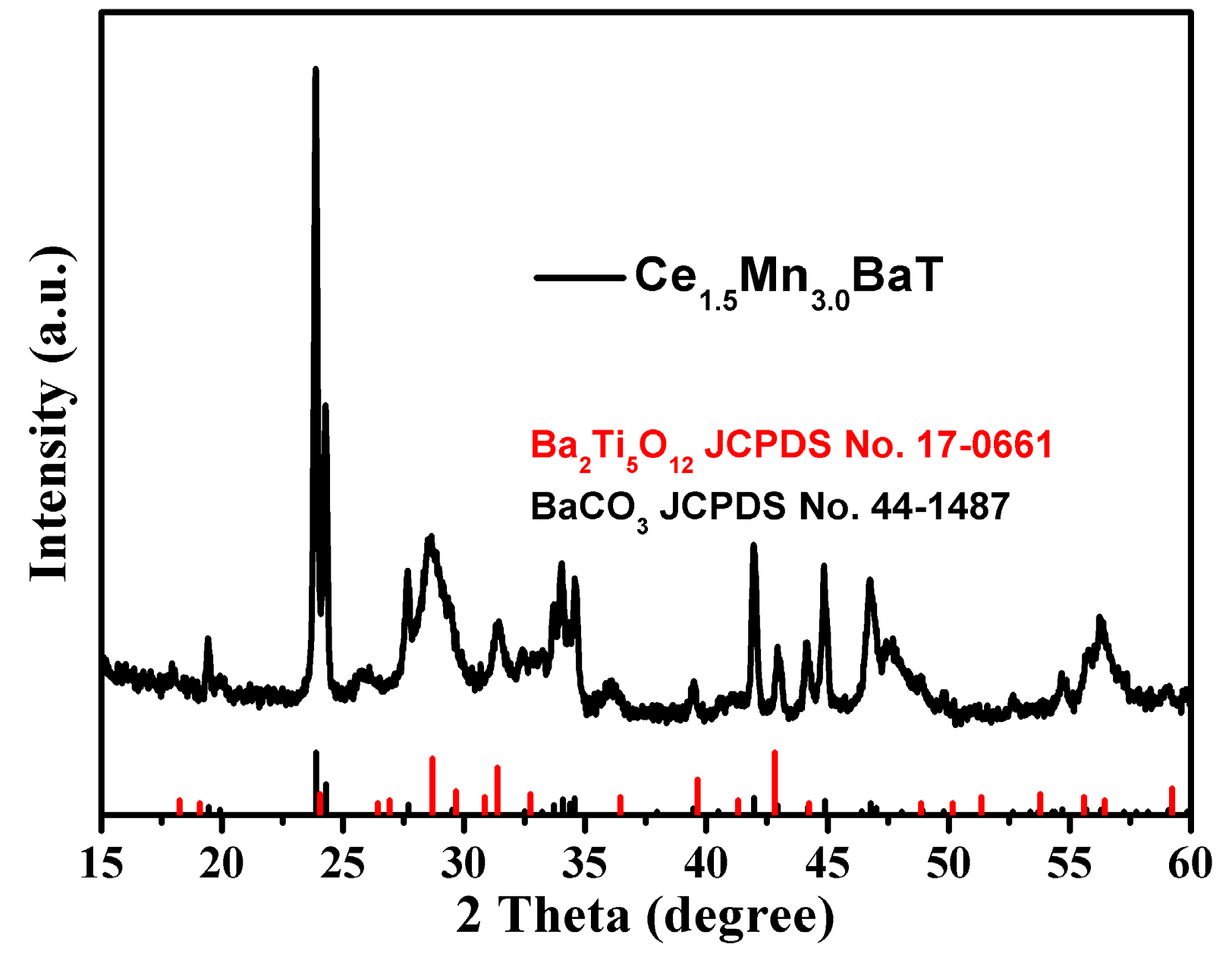
2.2. Phase Analysis of Catalysts
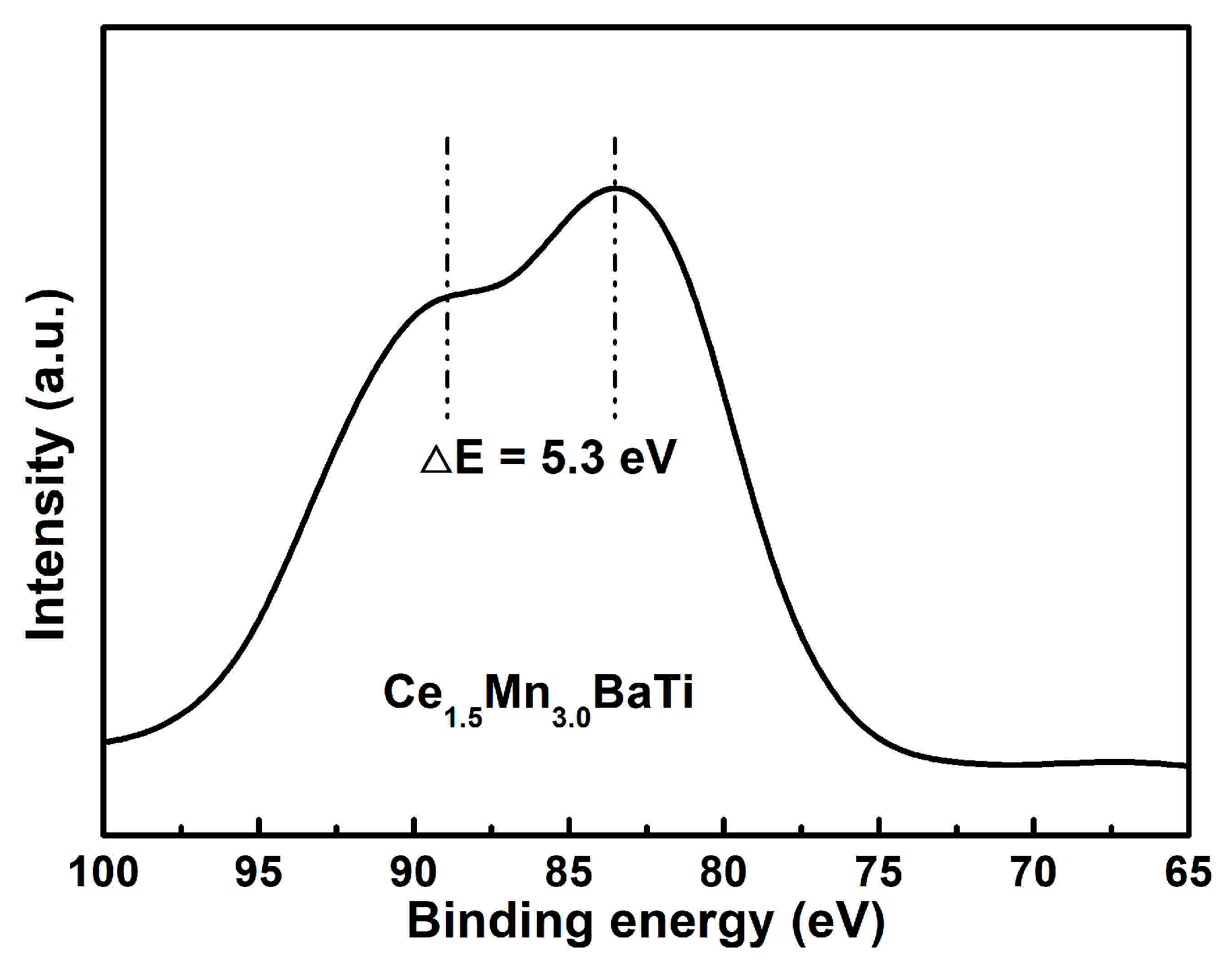
2.3. XPS Analysis
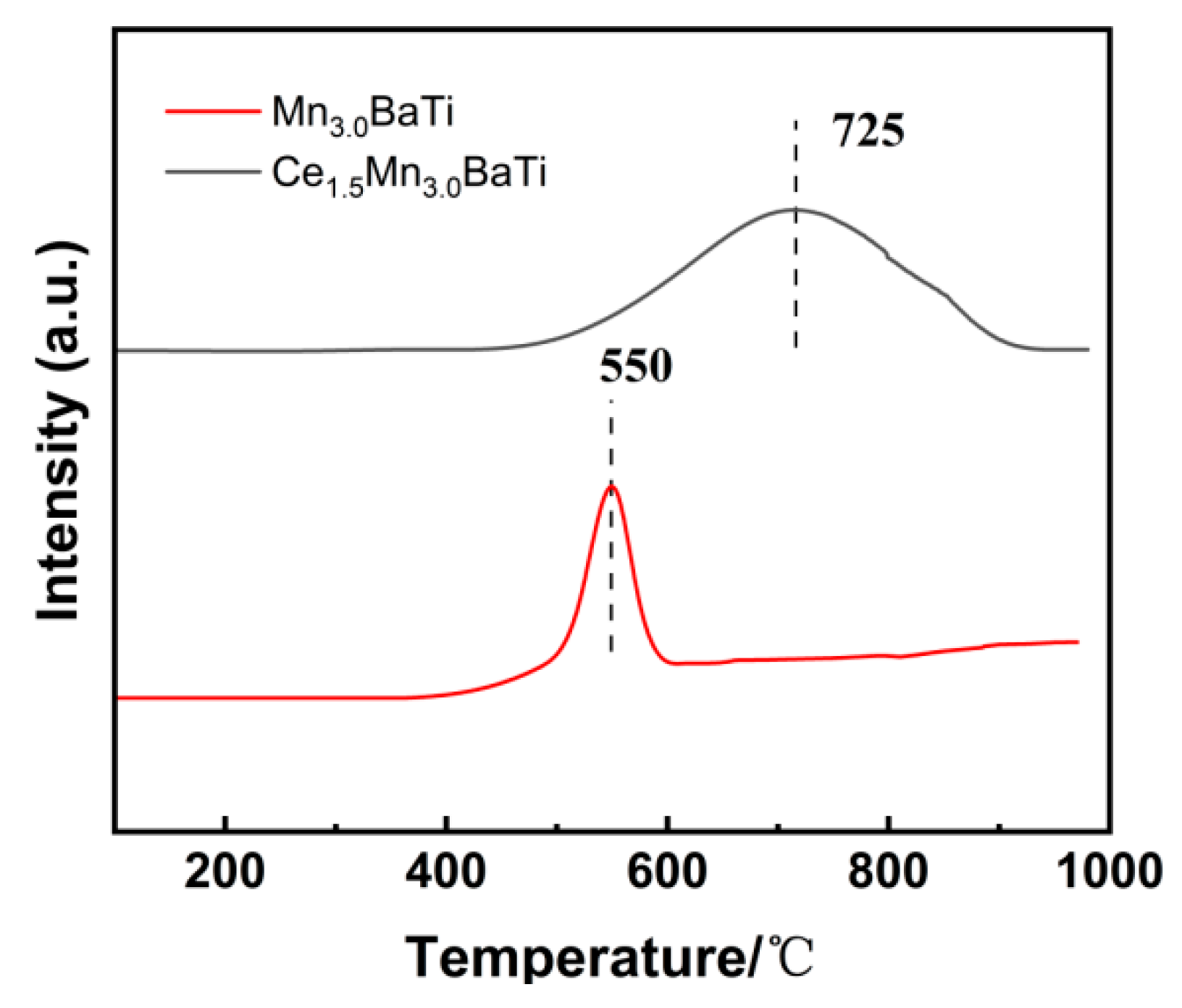
2.4. NH3-TPD
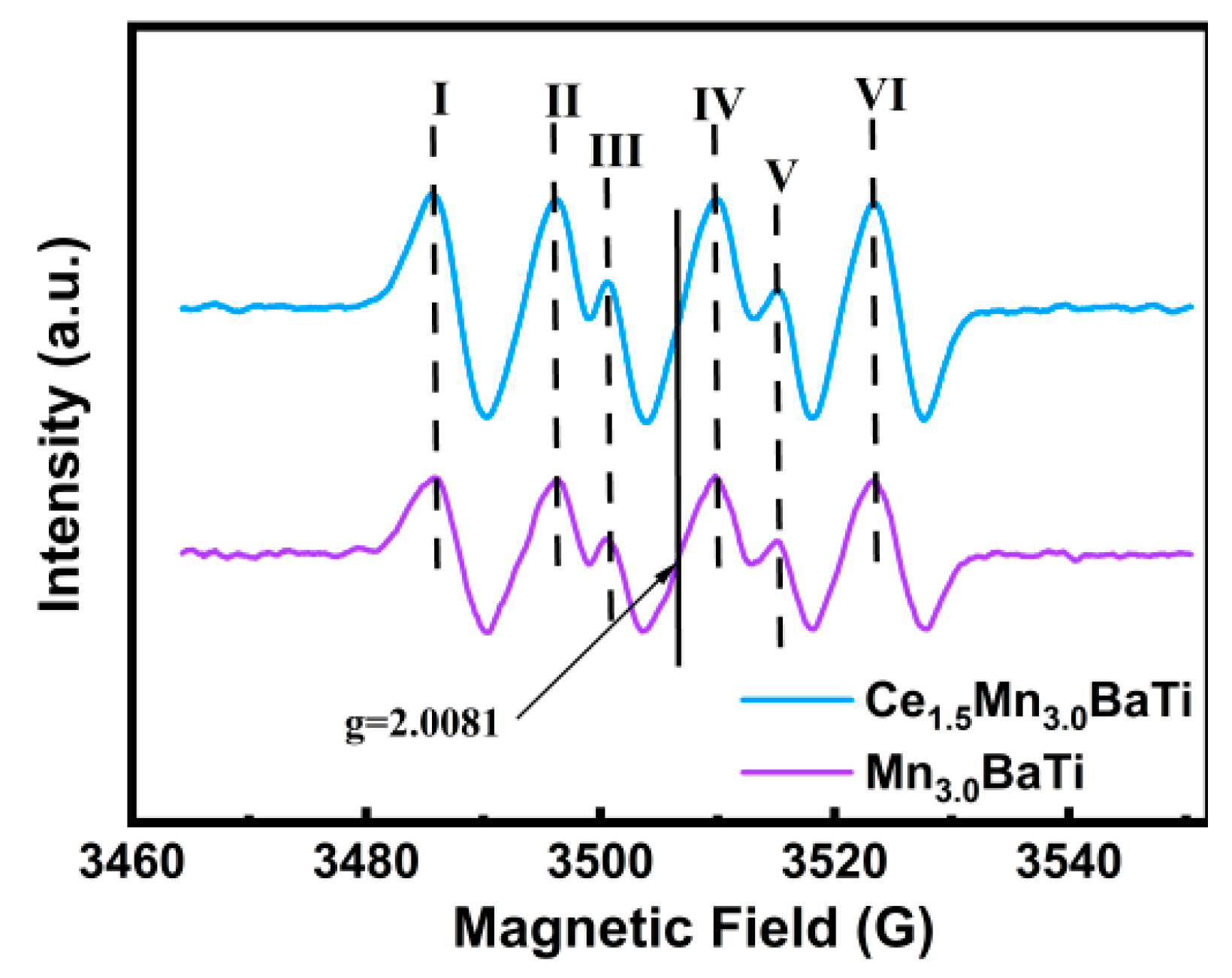
2.5. EPR Surface Features
3. Experimental Section
3.1. Samples Preparation
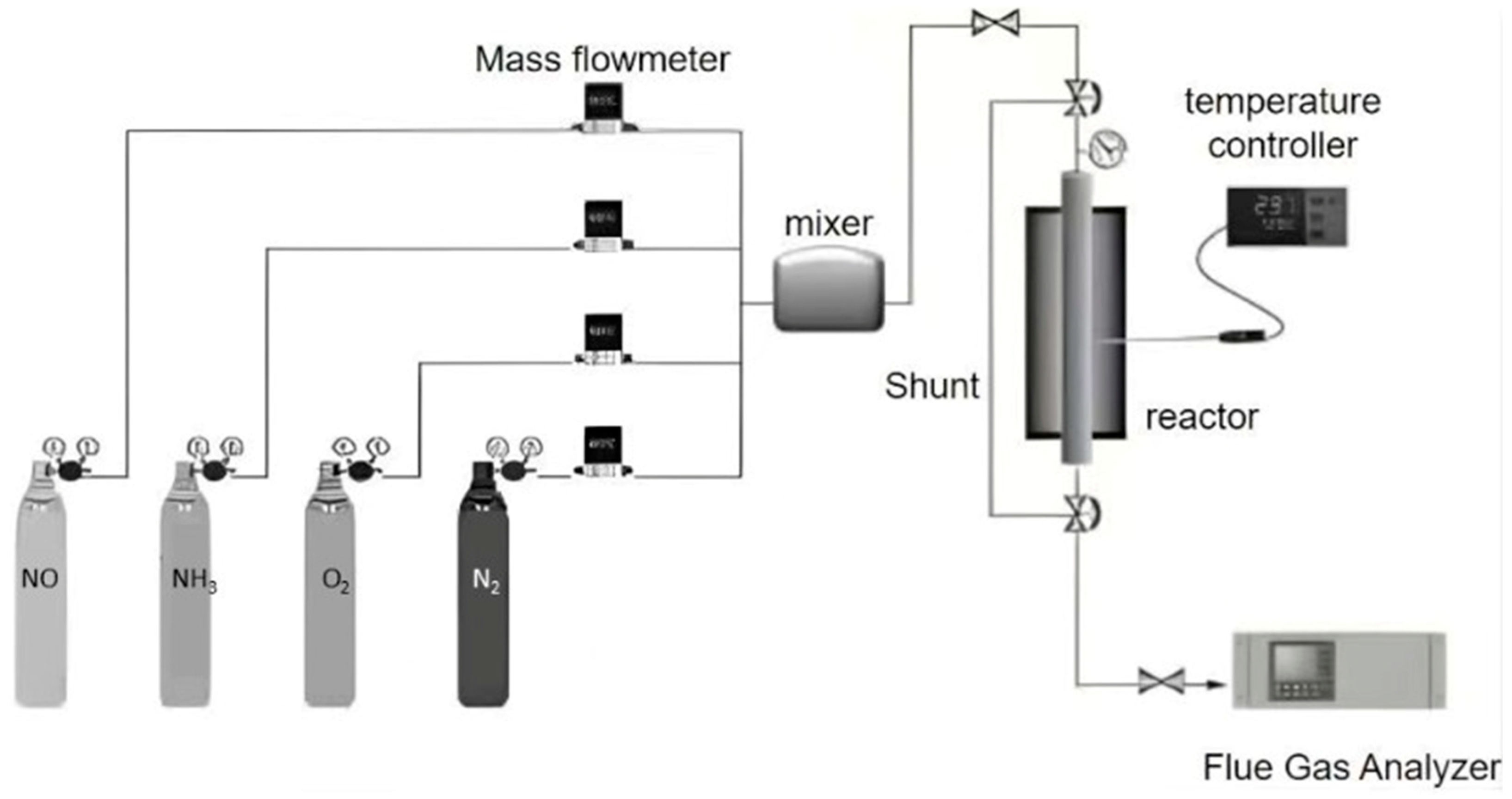
3.2. Activity Measurement
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Xing, Y.; Li, G.L.; Gao, J.J.; Li, R.; Liu, Q.; Yue, T. A comprehensive review of deactivation and modification of selective catalytic reaction catalysts installed in cement kilns. J. Environ. Sci. 2025, 148, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, S.; Tang, Y.; Wang, Y.; Lou, J.; Zhang, Q.; Zheng, X.; Li, J.; Iqbal, B.; Cheng, P.; et al. Spartina alterniflora invasion altered soil greenhouse gas emissions via affecting labile organic carbon in a coastal wetland. Appl. Soil Energy 2024, 203, 105615. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, H.; Wang, J.; Ji, C.; Liu, Y.; Chen, D.; Zhang, H.; Wang, J.; Zhang, Y. Fertilizer N triggers native soil N-derived N2O emission by priming gross N mineralization. Soil Biol. Biochem. 2023, 178, 108961. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Ahmed, A.A. Catalysts driving efficiency and innovation in thermal reactions: A comprehensive review. Green Technol. Sustain. 2024, 2, 100078. [Google Scholar] [CrossRef]
- Zhu, N.; Shan, W.P.; Lian, Z.H.; Zhang, Y.; Liu, K.; He, H. A superior Fe-V-Ti catalyst with high activity and SO2 resistance for the selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2020, 382, 120970. [Google Scholar] [CrossRef]
- Chen, Y.P.; Shang, C.Y.; Xiao, X.; Guo, W.H.; Xu, Q. Recent progress of electrocatalysts for acidic oxygen evolution reaction. Coord. Chem. Rev. 2024, 508, 215758. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Georgiadis, A.G.; Drosou, C.; Charisiou, N.D.; Goula, M.A. Selective catalytic reduction of NOx over perovskite-based catalysts using CXHY (OZ), H2 and CO as reducing agents-a review of the latest developments. Nanomaterials 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Ao, R.; Ma, L.P.; Guo, Z.Y.; Dai, Q.X.; Xie, L.G.; Yang, J. Positive effects of the Sr doping on LaCoO3 perovskites for simultaneous catalytic oxidation performances of NO and Hg0. Energy 2024, 290, 130196. [Google Scholar] [CrossRef]
- Xie, H.; Shu, D.B.; Chen, T.H.; Liu, H.B.; Zou, X.H.; Wang, C.; Han, Z.Y.; Chen, D. An in-situ DRIFTs study of Mn doped FeVO4 catalyst by one-pot synthesis for low-temperature NH3-SCR. Fuel 2022, 309, 122108. [Google Scholar] [CrossRef]
- Feng, X.D.; Liu, S.J.; Yue, K.; Wei, H.; Bi, D.M. Insight into the promotional effect of Mn-modified nitrogenous biochar on the NH3-SCR denitrification activity at low temperatures. Energy 2023, 285, 129323. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cao, J.; Tian, S.H.; Zhao, Y.C.; Long, L.L.; Yao, X.J. Mechanistic insights into the influence of preparation methods and Fe3+ doping on the low-temperature performance of MnCeOX catalyst for NH3-SCR reaction. Sep. Purif. Technol. 2024, 347, 127519. [Google Scholar] [CrossRef]
- Shi, X.K.; Guo, J.X.; Shen, T.; Fan, A.D.; Liu, Y.J.; Yuan, S.D. Improvement of NH3-SCR activity and resistance to SO2 and H2O by Ce modified La-Mn perovskite catalyst. J. Taiwan Inst. Chem. Eng. 2021, 126, 102–111. [Google Scholar] [CrossRef]
- Liu, J.Q.; Xiong, W.; Ren, X.; Ouyang, T.Y.; Zhang, Z.D.; Sun, N.; Tan, H.L.; Yan, C.X.; Cai, J.M. Study on the mechanism of NOx reduction by NH3-SCR over Mn and M (M = V, Ti) co-doped CoCr2O4 catalyst. Mol. Catal. 2022, 524, 112283. [Google Scholar] [CrossRef]
- Jin, L.Y.; Li, H.X.; Zhang, Y.; Peng, D.; Sun, Z.J.; Zhang, A.C. Enhanced activity of cerium-doped Co-Cr-O composite catalyst in selective catalytic reduction of NO with NH3. Chem. Sel. 2023, 13, 202204977. [Google Scholar] [CrossRef]
- Shao, S.; Sun, T.; Li, X.; Wang, Y.; Ma, L.; Liu, Z.; Wu, S. Preparation of heavy bio-oil-based porous carbon by pyrolysis gas activation and its performance in the aldol condensation for aviation fuel as catalyst carrier. Ind. Crop. Prod. 2024, 218, 118963. [Google Scholar] [CrossRef]
- Javed, M.; Huang, H.; Ma, Y.; Ettoumi, F.; Wang, L.; Xu, Y.; El-Seedi, H.R.; Ru, Q.; Luo, Z. Construction of self-assembled nano cellulose crystals/chitosan nanobubbles composite hydrogel with improved gallic acid release property. Food Chem. 2024, 438, 137948. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhou, X.; Zhang, C.; Yin, H.; Wang, A.; Wang, C. Functional characterization of bimetallic CuPdx nanoparticles in hydrothermal conversion of glycerol to lactic acid. J. Food Biochem. 2019, 43, e12931. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Z.; Mu, H.F.; Liu, L.L.; Wang, H.; Luan, J.D.; Ke, X. Promotional role of the TiOx nanorod arrays as a support to load MnOx for low-temperature NH3-Selective catalytic reduction of NOx: Comparison of two preparation strategies. Energy Fuels 2021, 36, 965–977. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.G.; Liu, B. Effect of oxygen vacancies on ceria catalyst for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2020, 529, 147068. [Google Scholar] [CrossRef]
- Yu, X.L.; Wu, X.M.; Chen, Z.Y.; Huang, Z.W.; Jing, G.H. Oxygen vacancy defect engineering in Mn-doped CeO2 nanostructures for nitrogen oxides emission abatement. Mol. Catal. 2019, 476, 110512. [Google Scholar] [CrossRef]
- Rong, J.; Zhao, W.X.; Luo, W.; Kang, K.K.; Long, L.L.; Chen, Y.; Yao, X.J. Doping effect of rare earth metal ions Sm3+, Nd3+ and Ce4+ on denitration performance of MnOX catalyst in low temperature NH3-SCR reaction. J. Rare Earths 2023, 41, 1323–1335. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Demeter, M.; Bartkowski, S.; Neumann, M.; Ovechkina, N.A.; Kurmaev, E.Z.; Lobachevskaya, N.I.; Mukovskii, Y.M.; Mitchell, J.; Ederer, D.L. Mn (formula presented) exchange splitting in mixed-valence manganites. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 65, 113102. [Google Scholar] [CrossRef]
- Yang, D.; Song, Y.; Zhang, M.Y.; Qin, Z.; Dong, R.; Li, C.; Liu, X.X. A Manganese Phosphate Cathode for Long-Life Aqueous Energy Storage. Adv. Funct. Mater. 2021, 31, 2100477. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Chen, W.; Chen, D.; Yu, S.; Liu, A.; Dong, L.; Feng, S. Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem. Eng. J. 2018, 347, 27–40. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Wei, L.; Hao, J. MnOx-SnO2 Catalysts Synthesized by a Redox Coprecipitation Method for Selective Catalytic Reduction of NO By NH3. Chin. J. Catal. 2008, 29, 531–536. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijin, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Zhou, Y.; Rong, S.P.; Xie, H.F.; Feng, Y.F.; Ding, D.N.; He, W.J.; Zhang, N.; Lu, J.L. Enhancement of acidic sites in layered MnO2 for the highly efficient selective catalytic oxidation of gaseous ammonia. J. Environ. Chem. Eng. 2023, 11, 109480. [Google Scholar] [CrossRef]
- Zhao, R.; Pang, R.; Wang, Y.; Zhao, Z.W. Effect of metal elements doping on the CePO4 catalysts for selective catalytic reduction of NO with NH3. Mol. Catal. 2022, 530, 112627. [Google Scholar] [CrossRef]
- Li, S.Y.; Liang, W.J.; Cai, J.Y. Promoting effects of cerium oxide on catalysts performance for selectivity catalytic oxidation of NH3 over RuOx/TiO2. J. Environ. Chem. Eng. 2024, 12, 113528. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Liu, L.; Zhao, Y.; Bai, F.; Wang, J.; Gao, R.; Xu, X. The influence mechanism of phospholipids structure and composition changes caused by oxidation on the formation of flavor substances in sturgeon caviar. Food Chem. 2024, 460, 140585. [Google Scholar] [CrossRef]
- Tan, T.; Chen, M.; Su, J.H.; Du, J.F. Temperature-dependent formation of redox sites in molybdenum trioxide studied by electron paramagnetic resonance spectroscopy. Chin. J. Chem. Phys. 2019, 32, 657–660. [Google Scholar] [CrossRef]
- Zhang, C.C.; Liu, X.Y.; Jiang, M.; Wen, Y.L.; Zhang, J.; Qian, G.R. A review on identification, quantification, and transformation of active species in SCR by EPR spectroscopy. Environ. Sci. Pollut. Res. 2023, 30, 28550–28562. [Google Scholar] [CrossRef] [PubMed]
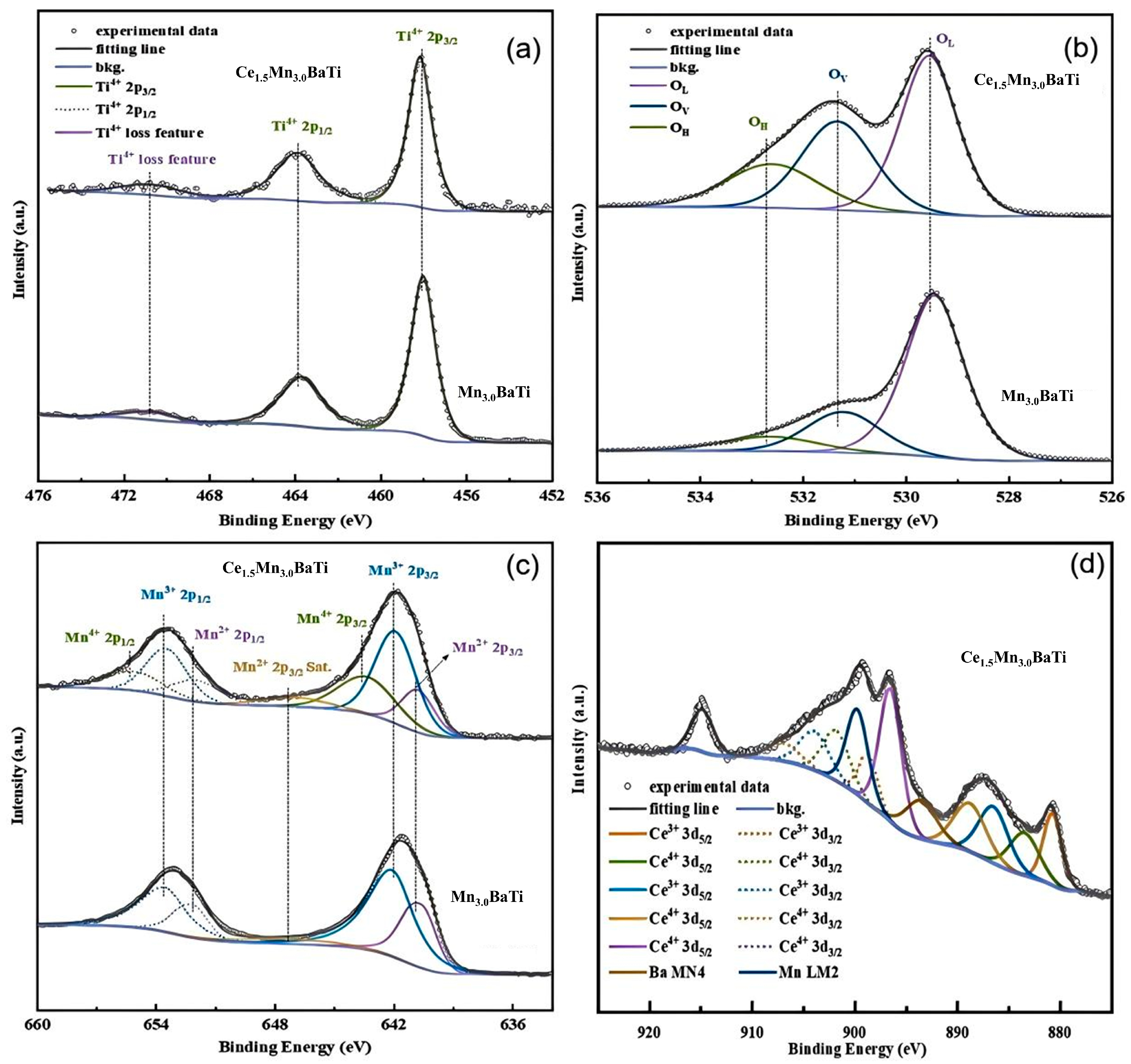
| Samples | Lattice Oxygen (OL)/% | Surface Adsorption Oxygen (OV)/% | Hydroxyl Oxygen (OH)/% | Mn2+/% | Mn3+/% | Mn4+/% | Mn4++Mn3+/Mnn+ | Ce3+/% | Ce4+/% |
|---|---|---|---|---|---|---|---|---|---|
| Mn3.0BaTi | 69.21 | 21.13 | 9.66 | 33.28 | 66.72 | 0 | 66.72 | 0 | 0 |
| Ce1.5Mn3.0BaTi | 46.66 | 32.71 | 20.63 | 13.72 | 55.17 | 31.11 | 86.28 | 34.06 | 65.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhao, W.; Wang, H.; Zhang, D.; Wang, Q.; Wang, A.; Shang, D.; Zhong, Q. Ce/Mn Co-Doping Induces Synergistic Effects for Low-Temperature NH3-SCR over Ba2Ti5O12 Catalysts. Catalysts 2025, 15, 593. https://doi.org/10.3390/catal15060593
Zhao W, Zhao W, Wang H, Zhang D, Wang Q, Wang A, Shang D, Zhong Q. Ce/Mn Co-Doping Induces Synergistic Effects for Low-Temperature NH3-SCR over Ba2Ti5O12 Catalysts. Catalysts. 2025; 15(6):593. https://doi.org/10.3390/catal15060593
Chicago/Turabian StyleZhao, Wei, Wang Zhao, Haiwen Wang, Dingwen Zhang, Qian Wang, Aijian Wang, Danhong Shang, and Qin Zhong. 2025. "Ce/Mn Co-Doping Induces Synergistic Effects for Low-Temperature NH3-SCR over Ba2Ti5O12 Catalysts" Catalysts 15, no. 6: 593. https://doi.org/10.3390/catal15060593
APA StyleZhao, W., Zhao, W., Wang, H., Zhang, D., Wang, Q., Wang, A., Shang, D., & Zhong, Q. (2025). Ce/Mn Co-Doping Induces Synergistic Effects for Low-Temperature NH3-SCR over Ba2Ti5O12 Catalysts. Catalysts, 15(6), 593. https://doi.org/10.3390/catal15060593






