Graphitic Carbon Nitride-Based S-Scheme Heterojunctions: Recent Advances in Photocatalytic Dye Degradation
Abstract
1. Introduction
2. S-Scheme Heterojunctions: Proposal and Principles
2.1. Proposal of S-Scheme Heterojunctions
2.2. Basic Principles of S-Scheme Heterojunctions
3. g-C3N4’s Role in S-Scheme Heterojunctions and Typical Systems
3.1. g-C3N4’s Role in S-Scheme Heterojunctions
3.2. Typical Systems of g-C3N4-Based S-Scheme Heterojunctions
4. Application of Graphitic Carbon Nitride-Based S-Scheme Heterojunctions in Dye Degradation
4.1. Application of Graphitic Carbon Nitride-Based S-Scheme Heterojunctions in Cationic Dye Degradation
4.1.1. Methylene Blue
| Photocatalyst | Synthesis Method | Amount of Catalyst | Light Source | Amount of MB | Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| GO/g-C3N4/TiO2 | Calcination In situ crystallization | 10 mg | 350 W Xenon lamp, λ ≥ 420 nm | 100 mL, 10 mg/L | 240 | 98.8 | [29] |
| Ag/AgI/g-C3N4 | Hydrothermal Calcination Photoreduction | / | Simulated sunlight | / | 50 | 97.0 | [48] |
| g-C3N4/Co/ZnO | Ultrasound Sol–gel | 20 mg | 300 W Xenon lamp, λ ≥ 420 nm | 50 mL, 15 ppm | 120 | 96.3 | [27] |
| CaSnO3/g-C3N4 | Solid-state route | 50 mg | 500 W Halogen lamp | 1 g/L | 120 | 95.0 | [55] |
| PbTiO3/g-C3N4 | Ultrasound Hydrothermal | 50 mg | 100 W Halogen lamp | 50 mL, 30 mg/L | 90 | 99.4 | [56] |
| Sm2CeMnO6/g-C3N4 | Sol–gel | 150 mg | 9 W three LED lamp, λ > 365 nm | 100 mL, 8 mg/L | 120 | 96.1 | [57] |
| TCN/TiO2 | Precursor reconstruction, Hydrothermal, Calcination | / | Visible | / | 60 | 96.6 | [58] |
| g-C3N4/TiO2 | Sol–gel | 20 mg | 14.4 W/m LED lamp (SMD 5050 Flexible Strips, Ltd. China), λ = 460 nm | 10 mg/L | 80 | 94.9 | [60] |
| g-C3N4/CuO | In situ synthesis | 200 mg | 150 W visible light source (Osram, Munich, Germany) | 50 mL, 2 mg/L | 180 | 93.0 | [61] |
| CoFe2O4/g-C3N4 | In situ deposition | 200 mg/L | 150 W visible light source (Osram, Munich, Germany), λ > 420 nm | 50 mL, 2 mg/L | 180 | 100.0 | [62] |

4.1.2. Rhodamine B
| Photocatalyst | Synthesis Method | Light Source | Amount of RhB | Amount of Catalyst | Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| g-C3N4/Co/ZnO | Ultrasound, sol–gel | 300 W Xenon lamp, λ > 420 nm | 50 mL, 15 ppm | 20 mg | 120 | 75.1 | [27] |
| Ag/AgI/g-C3N4 | Hydrothermal, calcination, photoreduction | Simulated sunlight | / | / | 50 | 95.6 | [48] |
| Zr(HPO4)2/g-C3N4 | Ultrasonic chemical coupling | 1000 W sunlight | 100 mL, 2×10−5 M | 10 mg | 180 | 98.0 | [63] |
| g-C3N4/PPy/ZnO | Hydrothermal, calcination, polymerization | 125 W LED lamp, λ ≥ 420 nm | 100 mL, 50 mg/L | 100 mg | 60 | 99.0 | [64] |
| CdS/TiO2/g-C3N4 | Hydrothermal | 50 W LED lamp | 50 mL, 25 mg/L | 10 mg | 180 | 99.4 | [65] |
| PbTiO3/g-C3N4 | Ultrasound, hydrothermal | 100 W halogen lamp | 50 mL, 10 mg/L | 50 mg | 60 | 99.8 | [56] |
| g-C3N4/TiO2 | Ultrasound | 14.4 W/m LED lamp (SMD 5050 Flexible Strips, Ltd. China), λ = 460 nm | 15 mg/L | 20 mg | 80 | 93.1 | [60] |
| Ag2CrO4/g-C3N4 | Ultrasound | 300 W solar simulator | 100 mL, 2 × 10−5 M | 50 mg | 120 | 96.0 | [66] |
| AgI/g-C3N4 | Ultrasound | 300 W solar simulator | 100 mL, 2 × 10−5 M | 100 mg | 120 | 96.0 | [67] |
| g-C3N4/TiO2/CuO | Hydrothermal | 500 W Xenon lamp | 100 mL, 30 ppm | 50 mg | 120 | 90.3 | [68] |
| CeO2/g-C3N4 | Ultrasound, sol–gel | 110 K to 90 K lumen, natural solar | 100 mL, 2 × 10−5 M | 20 mg | 120 | 98.9 | [69] |
| SnS2/g-C3N4 | Ultrasound | 1000 W natural solar | 100 mL, 2 × 10−5 M | 50 mg | 120 | 98.0 | [70] |
| Ag2CO3/g-C3N4 | Ultrasound | 450 W natural sunlight | 100 mL, 10 mg/L | 100 mg | 120 | 95.0 | [71] |
| HKUST-1/g-C3N4 | Ultrasound | Ultraviolet–visible light source | 50 mL, 10 mg/L | 50 mg | 120 | 94.4 | [72] |
| Bi2S3/g-C3N4 | Ultrasound | 500 W natural sunlight | / | / | 120 | 90.0 | [73] |
| g-C3N4/rGO/ZnO-Ag | Hydrothermal | Visible | 100 mL, 40 ppm | 60 mg | 100 | 83.4 | [74] |
| α-Fe2O3/g-C3N4/SiO2 | Hydrothermal, calcination | 100 W LED lamp, λ = 420 nm | 100 mL, 10 ppm | 60 mg | 120 | 97.0 | [78] |
| SnO2₋ₓ/g-C3N4 | Calcination, hydrothermal | Visible light source, λ > 420 nm | 20 mg/L | 0.33 g/L | 150 | 99.8 | [79] |
| g-C3N4/NiFe2O4 | Sol–gel combustion, calcination | Sunlight | 50 mL, 20 mg/L | 20 mg | 60 | 98.6 | [80] |
| K-g-C3N4/ZnO | High-temperature melting | Visible | / | / | 120 | 92.0 | [81] |
| β-Cu2V2O₇/Ni/Pg-C3N4 | Co-precipitation | Sunlight | 60 mL, 25 ppm | 20 mg | 60 | 98.6 | [90] |
| TiO2/g-C3N4 | Calcination | 300 W Xenon lamp (Asahi HAL-320) | 100 mL, 10 mg/L | 50 mg | 120 | 96.0 | [91] |
| g-C3N4/ZnCr-LDH | Hydrothermal, reflux | LED lamp | 100 mL, 5 ppm | 100 mg | 90 | 99.8 | [92] |
| g-C3N4/Bi/BiVO4 | Deposition, in situ reduction | 350 W Xenon lamp (Changzhou Siyu, China), λ > 420 nm | 50 mL, 10 mg/L | 50 mg | 70 | 99.0% | [93] |
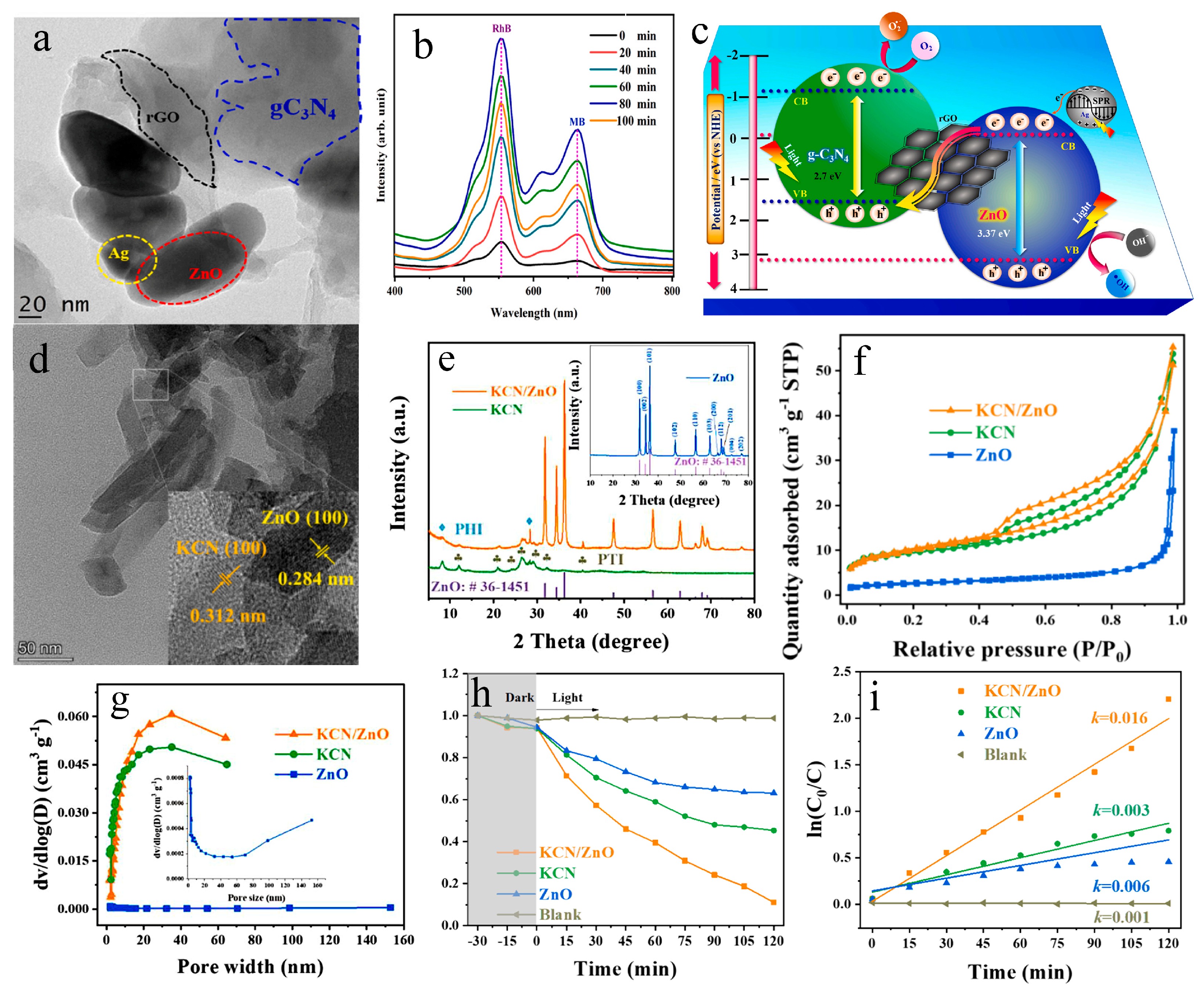
4.1.3. Other Cationic Dyes
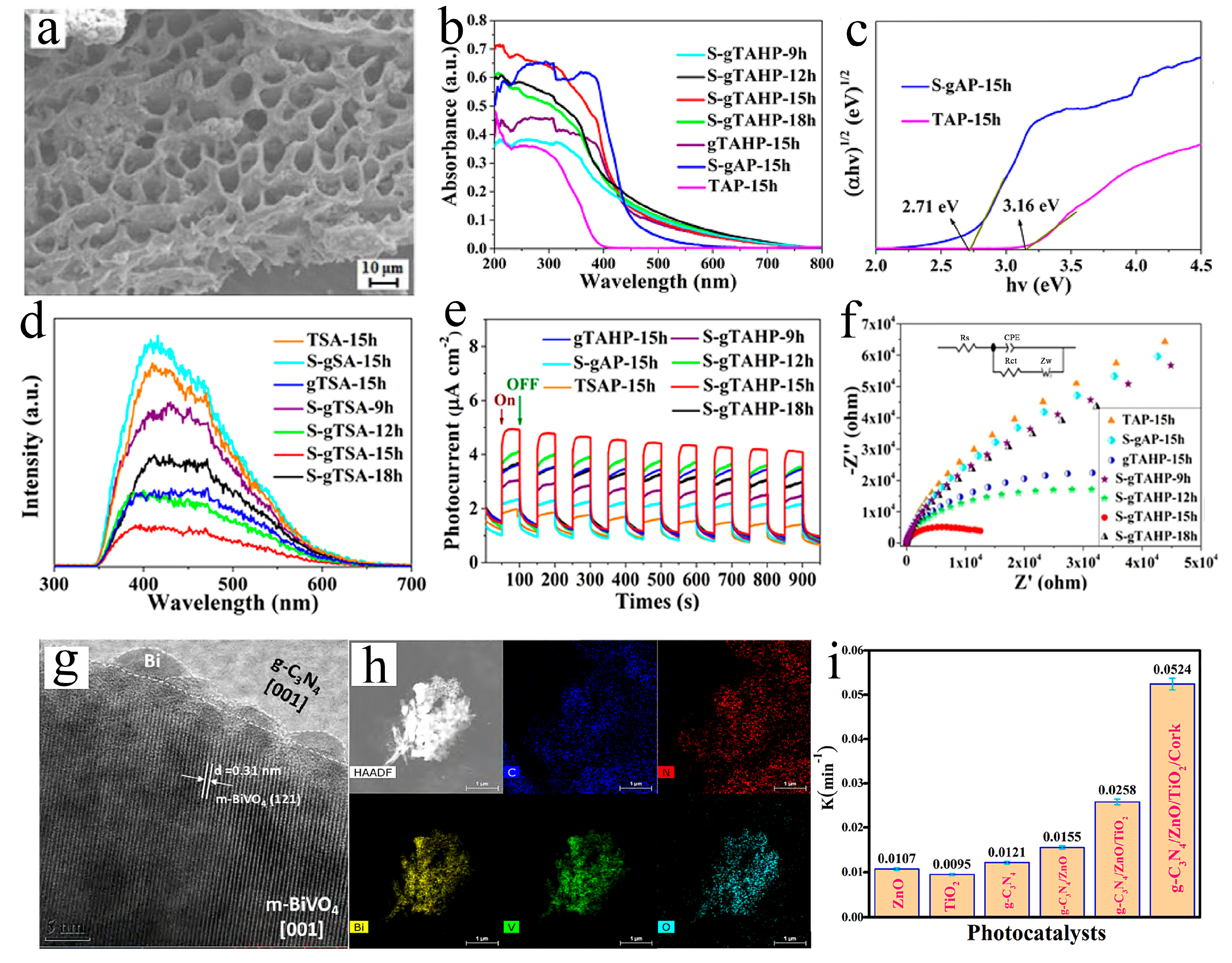
4.2. The Application of S-Scheme Heterojunction g-C3N4 Structures in the Degradation of Anionic Dyes
4.2.1. Methyl Orange
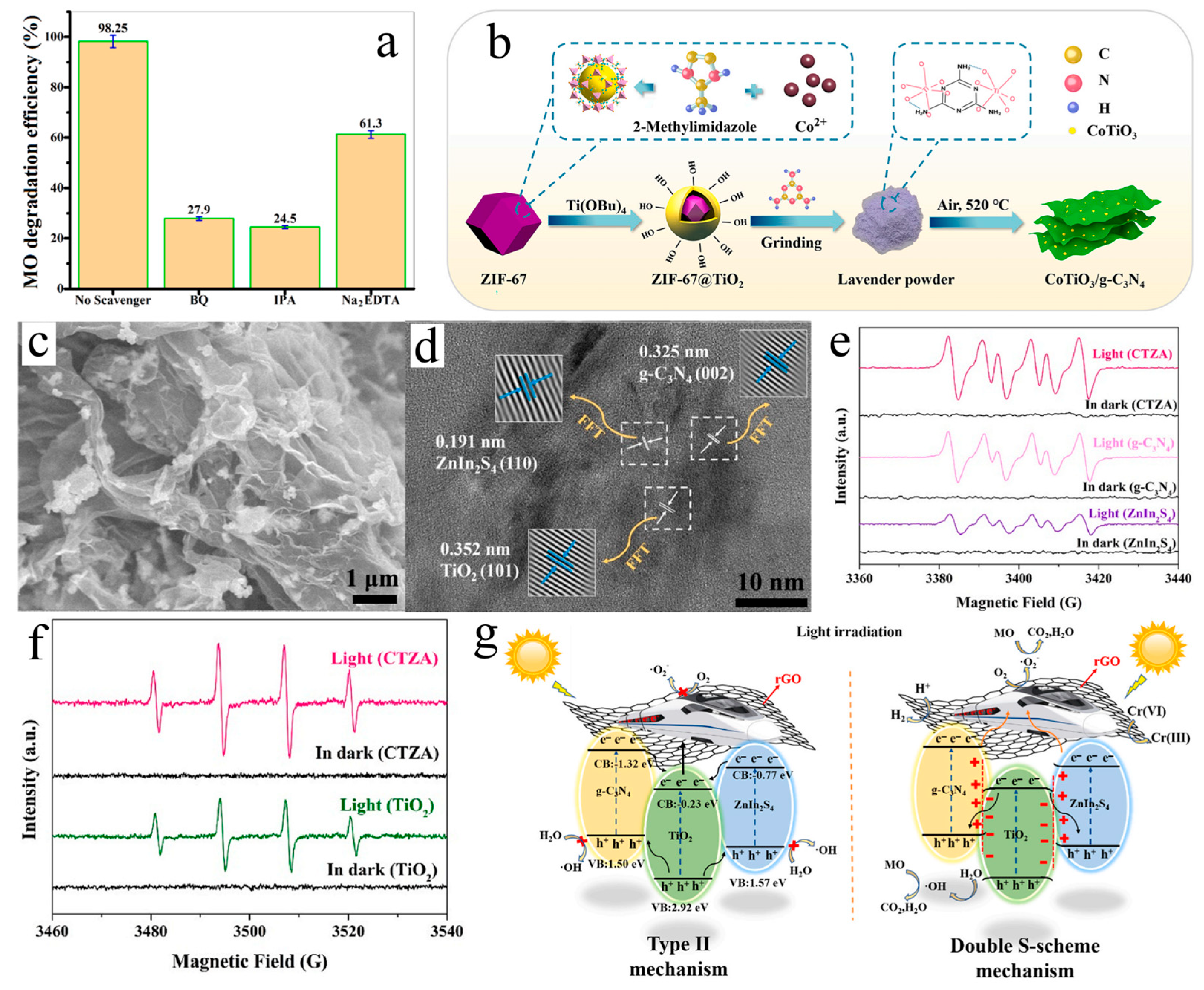
| Photocatalyst | Synthesis Method | Amount of Catalyst | Light Source | Dye | Amount of Dye | Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| g-C3N4/Co/ZnO | Ultrasound, sol–gel | 20 mg | 300 W Xenon lamp, λ > 420 nm | CV | 50 mL, 15 ppm | 120 | 74.5 | [27] |
| Ag/AgI/g-C3N4 | Hydrothermal | / | Simulated sunlight | CV | / | 60 | 100.0 | [48] |
| Ca@TiO2@g-C3N4 | Thermal polymerization, Ultrasound | 50 mg | 500W Xenon lamp | MG | 100 mL, 30 mg/L | 60 | 99.9 | [94] |
| C-CeO2/g-C3N4 | Hydrothermal | 50 mg | solar | MG | 100 mL, 30 mg/L | 150 | 91.9 | [95] |
| g-C3N4/ZnO/TiO2/Cork | Co-precipitation | 60 mg | 250 W halogen lamp | MO | 100 mL, 1 × 10−5 M | 60 | 98.3 | [96] |
| CoTiO3/g-C3N4 | In situ calcination | / | Visible, λ > 420 nm | MO | / | 240 | 99.7 | [97] |
| g-C3N4/Bi2S3/CuS | Hydrothermal | 10 mg | Visible | MO | 100 mL, 0.1 mg/L | 60 | 98.0 | [98] |
| CdS/GCNS | Solid-state diffusion | / | Visible | MO | / | 60 | 100.0 | [99] |
| CTZA | Isoelectric point calcination | / | Simulated sunlight | MO | / | 30 | 97.5 | [100] |
| g-C3N4/Ag2WO4/Bi2S3 | In situ growth | 50 mg | 140 W LED lamp | CR | 250 mL, 20 mg/L | 60 | 98.0 | [106] |
| R.palustris/RCM@CPU | Hydrothermal | / | 100 W light | CR | 50 mg/L | 480 | 99.5 | [107] |
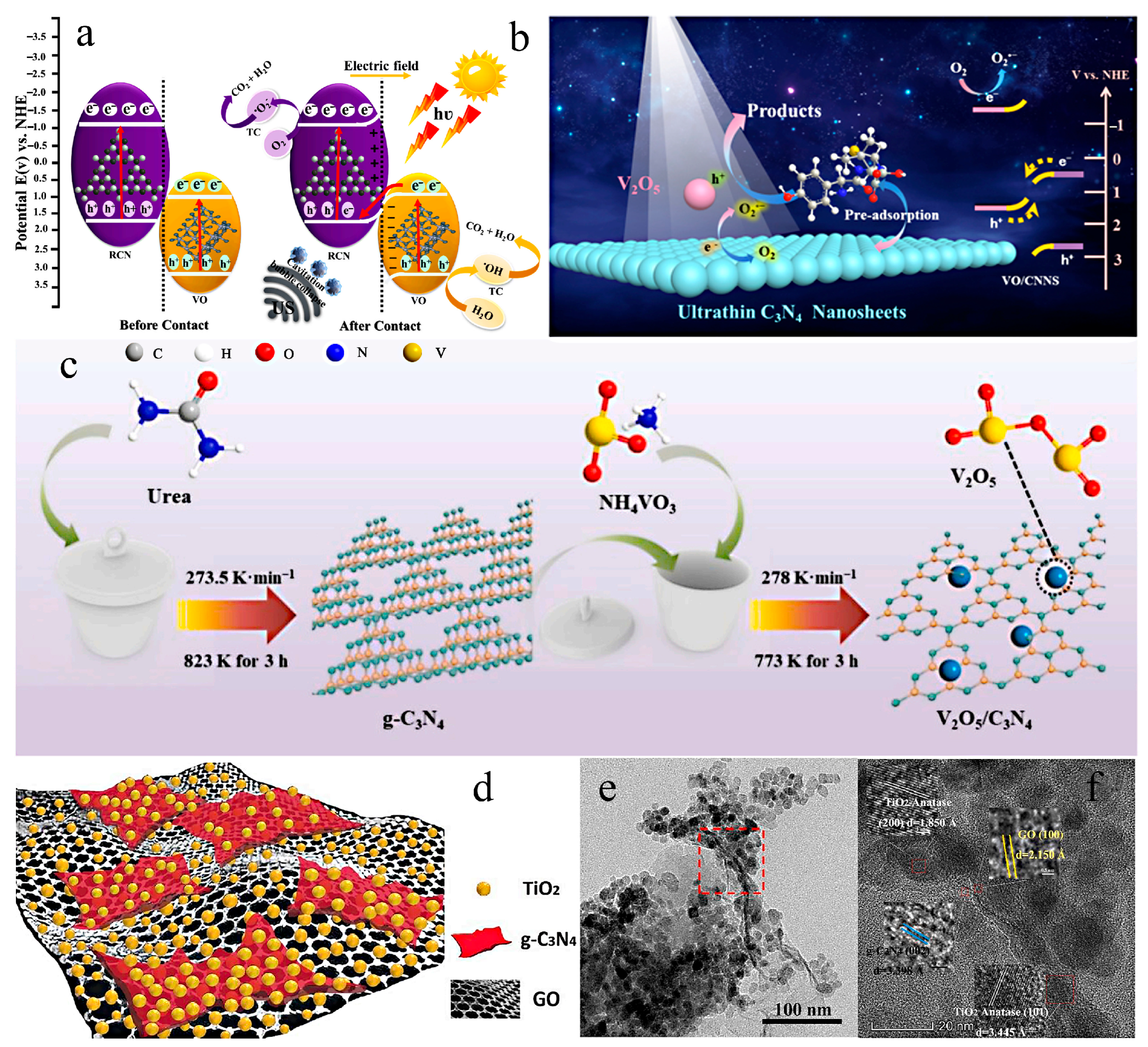
| V2O5/Ndef-g-C3N4 | Controllable pyrolysis | 5 mg | 18 W LED lamp | IC | 25 mL, 20 ppm | 35 | 98.2 | [108] |
| NiMn2O4/g-C3N4 | Ultrasonic co-precipitation | 50 mg | 400 W lamp (Osram, Munich, Germany) | EBT | 50 mL, 10 ppm | 120 | 96.4 | [109] |
| MgO-TiO2@g-C3N4 | Ultrasound | 50 mg | 500 W Xenon lamp, λ > 420 nm | ARS | 100 mL, 30 mg/L | 60 | 94.0 | [110] |
| CaSnO3/g-C3N4 | Ultrasonic co-precipitation | 45 mg | 400 W lamp (Osram, Munich, Germany) | ER | 45 mL, 10 ppm | 90 | 86.2 | [111] |
4.2.2. Congo Red
4.2.3. Other Anionic Dyes
5. Conclusions
6. Prospects
6.1. Development and Optimization of Novel Heterojunction Systems
6.2. Coupling of Multiple Technologies and Intelligent Control
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elumalai, P.; Gao, X.; Parthipan, P.; Luo, J.; Cui, J. Agrochemical pollution: A serious threat to environmental health. Curr. Opin. Environ. Sci. Health 2025, 43, 100597. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Lima, J.P.; Alvarenga, G.; Goszczynski, A.C.; Rosa, G.R.; Lopes, T.J. Batch adsorption of methylene blue dye using Enterolobium contortisiliquum as bioadsorbent: Experimental, mathematical modeling and simulation. J. Ind. Eng. Chem. 2020, 91, 362–371. [Google Scholar] [CrossRef]
- Naddeo, V.; Secondes, M.F.N.; Borea, L.; Hasan, S.W.; Ballesteros, F., Jr.; Belgiorno, V. Removal of contaminants of emerging concern from real wastewater by an innovative hybrid membrane process—UltraSound, Adsorption, and Membrane ultrafiltration. Ultrason. Sonochem. 2020, 68, 105237. [Google Scholar] [CrossRef] [PubMed]
- Geed, S.R.; Samal, K.; Tagade, A.J. Development of adsorption-biodegradation hybrid process for removal of methylene blue from wastewater. J. Environ. Chem. Eng. 2019, 7, 103439. [Google Scholar] [CrossRef]
- Rodriguez, N.O.M.; Picos, A.R.; Bravo, Y.N.; Pacheco, A.M.; Martínez, H.C.A.; Peralta-Hernández, J.M. Electrochemical oxidation technology to treat textile wastewaters. Curr. Opin. Electrochem. 2021, 29, 100806. [Google Scholar] [CrossRef]
- Liu, C.; Dong, S.; Chen, Y. Enhancement of visible-light-driven photocatalytic activity of carbon plane/g-C3N4/TiO2 nanocomposite by improving heterojunction contact. Chem. Eng. J. 2019, 371, 706–718. [Google Scholar] [CrossRef]
- Do, H.T.; Phan Thi, L.A.; Dao Nguyen, N.H.; Huang, C.W.; Le, Q.V.; Nguyen, V.H. Tailoring photocatalysts and elucidating mechanisms of photocatalytic degradation of perfluorocarboxylic acids (PFCAs) in water: A comparative overview. J. Chem. Technol. Biotechnol. 2020, 95, 2569–2578. [Google Scholar] [CrossRef]
- Xie, Z.; Saad, A.; Shang, Y.; Wang, Y.; Luo, S.; Wei, Z. Enhanced degradation of micropollutants by visible light photocatalysts with strong oxygen activation ability. Water Res. 2023, 247, 120785. [Google Scholar] [CrossRef]
- Sacco, O.; Mancuso, A.; Venditto, V.; Pragliola, S.; Vaiano, V. Behavior of N-doped TiO2 and N-doped ZnO in photocatalytic azo dye degradation under UV and visible light irradiation: A preliminary investigation. Catalysts 2022, 12, 1208. [Google Scholar] [CrossRef]
- Sun, F.; Qi, H.; Xie, Y.; Ma, Q.; He, W.; Xu, D.; Wang, G.; Yu, W.; Wang, T.; Dong, X. Flexible self-supporting bifunctional [TiO2/C]//[Bi2WO6/C] carbon-based Janus nanofiber heterojunction photocatalysts for efficient hydrogen evolution and degradation of organic pollutant. J. Alloys Compd. 2020, 830, 154673. [Google Scholar] [CrossRef]
- Zia, J.; Riaz, U. Photocatalytic degradation of water pollutants using conducting polymer-based nanohybrids: A review on recent trends and future prospects. J. Mol. Liq. 2021, 340, 117162. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.N.; Shen, C.C.; Xu, A.W. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Jiang, G.; Zheng, C.; Yan, T.; Jin, Z. Cd0.8Mn0.2S/MoO3 composites with an S-scheme heterojunction for efficient photocatalytic hydrogen evolution. Dalton Trans. 2021, 50, 5360–5369. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Vinesh, V.; Neppolian, B. Construction of S-scheme 1D/2D rod-like g-C3N4/V2O5 heterostructure with enhanced sonophotocatalytic degradation for Tetracycline antibiotics. Chemosphere 2022, 287, 132380. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ irradiated XPS investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef]
- Feng, K.; Tian, J.; Hu, X.; Fan, J.; Liu, E. Active-center-enriched Ni0.85Se/g-C3N4 S-scheme heterojunction for efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2022, 47, 4601–4613. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Huang, H.; Kou, J.; Lu, C.; Xu, Z. Effective solar driven H2 production by Mn0.5Cd0.5Se/g-C3N4 S-scheme heterojunction photocatalysts. Int. J. Hydrogen Energy 2021, 46, 32514–32522. [Google Scholar] [CrossRef]
- Wang, L.; Chuanbiao, B.; Yu, J. Challenges of Z-scheme photocatalytic mechanisms. Trends Chem. 2022, 4, 973–983. [Google Scholar] [CrossRef]
- Han, T.; Shi, H.; Chen, Y. Facet-dependent CuO/{010} BiVO4 S-scheme photocatalyst enhanced peroxymonosulfate activation for efficient norfloxacin removal. J. Mater. Sci. Technol. 2024, 174, 30–43. [Google Scholar] [CrossRef]
- Chen, R.; Xia, J.; Chen, Y.; Shi, H. S-scheme-enhanced PMS activation for rapidly degrading tetracycline using CuWO4−x/Bi12O17Cl2 heterostructures. Acta Phys. Chim. Sin. 2023, 39, 2209012. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; He, R.; Wan, L.; Zhang, S. In2O3-x(OH)y/Bi2MoO6 S-scheme heterojunction for enhanced photocatalytic performance. J. Mater. Sci. Technol. 2020, 56, 151–161. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Liu, E.; Fan, J. Novel S-scheme 2D/2D BiOBr/g-C3N4 heterojunctions with enhanced photocatalytic activity. Chin. J. Catal. 2021, 42, 1519–1529. [Google Scholar] [CrossRef]
- Leelavathi, H.; Muralidharan, R.; Abirami, N.; Tamizharasan, S.; Sankeetha, S.; Kumarasamy, A.; Arulmozhi, R. Construction of step-scheme g-C3N4/Co/ZnO heterojunction photocatalyst for aerobic photocatalytic degradation of synthetic wastewater. Colloids Surf. A 2023, 656, 130449. [Google Scholar] [CrossRef]
- Le, S.; Zhu, C.; Cao, Y.; Wang, P.; Liu, Q.; Zhou, H.; Chen, C.; Wang, S.; Duan, X. V2O5 nanodot-decorated laminar C3N4 for sustainable photodegradation of amoxicillin under solar light. Appl. Catal. B Environ. 2022, 303, 120903. [Google Scholar] [CrossRef]
- Ren, X.; Guo, M.; Xue, L.; Xu, L.; Li, L.; Yang, L.; Wang, M.; Xin, Y.; Ding, F.; Wang, Y. Photoelectrochemical performance and S-scheme mechanism of ternary GO/g-C3N4/TiO2 heterojunction photocatalyst for photocatalytic antibiosis and dye degradation under visible light. Appl. Surf. Sci. 2023, 630, 157446. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient photocatalytic overall water splitting induced by the giant internal electric field of a g-C3N4/rGO/PDIP Z-scheme heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, H.; Li, X.; Fan, J.; Xiang, Q. Carbon-graphitic carbon nitride hybrids for heterogeneous photocatalysis. Small 2021, 17, 2005231. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Chen, H.S.; Yang, P.; Jiang, S.P. Fusiform-Shaped g-C3N4 Capsules with Superior Photocatalytic Activity. Small 2020, 16, 2003910. [Google Scholar] [CrossRef]
- Yi, J.; El-Alami, W.; Song, Y.; Li, H.; Ajayan, P.M.; Xu, H. Emerging surface strategies on graphitic carbon nitride for solar driven water splitting. Chem. Eng. J. 2020, 382, 122812. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Benafqir, M.; Ajmal, Z.; Aarab, N.; Laabd, M.; Navío, J.; Puga, F.; Boukherroub, R.; Bakiz, B.; et al. Facile synthesis and characterization of a novel 1,2,4,5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water. J. Colloid Interface Sci. 2021, 585, 560–573. [Google Scholar] [CrossRef]
- Yang, W.; Kim, J.H.; Hutter, O.S.; Phillips, L.J.; Tan, J.; Park, J.; Lee, H.; Major, J.D.; Lee, J.S.; Moon, J.; et al. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat. Commun. 2020, 11, 861. [Google Scholar] [CrossRef]
- Kumar, P.; Laishram, D.; Sharma, R.K.; Vinu, A.; Hu, J.; Kibria, M.G. Boosting photocatalytic activity using carbon nitride based 2D/2D van der Waals heterojunctions. Chem. Mater. 2021, 33, 9012–9092. [Google Scholar] [CrossRef]
- Yaqoubi, M.; Salavati-Niasari, M.; Ghanbari, M. S-Scheme CuMn2O4/g-C3N4 heterojunction: Fabrication, characterization, and investigation of photodegradation potential of organic pollutants. Appl. Water Sci. 2025, 15, 13. [Google Scholar] [CrossRef]
- Tran Huu, H.; Thi, M.D.N.; Nguyen, V.P.; Thi, L.N.; Phan, T.T.T.; Hoang, Q.D.; Luc, H.H.; Kim, S.J.; Vo, V. One-pot synthesis of S-scheme MoS2/g-C3N4 heterojunction as effective visible light photocatalyst. Sci. Rep. 2021, 11, 14787. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Z.; Yang, Q.; Wang, L.; Xing, Y. Review on g-C3N4-based S-scheme heterojunction photocatalysts. J. Mater. Sci. Technol. 2022, 125, 128–144. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, W.; Zhai, Z.; Ren, K.; Wang, T.; Guan, H.; Shi, H. 2D/2D Bi2MoO6/g-C3N4 S-scheme heterojunction photocatalyst with enhanced visible-light activity by Au loading. J. Mater. Sci. Technol. 2020, 56, 216–226. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Peng, J.; Yu, X.; Tang, H.; Zhang, J. Hot-electron-assisted S-scheme heterojunction of tungsten oxide/graphitic carbon nitride for broad-spectrum photocatalytic H2 generation. Chin. J. Catal. 2021, 42, 1478–1487. [Google Scholar] [CrossRef]
- Guo, X.; Xi, Y.; Li, Y.; Zhu, J.; Yan, H.; Zha, F.; Tang, X.; Tian, H. Ag regulates the interfacial electric field of g-C3N4/FeVO4 S-scheme heterojunction for activated H2O2 degradation of organic pollutants in complex environments. Chem. Eng. J. 2025, 505, 159640. [Google Scholar] [CrossRef]
- Dai, Z.; Zhen, Y.; Sun, Y.; Li, L.; Ding, D. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal. Chem. Eng. J. 2021, 415, 129002. [Google Scholar] [CrossRef]
- Chu, Z.; Li, J.; Sohn, H.Y.; Chen, C.; Huang, X.; Lan, Y.; Murali, A.; Zhang, J. CeO2-g-C3N4 S-scheme heterojunctions for enhanced photocatalytic performance: Effects of surface C/N ratio on photocatalytic and adsorption properties. Compos. Part B Eng. 2023, 257, 110689. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Liu, S.; Zhao, M.; Duan, X.; Wu, H.; Liu, L.; Liu, W.; Li, J.; Ren, S. Constructing C–O bridged CeO2/g-C3N4 S-scheme heterojunction for methyl orange photodegradation: Experimental and theoretical calculation. J. Environ. Manag. 2023, 335, 117608. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, Z.; Liu, Z.; Liang, F.; Zhu, X.; Bin, Z.; Huang, F.; Wang, N.; Zhu, Y. Efficient removal of ciprofloxacin from water by BiOX/GaMOF S-scheme heterojunction: A synergistic effect of adsorption and photocatalysis. Chem. Eng. J. 2025, 506, 159689. [Google Scholar] [CrossRef]
- Sun, F.; Xu, Q.; Liu, H.; Xu, D.; Wang, X.; Luo, C.; Wang, T.; Yu, H.; Yu, W.; Dong, X. Plasmon and N-vacancy synergistically enhanced tubular carbon nitride-based S-scheme heterojunction photocatalyst with one stone five birds function: Pathways, DFT calculation and mechanism insight. J. Catal. 2024, 440, 115813. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, J.; Zhu, B.; Liang, G.; Zhang, L.; Yu, J. Verifying the charge-transfer mechanism in S-Scheme heterojunctions using femtosecond transient absorption spectroscopy. Angew. Chem. Int. Ed. 2023, 62, e202218688. [Google Scholar] [CrossRef]
- Guo, N.; Zeng, Y.; Li, H.; Xu, X.; Yu, H.; Han, X. Novel mesoporous TiO2@g-C3N4 hollow core@shell heterojunction with enhanced photocatalytic activity for water treatment and H2 production under simulated sunlight. J. Hazard. Mater. 2018, 353, 80–88. [Google Scholar] [CrossRef]
- Yan, Q.; Fu, Y.; Zhang, Y.; Wang, H.; Wang, S.; Cui, W. Ag/γ-AgI/Bi2O2CO3/Bi S-scheme heterojunction with enhanced photocatalyst performance. Sep. Purif. Technol. 2021, 263, 118389. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, Y.J.; Cho, I.S.; Park, S.; Lee, C.; Alvarez, P.J. Facile synthesis of N vacancy g-C3N4 using Mg-induced defect on the amine groups for enhanced photocatalytic •OH generation. J. Hazard. Mater. 2023, 449, 131046. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, B.; Chen, W.; Tao, Z.; Liu, J.; Wang, L. Biochar doped carbon nitride to enhance the photocatalytic hydrogen evolution through synergy of nitrogen vacancies and bridging carbon structure: Nanoarchitectonics and first-principles calculation. Carbon 2023, 209, 117988. [Google Scholar] [CrossRef]
- Fang, H.; Guo, H.; Niu, C.; Liang, C.; Huang, D.-W.; Tang, N.; Liu, H.; Yang, Y.; Li, L. Hollow tubular graphitic carbon nitride catalyst with adjustable nitrogen vacancy: Enhanced optical absorption and carrier separation for improving photocatalytic activity. Chem. Eng. J. 2020, 402, 126185. [Google Scholar] [CrossRef]
- Venkatesh, G.; Palanisamy, G.; Srinivasan, M.; Vignesh, S.; Elavarasan, N.; Pazhanivel, T.; Al-Enizi, A.M.; Ubaidullah, M.; Karim, A.; Prabu, K.M. CaSnO3 coupled g-C3N4 S-scheme heterostructure photocatalyst for efficient pollutant degradation. Diam. Relat. Mater. 2022, 124, 108873. [Google Scholar] [CrossRef]
- Patra, R.; Panda, P.K.; Lin, T.; Wu, M.; Yang, P. Graphitic carbon nitride nanosheet and ferroelectric PbTiO3 nanoplates S-scheme heterostructure for enhancing hydrogen production and textile dye degradation. Chem. Eng. Sci. 2024, 295, 120133. [Google Scholar] [CrossRef]
- Abedini, E.; Roudgar-Amoli, M.; Alizadeh, A.; Shariatinia, Z. S-scheme heterojunctions based on novel Sm2CeMnO6 double perovskite oxide and g-C3N4 with excellent photocatalytic dye degradation performances. Environ. Sci. Pollut. Res. 2023, 30, 114956–114984. [Google Scholar] [CrossRef]
- Wang, J.; Ren, P.; Du, Y.; Zhao, X.; Chen, Z.; Pei, L.; Jin, Y. Construction of tubular g-C3N4/TiO2 S-scheme photocatalyst for high-efficiency degradation of organic pollutants under visible light. J. Alloys Compd. 2023, 947, 169659. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Zhou, C.; Xiong, W.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132. [Google Scholar] [CrossRef]
- Barzegar, M.H.; Sabzehmeidani, M.M.; Ghaedi, M.; Avargani, V.M.; Moradi, Z.; Roy, V.A.L.; Heidari, H. S-scheme heterojunction g-C3N4/TiO2 with enhanced photocatalytic activity for degradation of a binary mixture of cationic dyes using solar parabolic trough reactor. Chem. Eng. Res. Des. 2021, 174, 307–318. [Google Scholar] [CrossRef]
- Riazati, P.; Sheibani, S. Enhancing photocatalytic water remediation by g-C3N4 through controlled CuO content in an S-scheme g-C3N4/CuO nanocomposite. J. Alloys Compd. 2025, 1016, 179008. [Google Scholar] [CrossRef]
- Ajami, A.; Sheibani, S.; Ataie, A. S-scheme CoFe2O4/g-C3N4 nanocomposite with high photocatalytic activity and antibacterial capability under visible light irradiation. J. Mater. Res. Technol. 2024, 30, 2168–2185. [Google Scholar] [CrossRef]
- Alsalme, A.; Alsaeedi, H.; Fayez, M.; Elmoneim, K.M.A.; Soltan, A.; Fahmy, M.; Ahmed, M.A. Sonochemical preparation of powerful S-scheme Zr(HPO4)2/g-C3N4 heterojunction for photocatalytic degradation of rhodamine B under natural solar radiations. J. Phys. Chem. Solids 2025, 196, 112392. [Google Scholar] [CrossRef]
- Chopan, N.A.; Chishti, H.T.N. Polypyrrole-decorated ZnO/g-C3N4 S-scheme photocatalyst for rhodamine B dye degradation: Mechanism and antibacterial activity. Mater. Today Chem. 2023, 32, 101643. [Google Scholar] [CrossRef]
- Shoaib, M.; Naz, M.Y.; Shukrullah, S.; Munir, M.A.; Irfan, M.; Rahman, S.; Ghanim, A.A. Dual S-Scheme Heterojunction CdS/TiO2/g-C3N4 Photocatalyst for Hydrogen Production and Dye Degradation Applications. ACS Omega 2023, 8, 43139–43150. [Google Scholar] [CrossRef]
- Alsalme, A.; Hassan, M.M.; Eltawil, M.A.; Amin, A.E.; Soltan, A.; Messih, M.F.A.; Ahmed, M.A. Rational sonochemical engineering of Ag2CrO4/g-C3N4 heterojunction for eradicating RhB dye under full broad spectrum. Heliyon 2024, 10, e31221. [Google Scholar] [CrossRef]
- Alsulmi, A.; Hussein, I.A.; Nasherty, M.; Hesham, M.; Soltan, A.; Messih, M.F.A.; Ahmed, M.A. Sonochemical Fabrication of S-Scheme AgI/g-C3N4 Heterojunction for Efficient Photocatalytic Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2023, 34, 640–654. [Google Scholar] [CrossRef]
- Rajendran, R.; Vignesh, S.; Suganthi, S.; Raj, V.; Kavitha, G.; Palanivel, B.; Shkir, M.; Algarni, H. g-C3N4/TiO2/CuO S-scheme heterostructure photocatalysts for enhancing organic pollutant degradation. J. Phys. Chem. Solids 2022, 161, 110391. [Google Scholar] [CrossRef]
- Alsulmi, A.; Mohammed, N.N.; Hassan, M.M.; Eltawil, M.A.; Amin, A.E.; Fahmy, M.; Sultan, A.; Ahmed, M.A. Rational engineering of S-scheme CeO2/g-C3N4 heterojunctions for effective photocatalytic destruction of rhodamine B dye under natural solar radiations. Colloids Surf. A 2024, 689, 133683. [Google Scholar] [CrossRef]
- Alsalme, A.; Hesham, M.; Soltan, A.; Mohammed, N.N.; Nejm, A.Q.; Messih, M.F.A.; Hussein, I.A.; Ahmed, M.A. Pioneering the design of S-scheme SnS2/g-C3N4 nanocomposites via sonochemical and physical mixing methods for solar degradation of cationic rhodamine B dye. Mater. Adv. 2024, 5, 7278–7295. [Google Scholar] [CrossRef]
- Alsulmi, A.; Shaker, M.H.; Basely, A.M.; Abdel-Messih, M.F.; Sultan, A.; Ahmed, M.A. Engineering S-scheme Ag2CO3/g-C3N4 heterojunctions sonochemically to eradicate Rhodamine B dye under solar irradiation. RSC Adv. 2023, 13, 12229–12243. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhu, F.; Xiao, F.; Zhang, G.; Hou, H.; Bi, J.; Yan, S.; Hao, H. Construction of S-scheme heterogeneous HKUST-1/g-C3N4 with the piezoelectric effect for enhanced piezo-photocatalytic performance. Solid State Sci. 2023, 144, 107303. [Google Scholar] [CrossRef]
- Basely, A.M.; Shaker, M.H.; Helmy, F.M.; Abdel-Messih, M.F.; Ahmed, M.A. Construction of Bi2S3/g-C3N4 step S-scheme heterojunctions for photothermal decomposition of rhodamine B dye under natural sunlight radiations. Inorg. Chem. Commun. 2023, 148, 110300. [Google Scholar] [CrossRef]
- Elavarasan, N.; Vignesh, S.; Srinivasan, M.; Venkatesh, G.; Palanisamy, G.; Ramasamy, P.; Palanivel, B.; Al-Enizi, A.M.; Ubaidullah, M.; Minnam Reddy, V.R.; et al. Synergistic S-Scheme mechanism insights of g-C3N4 and rGO combined ZnO-Ag heterostructure nanocomposite for efficient photocatalytic and anticancer activities. J. Alloys Compd. 2022, 906, 164255. [Google Scholar] [CrossRef]
- Rajendran, R.; Mani, A. Photocatalytic, antibacterial and anticancer activity of silver-doped zinc oxide nanoparticles. J. Saudi Chem. Soc. 2020, 24, 1010–1024. [Google Scholar] [CrossRef]
- Lee, S.J.; Begildayeva, T.; Jung, H.J.; Koutavarapu, R.; Yu, Y.; Choi, M.; Choi, M.Y. Plasmonic ZnO/Au/g-C3N4 nanocomposites as solar light active photocatalysts for degradation of organic contaminants in wastewater. Chemosphere 2021, 263, 128262. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. One pot synthesis and characterization of Ag-ZnO/g-C3N4 photocatalyst with improved photoactivity and antibacterial properties. Colloids Surf. A 2015, 482, 477–484. [Google Scholar] [CrossRef]
- Sharma, K.; Sudhaik, A.; Raizada, P.; Thakur, P.; Pham, X.M.; Van Le, Q.; Nguyen, V.; Ahamad, T.; Thakur, S.; Singh, P. Constructing α-Fe2O3/g-C3N4/SiO2 S-scheme-based heterostructure for photo-Fenton like degradation of rhodamine B dye in aqueous solution. Environ. Sci. Pollut. Res. 2023, 30, 124902–124920. [Google Scholar] [CrossRef]
- Bui, D.; Nguyen, T.; Le Vo, T.T.; Cao, T.M.; You, S.; Pham, V.V. SnO2–x Nanoparticles Decorated on Graphitic Carbon Nitride as S-Scheme Photocatalysts for Activation of Peroxymonosulfate. ACS Appl. Nano Mater. 2021, 4, 9333–9343. [Google Scholar] [CrossRef]
- Mishra, S.; Acharya, L.; Sharmila, S.; Sanjay, K.; Acharya, R. Designing g-C3N4/NiFe2O4 S-scheme heterojunctions for efficient photocatalytic degradation of Rhodamine B and tetracycline hydrochloride. Appl. Surf. Sci. Adv. 2024, 24, 100647. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, J.; Brouzgou, A.; Wang, A.; Jing, S.; Kannan, P.; Chen, F.; Tsiakaras, P. Efficient photocatalytic H2O2 production and photodegradation of RhB over K-doped g-C3N4/ZnO S-scheme heterojunction. J. Colloid Interface Sci. 2025, 677, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Qin, X.; Cheng, S.; Liu, S. ZnO/g-C3N4 S scheme photocatalytic material with visible light response and enhanced photocatalytic performance. Diam. Relat. Mater. 2023, 137, 110143. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging chemical functionalization of g-C3N4: Covalent/noncovalent modifications and applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]
- Sun, K.; Wang, Y.; Chang, C.; Yang, S.; Di, S.; Niu, P.; Wang, S.; Li, L. Molten-salt synthesis of crystalline C3N4/C nanosheet with high sodium storage capability. Chem. Eng. J. 2021, 425, 131591. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Raju, G.S.R.; Pavitra, E.; Kwak, C.H.; Han, Y.-K.; Huh, Y.S. Ultrasound-assisted heterogeneous degradation of tetracycline over flower-like rGO/CdWO4 hierarchical structures as robust solar-light-responsive photocatalysts: Optimization, kinetics, and mechanism. Appl. Surf. Sci. 2019, 489, 110–122. [Google Scholar] [CrossRef]
- Sun, F.; Xu, D.; Xie, Y.; Liu, F.; Wang, W.; Shao, H.; Ma, Q.; Yu, H.; Yu, W.; Dong, X. Tri-functional aerogel photocatalyst with an S-scheme heterojunction for the efficient removal of dyes and antibiotic and hydrogen generation. J. Colloid Interface Sci. 2022, 628, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.L.; Matter, F.; Schreck, M.; Wohlwend, J.; Tervoort, E.; Colbeau-Justin, C.; Niederberger, M. Monolithic metal-containing TiO2 aerogels assembled from crystalline pre-formed nanoparticles as efficient photocatalysts for H2 generation. Appl. Catal. B Environ. 2020, 267, 118660. [Google Scholar] [CrossRef]
- Sun, F.; Qi, H.; Xie, Y.; Xu, D.; Yu, W.; Ma, Q.; Yang, Y.; Yu, H.; Dong, X. Self-standing Janus nanofiber heterostructure photocatalyst with hydrogen production and degradation of methylene blue. J. Am. Ceram. Soc. 2022, 105, 1428–1441. [Google Scholar] [CrossRef]
- Pitcheri, R.; Mooni, S.P.; Harikrishnan, L.; Raghav, J.; Roy, S.; Maaouni, N.; Radhalayam, D.; Alothman, A.A.; Alharbi, A.F.; Al, Z.F.A.M.; et al. Novel S-scheme β-Cu2V2O7/Ni/Pg-C3N4 heterojunction photocatalyst for sunlight-induced degradation of RhB. Surf. Interfaces 2024, 52, 104950. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K. TiO2-modified g-C3N4 nanocomposite for photocatalytic degradation of organic dyes in aqueous solution. Heliyon 2022, 8, e10683. [Google Scholar] [CrossRef] [PubMed]
- Khamesan, A.; Esfahani, M.M.; Ghasemi, J.B.; Farzin, F.; Parsaei-Khomami, A.; Mousavi, M. Graphitic-C3N4/ZnCr-layered double hydroxide 2D/2D nanosheet heterojunction: Mesoporous photocatalyst for advanced oxidation of azo dyes with in situ produced H2O2. Adv. Powder Technol. 2022, 33, 103777. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Mallah, A.; Abdelrahman, E.A.; Raza, N.; Alqarni, L.S.; Ismail, M.; Modwi, A. Boosting of photocatalytic degradation of Malachite green dye on facile synthesized highly active Ca@TiO2@g-C3N4 photocatalyst: Photocatalytic mechanism and kinetics. Inorg. Chem. Commun. 2024, 170, 113464. [Google Scholar] [CrossRef]
- Madona, J.; Sridevi, C.; Indumathi, N.; Gokulavani, G.; Velraj, G. A novel carbon doped CeO2/g-C3N4 heterostructure for disinfection of microorganisms and degradation of Malachite green and Amoxicillin under sunlight. Surf. Interfaces 2024, 44, 103803. [Google Scholar] [CrossRef]
- Rana, A.; Sonu, S.; Sudhaik, A.; Kumar, R.; Chawla, A.; Raizada, P.; Chaudhary, V.; Ahamad, T.; Kaya, S.; Kumar, N.; et al. Tailoring dual S-Scheme based g-C3N4/ZnO/TiO2 ternary photocatalytic system immobilized on floating cork for environmental remediation. J. Taiwan Inst. Chem. Eng. 2025, 168, 105914. [Google Scholar] [CrossRef]
- Gou, L.; Wang, W.; Liu, E.-Z.; Xu, L.; He, R.; Yang, Y. Fabrication of MOF-derived CoTiO3/g-C3N4 S-scheme heterojunction for photocatalyst wastewater treatment. J. Alloys Compd. 2022, 918, 165698. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Olatunde, O.C.; Nkwe, V.M.; Ben Smida, Y.; Ferjani, H. Dual S-scheme heterojunction g-C3N4/Bi2S3/CuS composite with enhanced photocatalytic activity for methyl orange degradation. Inorg. Chem. Commun. 2023, 155, 111075. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Zhu, J.; Li, H.; Fang, Y.; Liu, R.; Yang, C.; Liu, W.; Ding, C.; Liu, Q.; et al. Activated CdS/sulfur doped g-C3N4 photocatalyst for dye and antibiotic degradation: Experimental and DFT verification of S-scheme heterojunction. Environ. Res. 2025, 266, 120487. [Google Scholar] [CrossRef]
- Liu, H.; Sun, F.; Li, X.; Ma, Q.; Liu, G.; Yu, H.; Yu, W.; Dong, X.; Su, Z. g-C3N4/TiO2/ZnIn2S4 graphene aerogel photocatalysts with double S-scheme heterostructure for improving photocatalytic multifunctional performances. Compos. Part B Eng. 2023, 259, 110746. [Google Scholar] [CrossRef]
- Yang, W.; Tang, S.; Wei, Z.; Chen, X.; Ma, C.; Duan, J.; Tan, R. Separate-free BiPO4/graphene aerogel with 3D network structure for efficient photocatalytic mineralization by adsorption enrichment and photocatalytic degradation. Chem. Eng. J. 2021, 421, 129720. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.; Wang, Y.; Zhang, H.; Tang, H.; Zhang, H.; Zhang, G.; Wang, Y.; Zhang, F.; Yan, H. Photo-induced synthesis of ternary Pt/rGO/COF photocatalyst with Pt nanoparticles precisely anchored on rGO for efficient visible-light-driven H2 evolution. J. Colloid Interface Sci. 2022, 608, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.; Cihlar, J.; Chang, C.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Tian, Y.; Xia, L.; Wu, Y.; Yu, J.; Bagley, D.M.; Sun, J.; Fan, M. A novel and high-performance double Z-scheme photocatalyst ZnO-SnO2-Zn2SnO4 for effective removal of the biological toxicity of antibiotics. J. Hazard. Mater. 2020, 399, 123017. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, X.; Ma, L.; Sun, F.; Yue, B.; Ma, Q.; Wang, J.; Liu, G.; Yu, H.; Yu, W.; et al. A three-dimensional TiO2/C/BiOBr graphene aerogel for enhancing photocatalysis and bidirectional sulfur conversion reactions in lithium-sulfur batteries. J. Environ. Chem. Eng. 2022, 10, 108606. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Okab, A.A.; Graimed, B.H.; Issa, M.A.; Ammar, S.H. Photocatalytic destruction of Congo red dye in wastewater using a novel Ag2WO4/Bi2S3 nanocomposite decorated g-C3N4 nanosheet as ternary S-scheme heterojunction: Improving the charge transfer efficiency. Diam. Relat. Mater. 2023, 133, 109711. [Google Scholar] [CrossRef]
- Liu, K.; Chen, J.; Sun, F.; Yu, J.; Zhang, X.; Xu, Y.; Liu, Y.; Tang, M.; Yang, Y. Enhanced degradation of azo dyes wastewater by S-scheme heterojunctions photocatalyst g-C3N4/MoS2 intimately coupled Rhodopseudomonas palustris with chitosan modified polyurethane sponge carrier. Int. J. Hydrogen Energy 2023, 48, 22319–22333. [Google Scholar] [CrossRef]
- Hassan, A.E.; Elsayed, M.H.; Hussien, M.S.A.; Mohamed, M.G.; Kuo, S.; Chou, H.; Yahia, I.S.; Mohamed, T.A.; Wen, Z. V2O5 nanoribbons/N-deficient g-C3N4 heterostructure for enhanced visible-light photocatalytic performance. Int. J. Hydrogen Energy 2023, 48, 9620–9635. [Google Scholar] [CrossRef]
- Yaqoubi, M.; Ghanbari, M.; Maya, R.W.; Jasim, H.A.; Salavati-Niasari, M. Synthesis and characterization of S-Scheme NiMn2O4/g-C3N4 nanocomposites heterojunction photocatalyst for effective degradation of organic pollutants under visible light. Results Eng. 2025, 25, 104037. [Google Scholar] [CrossRef]
- Alqarni, L.S.; Alghamdi, M.D.; Alhussain, H.; Elamin, N.Y.; Taha, K.K.; Modwi, A. S-scheme MgO-TiO2@g-C3N4 nanostructures as efficient photocatalyst for alizarin red S photodegradation. J. Mater. Sci. Mater. Electron. 2024, 35, 11996. [Google Scholar] [CrossRef]
- Hosseini, M.; Ghanbari, M.; Dawi, E.A.; Shuhata Alubiady, M.H.; Al-Ani, A.M.; Alkaim, A.F.; Salavati-Niasari, M. CaSnO3/g-C3N4 S-scheme heterojunction photocatalyst for the elimination of erythrosine and eriochrome black T from water under visible light. Results Eng. 2024, 21, 101903. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Ma, Z.; Wang, Z.; Jin, S.; Hu, J.; Xu, P.; Chen, Y. Graphitic Carbon Nitride-Based S-Scheme Heterojunctions: Recent Advances in Photocatalytic Dye Degradation. Catalysts 2025, 15, 592. https://doi.org/10.3390/catal15060592
Song X, Ma Z, Wang Z, Jin S, Hu J, Xu P, Chen Y. Graphitic Carbon Nitride-Based S-Scheme Heterojunctions: Recent Advances in Photocatalytic Dye Degradation. Catalysts. 2025; 15(6):592. https://doi.org/10.3390/catal15060592
Chicago/Turabian StyleSong, Xiaofang, Zhenxing Ma, Zhiyong Wang, Shiyi Jin, Jingding Hu, Penghui Xu, and Yijiang Chen. 2025. "Graphitic Carbon Nitride-Based S-Scheme Heterojunctions: Recent Advances in Photocatalytic Dye Degradation" Catalysts 15, no. 6: 592. https://doi.org/10.3390/catal15060592
APA StyleSong, X., Ma, Z., Wang, Z., Jin, S., Hu, J., Xu, P., & Chen, Y. (2025). Graphitic Carbon Nitride-Based S-Scheme Heterojunctions: Recent Advances in Photocatalytic Dye Degradation. Catalysts, 15(6), 592. https://doi.org/10.3390/catal15060592







