Abstract
The development of photothermal-assisted photocatalytic systems with broad-spectrum solar utilization and high charge separation efficiency remains a critical challenge for antibiotic degradation. Herein, we report novel black g-C3N4 (BCN) materials synthesized via a one-step thermal copolymerization strategy using C/N precursors and tetrachlorofluorescein. After the introduction of tetrachlorofluorescein, the color of the sample changes, which gives BCN enhanced light absorption and a significant photothermal effect for poorly heating-assisted photocatalysis. The synergistic coupling of photothermal and photocatalytic processes enabled the optimal BCN-U sample to achieve exceptional degradation efficiency (89% within 120 min) for a typical antibiotic (e.g., tetracycline) under an LED lamp as the visible light source, outperforming conventional yellow g-C3N4 (YCN-U) by a factor of 1.37. Mechanistic studies revealed that the photothermal effect facilitates carrier separation via thermal-driven electron excitation while accelerating reactive oxygen species (•OH and •O2−) generation. The synergistic interplay between photocatalysis and photothermal effects, which improved mass transfer, ensures robust stability, which provides new insights into designing dual-functional carbon nitride-based materials for sustainable environmental remediation.
1. Introduction
The pervasive contamination of aquatic ecosystems by antibiotic residues has emerged as a global environmental crisis, threatening biodiversity and human health [1]. Over 100,000 tons of antibiotics are discharged annually into water bodies, with concentrations detected up to μg/L levels in rivers near pharmaceutical industries and agricultural zones [2]. These residues not only foster antibiotic-resistant bacteria but also disrupt aquatic microbiota, leading to irreversible ecological imbalances [3]. The widespread presence of antibiotic residues in aquatic environments has triggered severe ecological and health risks, driving urgent demands for advanced remediation technologies [4]. Conventional remediation methods, such as adsorption, chlorination, and biological treatment, face limitations in selectivity, secondary pollution, and inefficiency against biorecalcitrant antibiotics. In contrast, photocatalysis offers a solar-powered, chemical-free solution for antibiotic mineralization by generating reactive oxygen species (ROS) capable of breaking complex molecular structures [5].
Graphitic carbon nitride (g-C3N4), a metal-free semiconductor, has garnered attention for its tunable electronic structure and chemical stability [6]. However, pristine g-C3N4 exhibits restricted light absorption and sluggish charge kinetics, hindering its practical application [7,8]. Recent advances propose integrating photothermal effects with photocatalysis to enhance solar conversion efficiency, where localized heat promotes charge separation and accelerates surface reactions [9,10]. Nevertheless, designing such materials with broad-spectrum response and optimized energy band alignment remains challenging.
In this work, we engineer a black g-C3N4 (BCN) material via a facile thermal copolymerization approach, employing melamine as C/N precursors and tetrachlorofluorescein as a synergistic modifier. The tetrachlorofluorescein incorporation induces surface carbonization to create a photothermal layer, which boosts light-to-heat conversion. Compared with yellow g-C3N4 (YCN), the BCN enhanced carrier mobility and utilization of full-spectrum solar energy for poorly heating-assisted photocatalysis. Combined experimental and theoretical analyses elucidate how photothermal energy alleviates recombination losses by thermally exciting trapped electrons and lowering activation barriers for reactive oxygen species (ROS) generation. This study not only demonstrates a rational strategy to reconcile photocatalysis and photothermal effects in carbon nitride-based systems but also offers mechanistic insights into their synergism for high-performance antibiotic degradation.
2. Results and Discussion
X-ray diffraction (XRD) was employed to measure and compare the prepared materials (Figure 1a), and, as the figure indicates, two diffraction peaks at 13.1° and 27.4° were detected in all four samples. By comparing with the existing literature, two distinct characteristic diffraction peaks were identified for the original CN sample: The first peak at 13.1° corresponds to the (001) plane structure, which specifically represents the arrangement of triazine units within the g-C3N4 network [11]. The second peak at 27.4° correlates with the (002) plane, indicating the periodic accumulation of carbon nitride nanosheets along the C-axis [12,13]. The presence of these characteristic peaks confirms that there was no significant alteration in the preparation of BCN. Fourier transform infrared spectroscopy (FT-IR) was further utilized to ascertain the functional groups of the synthesized samples. The spectral analysis of the prepared samples (Figure 1b) demonstrated that the FT-IR spectra of BCN-U, BCN-D, and BCN-M were consistent with the typical vibrational modes of YCN-U, indicating that the addition of tetrachlorofluorescein to different precursors did not significantly alter the functional group structure of CN. The typical absorption band in the range of 1200–1700 cm−1 represents the stretching pattern of the distinctive aromatic CN heterocyclic ring [14], while the broad band in the 3000–3500 cm−1 range may correspond to the stretching vibrations of the O-H and N-H bonds adsorbed on the CN surface [15]. The peak signal at 810 cm−1 is attributed to the vibrational peak of the triazine unit in CN, resulting from characteristic stretching [16]. The characterization results obtained from XRD and FT-IR reveal that following calcination, both tetrachlorofluorescein and the CN precursor retain their inherent carbon nitride structure; however, a notable alteration in color is observed.
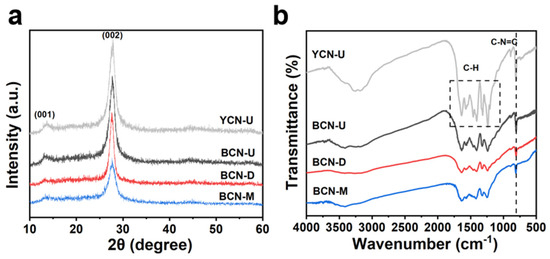
Figure 1.
(a) XRD patterns and (b) FT-IR spectra of YCN-U, BCN-U, BCN-D, and BCN-M.
The chemical composition and state of YCN and BCN samples were elucidated through X-ray photoelectron spectroscopy (XPS) analysis (Figure 2a). As illustrated in the XPS spectra presented in Figure 2b, the high-resolution C 1s spectra of the BCN photocatalyst and YCN exhibit three prominent peaks at 284.7 and 287.6 eV, which correspond to C-C and C-N-H bonds, respectively [17,18]. Furthermore, Figure 2c depicts the high-resolution N 1s spectra of BCN and YCN, revealing three peaks at 397.5 eV, 398.7 eV, and 400.3 eV, attributed to the triazine ring (C-N=C, N2c), the N-(C)3 group, and the N-H bond, respectively [19]. In addition, the high-resolution O 1s spectra for BCN and YCN exhibit two peaks at binding energies of 529.9 eV and 531.3 eV, which are associated with the C=O and C-O-H bonds, respectively (Figure 2d) [20,21]. In summary, the XPS analysis tests provide strong evidence for the successful preparation of BCN with CN characteristics.
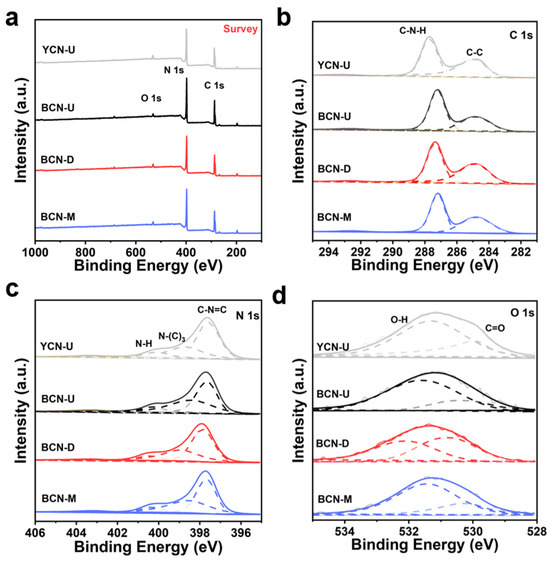
Figure 2.
(a) XPS survey spectra and high-resolution XPS spectra of (b) C 1s, (c) N 1s, and (d) O 1s for YCN-U, BCN-U, BCN-D, and BCN-M.
To evaluate the photocatalytic degradation efficiency of the synthesized photocatalyst, a series of experiments were conducted under visible light irradiation using tetracycline (TC) at a concentration of 30 mg/L as the target pollutant. To ensure an accurate assessment of the degradation performance, a standardized operational experiment was implemented. Initially, 20 mg of catalyst was dispersed in 50 mL of TC solution and continuously stirred for 30 min in a dark environment to establish dynamic equilibrium between adsorption and desorption on the catalyst surface. Following this equilibration period, illumination was initiated, and changes in TC concentration were quantitatively monitored using a UV-Vis spectrophotometer by detecting the characteristic absorption peak at a wavelength of 357 nm through timed sampling. As illustrated in Figure 3a, the degradation efficiency of the original yellow carbon nitride (YCN-U) reached only 65% after 120 min. In contrast, samples from the black carbon nitride series exhibited significantly enhanced photocatalytic performance (BCN-U: 89%, BCN-D: 86%, BCN-M: 80%). Notably, the superior degradation capability observed in BCN-U samples can be attributed to their modification into carbon nitride with color change, which results in higher charge transfer efficiency under visible light irradiation compared to those seen with YCN-U. This photothermal synergistic effect facilitates effective charge separation and enhances photo-generated carrier dynamics. The pseudo-first-order kinetic model was employed to analyze the photocatalytic degradation kinetics of TC, yielding a corresponding kinetic constant for the synthesized photocatalyst (Figure 3b,c). From the linear equation fit in Table S1, we can see that BCN-U exhibited a kinetic constant of 0.0166 min−1, which is 1.86 times greater than that of YCN-U (0.0089 min−1), which further substantiates the high degradation rate associated with black carbon nitride. Furthermore, to assess its practical application potential, a cyclic degradation test was conducted involving four consecutive cycles (Figure 3d). Following these repeated experiments, the degradation rate remained consistently high, thereby confirming the sustained effectiveness of BCN-U, as well as highlighting its superiority as a photocatalyst and operational stability in practical applications.
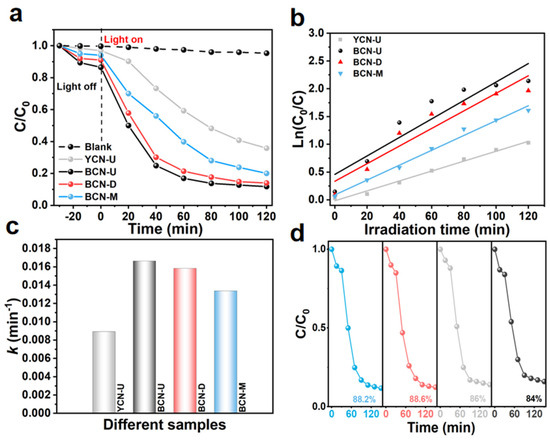
Figure 3.
(a) Photocatalytic degradation of TC over the as-prepared samples. (b) First-order degradation kinetic curves for the prepared samples and (c) the corresponding degradation rate constants. (d) Results from four-cycle experiments assessing the photocatalytic degradation of TC using the BCN-U photocatalyst.
The solar energy absorption capacity of the photocatalyst is critical in the photocatalytic degradation process. The optical properties of the YCN-U, BCN-U, BCN-D, and BCN-M photocatalysts were characterized using UV-Vis diffuse reflection spectroscopy (DRS) within the absorption wavelength range of 250–800 nm. As illustrated in Figure 4a, it is evident that YCN-U exhibits limited solar absorption; conversely, BCN-U, BCN-D, and BCN-M demonstrate significantly enhanced absorption in the visible range due to their strong light-absorbing characteristics associated with black materials [22]. Figure 4b presents a digital photograph of sample preparation, and YCN-U appears light yellow, but upon adding tetrachlorofluorescein to various precursors, the color of the resulting photocatalyst shifts to black, which enhances visible light absorption and improves the photothermal properties of the catalyst. Meanwhile, using the Kubelka–Munk model, the band gap values of YCN-U and BCN-U samples were calculated to be 2.84 eV and 2.76 eV, respectively (Figure S1). To elucidate how introducing tetrachlorofluorescein and different precursors contributes to enhanced photocatalytic degradation activity, powders of YCN-U, BCN-U, BCN-D, and BCN-M were irradiated for 90 s using visible light irradiation. Surface temperature measurements were recorded at intervals of 30 s utilizing an infrared thermal camera. The temperature changes observed for YCN-U, BCN-U, BCN-D, and BCN-M are depicted in Figure 4c,d. Notably, the temperature of BCN-U rises rapidly from 33.7 to 57.5 °C, significantly higher than that observed for YCN-U (41.3 °C), which can be attributed to the modification induced by tetrachlorofluorescein in transforming YCN-U into a black material, which subsequently enhances its poorly heating-assisted photocatalysis. In Figure 4e, it is shown that BCN-U exhibits a high intensity response in terms of photocurrent generation; this finding substantiates that the modified photocatalyst possesses robust electron transfer capabilities. Furthermore, as ambient temperature increases, the intensity response of BCN-U’s photocurrent also escalates, indicating that elevated temperatures further facilitate electron migration and transfer, thereby enhancing catalytic degradation activity. From the electrochemical impedance spectroscopy (EIS) plot presented in Figure 4f, it can be seen that the arc radius of the BCN-U sample is smaller than that of the YCN-U sample and exhibits a decrease with increasing ambient temperature, which further substantiates the notion that temperature exerts a facilitating effect on electron transfer.
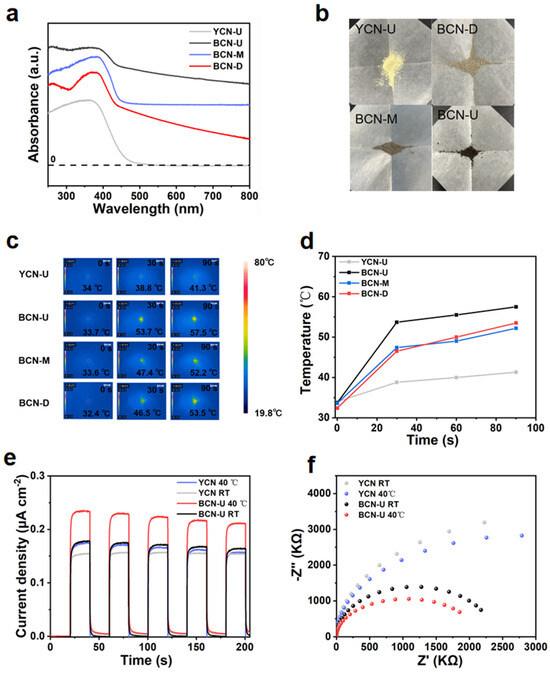
Figure 4.
(a) UV-Vis spectra of YCN-U, BCN-U, BCN-D, and BCN-M photocatalysts. (b) Digital photographs of YCN-U, BCN-U, BCN-D, and BCN-M powders. (c) Photothermal infrared thermal images of YCN-U, BCN-U, BCN-D, and BCN-M, along with (d) the corresponding temperature curves within 90 s irradiation. (e) Transient photocurrent response curves and (f) EIS plot for YCN-U and BCN-U at RT and 40 °C conditions.
The radical quenching experiment elucidated the predominant types of active free radicals generated during the photocatalytic degradation of TC (Figure 5a,b). The superoxide free radical (•O2−), hole (h+), and hydroxyl free radical (•OH) were effectively quenched by 1,4-benzoquinone (BQ), triethanolamine (TEOA), and isopropyl alcohol (IPA), respectively [23]. In comparison to the control group, the removal rate of TC reached an impressive 89%, while the introduction of IPA as a quencher resulted in a reduction of TC degradation efficiency to 60%, indicating the significant involvement of •OH in this process. Furthermore, when BQ was utilized as a quencher for the •O2−, it markedly inhibited the overall degradation efficiency of TC, suggesting that •O2− is the principal active radical responsible for its degradation. Notably, it is essential to emphasize that the addition of TEOA led to a discernible decrease in TC degradation efficiency, implying that h+ plays a secondary role in this complex degradation mechanism.
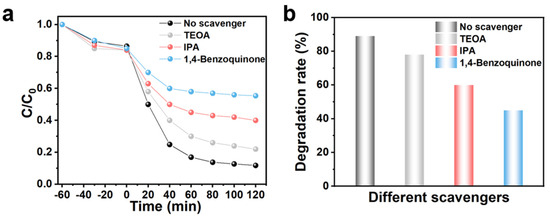
Figure 5.
(a) Degradation curves of BCN-U as a photocatalyst after adding different radical scavengers and (b) corresponding degradation rate.
Incorporating the previously discussed analysis, this study explores the mechanism by which photothermally assisted photocatalytic degradation of TC occurs utilizing a BCN-U photocatalyst under visible light exposure (Figure 6). As a cutting-edge photothermal material, BCN-U presents notable benefits in promoting effective photocatalytic degradation, due to its exceptional light absorption features and remarkable capabilities for photothermal conversion, which provide the BCN-U photocatalyst with considerable efficacy in the degradation process (Figure S2). When subjected to visible light, the BCN-U photocatalyst produces electrons and holes, which aids in the separation of charge carriers. Interestingly, the BCN-U material exhibits significant color shading, leading to an improvement in its photothermal effect as well as an increased reaction temperature, which further accelerates the efficient separation of the photo-generated charges. Subsequently, the generated electrons may be transferred to the conduction band (CB) of BCN and interact with dissolved oxygen in water to create •O2−. Meanwhile, the holes in the valence band (VB) react with water molecules, resulting in the formation of •OH. Ultimately, these reactive species (•O2−, •OH, and h+) proficiently decompose TC into harmless and non-toxic smaller molecules, such as CO2 and H2O.
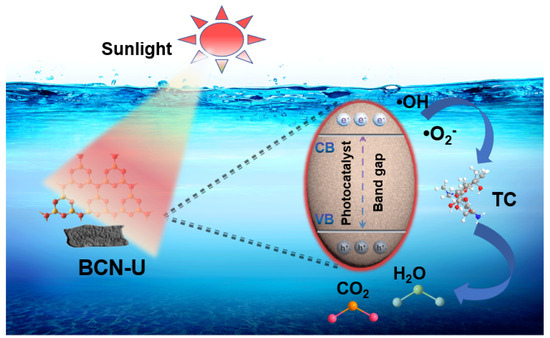
Figure 6.
Proposed mechanism of BCN-U photocatalyst for photothermal-enhanced photocatalytic TC degradation under light irradiation.
3. Experimental Section
3.1. Materials
Tetrachlorofluorescein (C20H4Br4Cl4O5), dicyandiamide (C2H4N4), melamine (C3H6N6), and urea (CH4N2O) were supplied by Shanghai Aladdin Biochemical Technology Co. Ltd. (Shanghai, China).
3.2. Synthesis of BCN Samples
The black g-C3N4 (denoted as BCN) samples were prepared by a simple mixing and calcination method of C/N precursors and tetrachlorofluorescein (Scheme 1). Specifically, the 3 g of C/N precursors (such as urea, dicyandiamide, and melamine) were finely ground with 0.03 g of tetrachlorofluorescein and subsequently transferred to a crucible for calcination in a muffle furnace. The mixture was heated to 520 °C at a rate of 5 °C per minute and maintained at this temperature for a duration of 2 h. Following the heating process, the crucible was allowed to cool naturally, and the resulting black powders were rinsed with water and anhydrous ethanol. The powders were then dried and collected for further use; the resultant samples were designated BCN-U, BCN-D, and BCN-M, respectively. For the comparison, the yellow g-C3N4 was also prepared by a calcination method of urea precursors based on the similar synthesis method described above, except without tetrachlorofluorescein and denoted as YCN-U.
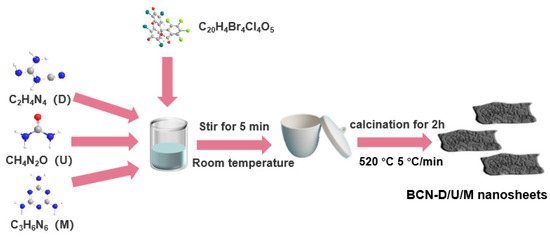
Scheme 1.
Diagrammatic sketch of the synthesis process of BCN samples.
3.3. Photocatalytic Activity Experiments
The photocatalytic degradation performance of the synthesized sample was assessed by evaluating its degradation efficiency for tetracycline (TC) solution under visible light radiation. Degradation experiments were conducted using a parallel photochemical reaction instrument (CEL-LAB200E7, Beijing Zhongqiao Jinyuan Technology Co., Ltd., Beijing, China). A specific amount of photocatalyst powder was dispersed into 50 mL of TC aqueous solution at a concentration of 30 mg/L and stirred in a dark environment for 30 min to achieve adsorption-desorption equilibrium. An LED lamp served as the visible light source during the photocatalytic degradation experiment, which lasted for 60 min. Every 20 min, a 2 mL aliquot of the reaction solution was taken, and after centrifugation, the degree of photocatalytic degradation of TC was measured using a UV-2450 spectrophotometer (Shimadzu, Tokyo, Japan) at a maximum wavelength of 357 nm.
3.4. Characterizations
X-ray diffraction (XRD) analysis was conducted to ascertain the lattice parameters and structural details, employing measurements of X-ray scattering angles and intensities. Fourier transformed infrared (FT-IR) spectroscopy (Nicolet 6700, Waltham, MA, USA) was employed to investigate infrared spectra within the 4000–500 cm−1 range. X-ray photoelectron spectroscopy (XPS) analysis was utilized for characterizing the chemical states of constituent elements. UV-vis diffuse reflectance spectroscopy was utilized to explore absorption, reflection, and transmission properties, encompassing absorption spectra, reflectance, and diffuse reflectance spectra. The thermal distribution within the evaporation unit was monitored via an FL-IR E2 infrared thermographic camera (Thermo Scientific™, Waltham, M, USA).
3.5. Photoelectrochemical Properties Measurements
Photoelectrochemical tests were conducted using a CHI660 workstation (CH Instruments Inc., Bee Cave, TX, USA) with a 0.5 M sodium sulfate solution. A saturated calomel electrode, the photocatalyst, and a platinum wire served as the reference electrode, working electrode, and counter electrode, respectively. The alternating current (AC) voltage ranged from 10 Hz to 105 Hz, while the open circuit voltage was maintained at 5 mV. The transient photocurrent response and electrochemical impedance spectrum of the sample were measured accordingly.
4. Conclusions
In this study, black g-C3N4 materials were successfully synthesized via a facile thermal polymerization strategy using C/N-rich precursors and tetrachlorofluorescein as a molecular modifier. The incorporation of tetrachlorofluorescein significantly enhanced the photothermal and photocatalytic properties of the material by broadening visible-light absorption and optimizing charge carrier separation. The synergistic coupling of photothermal and photocatalytic processes enabled the black g-C3N4 (BCN-U) to achieve exceptional degradation efficiency (89% within 120 min) for a typical antibiotic (e.g., tetracycline) under an LED lamp as the visible light source, outperforming conventional yellow g-C3N4 (YCN-U) by a factor of 1.37. Mechanistic studies revealed that the photothermal effect elevated the reaction temperature, accelerating charge transfer, while the narrowed bandgap and improved electron-hole separation facilitated the generation of reactive oxygen species (ROS), including •OH and •O2–, responsible for TC degradation. The BCN-U material also demonstrated robust stability over multiple cycles, with negligible performance loss, highlighting its practical potential. This work provides a strategy for designing high-performance photocatalysts by integrating a photothermal-assisted photocatalytic mechanism, offering new insights into addressing antibiotic contamination in aquatic environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15050504/s1, Figure S1. Band gap values of YCN-U and BCN-U samples by using the Kubelka-Munk model. Figure S2. Photothermal infrared thermal images of TC solution, YCN-U/TC solution, and BCN-U/TC solution during the real reaction process under LED irradiation. Table S1. Slopes and R2 of as-prepared samples.
Author Contributions
Conceptualization, W.S. and X.G.; methodology, X.G.; software, X.G.; validation, X.G., W.S., and F.G.; formal analysis, W.S.; investigation, W.S.; resources, W.S.; data curation, P.S.; writing—original draft preparation, X.G.; writing—review and editing, X.G.; visualization, W.S.; supervision, W.S.; project administration, F.G.; funding acquisition, F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the open fund of the Key Laboratory of Green Extraction & Efficient Utilization of Light Rare-Earth Resources (KLRE-KF-008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, W.; Zhang, H.; Chen, Y.; Shi, H. Efficient Degradation of Tetracycline via Coupling of Photocatalysis and Photo-Fenton Processes over a 2D/2D α-Fe2O3/g-C3N4 S-scheme Heterojunction Catalyst. Acta Phys. Chim. Sin. 2022, 38, 2201008. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, D.; Wang, Z.; Yi, L.; Sun, J.; Liu, D.; Yu, X.; Chen, Y. Bandgap engineering of carbon nitride by formic acid assisted thermal treatment for photocatalytic degradation of tetracycline hydrochloride. Chem. Eng. J. 2024, 485, 149830. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y.; Zheng, Y.; Meng, F. Antibiotics in mariculture systems: A review of occurrence, environmental behavior, and ecological effects. Environ. Pollut. 2022, 293, 118541. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Qiao, X.; Wang, Y.; Cai, X.; Zhou, C.; Zhang, Y.; Ding, G. DOM from mariculture ponds exhibits higher reactivity on photodegradation of sulfonamide antibiotics than from offshore seawaters. Water Res. 2018, 144, 365–372. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Barroga, S.Q.; Mathew, R.A.; Peña-Bahamonde, J.; Louie, S.M.; Perez, J.V.D.; Rodrigues, D.F. Use of polyaniline coating on magnetic MoO3 and its effects on material stability and visible-light photocatalysis of tetracycline. J. Environ. Chem. Eng. 2022, 10, 107635. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, C.; Xu, N.; Yang, J.; Gao, G.; Ding, J. A Floating Integrated Solar Micro-Evaporator for Self-Cleaning Desalination and Organic Degradation. Adv. Funct. Mater. 2023, 33, 2214769. [Google Scholar] [CrossRef]
- Ko, J.W.; Choi, W.S.; Kim, J.; Kuk, S.K.; Lee, S.H.; Park, C.B. Self-Assembled Peptide-Carbon Nitride Hydrogel as a Light-Responsive Scaffold Material. Biomacromolecules 2017, 18, 3551–3556. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Geng, L.; Tan, X.; Hu, Y.; Mu, P.; Li, J. Reduced graphene oxide/carbon nitride composite sponge for interfacial solar water evaporation and wastewater treatment. Chemosphere 2023, 311, 137163. [Google Scholar] [CrossRef]
- Shan, P.; Hao, P.; Geng, K.; Xue, W.; Xiong, B.; Hou, J.; Guo, F.; Sun, Y.; Shi, W. Hierarchical confinement effect of Co-Co Prussian blue analogues for photothermal-assisted photocatalytic H2 production. Chem. Eng. J. 2025, 509, 161502–161512. [Google Scholar] [CrossRef]
- Shan, P.; Geng, K.; Guo, L.; Kuang, L.; Shen, Y.; Xiong, B.; Hou, J.; Guo, F.; Wang, G.; Shi, W. Synergistic effects of photothermal response and Schottky junction for enhanced photothermal-assisted photocatalytic hydrogen production. Chem. Eng. J. 2025, 513, 162801–162811. [Google Scholar] [CrossRef]
- Fang, K.; Du, C.; Zhang, J.; Zhou, C.; Yang, S. Molecular engineering of a synergistic photocatalytic and photothermal membrane for highly efficient and durable solar water purification. J. Membr. Sci. 2022, 663, 121037. [Google Scholar] [CrossRef]
- Zhou, A.; Yang, K.; Wu, X.; Liu, G.; Zhang, T.C.; Wang, Q.; Luo, F. Functionally-Designed Chitosan-based hydrogel beads for adsorption of sulfamethoxazole with light regeneration. Sep. Purif. Technol. 2022, 293, 120973. [Google Scholar] [CrossRef]
- Tessema, A.A.; Wu, C.-M.; Motora, K.G.; Naseem, S. Highly-efficient and salt-resistant CsxWO3@g-C3N4/PVDF fiber membranes for interfacial water evaporation, desalination, and sewage treatment. Compos. Sci. Technol. 2021, 211, 108865. [Google Scholar] [CrossRef]
- Su, H.; Zhou, J.; Miao, L.; Shi, J.; Gu, Y.; Wang, P.; Tian, Y.; Mu, X.; Wei, A.; Huang, L.; et al. A hybrid hydrogel with protonated g-C3N4 and graphene oxide as an efficient absorber for solar steam evaporation. Sustain. Mater. Technol. 2019, 20, e00095. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, W.; Du, Y.; Shi, H.; Zeng, G.; Yan, X.; Li, X. The g-C3N4 decorated carbon aerogel with integrated solar steam generation and photocatalysis for effective desalination and water purification. Desalination 2023, 564, 116821. [Google Scholar] [CrossRef]
- Ginting, R.T.; Abdullah, H.; Fauzia, V. Facile preparation of MXene and protonated-g-C3N4 on natural latex foam for highly efficient solar steam generation. Mater. Lett. 2022, 313, 131779. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Kunnel, E.S.; Sasidharan, R.; Chinthala, M.; Kumar, A. 3D black g-C3N4 isotype heterojunction hydrogels as a sustainable photocatalyst for tetracycline degradation and H2O2 production. Chem. Eng. J. 2023, 475, 146163. [Google Scholar] [CrossRef]
- Gan, Q.; Xiao, Y.; Li, C.; Peng, H.; Zhang, T.; Ye, M. g-C3N4/MoS2 based floating solar still for clean water production by thermal/light activation of persulfate. Chemosphere 2021, 280, 130618. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, R.; Shan, P.; Zhang, S.; Sun, L.; Xie, H.; Guo, F.; Li, C.; Shi, W. Abundant Edge Active Sites-Modified High-Crystalline g-C3N5 for Hydrogen Peroxide Production from Pure-Water via a Quasi-Homogeneous Photocatalytic Process. Small 2024, 20, e2401566. [Google Scholar] [CrossRef]
- Sun, X.; Shi, Y.; Lu, J.; Shi, W.; Guo, F. Template-free self-assembly of three-dimensional porous graphitic carbon nitride nanovesicles with size-dependent photocatalytic activity for hydrogen evolution. Appl. Surf. Sci. 2022, 606, 154841. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Chen, Z.; Cai, Y.; Guo, F.; Du, X. Environmentally friendly supermolecule self-assembly preparation of S-doped hollow porous tubular g-C3N4 for boosted photocatalytic H2 production. Ceram. Int. 2023, 49, 11989–11998. [Google Scholar] [CrossRef]
- Ying, P.; Li, M.; Yu, F.; Geng, Y.; Zhang, L.; He, J.; Zheng, Y.; Chen, R. Band Gap Engineering in an Efficient Solar-Driven Interfacial Evaporation System. ACS Appl. Mater. Interfaces 2020, 12, 32880–32887. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, Z.; Zhang, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Zhou, C.; Guo, H.; Xue, W.; et al. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight. Chem. Eng. J. 2018, 349, 808–821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).