Abstract
Aromatic volatile organic compounds (VOCs) pose significant environmental and public health risks due to their toxicity, carcinogenicity, and role as precursors of hazardous secondary pollutants. Zeolite-based metal catalysts, with their well-defined microporous structures, tunable acidity, and high thermal stability, have shown promise in the catalytic oxidation of aromatic VOCs. However, the influence of the zeolite microenvironment on supported metal active sites remains insufficiently understood, limiting the rational design of advanced catalysts. This review highlights how microenvironmental parameters—including pore architecture, acid site distribution, framework composition, and surface/interface engineering—can be modulated to enhance adsorption, oxygen activation, and metal–support interactions. Advances in hierarchical porosity, heteroatom substitution, and surface hydrophobicity are discussed. This review provides a framework for the development of next-generation zeolite-based catalysts and offers strategic guidance for advancing microenvironment-controlled catalysis in sustainable environmental remediation.
1. Introduction
Aromatic volatile organic compounds (VOCs), including benzene, toluene, and xylene (BTX), are among the most hazardous air pollutants, primarily emitted from industrial production and vehicle exhaust. Their strong toxicity, carcinogenicity, and role in secondary pollution formation through photochemical reactions pose severe environmental and public health risks [1]. The efficient removal of BTX compounds is therefore a critical challenge in atmospheric pollution control. Among the various abatement strategies, catalytic oxidation has emerged as one of the most effective approaches due to its high efficiency, moderate reaction temperatures, and minimal by-product formation. However, the development of highly active, durable, and selective catalysts remains a formidable challenge, requiring innovative material design strategies that optimize reaction pathways and stability.
Zeolites, as crystalline microporous aluminosilicates, offer a unique platform for catalytic oxidation due to their precisely defined microporous structures, high surface areas, tunable acidity, and exceptional thermal stability [2,3,4]. Their confined pore networks enable efficient dispersion of active sites, preventing nanoparticle agglomeration while providing tailored adsorption environments for reactants [5]. Moreover, their ability to modulate reactant transport, metal–support interactions, and reaction intermediates makes them attractive candidates for selective VOC oxidation [6]. Despite these advantages, zeolite-supported catalysts often face limitations, including restricted molecular diffusion, suboptimal active site accessibility, and inadequate tuning of surface acidity, which hinder their overall catalytic efficiency.
Current research efforts have primarily focused on zeolite-supported noble metals (Pt, Pd) and transition metal oxides (MnOx, CoOx, CuOx), exploring synthesis strategies, hierarchical pore engineering, and advanced characterization techniques to probe catalytic active sites [7,8]. Despite substantial progress in optimizing zeolite structures for VOC oxidation, the fundamental influence of the zeolite microenvironment on catalytic activity and selectivity remains insufficiently understood. Previous studies have highlighted the importance of pore architectures and acidity [9], yet a systematic understanding of their direct impact on catalytic mechanisms is lacking. The interplay between zeolite framework properties and oxidation activity is not fully understood, limiting the rational design of next-generation catalysts.
While recent advances in zeolite-based VOC catalysts have demonstrated notable improvements in activity and durability, the underlying influence of the zeolite microenvironment on metal site behavior remains incompletely understood. This review seeks to provide a structured overview of how these interconnected parameters can be modulated to optimize adsorption characteristics, oxygen activation, and metal–support interactions. We summarize recent progress in pore architecture engineering, acid site regulation, heteroatom incorporation, and surface/interface design, with an emphasis on their role in shaping the catalytic microenvironment. By integrating catalytic performance with evolving material design strategies, this work aims to offer a foundation for future efforts toward the rational development of zeolite-based catalysts for aromatic VOC abatement and potentially broader applications in selective oxidation chemistry.
2. Zeolite Pore Architecture
2.1. Influence of Zeolite Topology on Catalytic Oxidation of Aromatic VOCs
Zeolite topology fundamentally governs the performance of VOC oxidation catalysts by dictating pore geometry, mass transport, active site accessibility, and metal dispersion behavior. Specifically, the pore dimensions, connectivity, and framework flexibility influence not only the diffusion and stabilization of aromatic intermediates but also the degree of molecular confinement and catalyst deactivation resistance. Among the diverse topologies, MFI, *BEA, and FAU frameworks (Figure 1) have emerged as prototypical structures due to their optimized balance of pore size, structural robustness, and commercial scalability [9].
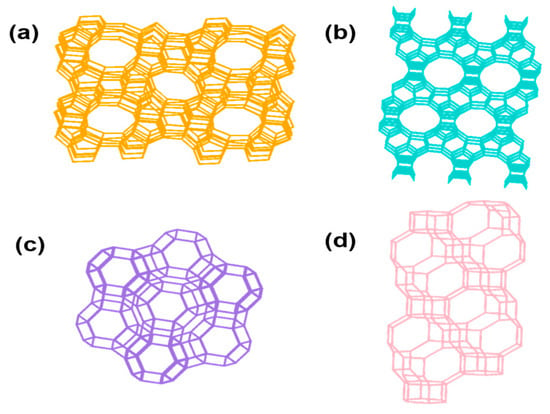
Figure 1.
MFI (a), *BEA (b), FAU (c), and CHA-type (d) zeolite frameworks (source from IZA).
MFI-type zeolites (e.g., ZSM-5) possess an anisotropic 3D microporous framework consisting of intersecting 10-membered ring (10-MR) channels, with pore dimensions of 5.3 × 5.6 Å and 5.1 × 5.5 Å. This dual-channel topology affords a moderate molecular confinement that promotes transition state stabilization without incurring excessive diffusion resistance. For Pt/ZSM-5 catalysts, this topology facilitates the uniform distribution of Pt nanoparticles and stabilizes active Pt0 species, enhancing catalytic efficiency in toluene oxidation [10]. Additionally, ZSM-5 synthesized from biomass-derived silica (e.g., rice husk ash) demonstrated enhanced surface roughness and hierarchical defect structures, further promoting metal dispersion and redox cycling during VOC oxidation [11]. Emerging tandem catalyst designs utilizing functionally decoupled topologies—for instance, combining Pt on inert oxides (e.g., SiO2) with acidic ZSM-5 domains—have demonstrated enhanced oxidation of multicomponent VOC mixtures by separating redox and acid functions within the catalytic architecture [12].
In comparison, *BEA-type zeolites feature larger 12-MR pores (~6.6–6.7 Å) and isotropic 3D connectivity, which make them particularly suitable for oxidizing bulkier aromatic molecules. Steamed Pt/Beta catalysts, with partially dealuminated frameworks, exhibited reduced Brønsted acidity and improved coke resistance while maintaining high Pt dispersion and stable toluene oxidation performance [13]. The more open channel system of *BEA also promotes the co-adsorption of oxygen and hydrocarbon species, facilitating oxidative reactions under lower temperature conditions.
FAU-type zeolites (e.g., Y) possess supercages of ~11.2 Å diameter, interconnected through 12-MR windows (~7.4 Å). These large cavities afford excellent diffusivity for VOCs, yet their high Brønsted acid site density often leads to over-adsorption and carbonaceous buildup. Ion exchange with alkali cations, such as Na+, has been shown to suppress excessive acidity, reduce coke formation, and stabilize Pt species, thereby improving catalytic durability [14].
By contrast, zeolites with narrower channels, such as CHA-type zeolites (e.g., SSZ-13), contain 8-MR windows (~3.8 × 3.8 Å) that severely limit the diffusion of bulky VOCs. Although CHA frameworks offer superior hydrothermal stability, their confined microporosity results in the low accessibility of internal acid or metal sites, which restricts their application in aromatic VOC catalysis.
These comparative insights highlight how zeolite topology not only determines physical accessibility and diffusion properties but also tunes metal–support interactions and intermediate stabilization. The widely employed FAU, MFI, and *BEA frameworks exemplify this topological balance, providing effective platforms for VOC catalysis across a range of molecular sizes and reaction conditions.
2.2. Hierarchical Porosity for Enhanced Diffusion and Metal Dispersion
Although conventional zeolites provide size- and shape-selective microporous environments, their narrow channels often limit both mass transport and the effective dispersion of metal clusters. The introduction of hierarchical porosity—by incorporating mesopores or macropores into the crystalline zeolite matrix—has emerged as an effective strategy to address these limitations. Hierarchical zeolites not only facilitate reactant/product diffusion but also create favorable environments for high metal dispersion and accessibility.
Recent advances in hierarchical zeolite engineering have enabled significant improvements in the catalytic oxidation of VOCs, particularly through the integration of mesoporous architectures into microporous ZSM-5 frameworks. In a study by Yang et al. [15], hierarchical ZSM-5 supports with tunable mesopore volumes were synthesized via a sol–gel method by varying gel aging times, followed by Pt loading via incipient wetness impregnation. Among the catalysts tested, Pt/mZSM-5-A8 exhibited optimal performance for toluene oxidation, achieving a T90 of 159 °C and a low activation energy (ΔEa = 40.0 kJ/mol) at 500 ppm toluene and GHSV of 80,000 mL·g−1·h−1. The superior activity was attributed to a high mesopore volume, elevated external surface area, and fine Pt dispersion (~2 nm), which enhanced oxygen activation and toluene adsorption while facilitating rapid mass transport.
Complementary work by Wang et al. [16] employed a post-synthetic alkali treatment with TPAOH to fabricate micro-mesoporous ZSM-5 supports. Pt nanoparticles (~1.8 nm) were anchored via ethylene glycol reduction, yielding Pt/ZSM-5 (0.1), which demonstrated excellent low-temperature activity with a T90 of 128 °C for benzene oxidation (Figure 2a). Notably, this catalyst also showed resistance to water vapor deactivation and sustained high conversion over 50 h. In situ CO adsorbed diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), and H2-TPR revealed that optimal desilication facilitated the formation of abundant mesopores and exposed strong Pt–zeolite interactions, increasing the proportion of catalytically active Pt0 species. These findings underscore the critical role of hierarchical porosity in balancing active site accessibility, Pt dispersion, and hydrothermal stability. In parallel, Wang et al. reported a hierarchical HZSM-5 catalyst loaded with Ru, which exhibited superior performance in multicomponent VOC oxidation due to the synergistic contribution of macropore accessibility, mesopore diffusion, and micropore–acid functionality [17].
The benefits of hierarchical structuring extend beyond MFI-type zeolites. In mesoporous Beta (Beta-H) systems templated with PDADMAC, enhanced external surface area and improved Pt dispersion led to lower T98 values in toluene oxidation and reduced coke formation. Likewise, Pt/meso-HZSM-5 achieved a T98 of 190 °C, demonstrating the generality of hierarchical architectures in improving both mass transfer and metal–support interaction [18].
Additionally, Zhang et al. [19] successfully introduced mesoporosity into MOR-type zeolites via acid–alkali post-treatment, creating Pt/HPMOR catalysts with smaller Pt particles and a greater external surface area (Figure 2b). The resulting catalyst delivered a T90 of 190 °C, matching the activity of more open-channeled Beta and ZSM-5 analogues.
The integration of bimetallic functionality within hierarchical matrices further amplifies these benefits. Li et al. developed a PtPd/5A zeolite with a hierarchical micro–mesoporous architecture via incipient wetness impregnation. The mesopores (~3.8 nm) enhanced toluene diffusion, while the micropores promoted VOC enrichment near active sites. Uniformly dispersed PtO2 and PdO (~2–3 nm) facilitated low-temperature oxidation, achieving 38.3% conversion at 100 °C and full conversion at 200 °C. High Pd2+ content improved oxygen activation, as evidenced by XPS and H2-TPR. The integrated porous framework enabled efficient adsorption–oxidation under humid conditions, with in situ DRIFTS confirming a stepwise oxidation mechanism.
In summary, hierarchical porous zeolites offer a structurally integrated solution to the limitations of microporous frameworks. By reconciling the confinement benefits of micropores with the transport efficiency of meso/macropores, these materials enhance metal dispersion, reduce diffusion resistance, and maintain high catalytic activity across complex VOC mixtures.
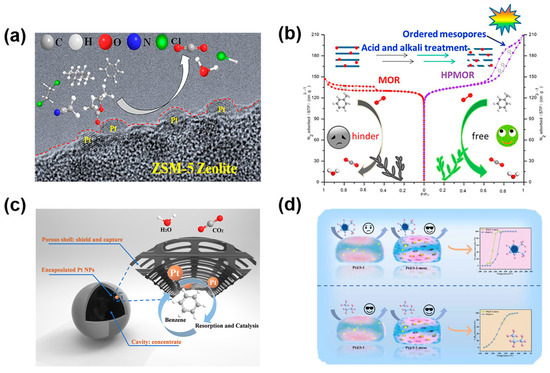
Figure 2.
(a) Post-synthetic alkali treatment using TPAOH to construct micro–mesoporous ZSM-5 frameworks supporting well-dispersed Pt nanoparticles; (b) Pt/HPMOR catalysts exhibiting reduced Pt particle sizes and enhanced external surface area due to hierarchical pore formation; (c) hollow Pt@ZSM-5 catalysts featuring a mesoporous shell and internal cavity; (d) Pt nanoparticles confined within the intracrystalline mesoporous framework of silicalite-1. Reprinted from Refs. [16,19,20,21] with permission from the respective publishers.
2.3. Confinement Effects of Zeolitic Voids on Metal Site Functionality
The spatial confinement of noble metals within tailored zeolite microstructures has emerged as a pivotal strategy for optimizing the catalytic oxidation of aromatic VOCs. By regulating the local coordination environment, confinement not only stabilizes active metal species against sintering or migration but also modulates adsorption–desorption dynamics, oxygen activation, and intermediate selectivity [22]. Especially in microporous and hierarchical zeolites, confining metal sites within cages, hollow cavities, or mesoporous shells enables fine control over both catalytic microenvironments and metal–support interfaces. The following studies exemplify how rational confinement architectures can dramatically enhance activity, stability, and water resistance during VOC oxidation.
Tian et al. [20] demonstrated the concept of spatial confinement by synthesizing a hollow Pt@ZSM-5 catalyst with a mesoporous shell and central cavity via a TPAOH-mediated etching–recrystallization process. The resulting architecture confined highly dispersed Pt nanoparticles within the ZSM-5 matrix, facilitating molecular transport and enhancing benzene adsorption through the exposure of internal acid sites (Figure 2c). This combination improved benzene oxidation activity and long-term stability by mitigating diffusion limitations and site blockage effects. To further expand this strategy, the same group introduced an outer hydrophobic octadecyltrichlorosilane (OTS) coating to form Pt@O-ZSM-5@OTS, creating a dual-shelled structure with hydrophobicity-induced water repellency. DFT calculations and 120 h humid reaction data confirmed that the OTS shell enhanced oxygen activation while suppressing water poisoning, thus preserving high benzene conversion [23].
Extending this strategy, Tang et al. extended the concept of spatial confinement by embedding Pt nanoparticles into a multi-hollow silicalite-1 (MH-S-1) framework through alkali etching and recrystallization [24]. The multi-hollow zeolite exhibited a hierarchical micro–mesoporous structure with a high external surface area and mesopore volume, ensuring excellent Pt dispersion and accessibility. The confined Pt species remained highly dispersed and catalytically active, achieving 100% toluene conversion at just 137 °C. The structure also suppressed Pt aggregation and facilitated the rapid diffusion of VOCs to the active sites. This combination of spatial restriction and hierarchical porosity enhanced thermal stability and minimized deactivation under operating conditions.
Li et al. [25] reported a confined Pt@D-Beta catalyst synthesized by combining dealumination and the secondary recrystallization of the Beta zeolite. This approach enhanced Pt dispersion, suppressed sintering, and enriched surface hydroxyl groups, promoting lattice oxygen activation. In situ DRIFTS and DFT simulations demonstrated that these structural features facilitated the Mars–van Krevelen oxidation pathway, where adsorbed VOC molecules react directly with lattice oxygen on the catalyst surface to form CO2 and H2O, resulting in the generation of oxygen vacancies. These vacancies are subsequently replenished by gaseous O2, which reoxidizes the reduced metal oxides on the catalyst surface. The catalyst achieved a low T90 of 158 °C and maintained ~85% toluene conversion over 50 h in the presence of water vapor.
Further advancing the confinement design, He et al. [21] applied a dry gel conversion method to transform an amorphous SiO2 shell into a mesoporous silicalite-1. Pt nanoparticles (~5 nm) were encapsulated within the intracrystalline framework, which featured silanol nests [Si–OH] and well-defined mesopores (Figure 2d). This configuration facilitated the deep oxidation of bulky VOCs, such as o-Xylene at low temperatures, while exhibiting excellent resistance to moisture-induced deactivation. XPS and H2-TPR analyses confirmed enhanced oxygen activation and electron enrichment at Pt surfaces, validating the confinement effect on catalytic performance.
Beyond monometallic systems, Wang et al. [26] developed a Pt/Ce-ZSM-5 catalyst using a confined encapsulation strategy combined with cerium incorporation. The introduction of Ce into mesoporous ZSM-5 increased lattice oxygen content and created abundant oxygen vacancies, which enhanced oxygen activation and VOC adsorption. Pt dispersion was improved through strong metal–support interactions and confinement within the zeolite framework. The synergistic interaction among Pt, Ce, and the mesoporous ZSM-5 carrier significantly improved toluene conversion and catalyst durability. This strategy integrated spatial control with redox-active dopants to simultaneously boost activity, reduce Pt loading, and extend the catalyst lifespan.
Collectively, these studies underscore the multifaceted role of spatial confinement in optimizing the catalytic oxidation of aromatic VOCs. From core–shell structures and hollow zeolites to intracrystalline mesopores and surface hydrophobic coatings, confinement not only ensures metal dispersion and resistance to deactivation but also enables precise control over reaction microenvironments. These effects are particularly critical in humid and thermally demanding conditions, where confined geometries facilitate oxygen activation, suppress undesired side reactions, and enhance intermediate stabilization.
3. Zeolitic Acidity Engineering
Zeolitic acidity, defined by the nature, density, and distribution of Brønsted and Lewis acid sites, plays a pivotal role in shaping the catalytic behavior of zeolite-based materials for VOC oxidation. Rational acidity modulation allows precise tuning of adsorption strength, intermediate stabilization, and metal–support interactions. This section outlines current strategies and mechanistic insights into tailoring acid properties through framework composition and extraframework cation engineering.
3.1. Acid Site Characteristics of Zeolite Support
In zeolite frameworks, the substitution of Si4+ by Al3+ introduces negative charges that must be balanced by extraframework cations, typically protons (H+) or alkali cations such as Na+, K+, and Cs+. These cations significantly influence zeolite acidity, particularly Brønsted acidity, crucial in catalytic oxidation reactions by affecting adsorption behaviors and the stability of reaction intermediates. Acidic site concentration primarily correlates with framework Al content, whereas acidic strength varies with zeolite topology [27]. Brønsted acidity continues to play a pivotal role in VOC activation and adsorption, significantly affecting reaction pathways and product selectivity. For instance, ZSM-5 exhibits relatively strong acidity, making it particularly suitable for activating challenging C–C and C–H bonds in aromatic VOC oxidation, whereas zeolites like Beta typically present moderate acidity [28].
A foundational study by Zhang et al. [29] systematically explored MnOx/HZSM-5 catalysts with varying Si/Al ratios to probe the impact of acid density on toluene oxidation. Catalysts with lower Si/Al ratios—therefore higher Brønsted acidity—demonstrated markedly enhanced catalytic performance despite similar redox and textural properties. A linear correlation between total acid site concentration and TOF for Mn confirmed that Brønsted acid sites facilitated both VOC adsorption and activation, positioning acidity as a true functional promoter in VOC catalysis.
Li et al. [13] provided mechanistic insight by tuning the Si/Al ratio of Beta zeolites (6, 25, 260) to study its influence on Pt speciation and reactivity. Lower Si/Al ratios stabilized isolated [Pt2+(OH)]+ species on Brønsted acid sites, whereas high-silica Beta favored metallic Pt0 formation. The latter demonstrated higher activity due to superior O2 activation. In situ DRIFTS and DFT calculations further revealed that while all catalysts followed similar oxidation pathways, acid-stabilized Pt2+ promoted undesired benzene release, demonstrating how acid strength and speciation co-regulate selectivity and efficiency.
Beyond activity, acidity also plays a compensatory role in catalyst durability under harsh conditions. Xie et al. [30] investigated Pd/Beta systems exposed to SO2, where active Pd0 was oxidized to PdSO4, reducing redox activity. Remarkably, the Beta support formed new acid sites via SO2 binding to AlO4 units, generating Al2(SO4)3 domains that partially restored performance. This demonstrates the dynamic nature of zeolitic acidity and its capacity to sustain catalysis during chemical perturbation.
A distinct yet complementary case was presented by Wang et al. [31], who employed untreated NaA zeolites loaded with ultralow Pt (0.22 wt%). Despite modest Brønsted acidity, the Pt/NaA catalyst achieved full toluene conversion at 160 °C, outperforming analogues such as NaY and ZSM-5. The high activity was linked to a combination of moderate Lewis acidity, accessible surface oxygen, and well-dispersed Pt0. Although acid site types were not explicitly quantified, the result highlights that even minimal and non-protonic acid environments can synergize with metal species to drive low-temperature VOC oxidation.
Finally, Wang et al. [32] demonstrated that Beta zeolites could dynamically generate new acid sites under sulfur-rich conditions. In MnO2/Beta systems, SO2 exposure induced the formation of strong acidic Al2(SO4)3 species from extraframework Al, mitigating deactivation from Mn4+ reduction and preserving catalytic function. This work reveals a novel route for leveraging acid–gas interactions to regenerate activity during poisoning events.
3.2. Cation Regulation of Acidity and Metal–Support Interactions
In addition to framework-derived acidity, the incorporation of extraframework cations in zeolites, particularly alkali metal species such as Na+, K+, and Cs+, plays a dual role in tuning both acid site properties and the electronic structure of supported metal nanoparticles [33]. These cations are essential to balance the framework negative charge arising from tetrahedral Al incorporation. While protons (H+) typically impart Brønsted acidity, replacing H+ with alkali cations introduces basic character and modulates the strength and accessibility of residual acid sites.
The type and identity of the alkali cation exert a marked influence on acid site distribution and strength. Zeolites exchanged with larger, less electronegative cations (e.g., K+, Cs+) often exhibit weaker Brønsted acidity due to reduced protonation capability [34]. This attenuation in acidity can suppress undesired over-adsorption and enhance the desorption of reaction intermediates—especially beneficial in VOC oxidation processes where coking and diffusion limitations are prevalent [9]. Moreover, alkali cations can promote the formation of new Lewis acidic domains or modify the proximity between acid and metal sites, thereby affecting catalytic turnover [13].
Beyond acidity regulation, alkali cations significantly influence the electronic state and dispersion of supported metal species. As shown in Figure 3a, systematic studies on Pt/ZSM-5 catalysts have shown that substituting H+ with Na+, K+, or Cs+ progressively increases the proportion of metallic Pt0 species due to the enhanced electron donation from the zeolite framework [35]. This trend correlates with the decreasing electronegativity of the cations and reflects a stronger metal–support electronic interaction in the presence of more basic cations. Such electronic modulation lowers the barrier for O2 activation and improves the redox efficiency of noble metal sites, leading to significantly enhanced catalytic performance. For example, Pt/KZSM-5 and Pt/CsZSM-5 achieved T98 values as low as 170 °C for toluene oxidation, in contrast to 205 °C for the unexchanged Pt/HZSM-5 counterpart.
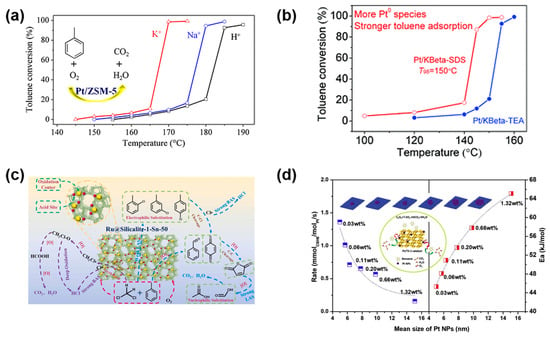
Figure 3.
(a) Alkali metal exchange promotes Pt0 formation via enhanced electron donation from the zeolite; (b) K+-exchanged Beta zeolite reduces Brønsted acidity, facilitating intermediate desorption; (c) Ru@Silicalite-1-Sn with dual acid sites enables efficient VOC oxidation and functional group transformation; (d) Pt/TS-1 with ultra-low Pt loading achieves high benzene oxidation activity and moisture resistance. Reprinted from Refs. [34,35,36,37] with permission from the respective publishers.
Similar activity enhancement has been observed in Pt-loaded Beta zeolites following cation exchange (Figure 3b), underscoring the generality of alkali cation-induced promotion [34]. Together, these findings suggest that the rational integration of basic cations can serve as an effective strategy to simultaneously modulate acidity, tailor metal site electronics, and construct spatially balanced acid–base microenvironments. Optimal performance can be achieved by designing systems wherein the cation type, acid site strength, and metal electronic state are co-engineered to synergistically enhance VOC activation and resist deactivation under operating conditions.
Taken together, the acidity of zeolite-based catalysts for VOC oxidation arises from both intrinsic framework characteristics and extrinsic ionic modifications. Framework-derived Brønsted and Lewis acid sites, determined by Al content, the Si/Al ratio, and topology, play a central role in substrate adsorption, bond activation, and intermediate stabilization. Meanwhile, the incorporation of alkali metal cations offers an additional layer of tunability, enabling fine control over acid site strength and distribution. Moreover, these basic cations actively influence the electronic structure of co-supported metal species, thereby bridging acid–metal functionalities at the nanoscale. This dual-axis approach—combining structural acidity with cation-mediated modulation—provides a versatile and synergistic strategy to construct optimal acid–base microenvironments.
4. Framework Composition Engineering: Heteroatom Substitution for Functional Modulation
The rational substitution of framework atoms with heteroatoms offers a powerful strategy to reconfigure the physicochemical microenvironment of zeolites for the catalytic oxidation of aromatic VOCs. Elements such as Ti, Sn, Fe, Mn, and Co can isomorphously replace Si or Al in tetrahedral positions, thereby introducing tailored acid sites, modifying electron density, and facilitating lattice oxygen dynamics (Figure 4). These changes reshape adsorption behavior, oxygen activation, and the stabilization of reaction intermediates, yielding pronounced effects on activity, selectivity, and durability in VOC abatement applications [28].
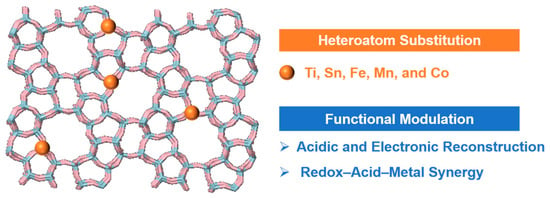
Figure 4.
Heteroatom substitution (Ti, Sn, Fe, Mn, or Co) in zeolites modulates acidity and electronic structure, enabling redox–acid–metal synergy for catalytic enhancement (source: the authors).
Heteroatom substitution directly alters the acidity and coordination environment of zeolite frameworks. Wu et al. [36] synthesized an Sn-doped silicalite-1-supported Ru single-atom catalyst (Ru@Silicalite-1-Sn) via the one-pot hydrothermal incorporation of Sn4+ into the lattice (Figure 3c). The resulting catalyst exhibited both Brønsted and Lewis acid sites and demonstrated superior activity for mixed VOCs (e.g., toluene and dichloromethane), owing to strong redox–acid site coupling. The oxygen vacancies created by Sn-induced lattice distortions enhanced O2 activation, while enriched acid sites facilitated the adsorption and activation of polar VOCs. DRIFTS and TPSR data confirmed the involvement of acid–vacancy ensembles in enabling selective oxidation and resistance to halogen poisoning under humid conditions.
Ti incorporation into the MFI-type TS-1 framework similarly generated weak Lewis acid sites and conferred hydrophobicity. As shown in Figure 3d, Pt/TS-1 catalysts with Pt loading as low as 0.03 wt% exhibited outstanding low-temperature benzene oxidation activity and moisture resistance [37]. This was attributed to the geometric matching between MFI pores and benzene, along with the Ti-mediated stabilization of Pt0 and promotion of surface-active oxygen species, as evidenced by XPS and TPSR. The study underscores the capacity of Ti to modulate the hydrophilicity and electronic landscape of the support, crucial for a low-Pt catalyst design.
Rokicińska et al. [38] introduced the Co species into BEA zeolites via dealumination followed by cobalt impregnation. Co incorporation into SiBEA restored catalytic functionality in the absence of conventional Brønsted acid sites. The formation of Co–pyridine-type Lewis acid centers allowed for selective toluene oxidation via Mars–van Krevelen pathways, enabled by lattice oxygen from Co3O4. This result highlights that even in dealuminated systems, appropriate heteroatom substitution can regenerate effective microenvironments for VOC activation and product selectivity control.
Incorporating redox-active metals such as Fe and Mn significantly improves lattice oxygen mobility and reshapes metal–support interactions. Peng et al. synthesized Pd/Fe-ZSM-5 catalysts via hydrothermal Fe doping, followed by Pd impregnation [39]. Despite lower Pd dispersion, the Fe-doped system displayed markedly higher activity and lower activation energy for toluene oxidation compared to Pd/Al-ZSM-5. This was linked to enhanced oxygen chemisorption and a stabilized Pd0 population. The framework Fe3+ centers served not only as structural modifiers but also as indirect promoters of noble metal redox cycling.
In a complementary approach, Meng et al. reported a one-step hydrothermal synthesis of Mn–ZSM-5, yielding a robust redox-active zeolite for aromatic VOC oxidation [40]. Mn substitution led to framework expansion, Mn–O–Si vibrational features, and dual oxygen species (adsorbed and lattice), as observed in CO-TPR profiles. The catalyst demonstrated high turnover frequencies and selective benzyl alcohol oxidation, suggesting that framework Mn3+ species functioned as lattice oxygen donors. Spectroscopic reversibility in UV–vis and colorimetric changes further evidenced dynamic Mn–O coordination, offering insights into autoreductive oxygen evolution under reaction conditions.
Taken together, these studies demonstrate that framework heteroatom substitution is a precise and structurally embedded strategy to reprogram zeolite microenvironments for selective VOC oxidation. These modifications not only influence the intrinsic reactivity of the support but also synergistically regulate the electronic structure, dispersion, and durability of supported metal nanoparticles.
5. Surface and Interface Engineering Strategies
The surface architecture of zeolite-based catalysts plays a pivotal role in determining their efficiency in VOC oxidation. Tailored structural features—including crystallite downsizing, nanosheet construction, and interlayer pillaring—enhance the external surface area, metal dispersion, and oxygen activation while alleviating diffusion and deactivation constraints. Surface chemical modification, such as hydrophobic functionalization and interface design, further stabilizes redox sites and improves durability under humid or reactive conditions.
5.1. Nanostructuring and Morphological Control
Reducing zeolite crystallite size to the nanoscale is a proven strategy to increase the external surface area and minimize diffusion constraints. A recent study demonstrated that downsizing ZSM-5 to ~100 nm via an amino acid-assisted route enhanced the performance of MnOx-based catalysts for toluene combustion [8]. Compared to the conventional micron-sized counterpart (~500 nm), the nanocrystalline ZSM-5 exhibited a higher external surface area (113 vs. 69 m2/g) and total surface area (354 vs. 309 m2/g), resulting in superior MnOx dispersion and smaller particle sizes (Figure 5a). These morphological improvements elevated surface oxygen vacancy density and low-temperature reducibility, lowering the T90 from 315 °C to 276 °C. The increased activity was attributed to improved oxygen activation and enhanced contact between MnOx clusters and the zeolite surface.
Zeolitic nanosheets offer another avenue for surface area enhancement and interface engineering. A sandwich-structured Pt@ZSM-5 nanosheet catalyst (Pt@PZN-2) was synthesized by intercalating Pt nanoparticles between ZSM-5 single layers using ammonium-ion-directed electrostatic assembly [41]. This architecture maintained mesoporosity during calcination by preventing sheet collapse and enabled Pt clusters (~4.3 nm) to serve as structural pillars. The resulting catalyst displayed significantly higher Pt dispersion (27.7%) and superior surface area compared to conventional Pt/ZSM-5 (Pt/CZ-500). Toluene conversion reached 98% at only 176 °C, with complete conversion sustained for 360 h at 230 °C (Figure 5b). In situ CO chemisorption and diffusion studies further confirmed the enhanced accessibility and sintering resistance of the nanosheet configuration.
Delaminated and pillared lamellar zeolites represent a third strategy for morphological optimization. One example involved the synthesis of a SiNb-MCM-36 structure from a lamellar MCM-22 precursor by co-pillaring with silicon and niobium oxide sols [42]. Structural analyses confirmed the formation of Nb–O–Si linkages and isolated NbOx species, resulting in a mesoporous matrix with a high external surface area (~300 m2/g). This architecture enabled efficient BTX oxidation (92% benzene, 69% toluene, 58% xylene conversion at 300 °C), driven by synergistic acid–redox interactions and improved thermal stability.
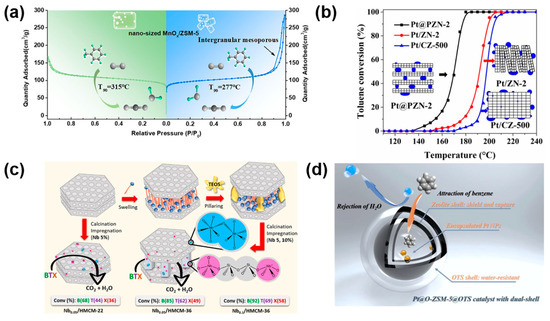
Figure 5.
(a) Downsizing ZSM-5 enhances catalytic activity of MnOx for toluene oxidation due to increased surface area and intergranular mesoporosity; (b) Pt/ZSM-5 nanosheet zeolite exhibits improved Pt dispersion and enlarged external surface area, boosting low-temperature performance; (c) lamellar MCM-36 zeolite with Nb5+ species incorporation exhibits enhanced Brønsted acidity, redox properties, and interlayer accessibility; (d) a dual-shell Pt@O-ZSM-5@OTS catalyst integrates zeolite confinement with a hydrophobic organosilane coating, achieving selective benzene adsorption and water resistance. Reprinted from Refs. [5,23,41,43] with permission from the respective publishers.
In a separate study, Nb-impregnated lamellar MCM-36 catalysts were synthesized via TEAOH intercalation followed by Nb loading (1–15 wt%) [43]. Increasing the Nb content led to more ordered pillared structures, elevated mesoporosity (~280 m2/g), and coexisting tetrahedral Nb5+ and Nb2O5 species (Figure 5c). The optimized catalyst (14.8 wt% Nb) achieved nearly complete toluene oxidation at 300 °C, with performance attributed to enhanced Brønsted acidity, redox capacity, and enlarged interlayer accessibility. These findings emphasize that tailored lamellar architectures not only provide high surface area but also serve as versatile platforms for integrating acid and redox functionalities.
Collectively, these surface engineering strategies—whether via particle downsizing, nanosheet construction, or lamellar pillaring—highlight the importance of accessible surface architecture in enhancing metal anchoring, redox reactivity, and VOC mass transport. By carefully optimizing crystallite geometry and interfacial connectivity, zeolite-based catalysts can achieve superior activity and durability, even under demanding oxidative conditions.
5.2. Hydrophobic Surface Modification
In zeolite-based catalytic systems, particularly under conditions involving aromatic VOC oxidation, the surface microenvironment plays a decisive role in dictating the adsorption behavior of reactants and the susceptibility to water vapor inhibition. To overcome the inherent hydrophilicity of many zeolite frameworks and enhance catalytic resilience, a variety of surface modification strategies have been developed. These include post-synthetic silylation, hydrophobicity enhancement via Si/Al ratio tuning, and functional group anchoring through sol–gel methods. Such surface engineering not only strengthens water repellency but also improves VOC affinity, metal dispersion, and active site accessibility. The following studies illustrate representative and complementary approaches to surface modification.
Zhao et al. [44] pioneered a targeted silylation method to simultaneously preserve acidity and induce hydrophobicity on low Si/Al ZSM-5. By grafting methyl groups via trimethoxymethylsilane, they achieved substantial surface hydrophobization without compromising the Brønsted acid site density. This modification enhanced toluene adsorption while facilitating the rapid desorption of co-adsorbed water, thereby minimizing competitive inhibition at oxidation sites. The resulting Pt/ZSM-5-Me catalyst delivered a low T90 of 144 °C and maintained catalytic stability for over 120 h. Their work underscores the dual benefit of combining acidity retention with surface polarity tuning to optimize VOC conversion under humid conditions.
As a complementary strategy that combines hydrophobicity enhancement with active site stabilization, Zhu et al. [45] employed a one-pot hydrothermal route to encapsulate Pt nanoparticles within ZSM-5 while simultaneously adjusting the Si/Al ratio. Higher Si/Al values yielded more hydrophobic surfaces that limited halogen poisoning and enhanced toluene adsorption. In particular, the Pt/Z-200-En catalyst (Si/Al = 200) exhibited 98% toluene conversion at 214 °C and showed excellent durability under 1,2-dichloroethane co-feeding. Compared to Zhao’s post-synthetic strategy, Zhu’s approach provides a framework-level solution that synergistically addresses both active metal stabilization and water tolerance, highlighting the versatility of hydrophobicity modulation across different synthesis routes.
An alternative surface engineering route utilizes organosilane functionalization not only for hydrophobic modulation but also for metal anchoring and redox tuning. Meng et al. [46] introduced chloropropyl groups into NaY zeolites during synthesis using 3-chloropropyltrimethoxysilane. This in situ functionalization improved the framework Si/Al ratio and enabled a better dispersion of subsequently loaded Cu species. The modified Cu/Y-CPT catalyst displayed uniformly dispersed CuO nanoparticles and a higher fraction of redox-active Cu2+ sites with mobile lattice oxygen. Toluene oxidation tests showed a dramatic T90 reduction from 375 °C (unmodified) to 296 °C, demonstrating that sol–gel-based ligand engineering can simultaneously optimize surface polarity, acid–redox synergy, and metal anchoring capacity. In contrast to the previous studies on noble metal catalysts, this work broadens the applicability of surface engineering to transition metal VOC systems.
Taken together, these studies highlight a coherent progression of surface modification strategies—ranging from post-synthetic silylation and framework-level hydrophobicity tuning to sol–gel functionalization—that converge on a common goal: enhancing water resistance, VOC adsorption, and redox accessibility through tailored surface microenvironments.
5.3. Structural Interface Architecture Engineering
Engineering the structural interfaces of zeolite-based catalysts offers a powerful means of tailoring microenvironments for selective VOC oxidation. Compared to simple surface modification, interface-oriented architectures—such as hollow structures, multi-shell frameworks, and spatially integrated redox domains—allow fine regulation of active site distribution, local reactant enrichment, and resistance to deactivation. These features are particularly critical for aromatic VOCs, which often require the simultaneous tuning of acid, redox, and mass transport properties.
A representative example is the RuOn/Ag@h-ZSM-5 system developed by Liu et al., which employed spatial segregation of catalytic functions within a hollow ZSM-5 framework [47]. Silver nanoparticles were localized within the internal void to enhance VOC adsorption through π-complexation, while an exterior layer of RuOx provided robust redox functionality. The interface design minimized mutual interference between the two components, yielding a stable catalytic pathway under chlorine-rich and humid conditions. The catalyst achieved a T90 of 265 °C for 600 ppm toluene under wet conditions, outperforming commercial Pt-based analogs. This work exemplifies how multi-domain interface organization can diversify and stabilize redox sites within a zeolite-derived framework.
Recognizing the challenge of moisture deactivation in VOC oxidation, an approach introduced by Tian et al. [23] involved constructing a dual-shell Pt@O-ZSM-5@OTS catalyst by grafting a hydrophobic organosilane layer atop a hollow ZSM-5–confined Pt system (Figure 5d). This configuration combined internal confinement benefits with external water resistance. DFT analysis supported that the OTS shell increased benzene affinity while simultaneously decreasing water interaction at the metal interface. Under 10 vol% H2O, the catalyst maintained full benzene conversion at 170 °C for 120 h, a performance unattainable by unmodified analogs. This work exemplifies how outer surface/interface engineering can complement internal confinement by reinforcing microenvironmental resilience and long-term performance under industrial VOC oxidation conditions.
Complementing noble metal examples, Zhang et al. [48] systematically compared MnOx supported on hollow, nano-, and micro-ZSM-5, providing insights into interface structure–activity correlations in redox-dominated systems. Among the tested morphologies, hollow ZSM-5 achieved the highest Mn4+ proportion, surface oxygen mobility, and coke resistance. The MnOx/H-Z5 catalyst also exhibited the lowest T50/T90 and facilitated a more complete transformation of benzyl-type intermediates, as verified by in situ DRIFTS. These results support that hollow structures improve redox accessibility and product desorption, enhancing both low-temperature activity and long-term durability in base metal VOC catalysts.
Taken together, these examples demonstrate that structural interface engineering offers a highly tunable platform for modulating zeolite-based catalytic environments. By integrating confinement effects, surface chemical modifications, and redox functionality into unified architectures, it becomes possible to construct catalysts with enhanced activity, selectivity, and operational robustness across a broad range of VOCs and reaction conditions. Representative examples highlighting the influence of key zeolite parameters on catalytic performance are summarized in Table 1, providing a comparative perspective on structure–function relationships.

Table 1.
Zeolite parameters governing catalytic performance in aromatic VOC oxidation, with corresponding feed compositions and T90 values for representative zeolite-based catalyst systems.
6. Challenges and Future Perspectives
6.1. Challenges in Microenvironment-Oriented Catalyst Design
Designing zeolite-based catalysts with tailored microenvironments for aromatic VOC oxidation offers considerable potential but entails complex challenges. Unlike conventional catalyst development focused on individual parameters such as composition or morphology, microenvironmental engineering demands the integration of multiple structural and chemical attributes—pore topology, acid site distribution, surface polarity, and metal–support interactions—within a confined framework.
A primary challenge lies in the spatial coordination of active components. Precisely aligning Brønsted acid sites, redox centers, and dispersed metal species within microporous or hierarchical structures requires sub-nanometer control over site distribution, orientation, and accessibility. However, current synthetic strategies typically result in stochastic or poorly defined arrangements, leading to functional mismatches and diminished catalytic synergy. These limitations are particularly pronounced under dynamic reaction conditions, where localized, cooperative interactions govern the selective transformation of structurally complex VOC molecules.
Beyond spatial precision, the structural and chemical stability of engineered microenvironments under realistic operating conditions remains a major bottleneck. Surface-functionalized zeolites, core–shell configurations, and organosilane-grafted interfaces are susceptible to hydrolysis, oxidation, and structural degradation under high-temperature, humid, and oxidative atmospheres typical of industrial VOC treatment. Preserving the integrity of active domains over extended time scales is essential but remains poorly understood.
Catalyst deactivation, primarily due to coke deposition and active site poisoning, remains a significant challenge in VOC oxidation. Recent advances have focused on innovative regeneration strategies, including controlled thermal treatments and mild oxidative protocols to restore catalytic activity. Hierarchical zeolites that integrate micro- and mesoporous architectures have shown particular promise by promoting coke oxidation at lower temperatures and minimizing sintering during regeneration. The introduction of secondary porosity also enhances metal dispersion and reactant diffusion, thereby suppressing coke formation. Furthermore, constructing a hydrophobic external shell can significantly improve the hydrothermal stability of zeolite-based catalysts under harsh operating conditions.
While hierarchical zeolites offer enhanced catalytic performance, their large-scale application is hindered by challenges in synthesis and stability. Methods such as soft- or hard-templating often rely on costly and less reproducible procedures, limiting scalability. Additionally, structural integrity under industrial conditions remains a concern, as hydrothermal aging can degrade mesostructures and sulfur species may poison active sites. These issues highlight the need for cost-effective, scalable synthesis routes and improved durability to enable practical implementation in VOC oxidation.
Furthermore, the mechanistic elucidation of confined catalytic environments is constrained by the limited resolution of current characterization and modeling approaches. Although operando techniques such as X-ray absorption spectroscopy (XAS), DRIFTS, and near ambient-pressure X-ray photoelectron spectroscopy (NAP-XPS) provide valuable insight into dynamic surface behavior, they rarely capture the spatially resolved evolution of multiple active domains within hierarchical structures. Similarly, most computational models assume idealized geometries and static conditions, overlooking the heterogeneity and temporal complexity of real catalytic systems. Addressing this gap will require the integration of spatially resolved spectroscopies, multi-scale in situ diagnostics, and data-driven modeling platforms capable of predicting microenvironmental behavior under operational conditions.
6.2. Future Perspectives: Toward Integrated Microenvironment–Metal Architectures
Addressing the complex challenges outlined above requires a fundamental shift from isolated structural tuning to the holistic design of catalytic microenvironments, where pore geometry, site distribution, surface properties, and metal integration are coordinated as a unified system.
Future developments should prioritize the spatially resolved construction of zeolitic microenvironments in which acidity, redox activity, and surface polarity are precisely engineered to direct VOC oxidation along desired pathways. This includes the integration of hierarchical pore networks with stabilized metal clusters and tailored surface hydrophobicity, while preserving oxygen activation capabilities and maintaining mass transport efficiency.
Emerging synthetic methodologies—such as confined crystallization, stepwise post-synthetic modification, and layered self-assembly—offer promising routes for constructing such multifunctional architectures with high structural fidelity and scalability. Coupled with advances in artificial intelligence, high-throughput screening, and operando characterization, these approaches will accelerate the discovery of non-obvious structure–function relationships and unlock previously inaccessible catalyst design spaces.
Looking ahead, the development of zeolite-based catalysts will increasingly depend on microenvironments that adapt dynamically to reaction conditions while preserving catalytic integrity. Embracing this integrated design paradigm will enable next-generation catalysts to meet the evolving demands of VOC abatement and extend the applicability of microenvironment engineering to broader domains, including selective oxidation, energy conversion, and sustainable synthesis.
7. Conclusions
The strategic modulation of zeolitic microenvironments has emerged as a powerful framework for designing next-generation catalysts for aromatic VOC oxidation. Through the integrated control of pore architecture, acidity, framework composition, and surface/interface features, recent advances have enabled the spatial and electronic tuning of active sites with unprecedented precision. These multifactorial designs enhance not only reactant diffusion and metal dispersion but also the stability and selectivity of oxidation pathways under realistic conditions. Yet, fundamental challenges persist, including the controlled spatial distribution of multifunctional sites, preservation of microenvironmental integrity under thermal and chemical stress, and limited mechanistic understanding of transient interfacial dynamics. Addressing these limitations will require new synthetic paradigms capable of hierarchical precision, in situ diagnostics with spatial and temporal resolution, and machine-learning-guided frameworks for rational catalyst discovery. Looking forward, the convergence of microenvironment engineering with data-driven design principles promises to redefine the scope of zeolite-based catalysis, advancing both environmental applications and broader domains of selective oxidation, energy conversion, and sustainable synthesis.
Author Contributions
Conceptualization, G.Y. and X.C.; validation, G.Y., X.C. and W.M.; writing—original draft preparation, X.C. and W.M.; writing—review and editing, G.Y.; supervision, G.Y.; project administration, G.Y.; funding acquisition, G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Province Science and Technology Project (Grant SKL202302019), the National Key Research and Development Program of China (Grant 2024YFC3714500, 2024YFE0201000 and 2023YFA1507703), and the National Natural Science Foundation of China (Grant 22371088). This work acknowledges the FAW Volkswagen China Environmental Protection Foundation Automotive Environmental Protection Innovation Leadership Program and the 111 Center (B17020). X.C. acknowledges the collaboration under the Sino-French International Research Network (IRN) “Zeolites”.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Verdoliva, V.; Saviano, M.; De Luca, S. Zeolites as Acid/Basic Solid Catalysts: Recent Synthetic Developments. Catalysts 2019, 9, 248. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Valtchev, V. Environmentally benign synthesis of crystalline nanosized molecular sieves. Green Energy Environ. 2020, 5, 394–404. [Google Scholar] [CrossRef]
- Yang, G.; Yu, J. Advancements in Basic Zeolites for Biodiesel Production via Transesterification. Chemistry 2023, 5, 438–451. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Yang, G. Advances and Challenges in Zeolite-Based Catalysts for the Selective Catalytic Oxidation of Ammonia. Catalysts 2025, 15, 204. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Zhao, S.; Meng, X.; Xiao, F.-S. Recent advances of zeolites in catalytic oxidations of volatile organic compounds. Catal. Today 2023, 410, 56–67. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Controlled fabrication of mesoporous ZSM-5 zeolite-supported PdCu alloy nanoparticles for complete oxidation of toluene. Appl. Catal. B 2020, 265, 118560. [Google Scholar] [CrossRef]
- Peng, S.; Yang, G.; Zhang, J. ZSM-5 nanocrystal promoted low-temperature activity of supported manganese oxides for catalytic oxidation of toluene. Inorg. Chem. Commun. 2022, 143, 109759. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.-S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.-S. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts. Chem. Commun. 2015, 51, 5936–5938. [Google Scholar] [CrossRef]
- Lan, W.; Shen, M.; Chen, Y.; Niu, K.; Zhou, W.; Xu, J.; Huang, B.; Cai, H.; Zuo, J.; Lin, D.; et al. Pt anchored in the skeleton of rice husk-based ZSM-5 for excellent catalytic VOC oxidation: Structure–activity relationship and environmental impact assessment. Green Chem. 2024, 26, 4051–4064. [Google Scholar] [CrossRef]
- Li, Z.; Gao, R.; Hou, Z.; Yu, X.; Dai, H.; Deng, J.; Liu, Y. Tandem supported Pt and ZSM-5 catalyst with separated catalytic functions for promoting multicomponent VOCs oxidation. Appl. Catal. B 2023, 339, 123131. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Lu, Y.; Deng, H.; Zhang, Z.; Wang, Y.; Ma, Y.; Pan, T.; Zhao, Q.; Shan, Y.; et al. New insights into the catalytic mechanism of VOCs abatement over Pt/Beta with active sites regulated by zeolite acidity. Appl. Catal. B 2023, 334, 122811. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, S. Enhanced Catalytic Oxidation of Toluene over Hierarchical Pt/Y Zeolite. Catalysts 2022, 12, 622. [Google Scholar] [CrossRef]
- Yang, D.; Fu, S.; Huang, S.; Deng, W.; Wang, Y.; Guo, L.; Ishihara, T. The preparation of hierarchical Pt/ZSM-5 catalysts and their performance for toluene catalytic combustion. Microporous Mesoporous Mater. 2020, 296, 109802. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Shi, Y.; Zhou, R. Synergistic effect of Pt nanoparticles and micro-mesoporous ZSM-5 in VOCs low-temperature removal. J. Environ. Sci. 2021, 107, 87–97. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Chen, M.; Guo, L.; Zhou, J. Ru/hierarchical HZSM-5 zeolite as efficient bi-functional adsorbent/catalyst for bulky aromatic VOCs elimination. Microporous Mesoporous Mater. 2018, 258, 17–25. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, J.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; Xiao, F.-S. Enhanced performance in catalytic combustion of toluene over mesoporous Beta zeolite-supported platinum catalyst. Appl. Catal. B 2013, 140–141, 199–205. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, C.; Peng, H.; Peng, C.; Zhang, L.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Enhanced toluene combustion performance over Pt loaded hierarchical porous MOR zeolite. Chem. Eng. J. 2018, 334, 10–18. [Google Scholar] [CrossRef]
- Tian, J.; Tan, K.B.; Liao, Y.; Sun, D.; Li, Q. Hollow ZSM-5 zeolite encapsulating Pt nanoparticles: Cage-confinement effects for the enhanced catalytic oxidation of benzene. Chemosphere 2022, 292, 133446. [Google Scholar] [CrossRef]
- He, T.; Li, G.; Ji, J.; Liu, W.; Zhang, H.; Xie, X.; Huang, S.; Huang, T.; Peng, H. Tailored engineering of intracrystalline mesopores for boosting multicomponent volatile organic compounds simultaneous deep oxidation. Appl. Catal. B-Environ. Energy 2025, 362, 124752. [Google Scholar] [CrossRef]
- Ding, S.; Wu, S.; Fang, N.; Chu, Y.; Wang, P.; Ding, L. Design and synthesis of porous nano-confined catalysts for VOCs oxidation: A critical review based on pollutant sorts. Sep. Purif. Technol. 2025, 352, 128158. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Wu, J.; Sun, D.; Li, Q. Enhancing water resistance of Pt nanoparticles by tailoring microenvironment of hollow ZSM-5 for efficient benzene oxidation. Chem. Eng. J. 2023, 451, 138351. [Google Scholar] [CrossRef]
- Tang, S.; Liu, H.; Li, T.; Wang, C.; Cui, Q.; Yue, Y.; Meng, X.; Bao, X. Encapsulating platinum nanoparticles into multi-hollow silicalite-1 zeolite for catalytic oxidation of volatile organic compounds. Chem. Eng. Sci. 2024, 286, 119674. [Google Scholar] [CrossRef]
- Li, C.; He, T.; Yan, J.; Li, G.; Liu, W.; Zhang, H.; Chen, J.; Lu, J.; Zhang, S.; Peng, H. An effective strategy for promoting toluene oxidation stability: Recrystallization Pt confinement and zeolite dealumination. Fuel 2024, 372, 132268. [Google Scholar] [CrossRef]
- Wang, L.; Dong, F.; Han, W.; Tang, Z. Engineering a Mesoporous Pt/Ce-ZSM-5 Catalyst by a Confined Encapsulation Strategy for Low-Temperature Catalytic Combustion of Toluene. Ind. Eng. Chem. Res. 2024, 63, 22354–22368. [Google Scholar] [CrossRef]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Meng, X.; Xiao, F.-S. Challenges and opportunities for zeolite-based catalysts in catalytic oxidation of volatile organic compounds. Catal. Sci. Technol. 2024, 14, 3277–3286. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Li, G.; Wang, L.; Song, L.; Li, X. Zeolitic acidity as a promoter for the catalytic oxidation of toluene over MnOx/HZSM-5 catalysts. Catal. Today 2019, 327, 374–381. [Google Scholar] [CrossRef]
- Xie, K.; Jiang, B.; Wang, Z.; Zuo, S.; Wang, Q. Study on the performance and sulfur tolerance mechanism of Pd/beta zeolite in catalytic combustion of toluene. J. Mater. Res. Technol. 2023, 25, 4543–4553. [Google Scholar] [CrossRef]
- Wang, J.; Hou, C.; Wang, X.; Xing, Y.; Xie, Y.; Wang, L.; Zhang, M. Low-content Pt loading on a post-treatment-free zeolite for efficient catalytic combustion of toluene. Microporous Mesoporous Mater. 2024, 363, 112832. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Z.; Ning, H.; Jiang, B.; Xie, K.; Zuo, S.; Wang, Q. Study on sulfur resistance of MnO2/Beta zeolite in toluene catalytic combustion: The effect of increased acidity on catalytic performance. Chin. J. Chem. Eng. 2025, 78, 187–195. [Google Scholar] [CrossRef]
- Tidahy, H.L.; Siffert, S.; Lamonier, J.F.; Cousin, R.; Zhilinskaya, E.A.; Aboukaïs, A.; Su, B.L.; Canet, X.; De Weireld, G.; Frère, M.; et al. Influence of the exchanged cation in Pd/BEA and Pd/FAU zeolites for catalytic oxidation of VOCs. Appl. Catal. B 2007, 70, 377–383. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Q.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.-S. Aluminium-rich Beta zeolite-supported platinum nanoparticles for the low-temperature catalytic removal of toluene. J. Mater. Chem. A 2015, 3, 5556–5562. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, J.; Pan, S.; Bian, C.; Wang, L.; Chen, F.; Meng, X.; Zheng, X.; Gao, X.; et al. Superior Performance in Catalytic Combustion of Toluene over KZSM-5 Zeolite Supported Platinum Catalyst. Catal. Lett. 2014, 144, 1851–1859. [Google Scholar] [CrossRef]
- Wu, L.; Deng, J.; Liu, Y.; Jing, L.; Yu, X.; Tao, J.; Gao, R.; Feng, Y.; Dai, H. Enhanced removal efficiency of multicomponent VOCs over the Sn-doped Silicalite-1-supported Ru single-atom catalysts by constructing tightly coupled redox and acidic sites. Appl. Catal. B-Environ. Energy 2024, 351, 123910. [Google Scholar] [CrossRef]
- Wu, B.; Chen, B.; Zhu, X.; Yu, L.; Shi, C. Lower loading of Pt on hydrophobic TS-1 zeolite: A high-efficiency catalyst for benzene oxidation at low temperature. Catal. Today 2020, 355, 512–517. [Google Scholar] [CrossRef]
- Rokicińska, A.; Drozdek, M.; Dudek, B.; Gil, B.; Michorczyk, P.; Brouri, D.; Dzwigaj, S.; Kuśtrowski, P. Cobalt-containing BEA zeolite for catalytic combustion of toluene. Appl. Catal. B 2017, 212, 59–67. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Jiang, Y.; Han, S.; Zhu, Q.; Meng, X.; Xiao, F.-S. Fe-ZSM-5 supported palladium nanoparticles as an efficient catalyst for toluene abatement. Catal. Today 2019, 332, 195–200. [Google Scholar] [CrossRef]
- Meng, Y.; Genuino, H.C.; Kuo, C.-H.; Huang, H.; Chen, S.-Y.; Zhang, L.; Rossi, A.; Suib, S.L. One-Step Hydrothermal Synthesis of Manganese-Containing MFI-Type Zeolite, Mn–ZSM-5, Characterization, and Catalytic Oxidation of Hydrocarbons. J. Am. Chem. Soc. 2013, 135, 8594–8605. [Google Scholar] [CrossRef]
- Liu, G.; Tian, Y.; Zhang, B.; Wang, L.; Zhang, X. Catalytic combustion of VOC on sandwich-structured Pt@ZSM-5 nanosheets prepared by controllable intercalation. J. Hazard. Mater. 2019, 367, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Schwanke, A.J.; Balzer, R.; Wittee Lopes, C.; Motta Meira, D.; Díaz, U.; Corma, A.; Pergher, S. A Lamellar MWW Zeolite With Silicon and Niobium Oxide Pillars: A Catalyst for the Oxidation of Volatile Organic Compounds. Chem. Eur. J. 2020, 26, 10459–10470. [Google Scholar] [CrossRef]
- Schwanke, A.J.; Balzer, R.; Lopes, C.W.; Meira, D.M.; Díaz, U.; Pergher, S.; Bernardo-Gusmão, K. Structure and reactive properties of Nb-impregnated two-dimensional pillared MWW zeolites for total oxidation of volatile organic compounds. Microporous Mesoporous Mater. 2021, 327, 111425. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, K.; Li, J.; Ma, Y.; Yang, F.; Meng, X.; Xiao, F.-S. Hydrophobic modification of zeolite-supported platinum catalysts for complete oxidation of toluene. Catal. Today 2024, 432, 114601. [Google Scholar] [CrossRef]
- Zhu, X.; He, X.; Guo, L.; Shi, Y.; Zhao, N.; Qiao, C.; Dai, L.; Tian, Y. Hydrophobic Modification of ZSM-5-Encapsulated Uniform Pt Nanoparticles for Catalytic Oxidation of Volatile Organic Compounds. ACS Appl. Nano Mater. 2022, 5, 3374–3385. [Google Scholar] [CrossRef]
- Meng, X.; Meng, L.; Gong, Y.; Li, Z.; Mo, G.; Zhang, J. Modifying Y zeolite with chloropropyl for improving Cu load on Y zeolite as a super Cu/Y catalyst for toluene oxidation. RSC Adv. 2021, 11, 37528–37539. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, D.; Wang, Y.; Chen, B.; Shi, C. Construction of sandwich type RuOn supported on Ag@hollow-zeolite catalysts for toluene enrichment and oxidation with superior chlorine resistance. Sep. Purif. Technol. 2025, 361, 131426. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Huang, H.; Zeng, K.; Wang, Z.; Jia, H.-P.; Li, X. Insights into the size and structural effects of zeolitic supports on gaseous toluene oxidation over MnOx/HZSM-5 catalysts. Appl. Surf. Sci. 2019, 486, 108–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).