MOF-Derived Electrocatalysts for High-Efficiency Hydrogen Production via Water Electrolysis
Abstract
1. Introduction
2. Experiments and Methods
2.1. Experimental Materials
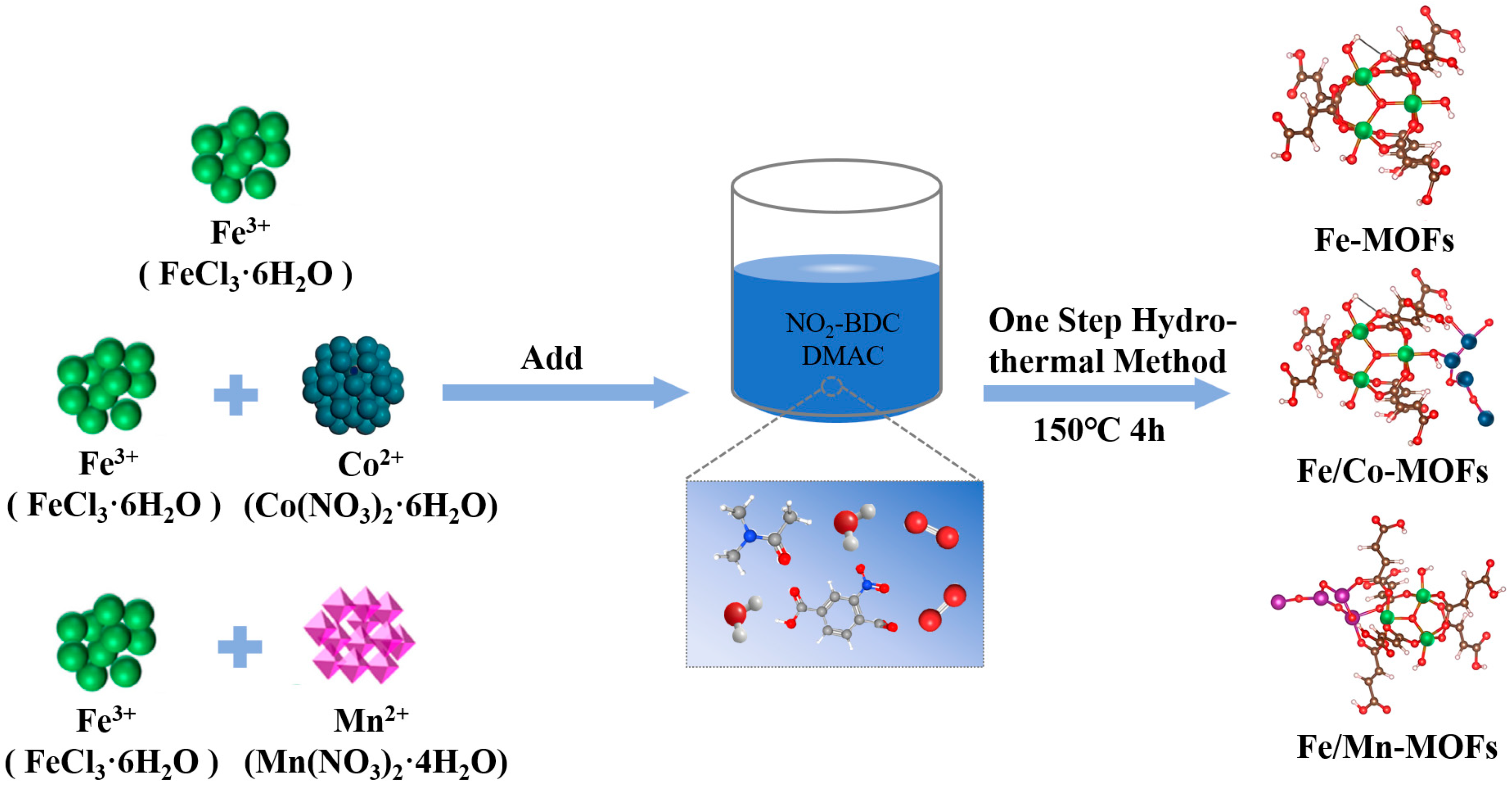
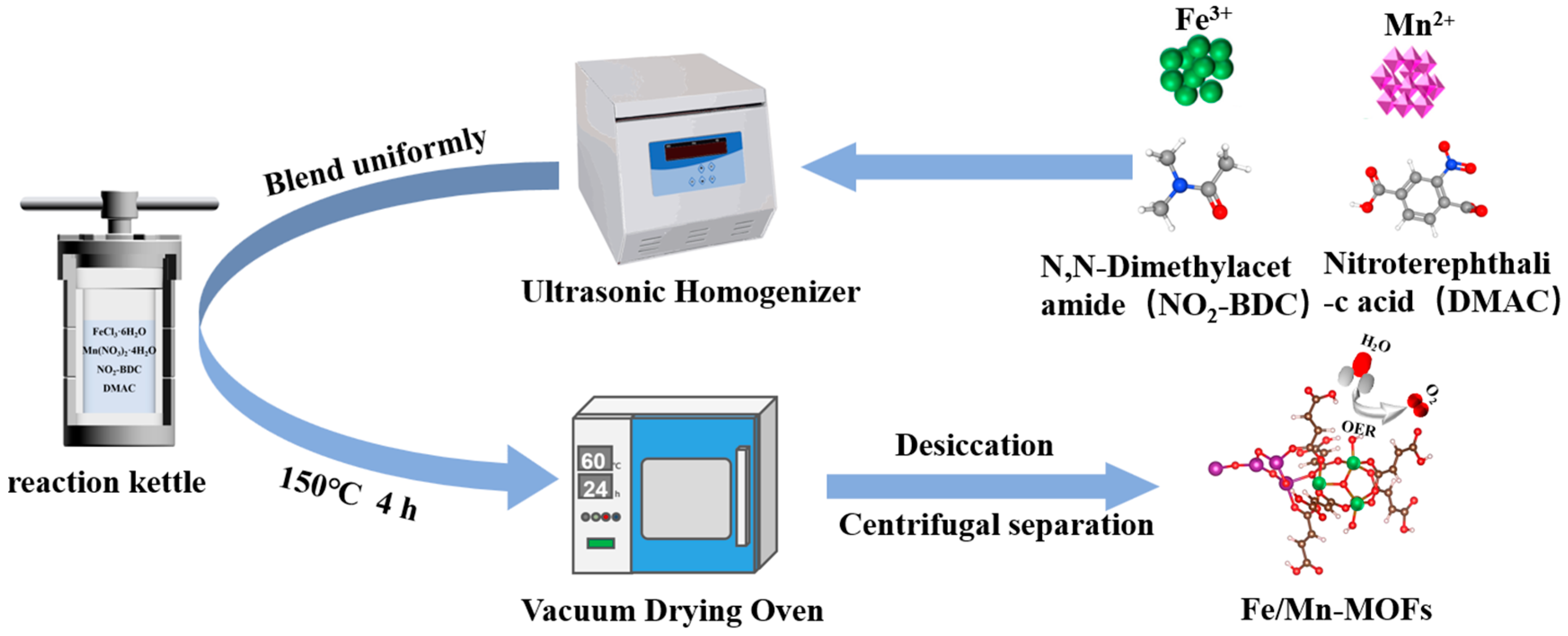
2.2. Preparation of Metal-Organic Frameworks Nanocatalysts
2.3. Electrode Preparation
2.4. Characterization and Electrochemical Evaluation of Synthesized MOFs Electrocatalysts
3. Results and Discussion
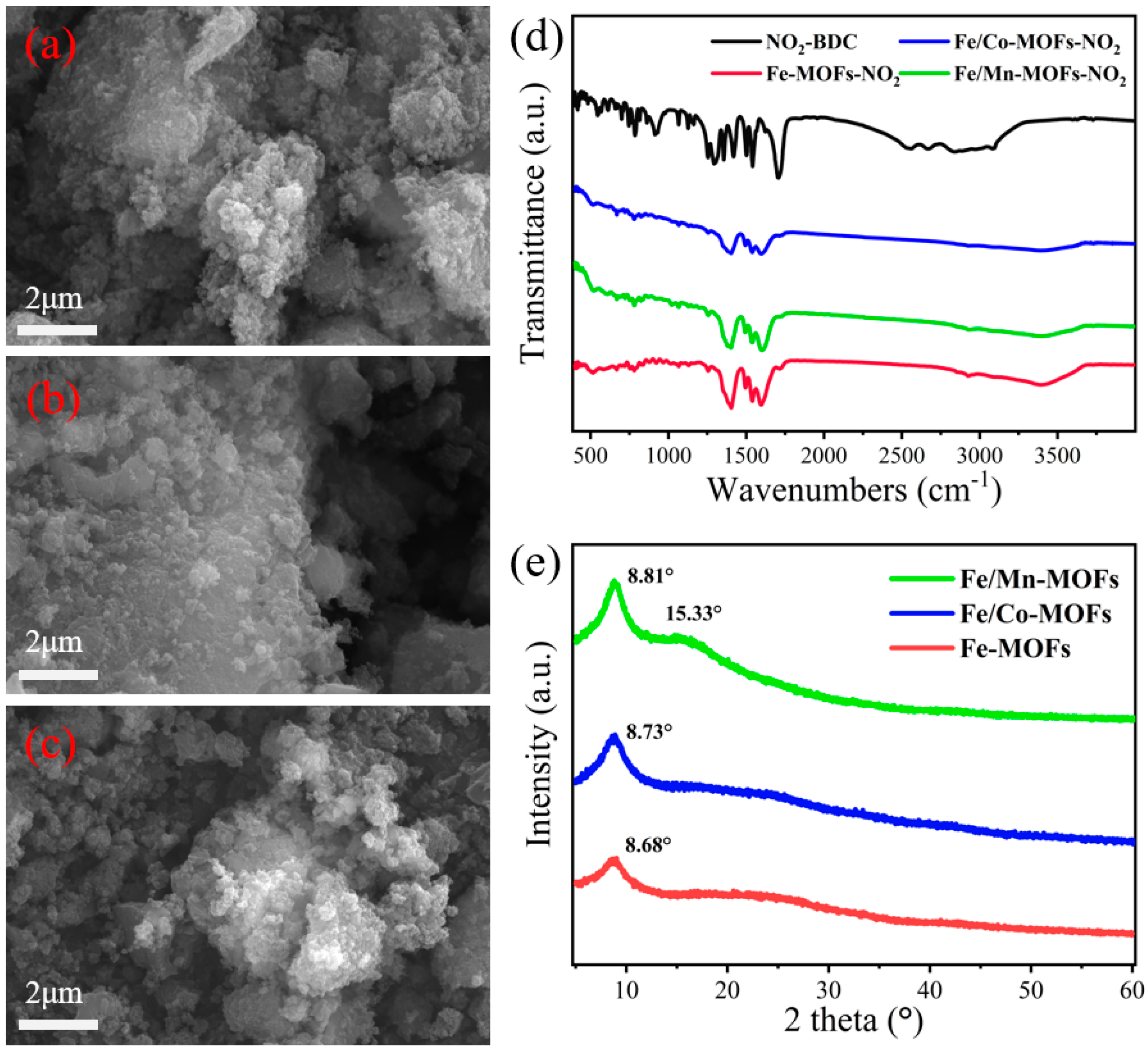
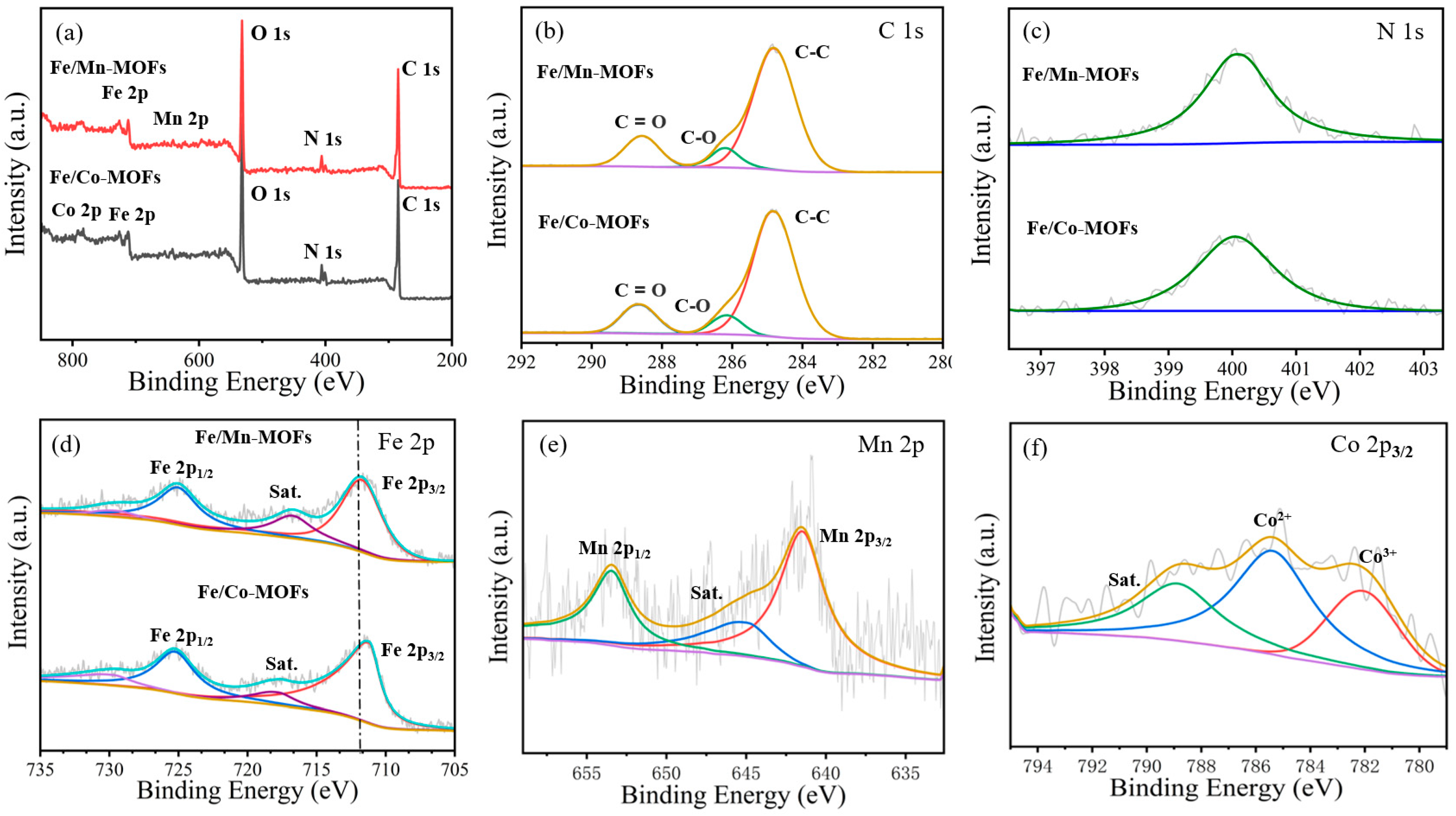
3.1. SYNTHESIS and Characterization of MOF-Derived Catalysts
3.2. Electrochemical Performance and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Long, Q.; Xiao, K.; Ouyang, T.; Li, N.; Ye, S.; Liu, Z.-Q. Vertically-interlaced NiFeP/MXene electrocatalyst with tunable electronic structure for high-efficiency oxygen evolution reaction. Sci. Bull. 2021, 66, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ci, S.; Ding, Y.; Wang, G.; Wen, Z. Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems. J. Mater. Chem. A 2019, 7, 8006–8029. [Google Scholar] [CrossRef]
- Li, Y.; Lu, M.; Wu, Y.; Ji, Q.; Xu, H.; Gao, J.; Qian, G.; Zhang, Q. Morphology regulation of metal-organic framework-derived nanostructures for efficient oxygen evolution electrocatalysis. J. Mater. Chem. A 2020, 8, 18215–18219. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, H.; Chang, J.; Tang, Z.; Lu, S. Progress on the mechanisms of Ru-based electrocatalysts for the oxygen evolution reaction in acidic media. J. Energy Chem. 2023, 85, 220–238. [Google Scholar] [CrossRef]
- Guo, B.; Ding, Y.; Huo, H.; Wen, X.; Ren, X.; Xu, P.; Li, S. Recent Advances of Transition Metal Basic Salts for Electrocatalytic Oxygen Evolution Reaction and Overall Water Electrolysis. Nano-Micro Lett. 2023, 15, 57. [Google Scholar] [CrossRef]
- Bhanja, P.; Kim, Y.; Paul, B.; Kaneti, Y.V.; Alothman, A.A.; Bhaumik, A.; Yamauchi, Y. Microporous nickel phosphonate derived heteroatom doped nickel oxide and nickel phosphide: Efficient electrocatalysts for oxygen evolution reaction. Chem. Eng. J. 2021, 405, 126803. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Fathoni, K.B.; Guo, Y.; Ide, Y.; Yuliarto, B.; Jiang, X.; Nugraha; Dipojono, H.K.; Golberg, D.; et al. Tailorable nanoarchitecturing of bimetallic nickel-cobalt hydrogen phosphate via the self-weaving of nanotubes for efficient oxygen evolution. J. Mater. Chem. A 2020, 8, 3035–3047. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Fathoni, K.B.; Kani, K.; Allah, A.E.; Yuliarto, B.; Nugraha; Dipojono, H.K.; Alothman, Z.A.; Golberg, D.; et al. Self-Assembly of Two-Dimensional Bimetallic Nickel-Cobalt Phosphate Nanoplates into One-Dimensional Porous Chainlike Architecture for Efficient Oxygen Evolution Reaction. Chem. Mater. 2020, 32, 7005–7018. [Google Scholar] [CrossRef]
- Chen, I.W.P.; Chen, W.-Y.; Liu, T.Y. Pioneering ultra-efficient oxygen evolution reaction: A breakthrough in tri-metallic organic frameworks synthesis. Mater. Today Chem. 2024, 35, 101873. [Google Scholar] [CrossRef]
- Hu, W.-C.; Shi, Y.; Zhou, Y.; Wang, C.; Younis, M.R.; Pang, J.; Wang, C.; Xia, X.-H. Plasmonic hot charge carriers activated Ni centres of metal- organic frameworks for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 10601–10609. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-P.; Lu, X.F.; Zang, S.-Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhu, H.-J.; Dong, L.-Z.; Zhang, A.M.; Li, S.-L.; Liu, J.; Lan, Y.-Q. Solid-phase hot-pressing of POMs-ZIFs precursor and derived phosphide for overall water splitting. Appl. Catal. B-Environ. 2019, 245, 528–535. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Yuan, W.; Li, C.M. Tannic Acid-Mediated In Situ Controlled Assembly of NiFe Alloy Nanoparticles on Pristine Graphene as a Superior Oxygen Evolution Catalyst. Acs Appl. Energy Mater. 2020, 3, 3966–3977. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, Y.; Lou, F.; Aslam, M.K.; Sun, Y.; Li, M.; Duan, J.; Li, Y.; Chen, S. “Uncapped” metal-organic framework (MOF) dispersions driven by O2 plasma towards superior oxygen evolution electrocatalysis. J. Mater. Chem. A 2022, 10, 20813–20818. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Wang, J.; Ren, D.; Li, B.-Q.; Zhang, Q. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts. Chem. Soc. Rev. 2021, 50, 7745–7778. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Ding, S.; Sun, Y.; Lou, F.; Yu, L.; Xia, B.; Duan, J.; Zhang, Y.; Chen, S. Plasma-regulated two-dimensional high entropy oxide arrays for synergistic hydrogen evolution: From theoretical prediction to electrocatalytic applications. J. Power Sources 2022, 520, 230873. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, W.; Jiao, L.; Yu, S.-H.; Jiang, H.-L. Template-Directed Growth of Well-Aligned MOF Arrays and Derived Self-Supporting Electrodes for Water Splitting. Chem 2017, 2, 791–802. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.; Ding, Y.; Yi, L.; Zhang, C.; Wen, Z. Nanohybrid photocatalysts with ZnIn2S4 nanosheets encapsulated UiO-66 octahedral nanoparticles for visible-light-driven hydrogen generation. Appl. Catal. B-Environ. 2020, 260, 118152. [Google Scholar] [CrossRef]
- Yang, M.; Jiao, L.; Dong, H.; Zhou, L.; Teng, C.; Yan, D.; Ye, T.-N.; Chen, X.; Liu, Y.; Jiang, H.-L. Conversion of bimetallic MOF to Ru-doped Cu electrocatalysts for efficient hydrogen evolution in alkaline media. Sci. Bull. 2021, 66, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Reghunath, B.S.; Devi, K.R.S.; Pinheiro, D. Designing coordinatively unsaturated metal sites in bimetallic organic frameworks for oxygen evolution reaction. Mater. Today Chem. 2023, 31, 101616. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Niu, S.; Li, C.; Chen, C.; Liu, Q.; Huo, J. Microwave-assisted rapid synthesis and activation of ultrathin trimetal-organic framework nanosheets for efficient electrocatalytic oxygen evolution. J. Colloid Interface Sci. 2021, 603, 148–156. [Google Scholar] [CrossRef]
- Xing, D.; Wang, H.; Cui, Z.; Lin, L.; Liu, Y.; Dai, Y.; Huang, B. A Conductive Two-dimensional Trimetallic FeCoNi-Benzenehexathiol π-d Conjugated Metal-organic Framework for Highly Efficient Oxygen Evolution Reaction. J. Colloid Interface Sci. 2024, 656, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yu, Q.; Liu, G.; Bi, J.; Zhang, Y.; Chi, J.; Lai, J.; Yang, B.; Wang, L. Hierarchical microsphere MOF arrays with ultralow Ir doping for efficient hydrogen evolution coupled with hydrazine oxidation in seawater. J. Mater. Chem. A 2021, 9, 27424–27433. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, Y.; Zheng, Y.; Pang, J.; Sun, K.; Hou, J.; Wang, G.; Guo, W.; Guo, X.; Chen, L. Self-supporting one-dimensional ZnFe-BDC for electrocatalysis oxygen evolution reaction in alkaline and natural seawater. Int. J. Hydrog. Energy 2022, 47, 35655–35665. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.; El Hankari, S.; Chen, R.; Wang, S. Plasma-Assisted Synthesis and Surface Modification of Electrode Materials for Renewable Energy. Adv. Mater. 2018, 30, e1705850. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. -Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Doughty, T.; Zingl, A.; Wunschek, M.; Pichler, C.M.; Watkins, M.B.; Roy, S. Structural Reconstruction of a Cobalt- and Ferrocene-Based Metal-Organic Framework during the Electrochemical Oxygen Evolution Reaction. Acs Appl. Mater. Interfaces 2024, 16, 40814–40824. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Mao, J.; Yan, J.; Song, W. Structural and electronic modulation of conductive MOFs for efficient oxygen evolution reaction electrocatalysis. J. Mater. Chem. A 2021, 9, 11248–11254. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, X.; Wang, Z.; Li, G.; Han, Z.; Yang, H.; Ren, X.; Zhang, Q.; Liu, J.; He, C. Unconventionally fabricating defect-rich NiO nanoparticles within ultrathin metal-organic framework nanosheets to enable high-output oxygen evolution. J. Mater. Chem. A 2020, 8, 2140–2146. [Google Scholar] [CrossRef]
- Kumar, S.; Weng, P.H.; Fu, Y.P. Core-shell-structured CuO@Ni-MOF: Bifunctional electrode toward battery-type supercapacitors and oxygen evolution reaction. Mater. Today Chem. 2022, 26, 101159. [Google Scholar] [CrossRef]
- Yu, R.; Wang, C.; Liu, D.; Wu, Z.; Li, J.; Du, Y. Bimetallic sulfide particles incorporated in Fe/Co-based metal-organic framework ultrathin nanosheets toward boosted electrocatalysis of the oxygen evolution reaction. Inorg. Chem. Front. 2022, 9, 3130–3137. [Google Scholar] [CrossRef]
- Ren, T.; Wang, J.; Yu, X.; Chen, Y.; Wu, Y.; Che, G.; Jiang, W.; Teng, H.; Liu, C. The electrocatalytic self-reconstruction of ultrathin 2D MOF nanoarrays supported on alloy foam improves the oxygen evolution reaction. Colloids Surf. A-Physicochem. Eng. Asp. 2024, 684, 133136. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Liu, S.; Zhao, Y.; Liu, L.; Zhang, D.; Zhao, S.; Liu, J.; Wang, J.; Liu, Y.; et al. Mesoporous Single-Crystalline Particles as Robust and Efficient Acidic Oxygen Evolution Catalysts. J. Am. Chem. Soc. 2025, 14, 13345–13355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, W.; Tan, L.; Yao, Z.; Yang, X.; Song, R.; Lan, S. Ultrafast Synthesis of Tetragonal-Distorted FeCoNiCuCr High-Entropy Alloy Nanoparticles for Enhanced OER Performance. Chin. Chem. Lett. 2025, 36, 110852. [Google Scholar] [CrossRef]
- Ding, W.; Saad, A.; Wu, Y.; Wang, Z.; Li, X. CNTs/CNF-Supported Multi-Active Components as Highly Efficient Bifunctional Oxygen Electrocatalysts and Their Applications in Zinc-Air Batteries. Nano Res. 2023, 16, 4793–4802. [Google Scholar] [CrossRef]
- Ni, H.; Xu, S.; Lin, R.; Ding, Y.; Qian, J. Ligand-Induced Hollow Binary Metal-Organic Framework Derived Fe-Doped Cobalt-Carbon Nanomaterials for Oxygen Evolution. J. Colloid Interface Sci. 2024, 671, 100–109. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, X.; Kwon, S.; Chen, K.; Liu, X.; Yang, J.; Kang, Y. Tantalum-Stabilized Ruthenium Oxide Electrocatalysts for Industrial Water Electrolysis. Science 2025, 387, 48–55. [Google Scholar] [CrossRef]
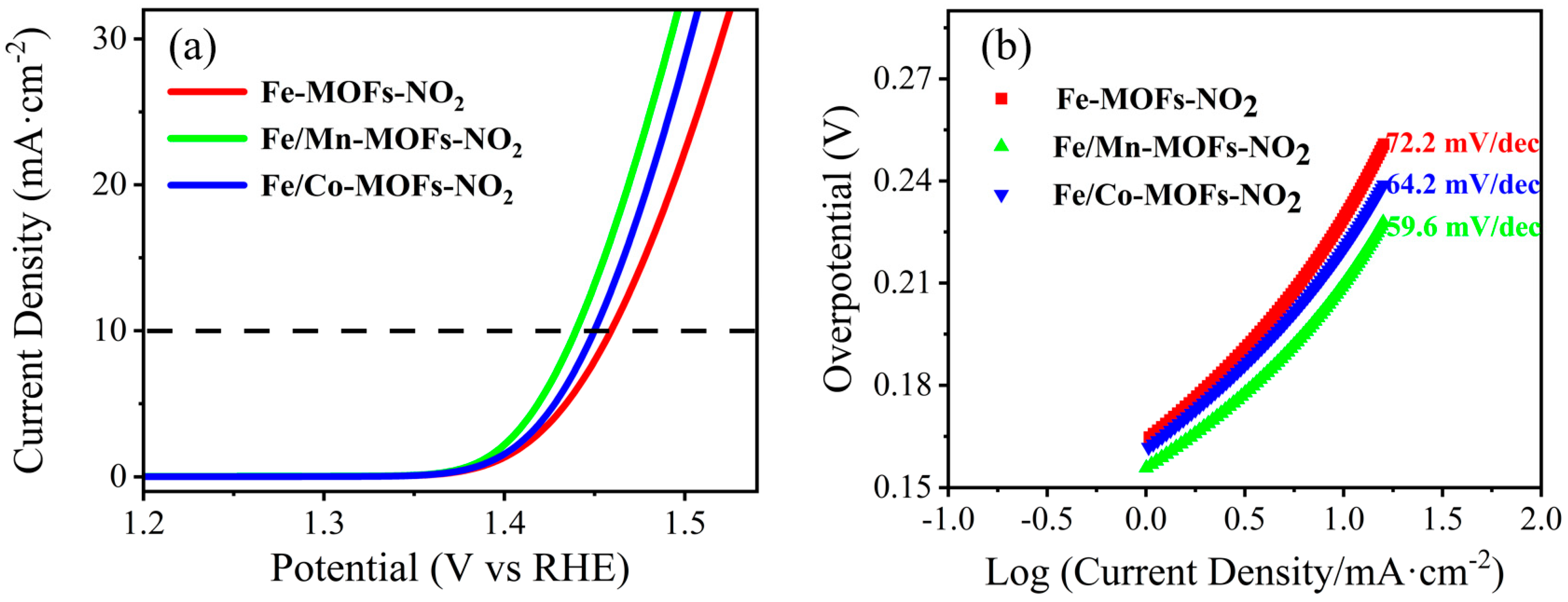
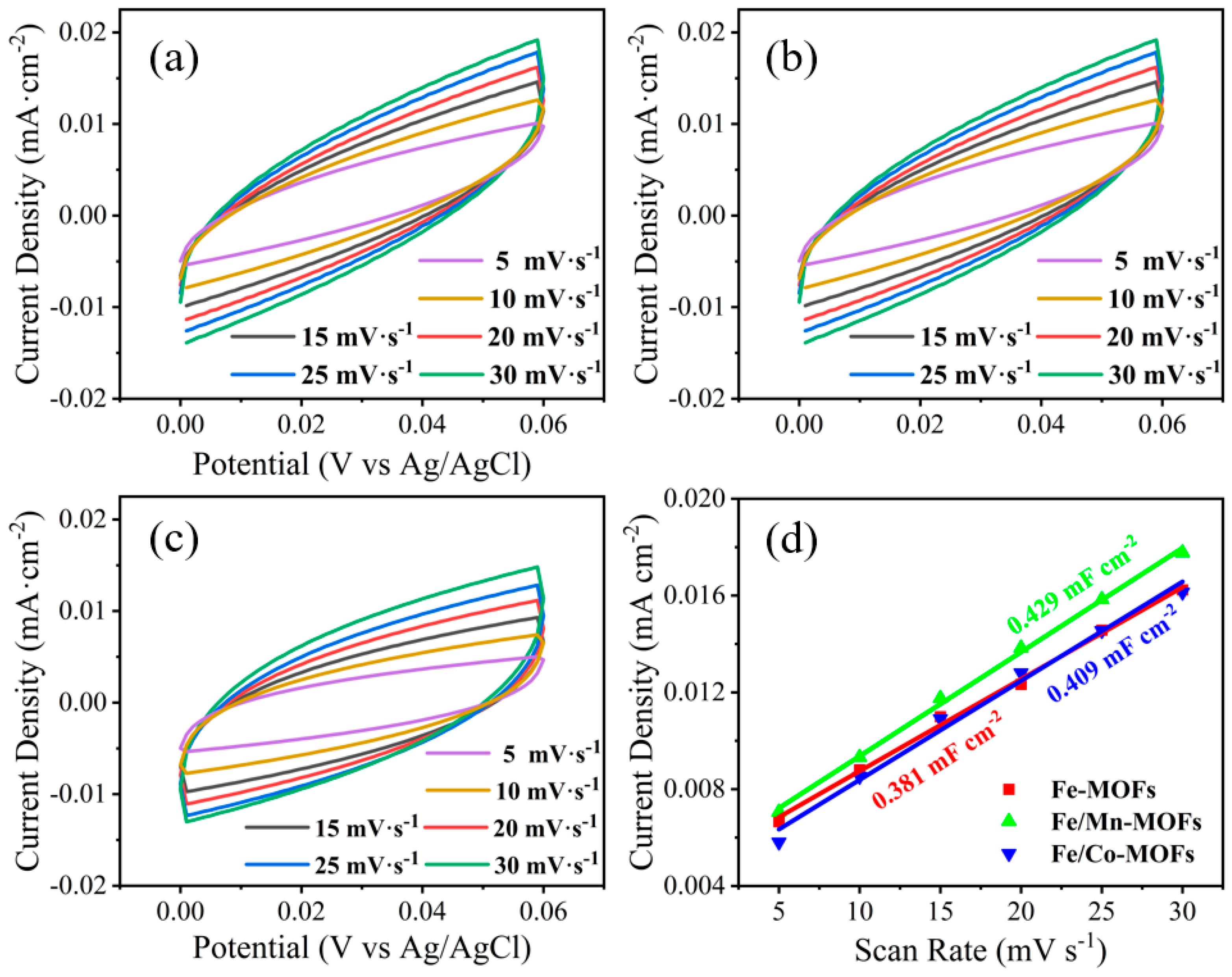
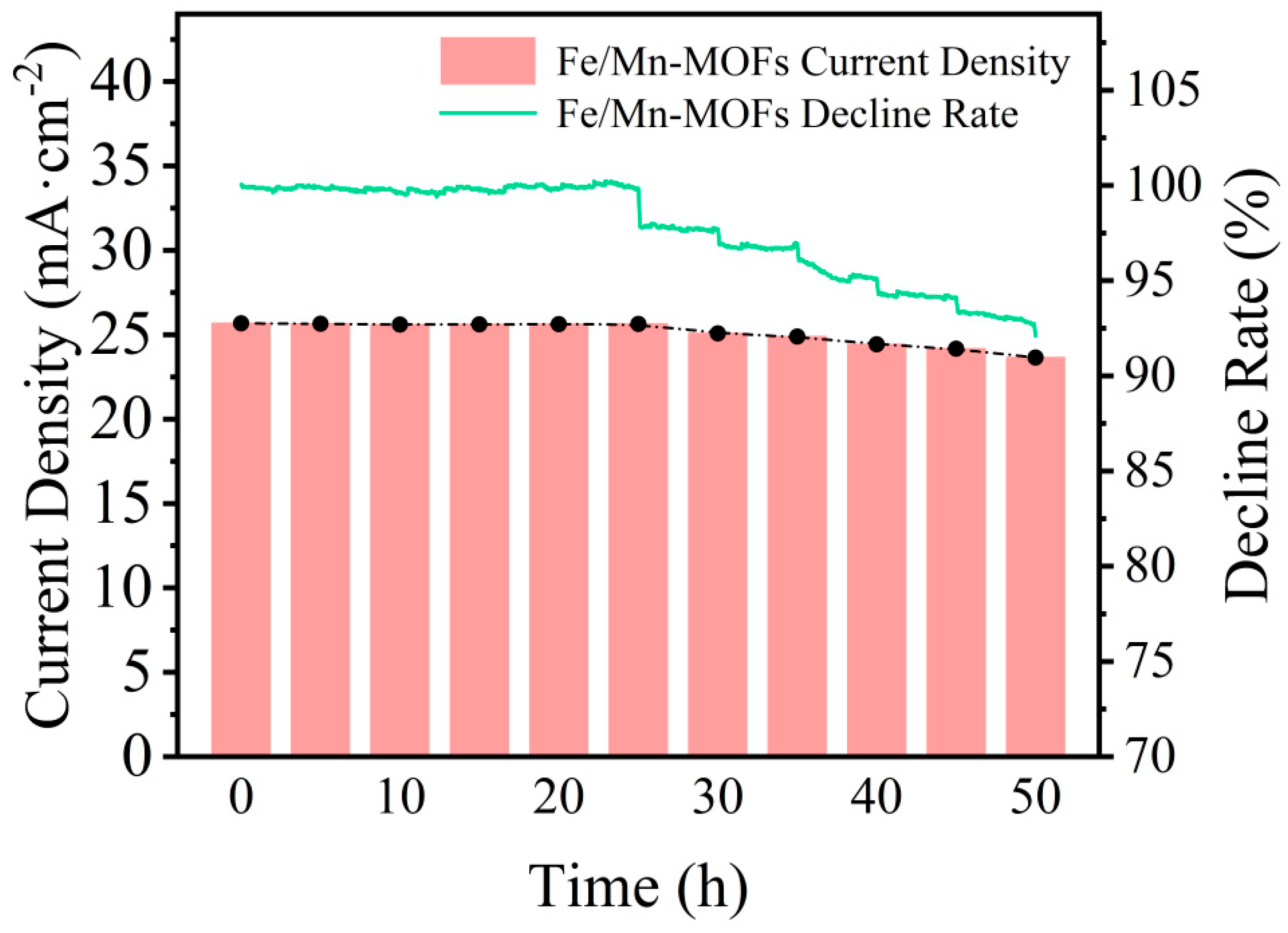
| Catalyst | Overpotential @10 mA cm−2 | Tafel Slope | Substrate |
|---|---|---|---|
| Fe/Mn-MOFs | 233 mV (alkaline) | 59.6 mV dec−1 | Glassy Carbon |
| Ir-MSC-Co3O4 | 248 mV (acidic) | 42.1 mV dec−1 | Glassy Carbon |
| FeCoNiCuCr HEA NPs | 272 mV (alkaline) | 58.3 mV dec−1 | Carbon Cloth |
| FeCo/FePx/MNx-CNTs/CNF | ~266 mV (alkaline) | 67.5 mV dec−1 | Carbon Fiber |
| Co0.3Fe-ZP | 284 mV (alkaline) | 71.2 mV dec−1 | Nickel Foam |
| Ta-RuO2 | 178 mV (acidic) | 40.2 mV dec−1 | Ti Mesh |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Cui, P.; Zhang, J.; Qiao, Y. MOF-Derived Electrocatalysts for High-Efficiency Hydrogen Production via Water Electrolysis. Catalysts 2025, 15, 579. https://doi.org/10.3390/catal15060579
Zhang N, Cui P, Zhang J, Qiao Y. MOF-Derived Electrocatalysts for High-Efficiency Hydrogen Production via Water Electrolysis. Catalysts. 2025; 15(6):579. https://doi.org/10.3390/catal15060579
Chicago/Turabian StyleZhang, Nan, Pengfei Cui, Jinrong Zhang, and Yang Qiao. 2025. "MOF-Derived Electrocatalysts for High-Efficiency Hydrogen Production via Water Electrolysis" Catalysts 15, no. 6: 579. https://doi.org/10.3390/catal15060579
APA StyleZhang, N., Cui, P., Zhang, J., & Qiao, Y. (2025). MOF-Derived Electrocatalysts for High-Efficiency Hydrogen Production via Water Electrolysis. Catalysts, 15(6), 579. https://doi.org/10.3390/catal15060579





