Abstract
The incorporation of Mg into PtCoAl catalysts was systematically investigated to elucidate its impact on the physicochemical properties and catalytic performance in CO2 hydrogenation. A series of x% Mg-PtCoAl catalysts with varying Mg contents were synthesized and evaluated for CO2 hydrogenation. The results demonstrate that Mg acts as a promoter by strengthening metal–support interactions, slightly suppressing reducibility while markedly enhancing hydrogen adsorption capacity and the number of basic sites. Additionally, an optimal Mg content increases the proportion of weak basic sites, which synergistically facilitates CO2 activation in conjunction with weakly adsorbed hydrogen. The effects of Pt and Mg loadings were further investigated using different precipitants, revealing that moderate loadings (Pt: 0.11–0.12 mmol gcat−1, Mg: 0.08–0.10 mmol gcat−1) most effectively enhance catalytic activity. Under an initial pressure of 4 MPa and 180 °C, the Mg-modified PtCoAl catalyst achieved a methane productivity of 51.4 mmol gcat−1 h−1 and an alcohol productivity of 0.895 mmol gcat−1 h−1, with higher alcohols accounting for 33.6% of the total alcohol products. This study underscores the pivotal role of Mg in tuning surface basicity and hydrogen adsorption properties, offering valuable insights for the rational design of efficient CO2 hydrogenation catalysts.
1. Introduction
Excessive consumption of fossil fuels has led to a significant increase in atmospheric CO2 concentrations, resulting in environmental issues such as the greenhouse effect and ocean acidification [1,2]. Mitigating the greenhouse effect to control the rise in global temperatures has become an urgent priority. Carbon capture and storage (CCS) technology can prevent CO2 from being released into the atmosphere. Meanwhile, carbon capture and utilization (CCU) technologies, particularly CO2 hydrogenation reactions, can convert CO2 into valuable chemicals or fuels, such as CO, methane, light olefins, alcohols, and carboxylic acids [3,4,5]. These technologies have shown promising prospects and have garnered significant interest.
Significant progress has been made in developing heterogeneous catalyst systems for CO2 hydrogenation. Current research focuses on two primary categories: non-noble metal-based catalysts (e.g., Fe, Co, Cu, Ni, Mo) [6,7,8,9] and noble metal-based catalysts (e.g., Pt, Pd, Ru, Rh) [6,10,11,12]. Among them, Pt is widely used in CO2 hydrogenation due to its excellent H dissociation activation and migration ability [13]. In addition, cobalt-based catalysts emerge as particularly promising candidates due to their favorable redox properties, tunable electronic structure, and operational stability under harsh reaction conditions [14,15,16,17].
Adsorption and activation of weak acidic CO2 molecules on the surface of catalysts, a key step in CO2 hydrogenation, can be effectively tailored by engineered basic sites on catalyst surfaces [18,19]. The strategic introduction of promoters enables precise engineering of these basic sites. For example, Gao et al. systematically investigated Cu/Zn/Al catalysts modified with Mn, La, Ce, Zr, and Y for CO2-to-methanol conversion, revealing a linear correlation between CO2 conversion efficiency and surface basic site density [20]. Similarly, Wang et al. demonstrated that the strength and distribution of basic sites critically regulate CO2 adsorption behavior during hydrogenation processes. By adjusting the Mg/Al ratio in Ni/MgAlOx catalysts, the synergistic effects of medium and weak basic sites are optimized, thus enhancing CO2 hydrogenation activity [21]. Furthermore, introducing a small amount of ZrO2 into Cu/ZnO catalysts markedly increased the methanol formation rate. This improvement is attributed to ZrO2 providing new medium-strength basic sites, which facilitate CO2 activation and methanol synthesis [22]. Therefore, selecting Co as the primary active component while utilizing Pt and Mg as promoters represents an effective strategy for achieving high catalytic performance while maintaining cost control.
In our previous work, we introduced Pt as a promoter into CoAl catalysts to tailor the hydrogen spillover effect, thereby optimizing H/CO2 adsorption and enhancing CO2 hydrogenation performance [23]. In this study, the basicity of the catalyst surface was further modulated by incorporating Mg as a promoter. The addition of Mg increased the density of surface basic sites, with the fraction of weak basic sites enhanced at a Mg loading of 0.7%. These weak basic sites facilitate CO2 adsorption, activation, and subsequent conversion. Furthermore, catalysts were synthesized using different types of precipitants to fine-tune the Pt and Mg contents in the CoAl framework. The results indicate that moderate Pt and Mg loadings (Pt: 0.12 to 0.15 mmol gcat−1, Mg (0.08 to 0.12 mmol gcat−1) are most effective in enhancing the CO2 hydrogenation performance of the CoAl catalyst. Under an initial pressure of 4 MPa and 180 °C, the Mg-modified catalyst exhibited a methane productivity of 51.4 mmol gcat−1 and an alcohol productivity of 0.895 mmol gcat−1, with higher alcohols accounting for 33.6% of the total alcohol products.
2. Results and Discussion
2.1. Structure and Performance of Mg-Modified Catalysts
The chemical composition of the calcined Mg-modified PtCoAl catalysts was analyzed using inductively coupled plasma-optical emission spectroscopy (ICP-OES, Varian 720ES, Palo Alto, CA, USA), as presented in Table 1 and Table S1. As the Mg dosage (α) increases during synthesis, the Mg content in the calcined catalysts rises from 0 to 0.497 mmol gcat−1. Consequently, the Mg/(Co+Al+Pt) molar content increases from 0% to 5.17%. Moreover, the crystal structure of the calcined x% Mg-PtCoAl catalysts was analyzed using X-ray diffraction (XRD), as presented in Figure 1a. The XRD patterns show diffraction peaks at 31.3°, 36.8°, 44.8°, 59.3°, and 65.2°, corresponding to the (022), (131), (040), (242), and (151) facets of the spinel structure [24]. This indicates the possible formation of Co3O4 and/or a CoAl2O4/MgAl2O4 structure. Notably, the signal noise ratio decreases at high Mg contents, suggesting the formation of a more amorphous structure or smaller crystallites. Additionally, the crystallite size, calculated based on the (131) reflex, initially increases, reaching 257 Å in the 0.7% Mg-PtCoAl catalyst, and then decreases with further increasing Mg content (Table 1).

Table 1.
Structural characteristics of calcined PtCoAl catalysts with various Mg contents.
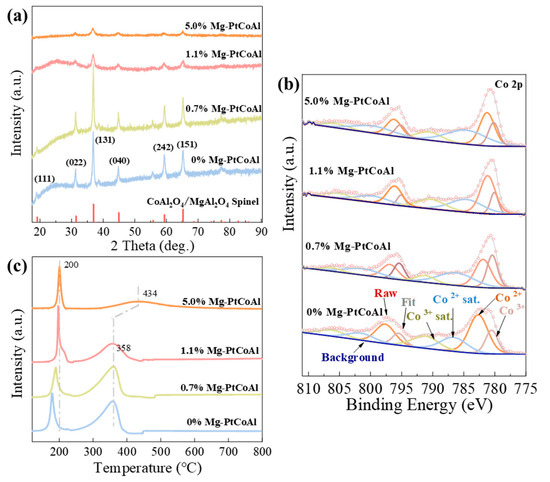
Figure 1.
(a) XRD patterns of calcined x% Mg-PtCoAl catalysts. (b) Co 2p XPS spectrum of the calcined x% Mg-PtCoAl catalysts. (c) H2-TPR profiles of the x% Mg-PtCoAl catalysts.
The textural properties of the calcined catalysts were investigated by N2 physisorption. The adsorption–desorption isotherms (Figure S1) show that the catalysts exhibit H3-type hysteresis loops, indicating the presence of slit-like pores or agglomerates of plate-like particles [25,26]. The variation trend of specific surface area with increasing Mg content in Table 1 exhibits two distinct stages. The initial stage shows a decline (0% to 0.7% Mg), where SBET decreases from 78 m2 g−1 to 53 m2 g−1, indicating that Mg incorporation initially promotes particle agglomeration. This phenomenon corresponds with the synchronous increase in L131 crystallite size (204 to 257 Å). Subsequently, an ascending stage emerges (0.7% to 5.0% Mg). When the Mg content reaches 5.0%, SBET significantly rebounds to 131 m2 g−1, accompanied by a notable reduction in crystallite size (L131 decreases to 93 Å). This may be attributed to high Mg content inhibiting crystal growth during calcination, along with the potential formation of Mg-rich surface phases with porous structures [27].
Figure 1b displays the Co 2p X-ray photoelectron spectroscopy (XPS) spectra of the calcined Mg-PtCoAl catalysts with varying Mg content. The spectra show peaks associated with the 2p 1/2 and 2p 3/2 orbitals of Co2+ and Co3+, along with their satellite peaks [28]. The deconvolution results in Table S2 reveal that the introduction of Mg into PtCoAl catalysts decreases the binding energies of Co3+ and Co2+ by 0.44 and 1.51 eV, respectively, for the 1.1% Mg-PtCoAl catalyst. This is because incorporating the less electronegative Mg induces electronic polarization, creating electron-rich Co centers [29]. However, at higher Mg levels, the binding energies of Co3+ and Co2+ increase to 780.20 and 781.24 eV, respectively, for the 5% Mg-PtCoAl catalyst. The aggregation of Mg species on the catalyst surface leads to a decrease in the electron density of Co. Furthermore, the incorporation of Mg reduces the fraction of Co3+ species from 35.7% to 29.7%, possibly due to the replacement of Co3+ by Mg2+ in the octahedral sites of the spinel structure [30].
The reduction behavior of Mg-PtCoAl catalysts was investigated using H2 temperature-programmed reduction (TPR), with the results presented in Figure 1c. All samples exhibit two distinct H2 reduction peaks. The low-temperature reduction peaks can be attributed to the two-step reduction processes of Co3O4 to CoO and CoO to Co0 (Equations (1) and (2)) [31], as well as the reduction of small amounts of Pt oxides and Pt-O-Co bonds. The high-temperature reduction peaks correspond to the reduction of the strongly interacted Co-Al -O species to Co0 [31].
Co3O4 + H2 → 3CoO + H2O
CoO + H2 → Co0 + H2O
It is noteworthy that the addition of Mg leads to an upshift in the reduction temperature. This shift is possibly due to the more electron-rich Co species induced by Mg addition [29,32], which makes it less favorable for this ion to gain additional electrons and be reduced to Co2+ or metallic Co, which leads to a higher reduction temperature and a potentially slower rate of reduction [33,34].
H2 temperature-programmed desorption (TPD) was employed to investigate the H2 activation capabilities of Mg-modified PtCoAl catalysts (Figure 2a and Table 2). The data reveal two distinct adsorption peaks: a low-temperature peak between 150 and 200 °C, representing weakly adsorbed hydrogen, and a peak between 300 and 350 °C, associated with strongly adsorbed hydrogen [35]. As the Mg content increases from 0% to 1.1% in the PtCoAl catalysts, the H2 uptake rises from 301.8 to 596.7 μmolH2 gcat−1 and starts to decrease with further increasing Mg content. This may be attributed to enhanced hydrogen adsorption/activation induced by the Mg promoter [36]. Even though the uptake of weakly adsorbed hydrogen (H2 uptake before 250 °C) follows a similar trend to the total H2 uptake, its fraction shows a decreasing trend.
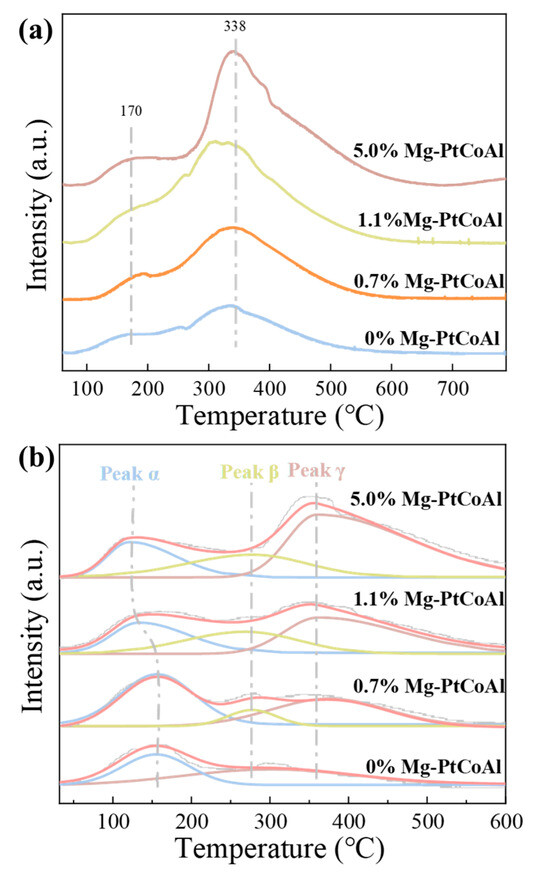
Figure 2.
(a) H2-TPD profiles of the x% Mg-PtCoAl catalysts. (b) CO2-TPD profiles of x% Mg-PtCoAl catalysts.

Table 2.
H2-TPD results of the x% Mg-PtCoAl catalysts.
CO2-TPD was employed to investigate CO2 activation and adsorption behavior on the surface of x% Mg-PtCoAl catalysts. The CO2 desorption profiles of the catalysts reduced at 250 °C are shown in Figure 2b, with the desorption peak temperatures and quantities of basic sites presented in Table 3. The desorption profiles indicate the formation of a series of adsorption sites possessing various adsorption strengths: weak basic sites (100–200 °C), medium basic sites (250–300 °C), and strong basic sites (>300 °C), labeled as α, β, and γ, respectively [37]. The weak basic sites are attributed to surface hydroxyl groups, also known as Brønsted basic sites. The medium basic sites are associated with metal–oxygen pairs, including Co-O, Mg-O, and Al-O pairs within the catalyst. The strong basic sites correspond to coordinatively unsaturated O2− ions, exhibiting pronounced Lewis basic characteristics [37,38]. The incorporation of Mg leads to an increase in the number of basic sites from 1004.8 to 1818.0 μmol gcat−1. Notably, the temperature corresponding to the weak basic sites, specifically the center of peak α, remains nearly constant, at approximately 150 °C, when the Mg content is within the range of 0% to 0.7%. With further addition of Mg, from 1.1% to 5.0%, the temperature of peak α decreases to around 120 °C. This observation suggests that Mg content exceeding 0.7% leads to a reduction in the strength of the weak basic sites. Moreover, the fraction of weak basic sites first increases to 46% but later significantly decreases to 16% with increasing Mg content.

Table 3.
CO2-TPD results of the x% Mg-PtCoAl catalysts.
The performance of Mg-modified PtCoAl catalysts for CO2 hydrogenation was evaluated. CO2 hydrogenation on Mg-modified PtCoAl catalysts primarily produces methane, with trace amounts of methanol, ethanol, and propanol (Figure 3). The reaction conditions were established based on previous studies: a reaction temperature of 180 °C, an initial pressure of 4 MPa, water as the solvent, and 50 mg of catalyst were used [23]. It was observed that, with increasing Mg concentration in the PtCoAl catalysts, the productivity of various products initially increases and then decreases with excessive Mg amounts. The highest productivities for methane (48.6 mmol gcat−1 h−1), methanol (549.0 μmol gcat−1 h−1), and higher alcohols (226.7 μmol gcat−1 h−1) are achieved with 0.7% Mg-PtCoAl. When the Mg content exceeds 1.1%, a significant decrease in the productivities of various products is observed. At 5.0% Mg content, the productivities for methane and methanol drop to 1.7 mmol gcat−1 h−1 and 12.3 μmol gcat−1 h−1, respectively, and no ethanol or propanol is formed.
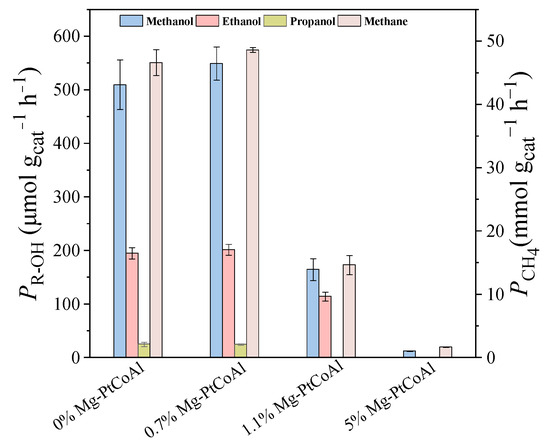
Figure 3.
The effect of Mg content on the performance of x% Mg-PtCoAl catalysts. 3 MPa, 180 °C, H2/CO2 = 3/1.
The role of Mg has been substantiated by multiple reports as being capable of constructing basic sites on the catalyst surface, thereby enhancing the catalyst’s adsorption and activation capacity for CO2 [36,39]. Additionally, Mg possesses the ability to promote H adsorption, optimize surface electronic structures to facilitate the reduction of active metals, and stabilize the catalyst structure [40,41,42]. The synergistic effects of these promotional functions further enhance the CO2 hydrogenation reaction. Next, we will conduct a detailed analysis of the influence of Mg incorporation on the adsorption behaviors of H and CO2 on these catalysts.
2.2. Effects of H and CO2 Adsorption on CO2 Hydrogenation
Previous studies have revealed that, on PtCoAl catalysts, the higher the fraction of weakly adsorbed H, the more H has the opportunity to spill over from the catalyst surface at low temperatures, thereby promoting CO2 hydrogenation [23]. Here, we further found that weak basic sites favor CO2 hydrogenation. In addition to regulating the Mg content of the catalysts, we also synthesized various Mg-PtCoAl-x (where x denotes the type of precipitant) catalysts using different precipitants. Their adsorption behaviors toward H and CO2 were analyzed via H2-TPD and CO2-TPD, with the results presented in Figure S3 and Tables S4 and S5. The fractions of weak H adsorption and weak basic sites were calculated and subsequently correlated with the turnover frequency (TOF).
Figure 4 shows the correlation for the fraction of weakly adsorbed H and the fraction of weak basic sites with CO2 hydrogenation TOFs. It can be observed that relatively low fractions of weak basic sites (<18.5%) and weakly adsorbed H (<21.1%) both lead to unsatisfactory TOF (<12.5 and 22.7 h−1, the blue area in the figure). A relatively high fraction of weak basic sites with relatively low fraction of weakly adsorbed H leads to a medium TOF level (54 h−1, the green area in the figure). However, when the catalyst has both a high fraction of weakly adsorbed H and a high fraction of weak basic sites, the highest TOF (up to 78 h−1, the red area in the figure) can be achieved. This indicates that both weakly adsorbed H and weak basic sites are of great importance for efficient CO2 hydrogenation. According to previous reports, weak basic sites are crucial for the adsorption and activation of CO2 [43,44]. Therefore, a relatively large number of weak basic sites is the key reason for enhancing the activity of the catalyst regarding CO2 hydrogenation. Even though Mg reduces the fraction of weakly adsorbed H, it optimizes the fraction of weak basic sites and increases the amount of active sites (Figure S2), leading to high apparent activity for 0.7% Mg-PtCoAl. However, a high Mg content, such as 5.0%, may lead to agglomeration of MgO on the catalyst surface, forming more sites with strong basicity and resulting in an increase in the total number of basic sites. The increase in the density of strong basic sites causes the catalyst to promote excessively strong CO2 adsorption, making it difficult to carry out subsequent reactions. Additionally, excessive Mg may cover the originally active weak basic sites, leading to a decrease in the density of surface weak basic sites and resulting in the lowest TOF and productivity.
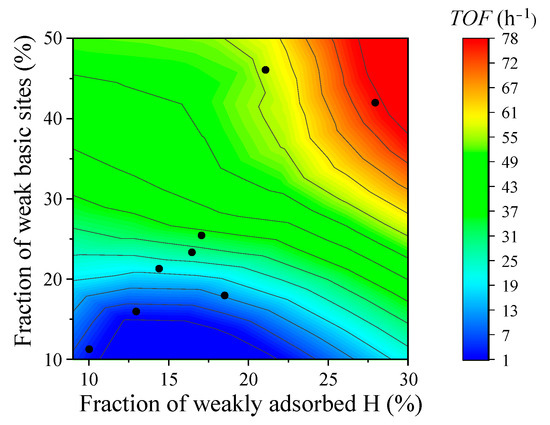
Figure 4.
Relationships among the fraction of weakly adsorbed H, the fraction of weak basic sites, and the TOFs of x% Mg-PtCoAl catalysts.
2.3. Synergistic Effect of Pt and Mg Promotors
To better understand the synergistic effects of Pt and Mg promoters on CoAl catalysts, we prepared catalysts using various precipitants and combinations thereof to achieve different Pt and Mg contents. Table S3 presents the ICP-OES results for the calcined catalysts. Elemental composition analysis reveals that the Co and Al contents of the catalysts synthesized with different precipitants are comparable, ranging from 8.422 to 9.665 mmol gcat−1 and 1.586 to 1.824 mmol gcat−1, respectively. However, the Pt content varies significantly across the catalysts. For instance, the two catalysts precipitated using carbonates contain only 0.031 mmol gcat−1 and 0.041 mmol gcat−1 of Pt, indicating a lower degree of Pt incorporation. In contrast, the catalyst precipitated with NaOH has the highest contents of Pt and Mg, which are 0.363 and 0.675 mmol gcat−1, respectively, whereas Mg content in other catalysts ranges from 0.08 to 0.101 mmol gcat−1. These variations in Pt and Mg contents among the catalysts synthesized using different precipitants are expected to significantly influence their structure and catalytic performance.
The catalysts’ precursors were analyzed using FT-IR (Thermo Scientific Nicolet iS20, Waltham, MA, USA) to investigate differences in chemical bonding. For the precipitated sample (Figure 5a), a broad absorption band at approximately 3479 cm−1 and a band at 1633 cm−1 are attributed to the stretching vibrations of bound water and hydroxyl groups (O–H) [45]. Absorption bands around 1483 cm−1 and 1383 cm−1 correspond to the stretching vibrations of carbonate (C=O) and nitrate (N=O), respectively [46,47]. Notably, in the precursor prepared with NaOH as the precipitant, the carbonate absorption band disappears. Furthermore, the samples synthesized using only NaOH as the precipitant exhibit a more intense peak at 1352 cm−1, likely due to the binding of metal to the OH group [48,49]. Absorption bands in the range of 500–1000 cm−1 are associated with the stretching and bending vibrations of metal-oxygen bonds, specifically Al–O at 572 cm−1 and Co–O at 667 cm−1 [50,51]. The precursors of the samples precipitated with NaOH are mainly hydroxides, while the precipitants containing carbonate will retain the intercalated carbonate species, thus forming an LDH-like structure.
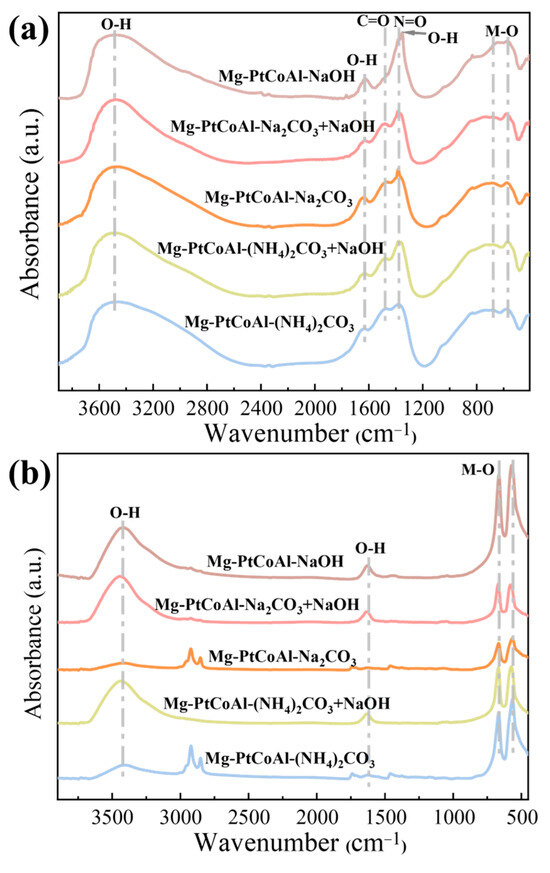
Figure 5.
(a) FT-IR spectra of the precipitated catalysts using different precipitants. (b) FT-IR spectra of calcined catalysts using different precipitants.
Figure 5b displays the Fourier-transform infrared spectroscopy (FT-IR) spectra of the catalysts after calcination, revealing significant changes in the absorption bands. The disappearance of the bands near 1483 cm−1 and 1383 cm−1 indicates the removal of carbonates and nitrates during high-temperature calcination. The broad absorption band in the range of 500–1000 cm−1 splits into two narrower bands, corresponding to the stretching vibrations of metal–oxygen bonds in spinel oxides, signifying the formation of spinel structures. The reduced intensity of the bands at 3479 cm−1 and 1633 cm−1 suggests that most bound water and hydroxyl groups are removed, although some residues remain [52]. The peak near 1629 cm−1 observed in catalysts synthesized using NaOH-containing precipitants likely corresponds to residual water [53]. These findings indicate that the chemical bond environment (hydroxyl/carbonate ratio) of the precursor directly influences the spinel structure, metal loading, and distribution of basic sites of the catalyst after calcination. The mixed precipitant optimizes the Pt/Mg loading, the proportion of surface weak basic sites, and the metal–support interaction by balancing the synergistic effect between hydroxyl and carbonate, thereby significantly enhancing CO2 hydrogenation activity.
Figure 6 illustrates the H2-TPR profiles of Mg-PtCoAl catalysts synthesized using different precipitants. The reduction peaks of the catalyst synthesized with only the carbonate precipitant occur at a higher temperature (214 °C) and exhibit a smaller peak area at low temperatures. This suggests that these two catalysts exhibit a lower degree of reduction, possibly due to their reduced hydrogen spillover on the catalyst surface. As demonstrated by the ICP-OES results in Table S3, the samples using only the carbonate precipitant had low Pt contents (0.041 and 0.031 mmol gcat−1, respectively). In contrast, the samples using the NaOH-containing precipitant had Pt contents exceeding 0.114 mmol gcat−1. The lower Pt content resulted in weaker hydrogen dissociation and spillover capacity and therefore lower catalyst reducibility [23]. The variation in reduction degree, as well as Pt and Mg contents, influences the activation and adsorption of H/CO2, accordingly leading to different CO2 hydrogenation activity. Figure 7 shows that the Mg-PtCoAl catalysts prepared using carbonate or NaOH precipitants exhibited low alcohol and methane productivity, while using mixed carbonate/NaOH as the precipitant leads to enhanced formation of alcohols and methane. Methane productivity of 51.4 mmol gcat−1 h−1 and alcohol productivity of 0.895 mmol gcat−1 h−1 were achieved, among which higher alcohols accounted for 33.6% of the total alcohol products.
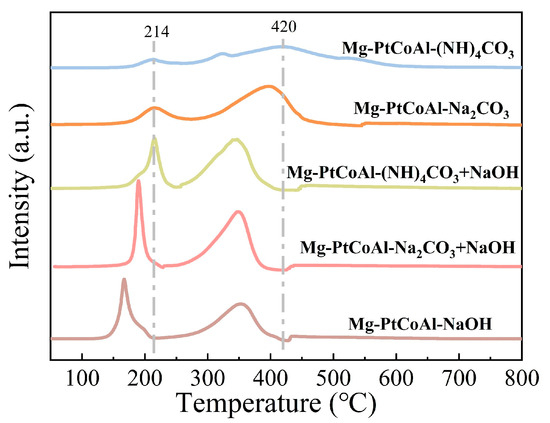
Figure 6.
H2-TPR profiles of catalysts synthesized with different precipitants.
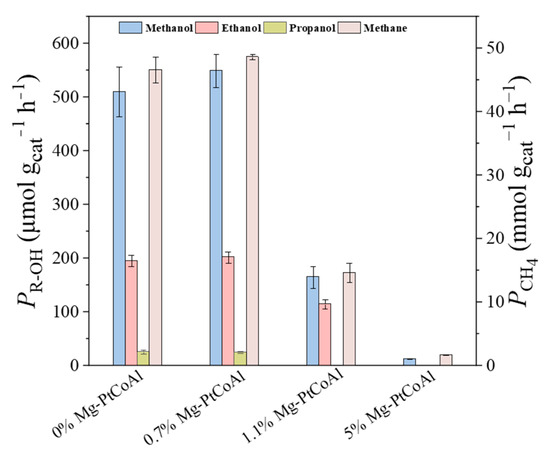
Figure 7.
CO2 hydrogenation performance of catalysts synthesized with different precipitants.
The catalysts synthesized by adopting different precipitants have different Mg and Pt contents, and controlling the appropriate Pt and Mg contents is crucial to enhance the activities of CoAl catalysts. Figure 8 offers a clear visualization of the relationship between the elemental composition (Pt and Mg contents) and the TOFs (calculated from H2-TPD, Table 2 and Table S4) of CO2 hydrogenation. It is found that catalysts with either excessively high or excessively low Pt and Mg contents disrupt the optimal Pt–Mg synergy, resulting in suboptimal TOFs. Conversely, catalysts within the moderate Pt and Mg content ranges (Pt: 0.11 to 0.12 mmol gcat−1, Mg: 0.08 to 0.10 mmol gcat−1) display the highest CO2 hydrogenation TOF. This optimal performance is attributed to a balanced catalyst surface. Weakly adsorbed H and weakly adsorbed CO2, together with an increased amount of active sites, lead to enhanced CO2 conversion.
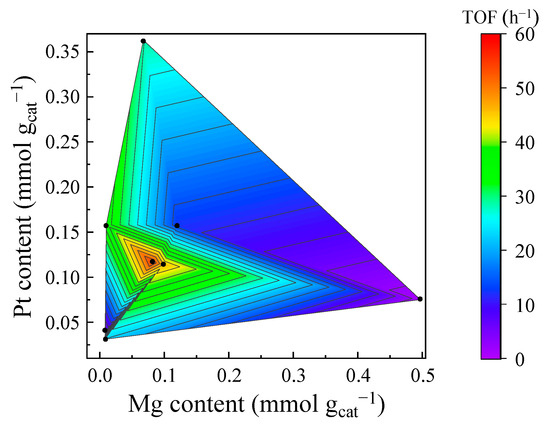
Figure 8.
Relationships among Mg, Pt content, and the TOF of CO2 hydrogenation for Mg-PtCoAl catalysts.
3. Materials and Methods
3.1. Synthesis of Catalysts
A series of Mg-modified PtCoAl catalysts were prepared using the co-precipitation method [54]. First, Solution A was prepared by dissolving 0.108 mol L−1 Co(NO3)2·6H2O, 0.012 mol L−1 Al(NO3)2·9H2O, 0.003 mol L−1 H2PtCl6·xH2O, and α mol L−1 Mg(NO3)2·6H2O (α = 0, 0.0012, 0.006, and 0.0084) in 100 mL of water. Then, Solution B, containing 0.12 mol L−1 Na2CO3 and 0.4 mol L−1 NaOH, was prepared. Solutions A and B were slowly added at a controlled rate using peristaltic pumps into a beaker placed in an ice bath, with continuous stirring to obtain a suspension. The pH of the suspension was maintained at 8.5. After precipitation, the suspension was filtered using a vacuum pump and Buchner funnel. The precipitate was continuously washed with water to remove impurity ions and then dried in an oven at 80 °C overnight. The dried precipitate was calcined at 400 °C in air for 4 h and subsequently reduced in an H2 atmosphere at 250 °C for 3 h. The catalysts with different Mg contents were labeled as x% Mg-PtCoAl catalysts, where x = 0, 0.7, 1.1, or 5.0. Notably, the characterization and catalytic results of 0% Mg-PtCoAl have been reported in our previous paper [23].
Additionally, catalysts synthesized with various precipitants were prepared using 0.0036 mol L−1 Mg(NO3)2·6H2O in Solution A. Solution B contained 0.4 mol L−1 NaOH, 0.4 mol L−1 NaOH with 0.12 mol L−1 Na2CO3, 0.4 mol L−1 NaOH with 0.12 mol L−1 (NH4)2CO3, 0.12 mol L−1 Na2CO3, or 0.12 mol L−1 (NH4)2CO3. These catalysts were labeled as Mg-PtCoAl-NaOH, Mg-PtCoAl-Na2CO3+NaOH, Mg-PtCoAl-(NH4)2CO3+NaOH, Mg-PtCoAl-Na2CO3, and Mg-PtCoAl-(NH4)2CO3, respectively.
3.2. Characterization of Catalysts
The crystal structures of the samples were analyzed using X-ray diffraction (XRD) on a Rigaku SmartLab (Rigaku, Tokyo, Japan) diffractometer equipped with a Cu Kα radiation source (λ = 1.5406 Å, 40 kV, 40 mA). Crystallite sizes were determined through the Scherrer equation. The amounts of Co, Al, Pt, and Mg in the catalysts were quantified using inductively coupled plasma-optical emission spectroscopy (ICP-OES, Varian 720ES, USA) with a Varian 720ES instrument. Fourier-transform infrared spectroscopy (FT-IR) was employed using a Thermo Scientific Nicolet iS20 (USA) instrument to characterize the chemical bonds present in the catalysts. X-ray photoelectron spectroscopy (XPS, Shimadzu Kratos Axis Supra, Kyoto, Japan) was conducted on a Kratos Axis Supra spectrometer, with binding energies calibrated using the C 1s peak at 284.8 eV as a reference. N2 physisorption was assessed with a Micromeritics 2460 instrument(Micromeritics, Norcross, GA, USA).
H2 temperature-programmed reduction (TPR), H2 temperature-programmed desorption (TPD), and CO2-TPD analyses were performed utilizing a Vodo VDSorb-9Xi chemisorption analyzer (Vodo, Quzhou, China). For H2-TPR, the catalysts underwent a preliminary drying stage under Ar flow (30 mL min−1) at 150 °C for 1 h. Subsequently, the system was cooled to 70 °C before being subjected to a temperature ramp of 10 °C min−1 to 800 °C, where it was isothermally maintained for 10 min under a 10% H2/Ar mixture (30 mL min−1). In the case of H2-TPD, the catalysts were initially reduced in a hydrogen atmosphere at 250 °C for 3 h. Following this, the system was cooled to 100 °C under an Ar environment and held at this temperature for 30 min. H2 adsorption was facilitated by exposing the catalyst to a 10% H2/Ar mixture at 100 °C for 1 h. An Ar purge (30 mL min−1) was conducted for 1 h before temperature-programmed desorption, wherein the catalysts were heated to 800 °C at a rate of 10 °C min−1 and held for 50 min to desorb adsorbed hydrogen. For CO2-TPD, the catalysts were subjected to H2 reduction at 250 °C for 3 h, followed by cooling to 50 °C under Ar and holding for 1 h. CO2 adsorption was achieved by introducing a 5% CO2/He mixture (30 mL min−1) at 50 °C for 1 h. After He purge for 1 h, the catalysts were heated to 800 °C at a rate of 10 °C min−1 and maintained at this temperature for 50 min to desorb adsorbed CO2. Changes in the thermal conductivity of the gas stream were detected using a thermal conductivity detector (GOWMAC, Yuma, AZ, USA).
3.3. Catalytic Tests
CO2 hydrogenation experiments were conducted in accordance with the methodology detailed in our previous work [15]. Although most current studies on CO2 hydrogenation employ plug-flow reactors, this work selects a stirred-tank slurry reactor (Zhengxin, China) based on the unique role of water molecules in facilitating proton transfer [10], which enhances hydrogenation efficiency and outperforms gas-phase reaction systems. A 50 mL Teflon-lined batch reactor equipped (Zhengxin China) with magnetic stirring was charged with 50 mg of pre-reduced catalyst and 15 mL of ultrapure water. The reactor was subjected to three consecutive purging cycles using 1 MPa of CO2 to remove residual atmospheric gases. Subsequently, an additional 1 MPa of CO2 and 3 MPa of H2 were introduced into the reactor. The reaction mixture was heated to 180 °C and agitated at 350 rpm for a duration of 1 h. Upon completion, the reactor was cooled in an ice bath, and the gaseous products were collected for offline analysis. Gas-phase product analysis was performed using an Agilent 8890 GC system (Agilent, Santa Clara, CA, USA) equipped with a thermal conductivity detector and HayeSep Q and MolSieve 5A columns. Methane concentrations were determined via the external standard method, with corresponding quantities calculated based on the ideal gas law. Liquid products were analyzed using an Agilent 8860 GC system (USA) equipped with a flame ionization detector and an HP-Innowax column, with tetrahydrofuran employed as an internal standard. The productivities, number of active sites, and turnover frequencies for methanol, ethanol, propanol, and methane were calculated according to Equations (3)–(5).
Here, Pi (mmol gcat−1 h−1, i denotes methanol, ethanol, propanol, or methane) represents the productivity of product i. The term ni (mmol, carbon-based) refers to the number of moles of product i. The variable t (h) indicates the reaction time, while mcat (g) denotes the mass of the catalyst used. TOFi (h−1) signifies the turnover frequency for product i, nAS (mmol gcat−1) indicates the number of active sites, and nH2 (mmol gcat−1) indicates the mole number of H2 adsorbed during H2-TPD.
4. Conclusions
A series of catalysts were prepared via a simple co-precipitation method, and the effects of a Mg promoter on CO2 hydrogenation on PtCoAl catalysts were systematically investigated. The addition of Mg slightly inhibits the reduction of Co but significantly enhances H2 and CO2 adsorption capacities and increases the number of active sites. Although Mg addition reduces the fraction of weakly adsorbed hydrogen—which is important for efficient CO2 hydrogenation—an appropriate Mg content increases the proportion of weak basic sites, thereby facilitating CO2 activation. For the 0.7% Mg-PtCoAl catalyst, the slight decrease in weakly adsorbed hydrogen, coupled with the increase in weak basic sites and active site density, leads to high apparent activity for CO2 hydrogenation. Furthermore, catalysts with either excessively high or excessively low Pt and Mg contents disrupt the optimal Pt–Mg synergy, resulting in reduced turnover frequencies. Moderate Pt and Mg loadings (Pt: 0.11 to 0.12 mmol gcat−1, Mg: 0.08 to 0.10 mmol gcat−1) are found to most effectively enhance the CO2 hydrogenation activity of the CoAl catalyst. Under an initial pressure of 4 MPa and 180 °C, the Mg-modified PtCoAl catalyst achieved a methane productivity of 51.4 mmol gcat−1 h−1 and an alcohol productivity of 0.895 mmol gcat−1 h−1, with higher alcohols accounting for 33.6% of the total alcohol products. In future work, we will investigate a broader range of transition metal and alkali metal promoters and explore tandem catalysis strategies to enhance both higher alcohol selectivity and CO2 conversion efficiency.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15060577/s1, Figure S1: N2 adsorption–desorption isotherms for calcined catalysts with various Mg contents; Figure S2: Relationship between the number of active sites of catalysts with different Mg contents and TOF; Figure S3: H2-TPD (a) and CO2-TPD (b) profiles of Mg-PtCoAl catalysts synthesized using different precipitants; Table S1: ICP-OES results of calcined PtCoAl catalysts with various Mg contents; Table S2: Deconvolution results of the Co 2p orbital; Table S3: ICP-OES results of catalysts synthesized using different precipitants; Table S4: H2-TPD results of catalysts synthesized with different precipitants; Table S5: CO2-TPD results of catalysts synthesized with different precipitants.
Author Contributions
Conceptualization, Y.H. and F.Z.; methodology, Y.H., J.C., Y.X., Q.H., L.W., Y.L., X.X. and B.X.; formal analysis, Y.H., J.C. and Y.X.; data curation, Y.H., J.C. and Y.X.; writing—original draft preparation, Y.H., J.C., Y.X. and F.Z.; writing—review and editing, Y.H., J.C., Y.X., Q.H., L.W., Y.L., X.X., B.X. and F.Z.; supervision, F.Z.; project administration, F.Z.; funding acquisition, F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Key Laboratory of Materials-Oriented Chemical Engineering, grant number ZK202101.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We acknowledge financial support from the State Key Laboratory of Materials-Oriented Chemical Engineering (ZK202101).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yoro, K.O.; Daramola, M.O. Chapter 1—CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–28. [Google Scholar]
- Olivier, J.G.; Schure, K.; Peters, J. Trends in Global CO2 and Total Greenhouse Gas Emissions. PBL Neth. Environ. Assess. Agency 2017, 5, 1–11. [Google Scholar]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Ding, M.; Hong, X.; Liu, G.; Tsang, S.C.E. Advances in Higher Alcohol Synthesis from CO2 Hydrogenation. Chem. 2021, 7, 849–881. [Google Scholar] [CrossRef]
- He, Y.; Müller, F.H.; Palkovits, R.; Zeng, F.; Mebrahtu, C. Tandem Ctalysis for CO2 Conversion to Higher Alcohols: A Review. Appl. Catal. B Environ. 2024, 345, 31. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Xi, X.; Liao, L.; Ren, J.; Xie, J.; Heeres, H.J.; Palkovits, R. Catalysts Design for Higher Alcohols Synthesis by CO2 Hydrogenation: Trends and Future Perspectives. Appl. Catal. B Environ. 2021, 291, 120073. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Fu, W.; Chen, J.; Ren, J.; Liao, L.; Sun, R.; Tang, Z.; Mebrahtu, C.; Zeng, F. Hetero-site Cobalt Catalysts for Higher Alcohols Synthesis by CO2 Hydrogenation: A Review. J. CO2 Util. 2023, 67, 102322. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Sun, K.; Jia, X.; Ye, J.; Liu, C.-j. Advances in studies of the structural effects of supported Ni catalysts for CO2 hydrogenation: From nanoparticle to single atom catalyst. J. Mater. Chem. A. 2022, 10, 5792–5812. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Guo, X.; Song, C.; Guo, X. Recent advances in application of iron-based catalysts for COx hydrogenation to value-added hydrocarbons. Chin. J. Catal. 2022, 43, 731–754. [Google Scholar] [CrossRef]
- He, Z.; Qian, Q.; Ma, J.; Meng, Q.; Zhou, H.; Song, J.; Liu, Z.; Han, B. Water-enhanced synthesis of higher alcohols from CO2 hydrogenation over a Pt/Co3O4 catalyst under Milder conditions. Angew. Chem. Int. Ed. 2016, 55, 737–741. [Google Scholar] [CrossRef]
- Lou, Y.; Zhu, W.; Wang, L.; Yao, T.; Wang, S.; Yang, B.; Yang, B.; Zhu, Y.; Liu, X. CeO2 supported Pd dimers boosting CO2 hydrogenation to ethanol. Appl. Catal. B Environ. 2021, 291, 120122. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Z.; Xiang, G.; Zhai, T.; Liu, Z.; Zhao, W.; Liang, X.; Wang, L. Interfacial compatibility critically controls Ru/TiO2 metal-support interaction modes in CO2 hydrogenation. Nat. Commun. 2022, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef]
- Fan, T.; Liu, H.; Shao, S.; Gong, Y.; Li, G.; Tang, Z. Cobalt Catalysts Enable Selective Hydrogenation of CO2 toward Diverse Products: Recent Progress and Perspective. J. Phys. Chem. Lett. 2021, 12, 10486–10496. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, Y.; Fu, W.; Ren, J.; Chen, J.; Chen, H.; Sun, R.; Tang, Z.; Mebrahtu, C.; Zeng, F. Synergy of Co0-Co2+ in Cobalt-based Catalysts for CO2 Hydrogenation: Quantifying via Reduced and Exposed Atoms Fraction. Appl. Catal. A Gen. 2024, 670, 119549. [Google Scholar] [CrossRef]
- Fu, W.; Liu, S.; He, Y.; Chen, J.; Ren, J.; Chen, H.; Sun, R.; Tang, Z.; Mebrahtu, C.; Zeng, F. CO2 hydrogenation over CoAl Based Catalysts: Effects of Cobalt-metal Oxide Interaction. Appl. Catal. A Gen. 2024, 678, 119720. [Google Scholar] [CrossRef]
- Fu, W.; He, Y.; Liu, S.; Chen, J.; Ren, J.; Sun, R.; Tang, Z.; Mebrahtu, C.; Chen, H.; Zeng, F. Inverse supported Al2O3/Co° catalysts for enhanced CO2 hydrogenation. Mol. Catal. 2024, 569, 114598. [Google Scholar] [CrossRef]
- Guo, X.; Gao, D.; He, H.; Traitangwong, A.; Gong, M.; Meeyoo, V.; Peng, Z.; Li, C. Promotion of CO2 Methanation at Low Temperature over Hydrotalcite-derived Catalysts-effect of the Tunable Metal Species and Basicity. Int. J. Hydrogen Energy 2021, 46, 518–530. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Wongsakulphasatch, S.; Vo, D.-V.N. Understanding the Role of Surface Basic Sites of Catalysts in CO2 Activation in Dry Reforming of Methane: A Short Review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Influence of Modifier (Mn, La, Ce, Zr and Y) on the Performance of Cu/Zn/Al Catalysts via Hydrotalcite-like Precursors for CO2 Hydrogenation to Methanol. Appl. Catal. A Gen. 2013, 468, 442–452. [Google Scholar] [CrossRef]
- Wang, Y.; Ban, H.; Wang, Y.; Yao, R.; Zhao, S.; Hu, J.; Li, C. Unraveling the Role of Basic Sites in the Hydrogenation of CO2 to Formic Acid over Ni-based Catalysts. J. Catal. 2024, 430, 115357. [Google Scholar] [CrossRef]
- Stangeland, K.; Navarro, H.H.; Huynh, H.L.; Tucho, W.M.; Yu, Z. Tuning the Interfacial Sites between Copper and Metal Oxides (Zn, Zr, In) for CO2 Hydrogenation to Methanol. Chem. Eng. Sci. 2021, 238, 116603. [Google Scholar] [CrossRef]
- He, Y.; Xu, B.; Liu, S.; Fu, W.; Chen, J.; Ren, J.; Sun, R.; Tang, Z.; Mebrahtu, C.; Chen, H.; et al. Effects of hydrogen spillover on CO2 hydrogenation over Pt-Co-Al based catalysts. Appl. Catal. A Gen. 2025, 691, 120051. [Google Scholar] [CrossRef]
- Bosi, F.; Hålenius, U.; D’Ippolito, V.; Andreozzi, G.B. Blue Spinel Crystals in the MgAl2O4-CoAl2O4 Series: Part II. Cation Ordering over Short-range and Long-range Scales. Am. Mineral. 2012, 97, 1834–1840. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Madani, M.; Mansour, H.; Alonizan, N.; El Mir, L. Effect of Mg and Cu co-doping on nanostructured TiO2 photocatalytic activity. J. Mater. Sci. Mater. Electron. 2024, 35, 1168. [Google Scholar] [CrossRef]
- Díaz-Fernández, D.; Méndez, J.; Bomatí-Miguel, O.; Yubero, F.; Mossanek, R.; Abbate, M.; Domínguez-Cañizares, G.; Gutiérrez, A.; Tougaard, S.; Soriano, L. The growth of cobalt oxides on HOPG and SiO2 surfaces: A comparative study. Surf. Sci. 2014, 624, 145–153. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wang, Z.; Feng, K.; Xu, S.; Li, X.; Yu, P.; Fan, X.; Zheng, H.; Sun, Y. Inert magnesium-doped Co3O4 spinel assembling catalytic membrane for instantaneous peroxymonosulfate activation and contaminants elimination. Chem. Eng. J. 2023, 477, 146987. [Google Scholar] [CrossRef]
- Dojcinovic, M.P.; Vasiljevic, Z.Z.; Pavlovic, V.P.; Barisic, D.; Pajic, D.; Tadic, N.B.; Nikolic, M.V. Mixed Mg–Co spinel ferrites: Structure, morphology, magnetic and photocatalytic properties. J. Alloys Compd. 2021, 855, 157429. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, Z.; Duan, A.; Jiang, G.; Liu, J. Comparative Study on the Formation and Reduction of Bulk and Al2O3-supported Cobalt Oxides by H2-TPR Technique. J. Phys. Chem. C 2009, 113, 7186–7199. [Google Scholar] [CrossRef]
- Wang, C.; Chang, L.; Zhang, X.; Chai, H.; Huang, Y. Promoting oxygen vacancies utility for tetracycline degradation via peroxymonosulfate activation by reduced Mg-doped Co3O4: Kinetics and key role of electron transfer pathway. Environ. Res. 2024, 252, 118892. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Xie, P.; Yong, X.; Li, Y.; Zhang, C. Tuning the Catalytic Activity of Complex Metal Oxides Prepared by a One-Pot Method for NO Direct Decomposition. Ind. Eng. Chem. Res. 2021, 60, 9399–9408. [Google Scholar] [CrossRef]
- Ribet, S.; Tichit, D.; Coq, B.; Ducourant, B.; Morato, F. Synthesis and Activation of Co–Mg–Al Layered Double Hydroxides. J. Solid State Chem. 1999, 142, 382–392. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Chen, S. Effect of Promoter SiO2, TiO2 or SiO2-TiO2 on the Performance of CuO-ZnO-Al2O3 Catalyst for Methanol Synthesis from CO2 Hydrogenation. Appl. Catal. A Gen. 2012, 415, 118–123. [Google Scholar] [CrossRef]
- He, Q.; Li, Z.; Li, D.; Ning, F.; Wang, Q.; Liu, W.; Zhang, W.; Cui, Y.; Zhang, J.; Liu, C. Mg Enhanced the Performance of Cu/ZnO/ZrO2 for CO2 Hydrogenation to Methanol and the Mechanism Investigation. Mol. Catal. 2024, 558, 114008. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Wang, H.; Sun, Y. Influence of Zr on the Performance of Cu/Zn/Al/Zr Catalysts via Hydrotalcite-like Precursors for CO2 Hydrogenation to Methanol. J. Catal. 2013, 298, 51–60. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Di Cosimo, J.I. Acid–base Properties and Active Site Requirements for Elimination Reactions on Alkali-promoted MgO Catalysts. Catal. Today 2000, 63, 53–62. [Google Scholar] [CrossRef]
- Fan, H.-X.; Cui, T.-Y.; Rajendran, A.; Yang, Q.; Feng, J.; Yue, X.-P.; Li, W.-Y. Comparative study on the activities of different MgO surfaces in CO2 activation and hydrogenation. Catal. Today 2020, 356, 535–543. [Google Scholar] [CrossRef]
- Ahmed, S.; Irshad, M.; Yoon, W.; Karanwal, N.; Sugiarto, J.R.; Khan, M.K.; Kim, S.K.; Kim, J. Evaluation of MgO as a promoter for the hydrogenation of CO2 to long-chain hydrocarbons over Fe-based catalysts. Appl. Catal. B Environ. 2023, 338, 123052. [Google Scholar] [CrossRef]
- Kim, S.-r.; Choi, Y.-N.; Park, K.; Lee, H.-G.; Lee, K.R.; Park, H.; Yoon, S.; Lee, K.Y.; Jung, K.-D. Magnesium-Promoted Catalytic Stability of the Cu/ZnO/ZrO2/Al2O3-MgO Catalyst in CO2 Hydrogenation to Methanol. Ind. Eng. Chem. Res. 2025, 64, 5903–5911. [Google Scholar] [CrossRef]
- Liu, K.; Xu, D.; Fan, H.; Hou, G.; Li, Y.; Huang, S.; Ding, M. Development of Mg-Modified Fe-Based Catalysts for Low-Concentration CO2 Hydrogenation to Olefins. ACS Sustain. Chem. Eng. 2024, 12, 2070–2079. [Google Scholar] [CrossRef]
- Liao, W.; Tang, C.; Zheng, H.; Ding, J.; Zhang, K.; Wang, H.; Lu, J.; Huang, W.; Zhang, Z. Tuning Activity and Selectivity of CO2 Hydrogenation via Metal-oxide Interfaces over ZnO-supported Metal Catalysts. J. Catal. 2022, 407, 126–140. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, L.; Zhao, F.; Fu, Y.; Lu, J.-Q.; Zhang, Z.; Teng, B.; Huang, W. Zinc Oxide Morphology-Dependent Pd/ZnO Catalysis in Base-Free CO2 Hydrogenation into Formic Acid. ChemCatChem 2020, 12, 5540–5547. [Google Scholar] [CrossRef]
- Mah, J.C.W.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J.; Raharjo, J. Formation of sol–gel derived (Cu,Mn,Co)3O4 spinel and its electrical properties. Ceram. Int. 2017, 43, 7641–7646. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Wei, M.; Wang, J.; He, J.; Evans, D.G.; Duan, X. In situ FT-IR, in situ HT-XRD and TPDE study of thermal decomposition of sulfated β-cyclodextrin intercalated in layered double hydroxides. Microporous Mesoporous Mater. 2005, 78, 53–61. [Google Scholar] [CrossRef]
- Charanya, C.; Sampathkrishnan, S.; Balamurugan, N. Quantum mechanical analysis, spectroscopic (FT-IR, FT-Raman, UV-Visible) study, and HOMO-LUMO analysis of (1S,2R)-2-amino-1-phenylpropan-1-ol using Density Functional Theory. J. Mol. Liq. 2017, 231, 116–125. [Google Scholar] [CrossRef]
- Gypser, S.; Hirsch, F.; Schleicher, A.M.; Freese, D. Impact of crystalline and amorphous iron- and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef]
- Lezcano, M.; Ribotta, A.; Miró, E.; Lombardo, E.; Petunchi, J.; Moreaux, C.; Dereppe, J.M. Spectroscopic Characterization of Dealuminated H-Mordenites: The Role of Different Aluminum Species on the SCR of NO with Methane. J. Catal. 1997, 168, 511–521. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Ta, N.; Shen, W. Co3O4 nanosheets: Synthesis and catalytic application for CO oxidation at room temperature. Sci. China Chem. 2014, 57, 873–880. [Google Scholar] [CrossRef]
- Kandori, H.; Maeda, A. FTIR spectroscopy reveals microscopic structural changes of the protein around the rhodopsin chromophore upon photoisomerization. Biochem. 1995, 34, 14220–14229. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Frei, H. Photochemical and FT-IR Probing of the Active Site of Hydrogen Peroxide in Ti Silicalite Sieve. J. Am. Chem. Soc. 2002, 124, 9292–9298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Gill, K.; Kumar, S.; Ganguly, S.K.; Jain, S.L. Magnetic Fe3O4@MgAl–LDH Composite Grafted with Cobalt Phthalocyanine as an Efficient Heterogeneous Catalyst for the Oxidation of Mercaptans. J. Mol. Catal. A Chem. 2015, 401, 48–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).