Effect of Copper Modification on Charge Carrier Transport and Defect Properties in Carbon-Doped TiO2 Nanotubes
Abstract
1. Introduction
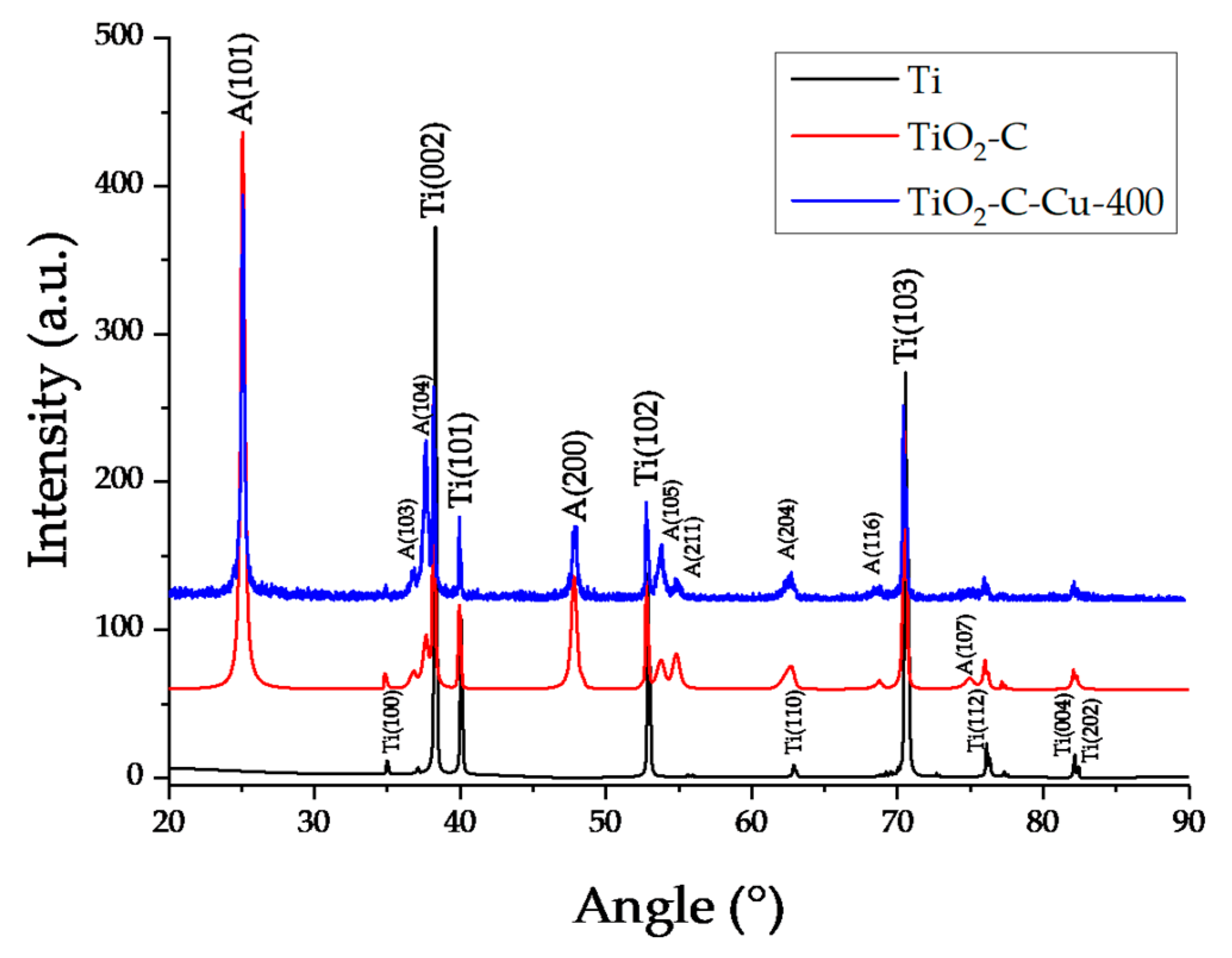
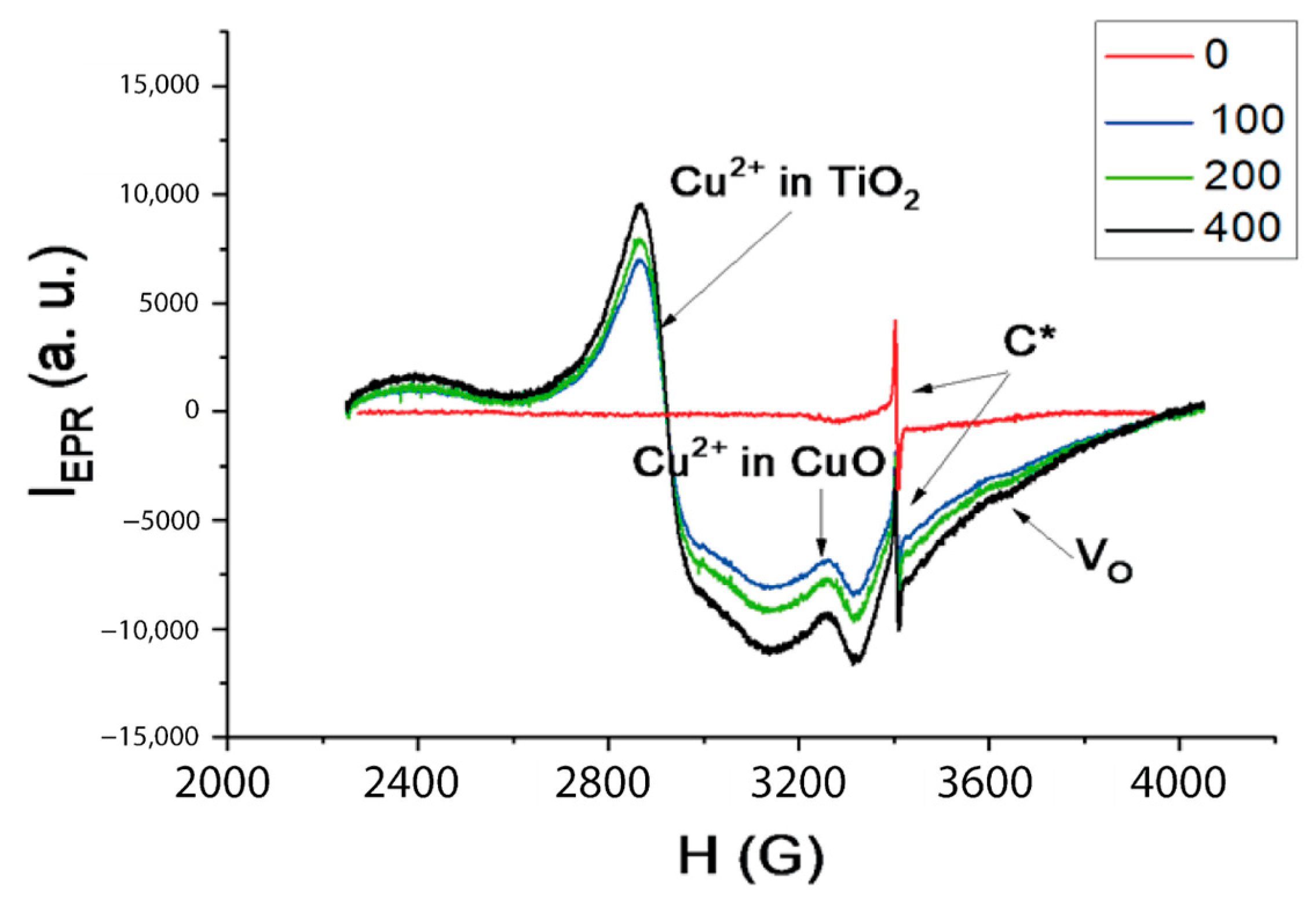
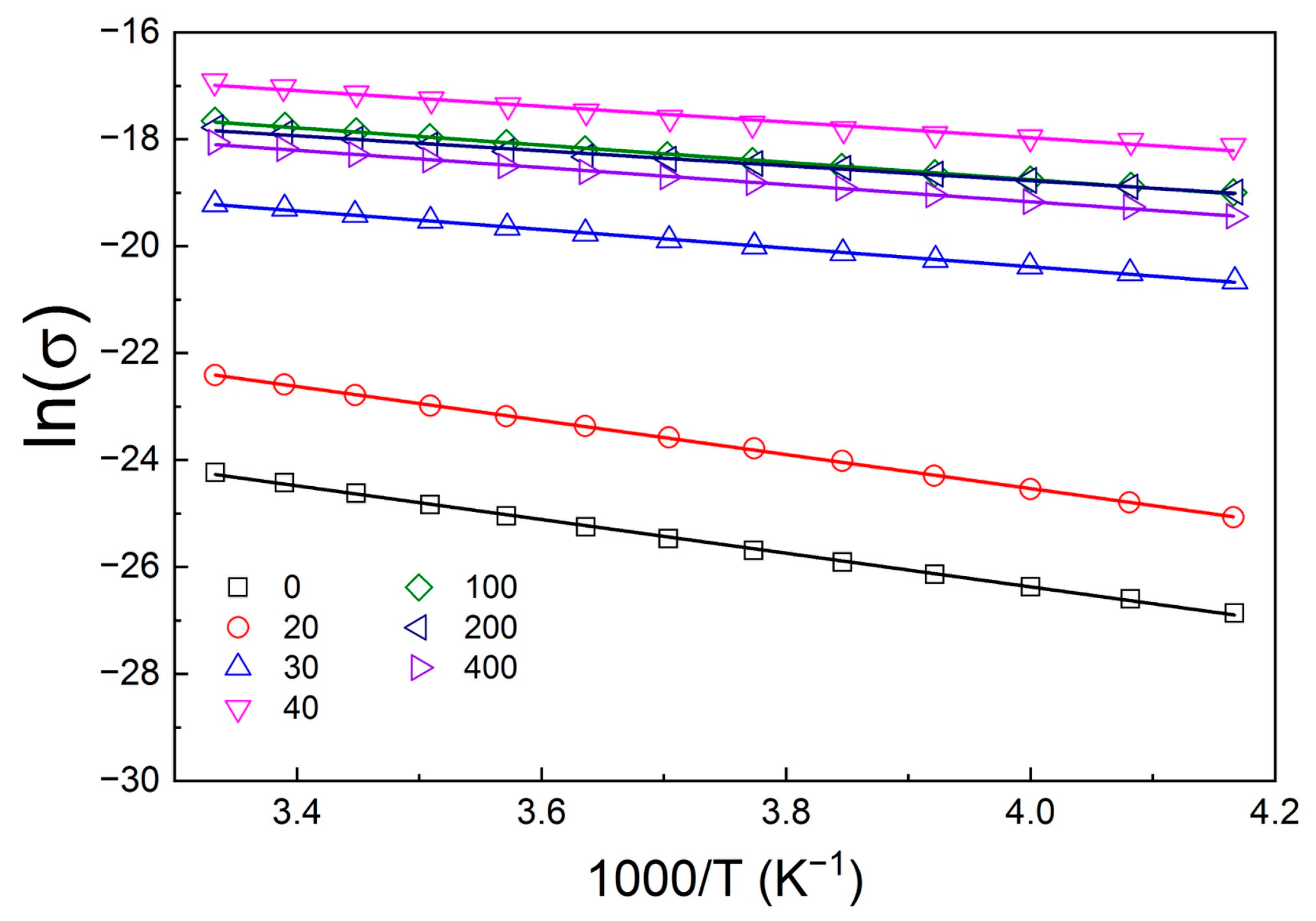
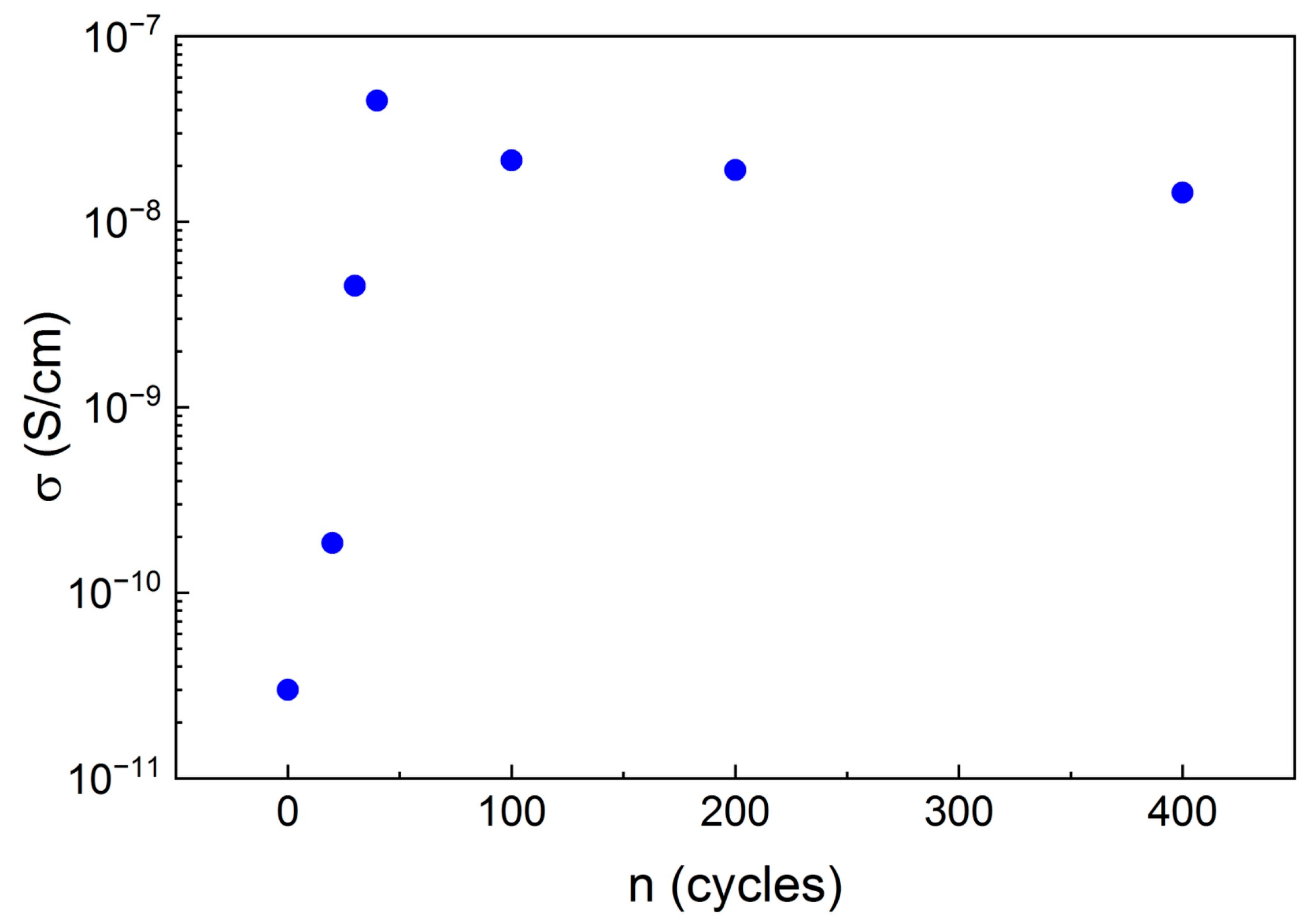
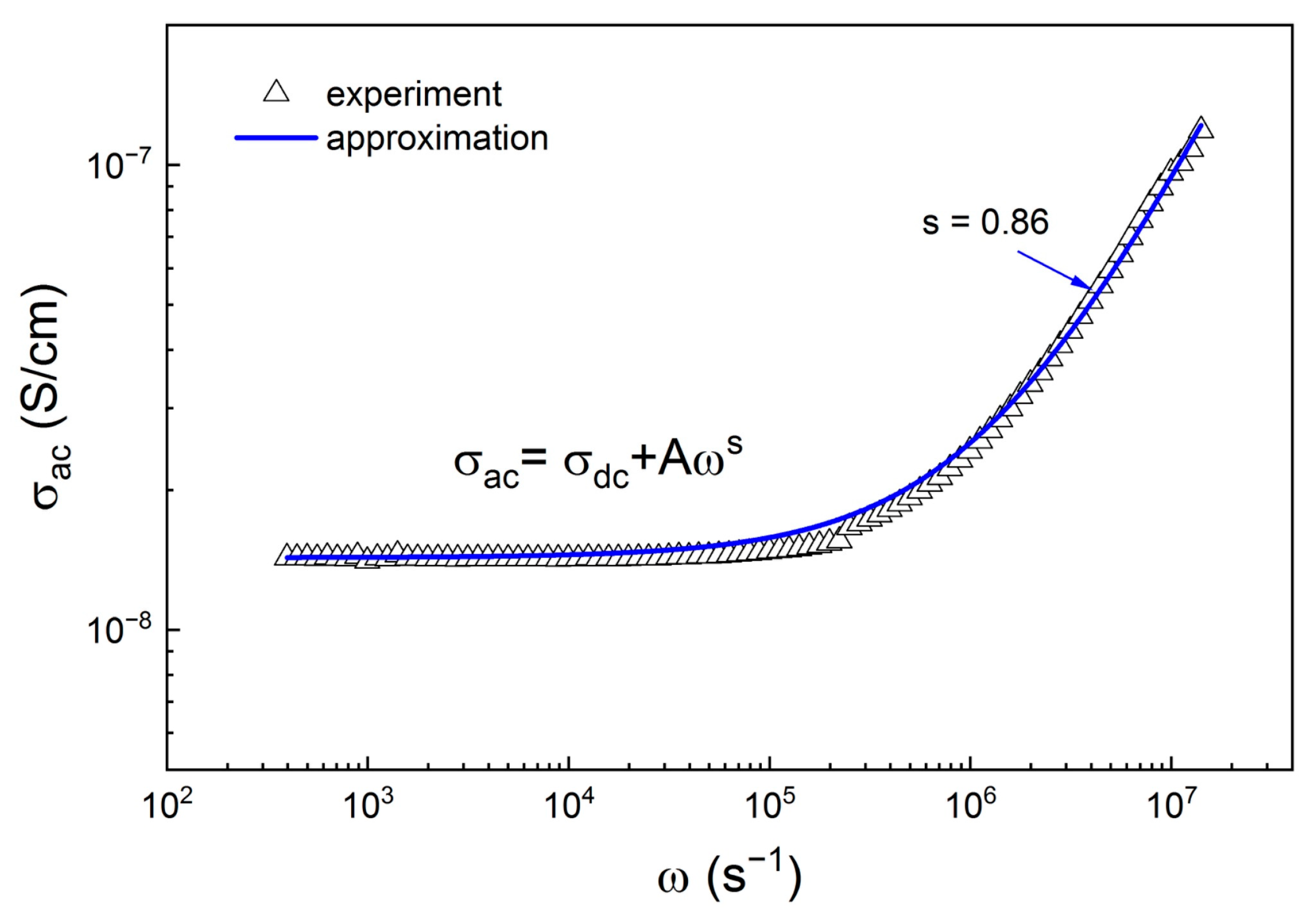
2. Results and Discussion
3. Materials and Methods
3.1. Formation of Carbon-Doped Titania Nanotubes
3.2. Synthesis of TiO2-C-Cu Nanocomposite by Electrochemical Deposition of Copper Nanoparticles
3.3. Microscopy, EDX, XRD
3.4. Secondary Ion Mass Spectrometry
3.5. Optical Measurement
3.6. EPR Spectroscopy
3.7. Conductivity Measurement
3.8. Photocurrent Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical Properties of TiO2 Photocatalyst and Its Applications for Environmental Purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 Nanotubes for Photoelectrochemical Water Splitting: Review. Int. J. Hydrogen Energy 2021, 46, 4998–5024. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, H.; Razzaq, A.; Grimes, C.A.; In, S.-I. Solar Spectrum Photocatalytic Conversion of CO2 to CH4 Utilizing TiO2 Nanotube Arrays Embedded with Graphene Quantum Dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Muiva, C.M.; Maabong, K.; Moditswe, C. CuO Nanostructured Thin Films Synthesised by Chemical Bath Deposition on Seed Layers Deposited by Successive Ionic Layer Adsorption and Reaction and Chemical Spray Pyrolysis Techniques. Thin Solid Films 2016, 616, 48–54. [Google Scholar] [CrossRef]
- Tang, X.; Ren, L.; Sun, L.; Tian, W.; Cao, M.; Hu, C. A Solvothermal Route to Cu2O Nanocubes and Cu Nanoparticles1. Chem. Res. Chin. Univ. 2006, 22, 547–551. [Google Scholar] [CrossRef]
- Rawool, S.A.; Yadav, K.K.; Polshettiwar, V. Defective TiO2 for Photocatalytic CO2 Conversion to Fuels and Chemicals. Chem. Sci. 2021, 12, 4267–4299. [Google Scholar] [CrossRef]
- Sviridova, T.V.; Sadovskaуa, L.Y.; Konstantinova, E.A.; Belyasova, N.A.; Kokorin, A.I.; Sviridov, D.V. Photoaccumulating TiO2-MoO3, TiO2-V2O5, and TiO2-WO3 Heterostructures for Self-Sterilizing Systems with the Prolonged Bactericidal Activity. Catal. Lett. 2019, 149, 1147–1153. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Zheng, H.; Zhang, X.; Liu, Y. Defect-Induced Yellow Color in Nb-Doped TiO2 and Its Impact on Visible-Light Photocatalysis. J. Phys. Chem. C 2015, 119, 16623–16632. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.; Fang, Y.; Zhao, Y.; Luo, D.; Guo, Q.; Fan, L.; Wu, J. Hollow Mesoporous TiO2/WO3 Sphere Heterojunction with High Visible-Light-Driven Photocatalytic Activity. Mater. Res. Bull. 2019, 119, 110571. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Mao, X.; Dong, Y.; Peng, L.; Sun-Waterhouse, D.; Kennedy, J.V.; Waterhouse, G.I.N. Recent Advances in Self-Supported Semiconductor Heterojunction Nanoarrays as Efficient Photoanodes for Photoelectrochemical Water Splitting. Small 2022, 18, 2204553. [Google Scholar] [CrossRef]
- Faisal, M.; Harraz, F.A.; Ismail, A.A.; El-Toni, A.M.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Novel Mesoporous NiO/TiO2 Nanocomposites with Enhanced Photocatalytic Activity under Visible Light Illumination. Ceram. Int. 2018, 44, 7047–7056. [Google Scholar] [CrossRef]
- Matos, J.; García, A.; Zhao, L.; Titirici, M.M. Solvothermal Carbon-Doped TiO2 Photocatalyst for the Enhanced Methylene Blue Degradation under Visible Light. Appl. Catal. A Gen. 2010, 390, 175–182. [Google Scholar] [CrossRef]
- Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Kacica, C.T.; Biswas, P. Improved Conductivity and Ionic Mobility in Nanostructured Thin Films via Aliovalent Doping for Ultra-High Rate Energy Storage. Nanoscale Adv. 2020, 2, 2160–2169. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Alakhras, L.A.; Al-Bataineh, Q.M.; Telfah, A. Impact of Metal Doping on the Physical Characteristics of Anatase Titanium Dioxide (TiO2) Films. J. Mater. Sci. Mater. Electron. 2023, 34, 1552. [Google Scholar] [CrossRef]
- Mohajernia, S.; Hejazi, S.; Andryskova, P.; Zoppellaro, G.; Tomanec, O.; Zboril, R.; Schmuki, P. Conductive Cu-Doped TiO2 Nanotubes for Enhanced Photoelectrochemical Methanol Oxidation and Concomitant Hydrogen Generation. ChemElectroChem 2019, 6, 1244–1249. [Google Scholar] [CrossRef]
- Su, J.; Li, Z.; Zhang, Y.; Wei, Y.; Wang, X. N-Doped and Cu-Doped TiO2-B Nanowires with Enhanced Photoelectrochemical Activity. RSC Adv. 2016, 6, 16177–16182. [Google Scholar] [CrossRef]
- Hua, Z.; Dai, Z.; Bai, X.; Ye, Z.; Wang, P.; Gu, H.; Huang, X. Copper Nanoparticles Sensitized TiO2 Nanotube Arrays Electrode with Enhanced Photoelectrocatalytic Activity for Diclofenac Degradation. Chem. Eng. J. 2016, 283, 514–523. [Google Scholar] [CrossRef]
- Miyauchi, M.; Sunada, K.; Hashimoto, K. Antiviral Effect of Visible Light-Sensitive CuxO/TiO2 Photocatalyst. Catalysts 2020, 10, 1093. [Google Scholar] [CrossRef]
- Motola, M.; Hromadko, L.; Prikryl, J.; Sopha, H.; Krbal, M.; Macak, J.M. Intrinsic Properties of High-Aspect Ratio Single- and Double-Wall Anodic TiO2 Nanotube Layers Annealed at Different Temperatures. Electrochim. Acta 2020, 352, 136479. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct Evidence and Enhancement of Surface Plasmon Resonance Effect on Ag-Loaded TiO2 Nanotube Arrays for Photocatalytic CO2 Reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Gazda, M.; Zaleska, A. Ordered TiO2 Nanotubes: The Effect of Preparation Parameters on the Photocatalytic Activity in Air Purification Process. Appl. Catal. B 2014, 144, 674–685. [Google Scholar] [CrossRef]
- Dronov, A.; Gavrilin, I.; Kirilenko, E.; Dronova, D.; Gavrilov, S. Investigation of Anodic TiO2 Nanotube Composition with High Spatial Resolution AES and ToF SIMS. Appl. Surf. Sci. 2018, 434, 148–154. [Google Scholar] [CrossRef]
- Gavrilin, I.; Dronov, A.; Volkov, R.; Savchuk, T.; Dronova, D.; Borgardt, N.; Pavlikov, A.; Gavrilov, S.; Gromov, D. Differences in the Local Structure and Composition of Anodic TiO2 Nanotubes Annealed in Vacuum and Air. Appl. Surf. Sci. 2020, 516, 146120. [Google Scholar] [CrossRef]
- Yuferov, Y.V.; Popov, I.D.; Zykov, F.M.; Suntsov, A.Y.; Baklanova, I.V.; Chukin, A.V.; Kukharenko, A.I.; Cholakh, S.O.; Zhidkov, I.S. Study of the Influence of Anodizing Parameters on the Photocatalytic Activity of Preferred Oriented TiO2 Nanotubes Self-Doped by Carbon. Appl. Surf. Sci. 2022, 573, 151366. [Google Scholar] [CrossRef]
- Savchuk, T.; Gavrilin, I.; Konstantinova, E.; Dronov, A.; Volkov, R.; Borgardt, N.; Maniecki, T.; Gavrilov, S.; Zaitsev, V. Anodic TiO2 Nanotube Arrays for Photocatalytic CO2 Conversion: Comparative Photocatalysis and EPR Study. Nanotechnology 2022, 33, 055706. [Google Scholar] [CrossRef]
- Ryu, M.-H.; Jung, K.-N.; Shin, K.-H.; Han, K.-S.; Yoon, S. High Performance N-Doped Mesoporous Carbon Decorated TiO2 Nanofibers as Anode Materials for Lithium-Ion Batteries. J. Phys. Chem. C 2013, 117, 8092–8098. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, X.; Xiao, M.; Liang, L.; Liu, C.; Liao, J.; Xing, W. The Construction of Nitrogen-Doped Graphitized Carbon–TiO2 Composite to Improve the Electrocatalyst for Methanol Oxidation. Carbon 2014, 72, 114–124. [Google Scholar] [CrossRef]
- Ghumro, S.S.; Lal, B.; Pirzada, T. Visible-Light-Driven Carbon-Doped TiO2-Based Nanocatalysts for Enhanced Activity toward Microbes and Removal of Dye. ACS Omega 2022, 7, 4333–4341. [Google Scholar] [CrossRef] [PubMed]
- Negi, C.; Kandwal, P.; Rawat, J.; Sharma, M.; Sharma, H.; Dalapati, G.; Dwivedi, C. Carbon-Doped Titanium Dioxide Nanoparticles for Visible Light Driven Photocatalytic Activity. Appl. Surf. Sci. 2021, 554, 149553. [Google Scholar] [CrossRef]
- Yang, Y.; Ni, D.; Yao, Y.; Zhong, Y.; Ma, Y.; Yao, J. High Photocatalytic Activity of Carbon Doped TiO2 Prepared by Fast Combustion of Organic Capping Ligands. RSC Adv. 2015, 5, 93635–93643. [Google Scholar] [CrossRef]
- Nogueira, M.V.; Lustosa, G.M.M.M.; Kobayakawa, Y.; Kogler, W.; Ruiz, M.; Monteiro Filho, E.S.; Zaghete, M.A.; Perazolli, L.A. Nb-Doped TiO2 Photocatalysts Used to Reduction of CO2 to Methanol. Adv. Mater. Sci. Eng. 2018, 2018, 7326240. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Li, Q.; Li, F. Recent Advances in Molecular Oxygen Activation via Photocatalysis and Its Application in Oxidation Reactions. Chem. Eng. J. 2021, 421, 129915. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, S.; Tong, Y.; An, X.; Long, J.; Fu, X.; Wang, X. Photocatalytic Reduction of CO2 with H2O to CH4 on Cu(i) Supported TiO2 Nanosheets with Defective {001} Facets. Phys. Chem. Chem. Phys. 2015, 17, 9761–9770. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Bahnemann, D. Chemical Physics of Nanostructured Semiconductors; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9781498708630. [Google Scholar]
- Bhattacharyya, K.; Mane, G.P.; Rane, V.; Tripathi, A.K.; Tyagi, A.K. Selective CO2 Photoreduction with Cu-Doped TiO2 Photocatalyst: Delineating the Crucial Role of Cu-Oxidation State and Oxygen Vacancies. J. Phys. Chem. C 2021, 125, 1793–1810. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of Metal Doping (Cu, Ag, Eu) on the Electronic and Optical Behavior of Nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A.; Borah, D. Interplay of Dopants and Defects in Making Cu Doped TiO2 Nanoparticle a Ferromagnetic Semiconductor. J. Alloys Compd. 2015, 646, 692–698. [Google Scholar] [CrossRef]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of Surface/Interfacial Cu 2+ Sites in the Photocatalytic Activity of Coupled CuO-TiO2 Nanocomposites. J. Phys. Chem. C 2008, 112, 19040–19044. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gómez, J.; Hidalgo-Carrillo, J.; Montes, V.; Estévez-Toledano, R.C.; Escamilla, J.C.; Marinas, A.; Urbano, F.J. EPR and CV Studies Cast Further Light on the Origin of the Enhanced Hydrogen Production through Glycerol Photoreforming on CuO:TiO2 Physical Mixtures. J. Environ. Chem. Eng. 2021, 9, 105336. [Google Scholar] [CrossRef]
- Lim, Y.-F.; Choi, J.J.; Hanrath, T. Facile Synthesis of Colloidal CuO Nanocrystals for Light-Harvesting Applications. J. Nanomater. 2012, 2012, 393160. [Google Scholar] [CrossRef]
- Wendlandt, W.; Hecht, H.G. Reflectance Spectroscopy; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Kim, B.-H.; Park, M.; Kim, G.; Hermansson, K.; Broqvist, P.; Choi, H.-J.; Lee, K.-R. Indirect-to-Direct Band Gap Transition of Si Nanosheets: Effect of Biaxial Strain. J. Phys. Chem. C 2018, 122, 15297–15303. [Google Scholar] [CrossRef]
- Martyshov, M.N.; Pavlikov, A.V.; Kytina, E.V.; Pinchuk, O.V.; Savchuk, T.P.; Konstantinova, E.A.; Zaitsev, V.B.; Kashkarov, P.K. Influence of Synthesis Conditions on the Structural, Optical, and Electrophysical Properties of TiO2/CuxO Nanocomposites. Tech. Phys. 2023, 68, 234. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Xu, F. Generating Oxygen Vacancies in Cu 2+ -doped TiO2 Hollow Spheres for Enhanced Photocatalytic Activity and Antimicrobial Activity. Micro Nano Lett. 2020, 15, 535–539. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- Khan, J.; Han, L. Oxygen Vacancy in TiO2: Production Methods and Properties. In Updates on Titanium Dioxide; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Jonscher, A.K. A New Understanding of the Dielectric Relaxation of Solids. J. Mater. Sci. 1981, 16, 2037–2060. [Google Scholar] [CrossRef]
- Dhiman, S.; Meena, R.; Manyani, N.; Tripathi, S.K. Investigating the Temperature and Frequency Dependence of Dielectric Response Using AC Impedance Spectroscopy on SnO2. Surf. Interfaces 2023, 42, 103362. [Google Scholar] [CrossRef]
- Benali, A.; Melo, B.M.G.; Prezas, P.R.; Bejar, M.; Dhahri, E.; Valente, M.A.; Graça, M.P.F.; Nogueira, B.A.; Costa, B.F.O. Structural, Morphological, Raman and Ac Electrical Properties of the Multiferroic Sol-Gel Made Bi0.8Er0.1Ba0.1Fe0.96Cr0.02Co0.02O3 Material. J. Alloys Compd. 2019, 775, 304–315. [Google Scholar] [CrossRef]
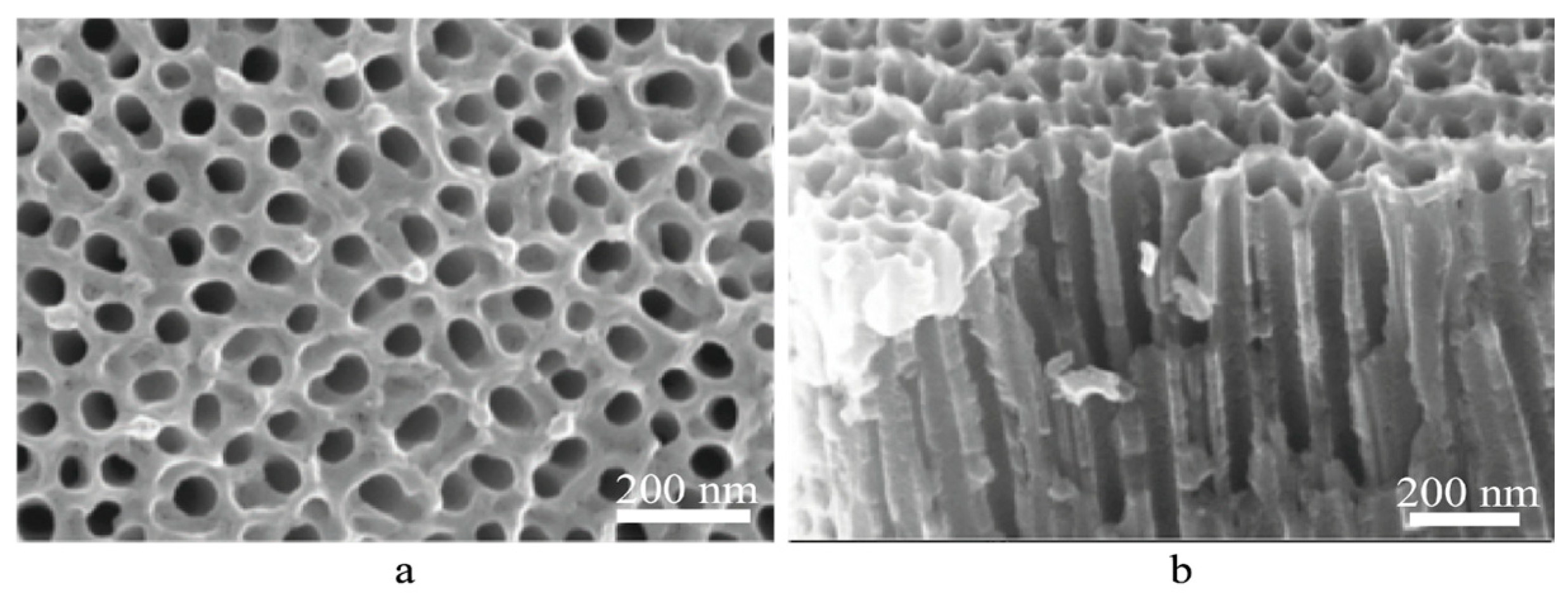
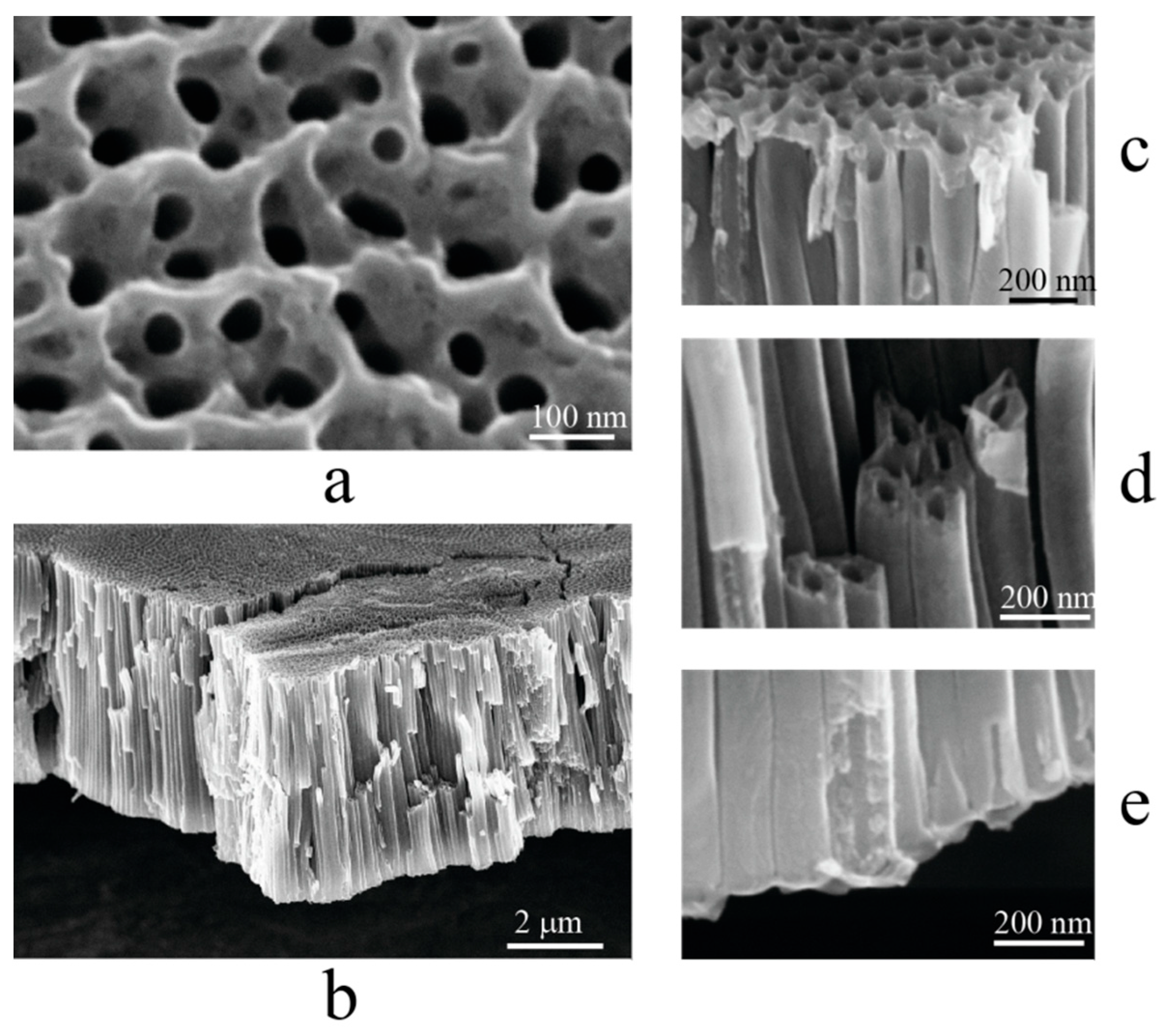



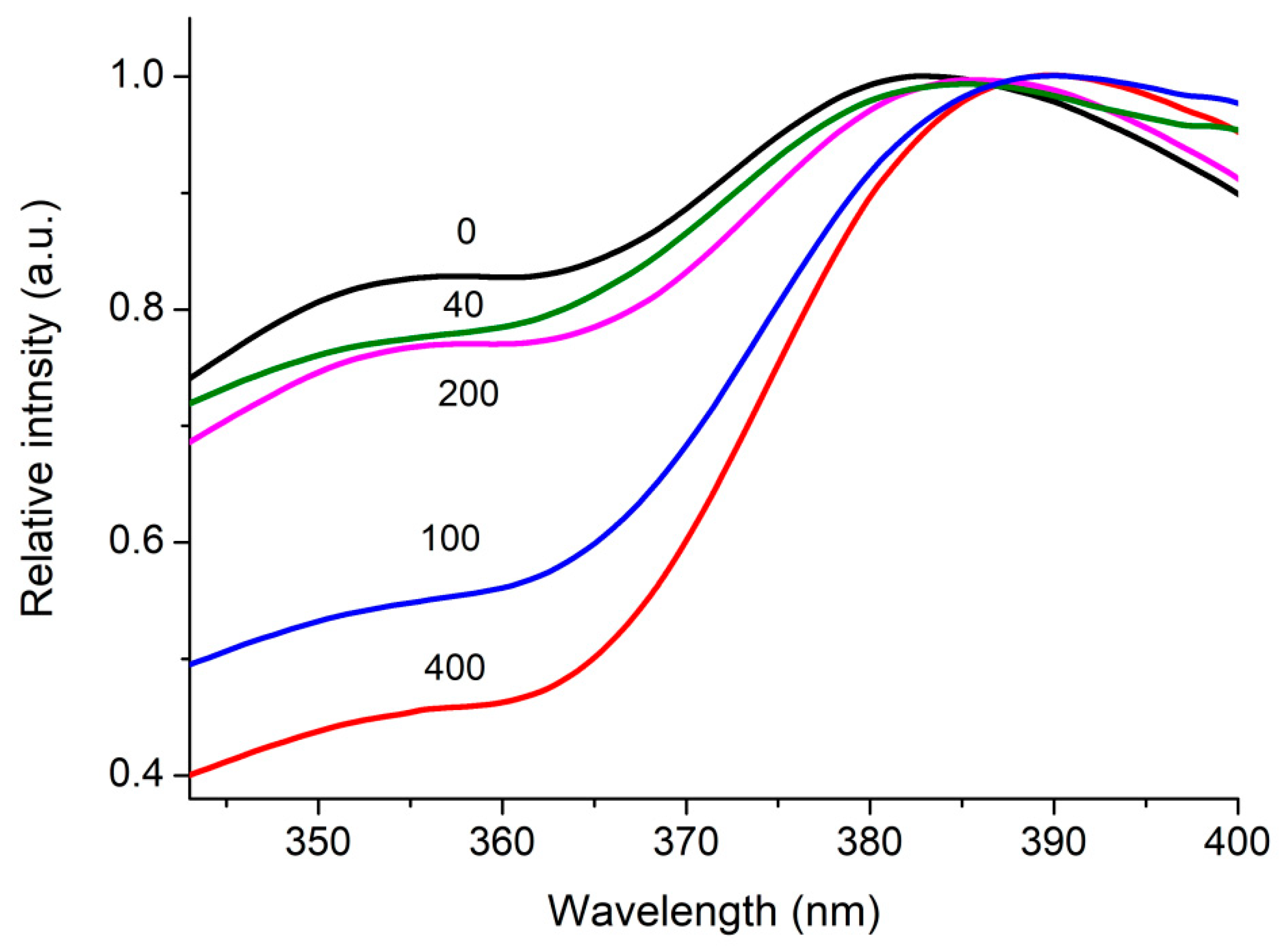
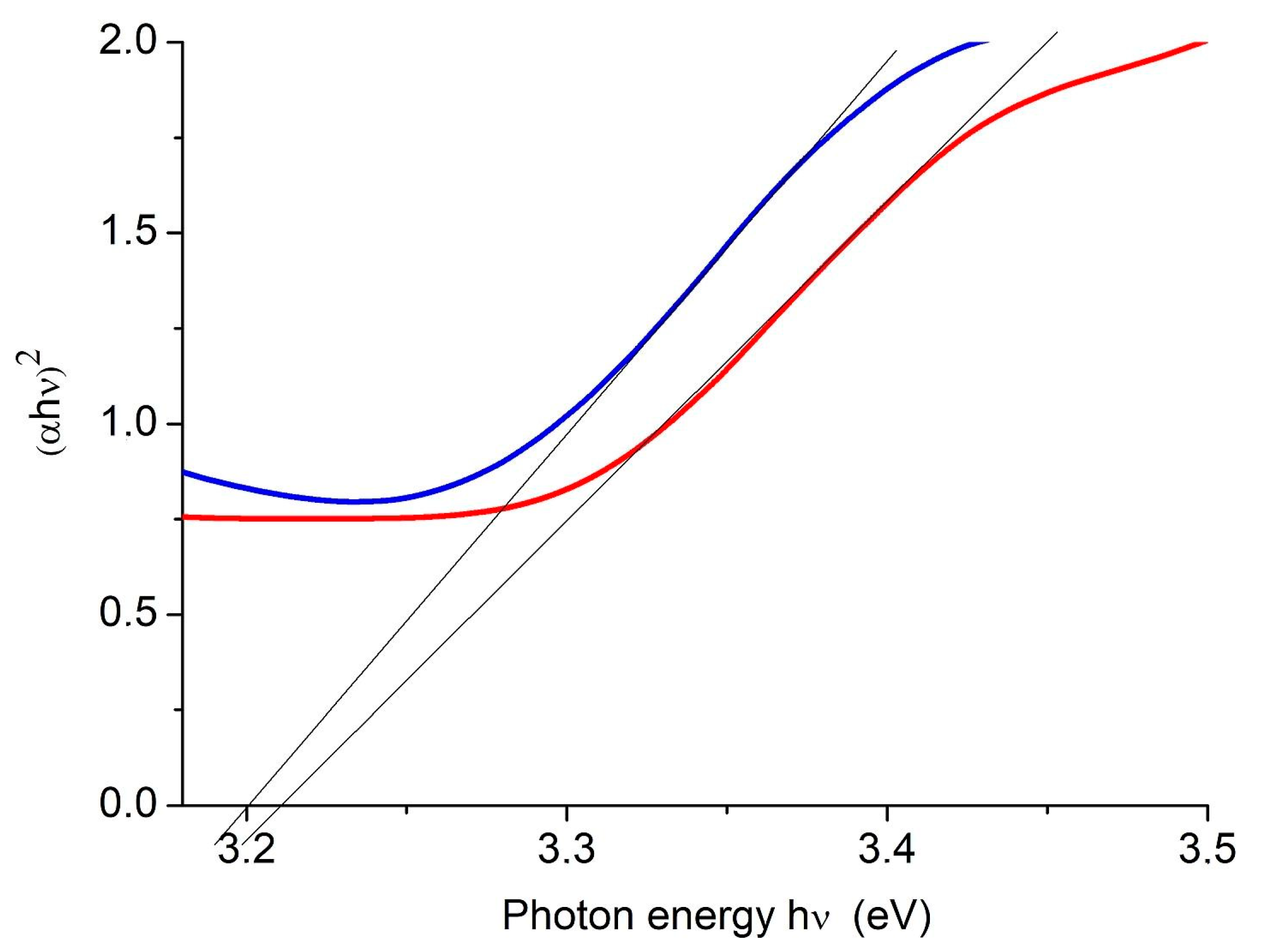

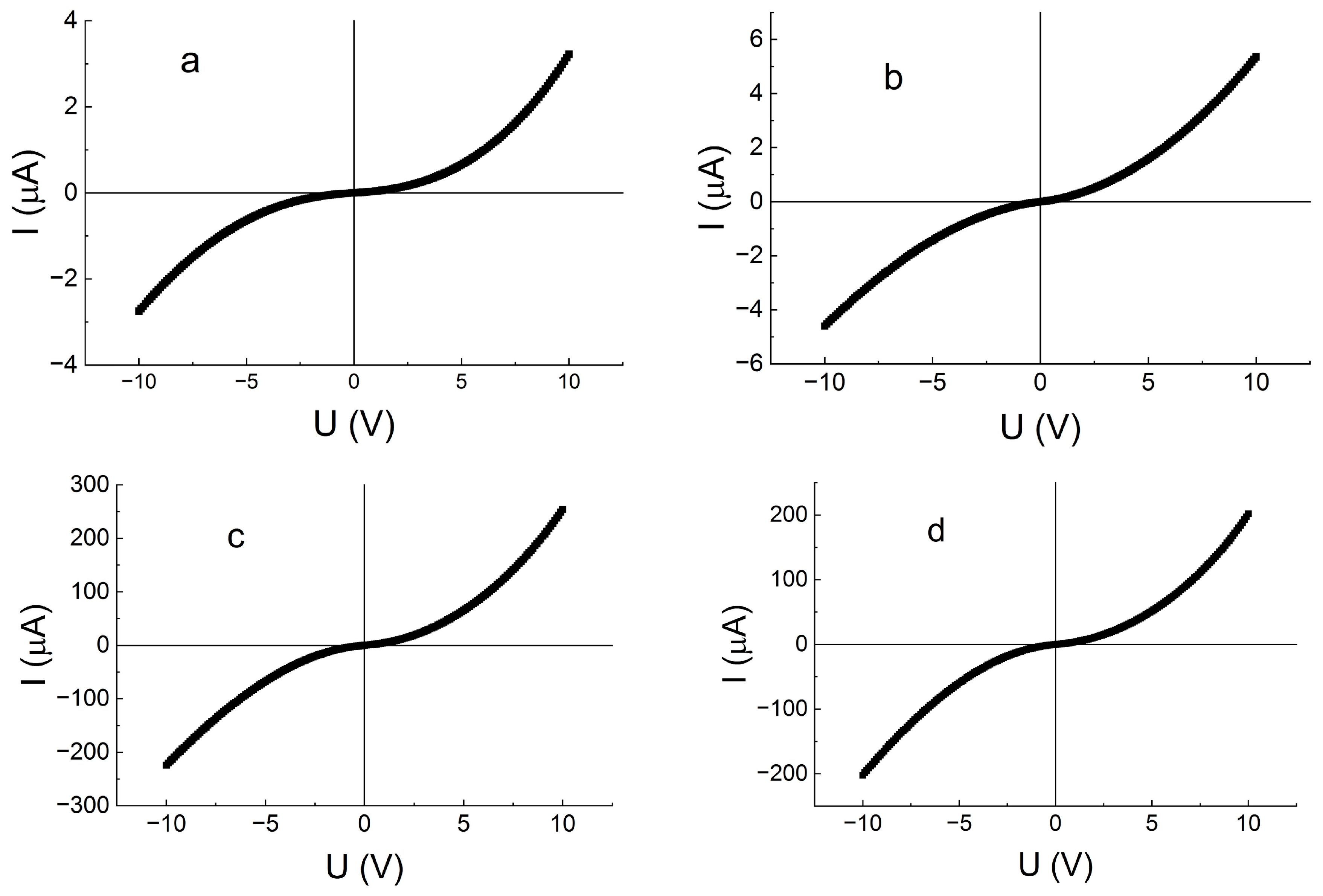
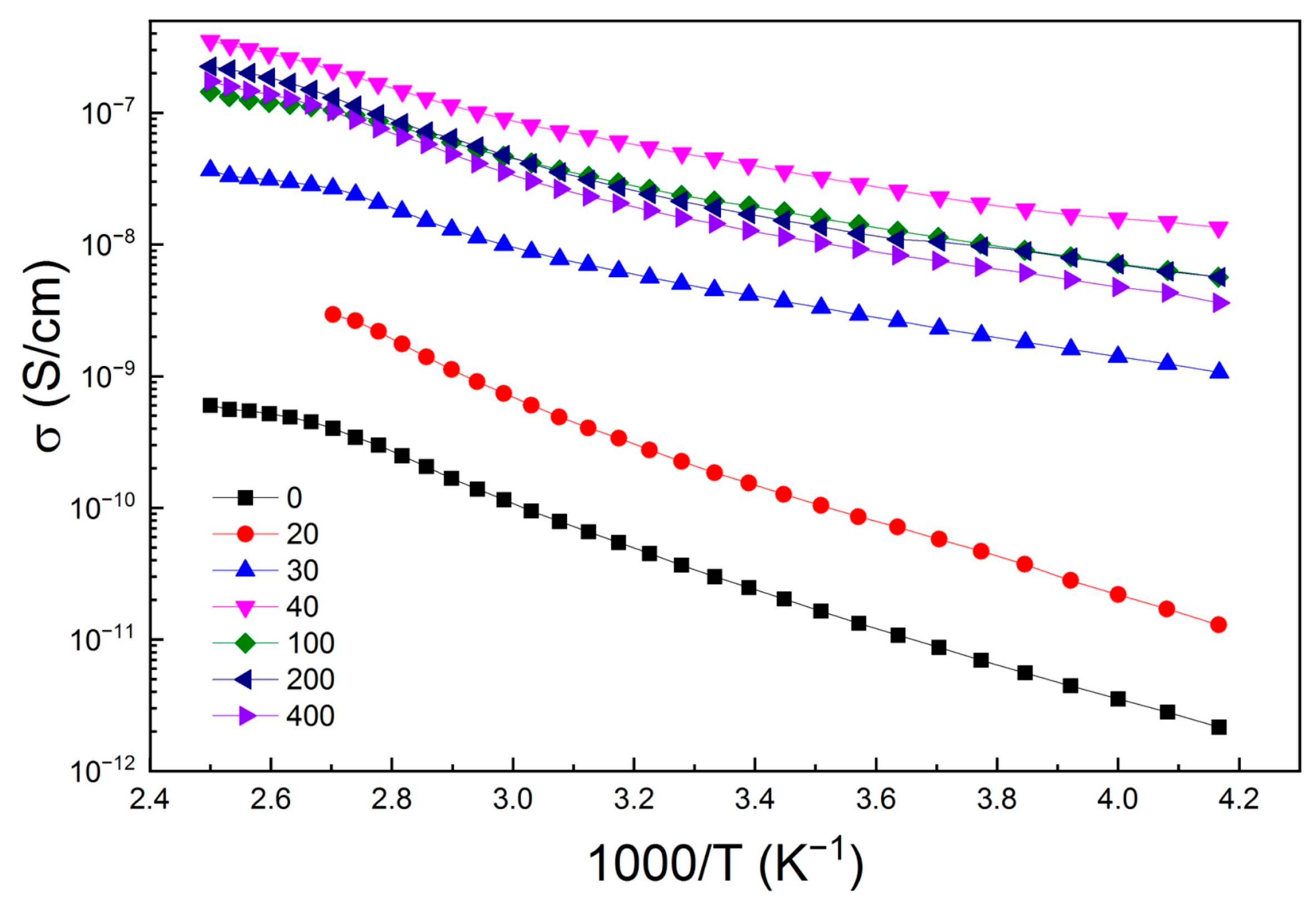


| Number of Copper Deposition Cycles | 0 | 40 | 100 | 200 | 400 |
|---|---|---|---|---|---|
| Eg, eV | 3.20 | 3.19 | 3.21 | 3.20 | 3.21 |
| № | Number of Copper Treatment Cycles | σ, S/cm (T = 300 K) | EA, eV |
|---|---|---|---|
| 1 | 0 | 3.0 × 10−11 | 0.27 ± 0.01 |
| 2 | 20 | 1.8 × 10−10 | 0.27 ± 0.01 |
| 3 | 30 | 4.5 × 10−9 | 0.15 ± 0.01 |
| 4 | 40 | 4.5 × 10−8 | 0.13 ± 0.01 |
| 5 | 100 | 2.1 × 10−8 | 0.14 ± 0.01 |
| 6 | 200 | 1.9 × 10−8 | 0.12 ± 0.01 |
| 7 | 400 | 1.4 × 10−8 | 0.14 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kytina, E.V.; Konstantinova, E.A.; Martyshov, M.N.; Savchuk, T.P.; Zaitsev, V.B.; Kokorin, A.I.; Ilin, A.S.; Trusov, G.V. Effect of Copper Modification on Charge Carrier Transport and Defect Properties in Carbon-Doped TiO2 Nanotubes. Catalysts 2025, 15, 572. https://doi.org/10.3390/catal15060572
Kytina EV, Konstantinova EA, Martyshov MN, Savchuk TP, Zaitsev VB, Kokorin AI, Ilin AS, Trusov GV. Effect of Copper Modification on Charge Carrier Transport and Defect Properties in Carbon-Doped TiO2 Nanotubes. Catalysts. 2025; 15(6):572. https://doi.org/10.3390/catal15060572
Chicago/Turabian StyleKytina, Ekaterina V., Elizaveta A. Konstantinova, Mikhail N. Martyshov, Timofey P. Savchuk, Vladimir B. Zaitsev, Alexander I. Kokorin, Alexander S. Ilin, and German V. Trusov. 2025. "Effect of Copper Modification on Charge Carrier Transport and Defect Properties in Carbon-Doped TiO2 Nanotubes" Catalysts 15, no. 6: 572. https://doi.org/10.3390/catal15060572
APA StyleKytina, E. V., Konstantinova, E. A., Martyshov, M. N., Savchuk, T. P., Zaitsev, V. B., Kokorin, A. I., Ilin, A. S., & Trusov, G. V. (2025). Effect of Copper Modification on Charge Carrier Transport and Defect Properties in Carbon-Doped TiO2 Nanotubes. Catalysts, 15(6), 572. https://doi.org/10.3390/catal15060572









