Abstract
Enzymes, as nature’s precision biocatalysts, hold transformative potential across industrial, environmental, and biomedical sectors. However, their instability, solvent sensitivity, and limited reusability in their free form necessitate advanced immobilization strategies to enhance their robustness and scalability. This review critically examines cutting-edge advancements in enzyme immobilization, focusing on the integration of artificial intelligence (AI), novel nanomaterials, and dynamic carrier systems to overcome the traditional limitations of mass transfer, enzyme leakage, and cost inefficiency. Key innovations such as metal–organic frameworks (MOFs), magnetic nanoparticles, self-healing hydrogels, and 3D-printed scaffolds are highlighted for their ability to optimize enzyme orientation, stability, and catalytic efficiency under extreme conditions. Moreover, AI-driven predictive modeling and machine learning emerge as pivotal tools for rationalizing nanomaterial synthesis, multi-enzyme cascade design, and toxicity assessment, while microfluidic systems enable precise biocatalyst fabrication. This review also explores emerging carrier-free strategies, including cross-linked enzyme aggregates (CLEAs) and DNA-directed immobilization, which minimize diffusion barriers and enhance substrate affinity. Despite progress, challenges persist in regards to eco-friendly nanomaterial production, industrial scalability, and real-world application viability. Future directions emphasize sustainable hybrid material design, AI-aided lifecycle assessments, and interdisciplinary synergies between synthetic biology, nanotechnology, and data analytics. By connecting laboratory innovation with industrial needs, this work provides a forward-thinking framework to harness immobilized enzymes for achieving global sustainability goals, particularly in bioremediation, bioenergy, and precision medicine.
1. Introduction
Enzymes play a crucial role in numerous biotechnological applications, including biosensors, biocatalysis, biomedicine, food processing, bioenergy, and agrochemicals [1]. However, natural enzymes face challenges including poor stability, solvent sensitivity, high costs, and limited reuse. Furthermore, the activities of natural enzymes are often influenced by environmental factors like temperature, pH, metal ions, and organic solvents, which restricts their wide application in industrial processes [2].
Enzyme immobilization has emerged as promising solution to address these challenges. This technique not only facilitates the easy separation and reuse of enzymes, thus reducing operational costs, but also enhances enzyme stability, allowing for more efficient and sustainable biocatalytic processes. The immobilization technique was initially reported in the early 1900s and was popularized in the 1960s [3].
Recently, numerous immobilization approaches, such as adsorption, entrapment, covalent binding, and cross-linking, have been examined in the literature [1,4,5,6]. These traditional immobilization techniques are often plagued by issues like enzyme leakage, instability, mass transfer limitations, and high operational costs. Additionally, these methods may result in enzyme inactivation, distortion of the enzyme shape, and the use of non-recyclable supports, complicating waste disposal. Steric hindrance and diffusional barriers can also impair the overall performance of the immobilized enzymes [7].
Recent advancements in enzyme immobilization technologies have focused on overcoming these limitations. Innovations such as mesoporous supports, hydrogels, cross-linked enzyme aggregates, microfluidics, 3D printing, and nanoparticle-based techniques have shown promise in enhancing enzyme activity, stability, and loading capacity, thus reducing the overall costs of biocatalysts [8]. The integration of machine learning into these methods further enhances their efficacy [9]. Nanomaterials, in particular, have proven effective in improving enzyme performance, while 3D printing allows for the precise design of immobilization carriers, offering eco-friendlier and cost-effective biocatalysts. The growing interest in enzyme immobilization, coupled with the push for sustainable industrial practices, has accelerated the research into novel carriers and immobilization techniques.
This review aims to provide a comprehensive overview of recent trends in enzyme immobilization research, highlighting the development of new carrier materials and innovative immobilization methods, especially AI-derived immobilization strategies. It will also explore the industrial applications of these advancements, focusing on their potential to improve biocatalytic processes and contribute to the achievement of global sustainability goals.
2. Traditional Approaches for Enzyme Immobilization
Enzyme immobilization improves reusability, stability, and catalytic efficiency [10]. Traditional methods include carrier-based (adsorption, affinity bonding, entrapment, encapsulation, ionic bonding) and carrier-free (covalent bonding, cross-linking) approaches, each with benefits and drawbacks. Among these, adsorption is simple and cost-effective, preserving enzyme structure but relying on weak interactions (e.g., hydrogen bonds, van der Waals forces), which may cause enzyme leakage under varying pH or temperature conditions [11]. However, its scalability for industrial applications be improved through carrier modifications. For instance, adsorbed laccase on granular activated carbon aids pollutant removal [12], while poly (methyl-methacrylate) (PMMA) microchannels improve horseradish peroxidase activity [13] and benzo[a]pyrene degradation in soil [14].
Entrapment immobilizes enzymes within polymeric matrices, offering high enzyme loading with minimal modification. However, pore-size constraints can lead to mass transfer limitations and enzyme leakage [8]. Optimizing material design can improve enzyme retention and substrate accessibility [9]. For example alkaline phosphatase entrapped in silica retained 30% of its activity over two months [15], while α-glucosidase in pHEMA maintained 90% of its activity after multiple uses [16].
Covalent bonding attaches enzymes to solid supports via stable functional groups, improving stability and reducing leakage. While this method may reduce activity due to conformational changes [17], it often preserves enzyme structure, enhancing catalytic performance. For instance, covalently immobilized cellulase retained 73% of its activity, while lipase on magnetic nanoparticles showed a 2.1-fold activity increase [18].
Cross-linking, often using bifunctional reagents like glutaraldehyde, enhances enzyme stability, but it may reduce activity if not controlled [19]. Cross-linked enzyme aggregates (CLEAs) offer benefits like scalability and low cost, though their small pores limit diffusion rates. For example, genipin-cross-linked laccase CLAEs show superior thermal stability and reusability compared to glutaraldehyde-based CLEAs [20], while magnetically separable CLEAs (Mp-CLEAs) further enhance catalytic efficiency and reusability [21].
In recent years, carrier-free methods like CLEAs have become popular due to their higher enzyme concentrations, stability, and reduced costs [22,23]. However, immobilization may decrease enzyme–substrate affinity and kcat due to diffusion barriers or conformational changes, depending on the method and materials used [24].
3. Major Factors Affecting Enzyme Immobilization Efficiency
Immobilization attaches enzymes to solid supports, forming a heterogeneous system that mimics the natural state of enzymes in living cells, where they bind to membranes or organelles. This enhances stability, making immobilized enzymes more resistant to environmental changes than are free enzymes [25]. Figure 1 shows a successful enzyme immobilization procedure, in which the following factors must be considered: substrate, carrier, enzyme, immobilization mechanisms, and carrier–enzyme interactions [26].
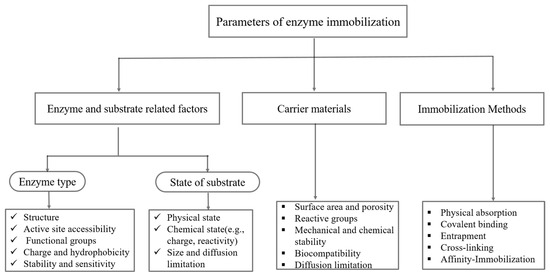
Figure 1.
Flow chart of parameters for enzyme immobilization.
Enzymes, substrates, and products exhibit diverse properties and applications, necessitating tailored immobilization strategies, each with distinct pros and cons. For instance, membrane reactor confinement, entrapment, and microencapsulation face diffusional constraints, whereas adsorption is simple, cost-effective, and efficient, but often reversible. In contrast, covalent attachment and cross-linking offer stability but may impair enzyme activity and incur higher costs [27].
The optimal immobilization technique depends on carrier properties such as porosity, chemical composition, and surface area [28]. Both the enzyme and the support must maintain stability during immobilization while ensuring maximum interaction. The enzyme–support affinity ultimately dictates the choice of the biocatalyst, support material, and immobilization method for a given process.
It is crucial to assess an enzyme’s chemical and physical stability prior to immobilization, since variables including byproduct production, adhesion to the support material, and environmental changes can drastically impact how well the enzyme functions [26]. Furthermore, maintaining the enzyme’s functioning requires protecting its active site and tertiary structure.
4. Matrixes for Enzyme Immobilization
The selection of a support material is essential in enzyme immobilization, requiring optimal particle size, surface area, surface charge, adjustable hydrophobic/hydrophilic properties, environmental sustainability, resistance to compaction and microbial attack, cost-effectiveness, and stability [29]. Support materials are categorized as inorganic and organic, with organic materials further divided into natural and synthetic types [30], as shown in Figure 2.
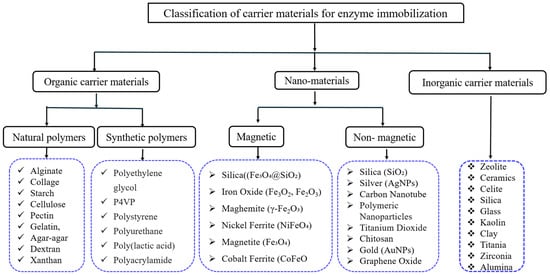
Figure 2.
Classification of carrier materials for enzyme immobilization.
Inorganic supports are preferred for their affordability, heat resistance, mechanical stability, microbial resistance [31,32], and high rigidity and porosity under stress. Despite being more sensitive to pH, temperature and pressure [33], organic materials allow functional changes, particularly the formation of polymers, which can be adjusted to enhance enzyme interactions [34].
4.1. Natural Organic Carriers
Natural biopolymers, such as cellulose, alginate, chitosan, starch, collagen, gelatin, and pectin, are sustainable, biodegradable alternatives to petroleum-based plastics [35,36]. Abundant, biocompatible, and rich in reactive site functionalizations, these versatile materials are widely used in medical, environmental, pharmaceutical, and food industries, as well as in biofuel/energy applications [37].
Among these, polysaccharides, e.g., cellulose, are particularly ideal for enzyme immobilization due to their non-toxicity, biodegradability, and modifiable surface hydroxyl groups. β (1→4)-linked D-glucose contains hydrophilic/hydrophobic regions and displays adaptability to chemical, enzymatic, or microbiological modifications, enhancing its application as a support [38].
Alginate is a water-soluble natural polymer derived from brown algae [39], and it is composed of α-L-guluronate and β-D-mannuronate blocks with functional groups (ether, carboxylate, and hydroxyl) that facilitate enzyme binding via cationic cross-linkers [40]. Its versatility allows it to form calcium alginate beads, hydrogels, and alginate–polyacrylamide composites, enhancing enzyme activity and reusability [41]. Due to tissue-like properties, alginate is also valuable in tissue engineering and cell delivery [42].
Chitosan, a mucopolysaccharide derived from chitin, contains abundant amine and hydroxyl groups, enabling direct enzyme binding without cross-linking [43]. Chitosan is widely favored for its availability, low toxicity, biodegradability, and biocompatibility, particularly for use in drug delivery systems [44]. For instance, chitosan nanoparticles and cationic polymer networks enhanced the stability, enzymatic activity, and reusability of protease and lipase, respectively, across a broad range of pH and temperatures [45,46]. Unlike alginate, chitosan reduces enzyme leaching and can be molded into beads, fibers, membranes, and gels [47]. The existence of numerous amine and hydroxyl groups in the chitosan structure enable the effective binding of different enzymes. While its low rigidity poses storage and catalysis challenges, chemical cross-linking can improve its mechanical strength [48]. Widely used in wastewater treatment, biomedicine, agriculture, and food processing, chitosan remains a robust alternative for enzyme immobilization [49].
Collagen, the most abundant protein in vertebrates, is widely used as a biomaterial due its excellent biological properties and functional groups (e.g., -OH, -COOH, CONH2 and NH2) which facilitate chemical reactions [50]. It has been employed in various forms, such as fibers, powders, and membranes, for enzyme immobilization. Among these, powdered collagen offers a larger surface area and greater versatility in bioreactors, making it particularly advantageous. Additionally, Fe3+-collagen fibers serve as effective supports for catalase immobilization, retaining significant enzyme activity even after 26 reuse cycles [51].
Gelatin, an affordable and widely available protein derived from collagen, retains biological properties similar to those of collagen [52]. Due to its low cost and biocompatibility, it is commonly used in drug delivery systems and tissue engineering [53]. Its reactive amino groups make it an effective support for enzyme immobilization. When attached to nanofiber surfaces, gelatin combines its biocompatibility with the nanofiber’s mechanical strength. In mixed carrier systems, gelatin enhances the cross-linkage with chromium (III) sulfate or potassium chromium (III) sulfate. Its abundant reactive groups provide strong bonding sites for enzymes. Additionally, gelatin improves enzyme loading efficiency; when paired with calcium alginate [54], it aids calcium phosphate deposition, and in combination with polyester films, it achieves a 75% loading efficiency, surpassing previous results of 50% [55].
Pectins is a complex polysaccharide that forms part of the plant cell walls and the middle lamellae. When combined with 0.2–0.7% glycerol, pectins act as plasticizers, reducing brittleness in support materials. This makes them useful for immobilizing enzymes like papain and for developing wound-healing treatments [56]. Due to their excellent gelling properties and biocompatibility, pectins are increasingly studied for biomedical and pharmaceutical applications [57].
Typically, pectins are used as microbeads to incapsulate biocatalysts within a hydrogel network. Cross-linking enzymes (e.g., peroxidase with ConA lectin) can increase their molecular weight [58]. Prokopijevic et al. [59] developed an oxidized pectin hydrogel modified with tyramine via reductive amination. Additionally, pectin–chitin and pectin calcium alginate support improved enzyme stability and catalytic efficiency by forming strong polyelectrolyte complexes [60].
Natural biopolymers like polysaccharides and proteins are prized for their biodegradability, biocompatibility, and adaptability, making them ideal for enzyme immobilization and other biotechnological applications and sustainable composite development. Their versatility meets the rising demand for eco-friendly technologies [61].
4.2. Synthetic Organic Carriers
Synthetic polymers, unlike natural biopolymers, are engineered for high purity, defined composition, and homogeneity [62]. They are ideal for enzyme immobilization, especially due to their tunable fiber synthesis via molecular weight control [63]. Common choices include polyvinyl alcohol (PVA), polyethylene oxide (PEO), polylactic acid (PLA), polyethylene glycol (PGA), polyacrylonitrile (PAN), polymethyl methacrylate (PMMA), polycaprolactone (PCL), polystyrene (PS), polysulfone (PSF), and polyurethane (PU) [64]. While most require organic solvents for electrospinning, PEO and PVA stand out for their water solubility and enzyme compatibility.
PVA is particularly favored for its low cost, hydrophilicity, and reactive hydroxyl groups, enabling efficient enzyme and cell immobilization [65]. When functionalized, PVA enhances the continuous processing of flexible carrier–enzyme composites and improves enzyme hydrolysis performance, and its fibers are effective in enzyme hydrolysis [63]. Similarly, P4VP (derived from pyridine), leverages vinyl groups and nitrogen atoms to form strong hydrogen bonds, enhancing enzyme attachment and stability [66]. PAM further excels with its high-water absorption, gel-forming, cross-linked structure, promoting enzyme stability and recyclability [67].
Polystyrene and polypropylene exhibit robust mechanical and chemical properties, making them ideal for industrial and bioreactor applications due to their high enzyme activity, stability, and reusability [68,69]. In tissue engineering, synthetic polymers such as PCL, PLA, and PLGA are widely used for their favorable mechanical properties, and cost-effectiveness [70]. However, their environmental persistence and potential to form microplastics raise significant concerns.
Studies have highlighted the environmental impacts of polyacrylates, PVP, PEG, and PAA on aquatic life [71,72], while under-researched polymers like polyethylene and polypropylene warrant further scrutiny [73]. These materials can also pose human health risks, including oxidative stress; apoptosis; and neurological, cardiovascular, and gastrointestinal effects [74,75]. To address these concerns, researchers are developing biodegradable alternatives, including hybrid systems employing biopolymers (e.g., starch or chitosan), although some composites may still generate microplastics [49].
4.3. Natural Inorganic Biopolymers
Inorganic carriers are ideal for enzyme immobilization due to their high thermal, mechanical, and microbial resistance [76]. Suitable materials must be water insoluble, mechanically strong, chemically stable, and have a large a large surface area [77], with examples including silica, silicates, borosilicates, aluminosilicates, alumina, zeolites, ceramics, titania, and other oxides [78].
Silica stands out due to its mechanical strength, thermal stability, and chemical resistance [79]. Its porous structure and high surface area minimize diffusional limitations, while facilitating enzyme attachment. Surface hydroxyl groups allow for easy functionalization with agents such as glutaraldehyde or 3-aminopropyltriethoxysilane (APTES), making silica suitable for accommodating diverse enzymes [80]. For example, silica-immobilized lipases retain 91–96% of their activity [81].
Other metal oxides such as titanium, aluminum, and zirconium oxides are also effective carriers for enzymes like lipase, urease, and α-amylase [29]. Their hydrophilic nature, due to their abundant hydroxyl groups, enhances enzyme attachment and stability [82].
Ceramics, including metal oxides (SnO2, Al2O3, TiO2, ZrO2) and silico-aluminates (kaolinite and montmorillonite), are particularly valuable due to their high thermal, chemical, and mechanical stability, along with their large surface areas [83]. These supports enhance enzyme activity and substrate conversion, making them valuable in food processing, biodiesel production, and biosensing [84,85,86].
The combination of inorganic and organic materials enhances their mechanical strength, controlled release, and functionalization, expanding their use in drug delivery and bioengineering [87]. Overall, inorganic carriers, especially silica, ceramics, and oxides, offer versatile and robust support for enzyme immobilization, with potential for further advancement in industrial and biomedical applications.
4.4. Hybrid and Composite Carriers
Hybrid and composite materials mix different components to produce improved qualities not present in each component alone. These substances provide biomolecules with the prefect environment, enhancing the stability, reusability, and resistance to structural alterations of the enzymes [88]. Therefore, for enzyme immobilization on hybrid or composite supports made from organic–organic, inorganic–inorganic, and organic–inorganic, these precursors are quite beneficial.
Synthetic polymers (like polyalanine and polyacrylonitrile) can be combined with biopolymers (like chitosan and cellulose) or two biopolymers (like alginate and lignin) to create organic–organic hybrids [89]. These produce long lasting, reusable biocatalytic devices with high enzyme retention by utilizing the mechanical stability of synthetic polymers and the biocompatibility of natural polymers [90].
Organic–inorganic hybrids combine natural or synthetic organic polymers with inorganic elements such as metal oxides, silica, or carbon-based compounds [91]. They work well for absorbing, covalently binding, or encasing enzymes like hydrolases and oxidoreductases because of their exceptional stability, mechanical strength, and high affinity for biomolecules [92].
Inorganic–inorganic hybrids, which are made entirely of inorganic elements, offer remarkable mechanical resilience, chemical inertness, and stability at high temperature and pH values [93]. They frequently have functional groups (such as carboxyl and hydroxyl) on their surfaces that improve biomolecule binding and hydrophilicity [94]. They are promising enzyme supports because of their low synthesis costs and good immobilization efficiency.
4.5. Semi-Synthetic Polymers
Semi-synthetic polymer hybrids of natural and synthetic materials are excellent for enzyme immobilization due to their mechanical strength, biocompatibility, and tunable properties. Natural components enhance biocompatibility and biodegradability, while synthetic components improve chemical stability and strength. Chemical modifications like cross-linking (glutaraldehyde, carbodiimide) or adding reactive groups (-NH2, -COOH, OH) boost enzyme binding. Common examples include chitosan-based hybrids (e.g., chitosan–alginate, chitosan glutaraldehyde, carboxymethyl chitosan), cellulose derivatives (e.g., carboxymethyl cellulose, cellulose with vinylpyrrolidone, cellulose acetate), starch derivatives (starch-polyacrylonitrile (PAN)), alginate-based hybrids (e.g., alginate–polylysine composites, alginate graft polyacrylamide), and other starch-, dextran-, protein-, and lignin-derived polymers.
Carboxymethyl cellulose (CMC) is the most common water-soluble cellulose derivative that enhances gel products by improving water retention through gel formation [95]. The addition of CMC to alginate gel boosts lactase immobilization efficiency. At a CMC-to-sodium alginate ratio of 1.0:1.5, the immobilization yield reached 58.2%, which is 14.2% higher than that for alginate alone [96]. The CMS–alginate gel also provided better thermal and pH stability for the enzyme. Similarly, carboxymethyl–chitosan (CMCht), derived from chitosan, is a biocompatible, water-soluble biopolymer. Its structure resembles that of glycosaminoglycans (GAGs), key components of articular cartilage. CMCht enhances water solubility and provides COOH groups for enzyme coupling. For example, Marina et al. developed biocatalysts using cysteine proteases (ficin, papain, and bromelain) bound to chitosan and carboxymethyl chitosan micro- and nanoparticles [97]. Molecular docking showed that these enzymes interact with chitosan derivatives via hydrogen bonding and hydrophobic interactions, particularly through active site residues. This complex formation enhances proteases activity and stability. Starch–polyacrylonitrile (PAN) copolymers combine natural starch with synthetic PAN, offering biocompatibility, mechanical stability, and easy modification for enzyme immobilization [98]. Graft copolymers (PAN chains on starch) and cross-linked networks (using glutaraldehyde or epichlorohydrin) provide a high surface area and functional groups (-COOH, -NH2) for covalent enzyme binding. For example, electrospun PAN/montmorillonite (O-MMT) membranes, after alkaline hydrolysis, immobilized laccase effectively, achieving high crystal violet removal (optimal pH 5, ~40 °C, 100 mg/L) [99]. The immobilized laccase outperformed both the free enzyme and the support alone, demonstrating synergetic enhancement.
4.6. Cross-Linked Polymers
Cross-linked polymers are formed by chemically bonding enzyme molecules to a 3D polymer network using cross-linking agents like glutaraldehyde. This method produces a stable, insoluble matrix that entraps enzymes while allowing substrate access to the active site. Compared to linear polymers, cross-linked polymers offer superior rigidity, durability, and thermal stability. Common examples include polyacrylamide gels, natural and synthetic hydrogels, polyethylene glycol (PEG), alginate, chitosan, cross-linked carrier free immobilized enzyme aggregates, etc. [100].
Polyacrylamide gel (PAG), composed of acrylamide monomers and bisacrylamide cross-linkers, is a widely used cross-linked polymer. Its porous structure immobilizes enzymes while facilitating substrate and product diffusion. The gel’s biocompatibility and protein compatibility make it nontoxic and suitable for enzymatic applications. To synthesize robust enzyme–polymer beads, optimal concentrations of enzymes, acrylamide monomers, bisacrylamide, and free radical initiators are required [101]. Free radical-induced polymerization encapsulates enzymes within gel, with the pore size critically influencing stability and reactivity, i.e., excessively small pores hinder substrate diffusion, while overly large pores risk enzyme leakage. For instance, it was demonstrated that maltase entrapped in PAG exhibited improved thermal stability, tolerating temperatures up to 55 °C (vs. 45 °C for free enzyme), while maintaining optimal pH and reaction time.
Polyethylene glycol diacrylate (PEGDA) is a derivative of PEG, a hydrophilic, biocompatible polymer widely used in biotechnology [102]. Laccase immobilized on PEGDA microspheres showed enhanced pH stability, retained 45% activity after six cycles, and maintained a 60.4% higher activity than that of free laccase after 25 days at 4 °C [103]. Similarly, glucose oxidase encapsulated in PEG hydrogels via physical entrapment and covalent binding retained activity for over a week without leakage, along with exhibiting higher activity [104].
5. Innovative Approaches for Enzyme Immobilization
Emerging technologies are driving the development of novel enzyme carriers and advanced immobilization techniques, yielding more robust, eco-friendly, and cost effective biocatalysts [18]. These innovations enhance applications in environmental remediation, pharmaceuticals, and biotechnology, driven by key trends such as improved immobilization efficiency, nanotechnology integration, and sustainable practices, addressing challenges like enzyme deactivation, versatility, and cost. Figure 3 summarizes the latest methods and the benefits of enzyme immobilization.
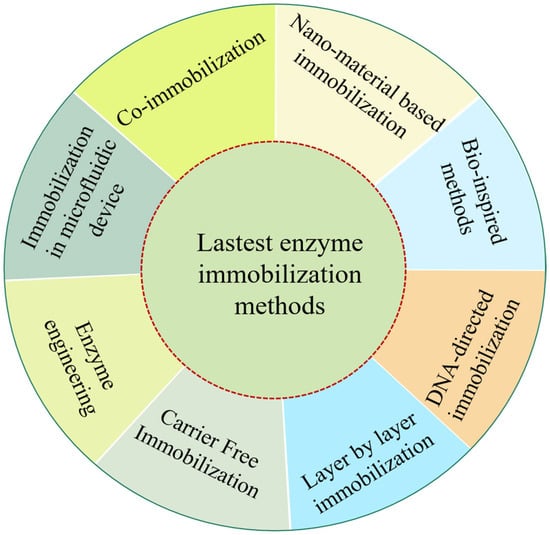
Figure 3.
Latest approaches of enzyme immobilization and their benefits.
5.1. Nanomaterial-Based Enzyme Immobilization
Nanotechnology has revolutionized chemistry, medicine, and pharmaceuticals by enabling advanced enzyme immobilization. Nanomaterials are ideal immobilization substrates because their high surface area maximizes enzyme loading, while their smaller size enhances efficacy by increasing binding sites, improving diffusion, and allowing functional customization [7,17]. Unlike bulk porous materials, nanomaterials minimize mass transfer resistance by shortening the pore pathway [105] and providing superior chemical/thermal stability, biocompatibility, and higher kinetic efficiency [106].
However challenges remain, including complex synthesis, stability, and specificity challenges [31], along with environmental concerns from toxic production chemicals [107]. Diverse nanostructures, such as particles, wires, tubes, sheets, and fibers, are employed, including carbon-based (graphene, carbon nanotubes), metals (titanium, gold, magnetite), and oxides (silica and alumina) [108]. Recent advances explore polymers, metal–organic frameworks, magnetic nanoparticles, and hybrid materials.
5.1.1. Polymer-Based Enzyme Immobilization
Immobilizing enzymes on polymer nanostructures creates an intermolecular network that can alter enzyme structure, metabolic pathways, and active site accessibility. Both natural (e.g., cellulose, chitosan, alginate) and synthetic (e.g., polypropylene, polyamide, polyethylene) polymers are used for this purpose [109]. Common synthetic polymers include polyvinyl alcohol (PVA), polyethylene oxide (PEO), polymethyl methacrylate (PMMA), and polylactic acid (PLA), which are often electrospun into fibers for enzyme immobilization.
Polymer-based enzyme immobilization enhances stability, catalytic efficiency, re-usability and storability. Studies show that nanofiber mats (NFM), PMMA/Fe3O4 nanofibers, spongin scaffolds, and polystyrene fibers effectively immobilize laccase and glucose oxidase [110,111,112,113].
Despite its benefits, polymer-based enzyme immobilization faces challenges including diffusion barriers that hinder substrate/product accessibility [114], the high costs of premium polymers and reagents, and potential enzyme performance impairment due to degradation or impurity accumulation [115]. Additionally, some polymers or cross-linkers pose biocompatibility concerns due to toxicity.
In conclusion, this approach is versatile, affordable, and biocompatible, with broad biotechnological and industrial potential, although efficiency, stability, and environmental impact challenges require further optimization.
5.1.2. Metal–Organic Frame (MOF)-Based Enzyme Immobilization
Metal–organic frameworks (MOFs) are porous crystalline hybrid materials formed by the self-assembly of metal ions and organic ligands. Their high surface area and adjustable pore sizes enhance the thermal and chemical stability of embedded enzymes, while facilitating substrate diffusion [116]. A dynamic defect production method using MOF degradation has been demonstrated to be an optimal enzyme immobilization [89], as shown in Figure 4.
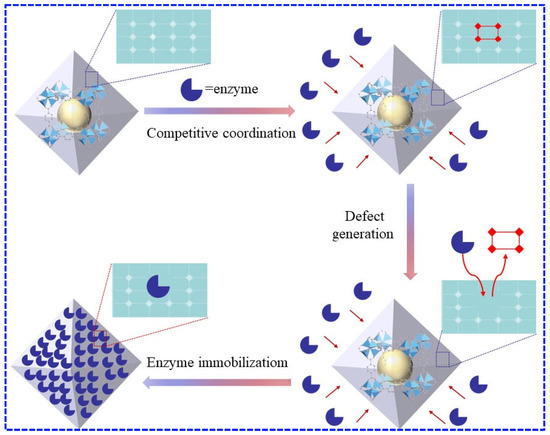
Figure 4.
Example of a dynamic defect production method using MOF degradation for optimal enzyme immobilization.
Zeolitic imidazolate frameworks (ZIFs), a subclass of MOFs, mimic traditional aluminosilicate zeolites in structure, with ZIF-8 (comprising zinc ions and imidazolate ligands) widely used due to its superior structural and textural properties [117]. Horseradish peroxidase (HRP) and glucose oxidase (Gox) were immobilized on a smart polymer membrane (PSMN), encapsulated in a hierarchical ZIF-8 (HZIF-8), showing 17.8-fold, 10.8-fold, and 6.0-fold higher catalytic activity than that of free enzymes and GOx-HRP@ZIF-8 at 37 °C, alongside enhanced stability, reusability, and efficiency [118,119]. Similarly, immobilizing cellulase and GOx on magnetic gold mesoporous silica nanoparticles (mAu@PSNs) with ZIF-8 improved thermal stability, storage life, reusability, and catalytic activity [118,120,121]. The abundant NH2 and COOH groups in MOFs further enhanced enzyme stabilization [121]. Table 1 summarizes the MOF-based immobilization methods, including the MOF types, enzyme reaction, and features of improved enzymes.
5.1.3. Magnetic Nanoparticles (MNP)-Based Enzyme Immobilization
Magnetic nanoparticles (MNPs), composed of iron, nickel, aluminum, and cobalt, are widely used due to their magnetic responsiveness [122]. Their large surface area, superparamagnetic properties, and adaptability make them ideal for industrial applications, while they also enhance enzyme activity and prevent denaturation, especially when encapsulated in biocompatible materials like polydopamine, chitosan, or silica [123]. Immobilized metal affinity magnetic nanotechnology (IMAN), based on IMAC, employs MNPs as carriers to chelate metal ions (e.g., Cu2+, Ni2+, Zn2+, Co2+) and bind proteases, maintaining enzyme activity capacity [124].
Magnetite (Fe3O4) is the most widely used MNP for enzyme immobilization due to its low toxicity, high magnetic susceptibility, large surface area, biocompatibility, and superparamagnetic properties at ambient temperatures [9]. Studies show that chitosan-coated MNPs retain 80% of their enzyme activity after 30 days at 4 °C and 48.8% after 13 cycles, yielding 64.45% glucose (21.84 g/L) [125], while glucosidase immobilized on MNPs achieved a 93% binding efficiency and maintained 50% of its activity after 16 cycles [126].
It has been reported that xylanase and laccase can be immobilized on magnetic supports such as chitosan beads with Fe3O4 nanoparticles, magnetic graphene oxide (GO-MNP), bioanodes with metals, and Fe3O4@NC [127,128]. These immobilized biocatalysts enhanced the efficiency, reusability, and storage stability for applications in cellulose/Xylan hydrolysis and phenolic compound degradation.
Reports indicate that MNPs such as magnetic Janus silica nanoparticles and Fe3O4 MNPs effectively immobilized protease, and cellulase exhibited stability under varying temperature and pH levels [129,130]. These immobilized enzymes showed potential for dye decolorization and juice clarification. MNPs-based immobilization offers advantages like high surface area, stability, low toxicity, and biocompatibility [131]. Cross-linking agents (e.g., glutaraldehyde) enhance enzyme thermostability by reducing steric hindrance and increasing flexibility [131]. Additionally, coating MNPs with organic and inorganic compounds can reduce toxicity, broadening their application potential.
In summary, MNP immobilization enhances enzyme stability and catalytic efficiency by optimizing active site orientation, minimizing diffusion barriers, and improving substrate accessibility [132]. It also simplifies enzyme separation and recycling, lowering costs for industrial and environmental applications.

Table 1.
Summary of MOF-immobilized enzyme reactions: immobilization methods, MOF types, enzyme reaction, and features of improved enzymes.
Table 1.
Summary of MOF-immobilized enzyme reactions: immobilization methods, MOF types, enzyme reaction, and features of improved enzymes.
| Enzyme | MOF | Immobilization Method | Enzyme Reaction | Improved Enzyme Application | Ref. |
|---|---|---|---|---|---|
| Horseradish peroxidase Lysozyme | UiO-66-F4 | Encapsulation | 3,3′,5,5′ tetramethylbenzidine (TMB) used as the substrate and H2O2 as an oxidizing agent in the presence of 1-phenyl-ethanol with vinyl acetate and chitosan. | Improved activity and stability, enhanced temperature tolerance over a wide range. Not exposed to digestive enzyme pepsin or long-term storage, providing protection from harsh conditions. | [133] |
| Lipase | UiO-66 | Encapsulation | For separation, 1-phenyl-ethanol racemates into (S)-enantiomers through chiral resolution of ((S)-phenyl ethanol) for production of critical chemicals and medicines. | Maintenance of enzyme activity, protective performance towards enzymes under harsh conditions, and excellent reusability. | [134] |
| β-glucosidase (β-G) | MOF-74-2-MI | Encapsulation | Cellobiose and cellulose hydrolysis to glucose. | Enzyme with a domain-limited space for long-term stability, low leaching ability, recyclability, and tolerance. | [135] |
| β-glucosidase (β-G) | (MCS/β-G) | Surface adsorption | Hydrolysis of cellobiose into glucose. | Maximized enzyme catalytic retention activity, stability under acidic conditions, structural stability, protective effect of the “core−shell” structure of Ca BDC on enzyme activity at higher temperatures and with denaturants. Maintain a stable active conformation during separation and washing. Improved enzyme affinity to the substrate, increased mass transfer resistance. | [136] |
| Laccase | Polydopamine-coated magnetic graphene (PDA-MGO) | Covalent binding | Enzyme system exhibits superior removal efficiencies for 2,4 DCP and BPA pollutant degradation. | More active than free laccase, with a wider pH and temperature range. Maintained approximately 80% of its initial activity, even after two hours of incubation at 50 °C. Exhibited remarkable removal efficiencies of 97.0% and 83.9% toward 2,4-DCP. High enzymatic activity, environmental stability, and excellent re-usability. The laccase ILs PDA MGO system demonstrated superior reusability. | [137] |
5.1.4. Carbon Nanotubes and Graphene-Based Enzyme Immobilization
The immobilization of enzymes onto nanomaterials, such as carbon nanotubes (CNTs) and graphene, has garnered attention for enhancing enzyme stability, activity, and reusability [138,139]. Graphene, a 2Dsp2-hybridized carbon material, offers exceptional mechanical, electrical, and chemical properties at low cost.
Both CNTs and graphene provide large surface areas, chemical stability, and superior electrical conductivity, making them ideal for biosensing and catalysis. Enzyme immobilization on these materials improves orientation, substrate interactions, and longevity while reducing diffusion barriers, benefiting industrial and medicinal applications. Recent progress in the development of biosensors, biofuel cells, and catalytic systems highlights the growing role of carbon-based nanomaterials in boosting enzyme performance [139,140].
Studies show that enzymes immobilized on 3D graphene, CNTs, graphene oxide, and multi-walled carbon nanotubes (MWCNTs) displayed enhanced efficiency and stability [141,142]. The CNT/3DG hybrid shows particular promise for biofuel cell production and biosensor development, offering extended enzyme lifespan, high stability, catalytic efficiency, and selectivity.
5.1.5. DNA-Directed Enzyme Immobilization
DNA-directed immobilization (DDI), introduced in the1990s, is a widely used, efficient, and safe method used in diagnostics, biosensing, and research. It leverages DNA-base pairing to immobilize DNA-tagged enzymes onto carriers, with hydrophilic DNA acting as spacer that preserves enzyme structure and improves catalytic performance [143,144].
Biomolecules like DNA, antibodies, and enzymes act as recognition elements for specific analytes. DNA identifies nucleic acids, antibodies detect antigens, and enzymes interact with their specific substrates [145]. Effective probe immobilization on electrodes is essential for DNA biosensors, since it has a direct impact on biosensor accuracy, sensitivity, selectivity, and longevity [146]. Nanomaterials improve DNA immobilization due to their large surface area and biocompatibility, preserving biological activity and expanding the sensing interface. Additionally, DNA can act as a bridging molecule, forming covalent and non-covalent linkages between enzymes and nanoparticles to ensure stable immobilization. This concept is illustrated in Figure 5.
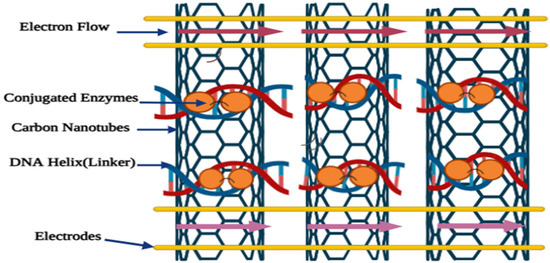
Figure 5.
Schematic representation of DNA serving as a catalytic tool in the modification of nanotubes.
Studies have indicated that a DNA double can serve as a flexible spacer to enhance enzyme flexibility. Enzymes were immobilized on supports such as TiO2 nanoparticles via a DNA helix and graphene/Au nanorod/polythionine (G/Au NR/PT) [146,147]. These composites, primarily used as sensors, exhibited excellent catalytic activity, stability, reusability, and sensitivity.
5.1.6. Organic–Inorganic Hybrid Nanomaterials-Based Enzyme Immobilization
Organic–inorganic hybrid nanoflowers (hNFs) have attracted significant attention for their simple synthesis, mild reaction conditions, high stability, and biocompatibility [148]. They are produced through biomimetic mineralization, where metal ions interact with protein solutions to form functional nanostructures without hazardous chemicals, even under challenging conditions [149].
Enzyme immobilization on hNFs significantly enhances catalytic activity and stability compared to the results using free enzyme or conventional methods. For instance, thermophilic lipase QLM from Alcaligenes sp. immobilized on Cu3(PO4)2-based hNFs exhibited excellent reusability and stability, retaining activity after eight cycles or 4 weeks at ambient temperature. After 10 min at 80 °C, the immobilized enzyme retained 51.6% of its activity, while the free enzyme kept only 19.8%. It also maintained 78.6% of its activity at pH 5.0 compared to 32.9% for the free form, and showed a lower Km value (15.54 mM vs. 24.42 mM), indicating stronger substrate affinity [150].
Collagenase immobilized on Col-Zn-hNFsand magnetic cross-linked hybrid nanoflowers (mcCNFs) also showed superior catalytic performance, with greater stability across wide pH and temperature ranges [149,151]. These immobilized enzymes also have promising medical and industrial applications, while their larger surface area increases substrate concentration at the enzyme’s active site, boosting efficiency [152,153].
5.2. Challenges and Limitations of Nanomaterial-Based Immobilization
Nanomaterial-based enzyme immobilization enhances traditional enzymatic catalysis by mitigating mass transfer resistance, enzyme instability, and inhibition [18,123]. This nano-biocatalyst approach integrates enzymes with functionalized nanocarriers, improving substrate specificity and catalytic efficiency. These nanomaterials offer large surface areas reusability, tunable surfaces, and options for magnetic separation [154]. However, broader adoption is limited by inconsistent material properties, high synthesis costs, and environmental concerns, i.e., toxicity and disposal, hinder wider use [155].
A major challenge lies in the development of cost-effective nanomaterial synthesis. Large-scale production demands precise control over material properties, especially in regards to 2D nanomaterials, where low-cost methods often introduce defects [156]. For carbon nanotubes, achieving uniform chirality, conductivity, and diameter remains technically difficult [157], and only structurally pure forms deliver optimal performance, requiring advanced fabrication techniques [158].
Additionally, nanoparticle agglomeration, driven by electrostatic forces and high surface energy, affects functionality. Liu et al. [159] emphasized that managing catalysts, solvents, and impurities is critical in nanoparticle production, particularly for pharmaceuticals. Inconsistent batches highlight the need for closed or automated systems to improve safety and cut costs.
Nanomaterials also pose significant toxicity concerns due to their small size, which facilitates penetration into human tissues and ecological systems [160]. Their toxic potential is influenced by their chemical composition, size, structure, and biological interactions. Accumulation in sensitive organs, like the lungs or brain, can lead substantial harm [161], including the generation of reactive oxygen species (ROS), oxidative stress, and cell damage [162]. For instance, silica nanoparticles may induce lipid peroxidation, membrane disruption, DNA damage, and even cell death [163].
Although polymeric nanoparticles are widely studied for medical applications, their neurotoxicity remains poorly understood. For example, Polysorbate 80 coated with chitosan nanoparticles accumulate in rat brains, causing neuronal death, inflammation, oxidative stress, and weight loss [164]. Likewise, gold nanoparticles contaminated with lipopolysaccharide disrupt human dendritic cell interactions [165]. Silver nanoparticles (AgNPs), widely utilized in pharmaceuticals and cosmetics, also warrant rigorous neurotoxicity assessment due to variable effects based on their size, morphology, surface chemistry, and cellular interactions [166]. Even bacterial responses to AgNP exhibit notable variability [167].
Nanomaterials pose potential risks to both human health and the environment. For instance, powdered carbon nanotubes (CNTs) are known to be hazardous [168], and toxic solvents used in metal-based catalysts can cause additional harm. Therefore, strict toxicity assessments and environmental monitoring throughout nanomaterial production are crucial [4].
Green synthesis, using bacteria, fungi, algae, and plants, offers a more eco-friendly and cost-effective alternative [169]. However, even green nanomaterials may mimic biomolecules and pose risks at high concentrations, with their toxicity still poorly understood. For example, AgNPs may damage tissue, DNA, and blood vessels [170].
Toxicity testing, both in vitro and in vivo, is crucial. Studies show that green-synthesized AgNPs and CuO NPs are often less toxic than their chemically synthesized counterparts, likely due to differences in size and composition [171,172]. To ensure safe use, regulators, industry experts, and researchers must collaborate to balance nanomaterial benefits against risks to safeguarding both public health and the environment.
5.3. Microfluidic Device for Enzyme Immobilization
Microfluidics (microreactors) are chip-based systems with micro- to nano-sized channels that process small chemical volumes [173]. They reduce reagent use, save space, and integrate multiple flow functions. Recent applications include enzyme-immobilized systems like wearable devices, microfluidic paper-based analytical devices (μPADs), and point-of-care testing for biomolecule detection, biocatalysis, and environmental monitoring [174]. However, industrial use is limited by complex cleanroom processes (e.g., photolithography, spin-coating) [175,176]. Emerging solutions include droplet parallelization [177] and soft lithography techniques.
Microfluidic enzyme reactors (μ-IMERs) provide key advantages over traditional systems, including faster mass/heat transfer, high efficiency, compact size, and enhanced safety [178]. Figure 6 shows the common types of enzyme-immobilized microfluidics, including wall-coated reactors, packed-bed reactors, and monolithic reactors, with enzymes immobilized in pores or channels [179]. These systems enable precise fluid control, improving biomolecule detection (e.g., nutrients, metabolites, contaminants) [180]. For example, a continuous-flow microreactor with poly-lysine-supported enzymes can detect hormones and drugs efficiently [181].
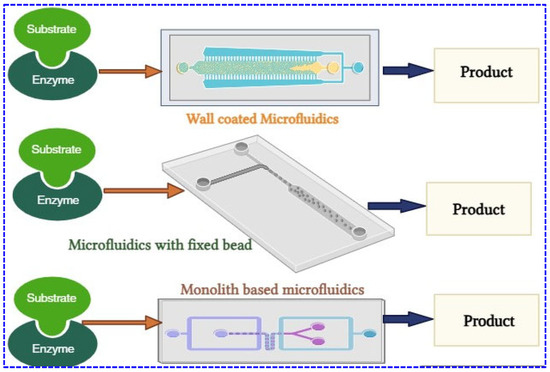
Figure 6.
Different types of enzyme-immobilized microfluidics.
Recent advances in enzyme immobilization and microfluidics have improved biosensors and yielded efficient bioremediation systems. For instance, cholesterol oxidase immobilized on gold electrodes using self-assembled monolayers (SAMs) achieved high sensitivity (93.75 mA/mM/cm2), a low detection limit (0.49 nM), and low sample use (<50 μL) [182]. Similarly, microfluidic epidermal patches with lactate oxidase biosensors accurately measured sweat lactate, while reducing pH and temperature interference [183]. Additionally, PEG hydrogel microstructures in microchannels yield fast enzyme assays, with response times under 10 min [184].
Wireless urea biosensors using nanocomposites (MBs-urease/GO/NiO) achieved high sensitivity (5.582 mV/(mg/dL), while paper-based microreactors (μPADs) detected trace pesticide residues (0.007 μg/mL) [185,186]. Immobilized enzymes also excel in environmental applications, e.g., a magnetic-nanoparticle microreactor removed 93% of dyes from waste water, and laccase-loaded microcapsules degraded 72% of acetaminophen in 30 min [187,188].
5.4. Self-Healing Hydrogels for Enzyme Immobilization
Hydrogels are 3D porous networks with high water absorption capacity due to their hydrophilic groups [189], making them ideal for enzymes in non-aqueous media [190]. For instance, enzyme can be encapsulated or covalently bound to polymers (e.g., cellulose, agarose, and dextran) via functional groups (e.g., amino, aldehyde, carboxyl, and epoxy), preventing leaching [191].
Hydrogels can be natural (hyaluronic acid, chitosan, heparin, alginate, gelatin) or synthetic; the natural hydrogels are biocompatible and biodegradable, and enhance tissue regeneration, but they lack the stability and strength of synthetic (PVA, polyethylene glycol, and sodium polyacrylate) types. Natural hydrogels are biocompatible and biodegradable but mechanically weaker, while synthetic variants offer greater rigidity and stability [192].
Hydrophilic polymers often expand in aqueous environments, leading to poor rigidity and enzyme leaching [114]. Cross-linkers like polyethylene glycol diacrylate (PEGDA) can mitigate these issues by forming highly cross-linked hydrogel networks under mild conditions, without requiring heat or solvents. High water content is beneficial for enzyme activity, but often leads to enzyme leakage, which can be reduced through co-polymerization with hydrophobic or covalent monomers [190].
Biobased hydrogels enhance enzyme immobilization, improving stability, reusability and catalytic activity. For example, sodium alginate grafted with acrylamide and glycidyl methacrylate immobilizes PersiXyn9 [193], while agarose–chitosan hydrogel cross-linked with N-hydroxy-succinimide (NHS) stabilizes horseradish peroxidase (HRP), doubling its activity [194]. Similarly, laccase [195] and alcohol dehydrogenase [196] show enhanced pollutant bioconversion and stability when immobilized on cross-linked hydrogels. Notably, SOD-coupled ferrocene in a PEGDA hydrogel enables precise tumor cell detection, with high stability and selectivity, in the monitoring of inflammatory byproducts [197]. Table 2 summarize the hydrogel-immobilized enzyme’s improved activities and applications.
5.5. Integration of Synthetic Biology and Immobilization
Enzyme immobilization improves traditional covalent immobilization by preventing random binding, thus safeguarding active sites. Strong chemical bonds reduce enzyme leakage and contamination [198]. However, only enzymes with reactive amino acids (e.g., Thr, Cys, and Lys) are suitable [199].

Table 2.
Summary of hydrogel-immobilized enzymes and their improved activities and applications.
Table 2.
Summary of hydrogel-immobilized enzymes and their improved activities and applications.
| Enzyme | Hydrogel | Improved Enzyme Activity | Application | Refs. |
|---|---|---|---|---|
| Xylanase | Superabsorbent hydrogels (SHs); carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide) hydrogel | Enhanced hydrolysis performance and reusability of the immobilized PersiXyn4. Improved the bleaching of paper pulp. | Hydrolysis of pulp paper to release reducing sugar. | [200] |
| Lipase | Bacterial cellulose (BC)–chitosan composite hydrogel | Highest thermal and operational stability and improved enzyme half-life time. | Hydrolysis of p-nitrophenyl butyrate to produce p-nitrophenol for drug, fungicide, and dye manufacturing. | [201] |
| Lipase | Polyvinyl alcohol/sodium alginate (PVA/Alg) hydrogel | Noticeable improvement in storage stability, limitation of enzyme leakage, improved enzyme affinity to the substrate, and catalytic efficiency. | Catalyzing ρ-nitrophenylphosphate(ρNPP) to produce p-nitrophenol, which is a chemical intermediate for the production of many substances. | [202] |
| Carbonyl reductase and lipase | Poly (2-hydroxyethyl methacrylate)–calcium magnesium carbonate (pHEMA-CaMg(CO3)2) hydrogel | Immobilized enzymes, maintaining over 80% catalytic activity after one month, with superior stability. | Aryl ketone reduction and esters hydrolysis. | [203] |
| β-mannanase | Sodium alginate-grafted-β-cyclodextrin | Enzyme was reused 15 times and retained its 70% activity, meanwhile it showed 60% activity after 30 days of storage at 4 °C. Increased the thermostability and half-life of the enzyme and reduced activation energy. | Enzymatic catalysis of locust bean gum (LBG) to produce mannose sugar. | [204] |
| Invertase | Poly (acrylamide/vinylsulfonic acid) PA/VSA and poly (acrylamide) PA hydrogels; | Maintained their stability over a wide pH and temperature range, with storage stability, after successive batch reaction. | Sucrose hydrolysis. | [205] |
| Cellulase | carboxymethyl cellulose grafted copolymers of 2-acrylamido-2methyl propane sulfonate and acrylamide (CMC-g-poly (AMPS-co-AAm)) hydrogels | Enzyme retained nearly 60% of its maximum activity at 90 °C, with storage stability. Enzyme showed 154.8% increase in conversion of alkaline-treated sugar beet pulp. | Hydrolyzing lignocellulosic biomass. | [206] |
| Cellulase | Carboxymethyl cellulose-based hydrogel | Stability in the presence of multiple chemicals, protected from enzyme aggregation, extending its industrial applications. | Lignocellulose hydrolysis of rice straw in harsh environment. Robust enzyme stability under extreme salt concentrations and high temperatures. | [207] |
Despite benefits like stability and reusability, challenges remain, including short half-lives, dependance on non-natural substrates, and decreased activity under harsh conditions. In situ immobilization, combining bioengineering and synthetic biology, offers a promising solution by enhancing the enzymes and carrier materials. Enzyme engineering further optimizes stability, activity, and resistance using protein engineering and recombinant DNA technology [208].
Advances in enzyme engineering, including directed evolution, metagenomics, rational design, and chemical modification, have optimized catalytic activity by targeting binding and active sites. Multi enzyme catalytic cascades further boost biosynthesis and biotransformation. For example, engineered xylanases exhibit improved thermal stability for use in animal feed and food [209].
Immobilization and site-directed mutagenesis enhance stability and enable controlled enzyme function [210]. A notable example is the engineering of 41 NOV1 dioxygenase variants, which achieved tenfold higher activity and greater stability than the native enzymes [211].
Recent advancements in enzyme engineering have been successfully integrated with immobilization techniques, enabling the development of more stable and active biocatalysts. Strain-promoted azide–alkyne cycloaddition (SPAAC) and artificial amino acid incorporation methods enhance enzyme performance, as demonstrated with lipase and amine transaminases (ATAs) [212,213]. Additionally, combining nanomaterials with novel vectors such as bacteriophage T4 capsid, directed evolution, and a chitin-functionalized cell-free protein synthesis systems improves enzyme stability and reusability for industrial applications [214,215,216].
Further advances include the development of robust biocatalysts for biodiesel production, such as a methanol-stable lipase from Geobacillus stearothermophilus T6 immobilized in ternary sol–gel matrices. This engineered mutant lipase showed improved thermal stability in methanol, with E. coli lysate proteins proving more effective than traditional stabilizers [217]. These innovations underscore the growing synergy between enzyme engineering and immobilization for industrial biocatalysis.
5.6. Carrier-Free Immobilized Enzymes
Carrier-free immobilized enzymes reduce costs by eliminating solid supports, using cross-linking agents to bind molecules, thereby enhancing enzyme stability and reusability [218]. These enzymes are typically smaller and more uniformly distributed in the solvent. The two primary carrier-free immobilization methods include cross-linked enzyme aggregates (CLEAs) and cross-linked enzyme crystals (CLECs) [219,220], with CLEAs being more widely applicable, as they do not require high enzyme purity.
In contrast to CLECs, CLEAs streamline the process by combining purification and immobilization. The preparation of CLEAs eliminates the need for solid supports by precipitating proteins and cross-linking them with a bifunctional chemical (such as glutaraldehyde) Despite being commonly utilized, glutaraldehyde can impair performance because of its toxicity and mass transfer limits [221]. Studies revealed that the carrier-free enzymes, such as type A feruloyl esterase, Candida rugosa lipase (CRL), and urase, exhibited high bioconversion rate, stability, and higher affinity and activity over a broad pH and temperature range [220,222,223,224]. Figure 7 illustrates two approaches for preparing CLEAs.
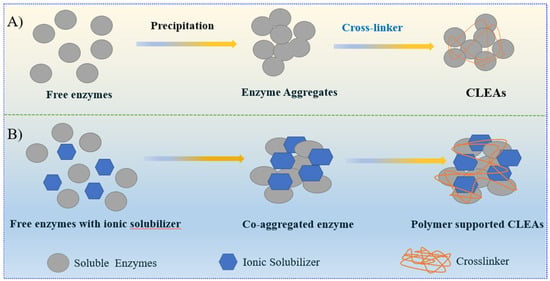
Figure 7.
Two different approaches for cross-linked enzyme aggregates. (A) Conventional CLEA formation; (B) Polymer-supported CLEA synthesis.
While CLEAs generally outperform carrier-bound enzymes in terms of activity, handling, and recovery, they face challenges such as poor reproducibility, mechanical instability, and difficulties in handling gelatinous forms [225]. Glutaraldehyde-based cross-linking can also reduce enzyme activity and environmental stability [226]. To address these limitations, hyperbranched polymer cross-linked laccase aggregates (HPCLEA) have been developed, which offer enhanced cross-linking sites and improved catalytic control due to their tunable internal structure [227].
Additional issues regarding CLEAs include their inconsistent particle size and cross-linking, which can limit enzyme performance. A Y-shaped millifluidic reactor was used to produce uniform cellulase CLEAs (200–400 nm) by optimizing reactant concentrations and flow rates [228]. Additionally, the dense structure of CLEAs limits their use with macromolecular substrates. To overcome this, porous co-precipitating enzymes with starch (later removed via amylase) or using dextran polyaldehyde as a cross-linker, significantly improved their activity toward macromolecular substrates [229].
5.7. 3D-Printing Technology for Enzyme Immobilization
3D printing (additive manufacturing) is an advanced fabrication method for creating complex, customizable structures with applications in tissue engineering, industrial design, and beyond [230]. This technology allows for precise control over enzyme loading and distribution, as well as the optimization of immobilization factors such as interconnectivity, surface contact, and porosity [231]. Additionally, it offers cost-effective production, intricate geometries, low material costs, reduced labor, scalability, and minimal waste, while avoiding toxic chemicals [232].
Extrusion-based printing, fused deposition modeling (FDM), and stereolithography are prominent 3D printing techniques for enzyme immobilization (SLA) [233]. Among these, FDM is widely favored due to its affordability, low material loss, and ease of material replacement [60]. Successful immobilization requires materials with chemical resistance, mechanical stability, non-toxicity, and enzyme compatibility [234].
The use of 3D printing is advancing enzyme immobilization across medical, biosensing, and industrial applications, including enzyme cascade reactions. For instance, FDM has been used to fabricate scaffolds for immobilizing four distinct enzymes [235], while 3D-printed nanocellulose scaffolds from soyabean and rice effectively immobilize sucrose synthase (SuSy) and C-glucosyltransferase (CGT) [236]. By optimizing scaffold properties of enzyme activity, efficiency and reproducibility can be significantly enhanced.
The integration of 3D printing with biocatalysis offers promising biotechnological applications. For instance, xylanase immobilized on 3D-printed sodium alginate (SA) microspheres efficiently degraded lignocellulose in corn cobs, demonstrating high catalytic activity and reusability, providing an ecofriendly alternative to conventional high-temperature acidic methods [237].
In environmental protection, 3D printing enables the creation of customized devices for removing toxic contaminants from water. Laccase has reportedly been immobilized by 3D printing using PLA scaffolds and an SLA-printed device [238]. The immobilized laccase showed promising potential, stability, and reusability in removing toxic pollutants like estrogen diclofenac and ethinylestradiol from waste water.
The use of 3D printing offers rapid, cost-effective bioelectrode production for medical applications. For example a 3D-printed microneedle biosensor enabled pain-free, continuous glucose monitoring in diabetic mice, a showing high correlation to commercial blood glucose meters [239]. Suitable materials, such as thermoplastics (e.g., PLA) and hydrogels (e.g., poly (ethylene glycol) diacrylate), enhance bioelectrode performance by supporting enzyme immobilization while maintaining activity and stability [240,241]. For instance, a hydrogel-based laccase immobilization system demonstrated remarkable stability and reusability in biodegradation processes [242].
5.8. AI-Based Enzyme Immobilization
Artificial intelligence (AI) and machine learning (ML) have been applied in pattern recognition, chemical process optimization, and the predictive modeling of unmeasured parameters [243]. Machine learning (ML), a subset of AI, enhances performance models by analyzing sample data and optimizing biocatalytic processes, predicting reaction conditions and designing enzyme variants [244].
The spatial and chemical distribution of immobilized enzymes is critical for performance, activity, and stability [245]. Imaging techniques such as atomic force microscopy, near-infrared fluorescence imaging, and mass spectrometry provide structural insights, but they also exhibit limitations. Near-infrared imaging requires labeling, which may alter the results, while mass spectroscopy can damage the samples [246]. ML aids in analyzing complex spectroscopic data, such as that obtained from label-free Raman microscopy, which captures vibrational data regarding enzymes and their supports. Raman hyperspectral imaging allows simultaneous chemical and spatial mapping, while unsupervised ML methods like non-negative matrix factorization (NMF) can resolve mixed chemical species [246,247].
Current techniques often fail to accurately assess the chemical and spatial distribution of immobilized enzymes, focusing only on surface visibility, stability, or activity. New analytical tools are needed, such as combining Raman hyperspectral imaging with principal component analysis (PCA) to study enzyme immobilization [248]. This approach enables the spatial and spectral resolution of enzymes on porous resins, distinguishing between enzyme, resin, and related chemical species, offering a novel approach to study immobilized biocatalysts [248].
Advances in proteomics, metabolomics, and genomics are accelerating ML integration into enzyme design. ML algorithms predict enzyme behavior, optimize enzyme immobilization conditions, and identify factors influencing enzymatic activity, aiding rational enzyme design [249,250]. For instance, lipase immobilization in microfluidic devices enhances stability, recyclability, and biodiesel production efficiency [250].
ML has also been used to predict enzyme MOF properties, such as reusability, activity retention, and enzyme loading. However, the diversity of MOFs and enzymes pose challenges. Random forest models improve predictions for immobilization yield and enzyme loading, although activity retention remains difficult to predict [251].
Raman hyperspectral imaging, combined with advanced analysis techniques like partial least squares discriminant analysis (PLS-DA) and NMF, has been used to identify variations in enzyme immobilization systems, with high accuracy in distinguishing samples [252,253].
ML uses sophisticated statistical techniques to refine models based on sample data; supervised, unsupervised, and reinforcement learning are among the common ML approaches [254]. Figure 8 explains how machine learning algorithms estimate the enzyme immobilization performance metrics of enzyme-nanocomposite biocatalysts. AI improves process control and automation by predicting unmeasured parameters, replacing hardware sensors, and enabling pattern recognition [243].
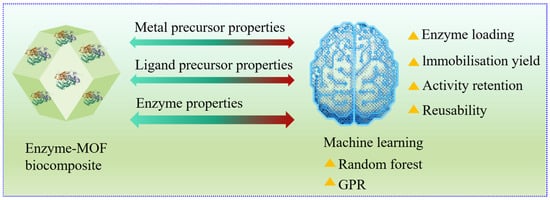
Figure 8.
The application of machine learning algorithms to estimate the enzyme immobilization performance metrics of enzyme-nanocomposite biocatalysts.
Supervised machine learning (ML) methods, such as artificial neural networks (ANN), help improve MOFs for adsorption and catalysis [255,256]. ML also aids in screening high performing MOFs and predicting unknown properties. For example, Ohno and Mukae [257] applied Gaussian process regression to link MOF structures with methane uptake, creating a model to identify the best structures for methane storage.
Recent progress involves automated ML tools like JAD Bio which analyze MOF structural features, such as linkers, metal clusters, and functional groups, alongside CO2 and H2 storage data [258]. These models predict storage capacities from structural traits, enabling efficient property estimation for new, untested MOFs.
Milton et al. [251] recently introduced ML to forecast critical enzyme–MOF composite properties, including enzyme loading, immobilization yield, activity retention, and reusability. Accurate prediction of these factors can streamline MOF substrate synthesis for enzyme immobilization, enhancing biocatalytic efficiency and reducing costs. The study also evaluated two ML models, the random forest method (RFM) and Gaussian process regression (GPR), demonstrating their ability to generalize predictions based on MOF metal/ligand properties and enzyme features. The sensitivity analysis identified the most influential parameters. The optimized model is then applied to predict outcomes for new experimental biocatalysis data outside the original training set.
ML also optimizes multienzyme configurations. For instance, Dixit et al. improved the combination of lipase, amylase, and endoglucanase (LIP-AMY-END) using an ANN with the Levenberg–Marquardt (LM) algorithm. This approach boosted endoglucanase activity by five-fold and enhanced deinking efficiency by 27.8% at 50 °C and 11% at 80 °C [259,260], respectively.
The enzymatic responses of Cndida antartica lipase B (calB) in porous acrylic resin were also investigated by Fard Masoumi et al. using a wavelet neural network (WNN) model [261]. The conversion rates were mostly influenced by the substrate molar ratio, and AI predictions highlighted how crucial it is to optimize the reaction parameters. In order to ensure maximum reaction efficiency, AI assisted in preventing the loss of immobilized enzyme activity.
The first ML-guided method for optimizing enzyme/zeolitic imidazolate framework (ZIF) synthesis for enhanced performance was most recently presented by Weibin L. et al. [262]. Their iterative ML workflow produced GOx/ZIF(G151) and HRP/ZIF (G150) composites, with overall performance indices (OPI = encapsulation efficiency × activity × stability) at least 1.3 times higher than prior benchmarks, using glucose oxidase (GOx) and horseradish peroxidase (HRP) as model enzymes and Zn (eIM)2 as the ZIF. The trained random forest model’s advanced statistical analysis also revealed important synthesis–structure performance correlations, offering vital information for next enzyme/ZIF research.
For enzyme immobilization, factor material selection, activation conditions, and reactor design significantly impact performance [263]. AI can optimize these parameters, enabling the design of more efficient immobilized enzymes. Additionally, AI can predict and adjust support materials or reaction conditions to maintain enzyme stability across cycles, minimizing activity loss [264]. Since immobilized enzymes often require different catalytic conditions, AI can also help predict optimal productivity and yield, advancing sustainable biocatalyst for biotech applications.
Overall, ML has significantly enhanced enzyme stability, effectiveness, and reusability in immobilization processes. However, challenges involving data quality, interpretability, and material limitations persist. Researchers are exploring various ML methods, including support vector machines (SVMs) and deep learning, to further improve immobilization techniques, while addressing concerns about generalization and algorithmic biases [249].
6. Concluding Remarks and Future Perspectives
Enzyme immobilization is advancing through innovations in nanomaterials, dynamic carrier systems, and artificial intelligence (AI), enhancing biocatalytic efficiency and sustainability. Technologies including metal–organic frameworks (MOFs), magnetic nanoparticles, and 3D-printed scaffolds improve enzyme stability and reusability, even under harsh conditions. AI and machine learning (ML) optimize enzyme–material interactions, immobilization protocols, and the screening of biocompatible nanomaterials. These advancements address challenges like mass transfer, enzyme leakage, and scalability, while enabling the creation of intelligent biocatalysts.
However, challenges remain, including nanomaterial toxicity, inconsistent synthesis, and environmental impact. The industrial scalability of microfluidics and AI-driven systems requires validation regarding cost-effectiveness and reproducibility. Future research should focus on eco-friendly hybrid materials, such as biodegradable polymer–MOF composites, to reduce ecological risks while maintaining catalytic performance. Optimizing multi-enzyme cascades with AI-driven simulations is also crucial for mimicking natural metabolic pathways.
Synthetic biology offers potential for engineering enzymes with specialized functions, enhanced through site-specific immobilization. AI-powered lifecycle assessments will be critical for evaluating sustainability. Interdisciplinary collaboration in computational biology, materials science, and green chemistry is essential to connect lab innovations with industry needs. The integration of 3D bioprinting, AI, and bio-based carriers will drive the development of biocatalytic platforms for precision medicine, biofuel production, and environmental detoxification, aligning with global sustainability goals.
Author Contributions
M.T.: writing—original draft. Y.L.: conceptualization, supervision, funding acquisition, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially funded by Entrusted projects by private enterprises (No. H2020111).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Kikani, B.; Patel, R.; Thumar, J.; Bhatt, H.; Rathore, D.S.; Koladiya, G.A.; Singh, S.P. Solvent tolerant enzymes in extremophiles: Adaptations and applications. Int. J. Biol. Macromol. 2023, 238, 124051. [Google Scholar] [CrossRef] [PubMed]
- Katchalski-Katzir, E. Immobilized enzymes—Learning from past successes and failures. Trends Biotechnol. 1993, 11, 471–478. [Google Scholar] [CrossRef]
- Khafaga, D.S.; Muteeb, G.; Elgarawany, A.; Aatif, M.; Farhan, M.; Allam, S.; Almatar, B.A.; Radwan, M.G. Green nanobiocatalysts: Enhancing enzyme immobilization for industrial and biomedical applications. PeerJ 2024, 12, e17589. [Google Scholar] [CrossRef]
- Robescu, M.S.; Bavaro, T. A comprehensive guide to enzyme immobilization: All you need to know. Molecules 2025, 30, 939. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.B. Recent advances in enzyme immobilisation strategies: An overview of techniques and composite carriers. J. Compos. Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Federsel, H.-J.; Moody, T.S.; Taylor, S.J. Recent trends in enzyme immobilization—Concepts for expanding the biocatalysis toolbox. Molecules 2021, 26, 2822. [Google Scholar] [CrossRef]
- Berillo, D.; Malika, T.; Baimakhanova, B.B.; Sadanov, A.K.; Berezin, V.E.; Trenozhnikova, L.P.; Baimakhanova, G.B.; Amangeldi, A.A.; Kerimzhanova, B. An Overview of Microorganisms Immobilized in a Gel Structure for the Production of Precursors, Antibiotics, and Valuable Products. Gels 2024, 10, 646. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Liu, Z. Enhanced activity of immobilized or chemically modified enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Khan, M.U.; Farid, A.; Liu, S.; Zhen, L.; Alahmad, K.; Chen, Z.; Kong, L. Innovative approaches for enzyme immobilization in milk processing: Advancements and industrial applications. Crit. Rev. Food Sci. Nutr. 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Al-sareji, O.J.; Meiczinger, M.; Somogyi, V.; Al-Juboori, R.A.; Grmasha, R.A.; Stenger-Kovács, C.; Jakab, M.; Hashim, K.S. Removal of emerging pollutants from water using enzyme-immobilized activated carbon from coconut shell. J. Environ. Chem. Eng. 2023, 11, 109803. [Google Scholar] [CrossRef]
- Vorvi, S.; Tsougeni, K.; Tserepi, A.; Kakabakos, S.; Petrou, P.; Gogolides, E. Enhanced Immobilization of Enzymes on Plasma Micro-Nanotextured Surfaces and Microfluidics: Application to HRP. Molecules 2024, 29, 4736. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, L.; Liang, H.; Chen, G.; Wu, J.; Xia, W.; Gao, D. Removal of benzo [a] pyrene from the soil by adsorption coupled with degradation on saponin-modified bentonite immobilized crude enzymes. Environ. Res. 2024, 261, 119716. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, M.; Yilmaz, S.; Karadag, E.; Sahiner, N. Enhanced enzymatic activity and stability by in situ entrapment of α-Glucosidase within super porous p (HEMA) cryogels during synthesis. Biotechnol. Rep. 2020, 28, e00534. [Google Scholar] [CrossRef]
- Ellis, G.A.; Díaz, S.A.; Medintz, I.L. Enhancing enzymatic performance with nanoparticle immobilization: Improved analytical and control capability for synthetic biochemistry. Curr. Opin. Biotechnol. 2021, 71, 77–90. [Google Scholar] [CrossRef]
- Cavalcante, F.T.; Cavalcante, A.L.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C. Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Attique, S.A.; Hussain, N.; Bilal, M.; Iqbal, H.M. Enzyme immobilization approaches. In Biocatalyst Immobilization; Elsevier: Amsterdam, The Netherlands, 2023; pp. 37–54. [Google Scholar]
- Hong, J.; Jung, D.; Park, S.; Oh, Y.; Oh, K.K.; Lee, S.H. Immobilization of laccase via cross-linked enzyme aggregates prepared using genipin as a natural cross-linker. Int. J. Biol. Macromol. 2021, 169, 541–550. [Google Scholar] [CrossRef]
- Venkataraman, S.; Vaidyanathan, V.K. Synthesis of magnetically recyclable porous cross-linked aggregates of Tramates versicolor MTCC 138 laccase for the efficient removal of pentachlorophenol from aqueous solution. Environ. Res. 2023, 229, 115899. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Rocha-Martin, J.; Santos, J.C.d. Designing carrier-free immobilized enzymes for biocatalysis. Front. Media SA 2022, 10, 924743. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Kaushal, D.; Dhiman, V.K.; Kanwar, S.S.; Singh, D.; Dhiman, V.K.; Pandey, H. An insight in developing carrier-free immobilized enzymes. Front. Bioeng. Biotechnol. 2022, 10, 794411. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.J.; Farajikhah, S.; Talebian, S.; Dehghani, F. Covalent immobilization: A review from an enzyme perspective. Chem. Eng. J. 2024, 503, 158054. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Remonatto, D.; Izidoro, B.F.; Mazziero, V.T.; Catarino, B.P.; do Nascimento, J.F.C.; Cerri, M.O.; Andrade, G.S.S.; de Paula, A.V. 3D printing and enzyme immobilization: An overview of current trends. Bioprinting 2023, 33, e00289. [Google Scholar] [CrossRef]
- Kujawa, J.; Głodek, M.; Li, G.; Al-Gharabli, S.; Knozowska, K.; Kujawski, W. Highly effective enzymes immobilization on ceramics: Requirements for supports and enzymes. Sci. Total Environ. 2021, 801, 149647. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Wang, X.; Xu, L.; Yan, Y. Biodiesel Synthesis Catalyzed by Burkholderia cenocepacia Lipase Supported on Macroporous Resin NKA in Solvent-Free and Isooctane Systems. Energy Fuels 2011, 25, 1206–1212. [Google Scholar] [CrossRef]
- Zahirinejad, S.; Hemmati, R.; Homaei, A.; Dinari, A.; Hosseinkhani, S.; Mohammadi, S.; Vianello, F. Nano-organic supports for enzyme immobilization: Scopes and perspectives. Colloids Surf. B Biointerfaces 2021, 204, 111774. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Devi, M.K.; Kumar, P.S. Advances in the application of immobilized enzyme for the remediation of hazardous pollutant: A review. Chemosphere 2022, 299, 134390. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.C.; de Souza e Castro, N.L.; Cipolatti, E.P.; Fernandez-Lafuente, R.; Manoel, E.A.; Freire, D.M.G.; Pinto, J.C. Effects of reaction operation policies on properties of core–shell polymer supports used for preparation of highly active biocatalysts. Macromol. React. Eng. 2019, 13, 1800055. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Zhang, J.; Hsu, Y.-C.; Lin, H.; Han, Z.; Pang, J.; Yang, Z.; Liang, R.-R.; Shi, W.; Zhou, H.-C. Bioinspired framework catalysts: From enzyme immobilization to biomimetic catalysis. Chem. Rev. 2023, 123, 5347–5420. [Google Scholar] [CrossRef] [PubMed]
- Prokopijević, M. Natural polymers: Suitable carriers for enzyme immobilization. Biol. Serbica 2021, 43, 43–49. [Google Scholar]
- Sasikanth, V.; Meganathan, B.; Rathinavel, T.; Seshachalam, S.; Nallappa, H.; Gopi, B. General overview of biopolymers: Structure and properties. Phys. Sci. Rev. 2024, 9, 1857–1883. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- MohammadKarimi, S.; Ershad-Langroudi, A.; Alizadegan, F. Surface analysis and water contact angle of modified natural biopolymers. In Handbook of Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2024; Volume 2, pp. 473–500. [Google Scholar]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R. A review on the modification of cellulose and its applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Jejurikar, A.; Seow, X.T.; Lawrie, G.; Martin, D.; Jayakrishnan, A.; Grøndahl, L. Degradable alginate hydrogels crosslinked by the macromolecular crosslinker alginate dialdehyde. J. Mater. Chem. 2012, 22, 9751–9758. [Google Scholar] [CrossRef]
- Intisar, A.; Hedar, M.; Sharif, A.; Ahmed, E.; Hussain, N.; Hadibarata, T.; Shariati, M.A.; Smaoui, S. Enzyme immobilization on alginate biopolymer for biotechnological applications. In Microbial Biomolecules; Elsevier: Amsterdam, The Netherlands, 2023; pp. 471–488. [Google Scholar]
- Bartolomeu, G.L. Alginate Hydrogels as a Substrate for Nerve Growth. Master’s Thesis, Universidade NOVA de Lisboa, Lisbon, Portugal, 2023. [Google Scholar]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Shojaei, F.; Homaei, A.; Taherizadeh, M.R.; Kamrani, E. Characterization of biosynthesized chitosan nanoparticles from Penaeus vannamei for the immobilization of P. vannamei protease: An eco-friendly nanobiocatalyst. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1413–1423. [Google Scholar]
- Wan, Y.; Zhou, J.; Ni, J.; Cai, Y.; Cohen Stuart, M.; Wang, J. Electrostatically mediated in situ polymerization for enzyme immobilization and activation. Biomacromolecules 2024, 25, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Bisht, M.; Thayallath, S.K.; Bharadwaj, P.; Franklin, G.; Mondal, D. Biomass-derived functional materials as carriers for enzymes: Towards sustainable and robust biocatalysts. Green Chem. 2023, 25, 4591–4624. [Google Scholar] [CrossRef]
- Kumar, N.K.M.F.; Meng, C.C.; Manas, N.H.A.; Ahmad, R.A.; Fuzi, S.F.Z.M.; Rahman, R.A.; Illias, R.M. Immobilization of maltogenic amylase in alginate-chitosan beads for improved enzyme retention and stability. Malays. J. Fundam. Appl. Sci. 2022, 18, 43–51. [Google Scholar] [CrossRef]
- Bai, Y.; Jing, Z.; Ma, R.; Wan, X.; Liu, J.; Huang, W. A critical review of enzymes immobilized on chitosan composites: Characterization and applications. Bioprocess Biosyst. Eng. 2023, 46, 1539–1567. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Chen, S.; Song, N.; Liao, X.; Shi, B. Immobilization of catalase on Fe (III) modified collagen fiber. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2011, 27, 1076–1081. [Google Scholar]
- Xin, M.; Zhang, X.; Yang, J.; Chen, M.; Ma, X.; Liu, J. Preparation and electrochemical sensing application of electrospun porous organosilica fibers. Electrochem. Commun. 2014, 48, 123–126. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef]
- Khan, R.S.; Rather, A.H.; Wani, T.U.; Rather, S.u.; Amna, T.; Hassan, M.S.; Sheikh, F.A. Recent trends using natural polymeric nanofibers as supports for enzyme immobilization and catalysis. Biotechnol. Bioeng. 2023, 120, 22–40. [Google Scholar] [CrossRef]
- Shen, Q.; Yang, R.; Hua, X.; Ye, F.; Zhang, W.; Zhao, W. Gelatin-templated biomimetic calcification for β-galactosidase immobilization. Process Biochem. 2011, 46, 1565–1571. [Google Scholar] [CrossRef]
- Ceniceros, E.S.; Ilyina, A.; Esquivel, J.C.; Menchaca, D.R.; Espinoza, J.F.; Rodriguez, O.M. Entrapment of enzymes in natural polymer extracted from residue of food industry: Preparation methods, partial characterisation and possible application. Becth Mock 2003, 44, 84–87. [Google Scholar]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Matto, M.; Husain, Q. Entrapment of porous and stable concanavalin A–peroxidase complex into hybrid calcium alginate–pectin gel. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 1316–1323. [Google Scholar] [CrossRef]
- Prokopijevic, M.; Prodanovic, O.; Spasojevic, D.; Kovacevic, G.; Polovic, N.; Radotic, K.; Prodanovic, R. Tyramine-modified pectins via periodate oxidation for soybean hull peroxidase induced hydrogel formation and immobilization. Appl. Microbiol. Biotechnol. 2017, 101, 2281–2290. [Google Scholar] [CrossRef]
- Satar, R.; Matto, M.; Husain, Q. Studies on calcium alginate-pectin gel entrapped concanavalin A-bitter gourd (Momordica charantia) peroxidase complex. J. Sci. Ind. Res. 2008, 67, 609–615. [Google Scholar]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Jung, K.; Corrigan, N.; Wong, E.H.; Boyer, C. Bioactive synthetic polymers. Adv. Mater. 2022, 34, 2105063. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef]
- Fromager, B.; Marhuenda, E.; Louis, B.; Bakalara, N.; Cambedouzou, J.; Cornu, D. Recent Advances in Electrospun Fibers for Biological Applications. Macromol 2023, 3, 569–613. [Google Scholar] [CrossRef]
- Guajardo, N. Immobilization of lipases using poly (vinyl) alcohol. Polymers 2023, 15, 2021. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of enzymes by polymeric materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Panatdasirisuk, W.; Faungnawakij, K.; Champreda, V. Novel fabrication via one-step polymerization and alginate cage-assisted shaping technique for polyacrylamide beads as a highly stable biocatalyst for xylan hydrolysis. React. Funct. Polym. 2023, 190, 105641. [Google Scholar] [CrossRef]
- Muthukumar, J.; Kandukuri, V.A.; Chidambaram, R. A critical review on various treatment, conversion, and disposal approaches of commonly used polystyrene. Polym. Bull. 2024, 81, 2819–2845. [Google Scholar] [CrossRef]
- Sánchez-Otero, M.G.; Quintana-Castro, R.; Rojas-Vázquez, A.S.; Badillo-Zeferino, G.L.; Mondragón-Vázquez, K.; Espinosa-Luna, G.; Kumar Nadda, A.; Oliart-Ros, R.M. Polypropylene as a selective support for the immobilization of lipolytic enzymes: Hyper-activation, purification and biotechnological applications. J. Chem. Technol. Biotechnol. 2022, 97, 436–445. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible synthetic polymers for tissue engineering purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef]
- Groh, K.J.; Arp, H.P.H.; MacLeod, M.; Wang, Z. Assessing and managing environmental hazards of polymers: Historical development, science advances and policy options. Environ. Sci. Process. Impacts 2023, 25, 10–25. [Google Scholar] [CrossRef]
- Nigro, L.; Magni, S.; Ortenzi, M.A.; Gazzotti, S.; Signorini, S.G.; Sbarberi, R.; Della Torre, C.; Binelli, A. Assessment of behavioural effects of three water-soluble polymers in zebrafish embryos. Sci. Total Environ. 2023, 893, 164843. [Google Scholar] [CrossRef]
- Mtibe, A.; Motloung, M.P.; Bandyopadhyay, J.; Ray, S.S. Synthetic biopolymers and their composites: Advantages and limitations—An overview. Macromol. Rapid Commun. 2021, 42, 2100130. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, D.Y.; Jeong, T.S.; Park, Y.S. Micro-and Nano-Plastic-Induced Adverse Health Effects on Lungs and Kidneys Linked to Oxidative Stress and Inflammation. Life 2025, 15, 392. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef] [PubMed]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [PubMed]
- Aaliya, B.; Sunooj, K.V.; Lackner, M. Biopolymer composites: A review. Int. J. Biobased Plast. 2021, 3, 40–84. [Google Scholar] [CrossRef]
- Das, B.; Patra, S. Some recent innovations related to enzyme immobilization. In Biocatalyst Immobilization; Elsevier: Amsterdam, The Netherlands, 2023; pp. 149–163. [Google Scholar]
- Lakhani, P.; Bhanderi, D.; Modi, C.K. Silica-supported ionic liquids as versatile catalysts: A case study. J. Mol. Liq. 2024, 408, 125306. [Google Scholar] [CrossRef]
- Telange, D.R.; Pethe, A.M.; Agrawal, S.S.; Telrandhe, U.B.; Bobade, P.S. Functionalized Magnetic Nanoparticles for Enzyme Immobilization. In Functionalized Magnetic Nanoparticles for Theranostic Applications; Wiley: Hoboken, NJ, USA, 2024; pp. 319–346. [Google Scholar]
- Zou, B.; Hu, Y.; Cui, F.; Jiang, L.; Yu, D.; Huang, H. Effect of surface modification of low cost mesoporous SiO2 carriers on the properties of immobilized lipase. J. Colloid Interface Sci. 2014, 417, 210–216. [Google Scholar] [CrossRef]
- Vallés, D.; Furtado, S.; Villadóniga, C.; Cantera, A.M.B. Adsorption onto alumina and stabilization of cysteine proteinases from crude extract of Solanum granuloso-leprosum fruits. Process Biochem. 2011, 46, 592–598. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds–current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Brask, J.; Mohammadi, M. Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem. Eng. J. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Thenmozhi, K.; Narayanan, S.S. Horseradish peroxidase and toluidine blue covalently immobilized leak-free sol-gel composite biosensor for hydrogen peroxide. Mater. Sci. Eng. C 2017, 70, 223–230. [Google Scholar] [CrossRef]
- Gao, J.; Feng, K.; Li, H.; Jiang, Y.; Zhou, L. Immobilized lipase on porous ceramic monoliths for the production of sugar-derived oil gelling agent. RSC Adv. 2015, 5, 68601–68609. [Google Scholar] [CrossRef]
- de la Fuente-Jiménez, J.L.; Korgel, B.A.; Ulises, A.; Sharmal, A. Drug-delivery using inorganic and organic nanoparticles. In Nanochemistry; CRC Press: Boca Raton, FL, USA, 2023; pp. 194–228. [Google Scholar]
- Yang, X.-Y.; Tian, G.; Jiang, N.; Su, B.-L. Immobilization technology: A sustainable solution for biofuel cell design. Energy Environ. Sci. 2012, 5, 5540–5563. [Google Scholar] [CrossRef]
- Riccardi, C.M.; Kasi, R.M.; Kumar, C.V. Nanoarmoring of enzymes by interlocking in cellulose fibers with poly (acrylic acid). In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 590, pp. 475–500. [Google Scholar]
- Kara, F.; Aksoy, E.A.; Calamak, S.; Hasirci, N.; Aksoy, S. Immobilization of heparin on chitosan-grafted polyurethane films to enhance anti-adhesive and antibacterial properties. J. Bioact. Compat. Polym. 2016, 31, 72–90. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liang, Y.; Jiang, Z.; Jiang, Y.; Zhang, L. Facile fabrication of organic–inorganic hybrid beads by aminated alginate enabled gelation and biomimetic mineralization. J. Biomater. Sci. Polym. Ed. 2013, 24, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Vatsyayan, P.; Bordoloi, S.; Goswami, P. Large catalase based bioelectrode for biosensor application. Biophys. Chem. 2010, 153, 36–42. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.-L.; Zink, J.I.; Stoddart, J.F. Mechanized silica nanoparticles: A new frontier in theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef]
- Yuce-Dursun, B.; Cigil, A.B.; Dongez, D.; Kahraman, M.V.; Ogan, A.; Demir, S. Preparation and characterization of sol–gel hybrid coating films for covalent immobilization of lipase enzyme. J. Mol. Catal. B Enzym. 2016, 127, 18–25. [Google Scholar] [CrossRef]
- Singthong, J.; Thongkaew, C. Using hydrocolloids to decrease oil absorption in banana chips. LWT-Food Sci. Technol. 2009, 42, 1199–1203. [Google Scholar] [CrossRef]
- Mai, T.H.A. Biochemical studies on the immobilized lactase in the combined alginate–carboxymethyl cellulose gel. Biochem. Eng. J. 2013, 74, 81–87. [Google Scholar] [CrossRef]
- Holyavka, M.; Redko, Y.; Goncharova, S.; Lavlinskaya, M.; Sorokin, A.; Kondratyev, M.; Artyukhov, V. Novel Hybrid Catalysts of Cysteine Proteases Enhanced by Chitosan and Carboxymethyl Chitosan Micro-and Nanoparticles. Polymers 2024, 16, 3111. [Google Scholar] [CrossRef]
- Selloum, D.; Abou Chaaya, A.; Bechelany, M.; Rouessac, V.; Miele, P.; Tingry, S. A highly efficient gold/electrospun PAN fiber material for improved laccase biocathodes for biofuel cell applications. J. Mater. Chem. A 2014, 2, 2794–2800. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Lu, K.; Krause, W.E.; Lucia, L.A.; Wei, Q. Laccase immobilized on PAN/O-MMT composite nanofibers support for substrate bioremediation: A de novo adsorption and biocatalytic synergy. RSC Adv. 2016, 6, 41420–41427. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Nawaz, M.A.; Pervez, S.; Jamal, M.; Attaullah, M.; Aman, A.; Qader, S.A.U. Encapsulation of pectinase within polyacrylamide gel: Characterization of its catalytic properties for continuous industrial uses. Heliyon 2020, 6, e04578. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly (ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Li, X.Y.; Yu, S.Y.; Park, H.J.; Zhao, M. Polyethyleneglycol diacrylate microspheres: A novel carrier for laccase immobilisation. J. Microencapsul. 2015, 32, 22–28. [Google Scholar] [CrossRef]
- Choi, D.; Lee, W.; Park, J.; Koh, W. Preparation of poly (ethylene glycol) hydrogels with different network structures for the application of enzyme immobilization. Bio-Med. Mater. Eng. 2008, 18, 345–356. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, A.; Wen, X. Enhanced stability and catalytic performance of immobilized phospholipase D on chitosan-encapsulated magnetic nanoparticles using oxidized dextran and glutaraldehyde as cross-linkers. Biochem. Eng. J. 2024, 212, 109499. [Google Scholar] [CrossRef]
- Zhang, J.; Lovell, J.F.; Shi, J.; Zhang, Y. Nanomaterials for co-immobilization of multiple enzymes. BMEMat 2024, 3, e12080. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.S.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef]
- Poorakbar, E.; Saboury, A.A.; Laame Rad, B.; Khoshnevisan, K. Immobilization of cellulase onto core-shell magnetic gold nanoparticles functionalized by aspartic acid and determination of its activity. Protein J. 2020, 39, 328–336. [Google Scholar] [CrossRef]
- Kumar, A.; Dutt, R.; Srivastava, A.; Kayastha, A.M. Immobilization of α-amylase onto functionalized molybdenum diselenide nanoflowers (MoSe2-NFs) as scaffolds: Characterization, kinetics, and potential applications in starch-based industries. Food Chem. 2024, 442, 138431. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Kaczorek, E.; Jesionowski, T. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: Effect of process conditions and catalytic pathways. Catal. Today 2020, 348, 127–136. [Google Scholar] [CrossRef]
- Chen, H.Y.; Cheng, K.C.; Hsu, R.J.; Hsieh, C.W.; Wang, H.T.; Ting, Y. Enzymatic degradation of ginkgolic acid by laccase immobilized on novel electrospun nanofiber mat. J. Sci. Food Agric. 2020, 100, 2705–2712. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef]
- Jankowska, K.; Su, Z.; Sigurdardóttir, S.B.; Staszak, M.; Pinelo, M.; Zdarta, J.; Jesionowski, T. Tailor-made novel electrospun polystyrene/poly (d, l-lactide-co-glycolide) for oxidoreductases immobilization: Improvement of catalytic properties under extreme reaction conditions. Bioorg. Chem. 2021, 114, 105036. [Google Scholar] [CrossRef]
- Xu, C.; Tong, S.; Sun, L.; Gu, X. Cellulase immobilization to enhance enzymatic hydrolysis of lignocellulosic biomass: An all-inclusive review. Carbohydr. Polym. 2023, 321, 121319. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-coated magnetite nanoparticles for protein immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. Exploring current tendencies in techniques and materials for immobilization of laccases–A review. Int. J. Biol. Macromol. 2021, 181, 683–696. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Bell, D.J.; Wiese, M.; Schönberger, A.A.; Wessling, M. Catalytically Active Hollow Fiber Membranes with Enzyme-Embedded Metal–Organic Framework Coating. Angew. Chem. 2020, 132, 16181–16187. [Google Scholar] [CrossRef]
- Jabeen, R.; Ali, N.; Tajwar, M.A.; Liu, Y.; Luo, D.; Li, D.; Qi, L. Encapsulation of an enzyme-immobilized smart polymer membrane in a metal–organic framework for enhancement of catalytic performance. J. Mater. Chem. B 2024, 12, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Poorakbar, E.; Shafiee, A.; Saboury, A.A.; Rad, B.L.; Khoshnevisan, K.; Ma’mani, L.; Derakhshankhah, H.; Ganjali, M.R.; Hosseini, M. Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: Improvement of enzymatic activity and thermal stability. Process Biochem. 2018, 71, 92–100. [Google Scholar] [CrossRef]
- Ahmed, I.N.; Yang, X.-L.; Dubale, A.A.; Li, R.-F.; Ma, Y.-M.; Wang, L.-M.; Hou, G.-H.; Guan, R.-F.; Xie, M.-H. Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks. Bioresour. Technol. 2018, 270, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.-J.; Lee, W.; Oh, Y.-S.; Lee, J.-K. Magnetic nanoparticles as a catalyst vehicle for simple and easy recycling. New J. Chem. 2003, 27, 227–229. [Google Scholar] [CrossRef]
- Oke, M.; Ojo, S.; Fasiku, S.; Adebayo, E. Nanotechnology and enzyme immobilization: A review. Nanotechnology 2023, 34, 385101. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, B.; Dong, S.; Tian, L.; Bai, Q. Preparation of core–shell structure Fe3O4@ SiO2 superparamagnetic microspheres immoblized with iminodiacetic acid as immobilized metal ion affinity adsorbents for his-tag protein purification. Biomed. Chromatogr. 2016, 30, 566–573. [Google Scholar] [CrossRef]
- Zanuso, E.; Ruiz, H.A.; Domingues, L.; Teixeira, J.A. Magnetic nanoparticles as support for cellulase immobilization strategy for enzymatic hydrolysis using hydrothermally pretreated corn cob biomass. BioEnergy Res. 2022, 15, 1946–1957. [Google Scholar] [CrossRef]
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Thermostable cellulose saccharifying microbial enzymes: Characteristics, recent advances and biotechnological applications. Int. J. Biol. Macromol. 2021, 188, 226–244. [Google Scholar] [CrossRef]
- Paz-Cedeno, F.R.; Carceller, J.M.; Iborra, S.; Donato, R.K.; Godoy, A.P.; de Paula, A.V.; Monti, R.; Corma, A.; Masarin, F. Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: Ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renew. Energy 2021, 164, 491–501. [Google Scholar] [CrossRef]
- Almajanni, Y.; Amiri, H.; Taheri-Kafrani, A. Efficient and cost-effective biodegradation of phenolic compounds in lignocellulosic biomasses using laccase immobilized onto magnetically recoverable nanocellulose-functionalized iron-oxide nanoparticles. Ind. Crops Prod. 2025, 223, 120256. [Google Scholar] [CrossRef]
- Herman, R.A.; Zhu, X.; Ayepa, E.; Khurshid, M.; Zhang, Z.-P.; You, S.; Qian, J.-F.; Wang, J. Magnetic Janus SiO2 nanoparticles immobilized protease mutant T70I as a novel clarification agent for juice processing. Int. J. Biol. Macromol. 2025, 292, 139327. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, M.; Habibi, A. Improved activity and stability of cellulase by immobilization on Fe3O4 nanoparticles functionalized with Reactive Red 120. Int. J. Biol. Macromol. 2025, 295, 139624. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; Ali, S.S.; Matter, I.A.; Elsamahy, T.; Mahmoud, Y.A. Enzymes immobilization onto magnetic nanoparticles to improve industrial and environmental applications. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 630, pp. 481–502. [Google Scholar]
- Cavalcante, A.L.G.; Dari, D.N.; da Silva Aires, F.I.; de Castro, E.C.; Dos Santos, K.M.; Dos Santos, J.C.S. Advancements in enzyme immobilization on magnetic nanomaterials: Toward sustainable industrial applications. RSC Adv. 2024, 14, 17946–17988. [Google Scholar] [CrossRef]
- Akpinar, I.; Wang, X.; Fahy, K.; Sha, F.; Yang, S.; Kwon, T.-w.; Das, P.J.; Islamoglu, T.; Farha, O.K.; Stoddart, J.F. Biomimetic Mineralization of Large Enzymes Utilizing a Stable Zirconium-Based Metal-Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 5108–5117. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, R.; Yang, M.; Zheng, Y.; Zhang, Z.; Chen, Y. A Dynamic Defect Generation Strategy for Efficient Enzyme Immobilization in Robust Metal-Organic Frameworks for Catalytic Hydrolysis and Chiral Resolution. Angew. Chem. Int. Ed. 2023, 62, e202302436. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Wang, Y.; Pang, Y.; Lou, H.; Li, Z.; Yang, D.; Qiu, X. Facile Construction of Stable Hierarchical Porous Metal–Organic Frameworks for In Situ Immobilization of β-Glucosidase Based on Competitive Coordination and Selective Etching Strategies. ACS Sustain. Chem. Eng. 2024, 12, 6728–6737. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Wang, X.; Li, Q.; Xu, S.; Sun, C.; Suo, H. Encapsulation of Immobilized β-Glucosidase with Calcium Metal–Organic Frameworks for Enhanced Stability in Hydrolysis of Cellobiose. Langmuir 2024, 40, 18727–18735. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Song, J.; Zhang, Y.; Nian, B.; Hu, Y. Spacer arm of ionic liquids facilitated laccase immobilization on magnetic graphene enhancing its stability and catalytic performance. Chemosphere 2024, 362, 142735. [Google Scholar] [CrossRef]
- Bilal, M.; Nguyen, T.A.; Iqbal, H.M. Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization–a paradigm shift in biocatalyst design. Coord. Chem. Rev. 2020, 422, 213475. [Google Scholar] [CrossRef]
- Kumari, A.; Rajeev, R.; Benny, L.; Sudhakar, Y.; Varghese, A.; Hegde, G. Recent advances in carbon nanotubes-based biocatalysts and their applications. Adv. Colloid Interface Sci. 2021, 297, 102542. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Gorab, M.G.; Hashemi, S.M.; Javanshir, S.; Cohan, R.A. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Babadi, A.A.; Fakhlaei, R.; Rahmati, S.; Wang, S.; Basirun, W.J. A high-power hybrid carbon nanotube/three-dimensional reduced graphene oxide glucose/O2 enzymatic biofuel cell. Electrochim. Acta 2024, 506, 145054. [Google Scholar] [CrossRef]
- Zhang, W.; Singh, R.; Wang, Z.; Li, G.; Xie, Y.; Jha, R.; Marques, C.; Zhang, B.; Kumar, S. Humanoid shaped optical fiber plasmon biosensor functionalized with graphene oxide/multi-walled carbon nanotubes for histamine detection. Opt. Express 2023, 31, 11788–11803. [Google Scholar] [CrossRef] [PubMed]
- Aparna, G.; Tetala, K.K. Recent progress in development and application of DNA, protein, peptide, glycan, antibody, and aptamer microarrays. Biomolecules 2023, 13, 602. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Gao, X.; Jiang, J.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y. DNA directed immobilization of horseradish peroxidase on phase-transitioned lysozyme modified TiO2 for efficient degradation of phenol in wastewater. Mater. Des. 2021, 201, 109463. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Khan, S.; Burciu, B.; Filipe, C.D.; Li, Y.; Dellinger, K.; Didar, T.F. DNAzyme-based biosensors: Immobilization strategies, applications, and future prospective. ACS Nano 2021, 15, 13943–13969. [Google Scholar] [CrossRef]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef]
- Mostafavi, M.; Poor, M.B.; Habibi, Z.; Mohammadi, M.; Yousefi, M. Hyperactivation of lipases by immobilization on superhydrophobic graphene quantum dots inorganic hybrid nanoflower. Int. J. Biol. Macromol. 2024, 254, 127817. [Google Scholar] [CrossRef]
- Jamal, H.S.; Raja, R.; Ahmed, S.; Yesiloz, G.; Ali, S.A. Immobilization of collagenase in inorganic hybrid nanoflowers with enhanced stability, proteolytic activity, and their anti-amyloid potential. Int. J. Biol. Macromol. 2024, 274, 133114. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, X.; Kong, D.; Li, G.; Li, Q. Immobilization of thermophilic lipase in inorganic hybrid nanoflower through biomimetic mineralization. Colloids Surf. B Biointerfaces 2021, 197, 111450. [Google Scholar] [CrossRef] [PubMed]
- Badoei-Dalfard, A.; Monemi, F.; Hassanshahian, M. One-pot synthesis and biochemical characterization of a magnetic collagenase nanoflower and evaluation of its biotechnological applications. Colloids Surf. B Biointerfaces 2022, 211, 112302. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Appiah, B.; Zhang, B.-W.; Yang, Z.-H.; Quan, C. Recent advances in enzyme immobilization based on nanoflowers. J. Catal. 2023, 418, 31–39. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Wang, A.; Li, N.; Chen, X.; Pei, X.; Zhang, P.; Wu, S.G. Dual-cycle immobilization to reuse both enzyme and support by reblossoming enzyme–inorganic hybrid nanoflowers. RSC Adv. 2018, 8, 16088–16094. [Google Scholar] [CrossRef]
- Bosu, S.; Pooja, R.; Rajasimman, M. Role of nanomaterials in enhanced ethanol production through biological methods–review on operating factors and machine learning applications. Fuel 2022, 320, 123905. [Google Scholar] [CrossRef]
- Pérez-Hernández, H.; Pérez-Moreno, A.; Sarabia-Castillo, C.; García-Mayagoitia, S.; Medina-Pérez, G.; López-Valdez, F.; Campos-Montiel, R.; Jayanta-Kumar, P.; Fernández-Luqueño, F. Ecological drawbacks of nanomaterials produced on an industrial scale: Collateral effect on human and environmental health. Water Air Soil Pollut. 2021, 232, 435. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Zhu, Z.; Cui, C.; Bai, Y.; Gao, J.; Jiang, Y.; Li, B.; Wang, Y.; Zhang, Q.; Qian, W.; Wei, F. Advances in precise structure control and assembly toward the carbon nanotube industry. Adv. Funct. Mater. 2022, 32, 2109401. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Lin, X.; Tang, Y.; Liu, Y.; Wang, T.; Zhu, H. The electronic properties of chiral carbon nanotubes. Comput. Mater. Sci. 2017, 129, 290–294. [Google Scholar] [CrossRef]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. View 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Mubeen, B.; Hasnain, A.; Wang, J.; Zheng, H.; Naqvi, S.A.H.; Prasad, R.; Rehman, A.u.; Sohail, M.A.; Hassan, M.Z.; Farhan, M. Current progress and open challenges for combined toxic effects of manufactured nano-sized objects (MNO’s) on soil biota and microbial community. Coatings 2023, 13, 212. [Google Scholar] [CrossRef]
- Gupta Ramesh, C. Veterinary Toxicology: Basic and Clinical Principles; Academic Press/Elsevier: San Diego, CA, USA, 2018. [Google Scholar]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Siddiqui, H.; Patil, G.; Ashquin, M.; Ahmad, I. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology 2010, 276, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-Y.; Hu, Y.-L.; Gao, J.-Q. Brain localization and neurotoxicity evaluation of polysorbate 80-modified chitosan nanoparticles in rats. PLoS ONE 2015, 10, e0134722. [Google Scholar] [CrossRef]
- Vallhov, H.; Qin, J.; Johansson, S.M.; Ahlborg, N.; Muhammed, M.A.; Scheynius, A.; Gabrielsson, S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006, 6, 1682–1686. [Google Scholar] [CrossRef]
- Strużyńska, L.; Skalska, J. Mechanisms underlying neurotoxicity of silver nanoparticles. In Cellular and Molecular Toxicology of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2018; pp. 227–250. [Google Scholar]
- Adegboyega, N.F.; Sharma, V.K.; Siskova, K.M.; Vecerova, R.; Kolar, M.; Zboril, R.; Gardea-Torresdey, J.L. Enhanced formation of silver nanoparticles in Ag+-NOM-iron (II, III) systems and antibacterial activity studies. Environ. Sci. Technol. 2014, 48, 3228–3235. [Google Scholar] [CrossRef]
- Rezaei Cherati, S.; Anas, M.; Liu, S.; Shanmugam, S.; Pandey, K.; Angtuaco, S.; Shelton, R.; Khalfaoui, A.N.; Alena, S.V.; Porter, E. Comprehensive risk assessment of carbon nanotubes used for agricultural applications. ACS Nano 2022, 16, 12061–12072. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Baadhe, R.R. Green synthesis of novel Ag–Cu and Ag–Znbimetallic nanoparticles and their in vitro biological, eco-toxicity and catalytic studies. Nano-Struct. Nano-Objects 2021, 26, 100687. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Green synthesis protocols, toxicity, and recent progress in nanomaterial-based for environmental chemical sensors applications. Trends Environ. Anal. Chem. 2023, 39, e00204. [Google Scholar] [CrossRef]
- Sabeena, G.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. Green and chemical synthesis of CuO nanoparticles: A comparative study for several in vitro bioactivities and in vivo toxicity in zebrafish embryos. J. King Saud Univ.-Sci. 2022, 34, 102092. [Google Scholar]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Miyazaki, M. Enzyme-immobilized microfluidic devices for biomolecule detection. TrAC Trends Anal. Chem. 2023, 159, 116908. [Google Scholar] [CrossRef]
- Enck, K.; Rajan, S.P.; Aleman, J.; Castagno, S.; Long, E.; Khalil, F.; Hall, A.R.; Opara, E.C. Design of an adhesive film-based microfluidic device for alginate hydrogel-based cell encapsulation. Ann. Biomed. Eng. 2020, 48, 1103–1111. [Google Scholar] [CrossRef]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.; Demello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Jeong, H.-H.; Issadore, D.; Lee, D. Recent developments in scale-up of microfluidic emulsion generation via parallelization. Korean J. Chem. Eng. 2016, 33, 1757–1766. [Google Scholar] [CrossRef]
- Tabeling, P. Introduction to Microfluidics; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Nagy, C.; Szabo, R.; Gaspar, A. Microfluidic immobilized enzymatic reactors for proteomic analyses—Recent developments and trends (2017–2021). Micromachines 2022, 13, 311. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Moreira, M.; Feijoo, G.; Lema, J.; Miyazaki, M. Improving the catalytic performance of laccase using a novel continuous-flow microreactor. Chem. Eng. J. 2013, 223, 497–506. [Google Scholar] [CrossRef]
- Honda, T.; Miyazaki, M.; Yamaguchi, Y.; Nakamura, H.; Maeda, H. Integrated microreaction system for optical resolution of racemic amino acids. Lab A Chip 2007, 7, 366–372. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asiri, A.M. One-step electrochemical detection of cholesterol in the presence of suitable K3Fe (CN) 6/phosphate buffer mediator by an electrochemical approach. Talanta 2015, 140, 96–101. [Google Scholar] [CrossRef]
- Xuan, X.; Perez-Rafols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate biosensing for reliable on-body sweat analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.-G.; Pishko, M. Immobilization of multi-enzyme microreactors inside microfluidic devices. Sens. Actuators B Chem. 2005, 106, 335–342. [Google Scholar] [CrossRef]
- Chou, J.-C.; Wu, C.-Y.; Lin, S.-H.; Kuo, P.-Y.; Lai, C.-H.; Nien, Y.-H.; Wu, Y.-X.; Lai, T.-Y. The analysis of the urea biosensors using different sensing matrices via wireless measurement system & microfluidic measurement system. Sensors 2019, 19, 3004. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, T.; Zheng, Z.; Jing, H.; Liu, C. Improving pesticide residue detection: Immobilized enzyme microreactor embedded in microfluidic paper-based analytical devices. Food Chem. 2024, 439, 138179. [Google Scholar] [CrossRef] [PubMed]
- Peñaranda, P.A.; Noguera, M.J.; Florez, S.L.; Husserl, J.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Treatment of wastewater, phenols and dyes using novel magnetic torus microreactors and laccase immobilized on magnetite nanoparticles. Nanomaterials 2022, 12, 1688. [Google Scholar] [CrossRef]
- Sotelo, L.D.; Sotelo, D.C.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Comparison of acetaminophen degradation by laccases immobilized by two different methods via a continuous flow microreactor process scheme. Membranes 2022, 12, 298. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional cross linked hydrophilic polymeric network “hydrogels”: An agriculture boom. Agric. Water Manag. 2021, 253, 106939. [Google Scholar] [CrossRef]
- Meyer, J.; Meyer, L.E.; Kara, S. Enzyme immobilization in hydrogels: A perfect liaison for efficient and sustainable biocatalysis. Eng. Life Sci. 2022, 22, 165–177. [Google Scholar] [CrossRef]
- Krishna, B.L.; Singh, A.N.; Patra, S.; Dubey, V.K. Purification, characterization and immobilization of urease from Momordica charantia seeds. Process Biochem. 2011, 46, 1486–1491. [Google Scholar] [CrossRef]
- Jeong, K.-H.; Park, D.; Lee, Y.-C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: A mini-review. J. Polym. Res. 2017, 24, 112. [Google Scholar] [CrossRef]
- Shakeri, F.; Ariaeenejad, S.; Ghollasi, M.; Motamedi, E. Synthesis of two novel bio-based hydrogels using sodium alginate and chitosan and their proficiency in physical immobilization of enzymes. Sci. Rep. 2022, 12, 2072. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M. Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. Int. J. Biol. Macromol. 2019, 124, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.; Pospiech, D.; Allertz, P.J.; Müller, M.; Salchert, K.; Hommel, R. Chemical design of hydrogels with immobilized laccase for the reduction of persistent trace compounds in wastewater. ACS Appl. Polym. Mater. 2021, 3, 2823–2834. [Google Scholar] [CrossRef]
- Dwamena, A.K.; Woo, S.H.; Kim, C.S. Enzyme immobilization on porous chitosan hydrogel capsules formed by anionic surfactant gelation. Biotechnol. Lett. 2020, 42, 845–852. [Google Scholar] [CrossRef]
- Crulhas, B.P.; Recco, L.C.; Delella, F.K.; Pedrosa, V.A. A novel superoxide anion biosensor for monitoring reactive species of oxygen released by cancer cells. Electroanalysis 2017, 29, 1252–1257. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Jayachandran, B.; Parvin, T.N.; Alam, M.M.; Chanda, K.; Mm, B. Insights on chemical crosslinking strategies for proteins. Molecules 2022, 27, 8124. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Lanjanian, H.; Motamedi, E.; Kavousi, K.; Moosavi-Movahedi, A.A.; Hosseini Salekdeh, G. The stabilizing mechanism of immobilized metagenomic xylanases on bio-based hydrogels to improve utilization performance: Computational and functional perspectives. Bioconjugate Chem. 2020, 31, 2158–2171. [Google Scholar] [CrossRef]
- Kim, H.J.; Jin, J.N.; Kan, E.; Kim, K.J.; Lee, S.H. Bacterial cellulose-chitosan composite hydrogel beads for enzyme immobilization. Biotechnol. Bioprocess Eng. 2017, 22, 89–94. [Google Scholar] [CrossRef]
- Mohammadi, N.S.; Khiabani, M.S.; Ghanbarzadeh, B.; Mokarram, R.R. Enhancement of biochemical aspects of lipase adsorbed on halloysite nanotubes and entrapped in a polyvinyl alcohol/alginate hydrogel: Strategies to reuse the most stable lipase. World J. Microbiol. Biotechnol. 2020, 36, 45. [Google Scholar] [CrossRef]
- Zhu, J.-Q.; Xu, J.-N.; Bian, X.-R.; Cheng, P.; Dou, Z.; Dai, W.-T.; Ju, S.-Y.; Wang, Y.-J. Enhancing the stability and activity of enzymes through layer-by-layer immobilization with nanocomposite hydrogel. Biochem. Eng. J. 2025, 215, 109604. [Google Scholar] [CrossRef]
- Dhiman, S.; Srivastava, B.; Singh, G.; Khatri, M.; Arya, S.K. Immobilization of mannanase on sodium alginate-grafted-β-cyclodextrin: An easy and cost effective approach for the improvement of enzyme properties. Int. J. Biol. Macromol. 2020, 156, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Öztop, H.N.; Akyildiz, F.; Saraydin, D. Poly (acrylamide/vinylsulfonic acid) hydrogel for invertase immobilization. Microsc. Res. Tech. 2020, 83, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Stable cellulase immobilized on graphene oxide@ CMC-g-poly (AMPS-co-AAm) hydrogel for enhanced enzymatic hydrolysis of lignocellulosic biomass. Carbohydr. Polym. 2020, 230, 115661. [Google Scholar] [CrossRef]
- Motamedi, E.; Sadeghian Motahar, S.F.; Maleki, M.; Kavousi, K.; Ariaeenejad, S.; Moosavi-Movahedi, A.A.; Hosseini Salekdeh, G. Upgrading the enzymatic hydrolysis of lignocellulosic biomass by immobilization of metagenome-derived novel halotolerant cellulase on the carboxymethyl cellulose-based hydrogel. Cellulose 2021, 28, 3485–3503. [Google Scholar] [CrossRef]
- Kapoor, S.; Rafiq, A.; Sharma, S. Protein engineering and its applications in food industry. Crit. Rev. Food Sci. Nutr. 2017, 57, 2321–2329. [Google Scholar] [CrossRef]
- Pandey, C.; Sharma, P.; Gupta, N. Engineering to enhance thermostability of xylanase: For the new era of biotechnology. J. Appl. Biol. Biotechnol. 2023, 11, 41–54. [Google Scholar] [CrossRef]
- Albayati, S.H.; Nezhad, N.G.; Taki, A.G.; Abd Rahman, R.N.Z.R. Efficient and feasible biocatalysts: Strategies for enzyme improvement. A review. Int. J. Biol. Macromol. 2024, 276, 133978. [Google Scholar] [CrossRef]
- De Simone, M.; Alonso-Cotchico, L.; Lucas, M.F.; Brissos, V.; Martins, L.O. Distal mutations enhance efficiency of free and immobilized NOV1 dioxygenase for vanillin synthesis. J. Biotechnol. 2024, 391, 92–98. [Google Scholar] [CrossRef]
- Wang, A.; Du, F.; Pei, X.; Chen, C.; Wu, S.G.; Zheng, Y. Rational immobilization of lipase by combining the structure analysis and unnatural amino acid insertion. J. Mol. Catal. B Enzym. 2016, 132, 54–60. [Google Scholar] [CrossRef]
- Czarnievicz, N.; Iturralde, M.; Comino, N.; Skolimowski, M.; López-Gallego, F. Surface engineering of amine transaminases to control their region-selective immobilization. Int. J. Biol. Macromol. 2025, 290, 138776. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, M.; Guo, B.; Luo, B.; Shao, X.; Wang, Y.; Cao, Y.; Zhang, D.; Ban, X.; Wei, L. Site-Directed Immobilization of An Engineered Cytidine Deaminase on a Magnetic Carrier with Bacteriophage-Aided Nanosurface for Multiround Biocatalysis. ACS Sustain. Chem. Eng. 2024, 12, 13438–13451. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, X.; Liu, W.-Q.; Ling, S.; Li, J. Chitin-Functionalized Cell-Free System Enables Sustainable Biocatalysis and Gene Expression. ACS Sustain. Chem. Eng. 2024, 12, 18219–18230. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Xu, Y.; Wu, J. Thermostability Enhancement of Tagatose 4-Epimerase through Protein Engineering and Whole-Cell Immobilization. J. Agric. Food Chem. 2025, 73, 1449–1457. [Google Scholar] [CrossRef]
- Gihaz, S.; Weiser, D.; Dror, A.; Sátorhelyi, P.; Jerabek-Willemsen, M.; Poppe, L.; Fishman, A. Creating an Efficient Methanol-Stable Biocatalyst by Protein and Immobilization Engineering Steps towards Efficient Biosynthesis of Biodiesel. ChemSusChem 2016, 9, 3161–3170. [Google Scholar] [CrossRef]
- Shukla, M.; Maiya, D.; Patel, T.; Pandya, A.; Tripathi, S. Nanozyme-Mediated Biosensing: An Insight into the Mechanisms Employed for the Continuous Analyte Monitoring. In Biosensors for Personalized Healthcare; Springer: Berlin/Heidelberg, Germany, 2024; pp. 253–278. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Jamwal, S.; Ranote, S.; Dautoo, U.; Chauhan, G.S. Improving activity and stabilization of urease by crosslinking to nanoaggregate forms for herbicide degradation. Int. J. Biol. Macromol. 2020, 158, 521–529. [Google Scholar] [CrossRef]
- Sheldon, R. Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Zerva, A.; Antonopoulou, I.; Enman, J.; Iancu, L.; Jütten, P.; Rova, U.; Christakopoulos, P. Optimization of transesterification reactions with CLEA-immobilized feruloyl esterases from Thermothelomyces thermophila and Talaromyces wortmannii. Molecules 2018, 23, 2403. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, L.; Liu, Y.; Zhang, X.; Yan, Y. Lipase-coated K2SO4 micro-crystals: Preparation, characterization, and application in biodiesel production using various oil feedstocks. Bioresour. Technol. 2012, 110, 224–231. [Google Scholar] [CrossRef]
- Xia, J.; Yan, Y.; Zou, B.; Liu, F. Improved catalytic performance of carrier-free immobilized lipase by advanced cross-linked enzyme aggregates technology. Bioprocess Biosyst. Eng. 2022, 45, 147–158. [Google Scholar]
- Matijošytė, I.; Arends, I.W.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Danielli, C.; van Langen, L.; Boes, D.; Asaro, F.; Anselmi, S.; Provenza, F.; Renzi, M.; Gardossi, L. 2, 5-Furandicarboxaldehyde as a bio-based crosslinking agent replacing glutaraldehyde for covalent enzyme immobilization. RSC Adv. 2022, 12, 35676–35684. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xiong, X.; Qiu, M.; Lu, Y.; Chen, T.; Xu, Z. Hyperbranched Polymer-Crosslinked Laccase Aggregates for Efficient Aerobic Oxidation of Alcohols. Enzym. Microb. Technol. 2025, 189, 110673. [Google Scholar] [CrossRef]
- Yang, K.-L. Uniform cross-linked cellulase aggregates prepared in millifluidic reactors. J. Colloid Interface Sci. 2014, 428, 146–151. [Google Scholar]
- Wang, M.; Jia, C.; Qi, W.; Yu, Q.; Peng, X.; Su, R.; He, Z. Porous-CLEAs of papain: Application to enzymatic hydrolysis of macromolecules. Bioresour. Technol. 2011, 102, 3541–3545. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Deane, J.K.; Rakes, T.R.; Rees, L.P. 3D printing technology and the market value of the firm. Inf. Syst. Front. 2022, 24, 1379–1392. [Google Scholar] [CrossRef]
- Liu, W.; Wu, N.; Pochiraju, K. Shape recovery characteristics of SiC/C/PLA composite filaments and 3D printed parts. Compos. Part A Appl. Sci. Manuf. 2018, 108, 1–11. [Google Scholar] [CrossRef]
- Pose-Boirazian, T.; Martínez-Costas, J.; Eibes, G. 3D Printing: An Emerging Technology for Biocatalyst Immobilization. Macromol. Biosci. 2022, 22, 2200110. [Google Scholar] [CrossRef]
- Shao, Y.; Liao, Z.; Gao, B.; He, B. Emerging 3D printing strategies for enzyme immobilization: Materials, methods, and applications. ACS Omega 2022, 7, 11530–11543. [Google Scholar] [CrossRef]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit. Manuf. 2022, 55, 102867. [Google Scholar] [CrossRef]
- Lackner, F.; Liu, H.; Štiglic, A.D.; Bračič, M.; Kargl, R.; Nidetzky, B.; Mohan, T.; Kleinschek, K.S. 3D printed porous nanocellulose-based scaffolds as carriers for immobilization of glycosyltransferases. ACS Appl. Bio Mater. 2022, 5, 5728–5740. [Google Scholar] [CrossRef]
- Ye, J.; Chu, T.; Chu, J.; Gao, B.; He, B. A versatile approach for enzyme immobilization using chemically modified 3D-printed scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 18048–18054. [Google Scholar] [CrossRef]
- Jiang, W.; Pei, R.; Zhou, S.-F. 3D-printed xylanase within biocompatible polymers as excellent catalyst for lignocellulose degradation. Chem. Eng. J. 2020, 400, 125920. [Google Scholar] [CrossRef]
- Xu, X.; Pose-Boirazian, T.; Eibes, G.; McCoubrey, L.E.; Martínez-Costas, J.; Gaisford, S.; Goyanes, A.; Basit, A.W. A customizable 3D printed device for enzymatic removal of drugs in water. Water Res. 2022, 208, 117861. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst. Nanoeng. 2021, 7, 75. [Google Scholar] [CrossRef]
- Manzanares-Palenzuela, C.L.; Hermanova, S.; Sofer, Z.; Pumera, M. Proteinase-sculptured 3D-printed graphene/polylactic acid electrodes as potential biosensing platforms: Towards enzymatic modeling of 3D-printed structures. Nanoscale 2019, 11, 12124–12131. [Google Scholar] [CrossRef]
- Schmieg, B.; Schimek, A.; Franzreb, M. Development and performance of a 3D-printable poly (ethylene glycol) diacrylate hydrogel suitable for enzyme entrapment and long-term biocatalytic applications. Eng. Life Sci. 2018, 18, 659–667. [Google Scholar] [CrossRef]
- Liu, J.; Shen, X.; Zheng, Z.; Li, M.; Zhu, X.; Cao, H.; Cui, C. Immobilization of laccase by 3D bioprinting and its application in the biodegradation of phenolic compounds. Int. J. Biol. Macromol. 2020, 164, 518–525. [Google Scholar] [CrossRef]
- Ali, J.M.; Hussain, M.A.; Tade, M.O.; Zhang, J. Artificial Intelligence techniques applied as estimator in chemical process systems–A literature survey. Expert Syst. Appl. 2015, 42, 5915–5931. [Google Scholar]
- Gulati, S.; Rouilly, V.; Niu, X.; Chappell, J.; Kitney, R.I.; Edel, J.B.; Freemont, P.S.; Demello, A.J. Opportunities for microfluidic technologies in synthetic biology. J. R. Soc. Interface 2009, 6 (Suppl. S4), S493–S506. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, M.; Saxena, S.K.; Kumar, R. Raman Spectromicroscopy: A tool to “see” subtle aspects in science, technology, and engineering. J. Phys. Chem. C 2022, 126, 4733–4743. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Guo, S.; Bocklitz, T.; Rösch, P.; Popp, J.; Yu, H.-Q. Nondestructive 3D imaging and quantification of hydrated biofilm matrix by confocal Raman microscopy coupled with non-negative matrix factorization. Water Res. 2022, 210, 117973. [Google Scholar] [CrossRef]
- Smith, J.P.; Liu, M.; Lauro, M.L.; Balasubramanian, M.; Forstater, J.H.; Grosser, S.T.; Dance, Z.E.; Rhodes, T.A.; Bu, X.; Booksh, K.S. Raman hyperspectral imaging with multivariate analysis for investigating enzyme immobilization. Analyst 2020, 145, 7571–7581. [Google Scholar] [CrossRef]
- Patil, P.D.; Gargate, N.; Dongarsane, K.; Jagtap, H.; Phirke, A.N.; Tiwari, M.S.; Nadar, S.S. Revolutionizing biocatalysis: A review on innovative design and applications of enzyme-immobilized microfluidic devices. Int. J. Biol. Macromol. 2024, 281, 136193. [Google Scholar] [CrossRef]
- Mazurenko, S. Predicting protein stability and solubility changes upon mutations: Data perspective. ChemCatChem 2020, 12, 5590–5598. [Google Scholar] [CrossRef]
- Chai, M.; Moradi, S.; Erfani, E.; Asadnia, M.; Chen, V.; Razmjou, A. Application of machine learning algorithms to estimate enzyme loading, immobilization yield, activity retention, and reusability of enzyme–metal–organic framework biocatalysts. Chem. Mater. 2021, 33, 8666–8676. [Google Scholar] [CrossRef]
- Ralbovsky, N.M.; Smith, J.P. Machine learning for prediction, classification, and identification of immobilized enzymes for biocatalysis. Pharm. Res. 2023, 40, 1479–1490. [Google Scholar] [CrossRef]
- Wei, H.; Smith, J.P. Modernized machine learning approach to illuminate enzyme immobilization for biocatalysis. ACS Cent. Sci. 2023, 9, 1913–1926. [Google Scholar] [CrossRef]
- Panteleev, J.; Gao, H.; Jia, L. Recent applications of machine learning in medicinal chemistry. Bioorg. Med. Chem. Lett. 2018, 28, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Ghaedi, M.; Dashtian, K.; Azghandi, M.H.A. Rapid and high-capacity ultrasonic assisted adsorption of ternary toxic anionic dyes onto MOF-5-activated carbon: Artificial neural networks, partial least squares, desirability function and isotherm and kinetic study. Ultrason. Sonochem. 2017, 37, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Parsazadeh, N.; Yousefi, F.; Ghaedi, M.; Dashtian, K.; Borousan, F. Preparation and characterization of monoliths HKUST-1 MOF via straightforward conversion of Cu(OH)2-based monoliths and its application for wastewater treatment: Artificial neural network and central composite design modeling. New J. Chem. 2018, 42, 10327–10336. [Google Scholar] [CrossRef]
- Ohno, H.; Mukae, Y. Machine learning approach for prediction and search: Application to methane storage in a metal–organic framework. J. Phys. Chem. C 2016, 120, 23963–23968. [Google Scholar] [CrossRef]
- Borboudakis, G.; Stergiannakos, T.; Frysali, M.; Klontzas, E.; Tsamardinos, I.; Froudakis, G.E. Chemically intuited, large-scale screening of MOFs by machine learning techniques. npj Comput. Mater. 2017, 3, 40. [Google Scholar] [CrossRef]
- Dixit, M.; Gupta, G.K.; Yadav, M.; Chhabra, D.; Kapoor, R.K.; Pathak, P.; Bhardwaj, N.K.; Shukla, P. Improved deinking and biobleaching efficiency of enzyme consortium from Thermomyces lanuginosus VAPS25 using genetic Algorithm-Artificial neural network based tools. Bioresour. Technol. 2022, 349, 126846. [Google Scholar] [CrossRef]
- Dixit, M.; Chhabra, D.; Shukla, P. Optimization of endoglucanase-lipase-amylase enzyme consortium from Thermomyces lanuginosus VAPS25 using Multi-Objective genetic algorithm and their bio-deinking applications. Bioresour. Technol. 2023, 370, 128467. [Google Scholar] [CrossRef]
- Fard Masoumi, H.R.; Basri, M.; Kassim, A.; Kuang Abdullah, D.; Abdollahi, Y.; Abd Gani, S.S. Comparison of estimation capabilities of the artificial neural network with the wavelet neural network in lipase-catalyzed synthesis of triethanolamine-based esterquats cationic surfactant. J. Surfactants Deterg. 2014, 17, 287–294. [Google Scholar] [CrossRef]
- Liang, W.; Zheng, S.; Shu, Y.; Huang, J. Machine Learning Optimizing Enzyme/ZIF Biocomposites for Enhanced Encapsulation Efficiency and Bioactivity. JACS Au 2024, 4, 3170–3182. [Google Scholar] [CrossRef]
- de Souza, T.C.; de Sousa Fonseca, T.; de Sousa Silva, J.; Lima, P.J.; Neto, C.A.; Monteiro, R.R.; Rocha, M.V.P.; de Mattos, M.C.; Dos Santos, J.C.; Gonçalves, L.R. Modulation of lipase B from Candida antarctica properties via covalent immobilization on eco-friendly support for enzymatic kinetic resolution of rac-indanyl acetate. Bioprocess Biosyst. Eng. 2020, 43, 2253–2268. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of lipases by the unfolding and refolding of covalently immobilized biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).