Biochar-Based Materials for Catalytic CO2 Valorization
Abstract
1. Introduction
2. Cycloaddition of CO2 to Epoxides
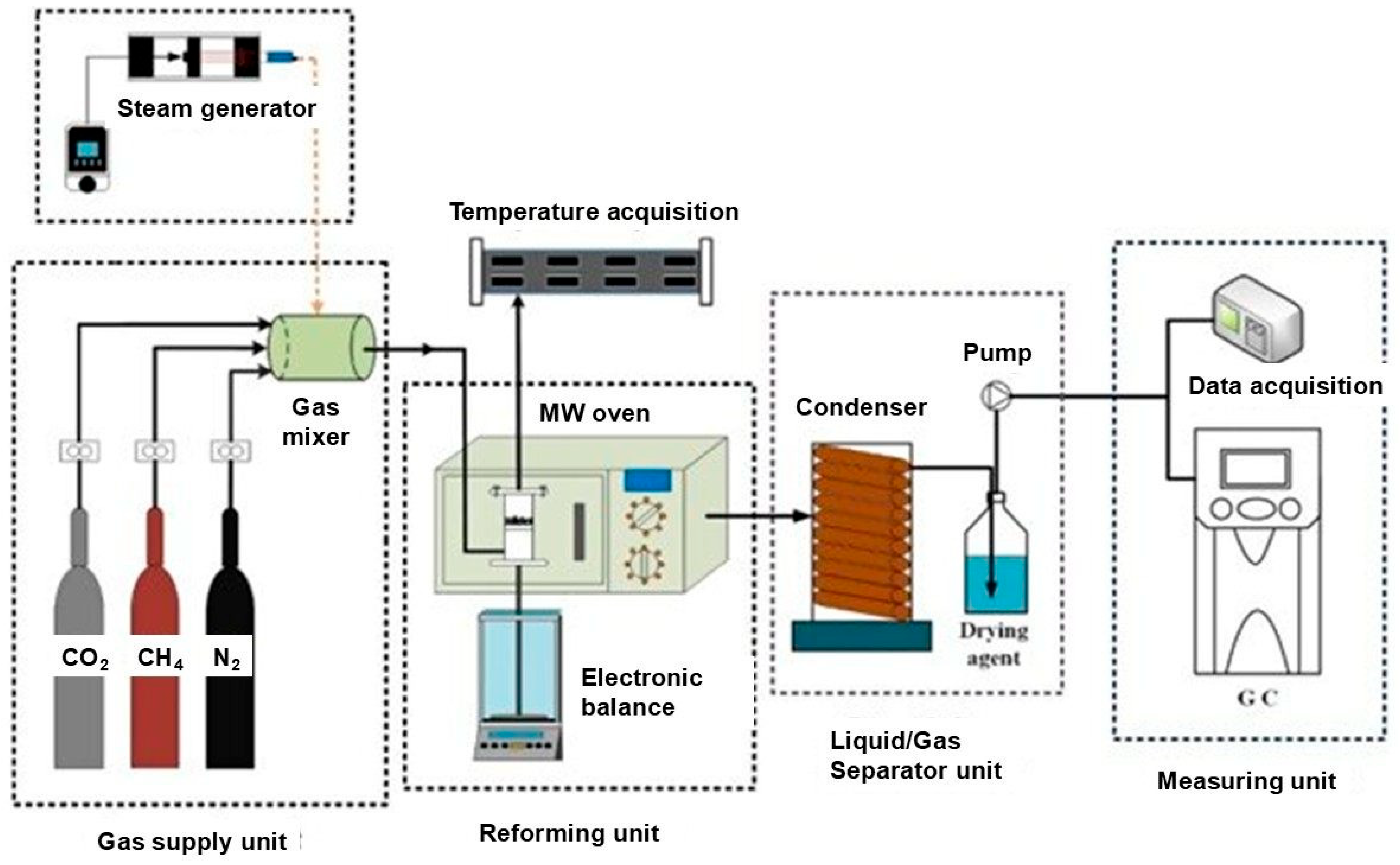
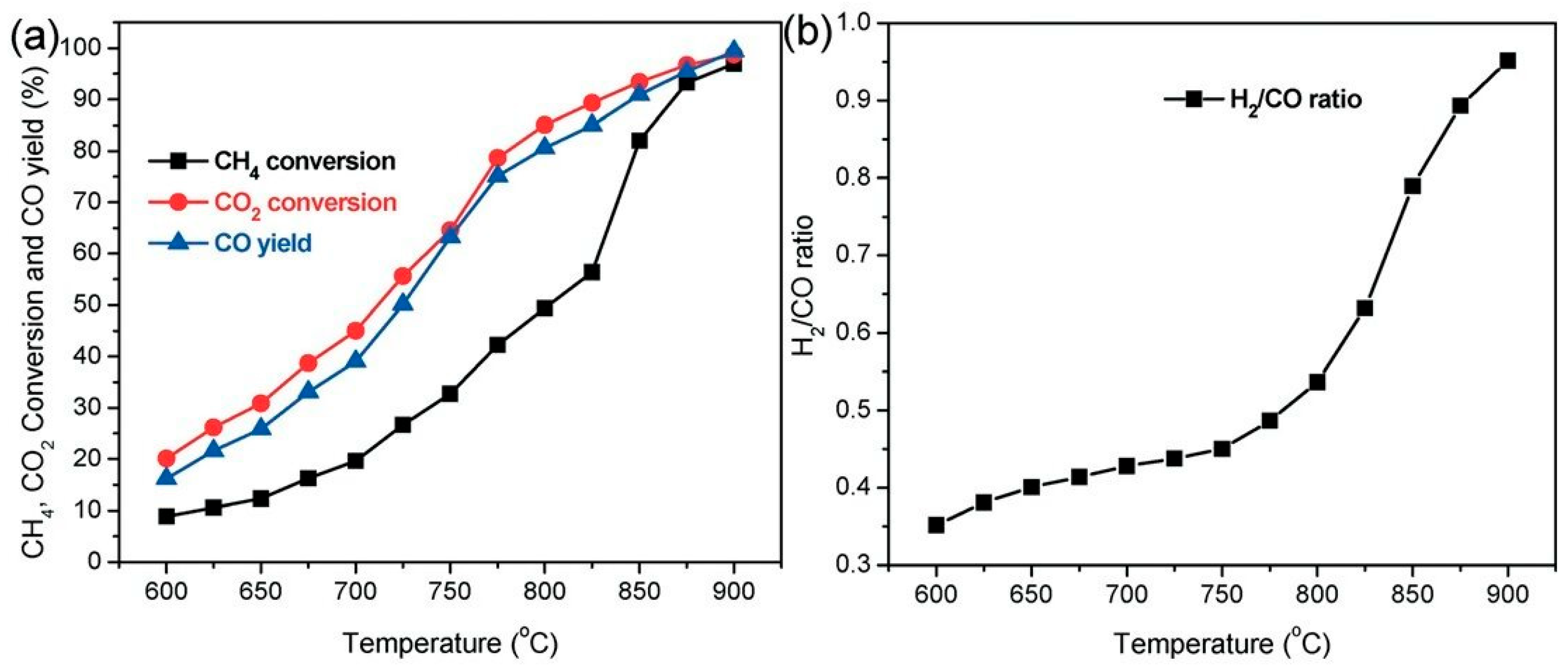
3. Dry Reforming of Methane
4. Catalytic Biomass Upgrading Reactions
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Catalysts 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Conte, P.; Bertani, R.; Sgarbossa, P.; Bambina, P.; Schmidt, H.-P.; Raga, R.; Lo Papa, G.; Chillura Martino, D.F.; Lo Meo, P. Recent Developments in Understanding Biochar’s Physical–Chemistry. Agronomy 2021, 11, 615. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen Containing Functional Groups of Biochar: An Overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, D.; Sharan, A.; Khare, R.; Rai, S.; Kumar, P.; Singh, V. Innovations in Biomass-Derived Materials for Efficient CO2 Sequestration. Chem. Afr. 2025, 8, 397–418. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, C. Nitrogen enriched biochar used as CO2 adsorbents: A brief review. Carbon Capture Sci. Technol. 2022, 2, 100018. [Google Scholar] [CrossRef]
- Kong, Z.; Zhang, H.; Zhou, T.; Xie, L.; Wang, B.; Jiang, X. Biomass-derived functional materials: Preparation, functionalization, and applications in adsorption and catalytic separation of carbon dioxide and other atmospheric pollutants. Sep. Purif. Technol. 2025, 354, 129099. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.-M.; Gong, X.-M.; Duan, A.; Zhang, Q.; et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Dutta Gupta, A.; Singh, H.; Varjani, S.; Awasthi, M.K.; Giri, B.S.; Pandey, A. A critical review on biochar-based catalysts for the abatement of toxic pollutants from water via advanced oxidation processes (AOPs). Sci. Total Environ. 2022, 849, 157831. [Google Scholar] [CrossRef]
- Legan, M.; Žgajnar Gotvajn, A.; Zupan, K. Potential of Biochar Use in Building Materials. J. Environ. Manag. 2022, 309, 114704. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable biochar for advanced electrochemical/energy storage applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Prabakar, P.; Mert, K.M.; Muruganandam, L.; Sivagami, K. A comprehensive review on biochar for electrochemical energy storage applications: An emerging sustainable technology. Front. Energy Res. 2024, 12, 1448520. [Google Scholar] [CrossRef]
- Ma, C.; Tang, L.; Cheng, H.; Li, Z.; Li, W.; He, G. Biochar for supercapacitor electrodes: Mechanisms in aqueous electrolytes. Battery Energy 2024, 3, 20230058. [Google Scholar] [CrossRef]
- Younis, S.A.; Kim, K.H. Recent Advances in Biochar-based Catalysts: Air Purification and Opportunities for Industrial Upscaling. Asian J. Atmos. Environ. 2022, 16, 2022117. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.-D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent advancements and challenges in emerging applications of biochar-based catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Tagliaferro, A. A Comprehensive Overview on Biochar-Based Materials for Catalytic Applications. Catalysts 2023, 13, 1336. [Google Scholar] [CrossRef]
- Khan, I.; Sadiq, S.; Wu, P.; Humayun, M.; Ullah, S.; Yaseen, W.; Khan, S.; Khan, A.; Abumousa, R.A.; Bououdina, M. Synergizing black gold and light: A comprehensive analysis of biochar-photocatalysis integration for green remediation. Carbon Capture Sci. Technol. 2024, 13, 100315. [Google Scholar] [CrossRef]
- Lopes, R.P.; Astruc, D. Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Ramos, R.; Abdelkader-Fernández, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts 2022, 12, 207. [Google Scholar] [CrossRef]
- Santos, J.L.; Mäki-Arvela, P.; Monzón, A.; Murzin, D.Y.; Centeno, M.Á. Metal catalysts supported on biochars: Part I synthesis and characterization. Appl. Catal. B Environ. 2020, 268, 118423. [Google Scholar] [CrossRef]
- Moradia, P.; Hajjami, M. Stabilization of ruthenium on biochar-nickel magnetic nanoparticles as a heterogeneous, practical, selective, and reusable nanocatalyst for the Suzuki C–C coupling reaction in water. RSC Adv. 2022, 12, 13523–13534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advanced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Kumar, R. Biochar Catalyst for Oxidation Reactions. In Biochar-Based Catalysts; Bhawani, S.A., Umar, K., Mohamad Ibrahim, M.N., Alotaibi, K.M., Eds.; Sustainable Materials and Technology; Springer: Singapore, 2024. [Google Scholar]
- Sharma, G.; Yadav, S.; das, R.; Kumari, I. Biochar-Based Catalysts for Reduction Reactions. In Biochar-Based Catalysts; Bhawani, S.A., Umar, K., Mohamad Ibrahim, M.N., Alotaibi, K.M., Eds.; Sustainable Materials and Technology; Springer: Singapore, 2024. [Google Scholar]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Cui, Z.-S.; Zhang, M.; Zhang, Z.-H. Biochar-based functional materials as heterogeneous catalysts for organic reactions. Curr. Opin. Green Sustain. Chem. 2022, 38, 100713. [Google Scholar] [CrossRef]
- Yang, H.; Cui, Y.; Jin, Y.; Lu, X.; Han, T.; Sandström, L.; Jönsson, P.G.; Yang, W. Evaluation of Engineered Biochar-Based Catalysts for Syngas Production in a Biomass Pyrolysis and Catalytic Reforming Process. Energy Fuels 2023, 37, 5942–5952. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Monfared, A.; Mohammadi, R.; Hosseinian, A.; Sarhandi, S.; Delir Kheirollahi Nezhad, P. Cycloaddition of atmospheric CO2 to epoxides under solvent-free conditions: A straightforward route to carbonates by green chemistry metrics. RSC Adv. 2019, 9, 3884–3899. [Google Scholar] [CrossRef]
- Yusuf, N.; Almomani, F.; Qiblawey, H. Catalytic CO2 conversion to C1 value-added products: Review on latest catalytic and process developments. Fuel 2023, 345, 128178. [Google Scholar] [CrossRef]
- Vega, L.F.; Bahamon, D.; Alkhatib, I.I.I. Perspectives on Advancing Sustainable CO2 Conversion Processes: Trinomial Technology, Environment, and Economy. ACS Sustain. Chem. Eng. 2024, 12, 5357–5382. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Tommasi, M.; Degerli, S.N.; Ramis, G.; Rossetti, I. Advancements in CO2 methanation: A comprehensive review of catalysis, reactor design and process optimization. Chem. Eng. Res. Des. 2024, 201, 457–482. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Zeng, Z.X.; Pan, W.G. Recent Progress of Catalysts for Synthesis of Cyclic Carbonates from CO2 and Epoxides. J. CO2 Util. 2023, 68, 102355. [Google Scholar] [CrossRef]
- Gulati, S.; Vijayan, S.; Mansi; Kumar, S.; Harikumar, B.; Trivedi, M.; Varma, R.S. Recent Advances in the Application of Metal-Organic Frameworks (MOFs)-Based Nanocatalysts for Direct Conversion of Carbon Dioxide (CO2) to Value-Added Chemicals. Coord. Chem. Rev. 2023, 474, 214853. [Google Scholar] [CrossRef]
- Guo, L.; Lamb, K.J.; North, M. Recent Developments in Organocatalysed Transformations of Epoxides and Carbon Dioxide into Cyclic Carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Jiang, Y.; Sun, J.; Arai, M. Hydrogen Bond Activation Strategy for Cyclic Carbonates Synthesis from Epoxides and CO2: Current State-of-the-Art of Catalyst Development and Reaction Analysis. Catal. Rev. 2019, 61, 214–269. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-Fixation into Cyclic and Polymeric Carbonates: Principles and Applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Gonzalez, A.C.S.; Felgueiras, A.P.; Aroso, R.T.; Carrilho, R.M.B.; Pereira, M.M. Al(III) Phthalocyanine Catalysts for CO2 Addition to Epoxides: Fine-Tunable Selectivity for Cyclic Carbonates versus Polycarbonates. J. Organomet. Chem. 2021, 950, 121979. [Google Scholar] [CrossRef]
- Carrilho, R.M.B.; Dias, L.D.; Rivas, R.; Pereira, M.M.; Claver, C.; Masdeu-Bultó, A.M. Solventless Coupling of Epoxides and CO2 in Compressed Medium Catalysed by Fluorinated Metalloporphyrins. Catalysts 2017, 7, 210. [Google Scholar] [CrossRef]
- Rong, W.; Ding, M.; Wang, Y.; Kong, S.; Yao, J. Porous biochar with a tubular structure for photothermal CO2 cycloaddition: One-step doping versus two-step doping. Sep. Purif. Technol. 2025, 353, 128427. [Google Scholar] [CrossRef]
- Gbe, J.-L.K.; Ravi, K.; Singh, M.; Neogi, S.; Grafouté, M.; Biradar, A.V. Hierarchical porous nitrogen-doped carbon supported MgO as an excellent composite for CO2 capture at atmospheric pressure and conversion to value-added products. J. CO2 Util. 2022, 65, 102222. [Google Scholar] [CrossRef]
- Vidal, J.L.; Andrea, V.P.; MacQuarrie, S.L.; Kerton, F.M. Oxidized Biochar as a Simple, Renewable Catalyst for the Production of Cyclic Carbonates from Carbon Dioxide and Epoxides. ChemCatChem 2019, 11, 4089–4095. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Deng, L.-J.; Jiang, J.; Jiang, H. Catalytic Cycloaddition of CO2 to Epoxides by the Synergistic Effect of Acidity and Alkalinity in a Functionalized Biochar. Chem. Eng. J. 2022, 442, 136265. [Google Scholar] [CrossRef]
- Parodi, A.; Vagnoni, M.; Frontali, L.; Albonetti, C.; De Giorgio, F.; Mezzi, A.; Petri, E.; Samorì, C.; Soavi, F.; Ruani, G.; et al. Bifunctional Heterogeneous Catalysts from Biomass and Waste Polysaccharides for the Conversion of CO2 into Cyclic Carbonates. J. Mater. Chem. A 2023, 11, 775–788. [Google Scholar] [CrossRef]
- Samikannu, A.; Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.-P. Renewable N-Doped Active Carbons as Efficient Catalysts for Direct Synthesis of Cyclic Carbonates from Epoxides and CO2. Appl. Catal. B Environ. 2019, 241, 41–51. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Cui, Z.; Fu, Z.; Yang, L.; Liu, G.; Li, M. Coffee Grounds Derived N Enriched Microporous Activated Carbons: Efficient Adsorbent for Post-Combustion CO2 Capture and Conversion. J. Colloid Interface Sci. 2020, 578, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zomorodbakhsh, S.; Gonzalez, A.C.S.; Cruz, I.G.; Piccirillo, G.; Maria, T.M.R.; Marques, I.S.; Peixoto, A.F.; Gil, J.M.; Ferreira, F.; Carrilho, R.M.B. Modified Biochar Materials from Eucalyptus globulus Wood as Efficient CO2 Adsorbents and Recyclable Catalysts. Adv. Sustain. Syst. 2025, 9, 2400431. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Hussain, I.; Taialla, O.A.; Awad, M.M.; Tanimu, A.; Alhooshani, K.; Ganiyu, S.A. Advances in Catalytic Dry Reforming of Methane (DRM): Emerging Trends, Current Challenges, and Future Perspectives. J. Clean. Prod. 2023, 423, 138638. [Google Scholar] [CrossRef]
- le Saché, E.; Reina, T.R. Analysis of Dry Reforming as Direct Route for Gas Phase CO2 Conversion. The Past, the Present and Future of Catalytic DRM Technologies. Prog. Energy Combust. Sci. 2022, 89, 100970. [Google Scholar] [CrossRef]
- Agún, B.; Abánades, A. Comprehensive Review on Dry Reforming of Methane: Challenges and Potential for Greenhouse Gas Mitigation. Int. J. Hydrogen Energy 2025, 103, 395–414. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Dentzer, J.; Gadiou, R.; Ouederni, A. Evaluation of activated carbons based on olive stones as catalysts during hydrogen production by thermocatalytic decomposition of methane. Int. J. Hydrogen Energy 2017, 42, 8712–8720. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, H.; Qi, M.; Zhang, G.; Hu, H.; Ma, X. Hydrogen production by catalytic methane decomposition: Carbon materials as catalysts or catalyst supports. Int. J. Hydrogen Energy 2017, 42, 19755–19775. [Google Scholar] [CrossRef]
- Nishii, H.; Miyamoto, D.; Umeda, Y.; Hamaguchi, H.; Suzuki, M.; Tanimoto, T.; Harigai, T.; Takikawa, H.; Suda, Y. Catalytic activity of several carbons with different structures for methane decomposition and by-produced carbons. Appl. Surf. Sci. 2019, 473, 291–297. [Google Scholar] [CrossRef]
- Di Stasi, C.; Greco, G.; Canevesi, R.L.S.; Izquierdo, M.T.; Fierro, V.; Celzard, A.; González, B.; Manyà, J.J. Influence of activation conditions on textural properties and performance of activated biochars for pyrolysis vapors upgrading. Fuel 2021, 289, 119759. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, S.; Li, L.; Meng, B.; Chen, J.; Yang, Z.; Yan, K.; Qin, X. Application of microwave heating for methane dry reforming catalyzed by activated carbon. Chem. Eng. Process. Process Intensif. 2019, 145, 107662. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Wang, S.; Tan, Y.; Meng, B.; Zou, G.; Wang, F.; Song, Z.; Ma, C. Utilization of biochar for a process of methane dry reforming coupled with steam gasification under microwave heating. J. Clean. Prod. 2019, 237, 117838. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, X.; Wang, G.; Zhou, P.; Wang, W.; Song, Z. Evaluating the effect of torrefaction on the pyrolysis of biomass and the biochar catalytic performance on dry reforming of methane. Renew. Energy 2022, 192, 313–325. [Google Scholar] [CrossRef]
- Jiang, M.; Su, Y.; Yang, L.; Qi, P.; Wang, J.; Xiong, Y. Study on H3PO4-activated carbon catalytic co-pyrolysis of bamboo and LDPE to poly-generation syngas and aromatics at low temperature. Fuel 2024, 369, 131737. [Google Scholar] [CrossRef]
- Yan, Q.; Lu, Y.; To, F.; Li, Y.; Yu, F. Synthesis of tungsten carbide nanoparticles in biochar matrix as a catalyst for dry reforming of methane to syngas. Catal. Sci. Technol. 2015, 5, 3270–3280. [Google Scholar] [CrossRef]
- Izhab, I.; Saidina Amin, N.A.; Asmadi, M. Dry reforming of methane over oil palm shell activated carbon and ZSM-5 supported cobalt catalysts. Int. J. Green Energy 2017, 14, 831–838. [Google Scholar] [CrossRef]
- Benedetti, V.; Ail, S.S.; Patuzzi, F.; Baratieri, M. Valorization of Char From Biomass Gasification as Catalyst Support in Dry Reforming of Methane. Front. Chem. 2019, 7, 119. [Google Scholar] [CrossRef]
- Liew, R.K.; Chong, M.Y.; Osazuwa, O.U.; Nam, W.L.; Phang, X.Y.; Su, M.H.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Production of activated carbon as catalyst support by microwave pyrolysis of palm kernel shell: A comparative study of chemical versus physical activation. Res. Chem. Intermed. 2018, 44, 3849–3865. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Zhang, Y.; Sun, J.; Zou, G. Ni-Co bimetallic catalysts on coconut shell activated carbon prepared using solid-phase method for highly efficient dry reforming of methane. Environ. Sci. Pollut. Res. 2022, 29, 37685–37699. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, P.; Shao, Q.; Ma, D.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B Environ. 2014, 152–153, 140–151. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S. Effects of H2O and CO2 on the Homogeneous Conversion and Heterogeneous Reforming of Biomass Tar over Biochar. Int. J. Hydrogen Energy 2017, 42, 13070–13084. [Google Scholar] [CrossRef]
- Collett, C.; Mašek, O.; Razali, N.; McGregor, J. Influence of Biochar Composition and Source Material on Catalytic Performance: The Carboxylation of Glycerol with CO2 as a Case Study. Catalysts 2020, 10, 1067. [Google Scholar] [CrossRef]




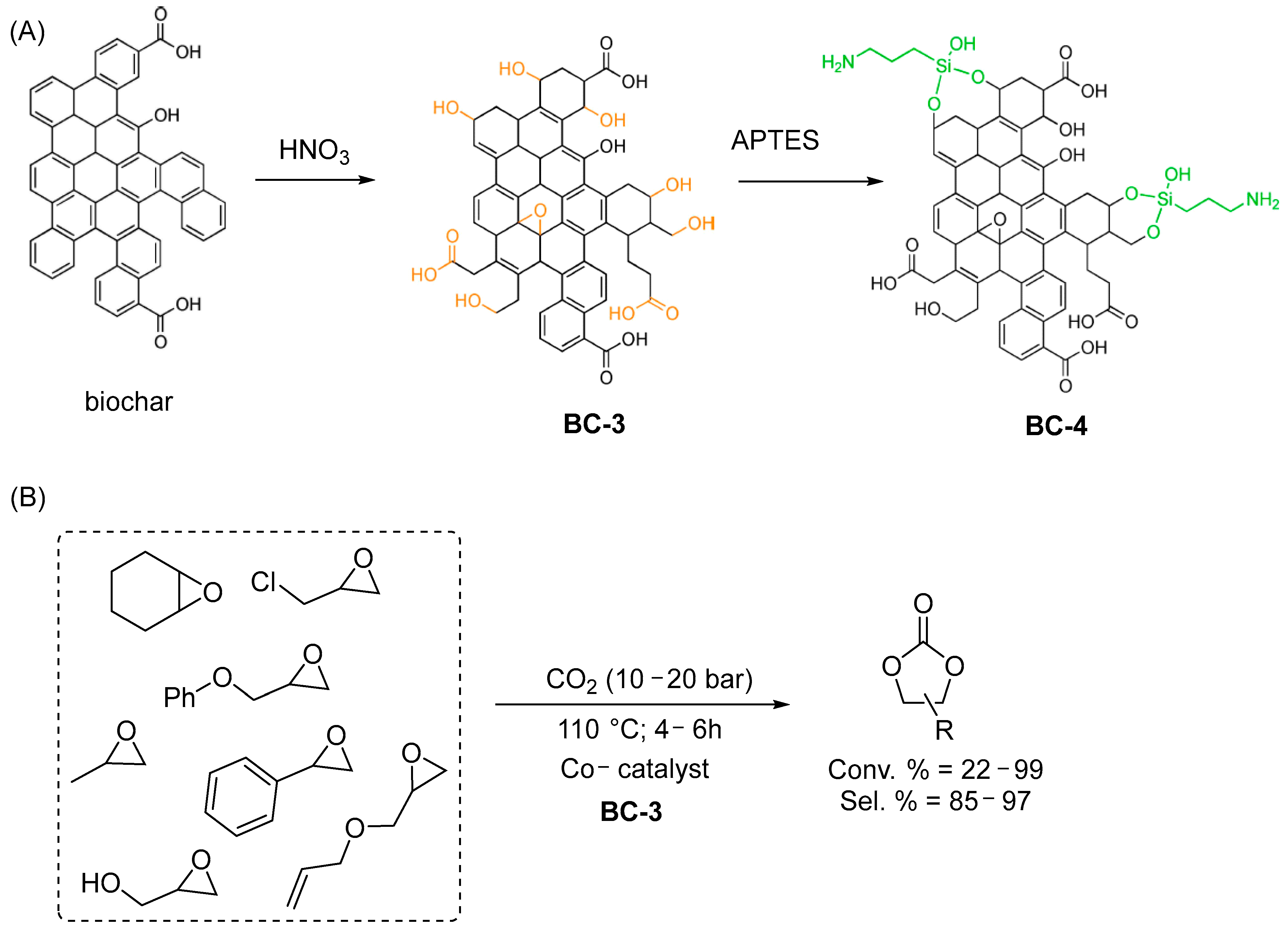
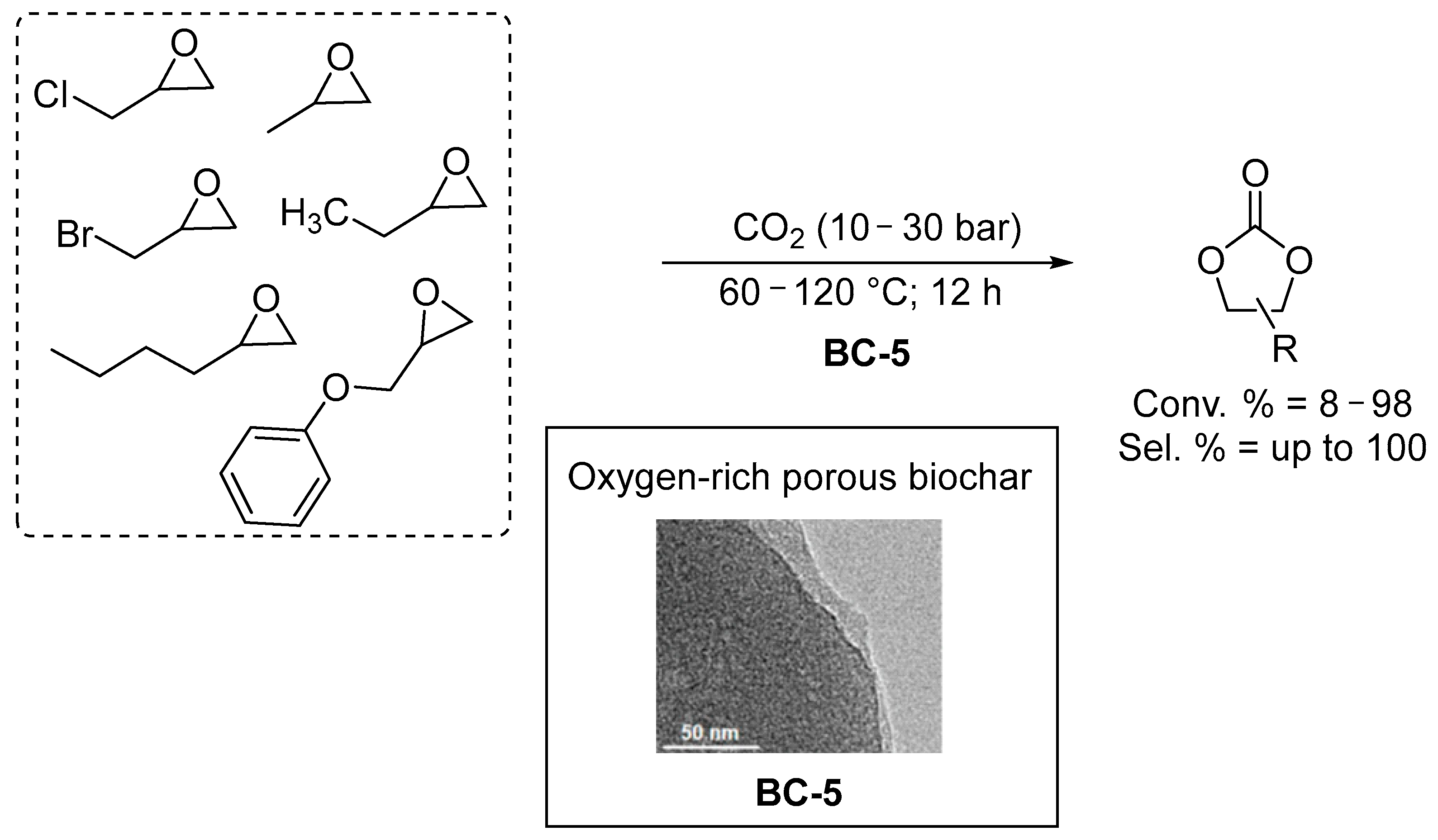



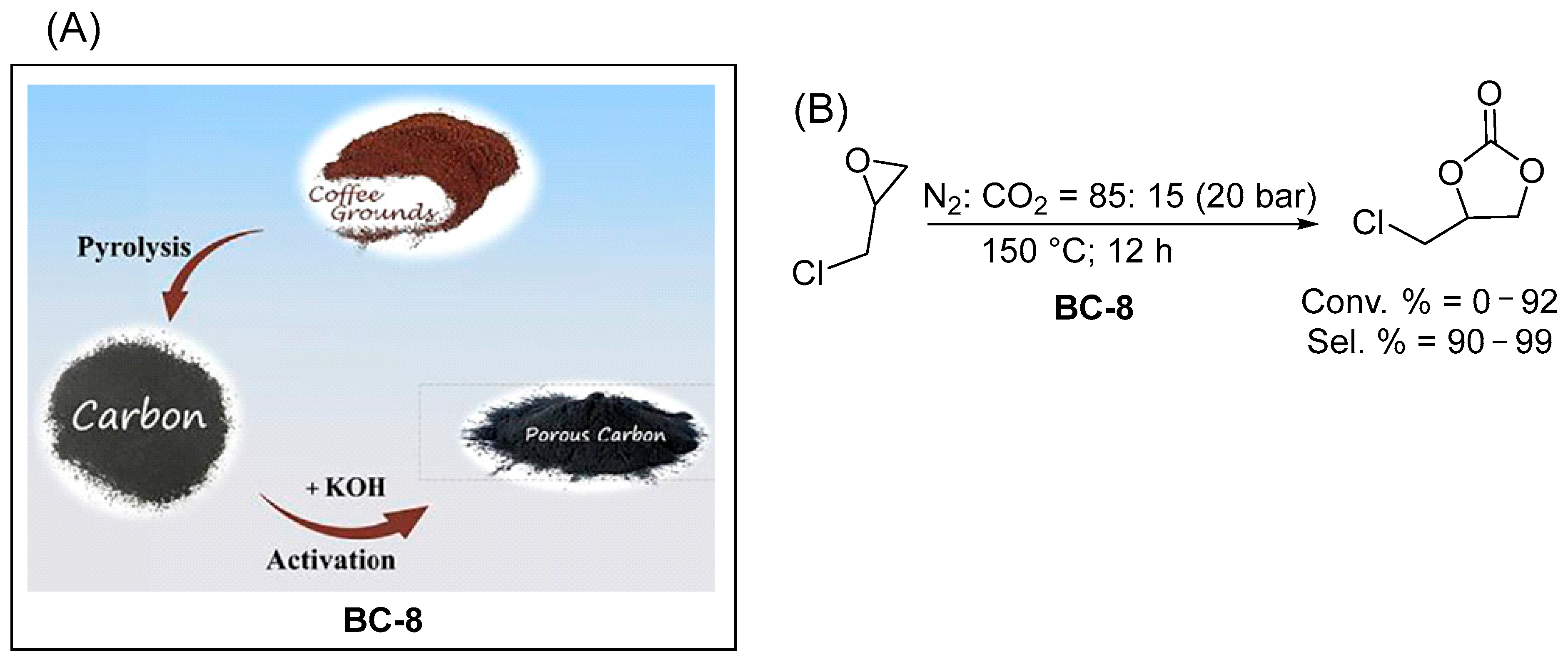
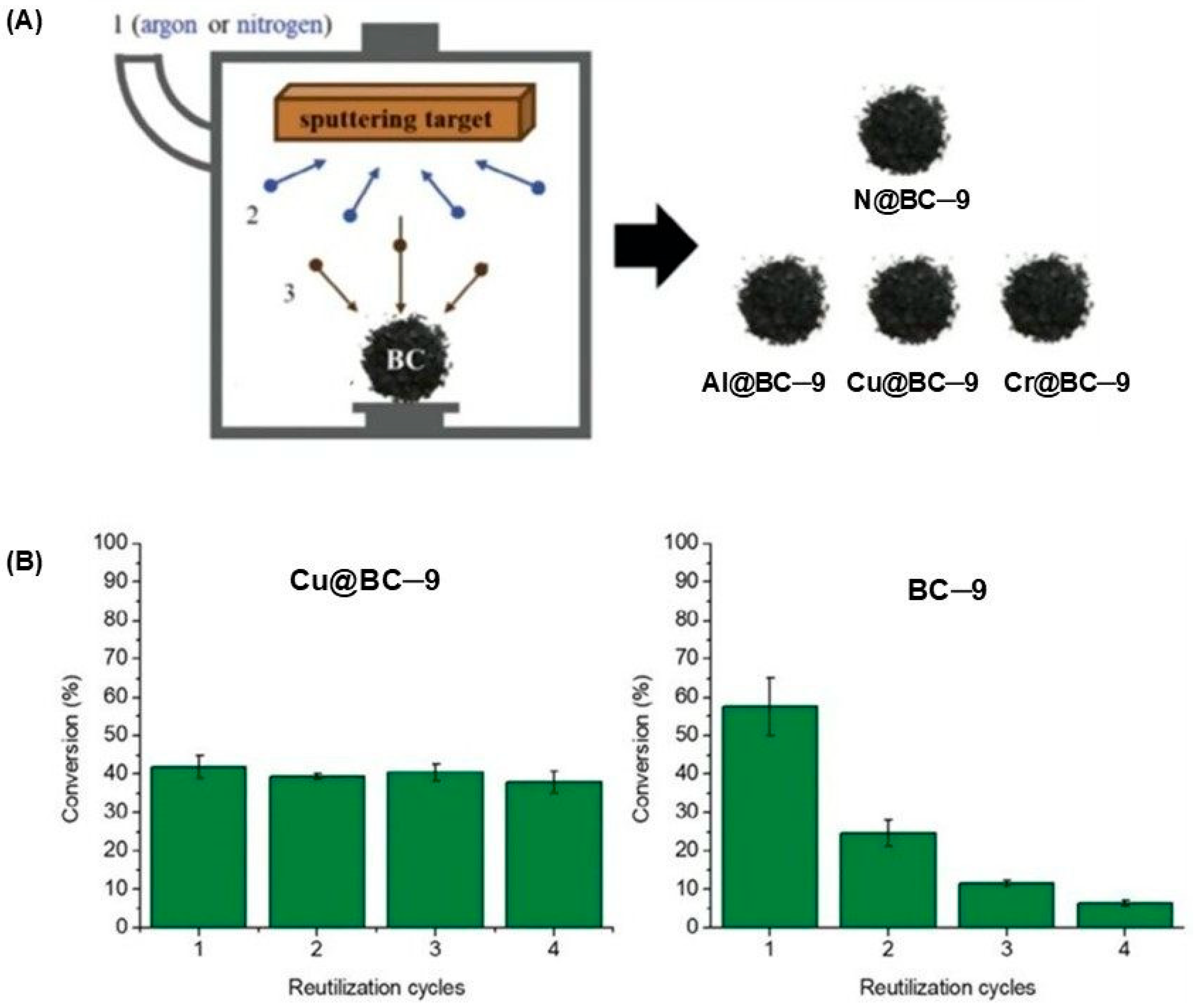



| Entry | CAT | BET Area (SBET) (m2/g) | Co-CAT | T (°C) | P (bar) | t (h) | Reuse Cycles | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Biochar prepared from corn stalk (BC-1) | 166 | TBAB | 60 | 1 | 6 | 10 | up to 100 a | [50] |
| 2 | Biochar prepared from chitosan (BC-2) | 259 | CTAB; NH4Cl; NaCl | 120–140 | 1 | 4–6 | 5 | 80–97 b | [51] |
| 3 | Biochar prepared from soft and hardwood (BC-3 and BC-4) | 231 | TBAB; PPNCl; PPNN3 | 110 | 10–20 | 4–16 | 5 | 22–100 c | [52] |
| Biochar prepared from chitosan/cellulose (BC-5) | 660 | none | 60–120 | 10–30 | 12 | 5 | 0–99 d | [53] | |
| 5 | Biochar prepared from natural polysaccharides (BC-6) | 442 | none | 70 | 3 | 7 | 5 | 0–96 e | [54] |
| 6 | Biochar prepared from chitosan (BC-7) | 635 | none | 150 | 15 | 15 | 3 | 80–99 f | [55] |
| 7 | Biochar prepared from coffee grounds (BC-8) | 654 | none | 150 | 20 | 12 | 5 | 95 g | [56] |
| 8 | Biochar prepared from eucalyptus waste (BC-9) | 527 | none | 80–120 | 20 | 24 | 4 | 22–58 h | [57] |
| Entry | Catalyst Source | Reaction Performance | Observations a | Ref. |
|---|---|---|---|---|
| 1 | MW-assisted biochar prepared from cotton stalk BC-10 and BC-10 (with Fe) | CO/H2 = 1.02 Syngas Production = 52.5 mL/min Syngas yield = 93.2% | Tconversion = 800 °C MW power = max 3 KW | [67] |
| 2 | Biochar prepared from hawthorn seed BC-11 | CO/H2 = 0.7 ΧCO2 = 68%; ΧCH4 = 84% Syngas yield = N.R. | Tconversion = N.R. (MW) MW power = 900 W | [68] |
| 3 | Biochar prepared from lotus stems BC-12 | CO/H2 = 0.33 Syngas yield = N.R. | Tconversion = 500 °C | [69] |
| 4 | W-modified biochar, prepared from pine wood W@BC-13 | CO/H2 = 1.08 ΧCO2 = 95%; ΧCH4 = 83% Syngas yield = N.R. | Tconversion = 850 °C | [70] |
| 5 | Co-modified biochar, prepared from oil palm shell Co@BC-14 | CO/H2 = 0.5 ΧCO2 = 17.5%; ΧCH4 = 15% Syngas yield = 60% | Tconversion = 750 °C | [71] |
| 6 | Co-modified biochar, prepared from char residues Co@BC-15 | CO/H2 = 0.45 ΧCO2 = 95%; ΧCH4 = 94% Syngas yield = 97% | Tconversion = 850 °C | [72] |
| 7 | Ni-modified biochar, prepared from palm kernel shell Ni@BC-16-P and Ni@BC-16-C | (For Ni@BC-16-C) CO/H2 = 4.6 ΧCO2 = 31%; ΧCH4 = 28% Syngas yield = 17% | Tconversion = 800 °C | [73] |
| 8 | Ni-Co-modified biochar, prepared from coconut shell Ni-Co@BC-17 | CO/H2 = N.R. ΧCO2 = 94%; ΧCH4 = 97.5% Syngas yield = N.R. | Tconversion = 900 °C | [74] |
| 9 | Ni-Fe-modified biochar, prepared from rice husks Ni-Fe@BC-18; Ni@BC-18; Fe@BC-18 | CO/H2 = 0.55 Syngas yield = 2.1 L/g cat | Tconversion = 850 °C | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zomorodbakhsh, S.; Dias, L.D.; Calvete, M.J.F.; Peixoto, A.F.; Carrilho, R.M.B.; Pereira, M.M. Biochar-Based Materials for Catalytic CO2 Valorization. Catalysts 2025, 15, 568. https://doi.org/10.3390/catal15060568
Zomorodbakhsh S, Dias LD, Calvete MJF, Peixoto AF, Carrilho RMB, Pereira MM. Biochar-Based Materials for Catalytic CO2 Valorization. Catalysts. 2025; 15(6):568. https://doi.org/10.3390/catal15060568
Chicago/Turabian StyleZomorodbakhsh, Shahab, Lucas D. Dias, Mário J. F. Calvete, Andreia F. Peixoto, Rui M. B. Carrilho, and Mariette M. Pereira. 2025. "Biochar-Based Materials for Catalytic CO2 Valorization" Catalysts 15, no. 6: 568. https://doi.org/10.3390/catal15060568
APA StyleZomorodbakhsh, S., Dias, L. D., Calvete, M. J. F., Peixoto, A. F., Carrilho, R. M. B., & Pereira, M. M. (2025). Biochar-Based Materials for Catalytic CO2 Valorization. Catalysts, 15(6), 568. https://doi.org/10.3390/catal15060568












