Facile and Low-Cost Fabrication of ZnO/Kaolinite Composites by Modifying the Kaolinite Composition for Efficient Degradation of Methylene Blue Under Sunlight Illumination
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure, Morphology, Surface Functional Group and Optical Studies
2.2. Photocatalytic Performance of As-Synthesized ZnO/Kaolinite Composites
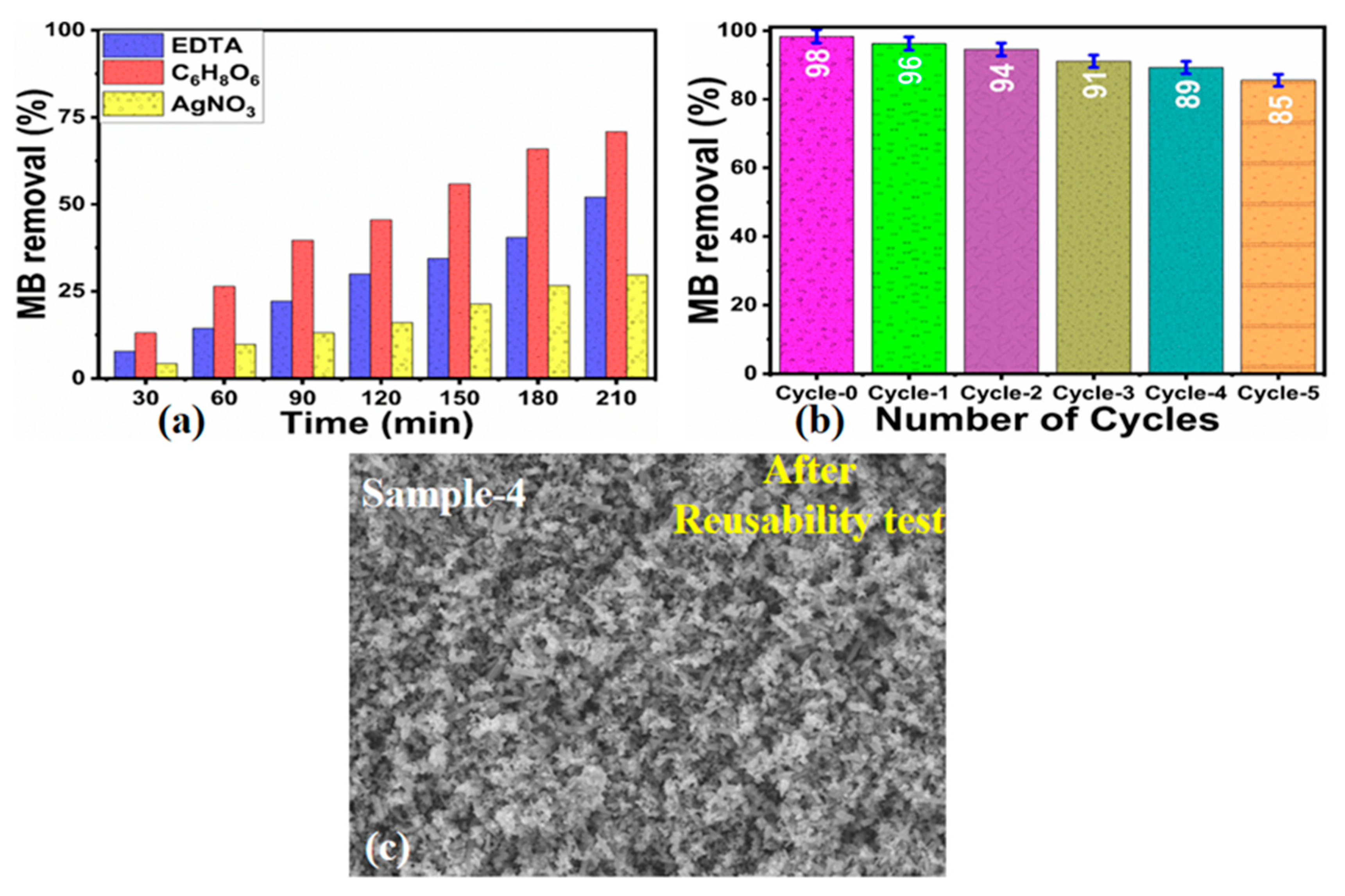
2.3. Scavenger and Reusability Tests
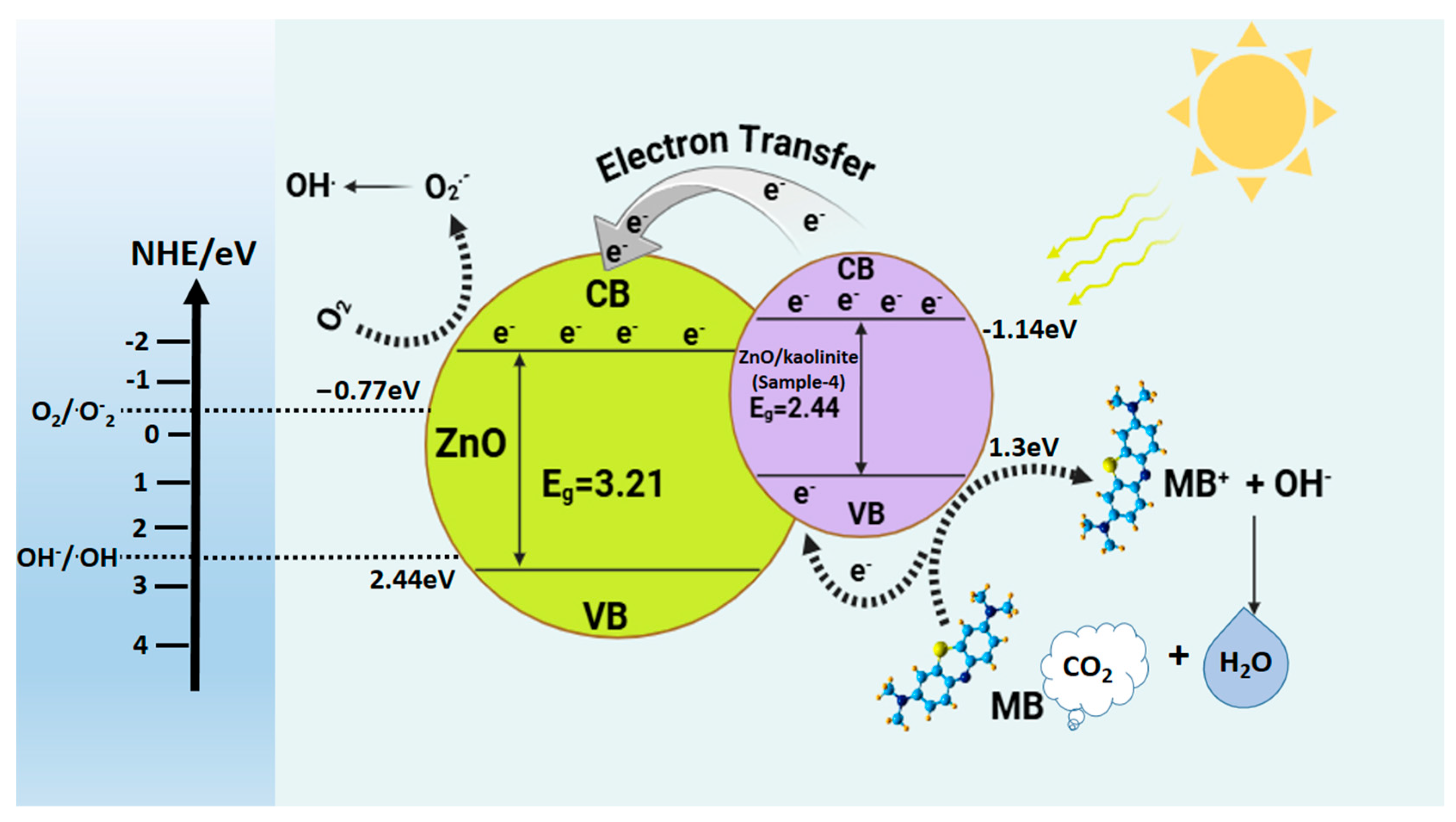
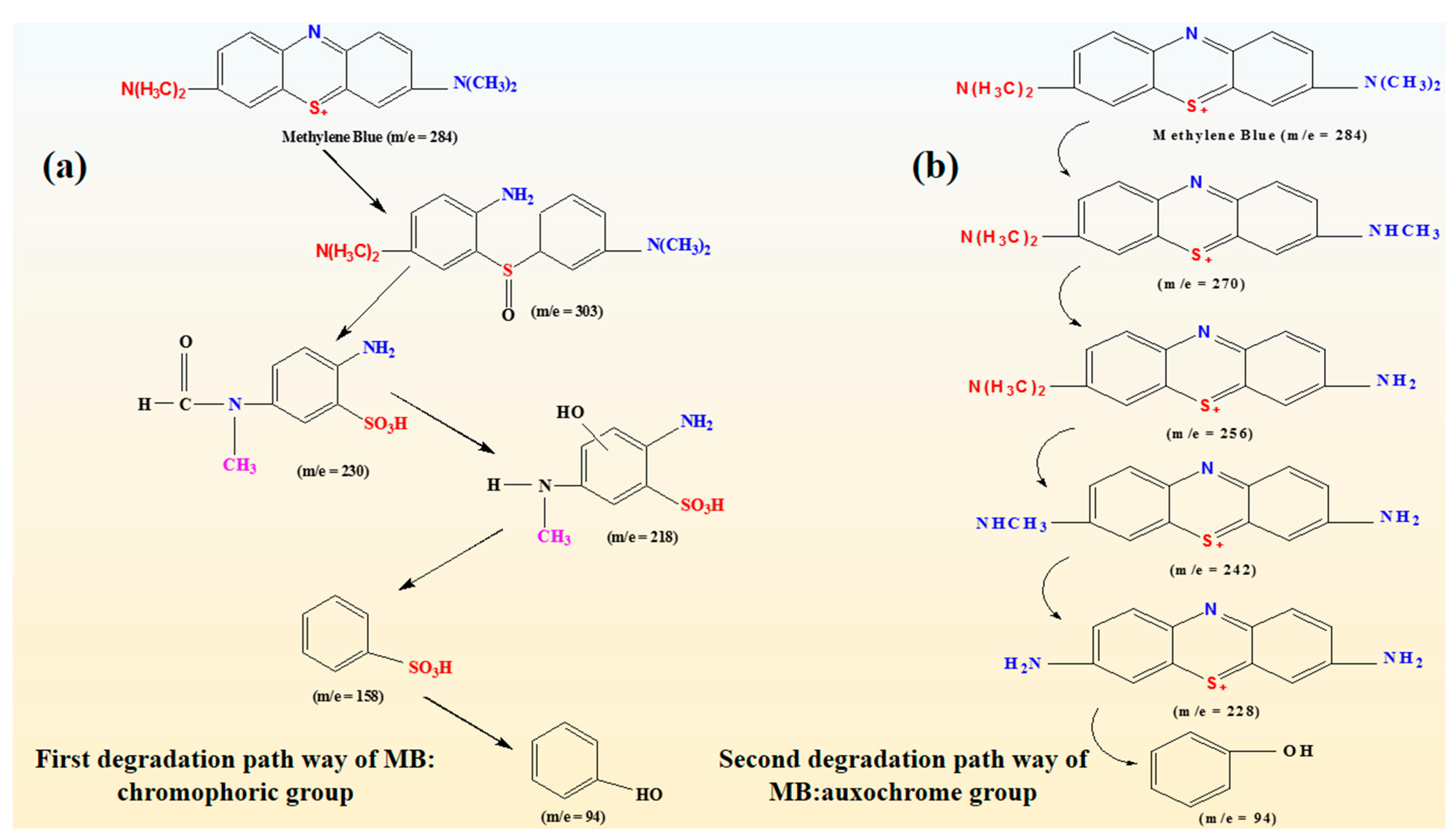
2.4. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemical Reagents
3.2. Synthesis of ZnO/Kaolinite Composites Through Hydrothermal Process
3.3. Structural Analysis
3.4. Photocatalytic Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, K.S.; Atlabachew, M.; Aragaw, B.A.; Asmare, Z.G. Synthesis of Kaolin-Supported Nickel Oxide Composites for the Catalytic Oxidative Degradation of Methylene Blue Dye. ACS Omega 2024, 9, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Mushtaq, A. Untreated wastewater reasons and causes: A review of most affected areas and cities. Int. J. Chem. Biochem. Sci. 2023, 23, 121–143. [Google Scholar]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 4, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cheng, L.; Ma, Z.; Weng, X.; Gao, J. Integrated nanostructures of TiO2/g-C3N4/diatomite based on low-grade diatomite as efficient catalyst for photocatalytic degradation of methylene blue: Performance and mechanism. Catalysts 2023, 13, 796. [Google Scholar] [CrossRef]
- Jida, S.M.; Zerefa, E.A. Preparation and photocatalysis of ZnO/bentonite based on adsorption and photocatalytic activity. Mater. Res. Express 2023, 10, 035502. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater. Sci. Eng. Int. J. 2017, 3, 1. [Google Scholar] [CrossRef]
- Tran, G.H.; Tran, T.K.; Leu, H.J.; Richards, D.; Lo, S.S. An integrated system combining electrochemical oxidation and filtration processes to remove chlorine from pharmaceutical industry wastewater. Arab. J. Chem. 2024, 17, 105611. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, G.; Ibhadon, A.O.; Salunke, D.B.; Sinha, A.S.; Kansal, S.K. A Facile synthesis of silver modified ZnO nanoplates for efficient removal of ofloxacin drug in aqueous phase under solar irradiation. J. Environ. Chem. Eng. 2018, 6, 3621–3630. [Google Scholar] [CrossRef]
- Iqbal, J.; Shah, N.S.; Sayed, M.; Khan, J.A.; Muhammad, N.; Khan, Z.U.; Naseem, M.; Howari, F.M.; Nazzal, Y.; Niazi, N.K.; et al. Synthesis of nitrogen-doped Ceria nanoparticles in deep eutectic solvent for the degradation of sulfamethaxazole under solar irradiation and additional antibacterial activities. Chem. Eng. J. 2020, 394, 124869. [Google Scholar] [CrossRef]
- Shaikh, B.; Bhatti, M.A.; Shah, A.A.; Tahira, A.; Shah, A.K.; Usto, A.; Aftab, U.; Bukhari, S.I.; Alshehri, S.; Shah Bukhari, S.N.; et al. Mn3O4@ ZnO hybrid material: An excellent photocatalyst for the degradation of synthetic dyes including methylene blue, methyl orange and malachite green. Nanomaterials 2022, 21, 3754. [Google Scholar] [CrossRef]
- Imgharn, A.; Hsini, A.; Naciri, Y.; Laabd, M.; Kabli, H.; Elamine, M.; Lakhmiri, R.; Souhail, B.; Albourine, A. Synthesis and characterization of polyaniline-based biocomposites and their application for effective removal of Orange G dye using adsorption in dynamic regime. Chem. Phys. Lett. 2021, 778, 138811. [Google Scholar] [CrossRef]
- Dutta, P.; Rabbi, M.; Sufian, M.; Mahjebin, S. Effects of textile dyeing effluent on the environment and its treatment: A review. Eng. Appl. Sci. Lett. (EASL) 2022, 5, 1–7. [Google Scholar] [CrossRef]
- Shaida, M.A.; Verma, S.; Talukdar, S.; Kumar, N.; Mahtab, M.S.; Naushad, M.; Farooqi, I.H. Critical analysis of the role of various iron-based heterogeneous catalysts for advanced oxidation processes: A state of the art review. J. Mol. Liquids. 2023, 374, 121259. [Google Scholar] [CrossRef]
- Freitas, W.; Trigueiro, P.; Marinho, T.; Honorio, L.M.; Silva-Filho, E.C.; Furtini, M.B.; Cecília, J.A.; Fonseca, M.G.; Osajima, J. The role of clay mineral-derived photocatalysts in insights of remediation. Ceramics 2022, 5, 862–882. [Google Scholar] [CrossRef]
- Alshgari, R.A.; Ujjan, Z.A.; Shah, A.A.; Bhatti, M.A.; Tahira, A.; Shaikh, N.M.; Kumar, S.; Ibupoto, M.H.; Elhawary, A.; Nafady, A.; et al. ZnO nanostructures doped with various chloride ion concentrations for efficient photocatalytic degradation of methylene blue in alkaline and acidic media. Molecules 2022, 27, 8726. [Google Scholar] [CrossRef]
- Fazli, A.; Brigante, M.; Khataee, A.; Mailhot, G. Fe2.5Co0.3Zn0.2O4/CuCr-LDH as a visible-light-responsive photocatalyst for the degradation of caffeine, bisphenol A, and simazine in pure water and real wastewater under photo-Fenton-like degradation process. Chemosphere 2022, 291, 132920. [Google Scholar] [CrossRef]
- Taylor, C.M.; Ramirez-Canon, A.; Wenk, J.; Mattia, D. Enhancing the photo-corrosion resistance of ZnO nanowire photocatalysts. J. Hazard. Mater. 2019, 378, 120799. [Google Scholar] [CrossRef]
- Chuaicham, C.; Trakulmututa, J.; Shu, K.; Shenoy, S.; Srikhaow, A.; Zhang, L.; Mohan, S.; Sekar, K.; Sasaki, K. Recent clay-based photocatalysts for wastewater treatment. Separations 2023, 10, 77. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Gilani, S.J.; Shah, A.A.; Channa, I.A.; Almani, K.F.; Chandio, A.D.; Halepoto, I.A.; Tahira, A.; Bin Jumah, M.N.; Ibupoto, Z.H. Effective removal of methylene blue by surface alteration of TiO2 with Ficus Carica leaf extract under visible light. Nanomaterials 2022, 12, 2766. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent advances in ZnO-based nanostructures for the photocatalytic degradation of hazardous, non-biodegradable medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Abid, M.; Ben Haj Amara, A.; Bechelany, M. Halloysite-TiO2 Nanocomposites for Water Treatment: A Review. Nanomaterials 2023, 13, 1578. [Google Scholar] [CrossRef]
- Choudhary, N.; Yadav, V.K.; Ali, H.; Ali, D.; Almutairi, B.O.; Cavalu, S.; Patel, A. Remediation of methylene blue dye from wastewater by using zinc oxide nanoparticles loaded on nanoclay. Water 2023, 7, 1427. [Google Scholar] [CrossRef]
- Buyondo, K.A.; Kasedde, H.; Kirabira, J.B. A comprehensive review on kaolin as pigment for paint and coating: Recent trends of chemical-based paints, their environmental impacts and regulation. Case Stud. Chem. Environ. Eng. 2022, 6, 100244. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Aamer, N.; Abdallah, H.M.; López-Maldonado, E.A.; Mohamed, Y.M.; El Nazer, H.A.; Mohamed, R.R. Novel high-efficient adsorbent based on modified gelatin/montmorillonite nanocomposite for removal of malachite green dye. Sci. Rep. 2024, 14, 1228. [Google Scholar] [CrossRef]
- Chellapandi, T.; Madhumitha, G.; Roopan, S.M.; Elamathi, M.; Leeladevi, K.; Nagarajan, E.R.; Vadivel, D.; Dondi, D. Construction of ZnO nanoparticles on the layered aluminosilicate Montmorillonite K30 nanocomposite and its enhanced photocatalytic removal performance. Opt. Mater. 2023, 142, 114099. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Wang, J.; Wang, Y.; Wei, J.; Zhan, H.; Guo, R.; Zhang, Y. Synthesis and characterization of rectorite/ZnO/TiO2 composites and their properties of adsorption and photocatalysis for the removal of methylene blue dye. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 729–735. [Google Scholar] [CrossRef]
- Vaizoğullar, A.İ. TiO2/ZnO supported on sepiolite: Preparation, structural characterization, and photocatalytic degradation of flumequine antibiotic in aqueous solution. Chem. Eng. Commun. 2017, 204, 689–697. [Google Scholar] [CrossRef]
- Mylarappa, M.; Raghavendra, N.; Bhumika, N.R.; Chaithra, C.H.; Nagalaxmi, B.N.; Kumara, K.S. Study of ZnO nanoparticle-supported clay minerals for electrochemical sensors, photocatalysis, and antioxidant applications. ChemPhysMater 2024, 3, 83–93. [Google Scholar]
- da Trindade, L.G.; Zanchet, L.; Souza, J.C.; Roveda, A.C., Jr.; Paiva, R.; Zanette, T.; Bernardo-Gusmão, K.; Martini, E.M.; Longo, E.; Ticianelli, E.A. Mesoporous ZrO2/C and ZnO/C nanocomposites derived from MOFs for SPEEK-based proton exchange membrane. Ionics 2024, 10, 6321–6339. [Google Scholar] [CrossRef]
- Freitas, W.A.; Soares, B.E.; Rodrigues, M.S.; Trigueiro, P.; Honorio, L.M.; Peña-Garcia, R.; Alcântara, A.C.; Silva-Filho, E.C.; Fonseca, M.G.; Furtini, M.B.; et al. Facile synthesis of ZnO-clay minerals composites using an ultrasonic approach for photocatalytic performance. J. Photochem. Photobiol. A Chem. 2022, 429, 113934. [Google Scholar] [CrossRef]
- Ighnih, H.; Haounati, R.; Malekshah, R.E.; Ouachtak, H.; Toubi, Y.; Alakhras, F.; Jada, A.; Ait Addi, A. Sunlight driven photocatalytic degradation of RhB dye using composite of bismuth oxy-bromide kaolinite BiOBr@ Kaol: Experimental and molecular dynamic simulation studies. J. Photochem. Photobiol. A Chem. 2023, 445, 115071. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.O.; Ndamitso, M.M.; Abdulkareem, A.S.; Shuaib, D.T.; Amigun, A.T.; Abubakar, H.L. Facile synthesis and characterization of TiO2 nanoparticles: X-ray peak profile analysis using Williamson–Hall and Debye–Scherrer methods. Int. Nano Lett. 2021, 3, 241–261. [Google Scholar] [CrossRef]
- Moya, J.S.; Cabal, B.; López-Esteban, S.; Bartolomé, J.F.; Sanz, J. Significance of the formation of pentahedral aluminum in the reactivity of calcined kaolin/metakaolin and its applications. Ceram. Int. 2024, 50, 1329–1340. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Cheng, H. Recent advances in kaolinite-based material for photocatalysts. Chin. Chem. Lett. 2021, 32, 2617–2628. [Google Scholar] [CrossRef]
- Alvarez-Coscojuela, A.; Marco-Gibert, J.; Mañosa, J.; Formosa, J.; Chimenos, J.M. Thermal activation of kaolinite through potassium acetate intercalation: A structural and reactivity study. Appl. Clay Sci. 2024, 259, 107515. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, S.; Devi, J.; Sharma, A.; Kumar, A. Decoration of 1, 3 oxazole modified g-C3N4 by Bio-synthesized Ag nanoparticle for the photodegradation of pharmaceutical effluent: Clotrimazole. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Devi, J.; Devi, S.; Sharma, A.; Kumar, A. Fabrication of CMC-PVP based RGO modified magnetic hydrogel for the adsorption and photo-reduction of hexavalent chromium from simulated waste water. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalination Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Qin, F.; Xia, Y.; Yang, D.; Xiao, T.; Zhu, X.; Feng, W.; Qi, Z. Enhanced photocatalytic activity of g-C3N4/Bi2WO6 heterojunction via Z-scheme charge-transfer mechanism. J. Mol. Struct. 2024, 1316, 139023. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 44, 27257–27266. [Google Scholar] [CrossRef] [PubMed]
- Gul, T.; Khan, I.; Ahmad, B.; Ahmad, S.; Alsaiari, A.A.; Almehmadi, M.; Abdulaziz, O.; Alsharif, A.; Khan, I.; Saeed, K. Efficient photodegradation of methyl red dye by kaolin clay supported zinc oxide nanoparticles with their antibacterial and antioxidant activities. Heliyon 2023, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.F.; Barai, H.R.; Islam, M.M.; Susan, M.A.; Joo, S.W.; Miran, M.S. ZnO nanocomposites supported by acid-activated kaolinite as photocatalysts for the enhanced photodegradation of an organic dye. Mater. Today Commun. 2023, 36, 106563. [Google Scholar] [CrossRef]
- Boutra, B.; Trari, M. Solar photodegradation of a textile azo dye using synthesized ZnO/Bentonite. Water Sci. Technol. 2017, 75, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; He, G.; Lei, Z.; Wu, W. Preparation and photocatalytic performance of ZnO/Sepiolite composite materials. Adv. Mater. Sci. Eng. 2021, 1, 5357843. [Google Scholar] [CrossRef]
- Fufa, P.A.; Feysia, G.B.; Gultom, N.S.; Kuo, D.H.; Chen, X.; Kabtamu, D.M.; Zelekew, O.A. Visible light-driven photocatalytic activity of Cu2O/ZnO/Kaolinite-based composite catalyst for the degradation of organic pollutant. Nanotechnology 2022, 33, 315601. [Google Scholar] [CrossRef]
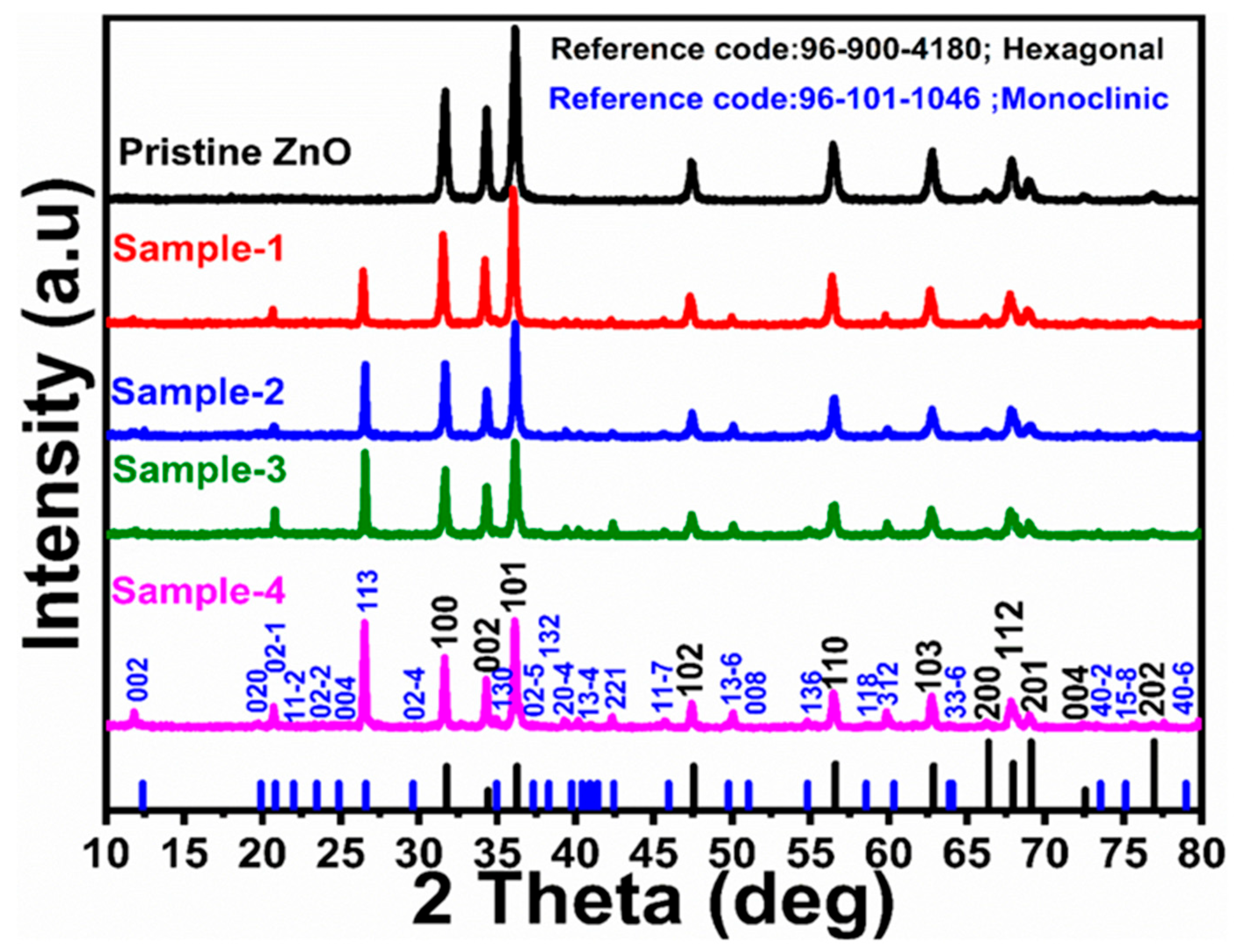


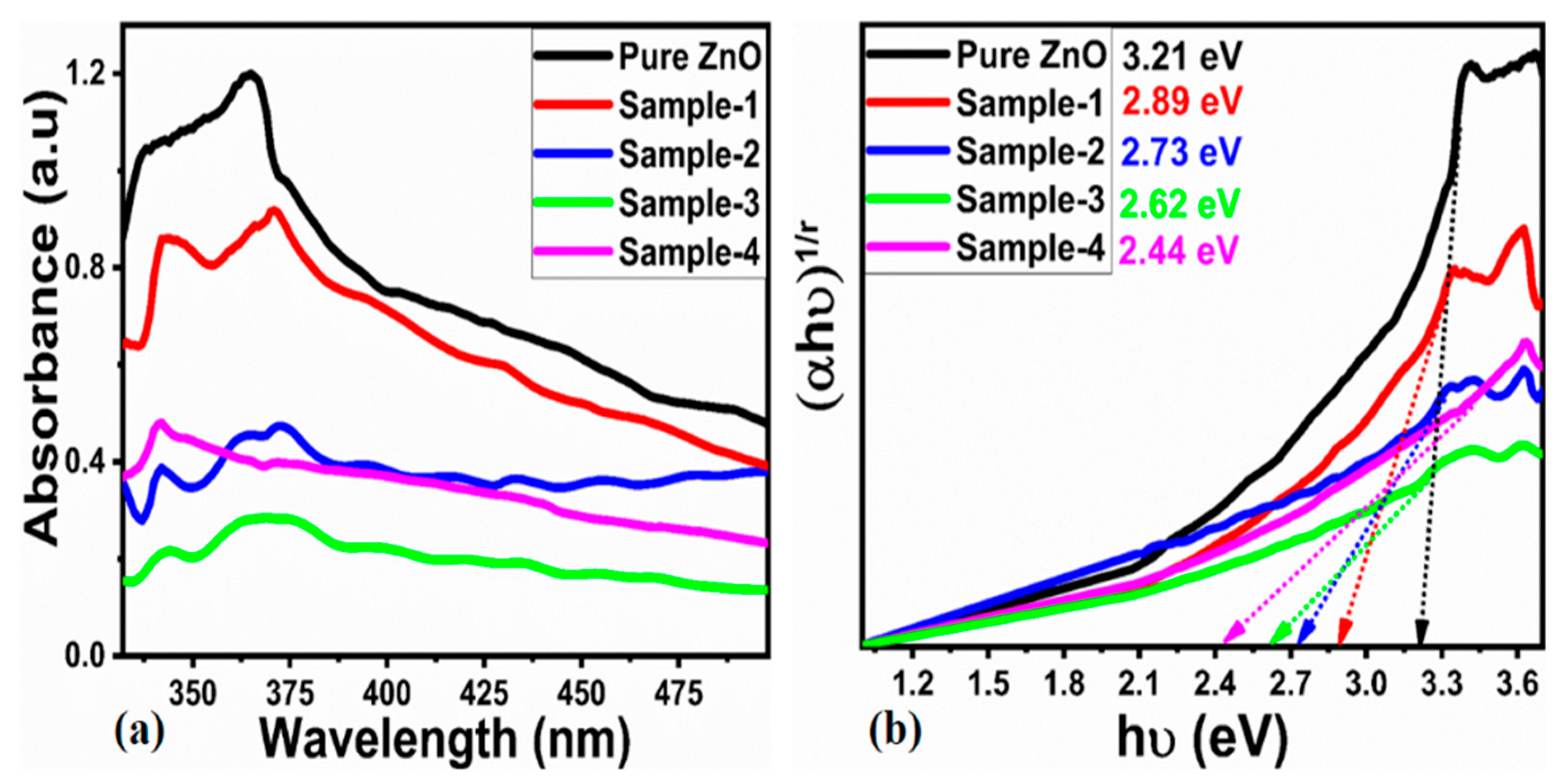


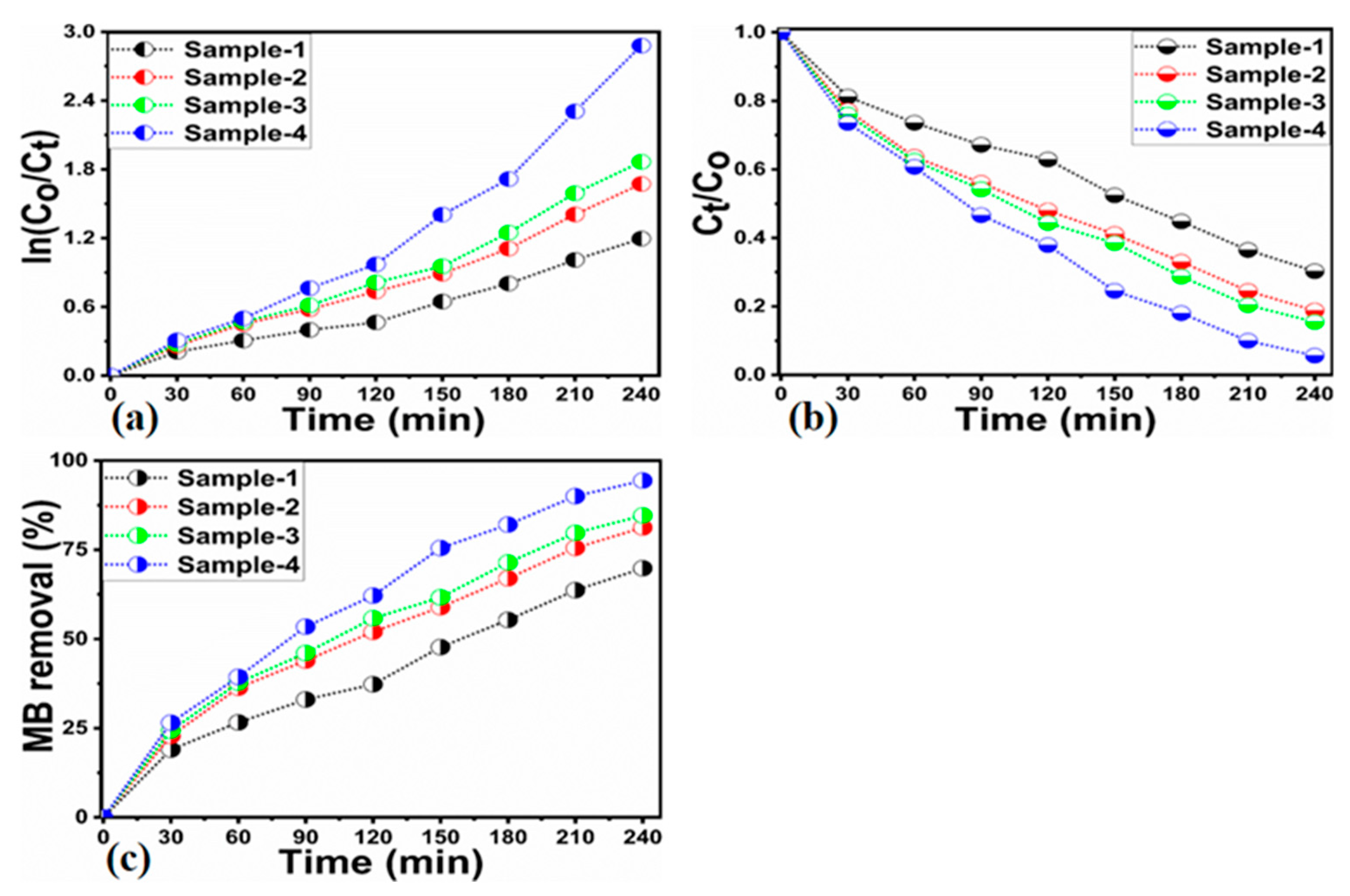

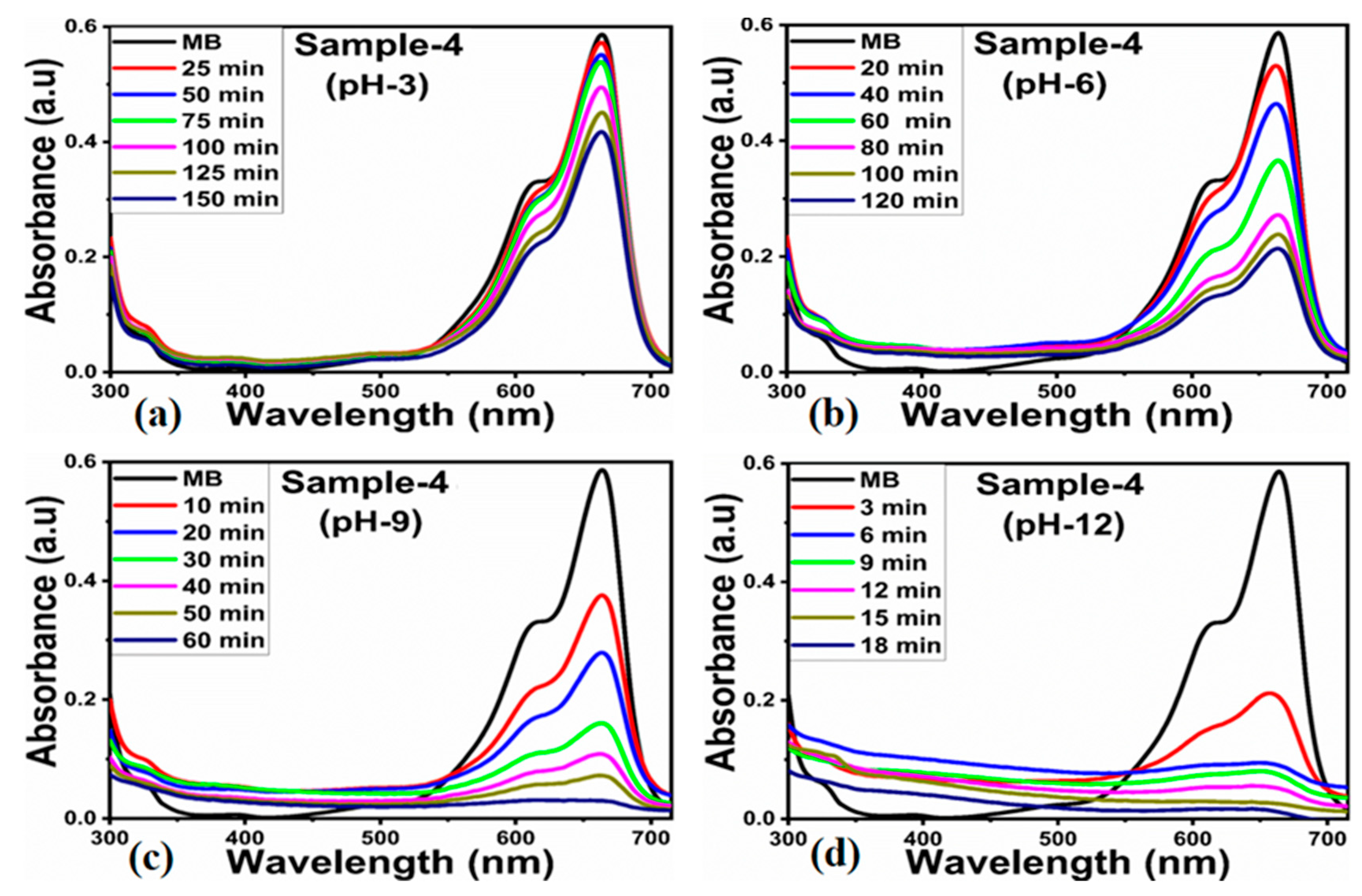

| Sample ID | 2 Theta (deg) | Peak Position | FWHM | Height | Average Size (nm) |
|---|---|---|---|---|---|
| Pristine ZnO | 100 | 31.68 | 0.31953 | 439 | 27 |
| 002 | 34.32 | 0.27503 | 363 | ||
| 101 | 36.14 | 0.31294 | 686 | ||
| Sample-1 | 100 | 31.54 | 0.24833 | 373 | 33 |
| 002 | 34.22 | 0.22486 | 259 | ||
| 101 | 36 | 0.27881 | 548 | ||
| Sample-2 | 100 | 31.7 | 0.13271 | 293 | 65 |
| 002 | 34.32 | 0.12765 | 189 | ||
| 101 | 36.14 | 0.12615 | 460 | ||
| Sample-3 | 100 | 31.68 | 0.21342 | 267 | 35 |
| 002 | 34.34 | 0.22506 | 200 | ||
| 101 | 36.12 | 0.27395 | 379 | ||
| Sample-4 | 100 | 31.64 | 0.20004 | 288 | 39 |
| 002 | 34.3 | 0.2103 | 192 | ||
| 101 | 36.12 | 0.23353 | 437 |
| Photocatalyst | Synthesis Method | Pollutant | Catalyst Dose | Removal (%) | References |
|---|---|---|---|---|---|
| ZnO/KC NCs | Chemical reduction method | MR (100 ppm) | 0.02 g | 90% | [42] |
| HC/ZnO NC | Chemical method | MB (25 ppm) | 20 mg | 90% to 97% | [22] |
| AAK/ZnO nanocomposites | Wet chemical precipitation method | MB | -------- | 98% | [43] |
| ZnO(7.5%)/Bentonite | Impregnation method | SR-3BL (5–75 mg/L) | 0.25–1 gL−1 | 70% | [44] |
| ZnO/MK30 nanocomposites | Ultrasonication technique | MB | -------- | 95.76% | [25] |
| ZnO/sepiolite photolytic composites | Sol–gel method | MB (0.015 g/L) | 20 mg | 93.5% | [45] |
| Cu2O/ZnO/Kaolinite-based composite | Co-precipitation method | MB | -------- | 93% | [46] |
| ZnO/Kaolinite Sample-4 | Hydrothermal method | MB (1.5 × 10−5 M) | 15 mg | 98% | Herein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, H.; Saleem, R.; Halepoto, I.A.; Barhaam, M.S.; Soomro, M.Y.; Abbasi, M.A.; Shaikh, N.M.; Bhatti, M.A.; Wassan, S.H.; Dawi, E.; et al. Facile and Low-Cost Fabrication of ZnO/Kaolinite Composites by Modifying the Kaolinite Composition for Efficient Degradation of Methylene Blue Under Sunlight Illumination. Catalysts 2025, 15, 566. https://doi.org/10.3390/catal15060566
Shaikh H, Saleem R, Halepoto IA, Barhaam MS, Soomro MY, Abbasi MA, Shaikh NM, Bhatti MA, Wassan SH, Dawi E, et al. Facile and Low-Cost Fabrication of ZnO/Kaolinite Composites by Modifying the Kaolinite Composition for Efficient Degradation of Methylene Blue Under Sunlight Illumination. Catalysts. 2025; 15(6):566. https://doi.org/10.3390/catal15060566
Chicago/Turabian StyleShaikh, Humera, Ramsha Saleem, Imran Ali Halepoto, Muhammad Saajan Barhaam, Muhammad Yousuf Soomro, Mazhar Ali Abbasi, Nek Muhammad Shaikh, Muhammad Ali Bhatti, Shoukat Hussain Wassan, Elmuez Dawi, and et al. 2025. "Facile and Low-Cost Fabrication of ZnO/Kaolinite Composites by Modifying the Kaolinite Composition for Efficient Degradation of Methylene Blue Under Sunlight Illumination" Catalysts 15, no. 6: 566. https://doi.org/10.3390/catal15060566
APA StyleShaikh, H., Saleem, R., Halepoto, I. A., Barhaam, M. S., Soomro, M. Y., Abbasi, M. A., Shaikh, N. M., Bhatti, M. A., Wassan, S. H., Dawi, E., Tahira, A., Tonezzer, M., & Ibupoto, Z. H. (2025). Facile and Low-Cost Fabrication of ZnO/Kaolinite Composites by Modifying the Kaolinite Composition for Efficient Degradation of Methylene Blue Under Sunlight Illumination. Catalysts, 15(6), 566. https://doi.org/10.3390/catal15060566








