The Construction and Photocatalytic Application of Covalent Triazine Framework (CTF)-Based Composites: A Brief Review
Abstract
1. Introduction
2. Composite Design
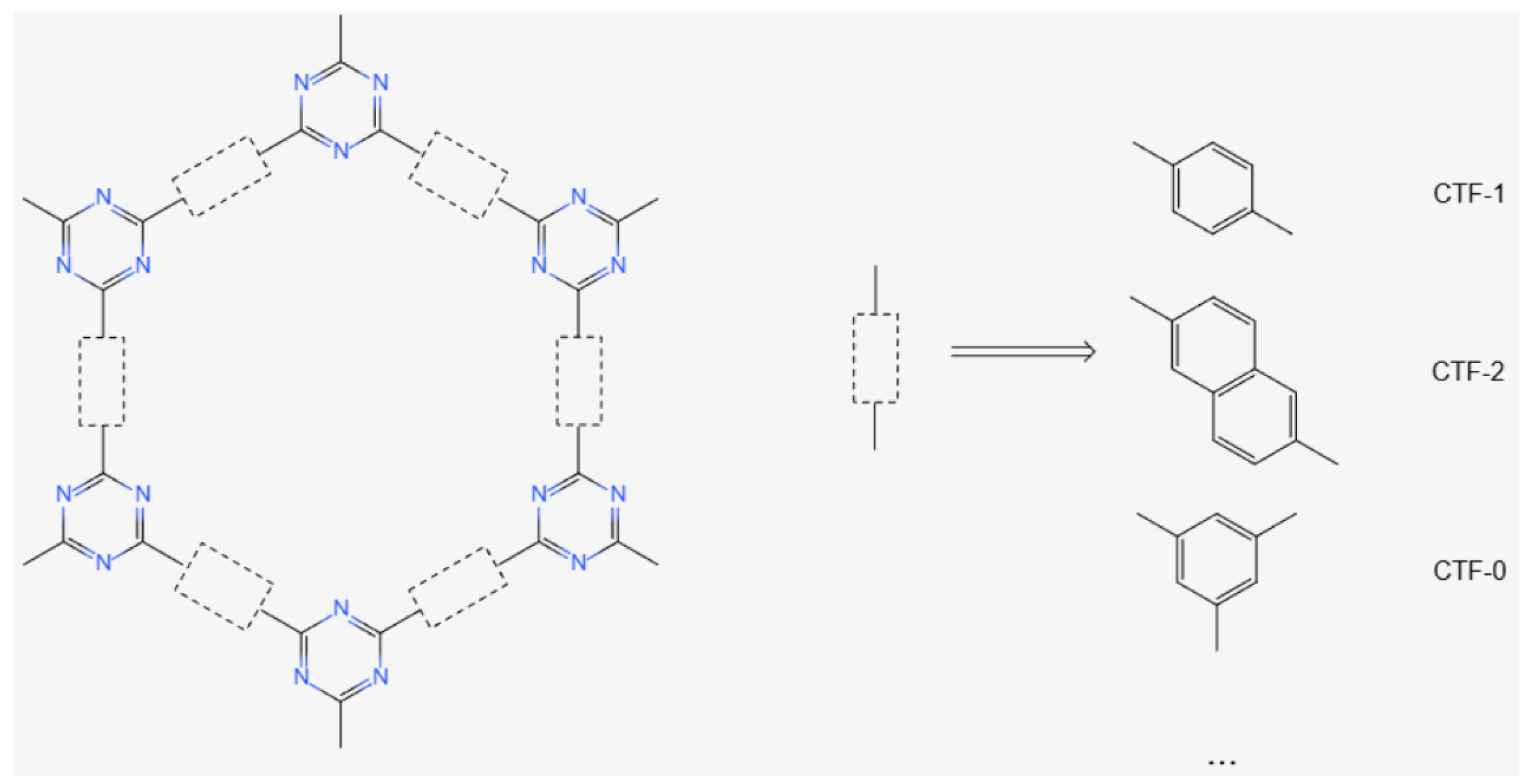
2.1. Structure and Synthesis of CTFs
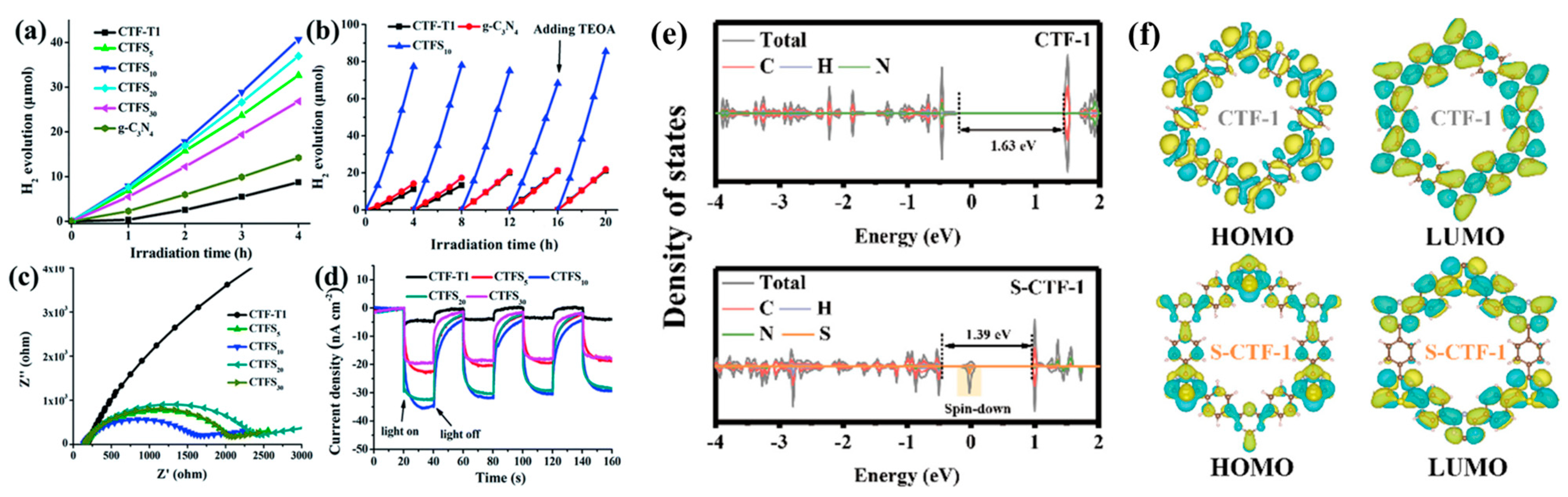
2.2. Chemical Doping
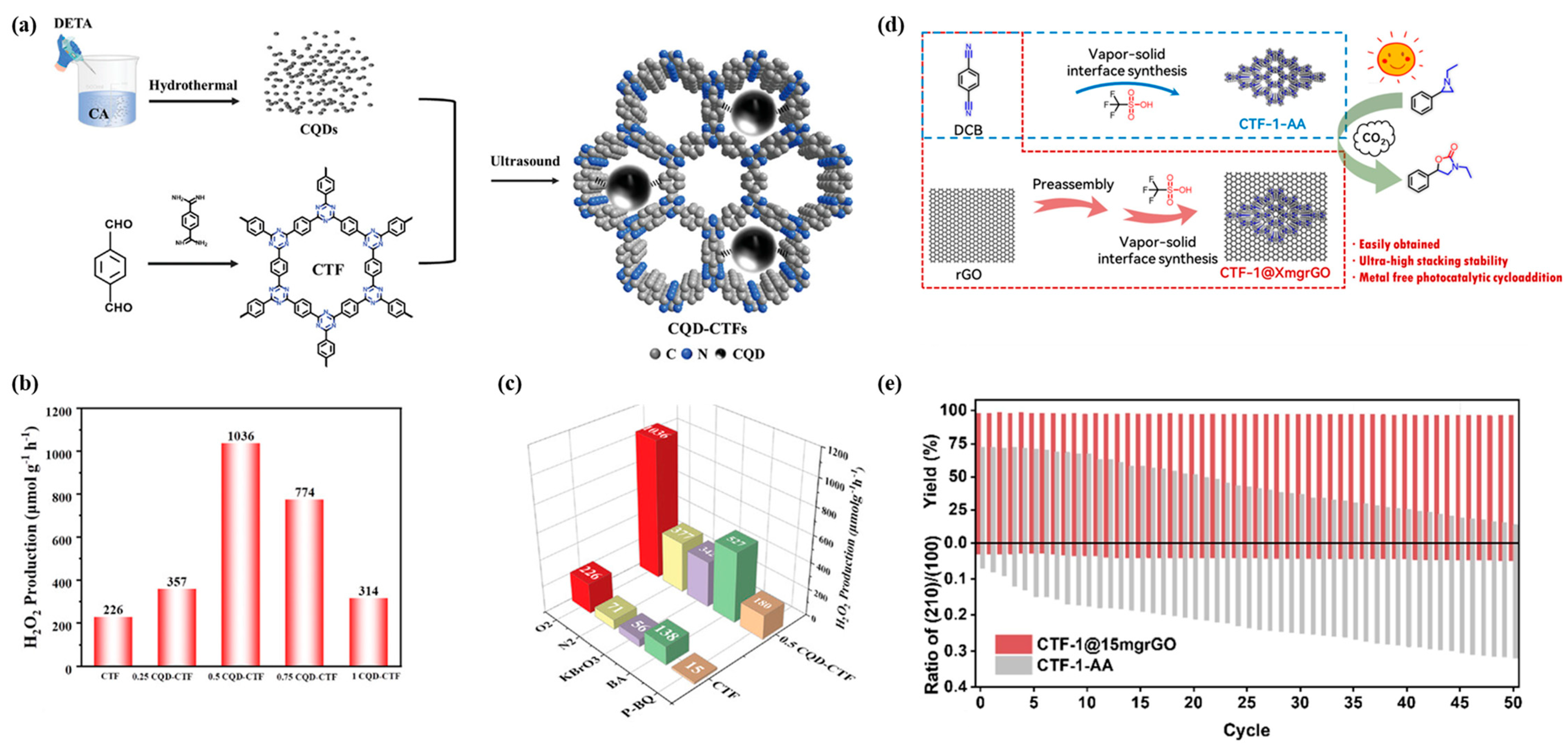
2.3. Composite with Non-Metallic Materials
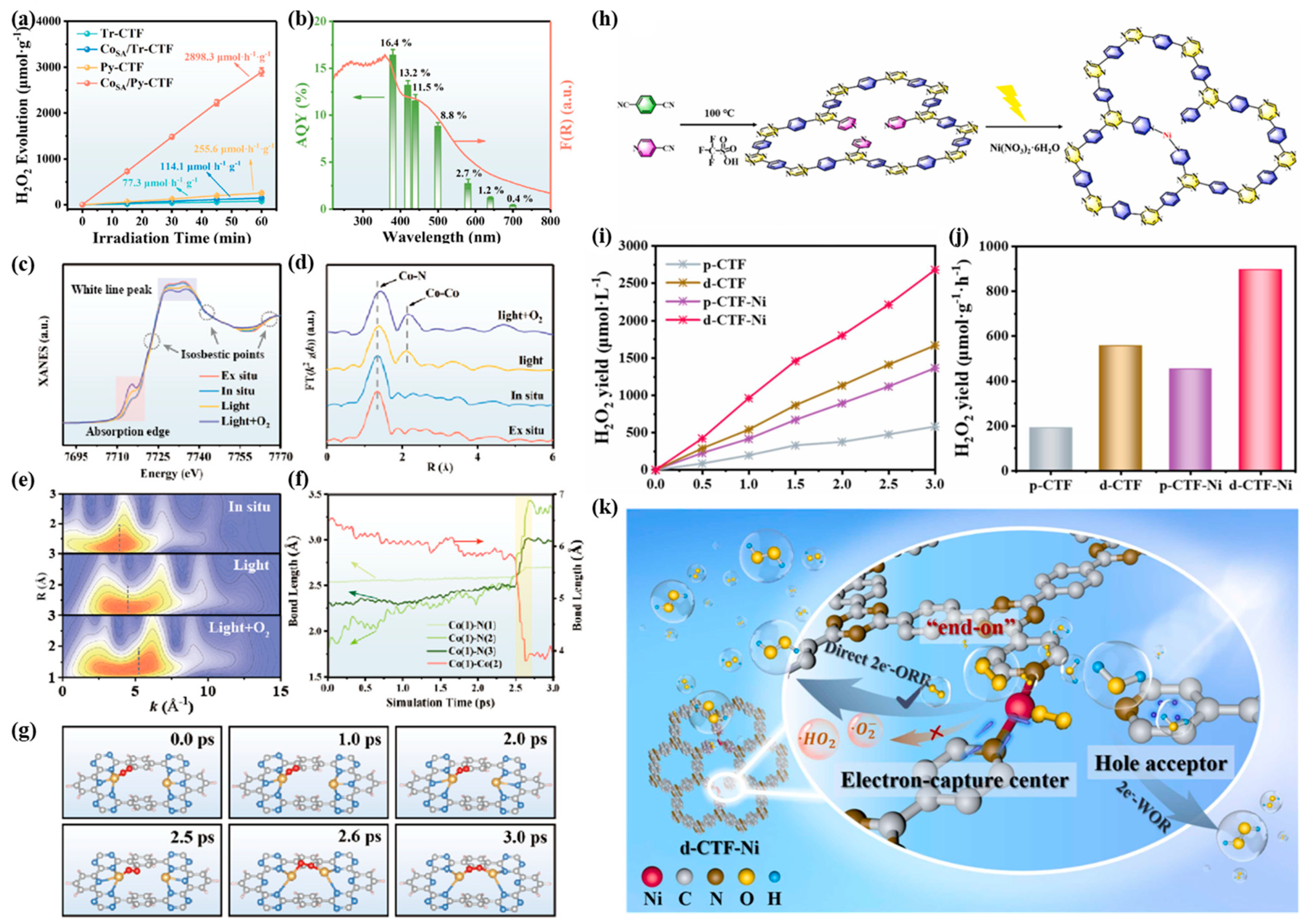
2.4. Single Atoms
2.5. Compounded with Metal Oxides
2.6. Compounded with Metal Sulfides
3. Photocatalytic Application of CTF-Based Composites
3.1. Photocatalytic Hydrogen Evolution
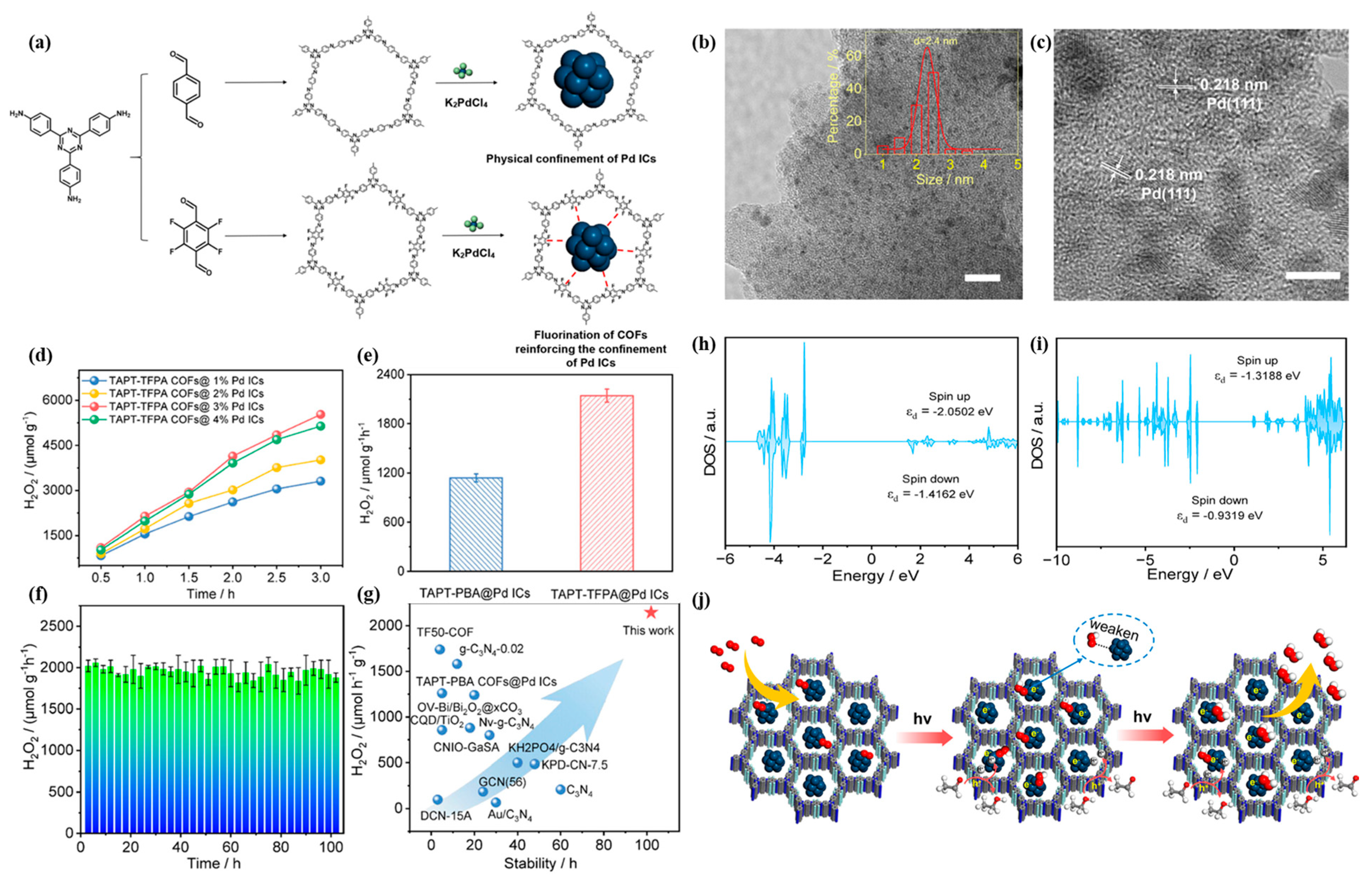
3.2. Photocatalytic Production of Hydrogen Peroxide
3.3. Photocatalytic Carbon Dioxide Reduction
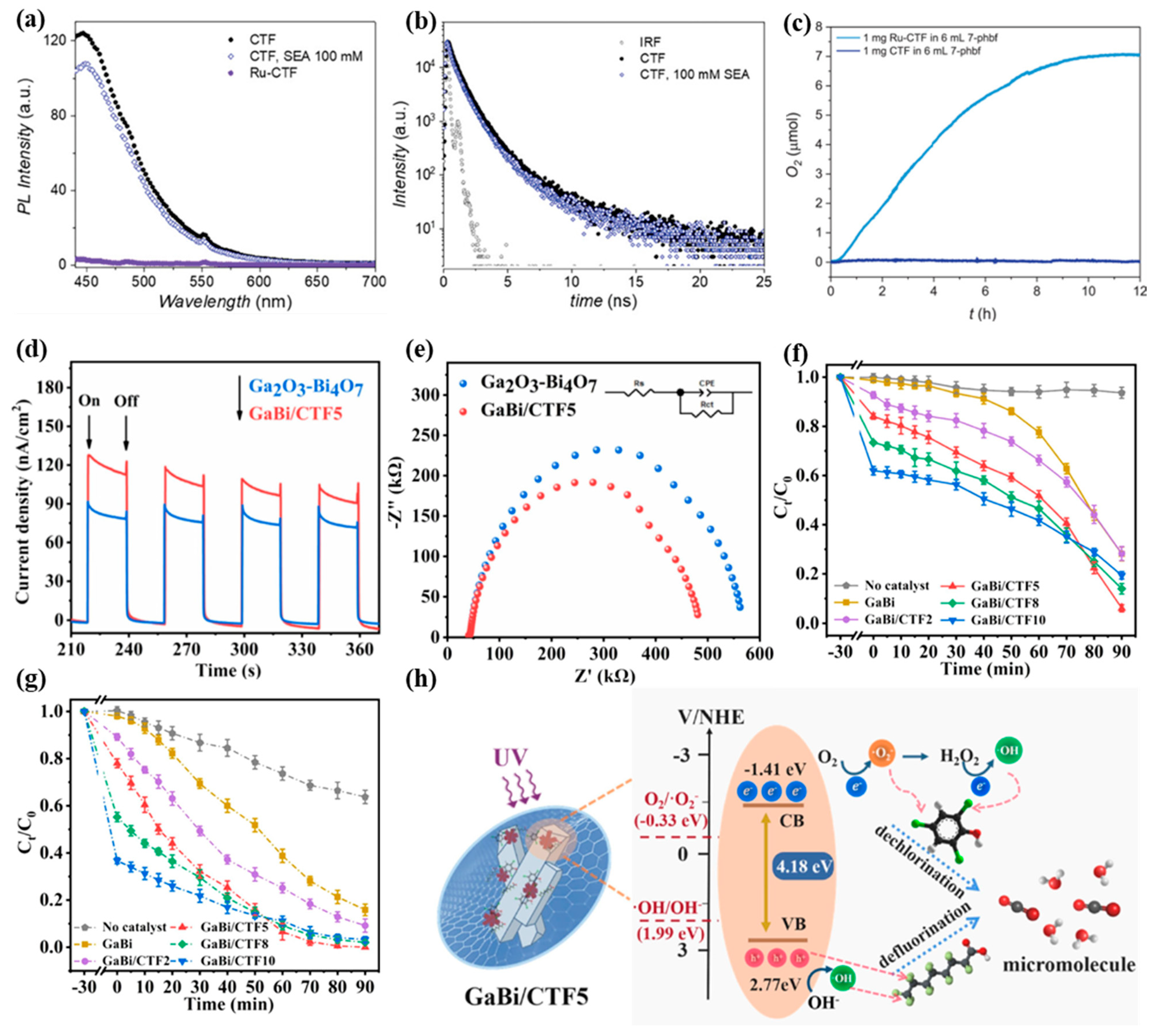
3.4. Other Photocatalytic Reactions
4. Conclusions and Outlook
4.1. Superior Composite Materials
4.2. Deeper Understanding of Reaction Mechanisms
4.3. Large-Scale Applications
Author Contributions
Funding
Conflicts of Interest
References
- Chen, J.; Xie, Q.; Shahbaz, M.; Song, M.; Wu, Y. The Fossil Energy Trade Relations Among BRICS Countries. Energy 2021, 217, 119383. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based Photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Qi, Y.; Zhang, J.; Wang, N.; Liu, M.; Zhang, B.; Cai, X.; Zhang, H.; Wei, S.-H.; et al. Etched BiVO4 Photocatalyst with Charge Separation Efficiency Exceeding 90%. Nat. Commun. 2025, 16, 3776. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Pei, L.; Maitra, S.; Mao, Q.; Zheng, R.; Liu, M.; Ng, Y.H.; Zhong, J.; Chen, L.-H.; et al. Emerging Heterostructured C3N4 Photocatalysts for Photocatalytic Environmental Pollutant Elimination and Sterilization. Inorg. Chem. Front. 2023, 10, 3756–3780. [Google Scholar] [CrossRef]
- Spies, L.; Carmo, M.E.G.; Döblinger, M.; Xu, Z.; Xue, T.; Hartschuh, A.; Bein, T.; Schneider, J.; Patrocinio, A.O.T. Designing Atomically Precise and Robust COF Hybrids for Efficient Photocatalytic CO₂ Reduction. Small 2025, 21, 2500550. [Google Scholar] [CrossRef]
- Doan, T.D.; Vu, N.-N.; Hoang, T.L.G.; Nguyen-Tri, P. Metal-Organic Framework (MOF)-Based Materials for Photocatalytic Antibacterial Applications. Coord. Chem. Rev. 2025, 523, 216298. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Q.; Wang, S.; Li, Z.; Li, B.; Jin, S.; Tan, B. Crystalline Covalent Triazine Frameworks by In Situ Oxidation of Alcohols to Aldehyde Monomers. Angew. Chem. Int. Ed. 2018, 57, 11968–11972. [Google Scholar] [CrossRef]
- Gao, Z.; Jian, Y.; Yang, S.; Xie, Q.; Ross Mcfadzean, C.J.; Wei, B.; Tang, J.; Yuan, J.; Pan, C.; Yu, G. Interfacial Ti−S Bond Modulated S-Scheme MOF/Covalent Triazine Framework Nanosheet Heterojunctions for Photocatalytic C−H Functionalization. Angew. Chem. Int. Ed. 2023, 62, e202304173. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Katekomol, P.; Roeser, J.; Bojdys, M.; Weber, J.; Thomas, A. Covalent Triazine Frameworks Prepared from 1,3,5-Tricyanobenzene. Chem. Mater. 2013, 25, 1542–1548. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Mahmood, J.; Noh, H.-J.; Seo, J.-M.; Jung, S.-M.; Shin, S.-H.; Im, Y.-K.; Jeon, I.-Y.; Baek, J.-B. Direct Synthesis of a Covalent Triazine-Based Framework from Aromatic Amides. Angew. Chem. Int. Ed. 2018, 57, 8438–8442. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, Fluorescent, Covalent Triazine-Based Frameworks via Room-Temperature and Microwave-Assisted Synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef]
- Wang, K.; Yang, L.-M.; Wang, X.; Guo, L.; Cheng, G.; Zhang, C.; Jin, S.; Tan, B.; Cooper, A. Covalent Triazine Frameworks via a Low-Temperature Polycondensation Approach. Angew. Chem. Int. Ed. 2017, 56, 14149–14153. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D. Synthesis of Luminescent Covalent–Organic Polymers for Detecting Nitroaromatic Explosives and Small Organic Molecules. Macromol. Rapid Commun. 2012, 33, 1184–1190. [Google Scholar] [CrossRef]
- Meier, C.B.; Sprick, R.S.; Monti, A.; Guiglion, P.; Lee, J.-S.M.; Zwijnenburg, M.A.; Cooper, A.I. Structure-property Relationships for Covalent Triazine-Based Frameworks: The Effect of Spacer Length on Photocatalytic Hydrogen Evolution from Water. Polymer 2017, 126, 283–290. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, J.; Zou, Y.; Di, C.-a.; Zhu, D. Chemical Doping of Organic Semiconductors for Thermoelectric Applications. Chem. Soc. Rev. 2020, 49, 7210–7228. [Google Scholar] [CrossRef]
- Lüssem, B.; Riede, M.; Leo, K. Doping of Organic Semiconductors. Phys. Status Solidi (A) 2013, 210, 9–43. [Google Scholar] [CrossRef]
- Cheng, Z.; Zheng, K.; Lin, G.; Fang, S.; Li, L.; Bi, J.; Shen, J.; Wu, L. Constructing a Novel Family of Halogen-Doped Covalent Triazine-Based Frameworks as Efficient Metal-Free Photocatalysts for Hydrogen Production. Nanoscale Adv. 2019, 1, 2674–2680. [Google Scholar] [CrossRef]
- Li, S.; Wu, M.-F.; Guo, T.; Zheng, L.-L.; Wang, D.; Mu, Y.; Xing, Q.-J.; Zou, J.-P. Chlorine-mediated Photocatalytic Hydrogen Production Based on Triazine Covalent Organic Framework. Appl. Catal. B Environ. 2020, 272, 118989. [Google Scholar] [CrossRef]
- Niu, Q.; Cheng, Z.; Chen, Q.; Huang, G.; Lin, J.; Bi, J.; Wu, L. Constructing Nitrogen Self-Doped Covalent Triazine-Based Frameworks for Visible-Light-Driven Photocatalytic Conversion of CO2 into CH4. ACS Sustain. Chem. Eng. 2021, 9, 1333–1340. [Google Scholar] [CrossRef]
- Han, X.; Zhao, F.; Shang, Q.; Zhao, J.; Zhong, X.; Zhang, J. Effect of Nitrogen Atom Introduction on the Photocatalytic Hydrogen Evolution Activity of Covalent Triazine Frameworks: Experimental and Theoretical Study. ChemSusChem 2022, 15, e202200828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Fang, W.; Zhao, T.; Fang, S.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution on Phosphorus-Doped Covalent Triazine-Based Frameworks. ACS Appl. Mater. Interfaces 2018, 10, 41415–41421. [Google Scholar] [CrossRef]
- Li, L.; Fang, W.; Zhang, P.; Bi, J.; He, Y.; Wang, J.; Su, W. Sulfur-doped Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution from Water Under Visible Light. J. Mater. Chem. A 2016, 4, 12402–12406. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, Q.; Liu, R.; Dong, W.; Song, S.; Shen, Y. Insights into the Crucial Role of Electron and Spin Structures in Heteroatom-Doped Covalent Triazine Frameworks for Removing Organic Micropollutants. Environ. Sci. Technol. 2022, 56, 6699–6709. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Jin, S.; Liu, Z.-Q.; Zhu, M. 2D Metal-Free Heterostructure of Covalent Triazine Framework/g-C3N4 for Enhanced Photocatalytic CO2 Reduction with High Selectivity. Chin. J. Catal. 2022, 43, 1306–1315. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Huang, X.; Tao, L.; Bi, Y. Direct Observation of Dynamic Interfacial Bonding and Charge Transfer in Metal-Free Photocatalysts for Efficient Hydrogen Evolution. Appl. Catal. B Environ. 2021, 283, 119633. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Q.; Li, Q.; Guo, L.; Chu, H.; Liao, L.; Wang, X.; Li, Z.; Zhou, W. Carbon Quantum Dots Confined into Covalent Triazine Frameworks for Efficient Overall Photocatalytic H2O2 Production. Adv. Funct. Mater. 2024, 34, 2400612. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Z.; Wei, T.; Zhang, B.; Guo, K.; Feng, Y.; Zhang, B. Highly Crystalline Covalent Triazine Framework@rGO Hybrids with Ultra-High Stacking Stability for Efficient Photocatalytic CO2 Fixation. Chem. Eng. J. 2025, 508, 160982. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Huang, C.; Li, L.; Yang, C.; Yu, Y. Encapsulation of Co Single Sites in Covalent Triazine Frameworks for Photocatalytic Production of Syngas. Chin. J. Catal. 2021, 42, 123–130. [Google Scholar] [CrossRef]
- Ran, L.; Li, Z.; Ran, B.; Cao, J.; Zhao, Y.; Shao, T.; Song, Y.; Leung, M.K.H.; Sun, L.; Hou, J. Engineering Single-Atom Active Sites on Covalent Organic Frameworks for Boosting CO2 Photoreduction. J. Am. Chem. Soc. 2022, 144, 17097–17109. [Google Scholar] [CrossRef]
- Huang, G.; Niu, Q.; He, Y.; Tian, J.; Gao, M.; Li, C.; An, N.; Bi, J.; Zhang, J. Spatial Confinement of Copper Single Atoms into Covalent Triazine-Based Frameworks for Highly Efficient and Selective Photocatalytic CO2 Reduction. Nano Res. 2022, 15, 8001–8009. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Tang, Y.; Huang, H.; Cui, H.; Mei, D.; Zhong, C. Single-Atom Pt–N3 Sites on the Stable Covalent Triazine Framework Nanosheets for Photocatalytic N2 Fixation. ACS Catal. 2020, 10, 2431–2442. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, P.; Huang, G.; Chen, Q.; Bi, J.; Wu, L. Band Gap Tuning of Covalent Triazine-Based Frameworks through Iron Doping for Visible-Light-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2021, 14, 3850–3857. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, S.; Song, Y.; Huang, S.; Gao, J.; Sun, L.; Hou, J. Engineering Single–Atom Active Sites Anchored Covalent Organic Frameworks for Efficient Metallaphotoredox CN Cross–coupling Reactions. Sci. Bull. 2022, 67, 1971–1981. [Google Scholar] [CrossRef]
- Xu, Z.; Cui, Y.; Guo, B.; Li, H.-Y.; Li, H.-X. Boosting Visible-Light-Driven H2 Evolution of Covalent Triazine Framework from Water by Modifying Ni(II) Pyrimidine-2-thiolate Cocatalyst. ChemCatChem 2021, 13, 958–965. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Golub, F.S.; Trubina, S.V.; Zvereva, V.V.; Bulusheva, L.G.; Gerasimov, E.Y.; Navlani-García, M.; Krot, A.D.; Jena, H.S. Single-Atom Pd Catalysts Supported on Covalent Triazine Frameworks for Hydrogen Production from Formic Acid. ACS Appl. Nano Mater. 2022, 5, 12887–12896. [Google Scholar] [CrossRef]
- Xu, N.; Diao, Y.; Xu, Z.; Ke, H.; Zhu, X. Covalent Triazine Frameworks Embedded with Ir Complexes for Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 7473–7478. [Google Scholar] [CrossRef]
- Xiong, Y.; Qin, Y.; Su, L.; Ye, F. Bioinspired Synthesis of Cu2+-Modified Covalent Triazine Framework: A New Highly Efficient and Promising Peroxidase Mimic. Chem. A Eur. J. 2017, 23, 11037–11045. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Lu, N.; Lin, H.; Zhou, S.; Liu, F. Anchoring Cobalt Single Atoms on 2D Covalent Triazine Framework with Charge Nanospatial Separation for Enhanced Photocatalytic Pollution Degradation. Mater. Today Chem. 2022, 24, 100832. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, L.; Fang, Q.; Song, S.; Chen, B.; Shen, Y. Unveiling Spin State-Dependent Micropollutant Removal using Single-Atom Covalent Triazine Framework. Adv. Funct. Mater. 2023, 33, 2210905. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, Y.; Fang, Q.; Song, S.; Chen, B.; Shen, Y. Unveiling the Dynamic Evolution of Single-Atom Co Sites in Covalent Triazine Frameworks for Enhanced H2O2 Photosynthesis. ACS Catal. 2024, 14, 2847–2858. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, C.; Xu, J.; Lu, L.; Fang, Q.; Xu, C.; Zheng, Y.; Song, S.; Shen, Y. Efficient Dual-pathway H2O2 Production Promoted by Covalent Triazine Frameworks with Integrated Dual Active Sites. Appl. Catal. B Environ. Energy 2024, 344, 123629. [Google Scholar] [CrossRef]
- Han, X.; Dong, Y.; Zhao, J.; Ming, S.; Xie, Y. Construction of Ternary Z-scheme Covalent Triazine Framework@Au@TiO2 for Enhanced Visible-Light-Driven Hydrogen Evolution activity. Int. J. Hydrog. Energy 2022, 47, 18334–18346. [Google Scholar] [CrossRef]
- Xu, Z.; Cui, Y.; Young, D.J.; Wang, J.; Li, H.-Y.; Bian, G.-Q.; Li, H.-X. Combination of Co2+-Immobilized Covalent Triazine Framework and TiO2 by Covalent Bonds to Enhance Photoreduction of CO2 to CO with H2O. J. CO2 Util. 2021, 49, 101561. [Google Scholar] [CrossRef]
- Chao, X.; Xu, Y.; Chen, H.; Feng, D.; Hu, J.; Yu, Y. TiO2-Based Photocatalyst Modified with a Covalent Triazine-Based Framework Organocatalyst for Carbamazepine Photodegradation. RSC Adv. 2021, 11, 6943–6951. [Google Scholar] [CrossRef]
- Oyegbeda, O.; Akpotu, S.O.; Moodley, B. Dual Functional Covalent Triazine Framework-TiO2 S-scheme Heterojunction for Efficient Sequestration of Ciprofloxacin: Mechanism and Degradation Products. Environ. Res. 2025, 266, 120501. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y.; Zhang, Z.; Zhao, X.; Wang, L.; Huang, J.; She, H.; Li, X.; Wang, Q. Regulating the Layer Stacking Configuration of CTF-TiO2 Heterostructure for Improving the Photocatalytic CO2 Reduction. Inorg. Chem. 2024, 63, 19344–19354. [Google Scholar] [CrossRef]
- Xia, B.; Liu, G.; Fan, K.; Chen, R.; Liu, X.; Li, L. Boosting Hydrogen Peroxide Photosynthesis via a 1D/2D S-scheme Heterojunction Constructed by a Covalent Triazine Framework with Dual O2 Reduction Centers. Chin. J. Catal. 2025, 69, 315–326. [Google Scholar] [CrossRef]
- Sayed, M.; Xu, F.; Kuang, P.; Low, J.; Wang, S.; Zhang, L.; Yu, J. Sustained CO2-Photoreduction Activity and High Selectivity over Mn, C-Codoped ZnO Core-Triple Shell Hollow Spheres. Nat. Commun. 2021, 12, 4936. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Cheng, B.; Zhang, L.; Zhang, Z.; Bie, C. A Review on ZnO-based S-scheme Heterojunction Photocatalysts. Chin. J. Catal. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, H.; Xiong, X.; Wu, M.-F.; Xia, M.; Xie, M.; Zou, J.-P.; Luo, S.-L. Highly Efficient Charge Transfer in CdS-Covalent Organic Framework Nanocomposites for Stable Photocatalytic Hydrogen Evolution under Visible Light. Sci. Bull. 2020, 65, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, P.; Zhao, Y.; Yu, X.; Wu, Y.; Zhang, H.; Yu, B.; Han, B.; George, M.W.; Liu, Z. Direct Z-Scheme Heterojunction of SnS2/Sulfur-Bridged Covalent Triazine Frameworks for Visible-Light-Driven CO2 Photoreduction. ChemSusChem 2020, 13, 6278–6283. [Google Scholar] [CrossRef]
- Yang, X.; Bai, X.; Ma, Y.; He, D.; Wang, X.; Guo, Y. A Solar Light Regenerated Adsorbent by Implanting CdS into An Active Covalent Triazine Framework to Decontaminate Tetracycline. Sep. Purif. Technol. 2021, 255, 117696. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, L.; Bi, J.; Liang, S.; Li, L.; Yu, Y.; Wu, L. MoS2 Quantum Dots-Modified Covalent Triazine-Based Frameworks for Enhanced Photocatalytic Hydrogen Evolution. ChemSusChem 2018, 11, 1108–1113. [Google Scholar] [CrossRef]
- Huang, H.; Xu, B.; Tan, Z.; Jiang, Q.; Fang, S.; Li, L.; Bi, J.; Wu, L. A Facile In Situ Growth of CdS Quantum Dots on Covalent Triazine-Based Frameworks for Photocatalytic H2 Production. J. Alloys Compd. 2020, 833, 155057. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; Lu, J. Internal Electric Field and Adsorption Effect Synergistically Boost Carbon Dioxide Conversion on Cadmium Sulfide@Covalent Triazine Frameworks Core–Shell Photocatalyst. Adv. Funct. Mater. 2023, 33, 2308553. [Google Scholar] [CrossRef]
- Zhou, T.; Han, X.; Shen, W.; Ji, F.; Liu, M.; Song, Y.; He, W.-W. Construction of NiS/CTF Heterojunction Photocatalyst with An Outstanding Photocatalytic Hydrogen Evolution Performance. Chin. Chem. Lett. 2024, 110415. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic Carbon Nitride (g–C3N4)–Based Metal-Free Photocatalysts for Water Splitting: A Review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, W.; Dang, L.; Mao, Y.; Wu, J.; Xu, K. Recent Progress in Doped g-C3N4 Photocatalyst for Solar Water Splitting: A Review. Front. Chem. 2022, 10, 955065. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Zhao, Y.; Yu, L.; Young, D.J.; Ren, Z.-G.; Li, H.-X. Covalent Organic Framework Spherical Nanofibers Bearing Carbon Quantum Dots for Boosting Photocatalytic Hydrogen Production. J. Mater. Chem. A 2025, 13, 1932–1941. [Google Scholar] [CrossRef]
- Chen, L.; Chen, G.; Gong, C.; Zhang, Y.; Xing, Z.; Li, J.; Xu, G.; Li, G.; Peng, Y. Low-Valence Platinum Single Atoms in Sulfur-Containing Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution. Nat. Commun. 2024, 15, 10501. [Google Scholar] [CrossRef]
- Huang, K.; Chen, D.; Zhang, X.; Shen, R.; Zhang, P.; Xu, D.; Li, X. Constructing Covalent Triazine Frameworks/N-Doped Carbon-Coated Cu2O S-Scheme Heterojunctions for Boosting Photocatalytic Hydrogen Production. Acta Phys. Chim. Sin. 2024, 40, 2407020. [Google Scholar] [CrossRef]
- Sun, Q.-G.; Yang, C.-L.; Li, X.; Liu, Y.; Zhao, W.; Ma, X. Photocatalytic Overall Water Splitting Z-Schemes for Hydrogen Production with the X@CTF-0/β-Sb (X = S, Se) Heterostructures. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135437. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, J.; Liu, S.; Zheng, Y.; Lu, L.; Fang, Q.; Song, S.; Zhu, C.; Shen, Y. A Pyridine-Woven Covalent Organic Framework Facilitating the Immobilization of Co Single Atoms towards Efficient Photocatalytic H2 Evolution. J. Mater. Chem. A 2024, 12, 31619–31629. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Yan, D.; Hu, H.; Song, Y.; Su, X.; Sun, J.; Xiao, S.; Gao, Y. Dipole Polarization Modulating of Vinylene-Linked Covalent Organic Frameworks for Efficient Photocatalytic Hydrogen Evolution. Chin. J. Catal. 2024, 65, 103–112. [Google Scholar] [CrossRef]
- Li, C.; Guan, L.; Zhang, J.; Cheng, C.; Guo, Z.; Tian, Z.; Yang, L.-M.; Jin, S. Regulating Pt-covalent Triazine Framework Schottky Junctions by Using Tailor-Made Nitrogen Sites towards Efficient Photocatalysis. J. Mater. Chem. A 2024, 12, 13876–13881. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, X.; Wang, H.; Cai, J.; Wang, H.; Bi, S.; Wei, R.; Yang, C.; Zheng, G.; Han, Q. p-π Conjugated Covalent Organic Frameworks Expedite Molecular Triplet Excitons for H2O2 Production Coupled with Biomass Upgrading. Adv. Mater. 2025, 37, 2502220. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Tan, H.; Ye, N.; Gu, Y.; Zhao, S.; Zhang, S.; Luo, M.; Guo, S. Fluorination of Covalent Organic Framework Reinforcing the Confinement of Pd Nanoclusters Enhances Hydrogen Peroxide Photosynthesis. J. Am. Chem. Soc. 2023, 145, 19877–19884. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, N.; Chang, Q.; Wu, J.; Yang, J.; Hu, S.; Kang, Z. Abstracting Photogenerated Holes from Covalent Triazine Frameworks through Carbon Dots for Overall Hydrogen Peroxide Photosynthesis. Chin. J. Catal. 2024, 62, 178–189. [Google Scholar] [CrossRef]
- Yang, L.; Lv, W.; Wang, Y.; Wang, Y. Carbon Nanotube Incorporation and Framework Protonation-Regulated Energy Band for Enhanced Photocatalytic Hydrogen Peroxide Production of COF. Nano Res. 2025, 18, 94907024. [Google Scholar] [CrossRef]
- Wang, H.; Yang, C.; Chen, F.; Zheng, G.; Han, Q. A Crystalline Partially Fluorinated Triazine Covalent Organic Framework for Efficient Photosynthesis of Hydrogen Peroxide. Angew. Chem. Int. Ed. 2022, 61, e202202328. [Google Scholar] [CrossRef]
- Wang, H.; Jin, S.; Zhang, X.; Xie, Y. Excitonic Effects in Polymeric Photocatalysts. Angew. Chem. Int. Ed. 2020, 59, 22828–22839. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Shen, F.; Zhang, Y.; Zhang, L.; Zang, L.; Sun, L. Engineered Covalent Triazine Framework Inverse Opal Beads for Enhanced Photocatalytic Carbon Dioxide Reduction. J. Colloid Interface Sci. 2025, 689, 137244. [Google Scholar] [CrossRef]
- Chen, S.; Huang, G.; Sheng, H.; Huang, G.; Sa, R.; Chen, Q.; Bi, J. Asymmetric Electronic Distribution Induced Enhancement in Photocatalytic CO2-to-CH4 Conversion via Boron-Doped Covalent Triazine Frameworks. J. Colloid Interface Sci. 2025, 685, 766–773. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Wei, S.; Li, M.; Wang, H.; Yu, B.; Li, J.; Huang, J. N-Bi Covalently Connected Z-Scheme Heterojunction by In Situ Anchoring Biocl on Triazine-Based Bromine-Substituted Covalent Organic Frameworks for the Enhanced Photocatalytic Reduction of CO2 and Cr (VI). Chem. Eng. J. 2025, 505, 159349. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Wu, Y.; Xiong, J.; Li, S.; Jiang, W.; Liu, Z.; Di, J. Strongly Coupled Interface in Electrostatic Self-Assembly Covalent Triazine Framework/Bi19S27Br3 for High-Efficiency CO2 Photoreduction. ACS Nano 2025, 19, 2759–2768. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, X.; Zhang, Q.; Guo, L.; Li, Z.; Wang, H.; Zhang, S.; Wang, J. Mimic Metalloenzymes with Atomically Dispersed Fe Sites in Covalent Organic Framework Membranes for Enhanced CO2 Photoreduction. Chem. Sci. 2025, 16, 1222–1232. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zheng, M.; Luo, F.; Zhang, L.; Zhang, B.; Jiang, B. Spatial Coupling of Photocatalytic CO2 Reduction and Selective Oxidation on Covalent Triazine Framework/ZnIn2S4 Core–Shell Structures. Adv. Funct. Mater. 2025, 35, 2417279. [Google Scholar] [CrossRef]
- Kong, K.; Zhong, H.; Zhang, F.; Lv, H.; Li, X.; Wang, R. The Reduced Barrier for the Photogenerated Charge Migration on Covalent Triazine-Based Frameworks for Boosting Photocatalytic CO2 Reduction into Syngas. Adv. Funct. Mater. 2025, 35, 2417109. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiong, L.; Guo, S.; Xu, C.; Wang, J.; Wu, X.; Xiao, Y.; Song, R. Installing Active Metal Species in a Covalent Triazine Framework for Highly Efficient and Selective Photocatalytic CO2 Reduction. J. Mater. Chem. A 2024, 12, 32045–32053. [Google Scholar] [CrossRef]
- Fu, P.; Chen, C.; Wu, C.; Meng, B.; Yue, Q.; Chen, T.; Yin, W.; Chi, X.; Yu, X.; Li, R.; et al. Covalent Organic Framework Stabilized Single CoN4Cl2 Site Boosts Photocatalytic CO2 Reduction into Tunable Syngas. Angew. Chem. Int. Ed. 2025, 64, e202415202. [Google Scholar] [CrossRef]
- Mandal, P.C.; Sherpa, N.D.; Sarkar, S.; Roy, C.K.; Ali, O.; Roy, N. CTF Stabilizes Truncated octahedral Cu2O Nanocrystals and SnO2 Nanoparticle Assisted Photocatalytic CO2 Reduction in Hybrid Ternary Cu2O/SnO2/CTF Nanostructures. CrystEngComm 2025, 27, 1427–1438. [Google Scholar] [CrossRef]
- Salati, M.; Dorchies, F.; Wang, J.-W.; Ventosa, M.; González-Carrero, S.; Bozal-Ginesta, C.; Holub, J.; Rüdiger, O.; DeBeer, S.; Gimbert-Suriñach, C.; et al. Covalent Triazine-Based Frameworks with Ru-tda Based Catalyst Anchored via Coordination Bond for Photoinduced Water Oxidation. Small 2025, 21, 2406375. [Google Scholar] [CrossRef] [PubMed]
- Sicignano, M.; Gobbato, T.; Bonetto, R.; Centomo, P.; Di Vizio, B.; De Biasi, F.; Rosa-Gastaldo, D.; Pierantoni, C.; Bonetto, A.; Glisenti, A.; et al. Synergistic Integration of a Ru(bda)-Based Catalyst in a Covalent Organic Framework for Enhanced Photocatalytic Water Oxidation. Adv. Sustain. Syst. 2025, 9, 2400653. [Google Scholar] [CrossRef]
- Sun, R.; Hu, X.; Shu, C.; Guo, Y.; Wang, X.; Tan, B. Covalent Triazine Frameworks with Ru Molecular Catalyst for Efficient Photocatalytic Oxygen Evolution Reaction. Sci. China Mater. 2024, 67, 642–649. [Google Scholar] [CrossRef]
- Chen, H.; Gardner, A.M.; Lin, G.; Zhao, W.; Bahri, M.; Browning, N.D.; Sprick, R.S.; Li, X.; Xu, X.; Cooper, A.I. Covalent Triazine-Based Frameworks with Cobalt-Loading for Visible Light-Driven Photocatalytic Water Oxidation. Catal. Sci. Technol. 2022, 12, 5442–5452. [Google Scholar] [CrossRef]
- Tang, Q.; Wan, Y.; Pan, Z.; Cheng, Q. Visible Light-Driven Triazine-Based S-scheme COF-TpTt@BiOBr Heterojunction with Oxygen Vacancy for Enhanced Photocatalytic Pollutants Removal and Hydrogen Production. Environ. Res. 2025, 269, 120901. [Google Scholar] [CrossRef]
- Moya, A.; Sánchez-Fuente, M.; Linde, M.; Cepa-López, V.; del Hierro, I.; Díaz-Sánchez, M.; Gómez-Ruiz, S.; Mas-Ballesté, R. Enhancing Photocatalytic Performance of F-Doped TiO2 through the Integration of Small Amounts of a Quinoline-Based Covalent Triazine Framework. Nanoscale 2025, 17, 8880–8891. [Google Scholar] [CrossRef] [PubMed]
- Zandipak, R.; Bahramifar, N.; Younesi, H.; Zolfigol, M.A. Decoration of Carbon Nanodots on Conjugated Triazine Framework Nanosheets as Z-Scheme Heterojunction for Boosting Opto-Electro Photocatalytic Degradation of Organic Hydrocarbons from Petrochemical Wastewater. J. Environ. Chem. Eng. 2025, 13, 115380. [Google Scholar] [CrossRef]
- Zandipak, R.; Bahramifar, N.; Younesi, H.; Zolfigol, M.A. Electro-Photocatalyst Effect of N-S-Doped Carbon Dots and Covalent Organic Triazine Framework Heterostructures for Boosting Photocatalytic Degradation of Phenanthrene in Water. Chemosphere 2024, 364, 142980. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, S.; Tang, R.; Liu, Y.; Chen, H. Removal of Mixed Pollutants of Perfluorooctanoic Acid and 2,4,6-Trichlorophenol via Adsorption-Photocatalysis Using Ga2O3-Bi4O7 Combining with Fluorine-Doped Covalent Triazine Framework: Role of Different Active Species. J. Colloid Interface Sci. 2024, 676, 959–973. [Google Scholar] [CrossRef]
- Qi, L.; Xiao, C.; Lu, W.; Zhang, H.; Zhou, Y.; Qi, J.; Yang, Y.; Zhu, Z.; Li, J. Triazine-based Covalent Organic Framework/g-C3N4 Heterojunction toward Highly Efficient Photoactivation of Peroxydisulfate for Sulfonamides Degradation. Sep. Purif. Technol. 2025, 354, 128758. [Google Scholar] [CrossRef]
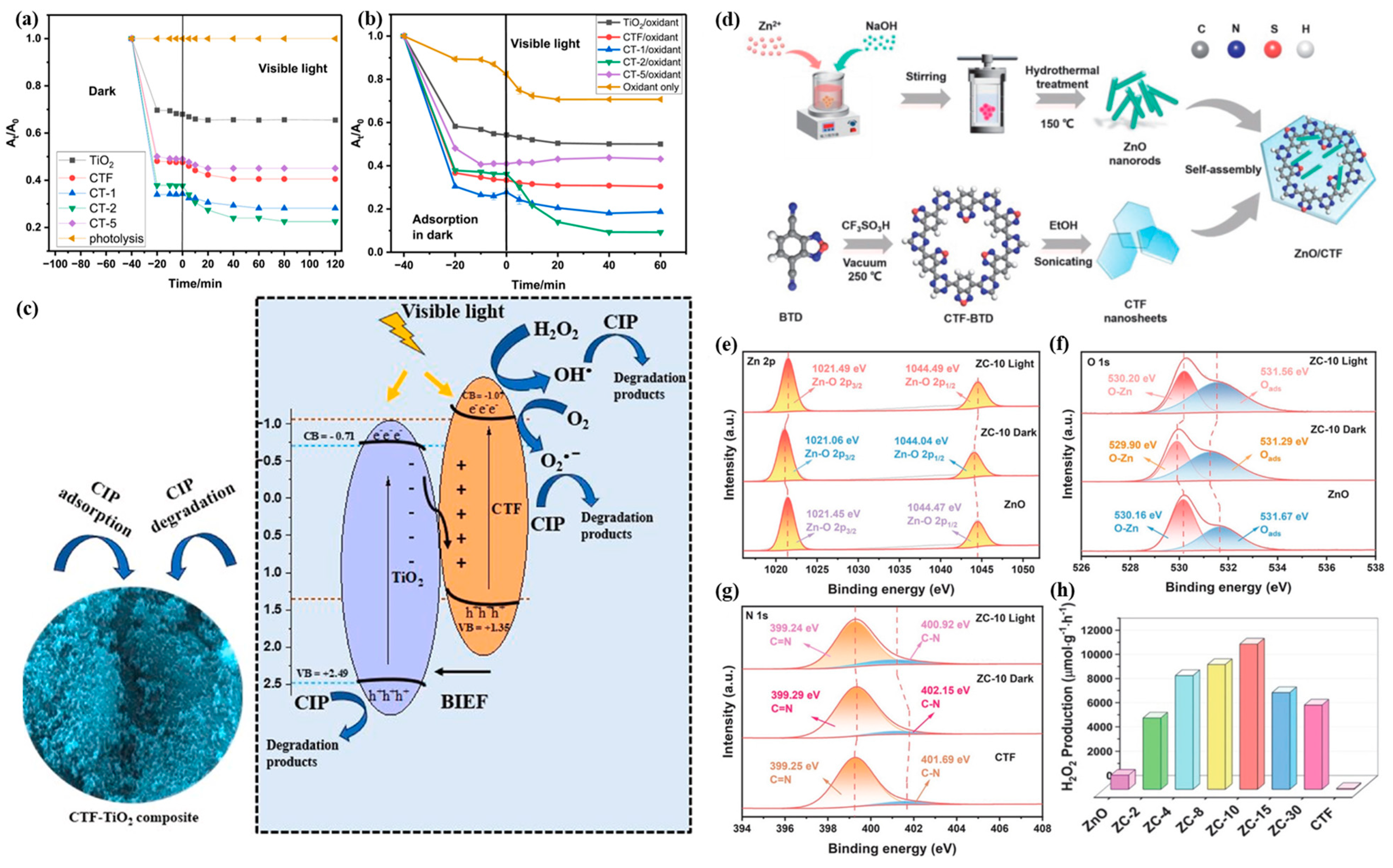
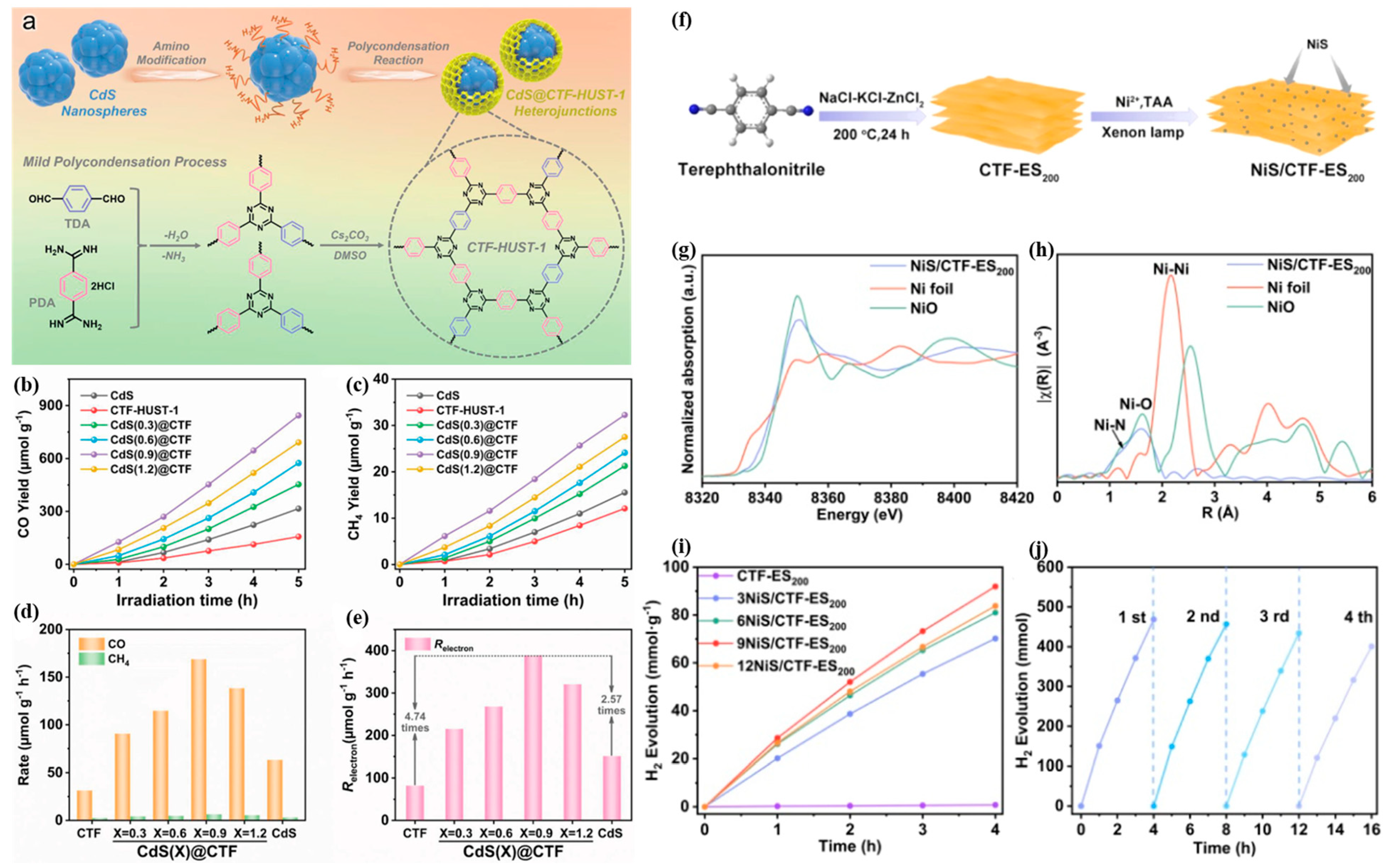
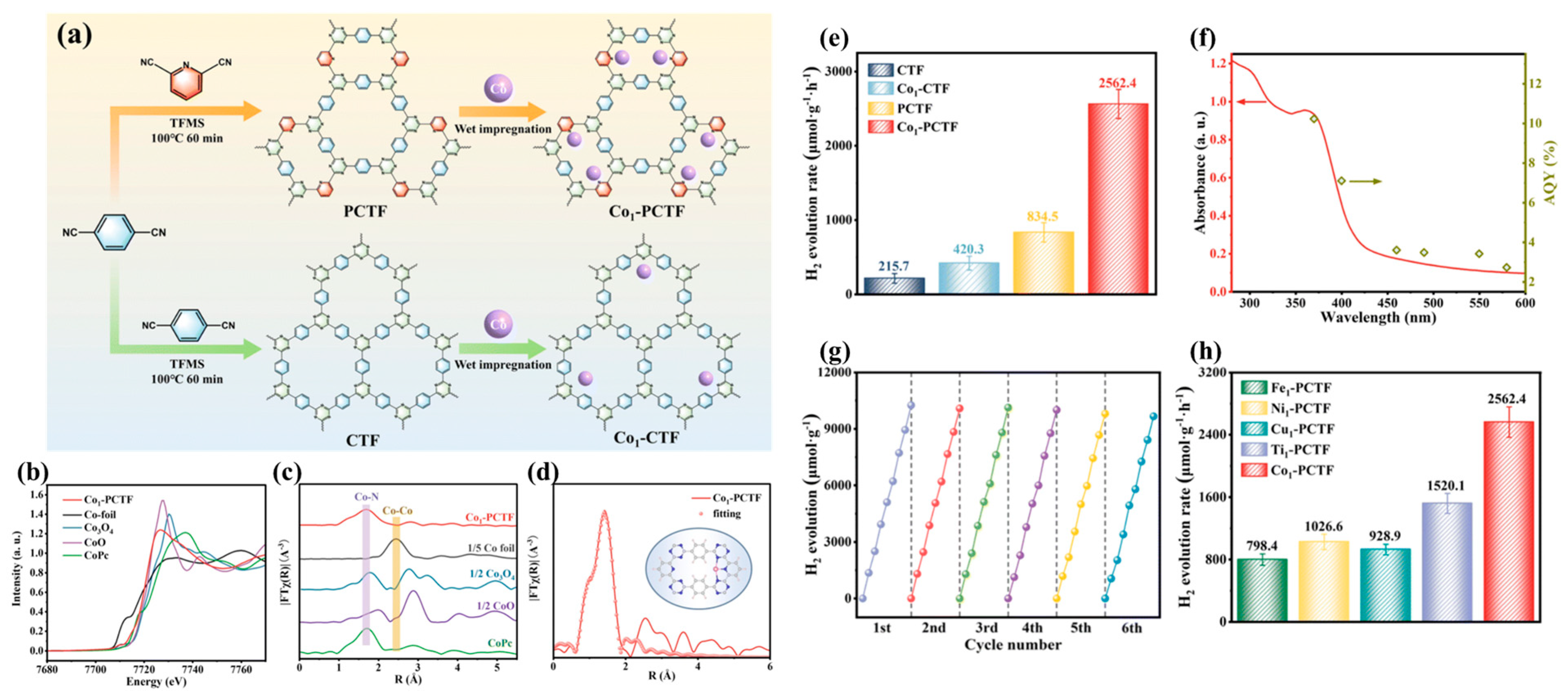
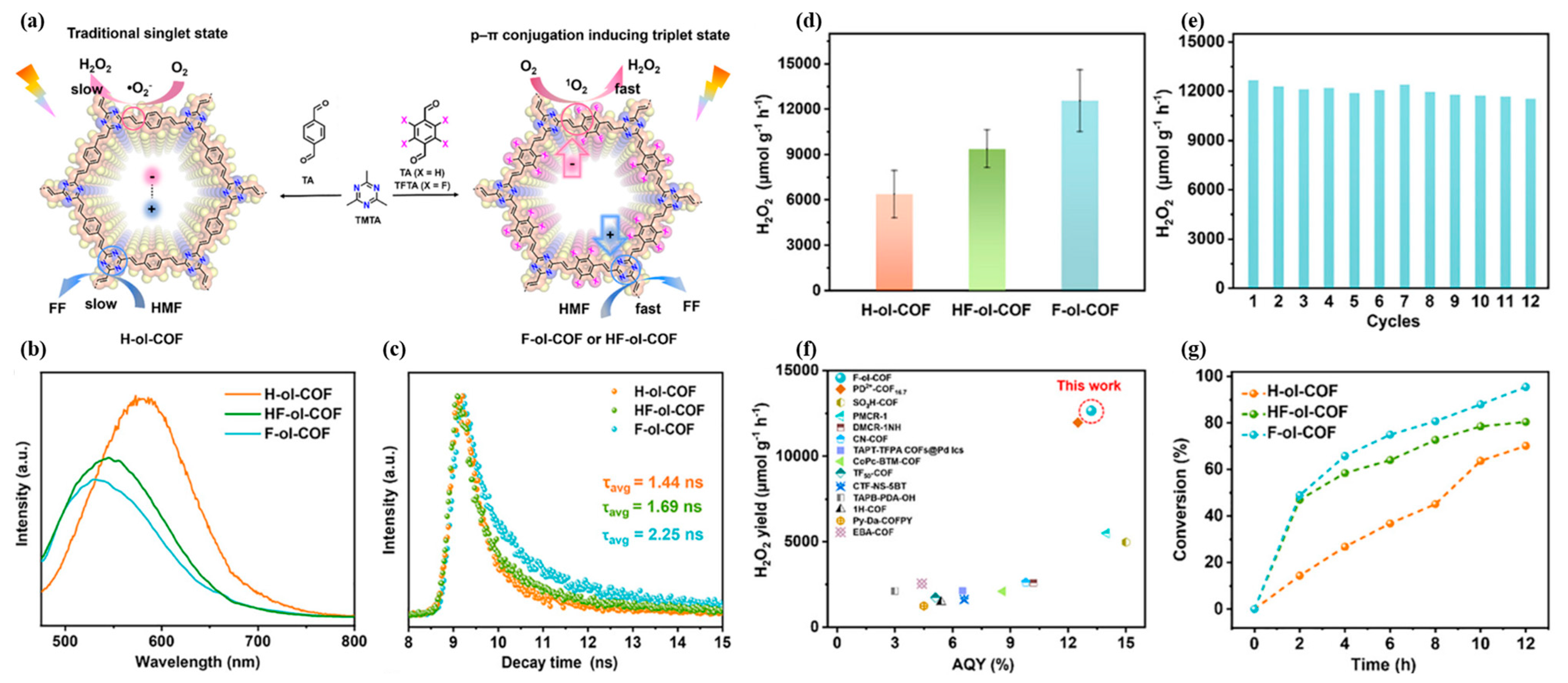

| Photocatalyst | Cocatalyst | Sacrificial Reagent | Light Source | HER (μmol g−1·h−1) | AQE (%) | Ref. |
|---|---|---|---|---|---|---|
| TAPT-COF-CQDs | Pt (3 wt%) | TEOA | 300 W Xe lamp (λ > 420 nm) | 69,570 | 9.5 (440 nm) | [63] |
| PtSA@S-TFPT | / | TEOA | 300 W Xe lamp (λ > 420 nm) | 11,400 | 4.65 (420 nm) | [64] |
| CTF-Cu2O@NC | Pt (3 wt%) | TEOA + MeOH | 300 W Xe lamp (λ > 420 nm) | 15,645 | 1.67 (420 nm) | [65] |
| Co1-PCTF | / | TEOA | 300 W Xe lamp (λ > 420 nm) | 2562.4 | 10.22 (365 nm) | [67] |
| TMT-BO-COF | Pt (5 wt%) | AA | 300 W Xe lamp | 23,700 | 0.11 (420 nm) | [68] |
| Pt@CTF-Py-1 | / | TEA | AM 1.5 G | 14,960 | 4.51 (420 nm) | [69] |
| Photocatalyst | Cocatalyst | Sacrificial Reagent | Light Source | H2O2 Yields (μmol g−1·h−1) | AQE (%) | Ref. |
|---|---|---|---|---|---|---|
| F-ol-COF | / | HMF | 300 W Xe lamp (λ > 420 nm) | 12,558 | 13.2 (420 nm) | [70] |
| TAPT-TFPA COFs@Pd IC | / | EtOH | 300 W Xe lamp (λ > 400 nm) | 2143 | 6.5 (400 nm) | [71] |
| CDs@CTFs CsCl | / | NaIO3 | AM 1.5 G | 2464 | 13.0 (500 nm) | [72] |
| CNT@COF-H | / | IPA | AM 1.5 G | 1581 | 34.0 (500 nm) | [73] |
| TF50-COF | / | EtOH | 300 W Xe lamp (λ > 400 nm) | 1739 | 5.1 (400 nm) | [74] |
| Photocatalyst | Photosensitizer | Sacrificial Reagent | Light Source | CO2RR Activity | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|
| CTF-240 | / | / | 300 W Xe lamp (λ > 420 nm) | 118.69 μmol·g−1·h−1 (CO) | 97.25 | [76] |
| CTFB10 | / | TEA | 300 W Xe lamp (λ > 420 nm) | 27.4 μmol·L−1 (CH4) | 90.3 | [77] |
| Br-COFs@BiOCl | / | / | 300 W Xe lamp (λ > 320 nm) | 27.4 μmol·g−1·h−1 (CO) | ≈100 | [78] |
| CTF/Bi19S27Br3 | / | / | AM 1.5 G | 572.2 μmol·g−1·h−1 (CO) | 99.9 | [79] |
| Fe-COF | / | / | 300 W Xe lamp (320 < λ < 780 nm) | 992 μmol·g−1·h−1 (CO) | ≈100 | [80] |
| SCTF/ZnIn2S4 | / | FFA | AM 1.5 G | 207.8 μmol·g−1·h−1 (CO) | ≈100 | [81] |
| DA-CTF@DPT-Co | / | TEOA | 300 W Xe lamp (λ > 420 nm) | 724 μmol·g−1·h−1 (CO) 695 μmol·g−1·h−1 (H2) | / | [82] |
| Ni-PT-CTF | / | TEOA | 300 W Xe lamp (λ > 420 nm) | 784.5 μmol·g−1·h−1 (CO) | 96.6 | [83] |
| TPy-COF-Co | [Ru(bpy)3]Cl2 | TEOA | 300 W Xe lamp (λ > 420 nm) | 426,000 μmol·g−1·h−1 (CO) 343,000 μmol·g−1·h−1 (H2) | / | [84] |
| Cu2O/SnO2/CTF | / | AA | 250 W high pressure Hg discharge lamp | 40.33 μmol·g−1·h−1 (CO) | ≈100 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhou, Q.; Wang, X.; Liao, Y.; Meng, J.; Huang, Y.; Gao, L.; Dai, W. The Construction and Photocatalytic Application of Covalent Triazine Framework (CTF)-Based Composites: A Brief Review. Catalysts 2025, 15, 562. https://doi.org/10.3390/catal15060562
Wei Y, Zhou Q, Wang X, Liao Y, Meng J, Huang Y, Gao L, Dai W. The Construction and Photocatalytic Application of Covalent Triazine Framework (CTF)-Based Composites: A Brief Review. Catalysts. 2025; 15(6):562. https://doi.org/10.3390/catal15060562
Chicago/Turabian StyleWei, Yuchen, Quanmei Zhou, Xinglin Wang, Yifan Liao, Jiayi Meng, Yamei Huang, Linlin Gao, and Weilin Dai. 2025. "The Construction and Photocatalytic Application of Covalent Triazine Framework (CTF)-Based Composites: A Brief Review" Catalysts 15, no. 6: 562. https://doi.org/10.3390/catal15060562
APA StyleWei, Y., Zhou, Q., Wang, X., Liao, Y., Meng, J., Huang, Y., Gao, L., & Dai, W. (2025). The Construction and Photocatalytic Application of Covalent Triazine Framework (CTF)-Based Composites: A Brief Review. Catalysts, 15(6), 562. https://doi.org/10.3390/catal15060562








