Tailoring Pore Size in Bimetallic Nb-Mn/MCM-41 Catalysts for Enhanced Plasma-Driven Catalytic Oxidation of Toluene
Abstract
1. Introduction
2. Results
2.1. Characterization of Catalysts
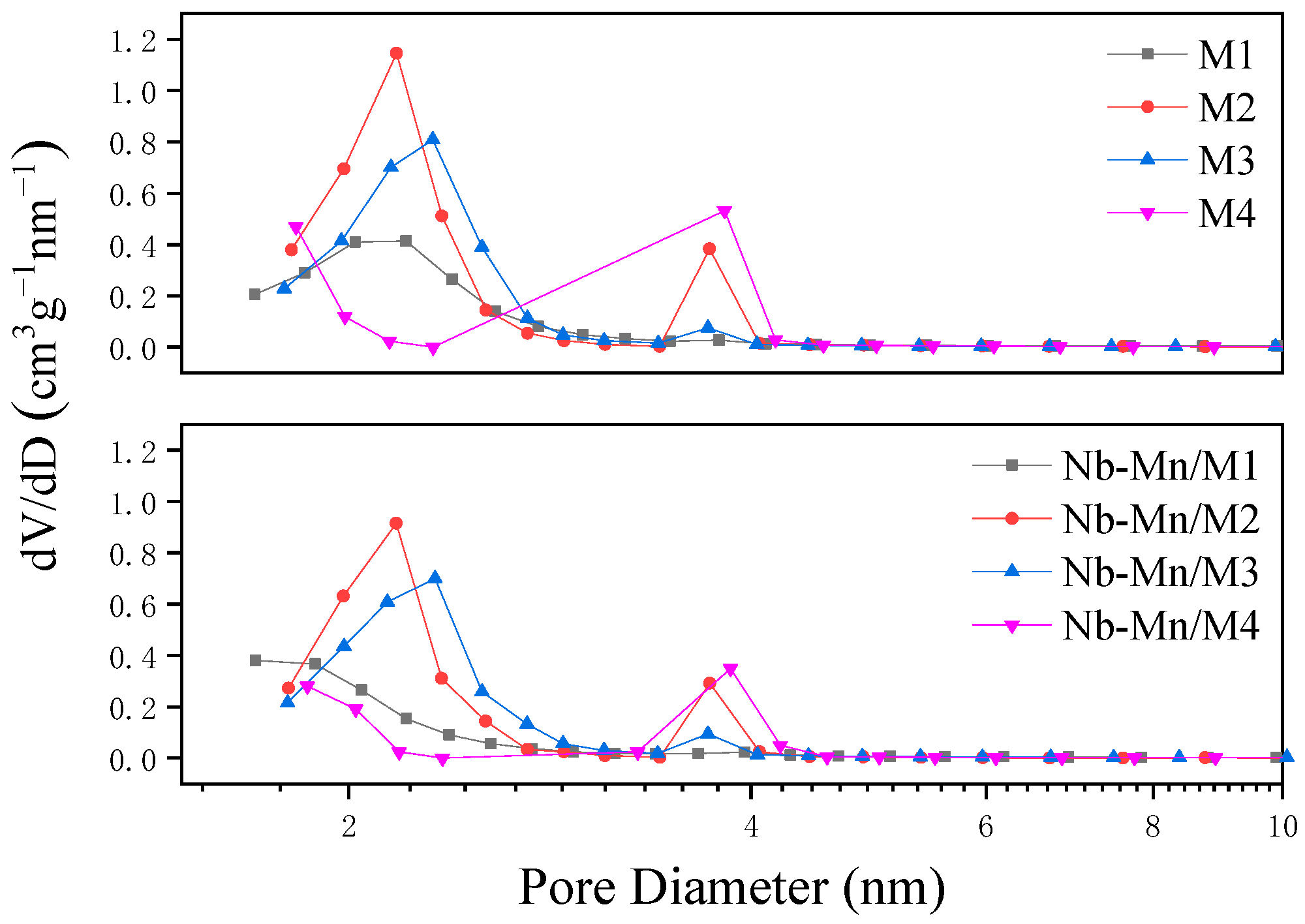
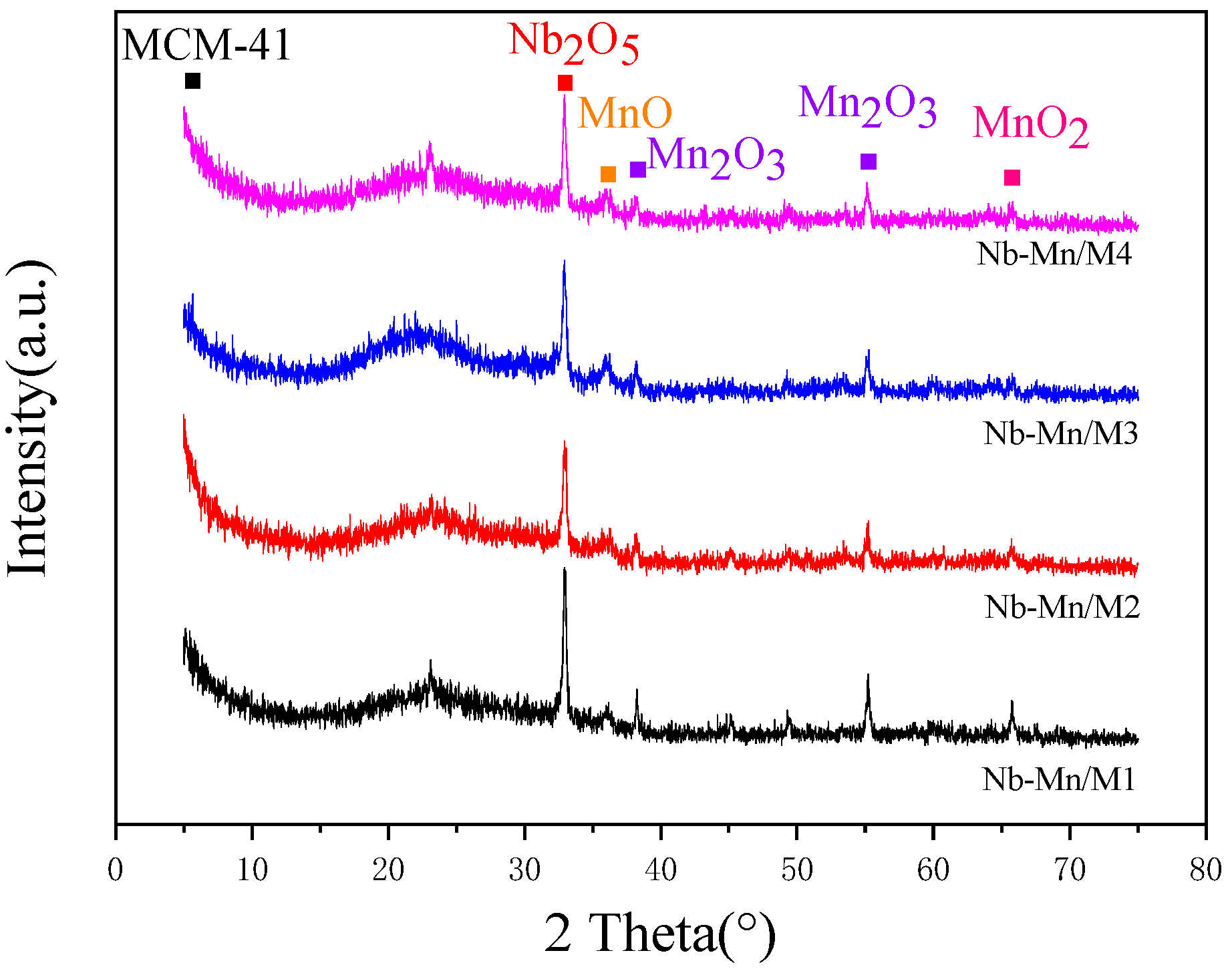
2.1.1. Structural Characterization
2.1.2. Surface and Redox Properties
- (1)
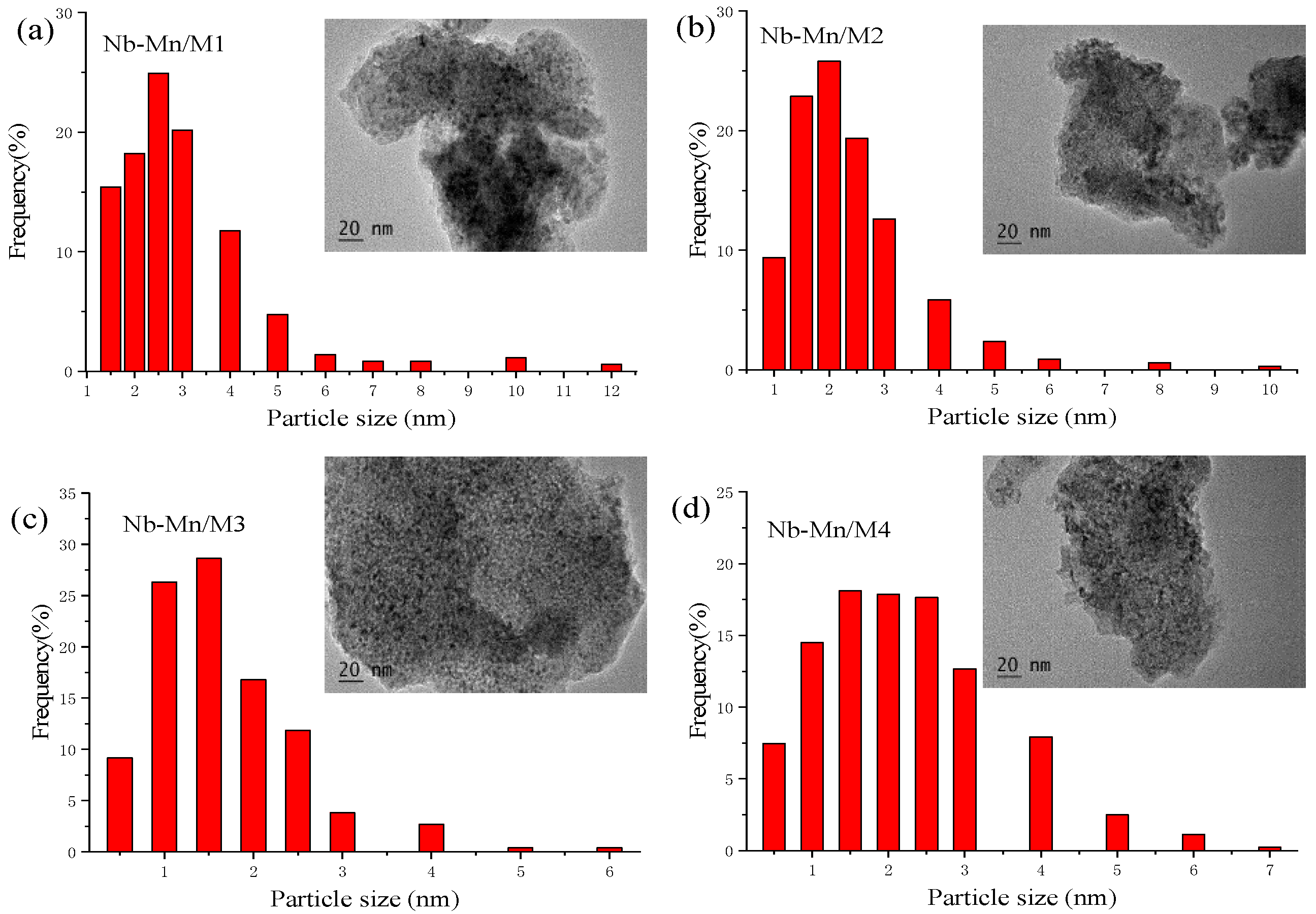
- TEM
- (2)
- XPS
- (3)
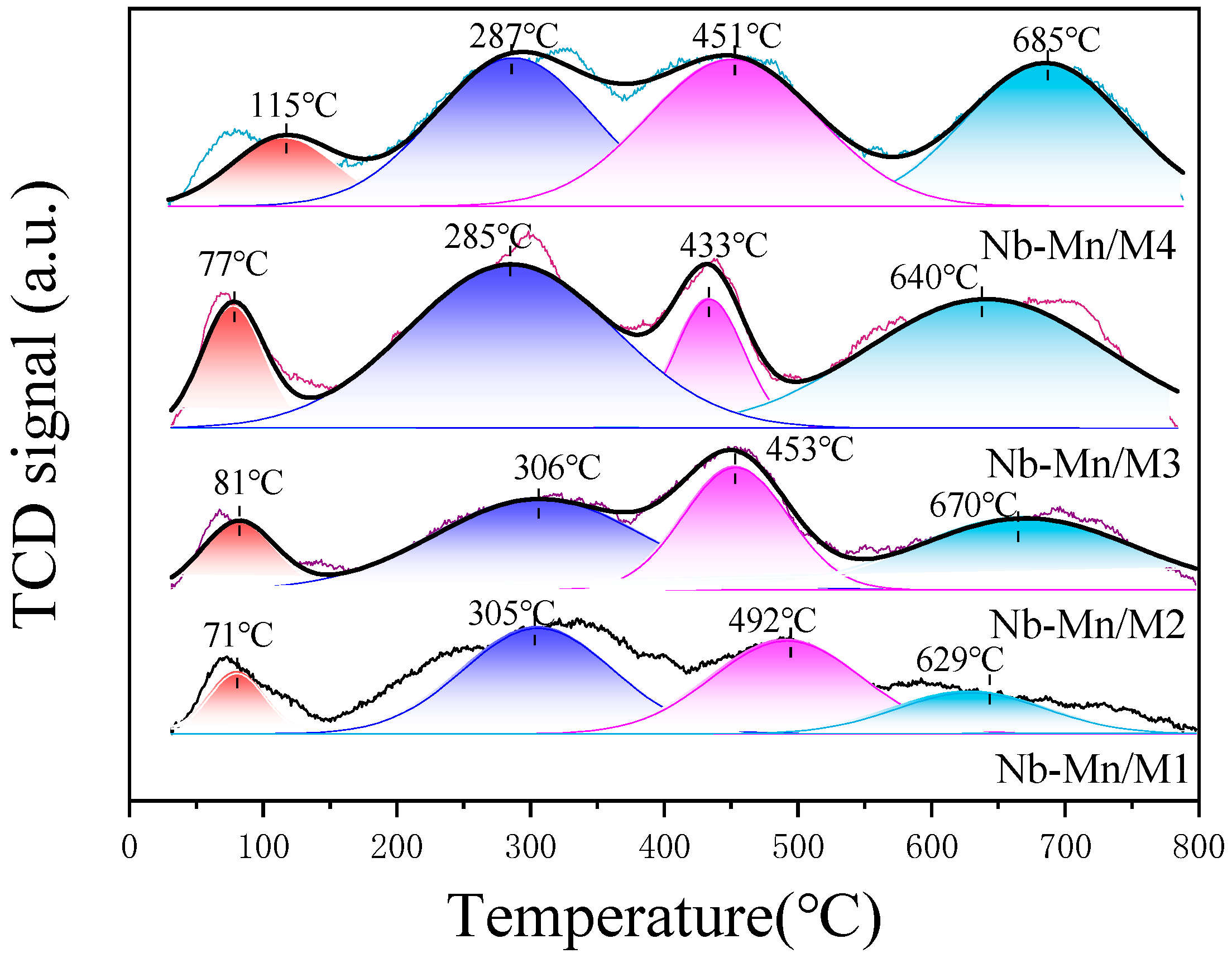
- O2-TPD
2.2. Toluene Degradation
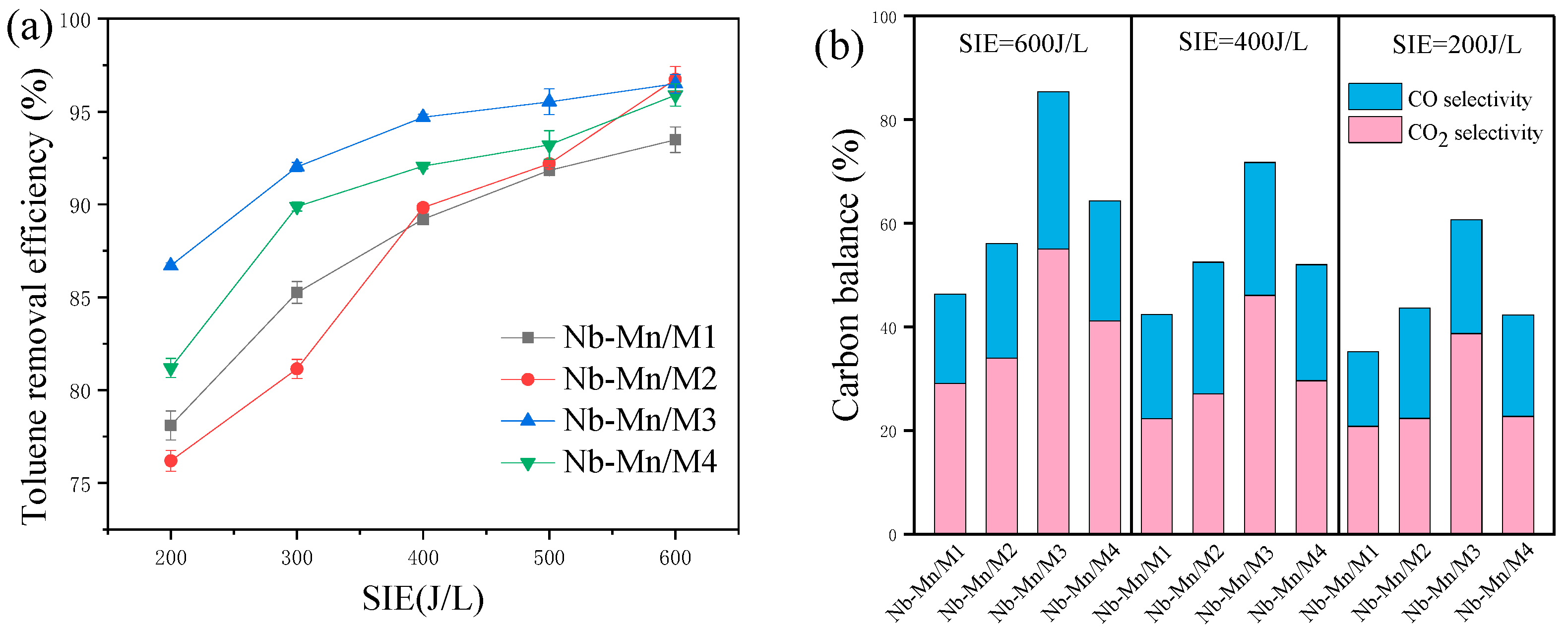
2.2.1. Effect of Plasma Discharge
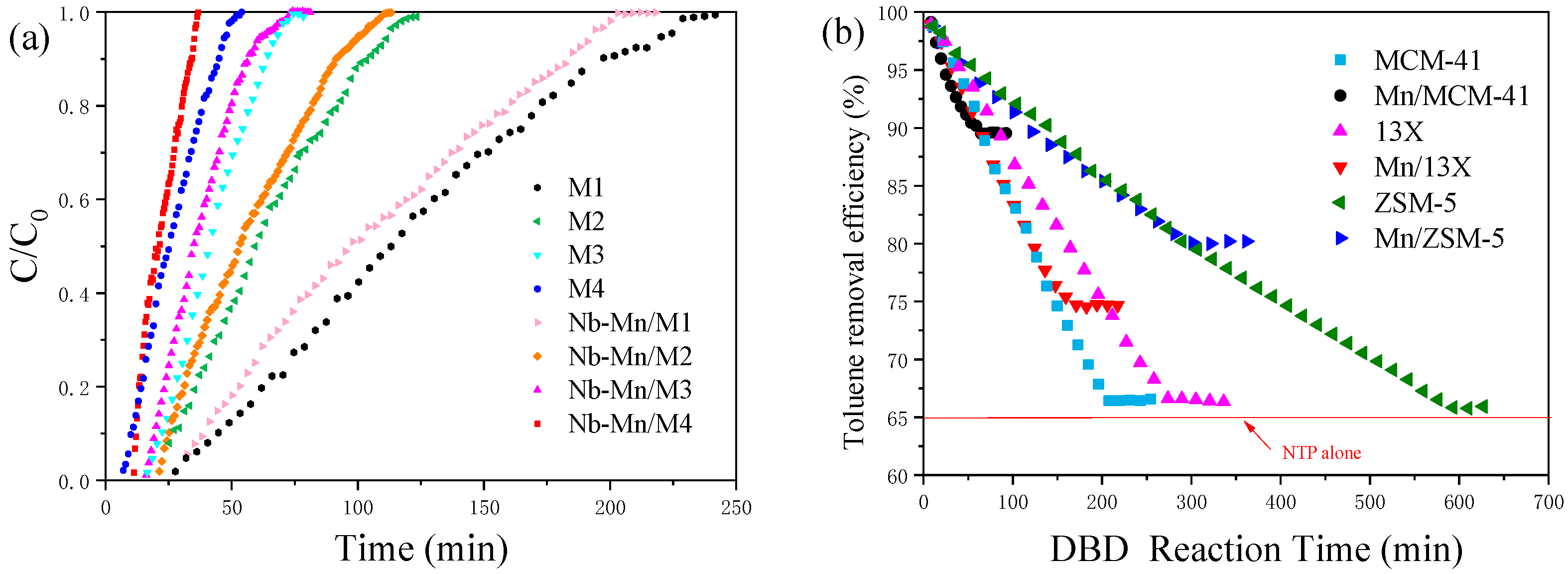
2.2.2. Effect of Adsorption
2.2.3. Effect of Pore Structure
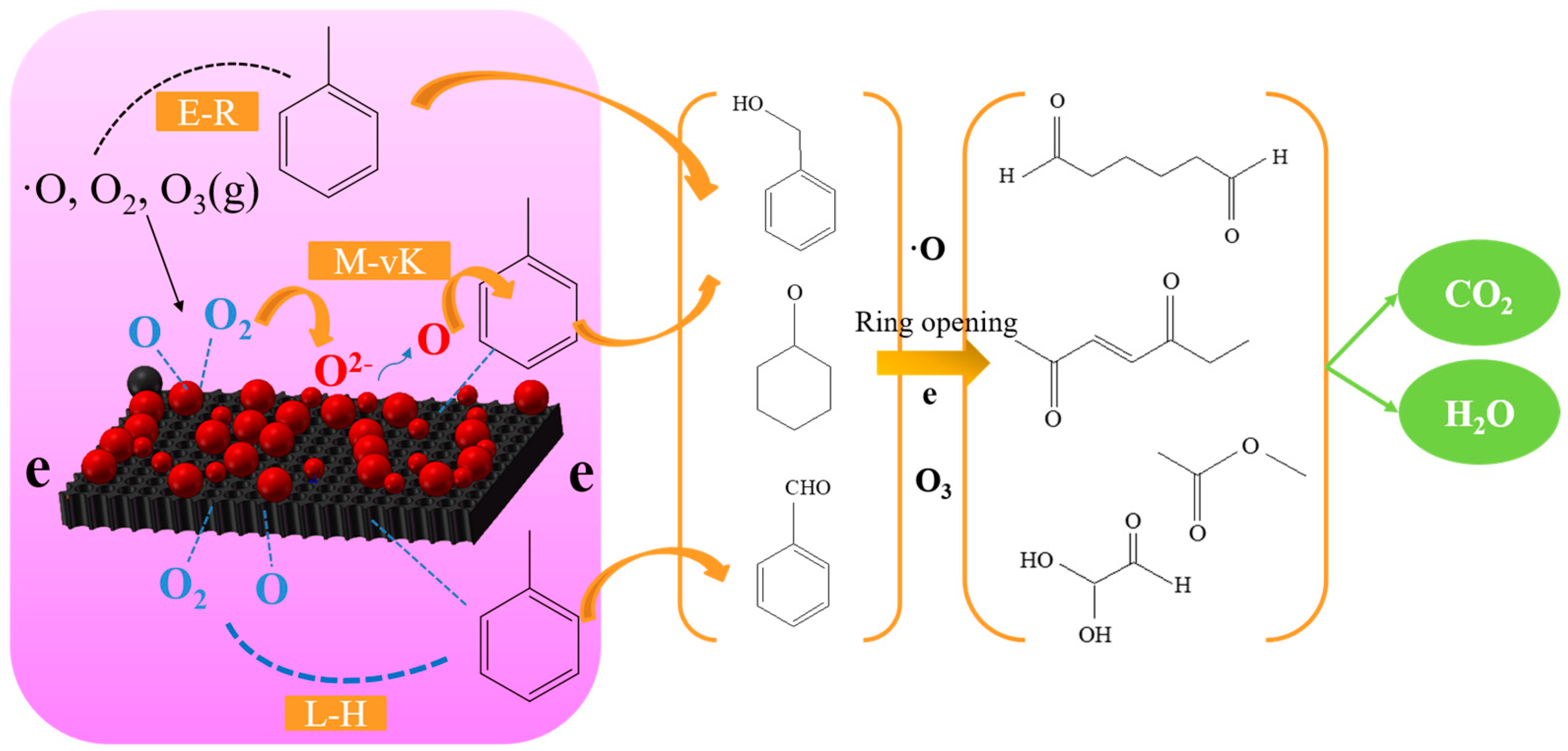
3. Discussion
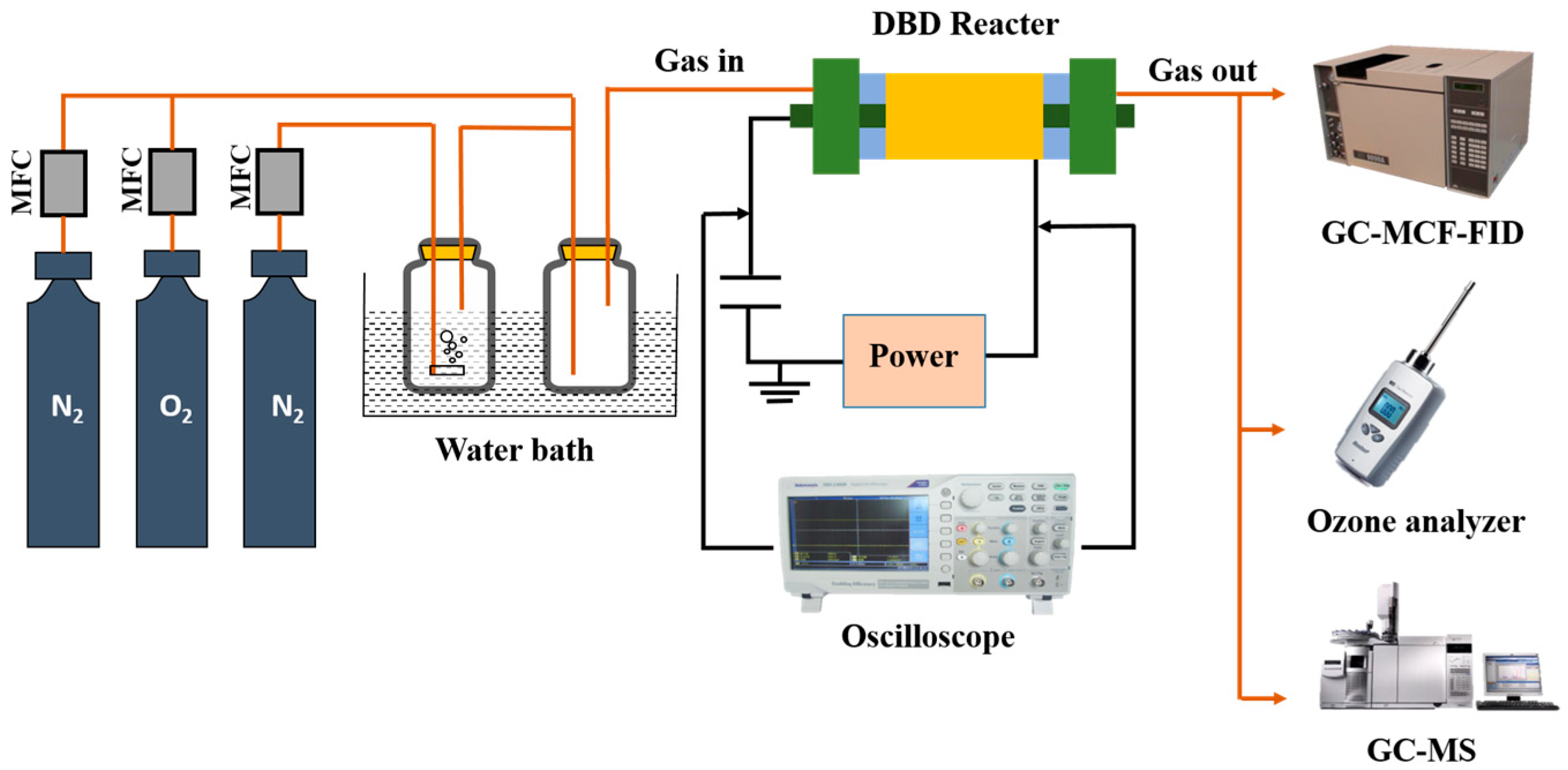
4. Experimental Instruments and Methods
4.1. Chemical Reagent
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Catalytic Performance Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.M.; Ren, Y.Q.; Deng, X.M.; Xu, M.L.; Chai, W.; Qian, X.F.; Bian, Z.F. Recent Developments on Gas-Phase Volatile Organic Compounds Abatement Based on Photocatalysis. Adv. Energy Sustain. Res. 2022, 3, 20. [Google Scholar] [CrossRef]
- Li, J.; Xia, T.T.; Xu, J.C.; Zhang, C.L.; Xu, L.; Wu, Z.L.; Yao, S.L. Boosting the plasma catalytic performance of CeO2/γ-Al2O3 in long-chain alkane VOCs via tuning the crystallite size. Appl. Surf. Sci. 2023, 611, 10. [Google Scholar] [CrossRef]
- Mu, Y.B.; Williams, P.T. Recent advances in the abatement of volatile organic compounds (VOCs) and chlorinated-VOCs by non-thermal plasma technology: A review. Chemosphere 2022, 308, 14. [Google Scholar] [CrossRef] [PubMed]
- Bal’zhinimaev, B.S.; Paukshtis, E.A.; Toktarev, A.V.; Kovalyov, E.V.; Yaranova, M.A.; Smirnov, A.E.; Stompel, S. Effect of water on toluene adsorption over high silica zeolites. Microporous Mesoporous Mat. 2019, 277, 70–77. [Google Scholar] [CrossRef]
- Zhu, L.L.; Shen, D.K.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 27. [Google Scholar] [CrossRef]
- Li, D.Q.; Wang, L.; Lu, Y.Q.; Deng, H.; Zhang, Z.L.; Wang, Y.J.; Ma, Y.; Pan, T.T.; Zhao, Q.; Shan, Y.L.; et al. New insights into the catalytic mechanism of VOCs abatement over Pt/Beta with active sites regulated by zeolite acidity. Appl. Catal. B-Environ. Energy 2023, 334, 13. [Google Scholar] [CrossRef]
- Abbas, Z.; Zaman, W.Q.; Danish, M.; Shan, A.; Ma, C.L.; Ayub, K.S.; Tariq, M.; Shen, Q.C.; Cao, L.M.; Yang, J. Catalytic nonthermal plasma using efficient cobalt oxide catalyst for complete mineralization of toluene. Res. Chem. Intermed. 2021, 47, 2407–2420. [Google Scholar] [CrossRef]
- Ge, G.W.; Lei, H.; Yao, X.M.; Fang, Y.B.; Cheng, X. Investigation of hybrid plasma-catalytic degradation of toluene over FeOOH/-Al2O3 catalysts. J. Environ. Chem. Eng. 2023, 11, 13. [Google Scholar] [CrossRef]
- Mok, Y.S.; Kim, S.G.; Nguyen, D.B.; Trinh, Q.H.; Lee, H.W.; Kim, S.B. Plasma-catalytic oxidation of ethylene over zeolite-supported catalysts to improve the storage stability of agricultural products. Catal. Today 2019, 337, 208–215. [Google Scholar] [CrossRef]
- Liu, L.; Shao, G.C.; Ma, C.L.; Nikiforov, A.; De Geyter, N.; Morent, R. Plasma-catalysis for VOCs decomposition: A review on micro- and macroscopic modeling. J. Hazard. Mater. 2023, 451, 15. [Google Scholar] [CrossRef]
- Lu, W.; Abbas, Y.; Mustafa, M.F.; Pan, C.; Wang, H.T. A review on application of dielectric barrier discharge plasma technology on the abatement of volatile organic compounds. Front. Env. Sci. Eng. 2019, 13, 19. [Google Scholar] [CrossRef]
- Xu, W.C.; Lin, K.C.; Ye, D.Q.; Jiang, X.D.; Liu, J.X.; Chen, Y.D. Performance of Toluene Removal in a Nonthermal Plasma Catalysis System over Flake-Like HZSM-5 Zeolite with Tunable Pore Size and Evaluation of Its Byproducts. Nanomaterials 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.Q.; Ji, J.F.; Chen, Y.; Zhao, G.S.; Jia, B.; Xu, L.; Cheng, P. By-product reduction for the non-thermal plasma removal of toluene using an α-MnO2/Cordierite honeycomb monolithic catalyst in a honeycomb structure. Appl. Catal. B-Environ. Energy 2024, 343, 11. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, Z.Y.; Ma, M.D.; He, C.; Yu, Y.K.; Liu, X.H.; Albilali, R. Revealing the unexpected promotion effect of diverse potassium precursors on α-MnO2 for the catalytic destruction of toluene. Catal. Sci. Technol. 2020, 10, 2100–2110. [Google Scholar] [CrossRef]
- Li, S.J.; Dang, X.Q.; Yu, X.; Abbas, G.; Zhang, Q.; Cao, L. The application of dielectric barrier discharge non -thermal plasma in VOCs abatement: A review. Chem. Eng. J. 2020, 388, 24. [Google Scholar] [CrossRef]
- Dong, S.L.; Wang, Y.F.; Yang, J.; Cao, J.; Su, L.; Wu, X.; Nengzi, L.C.; Liu, S.Y. Performance and mechanism analysis of degradation of toluene by DBD plasma-catalytic method with MnOx/Al2O3 catalyst. Fuel 2022, 319, 10. [Google Scholar] [CrossRef]
- Hoseini, S.; Rahemi, N.; Allahyari, S.; Tasbihi, M. Application of plasma technology in the removal of volatile organic compounds (BTX) using manganese oxide nano-catalysts synthesized from spent batteries. J. Clean Prod. 2019, 232, 1134–1147. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.Y.; Li, M.; Yan, Y.; Zhang, X.M.; Zhu, Y.M. High-performance of plasma-catalysis hybrid system for toluene removal in air using supported Au nanocatalysts. Chem. Eng. J. 2020, 381, 10. [Google Scholar] [CrossRef]
- Veerapandian, S.K.P.; De Geyter, N.; Giraudon, J.M.; Lamonier, J.F.; Morent, R. The Use of Zeolites for VOCs Abatement by Combining Non-Thermal Plasma, Adsorption, and/or Catalysis: A Review. Catalysts 2019, 9, 40. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.H.; Tang, X.L.; Zhao, S.Z.; Yang, Z.Y.; Ma, Y.Q.; Feng, T.C.; Cui, X.X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Shen, C.; Chen, Y.Q.; Zhao, W.N. Enhanced VOCs adsorption on Group VIII transition metal-doped MoS2: A DFT study. Chem. Phys. 2025, 589, 9. [Google Scholar] [CrossRef]
- Dong, Y.L.; Wang, F.; Song, W.A.; Wang, P.; Zhu, C.B.; Wang, X.T.; Li, S.; Li, X.W.; Li, W. Construction of surface oxygen vacancy active sites upon MnOx/AC composite catalysts for efficient coupling adsorption and oxidation of toluene. Chem. Eng. J. 2023, 464, 12. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, Z.R.; Lei, Z.; Jia, Y. Research on the Mechanism of Synergistic Treatment of VOCs-O3 by Low Temperature Plasma Catalysis Technology. Plasma Chem. Plasma Process. 2023, 43, 1651–1672. [Google Scholar] [CrossRef]
- Zhu, G.X.; Zhu, J.G.; Jiang, W.J.; Zhang, Z.J.; Wang, J.; Zhu, Y.F.; Zhang, Q.F. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B-Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Hong, W.; Shau, M.P.; Zhu, T.L.; Wang, H.N.; Sun, Y.; Shen, F.X.; Li, X. To Promote Ozone Catalytic Decomposition by Fabricating Manganese Vacancies in ε-MnO2 Catalyst via Selective Dissolution of Mn-Li Precursors. Appl. Catal. B-Environ. 2020, 274, 13. [Google Scholar] [CrossRef]
- Hu, X.Y.; Zhang, J.; Liu, Y.H.; Wen, T.C.; Yao, X.H.; Long, C. Regulating electronic metal-support interaction (EMSI) via pore-confinement to enhance non-thermal plasma catalytic oxidation of toluene. Sep. Purif. Technol. 2024, 336, 11. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Van Laer, K.; Neyts, E.C.; Bogaerts, A. Can plasma be formed in catalyst pores? A modeling investigation. Appl. Catal. B-Environ. 2016, 185, 56–67. [Google Scholar] [CrossRef]
- Jiang, Z.; Fang, D.X.; Liang, Y.T.; He, Y.Y.; Einaga, H.; Shangguan, W.F. Catalytic degradation of benzene over non-thermal plasma coupled Co-Ni binary metal oxide nanosheet catalysts. J. Environ. Sci. 2023, 132, 1–11. [Google Scholar] [CrossRef]
- Sun, Q.M.; Wang, N.; Yu, J.H. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 37. [Google Scholar] [CrossRef]
- Jiang, N.; Qiu, C.; Guo, L.J.; Shang, K.F.; Lu, N.; Li, J.; Zhang, Y.; Wu, Y. Plasma-catalytic destruction of xylene over Ag-Mn mixed oxides in a pulsed sliding discharge reactor. J. Hazard. Mater. 2019, 369, 611–620. [Google Scholar] [CrossRef]
- Zeng, X.L.; Li, B.; Liu, R.Q.; Li, X.; Zhu, T.L. Investigation of promotion effect of Cu doped MnO2 catalysts on ketone-type VOCs degradation in a one-stage plasma-catalysis system. Chem. Eng. J. 2020, 384, 11. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, B.; Xu, Y.H.; Li, X.; Yu, P.; Sun, Y.J.; Zheng, H.L. Catalytic oxidation of toluene in air using manganese incorporated catalyst by non-thermal plasma system. Sep. Purif. Technol. 2021, 257, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.S.; Hu, X.Y.; Shao, Q.; Xu, X.M.; Long, C. Balancing surface acidity, oxygen vacancies and Cu+ of CuOx CeO2 catalysts by Nb doping for enhancing CO oxidation and moisture resistance and lowering byproducts in plasma catalysis. J. Clean Prod. 2021, 318, 13. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, C.; Huang, H.; Zeng, K.; Wang, Z.; Jia, H.P.; Li, X.B. Insights into the size and structural effects of zeolitic supports on gaseous toluene oxidation over MnOx/HZSM-5 catalysts. Appl. Surf. Sci. 2019, 486, 108–120. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, J.; Liang, X.S.; Long, C. Plasma-catalytic removal of toluene over the supported manganese oxides in DBD reactor: Effect of the structure of zeolites support. Chemosphere 2018, 208, 922–930. [Google Scholar] [CrossRef]
- Hassan, M.A.; Mohamed, S.K.; Ibrahim, A.A.; Betiha, M.A.; El-Sharkawy, E.A.; Mousa, A.A. A comparative study of the incorporation of TiO2 into MCM-41 nanostructure via different approaches and its effect on the photocatalytic degradation of methylene blue and CO oxidation. React. Kinet. Mech. Catal. 2017, 120, 791–807. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, J.; Liang, X.Y.; Long, C. Niobium doping enhanced catalytic performance of Mn/MCM-41 for toluene degradation in the NTP-catalysis system. Chemosphere 2019, 230, 479–487. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; He, X.; Chen, X.; Miao, L.; Wang, Z.; Wang, X. Effect of interconnected mesoporous structure on HCHO catalytic oxidation over transition metal doped Ag/MCM-41. Surf. Interfaces 2023, 42, 103373. [Google Scholar] [CrossRef]
- Moosavifar, M.; Fathyunes, L. Influence of the post-synthesis method on the number and size of secondary mesoporous structure of NaY zeolite and its effect on catalyst loading. An efficient and eco-friendly catalyst for synthesis of xanthenes under conventional heating. J. Iran Chem. Soc. 2016, 13, 2113–2120. [Google Scholar] [CrossRef]
- Liu, L.J.; Wang, Z.M.; Fu, S.; Si, Z.B.; Huang, Z.; Liu, T.H.; Yang, H.Q.; Hu, C.W. Catalytic mechanism for the isomerization of glucose into fructose over an aluminium-MCM-41 framework. Catal. Sci. Technol. 2021, 11, 1537–1543. [Google Scholar] [CrossRef]
- Molaei, S.; Ghadermazi, M. Comparative study of two mesoporous magnetic and MCM-41 supported Cu complex: High-performance heterogeneous catalysts for aqueous synthesis of tetrazole. Inorg. Chem. Commun. 2024, 169, 10. [Google Scholar] [CrossRef]
- Kim, M.; Hong, S.A.; Shin, N.; Chae, K.H.; Lee, H.S.; Choi, S.; Shin, Y. Synthesis of manganese oxide microparticles using supercritical water. J. Supercrit. Fluids 2016, 112, 114–118. [Google Scholar] [CrossRef]
- Ali-Dahmane, T.; Brahmi, L.; Hamacha, R.; Hacini, S.; Bengueddach, A. Comparison of Lewis Acidity between Al-MCM-41 Pure Chemicals and Al-MCM-41 Synthesized from Bentonite. Bull. Chem. React. Eng. Catal. 2019, 14, 358–368. [Google Scholar] [CrossRef]
- Song, J.; Ren, Y.; Gao, X.; Fan, X.; Liu, B.; Zhao, Z. Ce-Driven Ce-MnOx/Na2WO4/SiO2 Composite Catalysts for Low-Temperature Oxidative Coupling of Methane. ACS Catal. 2024, 14, 5116–5131. [Google Scholar] [CrossRef]
- Chávez-Sifontes, M.; García, A.; Sanchis, R.; Furgeaud, C.; Mayoral, A.; Arenal, R.; Morgan, D.J.; Taylor, S.H.; López, J.M.; García, T.; et al. The promoter effect of Nb species on the catalytic performance of Ir-based catalysts for VOCs total oxidation. J. Environ. Chem. Eng. 2022, 10, 108261. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, S.; Lin, S.; Shan, B.; Zhou, X.; Zhao, J.; Qi, C.; Yang, P. Promoting the performance of Nb2O5 by doping transition metal oxide for catalytic degradation of monochlorobenzene and toluene. J. Mater. Res. Technol. 2023, 25, 3642–3653. [Google Scholar] [CrossRef]
- Batool, S.R.; Sushkevich, V.L.; van Bokhoven, J.A. Factors Affecting the Generation and Catalytic Activity of Extra-Framework Aluminum Lewis Acid Sites in Aluminum-Exchanged Zeolites. ACS Catal. 2024, 14, 678–690. [Google Scholar] [CrossRef]
- Yu, X.; Dang, X.Q.; Li, S.J.; Li, Y.; Wang, H.; Jing, K.R.; Dong, H.Y.; Liu, X. Enhanced activity of plasma catalysis for trichloroethylene decomposition via metal-support interaction of Si-O-Co/Mn bonds over CoMnOx/ZSM-5. Sep. Purif. Technol. 2023, 305, 15. [Google Scholar] [CrossRef]
- Zhu, G.X.; Zhu, J.G.; Li, W.L.; Yao, W.Q.; Zong, R.L.; Zhu, Y.F.; Zhang, Q.F. Tuning the K+ Concentration in the Tunnels of α-MnO2 To Increase the Content of Oxygen Vacancy for Ozone Elimination. Environ. Sci. Technol. 2018, 52, 8684–8692. [Google Scholar] [CrossRef]
- Si, W.Z.; Wang, Y.; Zhao, S.; Hu, F.Y.; Li, J.H. A Facile Method for in Situ Preparation of the MnO2/LaMnO3 Catalyst for the Removal of Toluene. Environ. Sci. Technol. 2016, 50, 4572–4578. [Google Scholar] [CrossRef]
- Chang, T.; Wang, Y.; Wang, Y.Q.; Zhao, Z.T.; Shen, Z.X.; Huang, Y.; Wang, C.Y.; Chen, Q.C.; Morent, R.; Veerapandian, S.K.P.; et al. A critical review on plasma-catalytic removal of VOCs: Catalyst development, process parameters and synergetic reaction mechanism. Sci. Total Environ. 2022, 828, 27. [Google Scholar] [CrossRef] [PubMed]
- Al Khudhair, A.; Bouchmella, K.; Mutin, P.H.; Hulea, V.; Gimello, O.; Mehdi, A. Hydrolytic vs. Nonhydrolytic Sol-Gel in Preparation of Mixed Oxide Silica-Alumina Catalysts for Esterification. Molecules 2022, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.C.; Jiang, X.D.; Chen, H.H.; Chen, X.; Chen, L.M.; Wu, J.L.; Fu, M.L.; Ye, D.Q. Adsorption-discharge plasma system for toluene decomposition over Ni-SBA catalyst: In situ observation and humidity influence study. Chem. Eng. J. 2020, 382, 10. [Google Scholar] [CrossRef]
- Zhuang, L.H.; Ge, L.; Yang, Y.S.; Li, M.R.; Jia, Y.; Yao, X.D.; Zhu, Z.H. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 7. [Google Scholar] [CrossRef]
- Fang, R.M.; Huang, J.; Huang, X.Y.; Luo, X.A.; Sun, Y.J.; Dong, F.; Huang, H.B. Reheat treatment under vacuum induces pre-calcined α-MnO2 with oxygen vacancy as efficient catalysts for toluene oxidation. Chemosphere 2022, 289, 10. [Google Scholar] [CrossRef]
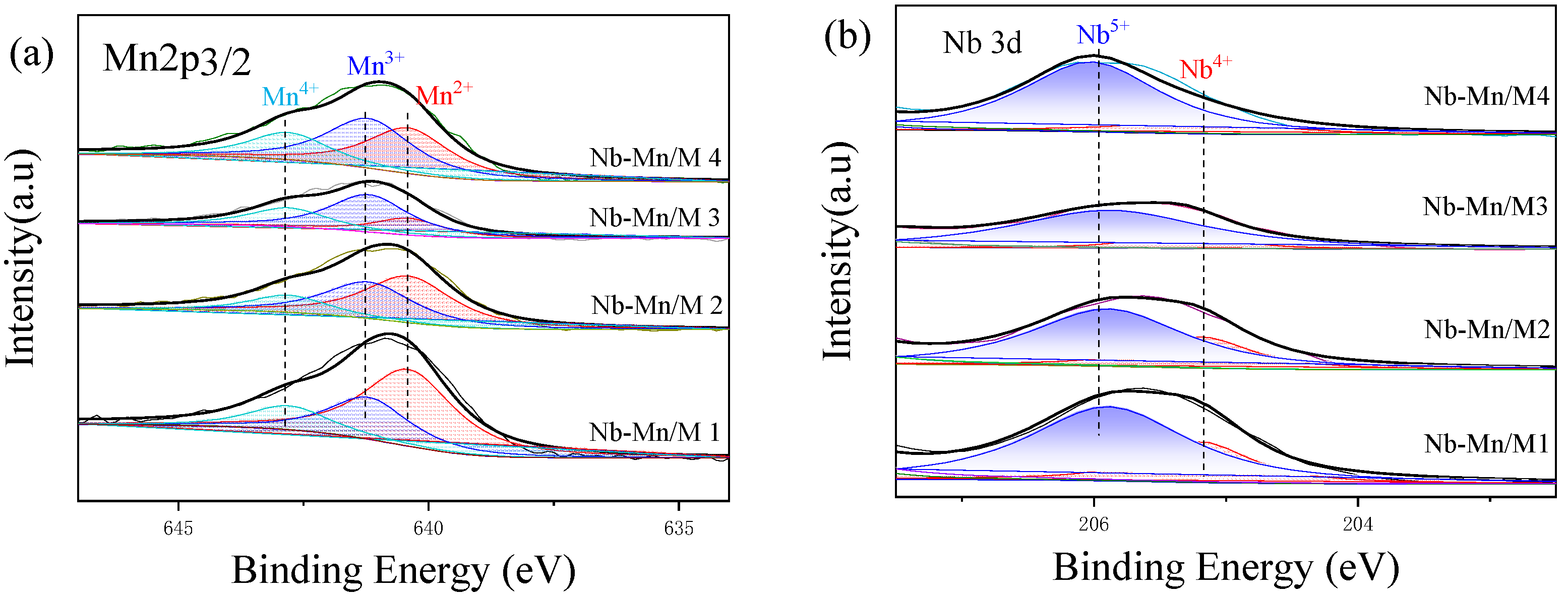

| Catalysts | Area (%) | Mn4+/ | Area (%) | Olat/ | Area (%) | Nb5+/ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn2+ | Mn3+ | Mn4+ | (Mn2+ + Mn3+) | Oad | Olat | Oad | Nb5+ | Nb4+ | Nb4+ | |
| Nb-Mn/M1 | 56.6 | 27.5 | 15.9 | 18.9% | 83.7 | 16.3 | 19.4% | 73.7 | 26.3 | 2.80 |
| Nb-Mn/M2 | 47.6 | 37.2 | 15.2 | 17.9% | 78.4 | 21.6 | 27.5% | 73.3 | 26.7 | 2.74 |
| Nb-Mn/M3 | 20.5 | 52.6 | 26.9 | 36.8% | 64.3 | 35.7 | 55.5% | 84.0 | 16.0 | 5.25 |
| Nb-Mn/M4 | 36.9 | 41.5 | 21.5 | 27.4% | 76.5 | 23.5 | 30.7% | 84.1 | 15.9 | 5.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Zhang, J.; Long, C. Tailoring Pore Size in Bimetallic Nb-Mn/MCM-41 Catalysts for Enhanced Plasma-Driven Catalytic Oxidation of Toluene. Catalysts 2025, 15, 545. https://doi.org/10.3390/catal15060545
Yao X, Zhang J, Long C. Tailoring Pore Size in Bimetallic Nb-Mn/MCM-41 Catalysts for Enhanced Plasma-Driven Catalytic Oxidation of Toluene. Catalysts. 2025; 15(6):545. https://doi.org/10.3390/catal15060545
Chicago/Turabian StyleYao, Xiaohong, Jian Zhang, and Chao Long. 2025. "Tailoring Pore Size in Bimetallic Nb-Mn/MCM-41 Catalysts for Enhanced Plasma-Driven Catalytic Oxidation of Toluene" Catalysts 15, no. 6: 545. https://doi.org/10.3390/catal15060545
APA StyleYao, X., Zhang, J., & Long, C. (2025). Tailoring Pore Size in Bimetallic Nb-Mn/MCM-41 Catalysts for Enhanced Plasma-Driven Catalytic Oxidation of Toluene. Catalysts, 15(6), 545. https://doi.org/10.3390/catal15060545






