Abstract
This work studies the effect of mesostructured silica–zirconia–tungstophosphoric acid (SiO2-ZrO2-TPA) composites used as catalysts in the synthesis of nifedipine by the Hantzsch methodology. The selectivity for nifedipine is determined, along with that of secondary products that may form depending on the reaction conditions. The materials were synthesized via the sol–gel method and characterized by N2 adsorption–desorption isotherms, infrared spectroscopy (FT-IR), 31P solid-state nuclear magnetic resonance (NMR-MAS), X-ray diffraction (XRD), thermogravimetric analysis (TGA), X-ray photoelectron spectra (XPS), and potentiometric titration. The characterization results from the XPS spectra showed that as the Si/Zr ratio drops, the Si-O-Si signal size decreases, while the Zr-O signal size increases. Characterization by titration indicated that an increase in the total acidity of the material, resulting from support modification with tungstophosphoric acid (H3PW12O40, TPA), enhances the reaction yield. The catalytic activity in the solvent-free Hantzsch reaction was evaluated under thermal heating and microwave irradiation. The experiments conducted at 80 °C achieved a maximum yield of 57% after 4 h of reaction using the Si20Zr80TPA30 catalyst (50 mg), while by microwave heating, the yield significantly improved, reaching 77% in only 1 h of reaction. This catalyst exhibited stability and reusability without significant loss of activity up to the third cycle. Depending on the type of material and the reaction conditions, it is possible to modify the selectivity of the reaction, obtaining a 1,2-dihydropyridine isomeric to nifedipine. Reaction intermediates and other minor secondary products that may be formed in the process were also evaluated.
1. Introduction
In the quest to find environmentally friendly methodologies that are in line with the principles of Green Chemistry, the pharmaceutical industry has directed all its efforts to synthesize molecules through innovative processes that minimize the use of toxic and hazardous solvents, use catalysts that have the necessary chemical characteristics, and reduce the energy cost of production [1,2,3,4,5].
The primary drawback of using homogeneous catalysts is that being in the same phase as the reactants and products, their separation from the reaction medium requires additional energy-intensive processes. Furthermore, some of the metals used are toxic, restricting their application in medical and food industries where pure, trace-free products that meet the stringent limits established by legislation are essential. The solution to these challenges lies in heterogeneous catalysis, where the catalyst is solid, allowing for easy separation from aqueous or gaseous systems. This advantage enables the catalyst to be reused multiple times without requiring extensive analysis or undergoing significant chemical changes [6].
One of the ways to achieve this objective consists of the use of multicomponent syntheses, which are methodologies that allow reactions to be carried out with advantages such as the elimination of toxic organic solvents and the replacement of homogeneous catalysts such as mineral acids. Unlike protocols that are carried out in several stages, multicomponent reactions (MCRs) are distinguished by high synthetic efficiency and a reduction in waste generation due to their high atom economy, which allows lower consumption of solvents in the production, extraction, and purification stages of the compounds of interest [4,7,8,9].
One of the most studied multicomponent reactions is the Hantzsch reaction [10], developed in 1881. This method enables the synthesis of biologically active compounds such as nifedipine, a 1,4-dihydropyridine classified as a calcium channel blocker with significant therapeutic applications in cardiovascular diseases as an antihypertensive [11]. It is also utilized to prevent vasospasms associated with conditions such as Raynaud’s phenomenon [12]. Raynaud’s phenomenon is defined as episodic artery spams in response to either cold exposure or emotional stress which manifests itself mainly as discoloration of the fingers but can also affect the toes, lips, ears, or nose. This phenomenon is more typical in women than men. Although the pathogenetic mechanisms of vasospasm in Raynaud’s phenomenon remain incompletely understood, the vasodilatory effects of calcium channel blockers—particularly nifedipine—and their demonstrated efficacy in treating coronary vasospasm have justified their evaluation in clinical trials involving patients with Raynaud’s phenomenon [12,13].
The synthesis involves the condensation of 2 equivalents of a methyl acetoacetate with 1 equivalent of 2-nitrobenzaldehyde, and 1 equivalent of ammonium acetate respectively, as illustrated in Scheme 1. Despite the importance of nifedipine, only a few studies have focused on its synthesis [14]. Moreover, research on using catalysts to optimize the reaction conditions remains limited, since not all the secondary products that can be formed are identified.
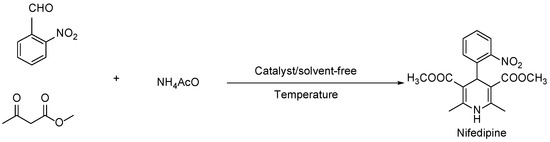
Scheme 1.
General synthesis of nifedipine by Hantzsch reaction.
Our research group has developed different multicomponent procedures for the synthesis of 1,4 dihydropyridines (DHP) [15,16,17], including nifedipine, for example, using micellar Keggin heteropolyacids (HPAs) in aqueous ethanol as solvent at 78 °C for 8 h, reaching a yield of 78%. Furthermore, it was shown that this type of catalyst is stable and can be used up to five times as heterogeneous catalysts without appreciable loss of catalytic activity. Using the same material, unsymmetrical 1,4-dihydropyridines such as nitrendipine can be obtained with a yield of 78%.
Micellar Keggin-type HPAs are supramolecular hybrids formed through the combination of a surfactant, such as CTAB (cetyltrimethylammonium bromide), which contains a long hydrophobic C16 chain, and a heteropolyacid (HPA) with Keggin structure. This interaction leads to the formation of organized micellar assemblies. Amphiphilic HPA-based catalysts have been successfully employed in various oxidation reactions carried out in emulsion and microemulsion systems [18]. Toufaily et al. [19] reported cetyltrimethylammonium bromide (CTAB) as a surfactant that plays a crucial role in the structural organization of the system. It enhances electrostatic interactions stronger than hydrogen bonding by compensating for the negative charges of the phosphotungstate (PW), thereby promoting the formation of a more ordered material.
One of the advantages of micellar Keggin catalysts [11] is their insolubility in polar media, which allows easy removal of the reaction products. Micellar Keggin heteropolyacids exhibit poor solubility in polar solvents due to their amphiphilic structure. Specifically, the HPA anion is stabilized or encapsulated by a cationic surfactant bearing a long hydrophobic alkyl chain. This micellar arrangement results in an outer surface dominated by aliphatic chains, significantly reducing the system’s affinity for polar media and promoting dispersion in less polar or non-polar environments. Additionally, the limited solubility arises from the unfavourable interactions between the hydrophobic domains of the micelles and the polar solvent molecules, which preferentially associate with each other rather than with the nonpolar regions of the micelle.
Heteropolyacids of the Keggin type are polyoxometalates with a general structure [XM12O40]n−, where X represents a central heteroatom (in this work, phosphorus P5+), and M indicates the metal ion (in this work, tungsten W6+). The structure consists of a central XO4 tetrahedron surrounded by twelve MO6 octahedra, which are arranged in four groups of three edge-sharing octahedra [20]. These are further connected through shared corners to form a compact. Keggin anions possess three types of external oxygen atoms as potential protonation centers: terminal M=O oxygens and two types of bridging M-O-M oxygens.
As shown in the previous example, it is necessary to modify HPAs in bulk [21] form due to their low surface area [20], rapid deactivation, and relatively poor stability, which makes them less effective when evaluating heterogeneous processes or in the absence of a solvent. To address these issues, efforts have been made to improve HPA performance by using different supports. Some of the materials mentioned in the literature include SiO2, Al2O3, ZrO2 [22], TUD-1 [23], MCM [24], SBA-15, zeolites, montmorillonite [25], and carbon. Anand Ramanathan et al. [26] reported that the incorporation of ZrO2 into a SiO2 matrix enhances chemical resistance to alkaline attack, increases fracture toughness, enhances their selectivity and improves surface acidity [27].
However, there is limited research on the use of hybrid silica and zirconia matrices, particularly with a focus on optimizing reaction conditions for the synthesis of nifedipine.
Zirconium as catalyst support [28,29] is particularly interesting because, when tetrahedrally coordinated, it acts solely as a Lewis acid, lacks Brønsted acidity, and is thermally stable in different atmospheres. One drawback of zirconia is its low surface area, which can be increased by supporting it on silica and synthesizing microporous and mesoporous materials, where zirconium partially replaces the structural components [26].
In this research, we report the synthesis and characterization of tungstophosphoric acid (H3PW12O40, TPA, a Keggin-type heteropolyacid) on silica and zirconia matrices. A series TPA immobilized on SiO2-ZrO2 mesoporous matrix was synthesized via the sol–gel method, using urea and triethanolamine as low-cost mesoporous template agents, varying the SiO2:ZrO2 ratios from 100:00 to 00:100 (wt/wt%). The TPA content in the final materials was 30 wt/wt% (Si100Zr00TPA30, Si80Zr20TPA30, Si20Zr80TPA30, and Si00Zr100TPA30 samples).
The catalysts were characterized using various physicochemical techniques, and the catalytic activity of the materials was studied in the multicomponent Hantzsch synthesis of nifedipine. The characteristics of the materials, together with the reaction conditions, showed that a variety of secondary products can be formed together with nifedipine.
2. Results and Discussion
2.1. Characterization of Materials
Figure 1 shows the adsorption–desorption isotherms and pore diameter distribution of SiXZrYTPA30 materials, and the key textural property data summarized in Table 1. The isotherms can be classified (according to IUPAC) as type IV, characteristic of mesoporous materials.
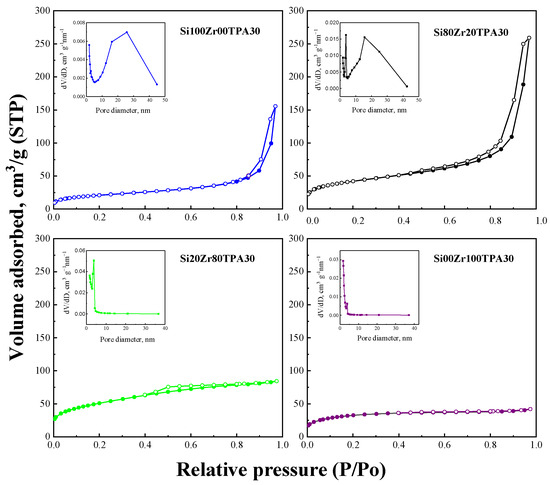
Figure 1.
N2 adsorption–desorption isotherms and pore size distribution (insert) of SiXZrYTPA30 samples.

Table 1.
Main textural properties of SiXZrYTPA30 materials.
The Si100Zr00TPA30 and Si80Zr20TPA30 sample isotherms show an increase of N2 adsorbed in the 0.8–1 range of P/Po, associated with the presence of large mesopores. The PSD obtained by the DFT method reveals the presence of small (in the range 2–4 nm) and large (in the range 10–40 nm) mesopores. In the case of samples with a higher Zr content (Si20Zr80TPA30 and Si00Zr100TPA30 samples), the contribution of large mesopores disappears. The pore size distribution (PSD) obtained by the DFT method reveals small mesopores centers at approximately 2.0 and 2.6 nm in the Si100Zr00TPA30 and Si80Zr20TPA30 samples, respectively. The other two samples were centered at 3.2–4.0 nm. The PSD also shows the existence of large mesopores (in the range 10–40 nm). However, in the case of samples with a higher Zr content (Si20Zr80TPA30 and Si00Zr100TPA30 samples), the contribution of large mesopores disappears. Despite the absence of mesopores in the range 10–40 nm, the amount increment of small mesopores keeps the pore volume almost constant. As can be observed (Table 1), in all the samples, SBET values are higher than 75 m2 g−1. In addition, Si100Zr00TPA30 and Si80Zr20TPA30 materials present isotherms with H1 type hysteresis loop, associated with porous materials consisting of agglomerates of uniform spheres (IUPAC). For Si20Zr80TPA30 and Si00Zr100TPA30 samples (materials with a higher Zr content), H3-H4 types of hysteresis loop (associated with narrow slit-like pores) can be seen.
On the other hand, SiXZrYTPA materials showed similar textural properties to the parent solids used as support. Thus, the impregnation with TPA solutions did not lead to a significant change in the textural characteristics of the SiXZrY materials used as support. However, although the mesoporous characteristics of the SiXZrYTPA solids look like those of the SiXZrY materials, their specific surface area decreased by about 25–30%. The drop in SBET values can be explained considering that the specific surface area of bulk TPA is very low (<5 m2 g−1), and that SiXZrYTPA materials are composed of 30% of TPA.
Figure 2a shows the XRD patterns of the SiXZrY solids used as support. The diffractogram corresponding to the Si100Zr00 solid shows a broad signal at 15 with a shoulder at ~25°, typical of amorphous silica. As the Zr content increases (Si80Zr20 sample) a shoulder at 2θ ≅ 30° appears due to the presence of zirconia noncrystalline phase. In parallel with the increase of Zr content, the shoulder became broad, and the signal assigned to amorphous silica disappeared. Neither the reflections corresponding to monoclinic (JCPDS 13-307) nor to the tetragonal ZrO2 crystalline phases (JCPSD 17-923) were present. On the other hand, any DRX peaks assigned to the ZrSiO4 (JCPDS No 06-0266) were not detectable.
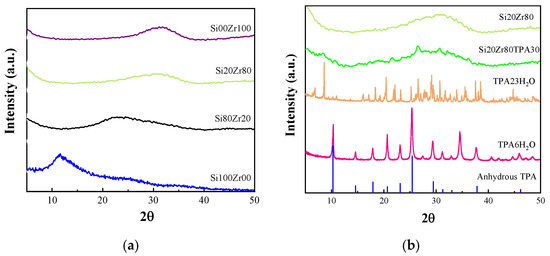
Figure 2.
(a) XDR patterns of SiXZrY; (b) XDR patterns of Si20Zr80TPA30, Si20Zr80 sample, TPA hydrated with 6 and 23 molecules, and anhydrous TPA (JCPDS No 50 0657).
The XRD patterns of the Si20Zr80TPA30 sample (Figure 2b) show a series of wide and weak peaks at 2θ = 21.6°, 26.4°, and 30.7°; neither the characteristic peaks of H3PW12O40·23H2O (JCPDS No 38-0178) or H3PW12O40·6H2O (JCPDS No 50-0304), nor those corresponding to H3PW12O40 anhydrous, appear. Considering the temperature used for SiXZrYTPA30 sample thermal treatment, these peaks could be assigned to the presence of H3PW12O40 crystals. XRD results suggest that the TPA is well dispersed mainly on the support as a noncrystalline phase [30].
Figure 3 displays the FT-IR spectra of Si100Zr00, Si80Zr20, Si20Zr80, and Si00Zr100 materials. The main SiO2 bands are summarized in Table 2. The series shows a decrease in the intensity of characteristic silica bands as the SiO2 content decreases, along with a shift of its maximum towards lower wavelengths. Concurrently, as the ZrO2 content increases, its characteristic bands become more visible. It is important to note that the stretching band at 980 cm−1, attributed to the Si–O–Zr bond, was not detected in either the Si80Zr20 or the Si20Zr80 spectrum [29,31].
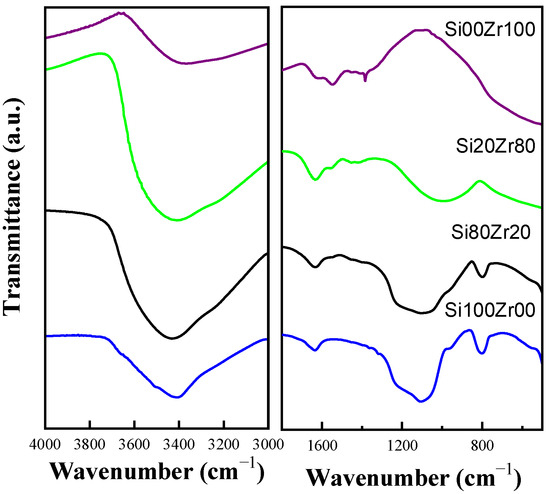
Figure 3.
FT-IR spectra of SiXZrY samples.
The Si00Zr100 spectrum reveals a broad band associated with the stretching vibrations of the -OH group (3600 and 3200 cm−1), as well as a pair of non-coincident bands with maxima at 1620 cm−1 and 1550 cm−1, assigned to the bending vibrations of (H-O-H) and (O–H–O), respectively [22]. Additionally, another broad band extending from 1060 cm−1 to 400 cm−1 is observed, which is characteristic of zirconia bonds.
The spectrum of the Si100Zr00 sample exhibits the characteristic bands of silica, including a broad band with a maximum at approximately 1100 cm−1 attributed to the asymmetric stretching vibration of the Si-O-Si group (siloxane), along with three bands of lower intensity at 966 cm−1, 810 cm−1, and 462 cm−1 assigned to the stretching of the Si-OH and Si-O-Si groups, and the bending of the O-Si-O group, respectively [30,32,33]. In the samples with the highest Zr content, the intensity of the band assigned to Si-O-Si groups (around 790–810 cm−1) is considerably lower than in the samples containing more Si. Typically, this band is used to characterize Zr-O-Si bond formation.

Table 2.
FT-IR characteristic bands of TPA, SiO2, ZrO2, and TEA materials.
Table 2.
FT-IR characteristic bands of TPA, SiO2, ZrO2, and TEA materials.
| Vibration Modes | Band (cm−1) | |
|---|---|---|
| Bending vibration Oa-P-Oa | 524 | [34,35,36] |
| Stretching vibrations W-OC-W | 793 | |
| Stretching vibrations W-Ob-W | 896 | |
| Stretching vibrations W=Od | 982 | |
| Stretching vibrations P-Oa | 1081 | |
| Bending vibration O-Si-O | 480 | |
| Stretching Si-O-Si group | 800 | |
| Stretching Si-OH group | 965 | |
| Angular vibration of H2O | 1650 | |
| Asymmetric stretching siloxane group | 1220–1100 | |
| Stretching OH group | 3700–3200 | |
| Stretching O-Zr-O | 460 | |
| Asymmetric stretching Si-O-Zr | 980 | |
| TEA C-H vibration | 1400 |
In the Si00Zr100 spectrum, a very low intensity band at 1400 cm−1, assigned to TEA C-H vibration, is observed, due to a remaining fraction of the structuring agent that could not be removed during ethanol washing and thermal treatment. This band is not visible in other materials.
The FT-IR spectrum of bulk TPA (Figure 4) reveals bands at 1081, 982, 896, 793, and 524 cm−1 [34,35,36]. These bands overlap with the characteristic bands of support.
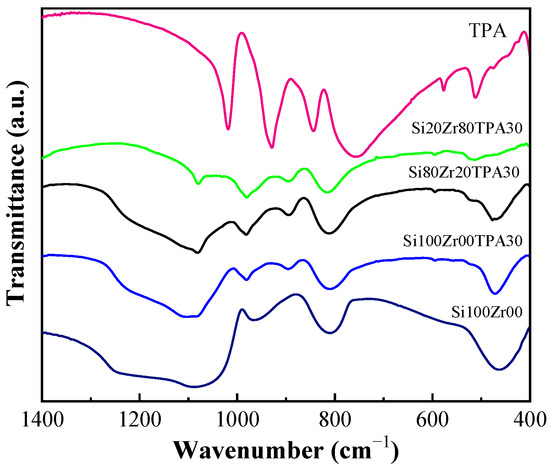
Figure 4.
FT-IR spectra of Si00Zr100, SiXZrYTPA30, and TPA samples.
In general, it is observed that the positions of the bands vary slightly with the change of the Si/Zr molar ratio. For example, in the Si80Zr20TPA30 material, the band around 500 cm−1 shifts from 470 cm−1 to 465 cm−1.
A high zirconium content in the materials can lead to less ordered structures, which results in a decrease of intensity in the band located between 790 and 810 cm−1 [37].
The TGA diagrams of SiXZrYTPA30 materials (Figure S1) show two weight losses at temperatures below 250 °C, corresponding to the elimination of moisture and absorbed water, which appear in the DTG (derivative thermogravimetry) curve as two peaks with maximum at ~100 and 200 °C respectively. For all the samples, the weight loss was lower than 6% (Table S1). No further significant weight changes take place in the range 200–400 °C. Thus, the synthesized materials are thermally stable until 400 °C and can be used as heterogeneous catalysts in a wide range of temperatures.
The surface composition, the oxidation states of tungsten on the surface, the possible formation of Zr-O-Si bonds, and the interaction between the heteropolyacid and the support were studied using XPS analysis. The high-resolution XPS spectra of the materials are shown in Figure 5, and the binding energy values depicted for Zr 3d, O1, Si 2p, and W 4f orbitals are listed in Table 3.
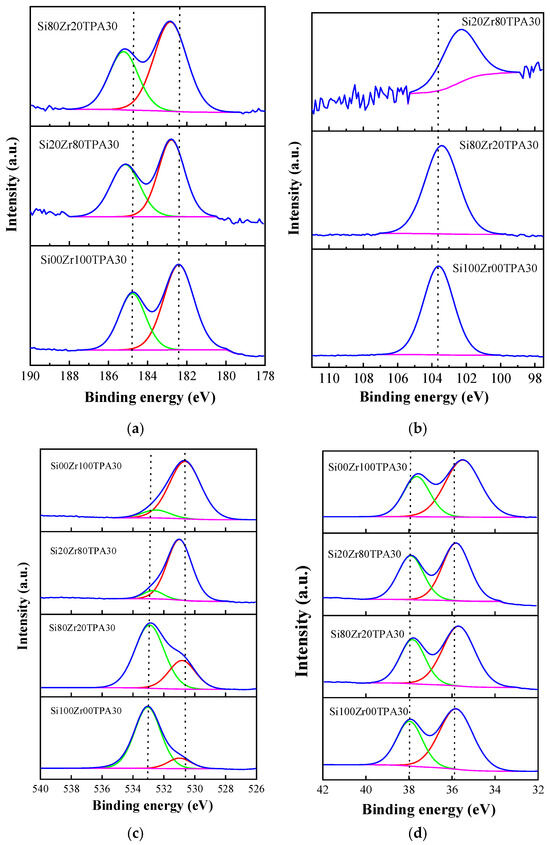
Figure 5.
Zr 3d (a), Si 2p (b), O 1s (c), and W 4f (d) X-ray photoelectron spectra of SiXXZrYYTPA30 samples. Blue curve: experimental results. Green, red, and pink: deconvolutions.

Table 3.
Binding energy (XPS) values of prepared materials.
The spectra of Zr 3d XPS (and their deconvolutions) of the samples Si80Zr20TPA30, Si20Zr80TPA30, and Si00Zr100TPA30 are shown in Figure 5a. The samples present signals at approximately 182 eV and 184 eV corresponding to the Zr 3d5/2 and Zr 3d3/2 levels, respectively [38,39]. The observed energy difference between Zr 3d5/2 and 3d3/2 (~2.4 eV) is consistent with the presence of ZrO2 type species [40]. The values found for the Zr 3d5/2 signal (182.4–182.8 eV) are close to those reported in the literature for ZrO2 (Zr4+) [41,42]. The deconvolution of the spectra did not give any evidence of the presence of Zr3+ signals associated with oxygen vacancies in the framework that appear at higher energy values (182.8–183.4 eV) [43].
With the increment of SiO2 content in the samples, the Zr 3d5/2 and Zr 3d3/2 level energy values slightly shift towards a higher binding energy. The largest variation (approximately 0.4 and 0.5 eV, respectively) is observed in the sample Si80Zr20TPA30 and Si20Zr80TPA30. The shift towards higher bond energies can be attributed to Si-O-Zr bond formation. Due to the Si (1.90 eV) higher electronegativity than that of Zr (1.33 eV), electrons around Zr are transferred to the Si atoms, thereby reducing the Zr electron cloud density and increasing Zr 3d5/2 binding energy [44]. However, considering that FT-IR spectra did not show the Zr-O-Si characteristic band at 1400 cm−1, we can suggest ZrO2 and SiO2 were mainly present as separate phases. Additionally, considering that W (1.70 eV) electronegativity is also higher than that of Zr, we must assume that W-O-Zr covalent bonds formed during thermal treatment can also be responsible for the Zr 3d5/2 binding energy shift.
Figure 5b shows the spectra of Si 2p along with their corresponding deconvolution, for the materials Si100Zr00TPA30, Si80Zr20TPA30, and Si20Zr80TPA30. The Si100Zr00TPA30 sample exhibits a peak at a binding energy of 103.6 eV, which, according to the literature, corresponds to the presence of silicon oxide in a tetrahedral structure [29,45]. In the case of samples containing Zr, a shift towards lower binding energies between 103.4 and 102.3 eV is evident. This shift increases as the Si/Zr ratio decreases, and is attributed to the formation of Si-O-Zr bonds [29]. As mentioned before, the electronegativity of Si is greater than that of Zr, therefore the Si electron cloud increases and the Si 2p binding energy decreases. Considering the small electronegativity difference between Si and W, the influence of W-O-Si covalent bonds formed during the thermal treatment over the Si 2p binding energy shift can be considered negligible. No evidence was found for the signals corresponding to Si(-OH)X that appear at energy values equal to 99.5 eV [46].
The high-resolution O 1s spectrum of TPA shows two distinct contributions at 531.2 eV and 532.5 eV, associated with W-O-W and W-O-P bonds respectively [34].
The Si100Zr00TPA30 O 1s spectrum exhibits signals at 533.0 and 530.9 eV, corresponding to Si-O-Si and W-O-W bonds [34]. No evidence was found for Si(OH)x species, which typically appear at ~531 eV [46].
In the spectrum of the Si00Zr100TPA30 sample, two signals appear at 532.5 and 530.6 eV. They can be attributed to Zr-O bonds such as those found in pure ZrO2 and TPA [29,45].
The spectra for the materials Si80Zr20TPA30 and Si20Zr80TPA30 show signals associated with Si-O and Zr-O bonds in the ranges of 532.9–532.5 eV and 530.9–530.6 eV, respectively. As the Si/Zr ratio drops, the Si-O-Si signal size decreases, while the Zr-O signal size increases, in agreement with the findings reported by Bosman et al. [45]. Additionally, the energy values for both signals slightly decrease as the Si/Zr ratio drops.
The XPS spectrum of W 4f from TPA shows a well-resolved doublet at 37.9 and 35.8 eV, corresponding to the W 4f5/2 and W 4f7/2 states [47,48], associated with the presence of W (VI) octahedrally coordinated by oxygen. In the spectra of the synthesized samples shown in Figure 5d, the signal assigned to W 4f5/2 and W 4f7/2 appear in the range 37.9–37.5 and 35.8–35.4 eV, respectively. The slight shift in energy values suggests a possible change or disturbance in the octahedron of oxygen atoms surrounding W [49]. This disturbance is attributed to the interaction of the Keggin structures of TPA with the support, which can be due to W-O-Si and W-O-Zr covalent bond formation or to electrostatic interaction of the terminal oxygens (W=O) [22].
The interaction between the TPA and the silica/zirconia matrix can be assumed to be of the electrostatic type due to the transfer of protons to Si/Zr–OH groups according to:
Si/Zr–OH + H3PW12O40 → [Si/Zr–OH2+]n[H3−nPW12O40]n−
However, interactions of hydrogen bond type between TPA terminal oxygen (W=O) atoms and hydroxyl groups could also take place. As a result of the thermal treatment at temperatures higher than 300 °C, Si/Zr-O-W bonds can be formed through [49]:
[Si/Zr–OH2+]n[H3−nPW12O40]n− → [Si/Zr-O-W-PW11O39]2− + H2O
31P magic angle spinning-nuclear magnetic resonance spectra of Si100Zr00TPA30, Si80Zr20TPA30, Si20Zr80TPA30, and Si00Zr100TPA30 samples (Figure 6) show a wide peak at -15.3 ppm, attributed to [PW12O40]3− (line width ~ 1.1 ppm) [50,51]. The increase of the line width observed, compared to the H3PW12O40∙6H2O (~0.04 ppm) (where [PW12O40]3− anion interacts with (H2O)2H+ species) [52], can be ascribed to the electrostatic interaction among the [PW12O40]3− anion and positively charged species (for example, ≡Si-OH2+, and ≡Zr-OH2+groups) present on the SiXZrY matrix surface.
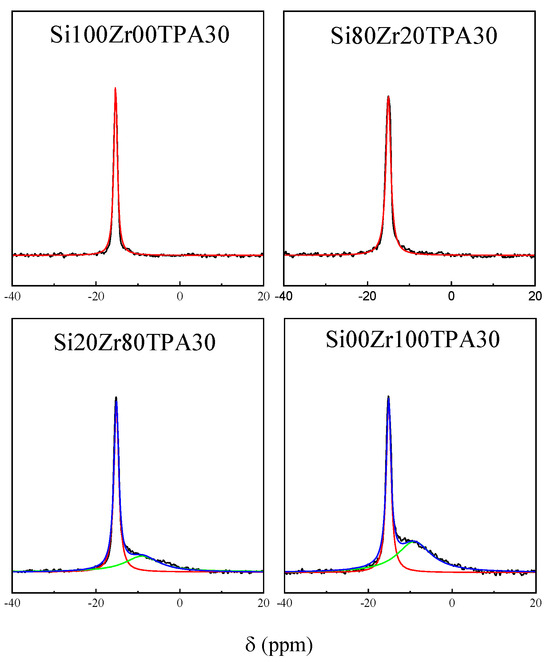
Figure 6.
31P MAS-NMR spectra of SiXZrYTPA30 materials.
Additionally, the line width increment can be due to W-O-Si and W-O-Zr covalent bond formation, which, according to Legagneux et al. [49], can be generated by thermal treatment at temperatures higher than 300 °C through the following path:
(≡M-OH2)+ [PW12O40]3− → (≡M-O-W-PW11O39)2− + H2O
An analogous behavior was suggested for the interaction of TPA with ZrO2, SiO2, and TiO2 [50,53,54]. For materials with lower SiO2 proportion (Si20Zr80TPA30 and Si00Zr100TPA30 samples), the additional presence of a shoulder at ~9 ppm, which is assigned to the [PW11O39]7− anion, was detected [55]. Moreover, its intensity increased with the increment of ZrO2 content in the sample.
According to 31P NMR and FT-IR results, the main species present in the SiXZrYTPA samples is the [PW12O40]3− anion, which partially transforms into [PW11O39]7− anion during synthesis and drying steps in Si20Zr80TPA30 and Si00Zr100TPA30 materials.
The [PW12O40]3− transformation into the lacunar [PW11O39]7− anion is generally assigned to its limited stability range in solution. At pH 1.5–2, [PW12O40]3− is reversibly and quickly transformed into the lacunar anion [55]. This transformation takes place according to the following scheme: [PW12O40]3− ⇔ [P2W21O71]6− ⇔ [PW11O39]7− because of the solution pH increment. We reported [22] the presence of both [P2W21O71]6− and [PW11O39]7− anions, together with [PW12O40]3− (the main species) on the ZrO2 impregnated with TPA solution. This transformation took place during the impregnation step and the subsequent thermal treatment.
The acidity properties of SiXZrYTPA30 materials, estimated from the potentiometric titration with n-butylamine curves (Figure 7), are listed in Table 4. Ei (the initial electrode potential) indicates the maximum acid strength and Ns (calculated as the area under the curve) the total number of acid sites. According to the potentiometric results, all the materials exhibit Ei values higher than 100 mV, assigned to the presence of very strong acid sites [56]. The acid strength values of SiXZrYTPA30 samples are higher 305 than in the SiXZrY solids used as support (Table S2) but lower than that 306 of H3PW12O40∙6H2O (Ei = 760 mV) [34].
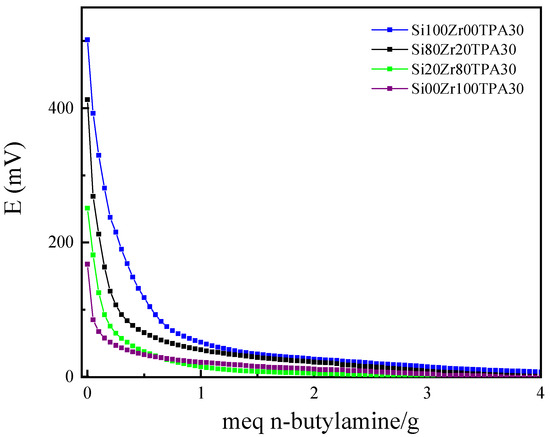
Figure 7.
Potentiometric titration curves of SiXZrYTPA30 samples.

Table 4.
Initial electrode potential (Ei) and total number of acid sites of catalysts with a 30% TPA loading.
The lower acid strength of SiXZrYTPA30 samples compared to hydrated TPA could be assigned to the fact that the protons in the latter are present as (H2O)2H+ species, whereas in the synthesized samples they are interacting with the oxygen of ≡M–OH groups.
2.2. Catalytic Test
The aim of this work is to evaluate the catalytic performance of synthesized Si/Zr/HPA-based materials in the synthesis of nifedipine through the Hantzsch multicomponent process by reaction of 2-nitrobenzaldehyde, methyl acetoacetate, and ammonium acetate. The selectivity towards nifedipine is evaluated, and the different secondary products obtained depending on the nature of the material and the reaction conditions are identified. Scheme 2 shows the classical process of nifedipine formation as well as the different routes that allow rationalization of intermediate and secondary products.
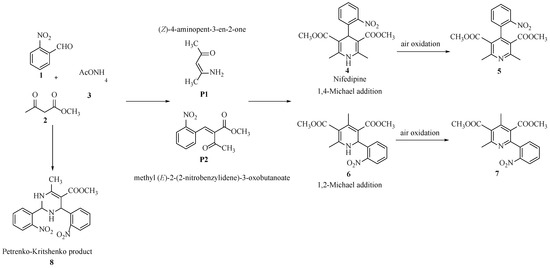
Scheme 2.
Classical process of nifedipine formation, as well as different pathways that elucidate formation of secondary products.
The Hantzsch pyridine synthesis, described in 1881 by Arthur Rudolph Hantzsch [10], is one of the most common methods for the synthesis of substituted dihydropyridines. In this process, two equivalents of a 1,3-dicarbonyl compound, an aldehyde and a nitrogen-containing molecule, such as ammonia or ammonium acetate, are combined to give a 1,4-dihydropyridine [11].
Nifedipine 4 (Scheme 2) is obtained as the main product under heating conditions and using an acid catalyst for a long time. The reaction proceeds via 1,4-addition (Michael-type), in which C-C bond formation is thermodynamically favorable and can occur under either basic conditions or using acidic catalysts [57,58].
In recent decades, it has been found that reaction conditions and catalyst type can alter the selectivity towards 1,4-dihydropyridine. However, no detailed study applied to the synthesis of nifedipine has been reported. 2-Phenylpyridine (the unsymmetrical 1,2-DHP) can be obtained as the main product under various conditions, for example, room temperature and long time periods [59], alumina-supported HPA [60], Mn and Ce oxides [15], and gold catalyst [61].
In all cases, the process involves a 1,2-Michael addition mechanism, which leads to the formation of 1,2-dihydropyridine 6 (Scheme 2), which is unstable and slowly transforms by aromatization into 2-phenylpyridine 7 in the presence of air, which acts as an oxidant.
Recently, To et al. [62] reported the synthesis of a new secondary product by varying the stoichiometry of the reactants, through a penta-component process. Studies of the new compound by X-ray crystallography analysis and DFT calculations confirmed the presence of a structure containing two groups at the cis position in the pyrimidine ring. When 2-nitrobenzaldehyde is used as substrate, the Petrenko–Kritschenko reaction yields product 8.
Catalytic evaluation of the synthesized materials (Si/Zr/HPA) was carried out by studying the multicomponent reaction between 2-nitrobenzaldehyde, methyl acetoacetate, and ammonium acetate to obtain nifedipine 4. The experimental conditions, the conversion of 2-nitrobenzaldehyde, and the selectivity towards nifedipine are summarized in Table 5 and Figure 8. In addition, the selectivity towards secondary products is shown depending on the material studied and the reaction conditions.

Table 5.
Hantzsch synthesis of nifedipine using different synthesized catalysts.
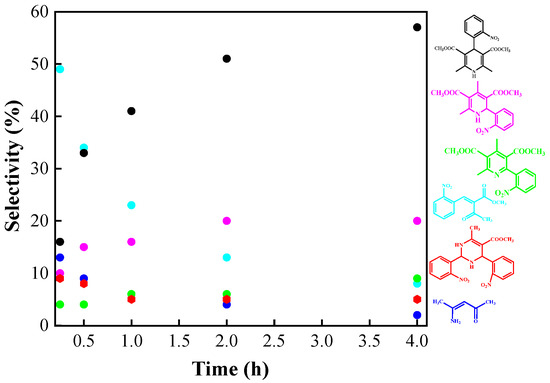
Figure 8.
Product selectivity yields (%). Reaction conditions: 1 mmol 2-nitrobenzaldehyde, 2 mmol methyl acetoacetate, 1 mmol ammonium acetate, 50 mg of Si20Zr80TPA30, 80 °C, 4 h.
The following are the formulas for determining the conversion of 2-nitrobenzaldehyde, the selectivity towards nifedipine, and R (which defines the ratio between the selectivity achieved by the 1,4-addition (products 4 and 5) and the 1,2-addition (products 6 and 7).
NBC% = 1/P1 + P2 + 4 + 5 + 6 + 7 + 8
NS = 4/P1 + P2 + 4 + 5 + 6 + 7 + 8
R = 1,4-Addition/1,2-Addition = 4 + 5/6 + 7
In the control experiment conducted in the absence of catalyst, a nifedipine selectivity of 35% and R = 1 was observed (Table 5, entry 1); however, the presence of the reaction intermediates P1 and P2 was detected by GC. The conversion of nitrobenzaldehyde was practically 100%, as in most of the following essays.
Entries 2 and 3 (Table 5) show that the selectivity to nifedipine 4 increases slightly when Si100Zr00 and Si00Zr100 supports were used as catalyst. The NS/Ns obtained using Si00Zr100TPA00 (50%) is a little bit higher than that obtained using Si100Zr00TPA00 (42%). Regarding R, it remains practically constant when compared to the reaction in the absence of catalyst, indicating a relationship/ratio close to 1 between the 1,4- and 1,2-acid products.
Experiments, entries 4 to 7 (Table 5), were carried out with the synthesized catalysts containing a constant amount of HPA (30 wt/wt%) (Si100Zr0TPA30, Si80Zr20TPA30, Si20Zr80TPA30, and Si00Zr100TPA30). The catalytic tests performed under identical reaction conditions, using a stoichiometric amount of reagents, at 80 °C for 4 h, under solvent-free conditions and with magnetic stirring, indicate that the selectivity towards nifedipine increases slightly, compared to pure supports (entries 2 and 3), reaching a maximum of 57% nifedipine selectivity using the Si20Zr80TPA30 catalyst. Moreover, the notable increase in the R ratio (1.9 vs. 1) is justified by an increase in selectivity towards a 1,4-addition of the reaction intermediates P1 and P2, and therefore there is a greater possibility of increasing the yield of nifedipine 4. This can be due to the acidic strength of the catalysts compared to that of the supports, which favors this type of addition (for example, support Si20Zr80 (Ei = 121 mV) vs. catalyst Si20Zr80TPA30 (Ei = 251 mV).
Both the results presented for nifedipine and those previously reported in the literature [59] for the Hantzsch reaction in general indicate that an increase in the acidity of the catalytic materials favors the formation of products from the 1,4-additions. The presence of a Bronsted acid in the reaction medium could protonate the carbonyl group of 2-nitrobenzaldehyde, increasing its electrophilicity. This increased electrophilicity facilitates the initial steps of the reaction, including the formation of imine intermediates and the nucleophilic attack by the β-dicarbonyl compound. However, high acidity also promotes secondary reactions, such as unwanted degradation or condensation of the reagents, complicating selectivity control and leading to the generation of by-products or undesirable modifications of nifedipine. Therefore, intermediate acidity is sufficient to improve the selectivity towards nifedipine 4.
Figure 8 shows the reaction selectivity as a function of time for the products formed at 80 °C. Conversion of 2-nitrobenzaldehyde is very rapid (100% in 15 min for most tests). Intermediate P1 and P2 transformation occurs at a slower rate. The selectivity of nifedipine 4 steadily increases over approximately 4 h of reaction time and then stabilizes, correlating with the consumption of intermediates P1 and P2. Notably, in these reaction conditions the formation of Michael 1,2-addition products 6 + 7 is approximately 28%. Furthermore, product 5 resulting from nifedipine oxidation was not detected, suggesting that the 1,2-addition product is more readily oxidized than nifedipine 4.
It is noteworthy that in all experiments, small amounts of the Petrenko–Kritschenko product 8 (<10%) were detected, particularly at shorter reaction times (15 min). The structures of products 7 (Figures S2–S6) and 8 (Figures S7–S11) were confirmed by 1H and 13C NMR, and the results are shown in the Supplementary Materials.
The following experiments were performed with the catalyst that gave the best results in nifedipine selectivity and a higher ratio between 1,4- and 1,2-addition (Si20Zr80TPA30), with the aim of further increasing the selectivity towards nifedipine and reducing the formation of secondary products.
To evaluate the influence of reaction temperature, experiments at 20 °C, 60 °C, 80 °C, and 120 °C were performed in identical reaction conditions (see Figure 9). No significant nifedipine formation was detected at 20 °C (less than 3% at 4 h). The selectivity of nifedipine increases significantly with rising reaction temperatures, reaching a maximum at 120 °C. For instance, a yield of 65% was achieved after 1 h of reaction, which increased slightly to 74% after 4 h. Under these conditions, the sample becomes slightly dark grey, and secondary products associated with possible decomposition begin to be qualitatively detected by TLC.
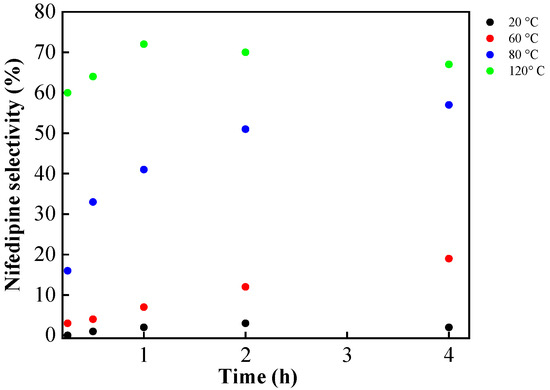
Figure 9.
Temperature effect on nifedipine selectivity (%). Reaction conditions: 1 mmol 2-nitrobenzaldehyde, 2 mmol methyl acetoacetate, 1.3 mmol ammonium acetate, 50 mg of Si20Zr80TPA30, 80 °C, 4 h.
The 2-nitrobenzaldehyde conversion together with its selectivity towards nifedipine and 6 + 7 Michael 1,2-addition products are shown as a function of temperature in Figure 10. The experiments performed at 20 °C show that products 6 and 7 can be obtained as the main products, in a long time (72 h). In this case, the reaction proceeds through a 1,2-addition (kinetic control) and the yield on products 6 and 7 is dependent on reaction time [15]. At 80 °C and 120 °C, a notable increase in nifedipine 4 selectivity was observed. In this case, the reaction proceeds via 1,4-addition (Michael-type), and nifedipine 4 is the most thermodynamically favorable compound.
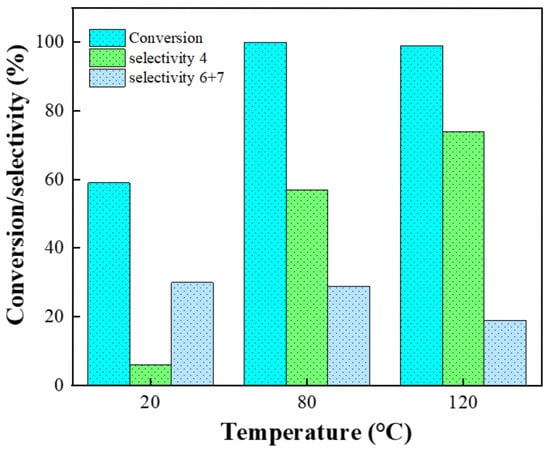
Figure 10.
Temperature effect on nifedipine selectivity (%). Reaction conditions: 1 mmol 2-nitrobenzaldehyde, 2 mmol methyl acetoacetate, 1 mmol ammonium acetate, and 50 mg Si20Zr80TPA30, 4 h of reaction.
The amount of Si20Zr80TPA30 catalyst was also evaluated in the next experiment. The test conducted at 80 °C demonstrates that nifedipine 4 can be obtained as the main product with a reaction time of 4 h. The reaction selectivity increased from 44% to 56% when the amount of catalyst was raised from 25 mg to 50 mg (reaction time: 4 h). However, a further increase of the catalyst amount to 75 mg resulted in a 45% decrease in nifedipine selectivity. This effect could be attributed to strong adsorption of the reaction components on the surface of the material. These results are consistent with the yields observed for 1,2-addition products: with 25 mg of catalyst, the combined selectivity of products (6 + 7) was 32%; with 50 mg, it was 29%; and with 75 mg, it increased to 35%.
The possibility of increasing the selectivity of nifedipine by adjusting the amount of one of the reagents was also evaluated. To this end, additional experiments were conducted using different molar ratios of 2-nitrobenzaldehyde, methyl acetoacetate, and ammonium acetate (in excess): 1:2:1, 1:2:1.3, and 1:2:1.5, respectively. No significant changes in nifedipine yields were observed across these variations, indicating that a stoichiometric ratio of reagents is optimal for this reaction. A stoichiometric amount of ammonium acetate decomposes quantitatively into ammonia, which reacts completely with the other two starting substrates. This result improves the atom economy of the process compared to other methodologies that indicate the need to use excess ammonium acetate.
Microwave radiation has proved to be a highly effective and uniform heating source in chemical reactions. It can also accelerate the reaction rate, provide better yields, achieve greater reproducibility of reactions, and help develop cleaner synthetic routes. Different reaction conditions were explored: temperature, time, molar ratio of reagents, and addition of a dehydrating agent (Figure 11) using Si20Zr80TPA30 as catalyst and microwave irradiation. Test (a), which was carried out at 100 °C using a stoichiometric ratio of substrates (1:2:1), led to 98% of 2-nitrobenzaldehyde conversion in 1 h of reaction. At this time and under such conditions, nifedipine yield was 45%, which increased to 54% in 2 h. Test (b) was carried out at the same temperature (100 °C) using a slight excess of ammonium acetate (1:2:1.3). The 2-nitrobenzaldehyde conversion achieved at 2 h was 99%, with a nifedipine yield of 56%. In the case of the reaction with microwaves, a slight increase in the selectivity towards nifedipine was observed when working with a slight excess of ammonium acetate. Test (c) was carried performed using the same slight excess of ammonium acetate at 120 °C. The maximum yield of nifedipine obtained was 77% in 1 h at 120 °C (with a 99% 2-nitrobenzaldehyde conversion). Since water is released during the reaction and the experimental conditions in the microwave require working in a closed container, a dehydrating agent (anhydrous CuSO4, 50 mg) was added to potentially shift the reaction equilibrium [63]. The effectiveness of this agent to improve yields was previously confirmed in esterification reactions using microwave irradiation conducted by our research group [64]. However, in this case (solvent-free conditions), no significant improvement in yield was observed, and even a decrease in selectivity was noted, which is attributed to the adsorption of products in the solid mixture formed by the catalyst and the drying agent (d experiment).
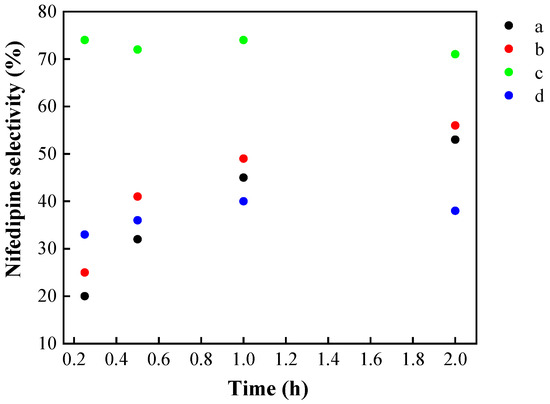
Figure 11.
Microwave effect on nifedipine selectivity (%). (a) 100 °C/1 mmol ammonium acetate, (b) 100 °C/1.3 mmol ammonium acetate, (c) 120 °C/1.3 mmol ammonium acetate, (d) 120 °C/1.3 mmol ammonium acetate/50 mg CuSO4.
Figure 12 shows all products formed under microwave irradiation at 120 °C as a function of time.
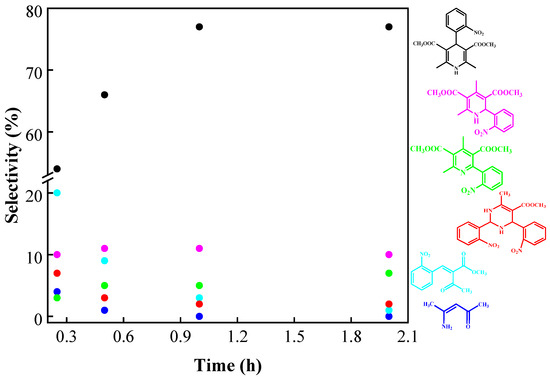
Figure 12.
Products formed under microwave irradiation at 120 °C. Reaction conditions: 1 mmol 2-nitrobenzaldehyde, 2 mmol methyl acetoacetate, 1.3 mmol ammonium acetate, Si20Zr80TPA30 as catalyst (50 mg), solvent-free.
Finally, the reuse of the catalyst over four consecutive reaction cycles was evaluated. After each cycle, the catalyst was recovered by filtration, thoroughly washed, and dried before being reused under the same reaction conditions. The results demonstrated that the catalyst maintained its catalytic activity across four cycles, yielding nifedipine at 77%, 74%, 74%, and 74%, respectively. The catalyst mass loss after the four cycles was less than 5%.
3. Materials and Methods
3.1. Reagents
TEA (triethanolamine) ≥ 99% (Sigma-Aldrich, St. Louis, MO, USA); TEOS (tetraethyl orthosilicate) (Sigma-Aldrich); Zirconium (IV) propoxide solution (70 wt.% in 1-propanol) (Sigma-Aldrich); urea (Cicarelli, Milan, Italy); and TPA (H3PW12O40 tungstophosphoric acid) 99% (Fluka, Buchs, Switzerland) were used for the synthesis of supports and catalysts. For the catalytic tests, 2-nitrobenzaldehyde 98% (Sigma-Aldrich); methyl acetoacetate 99% (Sigma-Aldrich), and ammonium acetate ≥ 97% (Biopack, Zibo, China) were employed.
3.2. Synthesis of Mesostructured SiO2-ZrO2-TPA Composites
A series of tungstophosphoric acid (TPA, H3PW12O40) immobilized on SiO2-ZrO2 mesoporous matrix was synthesized via the sol–gel method using urea and triethanolamine as low-cost mesoporous template agents [30,63], varying the SiO2:ZrO2 ratios from 100:00 to 00:100 (wt/wt%). The TPA content in the final materials was 30 wt/wt%. The materials were denoted as Si100Zr00TPA30, Si80Zr20TPA30, Si20Zr80TPA30, and Si00Zr100TPA30.
For instance, to synthesize 2 g of Si20Zr80TPA30, 1.5 mL of tetraethyl orthosilicate was mixed directly with 5.8 mL of Zirconium (IV) propoxide solution (70 wt% in 1-propanol). After mixing, a water/TEA solution (2.93 g in 2.6 mL of distilled water) was added under vigorous stirring, followed by a slow addition of a urea solution (0.8 g dissolved in 3 mL of distilled water) to obtain a gel. The mixture was stirred at room temperature for 2 h, followed by 24 h of gel aging, and then subjected to hydrothermal treatment in a stainless-steel hydrothermal reactor Teflon-lined jacket at 180 °C for 8 h. The resulting solid was transferred to a beaker, 50 mL of ethanol 96% was added, and was stirred for 8 h; the mixture was centrifuged, and the supernatant liquid was removed (repeated twice). The resulting solid was dried at 100 °C for 24 h and was used as TPA support. Impregnation experiments were performed by contacting, at room temperature, 0.7 g of the support with 0.3 g of tungstophosphoric acid (H3PW12O40) dissolved in 3 mL of 50% (v/v) ethanol–water solution to obtain a TPA concentration of 30% (w/w) in the final material. The solution and the support were allowed to stand in contact at room temperature until they were completely dry. The solids were then calcined at 350 °C under air atmosphere for 4 h to obtain the final material. The TPA content in the SiXZrYTPA30 materials was determined by atomic absorption spectrometry, as described in the Supplementary Materials.
3.3. Catalyst Characterization
Nitrogen adsorption/desorption measurements were carried out at liquid nitrogen temperature (77 K) using Micromeritics ASAP 2020 equipment (Norcross, GA, USA). From the obtained data, the specific surface area (SBET), using the Brunauer–Emmett–Teller model in the relative pressure range 0.05–0.3, the mean pore diameter (Dp) by the BJH method, and the pore size distribution (PSD) with the DFT density functional theory (DFT) method were estimated. The FT-IR spectra of the compounds were acquired using KBr pellets and recorded on a Bruker Equinox 55 spectrometer (Billerica, MA, USA) with a resolution of 4 cm−1 across the range of 4000 to 400 cm−1. The structural characteristics were determined by X-ray diffraction (XRD) using an automatic diffractometer PANalyical X’Pert PRO (Almelo, The Netherlands). The samples were measured on a Philips X’Pert diffractometer (Eindhoven, The Netherlands) at 45 Kv and 45 mA. The diffraction patterns were obtained in steps in the angular range 5–60° (2θ) with a speed of 2°/min. The CuKα radiation (1.542 Å) was monochromatized with a graphite monochromator. The diffractometer is equipped with a 1° divergence slit and a 1/2° dispersion slit. Acidic properties were evaluated through potentiometric titration with 0.05 N n-butylamine in acetonitrile using a Hanna 11 pH meter with a double junction electrode employing a Metrohm 794 Basic Titrino device (Herisau, Switzerland), with the variation of electrode potential (E) being recorded in millivolts (mV). X-ray photoelectron spectra were collected using a SPECS ProvenX system (Berlin, Germany) with a non-monochromatic Al Ka radiation source (hν = 1486.6 eV), combined with a Phoibos 150 hemispherical analyzer (SPECS Surface Nano Analysis GmbH, Berlin, Germany), operating at a pass energy of 20 eV. The instrument resolution was 0.1 eV. All binding energies (BE) were referred to the adventitious C1s peak at 284.6 eV, to correct spectral shifts due to charging effects. The chamber pressure was kept at <10−9 Torr during the measurements. The solids were analyzed by 31P magic angle spinning-nuclear magnetic resonance (MAS-NMR) spectroscopy. For this purpose, Bruker MSL-300 equipment was employed, using 5 ms pulses, a 60 s delay, and working at a frequency of 121.496 MHz for 31P at room temperature, the resolution being 3.052 Hz per point. A 5 mm diameter and 10 mm high sample holder was used; the spin rate was 2.1 kHz. Several hundred pulse responses were collected. Phosphoric acid 85% was employed as an external reference. Thermogravimetric analysis was performed using a Setaram thermobalance. Sixty milligrams of each sample was measured in an alumina crucible and heated using a 5 °C min−1 heating rate up to 800 °C, under an atmosphere of N2 flowing at 20 cm3/min.
3.4. Catalytic Test and Product Identification
Reactions were carried out by thermal heating using conventional equipment or assisted by microwaves. Microwave-assisted reactions were carried out in a glass vial G10, Anton Paar Monowave 400 Series (Graz, Austria, Instrument Software Version: 4.10.9376.7). The reactions were monitored by thin layer chromatography (TLC) and visualized by an ultraviolet (UV) lamp (254 or 365 nm). Also, the reaction products were analyzed using a Shimadzu gas chromatograph model 2014 (Kyoto, Japon) with an SPB-1 column (30 m, 32 mm, 1.00 μm), equipped with an FID detector. The carrier gas was high purity nitrogen (≥99.999%); splitless injection mode was used (1 μL), and the injector temperature was 280 °C. The oven temperature was kept at 70 °C for 1 min, then raised to 90 °C at 7 °C min−1, and subsequently to 250 °C at 20 °C min−1 and maintained for 20 min. Finally, it was raised to 280 °C and maintained for 1 min. The products and reaction intermediates were identified by GC–MS using a Shimadzu GCMS-QP2010SE gas chromatography–mass spectrometry system (see Supplementary Materials), and NMR. 1H NMR (300 MHz) and 13C NMR (75 MHz), 1H–1H COSY NMR (300 MHz) spectra in DMSO-d6 as solvent were recorded on a Bruker 300 Ultrashield (300 MHz) NMR spectrometer with Fourier transform. Coupling constants are given in Hz, and chemical shifts are reported in δ values in ppm. Data are reported as follows: chemical shift, multiplicity (s = singlet, br s = broad singlet, d = doublet, t = triplet, dd = double doublet, m = multiplet), coupling constants (Hz), and integration.
The multicomponent Hantzsch synthesis reaction of 2-nitrobenzaldehyde, methyl acetoacetate, ammonium acetate was chosen for evaluating the optimal reaction conditions in the absence of a catalyst using conventional heating or microwave irradiation as an alternative heat source, in solvent-free conditions. A mixture of methyl acetoacetate, 2-nitrobenzaldehyde, ammonium acetate, and the catalyst were heated with constant stirring. Different variables were evaluated such as the time, temperature, ratio of reagents, and amount of catalyst. A constant amount of 30% HPAs was set for all supports and for comparative purposes, since this is generally the optimal amount we have previously determined [34].
3.5. General Procedure for the Synthesis of Nifedipine (Thermal Procedure)
A mixture of 2-nitrobenzaldehyde (1 mmol), methyl acetoacetate (2 mmol), ammonium acetate (1 mmol), and Si20Zr80TPA30 (50 mg) were heated with constant stirring for 4 h at 80 °C. The reaction mixture was cooled to room temperature, and the crude product was crushed and washed with chilled water (3 × 5 mL), filtered, and dried under vacuum. To obtain a pure product, the final solid mass was recrystallized from acetonitrile.
3.6. General Procedure for the Synthesis of Nifedipine (Microwave Procedure)
A mixture of 2-nitrobenzaldehyde (1 mmol), methyl acetoacetate (2 mmol), ammonium acetate (1.3 mmol), and Si20Zr80TPA30 (50 mg) were subjected to the action of microwaves at 120 °C for 2 h. The reaction mixture was cooled to room temperature, and the crude product was crushed and washed with chilled water (3 × 5 mL), filtered, and dried under vacuum. To obtain a pure product, the final solid mass was recrystallized from acetonitrile.
3.7. Catalyst Recycling Under Microwave Irradiation
Reuse stability tests of the catalysts were carried out by running five consecutive experiments using the same ratios of reagents: 2-nitrobenzaldehyde (1 mmol), methyl acetoacetate (2 mmol), ammonium acetate (1.3 mmol), Si20Zr80TPA30 (50 mg). After each test, the catalyst was separated from the reaction mixture by filtration, washed with chloroform (3 × 2 mL), dried, and reused under microwave irradiation at 120 °C for 2 h.
4. Conclusions
In this study, mesoporous catalysts based on tungstophosphoric acid (TPA) supported on silica–zirconia matrices with varying compositions were synthesized using the sol–gel method. The materials were characterized by N2 adsorption–desorption isotherms, infrared spectroscopy (FT-IR), 31P solid-state nuclear magnetic resonance (NMR-MAS), X-ray diffraction (XRD), thermogravimetric analysis (TGA), X-ray photoelectron spectra (XPS), and potentiometric titration. The characterization results from the XPS spectra showed that as the Si/Zr ratio drops, the Si-O-Si signal size decreases, while the Zr-O signal size increases. Characterization by titration indicated an increase in the total acidity of the material.
The effect of mesostructured silica–zirconia–tungstophosphoric acid (SiO2-ZrO2-TPA) composites used as catalysts was studied in the synthesis of nifedipine by the Hantzsch methodology. The selectivity for nifedipine was determined, along with all potential secondary products that may form depending on the reaction conditions. It is noteworthy that although there are numerous procedures for synthesizing 1,4-dihydropyridines using acid catalysts under homogeneous and heterogeneous conditions, there are practically no reports on the study of reaction conditions in the specific synthesis of nifedipine.
The experiments conducted at 80 °C achieved a maximum yield of 57% of nifedipine 4 after 4 h at 80 °C using the Si20Zr80TPA30 catalyst (50 mg), while by microwave heating, the yield significantly improved, reaching 77% in only 1 h of reaction. This catalyst exhibited stability and reusability without significant loss of activity up to the third cycle.
Reaction intermediates and other minor by-products are formed in the process, which were also evaluated. Depending on the type of material and the reaction conditions, it is possible to modify the selectivity of the reaction, obtaining a 1,2-dihydropyridine isomeric to nifedipine (1,2-Michael addition). The formation of 1,2-dihydropyridine 6 is favored at lower temperatures and lower acidity of the catalyst. It is unstable and dehydrogenates, aromatizing to 2-pyridine 7.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15060537/s1, Figure S1: Thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG); Table S1: Weight loss; Table S2: Initial electrode potential (Ei) and total number of acid sites values of supports; Figure S2: Compound 7: 1H NMR; Figure S3. Compound 7: 13C NMR; Figure S4. Compound 7: 1H-1H COSY NMR; Figure S5: Compound 7: 1H-13C HSQC NMR; Figure S6: Compound 7: 1H-13C HMBC NMR; Figure S7. Compound 8: 1H NMR; Figure S8: Compound 8: 13C NMR; Figure S9: Compound 8: 1H-1H COSY; Figure S10: Compound 8: 1H-13C HSQC NMR; Figure S11: Compound 8: 1H-13C HMBC NMR.
Author Contributions
Conceptualization, L.R.P. and G.P.R.; methodology, E.X.A., A.S., L.R.P. and G.P.R.; formal analysis, E.X.A., Á.G.S., M.C.M., J.J.M., L.R.P. and G.P.R.; investigation, E.X.A. and A.S.; resources, M.C.M., L.R.P. and G.P.R.; data curation, E.X.A. and M.C.M.; writing—original draft preparation, E.X.A.; writing—review and editing, E.X.A., L.R.P. and G.P.R.; supervision, Á.G.S., L.R.P. and G.P.R.; project administration, L.R.P. and G.P.R.; funding acquisition, M.C.M., L.R.P. and G.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas CONICET (PIP 0111), (PIP 2255), and (PIP 1492); Universidad Nacional de La Plata UNLP (A349, X879), Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación ANPCyT (PICT-2021-00438), and Fundación Williams (P 101, 2024).
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
We gratefully acknowledge the financial and scientific support of La Universidad Nacional de La Plata (UNLP), Argentina; Universidad Nacional del Litoral (UNL); Concejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina; Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC-PBA), Argentina; Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina; Fundación Williams, Argentina; and Grupo de Catálisis de la Universidad Pedagógica y Tecnológica de Colombia.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| TPA | Tungstophosphoric acid |
| TEA | Triethanolamine |
| TEOS | Tetraethyl orthosilicate |
| HPA | Heteropolyacid |
| MCR | Reaction multicomponent |
| DHP | Dihydropyridines |
| DTG | Derivative thermogravimetry |
| SBET | Surface Area Brunauer–Emmett–Teller |
| Dp | Pore diameter |
| FT-IR | Infrared spectroscopy |
| (NMR-MAS) | 31P solid-state nuclear magnetic resonance |
| XRD | X-ray diffraction |
| TGA | Thermogravimetric analysis |
| XPS | X-ray photoelectron spectra |
References
- Kajal, K.; Shakya, R.; Rashid, M.; Nigam, V.; Das Kurmi, B.; Das Gupta, G.; Patel, P. Recent Green Chemistry Approaches for Pyrimidine Derivatives as a Potential Anti-Cancer Agent: An Overview (2013–2023). Sustain. Chem. Pharm. 2024, 37, 101374. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; El-Monaem, E.M.A.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review; Springer International Publishing: Cham, Switzerland, 2024; Volume 22, ISBN 0123456789. [Google Scholar]
- Nardi, M.; Cano, N.C.H.; Simeonov, S.; Bence, R.; Kurutos, A.; Scarpelli, R.; Wunderlin, D.; Procopio, A. A Review on the Green Synthesis of Benzimidazole Derivatives and Their Pharmacological Activities. Catalysts 2023, 13, 392. [Google Scholar] [CrossRef]
- Castiello, C.; Junghanns, P.; Mergel, A.; Jacob, C.; Ducho, C.; Valente, S.; Rotili, D.; Fioravanti, R.; Zwergel, C.; Mai, A. GreenMedChem: The Challenge in the next Decade toward Eco-Friendly Compounds and Processes in Drug Design. Green Chem. 2023, 25, 2109–2169. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a Green Chemistry Future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef]
- Zaera, F. Designing Sites in Heterogeneous Catalysis: Are We Reaching Selectivities Competitive with Those of Homogeneous Catalysts? Chem. Rev. 2022, 122, 8594–8757. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Júnior, E.N.d.S.; Neto, B.A.D. What Do We Know about Multicomponent Reactions? Mechanisms and Trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Younus, H.A.; Al-Rashida, M.; Hameed, A.; Uroos, M.; Salar, U.; Rana, S.; Khan, K.M. Multicomponent Reactions (MCR) in Medicinal Chemistry: A Patent Review (2010–2020). Expert Opin. Ther. Pat. 2021, 31, 267–289. [Google Scholar] [CrossRef]
- Mohlala, R.L.; Rashamuse, T.J.; Coyanis, E.M. Highlighting Multicomponent Reactions as an Efficient and Facile Alternative Route in the Chemical Synthesis of Organic-Based Molecules: A Tremendous Growth in the Past 5 Years. Front. Chem. 2024, 12, 1469677. [Google Scholar] [CrossRef]
- Hantzsch, A. Condensationsprodukte aus Aldehydammoniak und Ketonartigen Verbindungen. Berichte Dtsch. Chem. Ges. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- Palermo, V.; Sathicq, Á.G.; Constantieux, T.; Rodríguez, J.; Vázquez, P.G.; Romanelli, G.P. First Report About the Use of Micellar Keggin Heteropolyacids as Catalysts in the Green Multicomponent Synthesis of Nifedipine Derivatives. Catal. Lett. 2016, 146, 1634–1647. [Google Scholar] [CrossRef]
- Kiowski, W.; Erne, P.; Bühler, F.R. Use of Nifedipine in Hypertension and Raynaud’s Phenomenon. Cardiovasc. Drugs Ther. 1990, 4, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Block, J.A.; Sequeira, W. Raynaud’s Phenomenon. Lancet 2001, 357, 2042–2048. [Google Scholar] [CrossRef]
- Khrustalev, D.; Yedrissov, A.; Khrustaleva, A.; Mustafin, M.; Bekisheva, K. An Efficient Method for the Synthesis of 1,4-Dihydropyridine Derivatives in a Flow Microwave Reactor. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2023; Volume 81, pp. 1204–1208. [Google Scholar]
- D’Alessandro, O.; Sathicq, Á.G.; Sambeth, J.E.; Thomas, H.J.; Romanelli, G.P. A Study of the Temperature Effect on Hantzsch Reaction Selectivity Using Mn and Ce Oxides under Solvent-Free Conditions. Catal. Commun. 2015, 60, 65–69. [Google Scholar] [CrossRef]
- Vargas, A.Y.; Rojas, H.A.; Romanelli, G.P.; Martínez, J.J. Synthesis of 1,4-Dihydropyrimidines with Immobilized Urease: Effect of Method Immobilization on Magnetic Supports. Green Process. Synth. 2017, 6, 377–384. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Sathicq, Á.G.; Romanelli, G.P.; González, L.M.; Villa, A.L. Activity of Immobilized Metallic Phthalocyanines in the Multicomponent Synthesis of Dihydropyridine Derivatives and Their Subsequent Aromatization. Mol. Catal. 2017, 435, 1–12. [Google Scholar] [CrossRef]
- Cheng, M.; Shi, T.; Guan, H.; Wang, S.; Wang, X.; Jiang, Z. Clean Production of Glucose from Polysaccharides Using a Micellar Heteropolyacid as a Heterogeneous Catalyst. Appl. Catal. B Environ. 2011, 107, 104–109. [Google Scholar] [CrossRef]
- Toufaily, J.; Soulard, M.; Guth, J.L.; Patarin, J.; Delmote, L.; Hamieh, T.; Kodeih, M.; Naoufal, D.; Hamad, H. Synthesis and Characterization of New Catalysts Formed by Direct Incorporation of Heteropolyacids into Organized Mesoporous Silica. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 285–291. [Google Scholar] [CrossRef]
- Gorsd, M.; Sathicq, G.; Romanelli, G.; Pizzio, L.; Blanco, M. Tungstophosphoric acid supported on core-shell polystyrene-silica microspheres or hollow silica spheres catalyzed trisubstituted imidazole synthesis by multicomponent reaction. J. Mol. Catal. A Chem. 2016, 420, 294–302. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Cáceres, V.C.; Blanco, M.N. Adsorption of Tungstophosphoric or Tungstosilicic Acids from Ethanol–Water Solutions on Carbon. J. Colloid Interface Sci. 1997, 190, 318–326. [Google Scholar] [CrossRef]
- Rivera, T.S.; Sosa, A.; Romanelli, G.P.; Blanco, M.N.; Pizzio, L.R. Tungstophosphoric Acid/Zirconia Composites Prepared by the Sol-Gel Method: An Efficient and Recyclable Green Catalyst for the One-Pot Synthesis of 14-Aryl-14H-Dibenzo[a,j]Xanthenes. Appl. Catal. A Gen. 2012, 443–444, 207–213. [Google Scholar] [CrossRef]
- Méndez, F.J.; Llanos, A.; Echeverría, M.; Jáuregui, R.; Villasana, Y.; Díaz, Y.; Liendo-Polanco, G.; Ramos-García, M.A.; Zoltan, T.; Brito, J.L. Mesoporous Catalysts Based on Keggin-Type Heteropolyacids Supported on MCM-41 and Their Application in Thiophene Hydrodesulfurization. Fuel 2013, 110, 249–258. [Google Scholar] [CrossRef]
- Morey, M.S.; Stucky, G.D.; Schwarz, S.; Fröba, M. Isomorphic Substitution and Postsynthesis Incorporation of Zirconium into MCM-48 Mesoporous Silica. J. Phys. Chem. B 1999, 103, 2037–2041. [Google Scholar] [CrossRef]
- Lai, F.; Yan, F.; Wang, Y.; Li, C.; Cai, J.; Zhang, Z. Tungstophosphoric Acid Supported on Metal/Si-Pillared Montmorillonite for Conversion of Biomass-Derived Carbohydrates into Methyl Levulinate. J. Clean. Prod. 2021, 314, 128072. [Google Scholar] [CrossRef]
- Ramanathan, A.; Villalobos, M.C.C.; Kwakernaak, C.; Telalovic, S.; Hanefeld, U. Zr-TUD-1: A Lewis Acidic, Three-Dimensional, Mesoporous, Zirconium-Containing Catalyst. Chem. A Eur. J. 2008, 14, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Zeng, H.C. The Self-Catalytic Role of Zirconium w-Propoxide in Sol-Gel Syntheses of ZrO2-SiO2 Mixed Oxides. J. Mater. Chem. 1999, 9, 2647–2652. [Google Scholar] [CrossRef]
- Sawant, D.P.; Vinu, A.; Jacob, N.E.; Lefebvre, F.; Halligudi, S.B. Formation of Nanosized Zirconia-Supported 12-Tungstophosphoric Acid in Mesoporous Silica SBA-15: A Stable and Versatile Solid Acid Catalyst for Benzylation of Phenol. J. Catal. 2005, 235, 341–352. [Google Scholar] [CrossRef]
- Colmenares-Zerpa, J.; Gajardo, J.; Peixoto, A.F.; Silva, D.S.A.; Silva, J.A.; Gispert-Guirado, F.; Llorca, J.; Urquieta-Gonzalez, E.A.; Santos, J.B.O.; Chimentão, R.J. High Zirconium Loads in Zr-SBA-15 Mesoporous Materials Prepared by Direct-Synthesis and PH-Adjusting Approaches. J. Solid State Chem. 2022, 312, 123296. [Google Scholar] [CrossRef]
- Gorsd, M.N.; Sosa, A.A.; Frenzel, R.A.; Pizzio, L.R. Synthesis and Characterization of Tungstophosphoric Acid-Modified Mesoporous Sponge-like TUD-1 Materials. J. Sol-Gel Sci. Technol. 2018, 87, 204–215. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Xu, M.; Wang, S.; You, J. In Situ Spectroscopic Studies of Decomposition of ZrSiO4 during Alkali Fusion Process Using Various Hydroxides. RSC Adv. 2015, 5, 11658–11666. [Google Scholar] [CrossRef]
- Escobar, A.M.; Blanco, M.N.; Martínez, J.J.; Cubillos, J.A.; Romanelli, G.P.; Pizzio, L.R. Biomass Derivative Valorization Using Nano Core-Shell Magnetic Materials Based on Keggin-Heteropolyacids: Levulinic Acid Esterification Kinetic Study with n-Butanol. J. Nanomater. 2019, 2019, 5710708. [Google Scholar] [CrossRef]
- Sosa, A.A.; Gorsd, M.N.; Blanco, M.N.; Pizzio, L.R. Synthesis and Characterization of Tungstophosphoric Acid-Modified Mesoporous Silica Nanoparticles with Tuneable Diameter and Pore Size Distribution. J. Sol-Gel Sci. Technol. 2017, 83, 355–364. [Google Scholar] [CrossRef]
- Morales, M.D.; Infantes-Molina, A.; Lázaro-Martínez, J.M.; Romanelli, G.P.; Pizzio, L.R.; Rodríguez-Castellón, E. Heterogeneous Acid Catalysts Prepared by Immobilization of H3PW12O40 on Silica through Impregnation and Inclusion, Applied to the Synthesis of 3H-1,5-Benzodiazepines. Mol. Catal. 2020, 485, 110842. [Google Scholar] [CrossRef]
- Méndez, L.; Torviso, R.; Pizzio, L.; Blanco, M. 2-Methoxynaphthalene acylation using aluminum or copper salts of tungstophosphoric and tungstosilicic acids as catalysts. Catal. Today 2011, 173, 32–37. [Google Scholar] [CrossRef]
- Khder, A.E.R.S.; Hassan, H.M.A.; El-Shall, M.S. Acid Catalyzed Organic Transformations by Heteropoly Tungstophosphoric Acid Supported on MCM-41. Appl. Catal. A Gen. 2012, 411–412, 77–86. [Google Scholar] [CrossRef]
- Salas, P.; Wang, J.A.; Armendariz, H.; Angeles-Chavez, C.; Chen, L.F. Effect of the Si/Zr Molar Ratio on the Synthesis of Zr-Based Mesoporous Molecular Sieves. Mater. Chem. Phys. 2009, 114, 139–144. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Qin, X.; Qin, Z.; Lin, J.; Li, Z. Ni/SBA-15 Catalysts for CO Methanation: Effects of V, Ce, and Zr Promoters. RSC Adv. 2015, 5, 96504–96517. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J. Zirconia-Doped Methylated Silica Membranes via Sol-Gel Process: Microstructure and Hydrogen Permselectivity. Nanomaterials 2022, 12, 2159. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L. XPS Characterization of Sulphated Zirconia Catalysts: The Role of Iron. In Surface and Interface Analysis; Wiley Analytical Science: Weinheim, Germany, 2000; Volume 30. [Google Scholar]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using Photoelectron Spectroscopy to Observe Oxygen Spillover to Zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef]
- Yang, G.; Wang, L.; Jiang, H. Zr-Incorporating SBA-15 for Conversion of the Ethanol-Acetaldehyde Mixture to Butadiene. React. Chem. Eng. 2020, 5, 1833–1844. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 Support Imparts Superior Activity and Stability of Co Catalysts for CO2 Methanation. Appl. Catal. B Environ. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Gu, J.; Xin, Z.; Tao, M.; Lv, Y.; Gao, W.; Si, Q. Effect of Si-Modified Zirconia on the Properties of MoO3/Si-ZrO2 Catalysts for Sulfur-Resistant CO Methanation. Appl. Catal. A Gen. 2019, 575, 230–237. [Google Scholar] [CrossRef]
- Bosman, H.J.M.; Pijpers, A.P.; Jaspers, A.W.M.A. An X-Ray Photoelectron Spectroscopy Study of the Acidity of SiO2-ZrO2 Mixed Oxides. J. Catal. 1996, 161, 551–559. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. Oxygen Plasma and Humidity Dependent Surface Analysis of Silicon, Silicon Dioxide and Glass for Direct Wafer Bonding. ECS J. Solid State Sci. Technol. 2013, 2, P515–P523. [Google Scholar] [CrossRef]
- You, X.; Yu, L.-L.; Xiao, F.-F.; Wu, S.-C.; Yang, C.; Cheng, J.-H. Synthesis of Phosphotungstic Acid-Supported Bimodal Mesoporous Silica-Based Catalyst for Defluorination of Aqueous Perfluorooctanoic Acid under Vacuum UV Irradiation. Chem. Eng. J. 2018, 335, 812–821. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Blanco, M.; Wist, J.; Florian, P.; Pizzio, L.R. TiO2 Modified with Polyoxotungstates Should Induce Visible-Light Absorption and High Photocatalytic Activity through the Formation of Surface Complexes. Appl. Catal. B Environ. 2016, 189, 99–109. [Google Scholar] [CrossRef]
- Legagneux, N.; Basset, J.M.; Thomas, A.; Lefebvre, F.; Goguet, A.; Sá, J.; Hardacre, C. Characterization of Silica-Supported Dodecatungstic Heteropolyacids as a Function of Their Dehydroxylation Temperature. Dalton Trans. 2009, 2235–2240. [Google Scholar] [CrossRef]
- Fuchs, V.; Méndez, L.; Blanco, M.; Pizzio, L. Mesoporous Titania Directly Modified with Tungstophosphoric Acid: Synthesis, Characterization and Catalytic Evaluation. Appl. Catal. A Gen. 2009, 358, 73–78. [Google Scholar] [CrossRef]
- Massart, R.; Contant, R.; Fruchart, J.M.; Ciabrini, J.P.; Fournier, M. 31P NMR Studies on Molybdic and Tungstic Heteropolyanions. Correlation between Structure and Chemical Shift. Inorg. Chem. 1977, 16, 2916–2921. [Google Scholar] [CrossRef]
- Sosa, A.A.; Palermo, V.; Langer, P.; Luque, R.; Romanelli, G.P.; Pizzio, L.R. Tungstophosphoric Acid/Mesoporous Silicas as Suitable Catalysts in Quinoxaline Synthesis. Mol. Catal. 2022, 517, 112046. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Blanco, M.; Pizzio, L.R. Photocatalytic bleaching of aqueous malachite green solutions by UV-A and blue-light-illuminated TiO2 spherical nanoparticles modified with tungstophosphoric acid. Appl. Catal. B Environ. 2011, 110, 126–132. [Google Scholar] [CrossRef]
- Sosa, A.A.; Rivera, T.S.; Blanco, M.N.; Pizzio, L.R.; Romanelli, G.P. Tungstophosphoric Acid Supported on Zirconia: A Recyclable Catalyst for the Green Synthesis on Quinoxaline Derivatives under Solvent-Free Conditions. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1071–1079. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Cáceres, C.V.; Blanco, M.N. Equilibrium Adsorption of 11-Tungstophosphate Anion on Different Supports. Appl. Surf. Sci. 1999, 151, 91–101. [Google Scholar] [CrossRef]
- Escobar, A.; Sathicq, Á.; Pizzio, L.; Blanco, M.; Romanelli, G. Biomass Valorization Derivatives: Clean Esterification of 2-Furoic Acid Using Tungstophosphoric Acid/Zirconia Composites as Recyclable Catalyst. Process Saf. Environ. Prot. 2015, 98, 176–186. [Google Scholar] [CrossRef]
- Mokhtar, M.; Saleh, T.S.; Narasimharao, K.; Al-Mutairi, E. New Green Perspective to Dihydropyridines Synthesis Utilizing Modified Heteropoly Acid Catalysts. Catal. Today 2022, 397–399, 484–496. [Google Scholar] [CrossRef]
- Abdella, A.M.; Abdelmoniem, A.M.; Abdelhamid, I.A.; Elwahy, A.H.M. Synthesis of Heterocyclic Compounds via Michael and Hantzsch Reactions. J. Heterocycl. Chem. 2020, 57, 1476–1523. [Google Scholar] [CrossRef]
- Shen, L.; Cao, S.; Wu, J.; Zhang, J.; Li, H.; Liu, N.; Qian, X. A Revisit to the Hantzsch Reaction: Unexpected Products beyond 1,4-Dihydropyridines Supporting Information: Spectra of the Pyridine Derivatives: Diethyl 2-Phenyl-4, 6-Dimethylpyridine-3, 5-Dicarboxylate (3a). Green Chem. 2009, 11, 1414–1420. [Google Scholar] [CrossRef]
- Bosica, G.; Demanuele, K.; Padrón, J.M.; Puerta, A. One-Pot Multicomponent Green Hantzsch Synthesis of 1,2-Dihydropyridine Derivatives with Antiproliferative Activity. Beilstein J. Org. Chem. 2020, 16, 2862–2869. [Google Scholar] [CrossRef]
- Wei, J.; Xing, Y.; Ye, X.; Nguyen, B.; Wojtas, L.; Hong, X.; Shi, X. Gold-Catalyzed Amine Cascade Addition to Diyne-Ene: Enantioselective Synthesis of 1,2-Dihydropyridines. Angew. Chem. Int. Ed. 2023, 62, e202305409. [Google Scholar] [CrossRef]
- To, T.H.; Tran, D.B.; Pham, T.C.; Tran, P.D. A New Approach to Pyrimidine-Type Heterocycles Based on Petrenko–Kritschenko Synthesis. Chem. Heterocycl. Compd. 2022, 58, 608–614. [Google Scholar] [CrossRef]
- Kukreja, S. Copper-Catalyzed Ambient Synthesis of Functionalized 1,4-Dihydropyridines. J. Integr. Sci. Technol. 2023, 11, 4–8. [Google Scholar]
- Gallego-Villada, L.A.; Alarcón, E.A.; Cerrutti, C.; Blustein, G.; Sathicq, Á.G.; Romanelli, G.P. Levulinic Acid Esterification with N-Butanol over a Preyssler Catalyst in a Microwave-Assisted Batch Reactor: A Kinetic Study. Ind. Eng. Chem. Res. 2023, 62, 10915–10929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).