Palladium Nanoparticles Immobilized on the Amine-Functionalized Lumen of Halloysite for Catalytic Hydrogenation Reactions
Abstract
1. Introduction
2. Results and Discussion
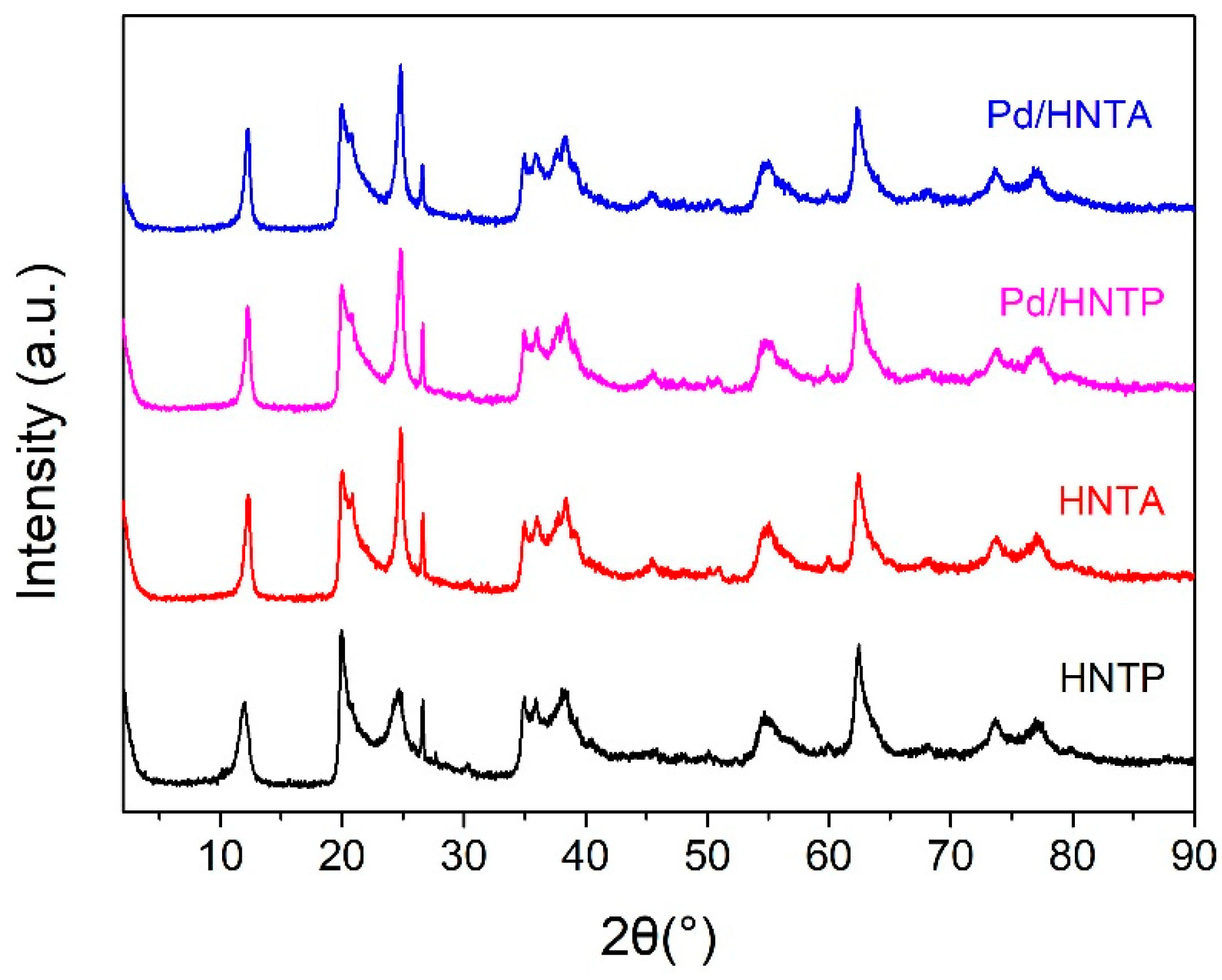
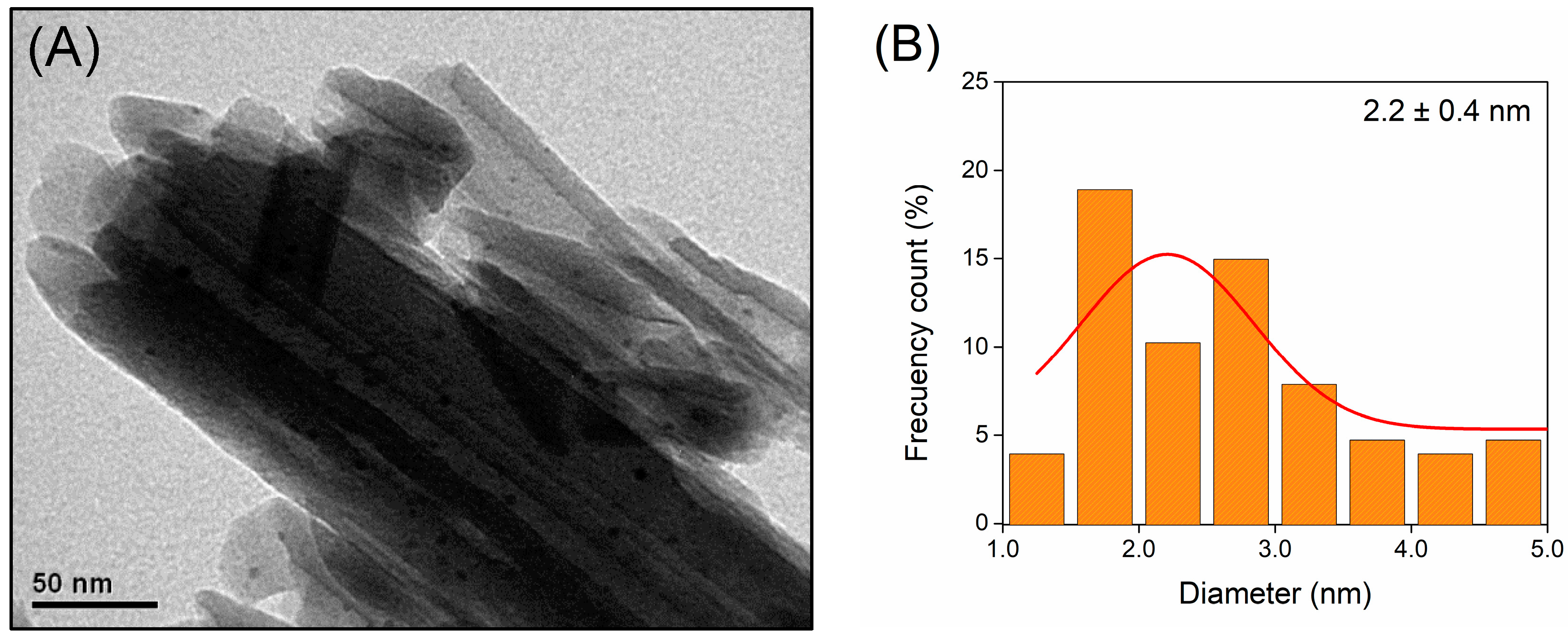
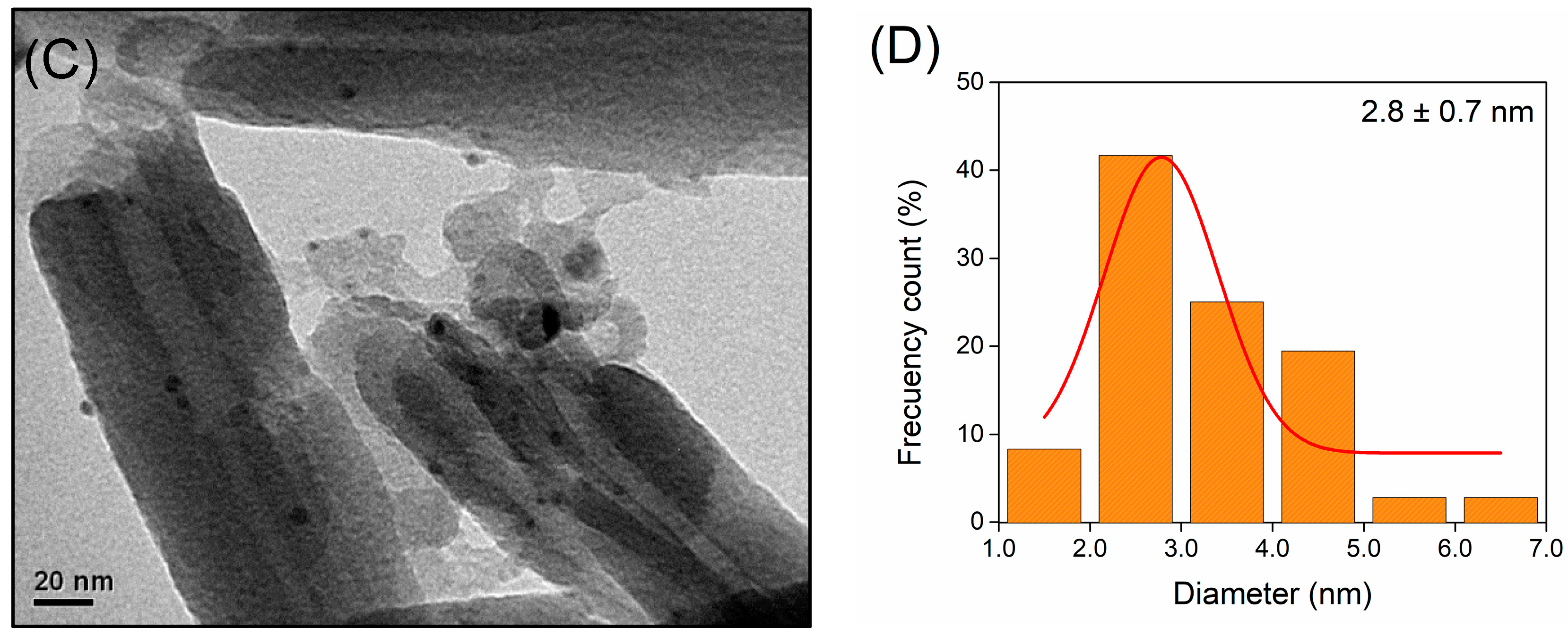
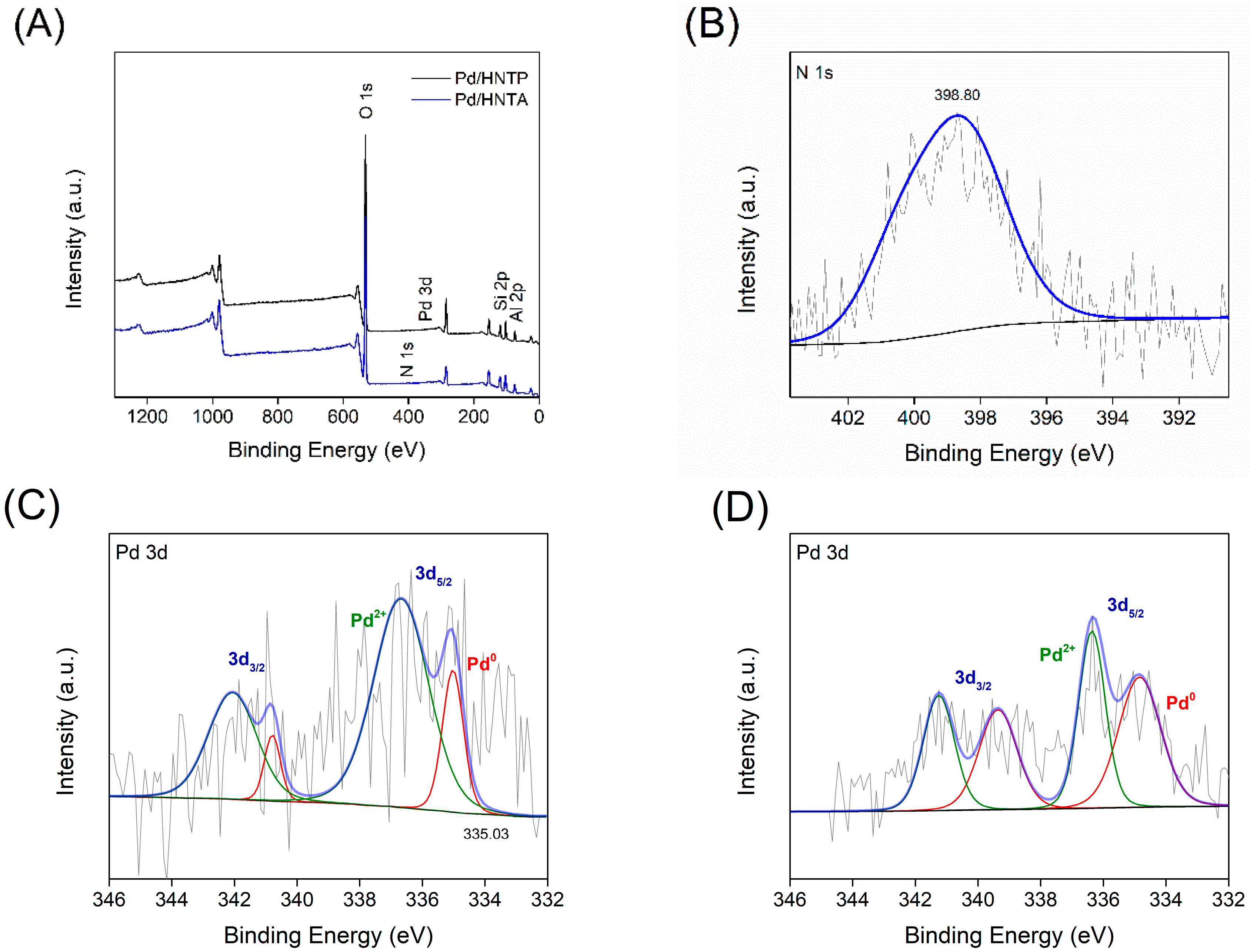
2.1. Characterization of Supports and Catalysts
2.2. Catalytic Activity
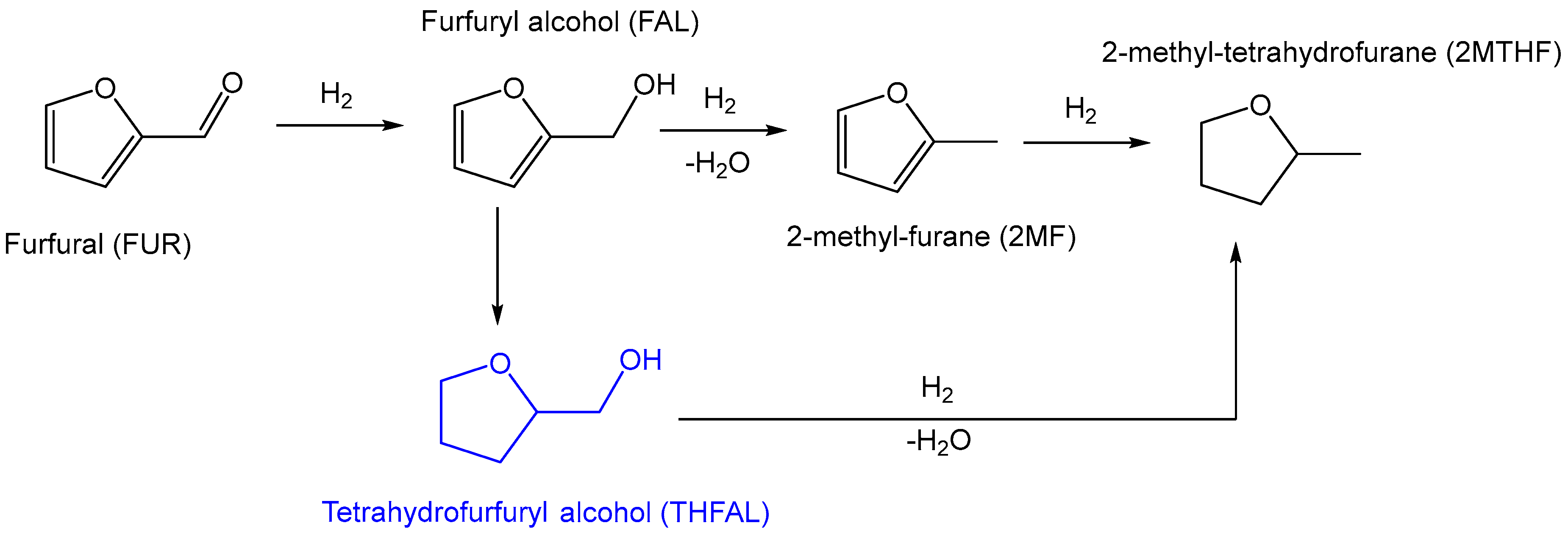
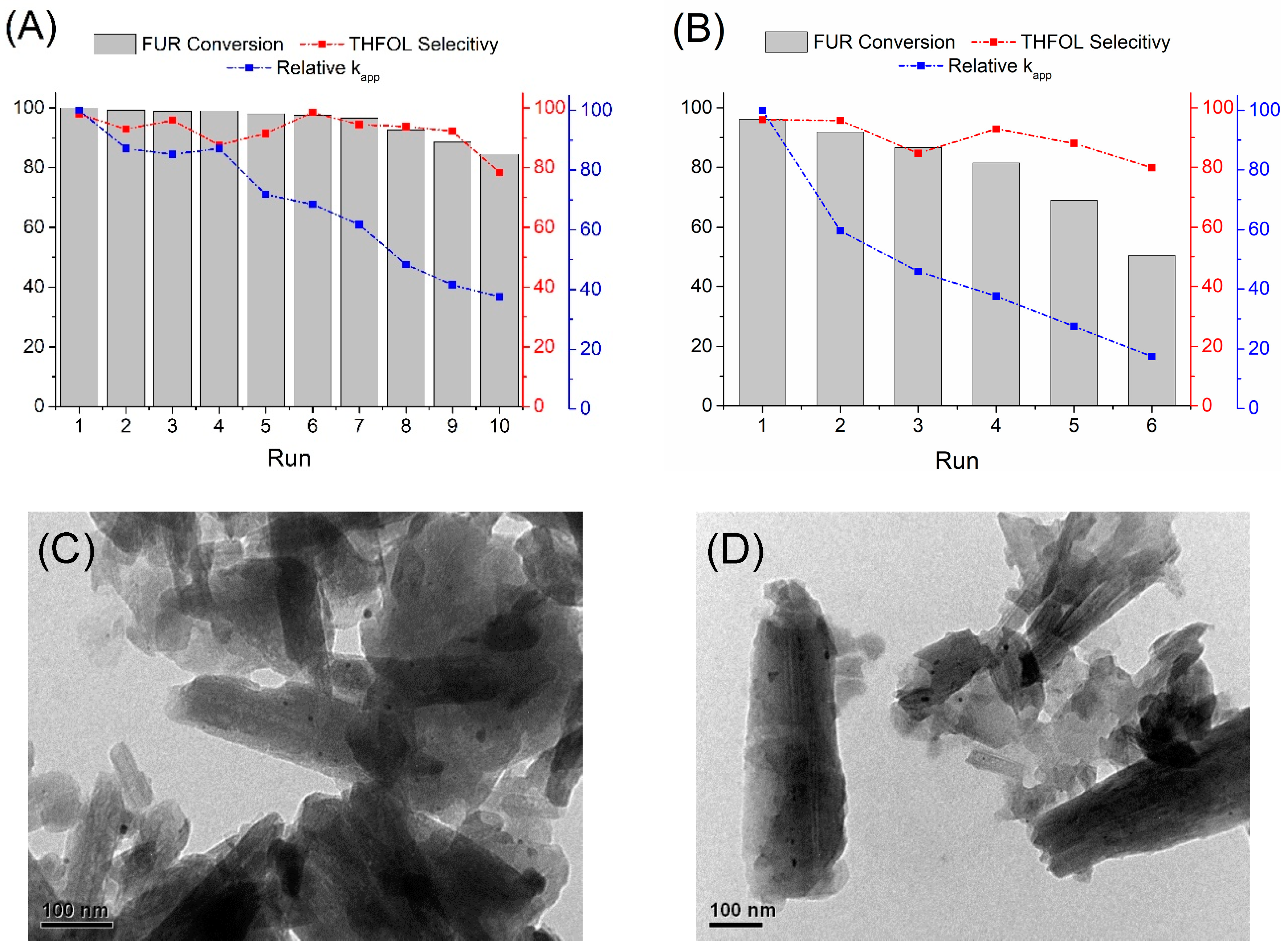
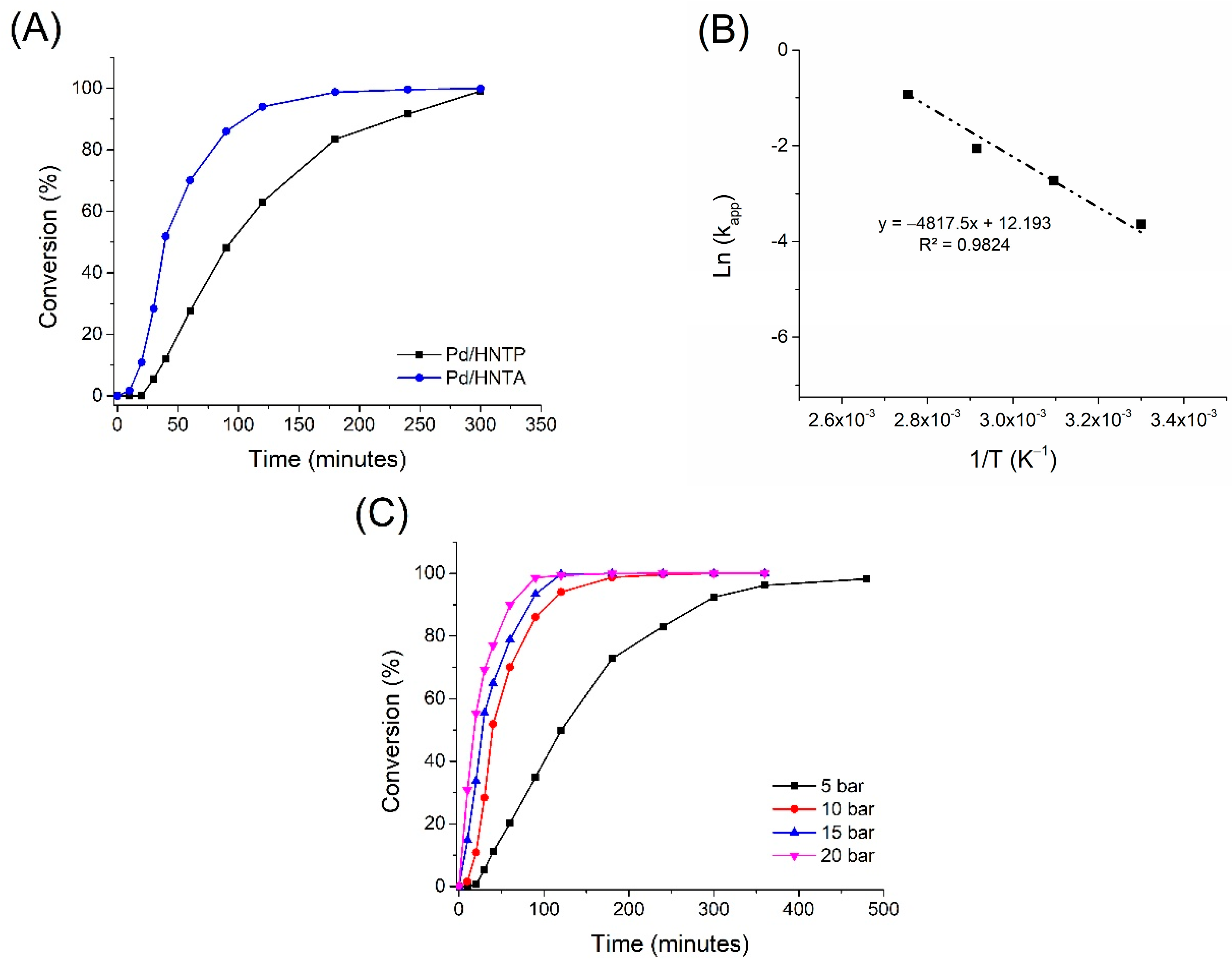
2.2.1. Hydrogenation of Furfural
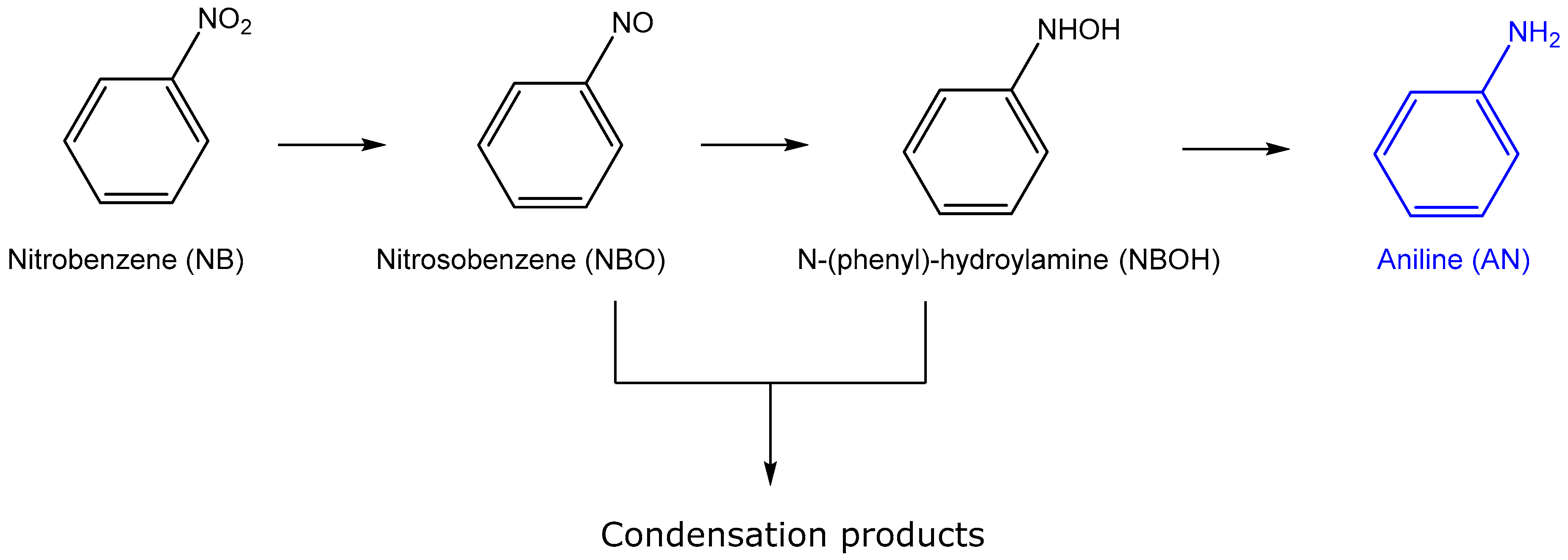
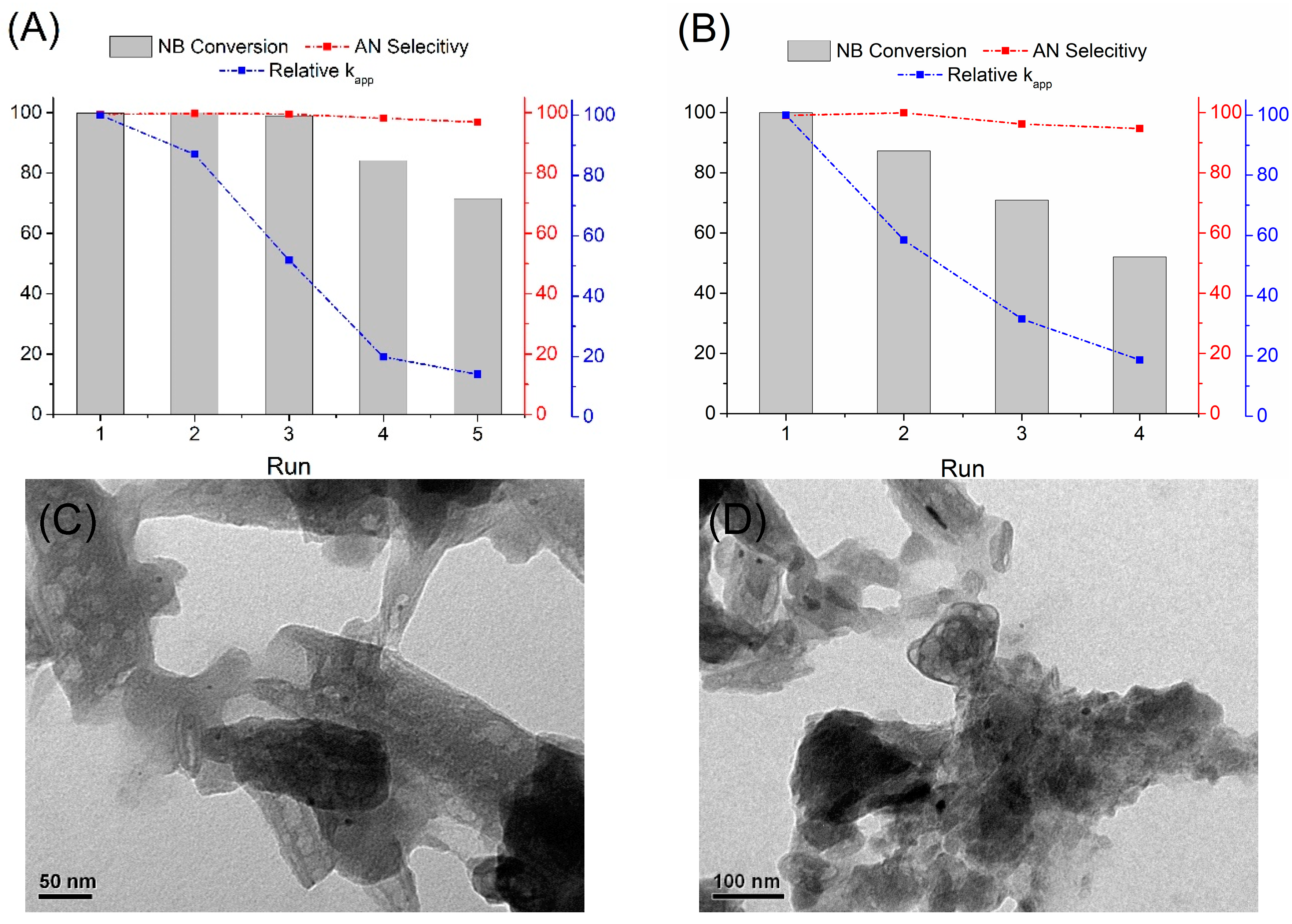
2.2.2. Hydrogenation of Nitrobenzene
3. Experimental Section
3.1. Preparation of Catalysts
3.2. Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zang, W.; Li, G.; Wang, L.; Zhang, X. Catalytic hydrogenation by noble-metal nanocrystals with well-defined facets: A review. Catal. Sci. Technol. 2015, 5, 2532–2553. [Google Scholar] [CrossRef]
- Lai, X.; Liang, X.; Wang, S.; Xu, Y. Surface Engineering of Noble Metal Nanocrystals for Selective Hydrogenation. ChemCatChem 2024, 16, e202400716. [Google Scholar]
- Mitsui, T.; Rose, M.K.; Fomin, E.; Ogletree, D.F.; Salmeron, M. Dissociative hydrogen adsorption on palladium requires aggregates of three or more vacancies. Nature 2003, 422, 705–707. [Google Scholar] [CrossRef]
- Zhao, X.; Chang, Y.; Chen, W.-J.; Wu, Q.; Pan, X.; Chen, K.; Weng, B. Recent Progress in Pd-Based Nanocatalysts for Selective Hydrogenation. ACS Omega 2022, 7, 17–31. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Approaches to the synthesis of Pd/C catalysts with controllable activity and selectivity in hydrogenation reactions. Catal. Today 2020, 357, 152–165. [Google Scholar]
- Wang, Y.; Meng, H.; Yu, R.; Hong, J.; Zhang, Y.; Xia, Z.; Wang, Y. Unconventional Interconnected High-Entropy Alloy Nanodendrites for Remarkably Efficient C−C Bond Cleavage toward Complete Ethanol Oxidation. Angew. Chem. Int. Ed. 2025, 64, e202420752. [Google Scholar]
- Liu, K.; Qin, R.; Zheng, N. Insights into the Interfacial Effects in Heterogeneous Metal Nanocatalysts toward Selective Hydrogenation. J. Am. Chem. Soc. 2021, 143, 4483–4499. [Google Scholar]
- Saikia, M.; Saikia, L. Palladium nanoparticles immobilized on an amine-functionalized MIL-101(Cr) as a highly active catalyst for oxidative amination of aldehydes. RSC Adv. 2016, 6, 14937–14947. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Sajiki, H. Recent Development of Palladium-Supported Catalysts for Chemoselective Hydrogenation. Chem. Pharm. Bull. 2017, 65, 2–9. [Google Scholar]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar]
- Serra, M.; Arenal, R.; Tenne, R. An overview of the recent advances in inorganic nanotubes. Nanoscale 2019, 11, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Kamarudin, S.K. Titanium dioxide nanotubes (TNT) in energy and environmental applications: An overview. Renew. Sustain. Energy Rev. 2017, 76, 212–225. [Google Scholar] [CrossRef]
- García-Merino, J.A.; Jiménez-Marín, E.; Mercado-Zúñiga, C.; Trejo-Valdez, M.; Vargas-García, J.R.; Torres-Torres, C. Quantum and bistable magneto-conductive signatures in multiwall carbon nanotubes decorated with bimetallic Ni and Pt nanoparticles driven by phonons. OSA Contin. 2019, 2, 1285–1295. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Tenne, R.; Rao, C.N.R. Inorganic nanotubes. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2004, 362, 2099–2125. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Jiang, L.; Dai, H.; Zhao, Y.; Guan, X.; Bai, J.; Wang, H. Manipulation of the halloysite clay nanotube lumen for environmental remediation: A review. Environ. Sci. Nano 2022, 9, 841–866. [Google Scholar] [CrossRef]
- Chadha, U.; Sinha, S.; Jonna, J.; Goswami, M.; Ghani, H.; Nair, K.; Pandey, N.; Kataray, T.; Selvaraj, S.K.; Bhardwaj, P.; et al. Review-Chemical Structures and Stability of Carbon-doped Graphene Nanomaterials and the Growth Temperature of Carbon Nanomaterials Grown by Chemical Vapor Deposition for Electrochemical Catalysis Reactions. ECS J. Solid State Sci. Technol. 2022, 11, 041003. [Google Scholar] [CrossRef]
- Zhang, H. Selective modification of inner surface of halloysite nanotubes: A review. Nanotechnol. Rev. 2017, 6, 573–581. [Google Scholar] [CrossRef]
- Melnikov, D.; Reshetina, M.; Novikov, A.; Cherednichenko, K.; Stavitskaya, A.; Stytsenko, V.; Vinokurov, V.; Huang, W.; Glotov, A. Strategies for palladium nanoparticles formation on halloysite nanotubes and their performance in acetylene semi-hydrogenation. Appl. Clay Sci. 2023, 232, 106763. [Google Scholar] [CrossRef]
- Asadi, Z.; Sadjadi, S.; Nekoomanesh-Haghighi, M.; Posada-Pérez, S.; Solà, M.; Bahri-Laleh, N.; Poater, A. Lubricant hydrogenation over a functionalized clay-based Pd catalyst: A combined computational and experimental study. Appl. Organomet. Chem. 2022, 36, e6850. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Yan, H.; Lian, L.; Si, J.; Long, Z.; Cui, X.; Wang, J.; Zhao, L.; Yang, C.; et al. Palladium-halloysite nanocomposites as an efficient heterogeneous catalyst for acetylene hydrochlorination. J. Mater. Res. Technol. 2021, 13, 2055–2065. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Xu, Y.; Zhang, P.; Gao, Q.; Liang, Q.; Zhang, W.; Li, W.; Guo, R.; Jin, B.; et al. Halloysite-Based Nanomotors with Embedded Palladium Nanoparticles for Selective Benzyl Alcohol Oxidation. ACS Appl. Nano Mater. 2022, 5, 12806–12816. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Zhong, F.; Cai, G.; Xiao, Y.; Jiang, L. Site-Oriented Design of High-Performance Halloysite-Supported Palladium Catalysts for Methane Combustion. Ind. Eng. Chem. Res. 2020, 59, 5636–5647. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Mahajani, V.V. Kinetics of Liquid-Phase Hydrogenation of Furfuraldehyde to Furfuryl Alcohol over a Pt/C Catalyst. Ind. Eng. Chem. Res. 2003, 42, 3881–3885. [Google Scholar] [CrossRef]
- Iroegbu, A.O.; Hlangothi, S.P. Furfuryl Alcohol a Versatile, Eco-Sustainable Compound in Perspective. Chem. Afr. 2019, 2, 223–239. [Google Scholar] [CrossRef]
- Chen, L.; Ye, J.; Yang, Y.; Yin, P.; Feng, H.; Chen, C.; Zhang, X.; Wei, M.; Truhlar, D.G. Catalytic Conversion Furfuryl Alcohol to Tetrahydrofurfuryl Alcohol and 2-Methylfuran at Terrace, Step, and Corner Sites on Ni. ACS Catal. 2020, 10, 7240–7249. [Google Scholar] [CrossRef]
- Grazia, L.; Lolli, A.; Folco, F.; Zhang, Y.; Albonetti, S.; Cavani, F. Gas-phase cascade upgrading of furfural to 2-methylfuran using methanol as a H-transfer reactant and MgO based catalysts. Catal. Sci. Technol. 2016, 6, 4418–4427. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Li, E.; Wang, M.; Jie, K.; Zhu, H.; Huang, F. Selective Separation of Methylfuran and Dimethylfuran by Nonporous Adaptive Crystals of Pillararenes. J. Am. Chem. Soc. 2020, 142, 19722–19730. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; de María, P.D.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Shen, C.; Li, Y.; Xu, Y.; Wang, C.; Liu, Q. Biomass-derived 2-methyltetrahydrofuran platform: A focus on precious and non-precious metal-based catalysts for the biorefinery. Green Chem. 2022, 24, 4201–4236. [Google Scholar]
- Hu, Z.; Cheng, Y.; Wu, M.; Duan, Y.; Yang, Y.; Lu, T. A Novel Strategy for the Preparation of Supported Pd as an Efficient Catalyst for the Hydrogenation of Nitrobenzene in Mild Conditions. Catalysts 2023, 13, 1438. [Google Scholar] [CrossRef]
- Hajdu, V.; Muránszky, G.; Nagy, M.; Kopcsik, E.; Kristály, F.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Development of High-Efficiency, Magnetically Separable Palladium-Decorated Manganese-Ferrite Catalyst for Nitrobenzene Hydrogenation. Int. J. Mol. Sci. 2022, 23, 6535. [Google Scholar] [CrossRef] [PubMed]
- Khormi, A.Y.; Al-Shehri, B.M.; Al-Zahrani, F.A.M.; Hamdy, M.S.; Fouda, A.; Shaaban, M.R. Palladium Nanoparticles Incorporated Fumed Silica as an Efficient Catalyst for Nitroarenes Reduction via Thermal and Microwave Heating. Catalysts 2023, 13, 445. [Google Scholar] [CrossRef]
- Campos, C.H.; Shanmugaraj, K.; Bustamante, T.M.; Leal-Villarroel, E.; Vinoth, V.; Aepuru, R.; Mangalaraja, R.V.; Torres, C.C. Catalytic production of anilines by nitro-compounds hydrogenation over highly recyclable platinum nanoparticles supported on halloysite nanotubes. Catal. Today 2022, 394–396, 510–523. [Google Scholar] [CrossRef]
- Sadjadi, S.; Akbari, M.; Léger, B.; Monflier, E.; Heravi, M.M. Eggplant-Derived Biochar-Halloysite Nanocomposite as Supports of Pd Nanoparticles for the Catalytic Hydrogenation of Nitroarenes in the Presence of Cyclodextrin. ACS Sustain. Chem. Eng. 2019, 7, 6720–6731. [Google Scholar] [CrossRef]
- Witońska, I.; Karski, S.; Frajtak, M.; Krawczyk, N.; Królak, A. Temperature-programmed desoprtion of H2 from the surfaces of pd/support and Pd-Ag/support catalysts (support = Al2O3, SiO2). React. Kinet. Catal. Lett. 2008, 93, 241–248. [Google Scholar] [CrossRef]
- Park, Y.H.; Price, G.L. Temperature-programmed-reaction study on the effect of carbon monoxide on the acetylene reaction over palladium/alumina. Ind. Eng. Chem. Res. 1991, 30, 1700–1707. [Google Scholar] [CrossRef]
- Song, L.; Tan, K.; Ye, Y.; Zhu, B.; Zhang, S.; Huang, W. Amine-Functionalized Natural Halloysite Nanotubes Supported Metallic (Pd, Au, Ag) Nanoparticles and Their Catalytic Performance for Dehydrogenation of Formic Acid. Nanomaterials 2022, 12, 2414. [Google Scholar] [CrossRef]
- Dong, C.; Gao, Z.; Li, Y.; Peng, M.; Wang, M.; Xu, Y.; Li, C.; Xu, M.; Deng, Y.; Qin, X.; et al. Fully exposed palladium cluster catalysts enable hydrogen production from nitrogen heterocycles. Nat. Catal. 2022, 5, 485–493. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Ouyang, J.; Yang, H. Palladium nanoparticles deposited on silanized halloysite nanotubes: Synthesis, characterization and enhanced catalytic property. Sci. Rep. 2013, 3, 2948. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; She, T.; Chen, G.; Sun, M.; Niu, L.; Bai, G. Pd nanoparticles supported on amine-functionalized SBA-15 for the selective hydrogenation of phenol. Mol. Catal. 2021, 504, 111493. [Google Scholar] [CrossRef]
- Date, N.S.; Biradar, N.S.; Chikate, R.C.; Rode, C.V. Effect of Reduction Protocol of Pd Catalysts on Product Distribution in Furfural Hydrogenation. ChemistrySelect 2017, 2, 24–32. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Bedoya, S.; González-Vera, D.; Mangalaraja, R.V.; Vigneshwaran, S.; de León, J.N.D.; Herrera, C.; Al-Sehemi, A.G.; Campos, C.H. Palladium nanoparticles immobilized on TiO2 nanosheets matrix for the valorization of furfural to produce tetrahydrofurfuryl alcohol. J. Environ. Chem. Eng. 2024, 12, 113442. [Google Scholar] [CrossRef]
- Byun, M.Y.; Park, D.-W.; Lee, M.S. Effect of Oxide Supports on the Activity of Pd Based Catalysts for Furfural Hydrogenation. Catalysts 2020, 10, 837. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Talsi, V.P.; Gulyaeva, T.I.; Trenikhin, M.V.; Belskaya, O.B. Aqueous-phase hydrogenation of furfural over supported palladium catalysts: Effect of the support on the reaction routes. React. Kinet. Mech. Catal. 2019, 126, 811–827. [Google Scholar] [CrossRef]
- Huang, R.; Cui, Q.; Yuan, Q.; Wu, H.; Guan, Y.; Wu, P. Total Hydrogenation of Furfural over Pd/Al2O3 and Ru/ZrO2 Mixture under Mild Conditions: Essential Role of Tetrahydrofurfural as an Intermediate and Support Effect. ACS Sustain. Chem. Eng. 2018, 6, 6957–6964. [Google Scholar] [CrossRef]
- Sohrabi, S.; Abasabadi, R.K.; Khodadadi, A.A.; Mortazavi, Y.; Hoseinzadeh, A. In-situ one-step deposition of highly dispersed palladium nanoparticles into zirconium metal–organic framework for selective hydrogenation of furfural. Mol. Catal. 2021, 514, 111859. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Romero, A.H. Reduction of Nitroarenes via Catalytic Transfer Hydrogenation Using Formic Acid as Hydrogen Source: A Comprehensive Review. ChemistrySelect 2020, 5, 13054–13075. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Bustamante, T.M.; de León, J.N.D.; Aepuru, R.; Mangalaraja, R.V.; Torres, C.C.; Campos, C.H. Noble metal nanoparticles supported on titanate nanotubes as catalysts for selective hydrogenation of nitroarenes. Catal. Today 2022, 392–393, 93–104. [Google Scholar] [CrossRef]
- Santagata, M.; Johnston, C.T. A study of nanoconfined water in halloysite. Appl. Clay Sci. 2022, 221, 106467. [Google Scholar]
- Agrahari, S.K.; Lande, S.; Balachandran, V.; Kalpana, G.; Jasra, R.V. Palladium Supported on Mesoporous Alumina Catalyst for Selective Hydrogenation. J. Nanosci. Curr. Res. 2017, 2, 1000114. [Google Scholar] [CrossRef]
- Jiang, T.; Du, S.; Jafari, T.; Zhong, W.; Sun, Y.; Song, W.; Luo, Z.; Hines, W.A.; Suib, S.L. Synthesis of mesoporous γ-Fe2O3 supported palladium nanoparticles and investigation of their roles as magnetically recyclable catalysts for nitrobenzene hydrogenation. Appl. Catal. A Gen. 2015, 502, 105–113. [Google Scholar]
- Harraz, F.A.; El-Hout, S.E.; Killa, H.M.; Ibrahim, I.A. Palladium nanoparticles stabilized by polyethylene glycol: Efficient, recyclable catalyst for hydrogenation of styrene and nitrobenzene. J. Catal. 2012, 286, 184–192. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, K.; Wu, C.; Zhou, C.; Yin, H.; Zhou, S. Size-Controlled Synthesis of Highly Stable and Active Pd@SiO2 Core–Shell Nanocatalysts for Hydrogenation of Nitrobenzene. J. Phys. Chem. C 2013, 117, 8974–8982. [Google Scholar] [CrossRef]
- Villarroel, E.A.L.; Marcelot, C.; Torres, C.C.; Soulantica, K.; Campos, C.H.; Serp, P. Toward decorrelation of surface oxygen groups from metal dispersion effects in Pd/C hydrogenation catalysts. Catal. Sci. Technol. 2025, 15, 2034–2048. [Google Scholar]
- Guzmán, C.; Del Angel, G.; Gómez, R.; Galindo, F.; Zanella, R.; Torres, G.; Ángeles-Chávez, C.; Fierro, J.L.G. Gold Particle Size Determination on Au/TiO2-CeO2 Catalysts by Means of Carbon Monoxide, Hydrogen Chemisorption and Transmission Electron Microscopy. J. Nano Res. 2009, 5, 13–23. [Google Scholar]



| Material | Elemental Analysis | ICP-OES (Pd%) | SBET (m2 g−1) | dpore (nm) | Pore Volume (cm3 g−1) | ||
|---|---|---|---|---|---|---|---|
| %N | %C | %H | |||||
| HNTP | 0.00 | 0.02 | 1.74 | -- | 50 | 14.2 | 0.16 |
| HNTA | 0.13 (0.16) | 0.86 | 1.55 | -- | 46 | 13.5 | 0.15 |
| Pd/HNTA | 0.14 (0.15) | 0.86 | 1.58 | 0.46 (0.50) | 35 | 11.5 | 0.14 |
| Pd/HNTP | 0.00 | 0.04 | 1.78 | 0.41 (0.50) | 42 | 12.8 | 0.15 |
| Sample | Pd5/2 | Pd3/2 | Atomic Ratio Pd2+/Pd0 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pd2+ | Pd0 | Pd2+ | Pd0 | ||||||
| BE (eV) | % | BE (eV) | % | BE (eV) | % | BE (eV) | % | ||
| Pd/HNTP | 336.7 | 48.9 | 335.0 | 12.7 | 342.1 | 31.8 | 340.8 | 6.6 | 3.9 |
| Pd/HNTA | 336.2 | 22.6 | 334.6 | 26.4 | 341.3 | 24.9 | 339.4 | 26.1 | 0.9 |
| Entry | Catalyst | Reaction Conditions | Recycles | TOF (h−1) | Ref. |
|---|---|---|---|---|---|
| 1 | 1.1%Pd/Al2O3 | 30 °C, 5 bar, 0.5 h, water | -- | 204 | [48] |
| 2 | 1.0%Pd/CNT | 150 °C, 30 bar, 1 h, water | -- | 14,040 | [47] |
| 3 | 1.0%Pd/UiO-66 | 110 °C, 20 bar, 2 h, isopropanol | 3 | 206 | [49] |
| 4 | 2.0%Pd/Al2O3 | 25 °C, 60 bar, 0.5 h, isopropanol | 5 | 1155 | [50] |
| 5 | 1.0%Pd/TiO2 nanosheets | 140 °C, 20 bar, 4 h, n-dodecane | 5 | 2898 | [7] |
| 6 | Pd/HNTA | 120 °C, 30 bar, 6 h, n-dodecane | 10 | 880 | This work |
| Entry | Catalyst | Reaction Conditions | Recycles | TOF (h−1) | Ref. |
|---|---|---|---|---|---|
| 1 | 0.5%Pd/γ-Al2O3 | 25 °C, 1 bar, 3 h, THF | 3 | 583 | [54] |
| 2 | 2.0%Pd/meso-γ-Fe2O3 | 50 °C, 1 bar, 2 h, ethanol | 4 | 626 | [55] |
| 3 | 3.75%Pd-NPs stabilized PEG-4000 | 25 °C, 1 bar, 3 h, ethanol | 10 | 126 | [56] |
| 4 | 1.0%Pd/TiO2 nanotubes | 25 °C, 20 bar, 6 h, ethanol | 7 | 342 | [52] |
| 5 | Pd@SiO2 core-shell | 45 °C, 1 bar, 2 h, ethanol | 10 | 6335 | [57] |
| 6 | Pd/HNTA | 30 °C, 10 bar, 6 h, ethanol | 10 | 946 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedoya, S.; González-Vera, D.; Leal-Villarroel, E.A.; Díaz de León, J.N.; Domine, M.E.; Pecchi, G.; Torres, C.C.; Campos, C.H. Palladium Nanoparticles Immobilized on the Amine-Functionalized Lumen of Halloysite for Catalytic Hydrogenation Reactions. Catalysts 2025, 15, 533. https://doi.org/10.3390/catal15060533
Bedoya S, González-Vera D, Leal-Villarroel EA, Díaz de León JN, Domine ME, Pecchi G, Torres CC, Campos CH. Palladium Nanoparticles Immobilized on the Amine-Functionalized Lumen of Halloysite for Catalytic Hydrogenation Reactions. Catalysts. 2025; 15(6):533. https://doi.org/10.3390/catal15060533
Chicago/Turabian StyleBedoya, Santiago, Daniela González-Vera, Edgardo A. Leal-Villarroel, J. N. Díaz de León, Marcelo E. Domine, Gina Pecchi, Cecilia C. Torres, and Cristian H. Campos. 2025. "Palladium Nanoparticles Immobilized on the Amine-Functionalized Lumen of Halloysite for Catalytic Hydrogenation Reactions" Catalysts 15, no. 6: 533. https://doi.org/10.3390/catal15060533
APA StyleBedoya, S., González-Vera, D., Leal-Villarroel, E. A., Díaz de León, J. N., Domine, M. E., Pecchi, G., Torres, C. C., & Campos, C. H. (2025). Palladium Nanoparticles Immobilized on the Amine-Functionalized Lumen of Halloysite for Catalytic Hydrogenation Reactions. Catalysts, 15(6), 533. https://doi.org/10.3390/catal15060533









