Catalytic Transformation of LDPE into Aromatic-Rich Fuel Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of LDPE
2.2. Characterization of Catalysts
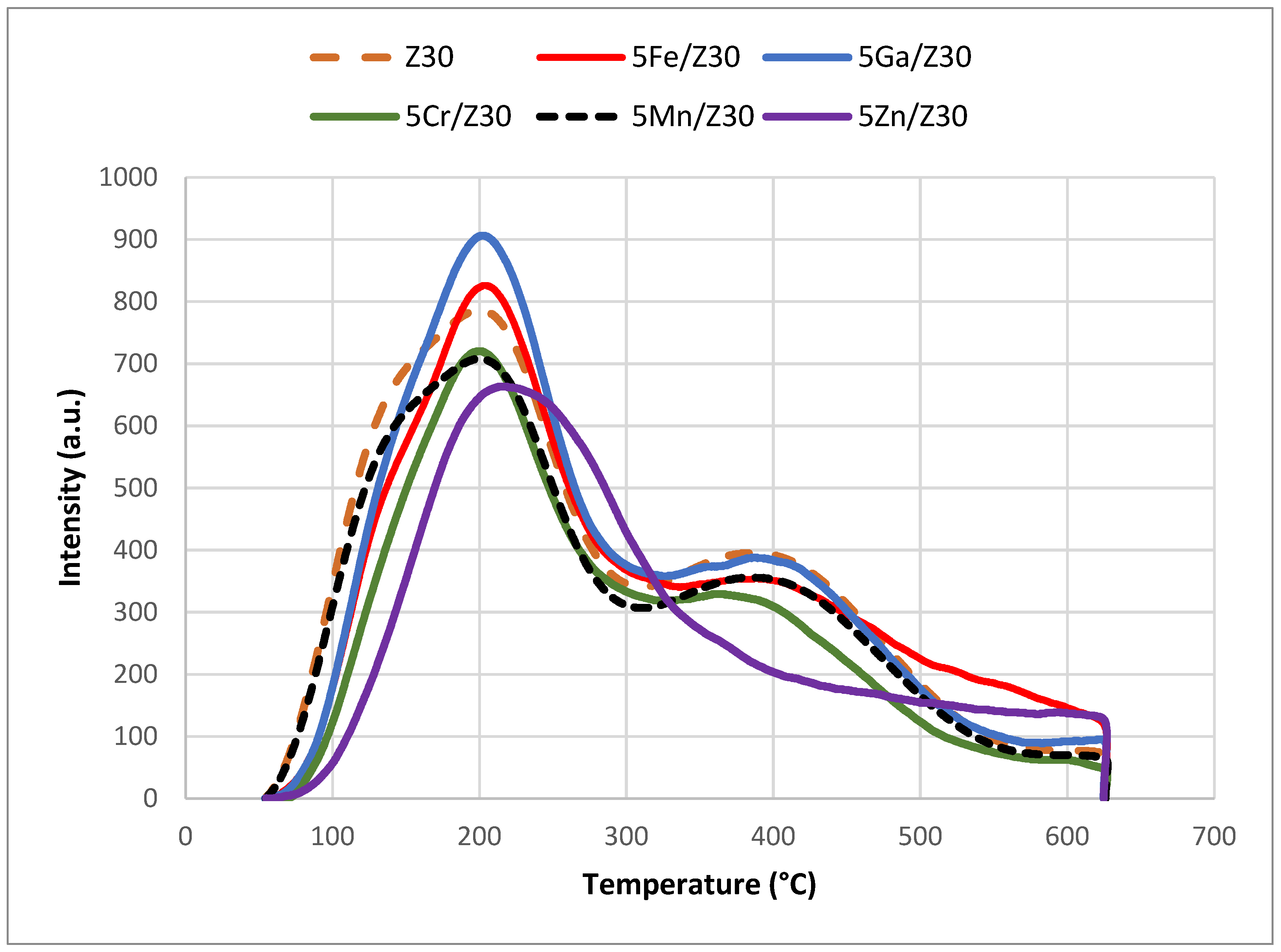
2.2.1. NH3-TPD Analysis
2.2.2. XRD Analysis
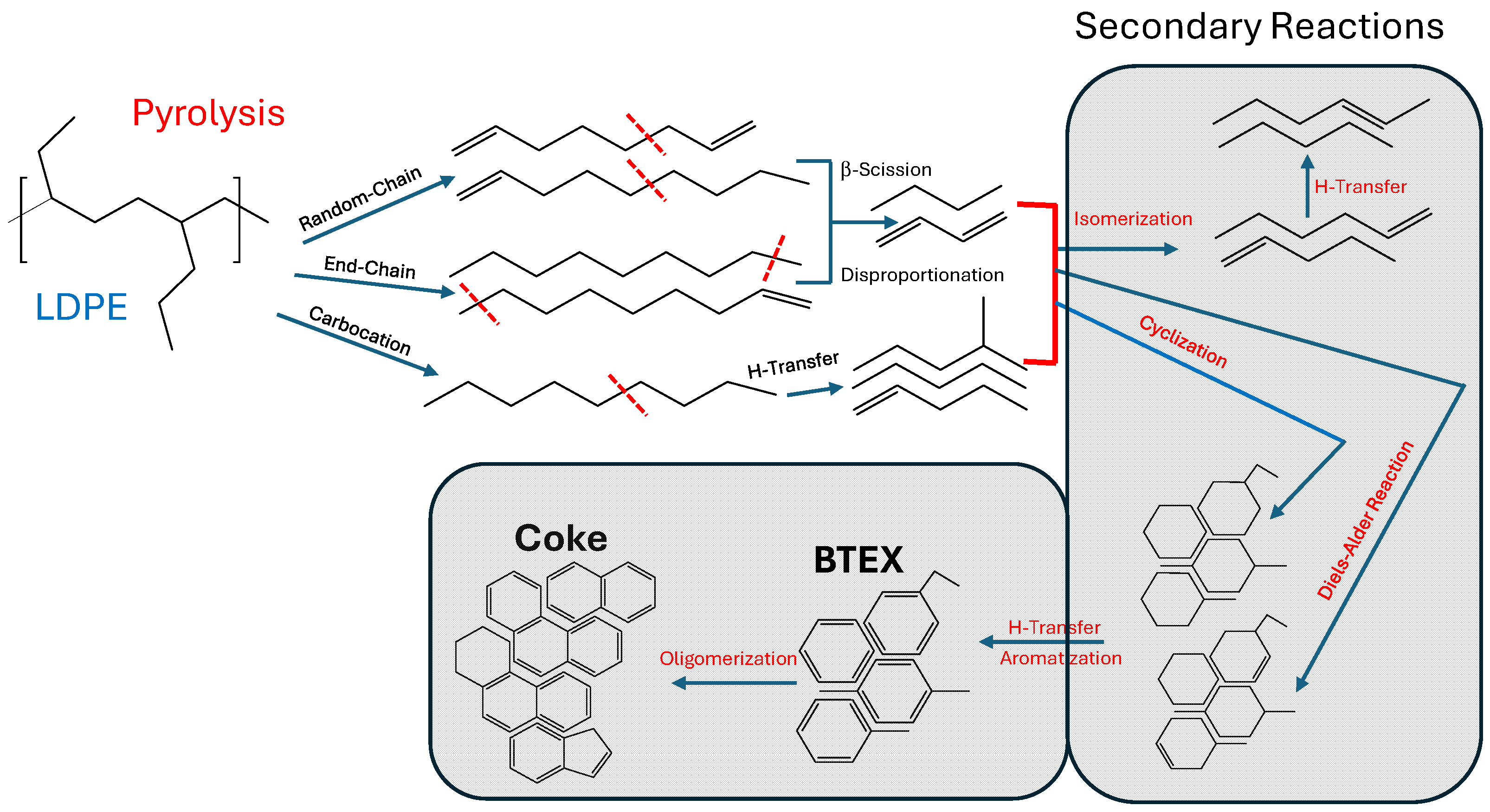
2.3. Catalytic Pyrolysis of LDPE
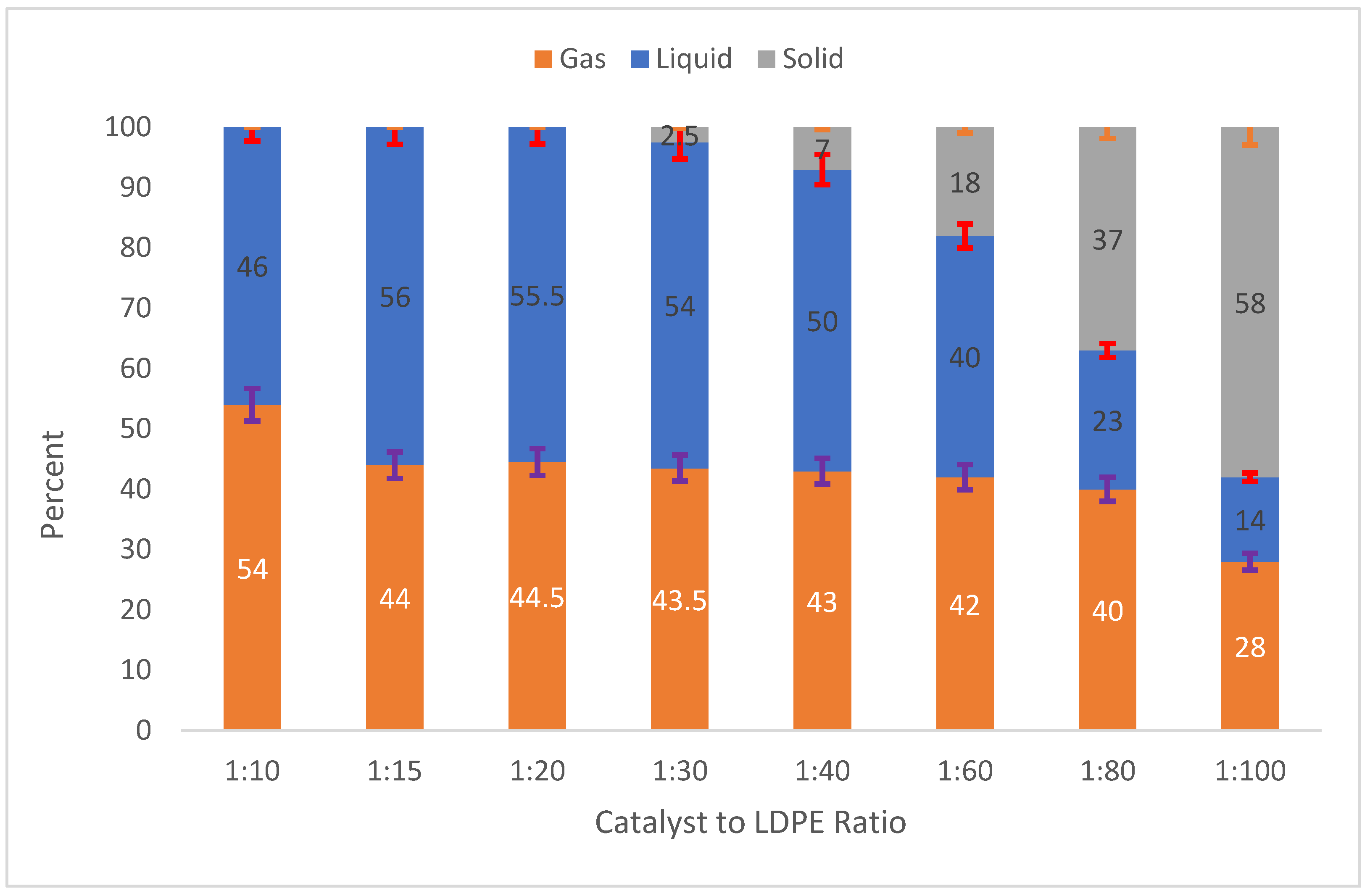
2.3.1. Effect of the Catalyst-to-Feed (Z30/LDPE) Ratio
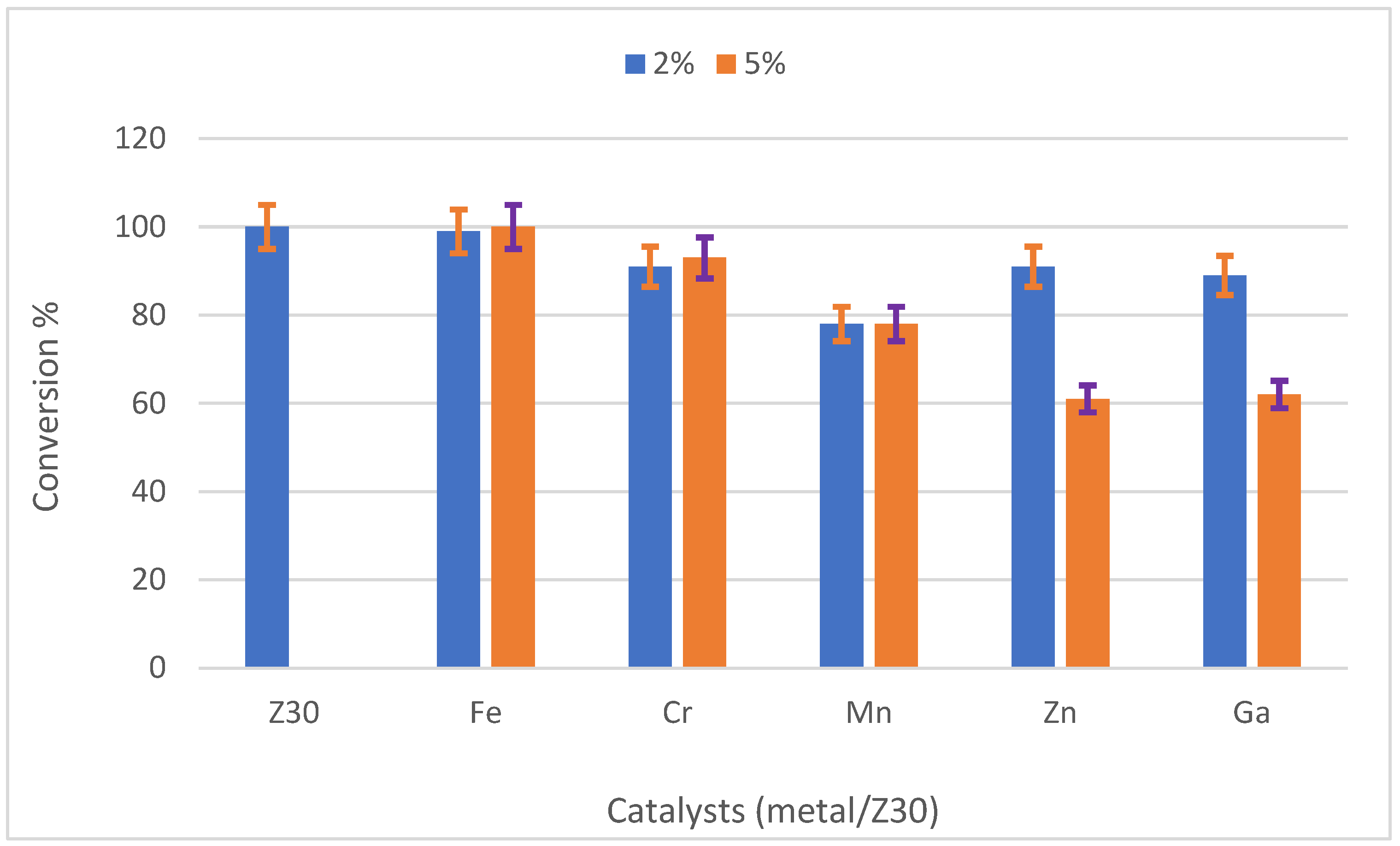
2.3.2. Catalytic Performance of Metal/Z30 Catalysts
2.4. Characterization of Pyrolytic Oil
2.4.1. Elemental Analysis, GCV, and Octane Number of Pyrolytic Oil Samples
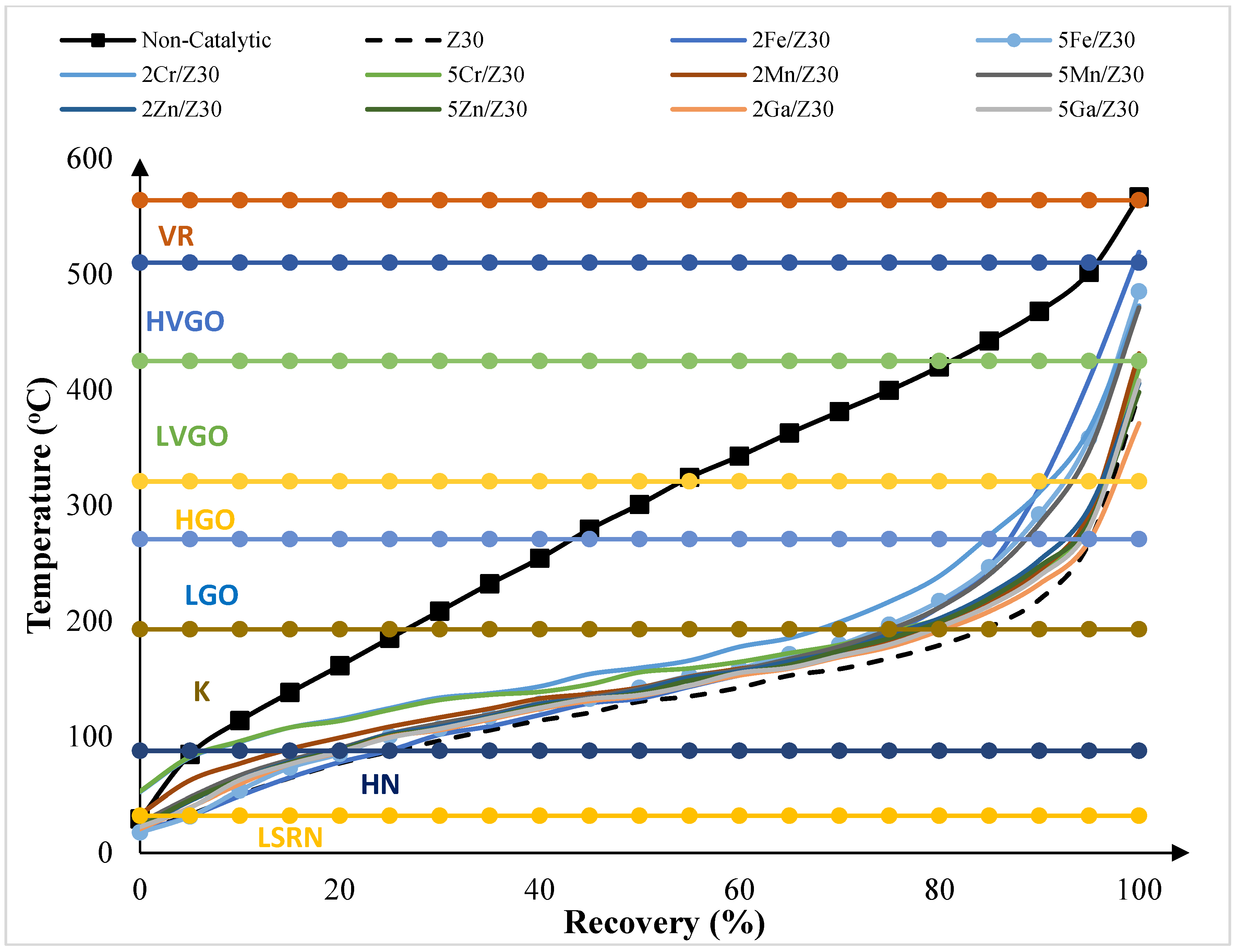
2.4.2. Simulated Distillation Analysis of Pyrolytic Oil Samples
2.4.3. GC-DHA Analysis of Pyrolytic Oil Samples
3. Experimental
3.1. Materials
3.2. Catalyst Synthesis
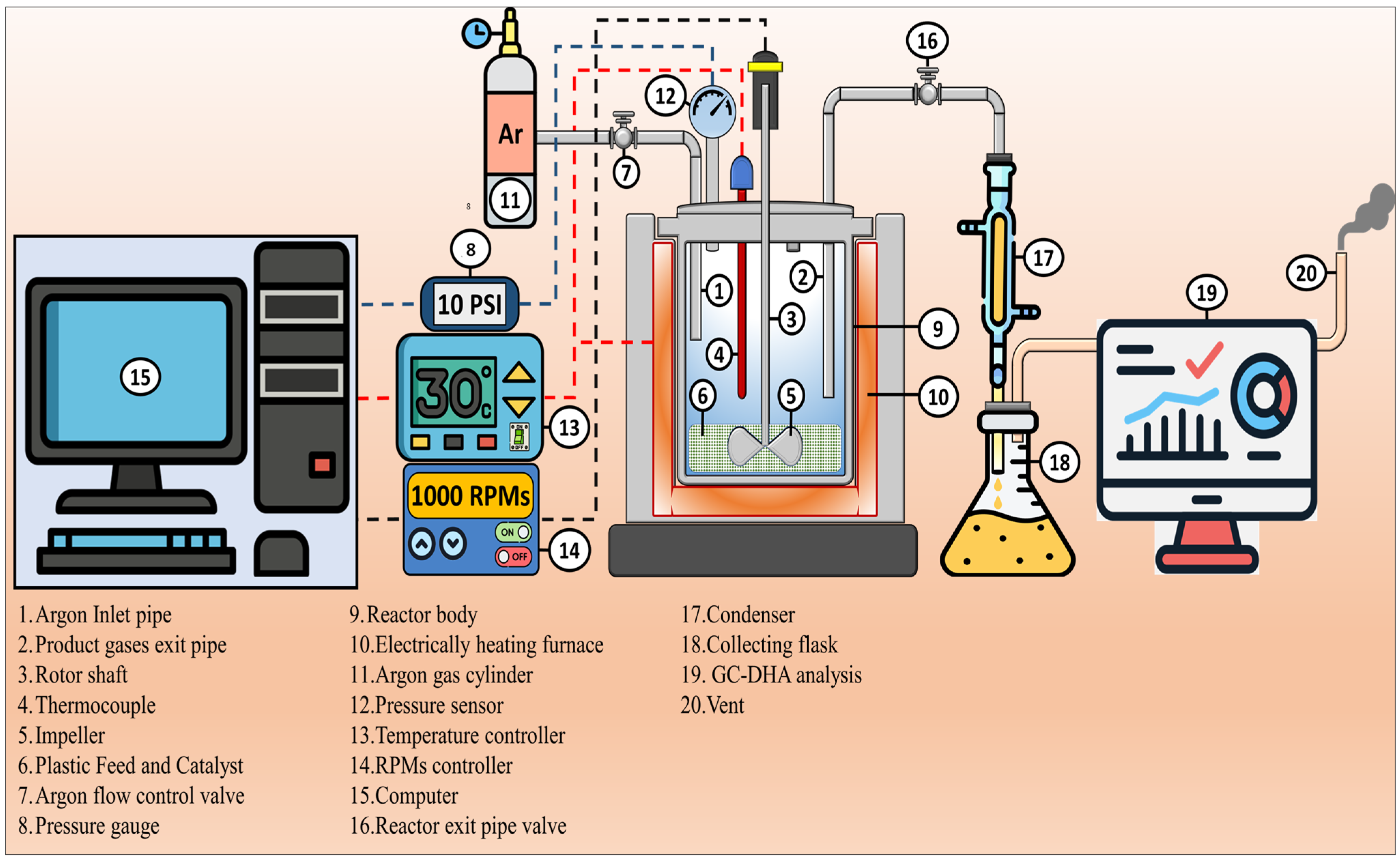
3.3. Pyrolysis Process
3.4. Characterization of Raw Materials, Catalysts and Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| LDPE | Low Density Polyethylene |
| Z30 | ZSM-5 (SiO2/Al2O3: 30) |
| CHNS analysis | Carbon, Hydrogen, Nitrogen and Sulphur analysis |
| GC-DHA | GC equipped with Detailed Hydrocarbon Analysis |
| BET | Brunauer-Emmett-Teller |
| ICP-OES | Inductively Coupled Plasma—Optical Emission Spectroscopy |
| XRD | X-ray diffraction |
| NH3-TPD | Ammonia Temperature-Programmed Desorption |
| TCD | Thermal Conductivity Detector |
| GCV | Gross Calorific Value |
| RTD | Resistance Temperature Detector |
| ASTM | The American Society for Testing and Materials |
| GPC | Gel Permeation Chromatograph |
| TGA | Thermogravimetric Analysis |
| SimDist | Simulated Distillation |
| FID | Flame Ionization Detector |
| VM | Volatile Matter |
| MC | Moisture Content |
| FC | Fixed Carbon |
| Mn | Number average molecular weight |
| Mw | Weight average molecular weight |
| LTP | Low-Temperature Peak |
| HTP | High-Temperature Peak |
| EIA | Environmental Impact Assessment |
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, K.; Wang, S.; Yu, J.; Luo, B.; Zhang, H. Tandem catalytic pyrolysis of mixed plastic packaging wastes to produce BTEX over dual catalysts. Fuel Process. Technol. 2023, 243, 107670. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime accumulation of microplastic in children and adults. Environ. Sci. Tech. 2021, 55, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Central Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef]
- Kots, P.A.; Liu, S.; Vance, B.C.; Wang, C.; Sheehan, J.D.; Vlachos, D.G. Polypropylene plastic waste conversion to lubricants over Ru/TiO2 catalysts. ACS Catal. 2021, 11, 8104–8115. [Google Scholar] [CrossRef]
- Wang, C.; Xie, T.; Kots, P.A.; Vance, B.C.; Yu, K.; Kumar, P.; Fu, J.; Liu, S.; Tsilomelekis, G.; Stach, E.A.; et al. Polyethylene Hydrogenolysis at Mild Conditions over Ruthenium on Tungstated Zirconia. JACS Au 2021, 1, 1422–1434. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Ebrahim, A.M.; Questell-Santiago, Y.; Zhu, J.; Troyano-Valls, C.; Asundi, A.S.; Brenner, A.E.; Bare, S.R.; Tassone, C.J.; Beckham, G.T.; et al. Role of Bifunctional Ru/Acid Catalysts in the Selective Hydrocracking of Polyethylene and Polypropylene Waste to Liquid Hydrocarbons. ACS Catal. 2022, 12, 13969–13979. [Google Scholar] [CrossRef]
- Sujithraj, A.; Tamizhdurai, P.; Mangesh, V.; Kavitha, C.; Subramani, A.; Saravanan, P.; Nisha, P.; Sasikumar, P.; Kumar, N.S.; Alreshaidan, S.B.; et al. Renewable energy from waste plastic: Hydroprocessing of mixed waste plastic to diesel fuel utilizing Fe/Mo-Al2O3 catalyst. Process. Saf. Environ. Prot. 2025, 193, 683–695. [Google Scholar] [CrossRef]
- Ke, L.; Wu, Q.; Zhou, N.; Li, H.; Zhang, Q.; Cui, X.; Fan, L.; Liu, Y.; Cobb, K.; Ruan, R.; et al. Polyethylene upcycling to aromatics by pulse pressurized catalytic pyrolysis. J. Hazard. Mater. 2024, 461, 132672. [Google Scholar] [CrossRef]
- Li, K.; Cai, C.; Zhou, W.; Wang, Y.; Amy, T.G.Y.; Sun, Z.; Min, Y. Tandem pyrolysis-catalytic upgrading of plastic waste towards kerosene-range products using Si-pillared vermiculite with transition metal modification. J. Hazard. Mater. 2024, 465, 133231. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zeng, L.; Yan, T.; Wang, C.; Wang, M.; Luo, L.; Wu, W.; Peng, Z.; Li, H.; Zeng, J. Efficient solvent- and hydrogen-free upcycling of high-density polyethylene into separable cyclic hydrocarbons. Nat. Nanotechnol. 2023, 18, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.M.O.; Ahmad, N.; Ahmed, U.; Siddiqui, M.N.; Ummer, A.C.; Abdul Jameel, A.G. Producing aromatic-rich oil through microwave-assisted catalytic pyrolysis of low-density polyethylene over Ni/Co/Cu-doped Ga/ZSM-5 catalysts. Biofuels Bioprod. Biorefining 2025, 19, 34–54. [Google Scholar] [CrossRef]
- Riaz, S.; Ahmad, N.; Farooq, W.; Ali, I.; Sajid, M.; Akhtar, M.N. Catalytic pyrolysis of HDPE for enhanced hy-drocarbon yield: A boosted regression tree assisted kinetics study for effective recycling of waste plastic. Digit. Chem. Eng. 2025, 14, 100213. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. Energy recovery from pyrolysis of plastic waste: Study on non-recycled plastics (NRP) data as the real measure of plastic waste. Energy Convers. Manag. 2017, 148, 925–934. [Google Scholar] [CrossRef]
- Chaudhary, A.; Lakhani, J.; Dalsaniya, P.; Chaudhary, P.; Trada, A.; Shah, N.K.; Upadhyay, D.S. Slow pyrolysis of low-density Poly-Ethylene (LDPE): A batch experiment and thermodynamic analysis. Energy 2023, 263, 125810. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Hu, J.; Li, S.; Chen, Y.; Yang, H.; Chen, H. Co-pyrolysis of microalgae with low-density polyethylene (LDPE) for deoxygenation and denitrification. Bioresour. Technol. 2020, 311, 123502. [Google Scholar] [CrossRef]
- Dubdub, I.; Al-Yaari, M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers 2020, 12, 891–905. [Google Scholar] [CrossRef]
- Contat-Rodrigo, L.; Ribes-Greus, A.; Imrie, C.T. Thermal analysis of high-density polyethylene and low-density polyethylene with enhanced biodegradability. J. Appl. Polym. Sci. 2002, 86, 764–772. [Google Scholar] [CrossRef]
- Kple, M.; Girods, P.; Fagla, B.; Anjorin, M.; Ziegler-Devin, I.; Rogaume, Y. Kinetic Study of Low Density Polyethylene Using Thermogravimetric Analysis, Part 2: Isothermal Study. Waste Biomass-Valorization 2017, 8, 707–719. [Google Scholar] [CrossRef]
- Oseke, G.; Atta, A.; Mukhtar, B.; El-Yakubu, B.; Aderemi, B. Increasing the catalytic stability of microporous Zn/ZSM-5 with copper for enhanced propane aromatization. J. King Saud Univ. Eng. Sci. 2021, 33, 531–538. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, A.; Wu, X.; Hai, G.; Hu, J.; Li, T.; Li, G. The preparation of nano-sized H[Zn, Al]ZSM-5 zeolite and its application in the aromatization of methanol. Microporous Mesoporous Mater. 2011, 143, 435–442. [Google Scholar] [CrossRef]
- Susanto, H.; Darmawan, A. Characteristic of ZSM-5 catalyst supported by nickel and molybdenum. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 509, p. 012138. [Google Scholar]
- Rahimi, S.; Rostamizadeh, M. Novel Fe/B-ZSM-5 nanocatalyst development for catalytic cracking of plastic to valuable products. J. Taiwan Inst. Chem. Eng. 2021, 118, 131–139. [Google Scholar] [CrossRef]
- Yuan, E.; Han, W.; Zhang, G.; Zhao, K.; Mo, Z.; Lu, G.; Tang, Z. Structural and Textural Characteristics of Zn-Containing ZSM-5 Zeolites and Application for the Selective Catalytic Reduction of NOx with NH3 at High Temperatures. Catal. Surv. Asia 2016, 20, 41–52. [Google Scholar] [CrossRef]
- Ling, R.; Chen, W.; Hou, J. Preparation of modified MFI (ZSM-5 and silicalite-1) zeolites for potassium extraction from seawater. Particuology 2018, 36, 190–192. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, M.; Chen, Z.; Zhang, X.; Yang, H.; Wang, X.; Feng, H.; Yu, J.; Gao, S. Production of monocyclic aromatics and light olefins through ex-situ catalytic pyrolysis of low-density polyethylene over Ga/P/ZSM-5 catalyst. J. Energy Inst. 2023, 108, 101235. [Google Scholar] [CrossRef]
- Xin, M.; Xing, E.; Gao, X.; Wang, Y.; Ouyang, Y.; Xu, G.; Luo, Y.; Shu, X. Ga Substitution during Modification of ZSM-5 and Its Influences on Catalytic Aromatization Performance. Ind. Eng. Chem. Res. 2019, 58, 6970–6981. [Google Scholar] [CrossRef]
- Iisa, K.; Kim, Y.; Orton, K.A.; Robichaud, D.J.; Katahira, R.; Watson, M.J.; Wegener, E.C.; Nimlos, M.R.; Schaidle, J.A.; Mukarakate, C.; et al. Ga/ZSM-5 catalyst improves hydrocarbon yields and increases alkene selectivity during catalytic fast pyrolysis of biomass with co-fed hydrogen. Green Chem. 2020, 22, 2403–2418. [Google Scholar] [CrossRef]
- Mohiuddin, E.; Mdleleni, M.M.; Key, D. Catalytic cracking of naphtha: The effect of Fe and Cr impregnated ZSM-5 on olefin selectivity. Appl. Petrochem. Res. 2018, 8, 119–129. [Google Scholar] [CrossRef]
- Calsavara, V.; Baesso, M.L.; Fernandes-Machado, N.R.C. Transformation of ethanol into hydrocarbons on ZSM-5 zeolites modified with iron in different ways. Fuel 2008, 87, 1628–1636. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Miskolczi, N.; Juzsakova, T.; Sója, J. Preparation and application of metal loaded ZSM-5 and y-zeolite catalysts for thermo-catalytic pyrolysis of real end of life vehicle plastics waste. J. Energy Inst. 2019, 92, 118–127. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. A novel approach of solid waste management via aromatization using multiphase catalytic pyrolysis of waste polyethylene. Waste Manag. 2018, 71, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Chen, C.-N.; Juang, R.-S. Structure and thermal stability of toxic chromium(VI) species doped onto TiO2 powders through heat treatment. J. Environ. Manag. 2009, 90, 1950–1955. [Google Scholar] [CrossRef]
- Alexzman, Z.; Annuar, N.; Salamun, N.; Yusoff, S.; Daud, A. Chromium oxide silica catalyst: Synthesis and characterization. Mater. Today Proc. 2022, 57, 1301–1305. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, G.; Chen, H.; Zhang, G.; Han, Y.; Chang, Y.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, N.; Maafa, I.M.; Ahmed, U.; Akhter, P.; Shehzad, N.; Amjad, U.-E.; Hussain, M. Thermal conversion of polystyrene plastic waste to liquid fuel via ethanolysis. Fuel 2020, 279, 118498. [Google Scholar] [CrossRef]
- Speight, J.G. Rules of Thumb for Petroleum Engineers; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Akhtar, M.N.; Riaz, S.; Ahmad, N.; Jaseer, E.A. Pioneering Aromatic Generation from Plastic Waste via Catalytic Thermolysis: A Minireview. Energy Fuels 2024, 38, 11363–11390. [Google Scholar] [CrossRef]
- Wang, J.; Shan, J.; Tian, Y.; Zhu, T.; Duan, H.; He, X.; Qiao, C.; Liu, G. Catalytic cracking of n-heptane over Fe modified HZSM-5 nanosheet to produce light olefins. Fuel 2021, 306, 121725. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, K.; Wei, B.; Yang, H.; Jin, L.; Hu, H. Pyrolysis behavior of low-density polyethylene over HZSM-5 via rapid infrared heating. Sci. Total. Environ. 2022, 806, 151287. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, Z.; Han, D.; Wang, C.; Zhang, J.; Sun, M.; Hao, Q.; Chen, H.; Ma, X. Effective regulation of Ga active species in mesoporous ZSM-5 for catalytic upgrading of coal pyrolysis volatiles. Fuel 2022, 321, 124105. [Google Scholar] [CrossRef]
- Nzihou, J.F.; Hamidou, S.; Bouda, M.; Koulidiati, J.; Segda, B.G. Using Dulong and Vandralek formulas to estimate the calorific heating value of a household waste model. Int. J. Sci. Eng. Res. 2014, 5, 1878–1883. [Google Scholar]
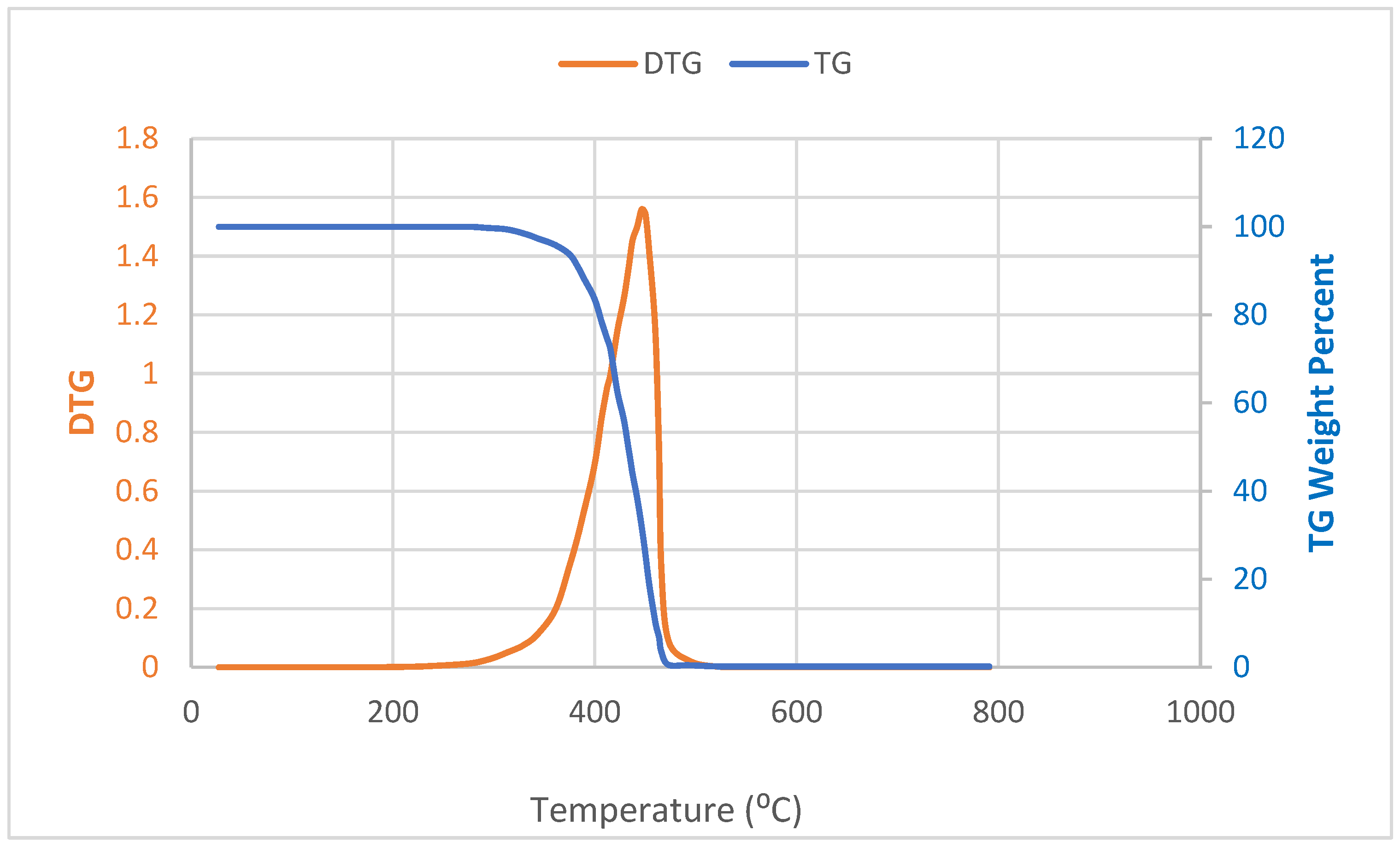


| Ultimate Analysis (%) | Proximate Analysis (%) | ||
|---|---|---|---|
| Carbon (C) | 86.05 | Volatile matter (VM) | 99.60 |
| Hydrogen (H) | 13.95 | Ash | 0.33 |
| Oxygen (O) | 0.00 | Fixed carbon (FC) | 0.00 |
| Nitrogen (N) | 0.00 | Moisture content (MC) | 0.07 |
| Sulphur (S) | 0.00 | ||
| GCV (MJ/kg) | 42.27 | ||
| Size of pellets (mm) | 2–4 | ||
| Mn (Daltons) | 5000 | Mw (Daltons) | 98,000 |
| Entry | Catalyst | Textural Properties | NH3-TPD Results (mmol NH3/g) | |||||
|---|---|---|---|---|---|---|---|---|
| SBET | PVtotal | PDavg | LTP | HTP | Total | HTP/LTP | ||
| (m2/g) | (cm3/g) | (nm) | (100–300 °C) | (300–625 °C) | (100–625 °C) | |||
| 1 | Z30 | 342.00 | 0.17 | 5.05 | 0.78 | 0.62 | 1.39 | 0.79 |
| 2 | 2Fe/Z30 | 333.00 | 0.14 | 6.41 | 0.62 | 0.55 | 1.17 | 0.88 |
| 3 | 5Fe/Z30 | 319.00 | 0.17 | 7.87 | 0.57 | 0.54 | 1.11 | 0.95 |
| 4 | 2Zn/Z30 | 332.00 | 0.15 | 7.30 | 0.68 | 0.15 | 0.83 | 0.22 |
| 5 | 5Zn/Z30 | 293.00 | 0.11 | 7.14 | 0.53 | 0.05 | 0.58 | 0.08 |
| 6 | 2Cr/Z30 | 320.00 | 0.18 | 8.00 | 0.63 | 0.52 | 1.15 | 0.84 |
| 7 | 5Cr/Z30 | 315.00 | 0.20 | 7.10 | 0.48 | 0.42 | 0.90 | 0.89 |
| 8 | 2Mn/Z30 | 317.00 | 0.14 | 7.00 | 0.74 | 0.60 | 1.34 | 0.81 |
| 9 | 5Mn/Z30 | 291.00 | 0.12 | 7.00 | 0.70 | 0.59 | 1.29 | 0.84 |
| 10 | 2Ga/Z30 | 325.00 | 0.16 | 7.57 | 0.76 | 0.52 | 1.28 | 0.68 |
| 11 | 5Ga/Z30 | 316.00 | 0.13 | 7.00 | 0.75 | 0.44 | 1.19 | 0.59 |
| Entry | Parent Catalyst | Metal Precursor | Metal Loading (wt%) | Catalyst Name | Octane Number | Elemental Analysis | GCV (MJ/kg) | ||
|---|---|---|---|---|---|---|---|---|---|
| Carbon (%) | Hydrogen (%) | Oxygen (%) | |||||||
| 1 | Non-Catalytic | ꟷ | ꟷ | Non-Cat | 75.20 | 85.26 | 14.74 | 0.00 | 42.81 |
| 2 | ZSM-5 | ꟷ | ꟷ | Z30 | 83.40 | 85.56 | 14.43 | 0.00 | 42.60 |
| 3 | ZSM-5 | Fe(NO3)3·9H2O | 2.00 | 2Fe/Z30 | 89.10 | 85.94 | 14.05 | 0.00 | 42.34 |
| 4 | ZSM-5 | 5.00 | 5Fe/Z30 | 91.00 | 86.02 | 13.92 | 0.05 | 42.22 | |
| 4 | ZSM-5 | Zn(NO3)2·6H2O | 2.00 | 2Zn/Z30 | 87.30 | 85.75 | 14.24 | 0.00 | 42.46 |
| 5 | ZSM-5 | 5.00 | 5Zn/Z30 | 88.40 | 86.15 | 13.79 | 0.05 | 42.13 | |
| 6 | ZSM-5 | Cr(NO3)3·9H2O | 2.00 | 2Cr/Z30 | 89.20 | 86.17 | 13.82 | 0.00 | 42.18 |
| 7 | ZSM-5 | 5.00 | 5Cr/Z30 | 90.20 | 86.45 | 13.51 | 0.03 | 41.96 | |
| 8 | ZSM-5 | MnCl2·4H2O | 2.00 | 2Mn/Z30 | 85.50 | 85.43 | 14.49 | 0.07 | 42.60 |
| 9 | ZSM-5 | 5.00 | 5Mn/Z30 | 84.30 | 85.58 | 14.41 | 0.00 | 42.58 | |
| 10 | ZSM-5 | Ga(NO3)3 xH2O | 2.00 | 2Ga/Z30 | 89.80 | 86.15 | 13.80 | 0.04 | 42.14 |
| 11 | ZSM-5 | 5.00 | 5Ga/Z30 | 90.10 | 86.21 | 13.78 | 0.00 | 42.15 | |
| Entry | Catalyst | Paraffins | Olefins | Naphthenes | Aromatics | C14+ |
|---|---|---|---|---|---|---|
| 1 | Non-catalyst | 19.47 | 54.60 | 0.00 | 12.00 | 13.90 |
| 2 | Z30 | 27.75 | 46.97 | 6.96 | 18.28 | 0.00 |
| 3 | 2Fe/Z30 | 23.65 | 35.60 | 7.48 | 32.70 | 0.60 |
| 4 | 5Fe/Z30 | 23.16 | 34.48 | 7.00 | 34.57 | 0.80 |
| 5 | 2Cr/Z30 | 24.50 | 36.00 | 7.20 | 31.90 | 0.40 |
| 6 | 5Cr/Z30 | 24.60 | 34.40 | 7.10 | 33.40 | 0.48 |
| 7 | 2Mn/Z30 | 23.00 | 45.40 | 6.60 | 24.80 | 0.16 |
| 8 | 5Mn/Z30 | 24.45 | 52.40 | 6.35 | 16.76 | 0.00 |
| 9 | 2Zn/Z30 | 23.85 | 43.06 | 7.29 | 25.60 | 0.20 |
| 10 | 5Zn/Z30 | 22.39 | 42.10 | 6.20 | 29.07 | 0.20 |
| 11 | 2Ga/Z30 | 22.59 | 37.39 | 8.00 | 31.68 | 0.30 |
| 12 | 5Ga/Z30 | 21.90 | 35.35 | 7.14 | 34.77 | 0.79 |
| Entry | Parent Catalyst | Metal Precursor | Metal Loading (wt. %) | Catalyst Name |
|---|---|---|---|---|
| 1 | ZSM-5 | ꟷ | ꟷ | Z30 |
| 2 | ZSM-5 | Fe(NO3)3·9H2O | 2.00 | 2Fe/Z30 |
| 3 | ZSM-5 | 5.00 | 5Fe/Z30 | |
| 4 | ZSM-5 | Zn(NO3)2·6H2O | 2.00 | 2Zn/Z30 |
| 5 | ZSM-5 | 5.00 | 5Zn/Z30 | |
| 6 | ZSM-5 | Cr(NO3)3·9H2O | 2.00 | 2Cr/Z30 |
| 7 | ZSM-5 | 5.00 | 5Cr/Z30 | |
| 8 | ZSM-5 | MnCl2·4H2O | 2.00 | 2Mn/Z30 |
| 9 | ZSM-5 | 5.00 | 5Mn/Z30 | |
| 10 | ZSM-5 | Ga(NO3)3 xH2O | 2.00 | 2Ga/Z30 |
| 11 | ZSM-5 | 5.00 | 5Ga/Z30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.N.; Ahmad, N.; Alqudayri, F. Catalytic Transformation of LDPE into Aromatic-Rich Fuel Oil. Catalysts 2025, 15, 532. https://doi.org/10.3390/catal15060532
Akhtar MN, Ahmad N, Alqudayri F. Catalytic Transformation of LDPE into Aromatic-Rich Fuel Oil. Catalysts. 2025; 15(6):532. https://doi.org/10.3390/catal15060532
Chicago/Turabian StyleAkhtar, Muhammad Naseem, Nabeel Ahmad, and Feras Alqudayri. 2025. "Catalytic Transformation of LDPE into Aromatic-Rich Fuel Oil" Catalysts 15, no. 6: 532. https://doi.org/10.3390/catal15060532
APA StyleAkhtar, M. N., Ahmad, N., & Alqudayri, F. (2025). Catalytic Transformation of LDPE into Aromatic-Rich Fuel Oil. Catalysts, 15(6), 532. https://doi.org/10.3390/catal15060532






