Current Strategies to Improve the Properties of Graphitic Carbon Nitride for Effective and Scalable Wastewater Pollutant Removal: A Critical Review
Abstract
1. Introduction
- Morphological modifications: Adjusting the morphology of gCN to create structures like 0D quantum dots (QDs); 1D nanorods, nanowires, or nanotubes (NRs, NWs, and NTs, respectively); 2D nanosheets (NSs); and 3D porous networks. These modifications mainly enhance the surface area as well as improve the mobility of the charge carriers (their diffusion to the surface) and may even reduce the band gap of the material by introducing defects in its morphology [4,6,13].
- Modification of band structure:Vacancies and defects: The introduction of defects such as nitrogen and carbon vacancies in gCN can significantly influence its photocatalytic performance. Nitrogen vacancies (Nv) can enhance charge separation and extend the lifetime of photogenerated excitons [4,6], while carbon vacancies (Cv) can serve as electron reservoirs, facilitating the generation of superoxide radicals (O2•−) [43,44].Metal, non-metal, and self-doping: Doping with metal and non-metal elements influences the electronic structure of gCN by not only improving the absorption range (due to the introduction of intermediate energy levels in the band gap, as well as vacancies) but also by increasing the lifetime of the charge carriers and improving their separation [1,13]. This doping can also correct the increased gap produced in nanostructures (e.g., nanosheets) by the quantum confinement effect [1,13].

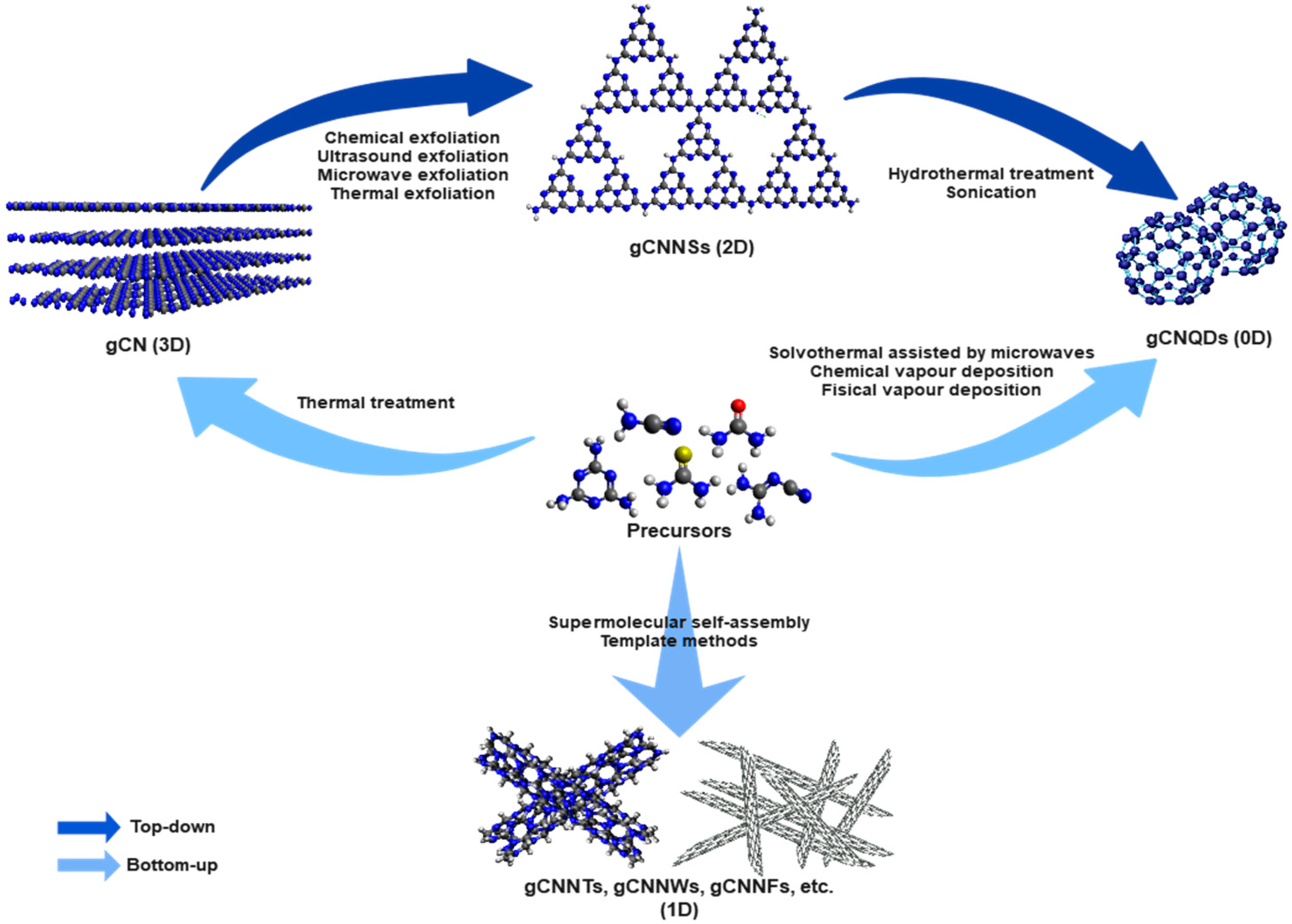
2. Nanoarchitecture Design of gCN
2.1. Zero-Dimensional Structures (0D)
- The fabrication of heterojunctions, mainly Z-type and II type (semiconductor/semiconductor);
2.2. One-Dimensional Structures (1D)
2.3. Two-Dimensional Structures (2D)
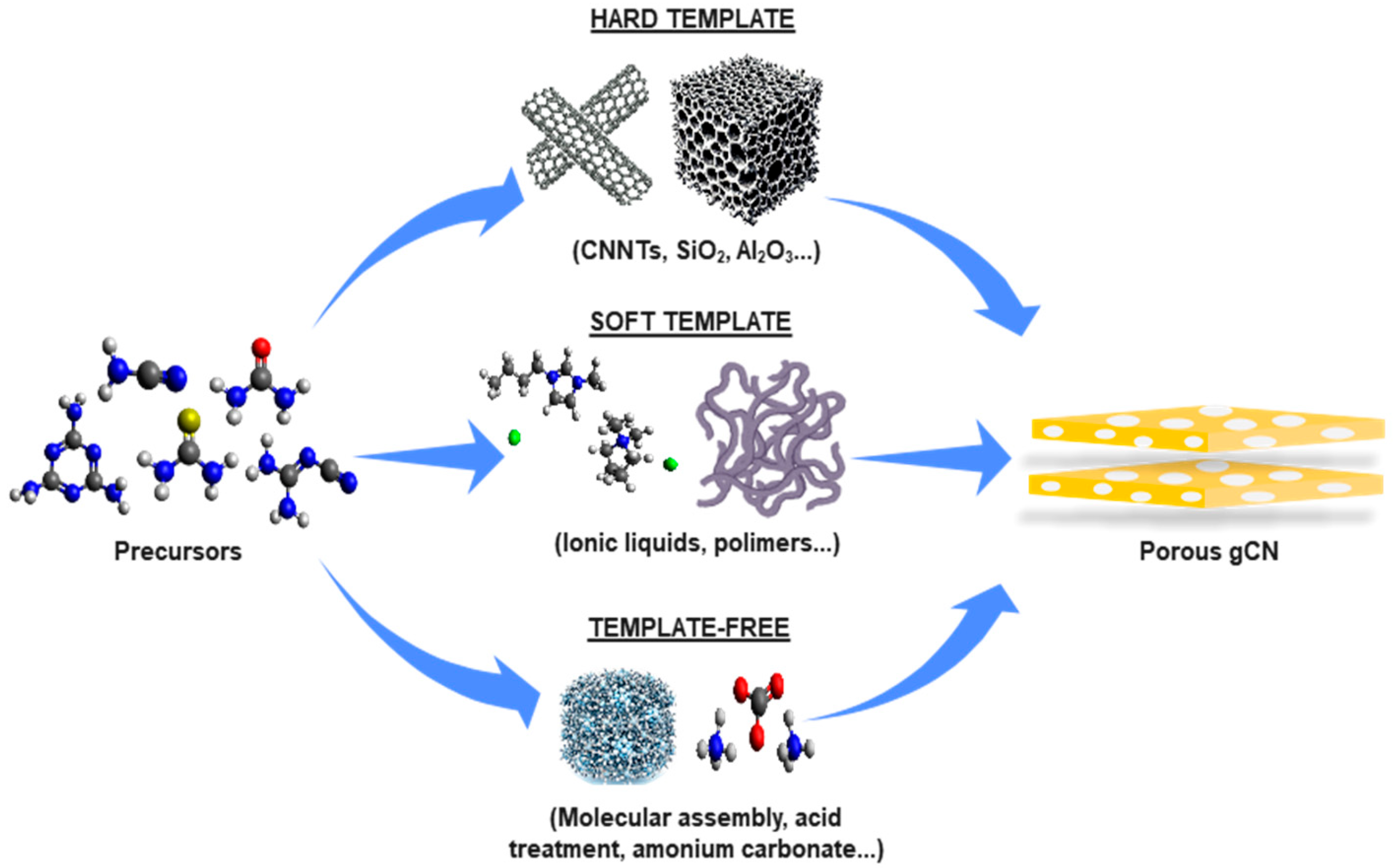
2.4. Three-Dimensional Porous Structures (3D)
2.4.1. Hard Template
- i.
- Coating the chosen hard template with the gCN precursor;
- ii.
- Treatment for conversion to gCN on the template;
- iii.
- Removal of the template.
2.4.2. Soft Template


2.4.3. Template Free
2.5. High Crystalline gCN Structures
3. Surface Functionalization
- Cyano groups (by treatment of gCN with NaBH4 and subsequent treatment at 150–350 °C, or with potassium thiocyanate (KSCN) and subsequent heat treatment at 500 °C, among other methods) [42,110]. These functional groups can considerably reduce the band gap (they are electron-accepting groups) [109]. The introduction of the cyano group into gCN structures increases O2 adsorption and introduces lone pair electrons into the structure that participate in photoexcitation [111].
- Ureido groups (introduced in the same way as cyano groups, with the addition of a subsequent treatment with HCL and stirring for a long time) also improve charge separation [42].
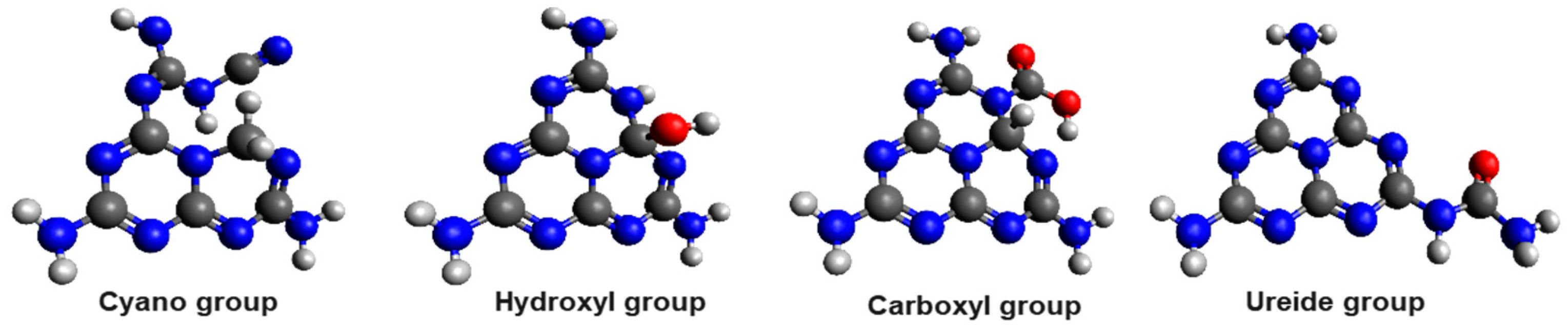
4. Electronic Structure Optimization
4.1. Defect Engineering
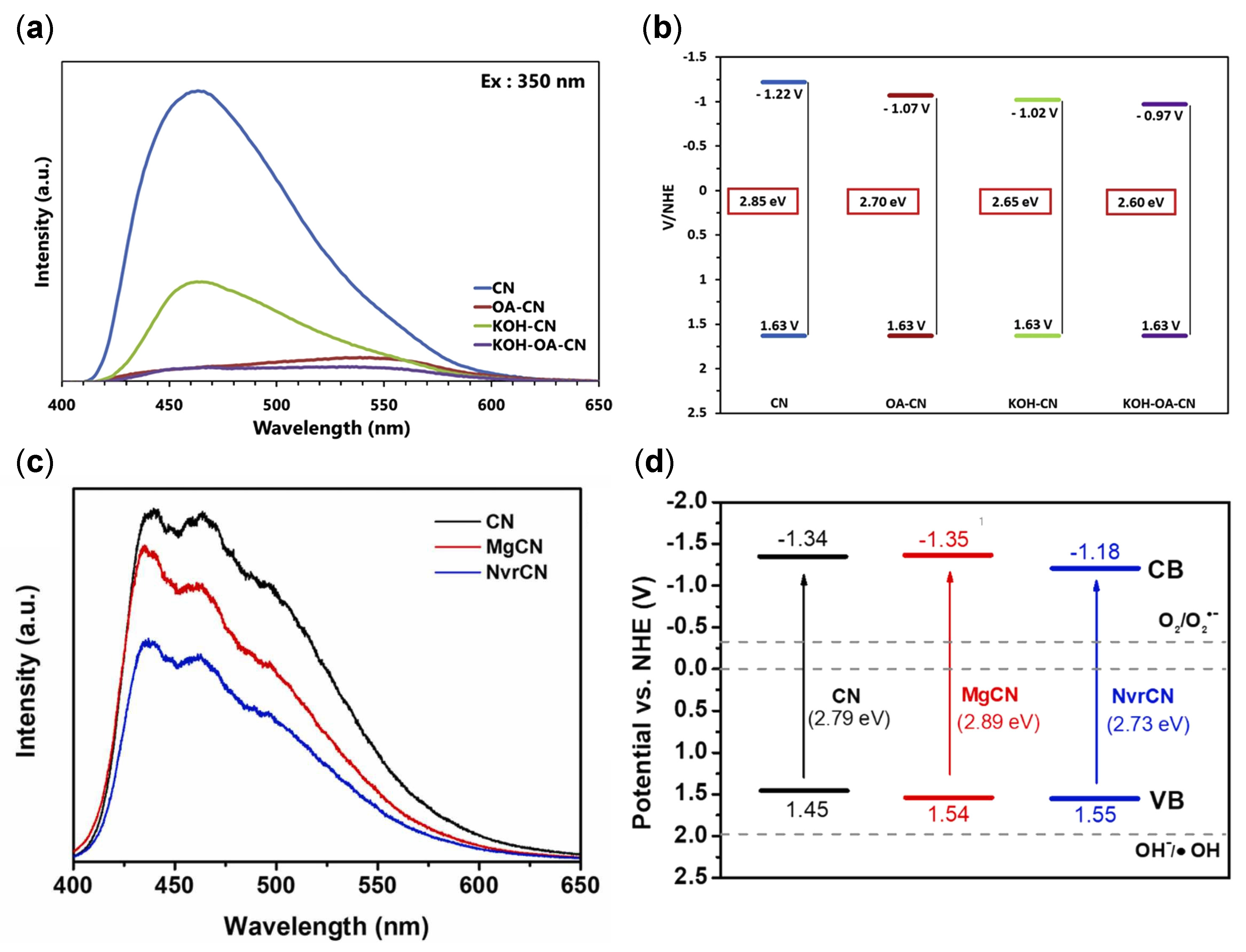
4.1.1. Nitrogen Vacancies (Nv)
4.1.2. Carbon Vacancies (Cv)
- i.
- Calcination in an air muffle furnace at 550 °C 2 h;
- ii.
- Pyrolysis at 520 °C in a tube furnace in Ar atmosphere (2 h).
4.2. Element Doping Strategies
4.2.1. Non-Metal Doping
Doping with O
Doping with P
Doping with S
Doping with B
Doping with Halogens
Self-Doping
4.2.2. Metal Doping
Alkaline and Alkaline Earth Metal Doping
Rare Earths (Ln) Doping
Noble Metals Doping
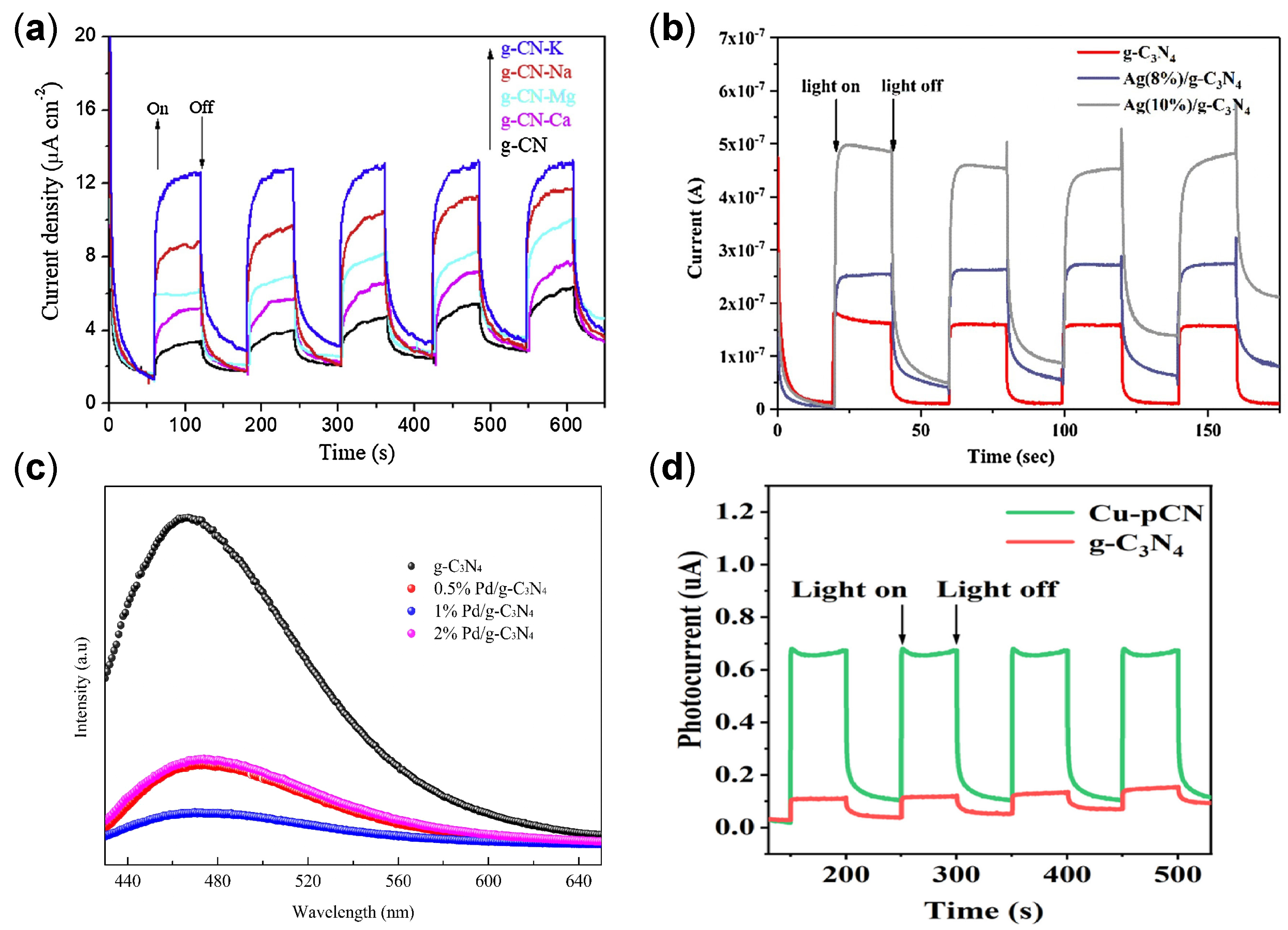
Transition Metal (Non-Noble) Doping
5. Conclusions
- The documented leaching of metals when doping gCN and taking place during degradation cycles could be an environmental and/or public health issue, and further research into doping techniques to overcome this effect would be desirable.
- The stability of the synthesized materials is a matter of concern and there is no consensus in this respect. Not all the studies present these stability studies, whether carried out with only 2 or 3 or up to 10 consecutive degradation cycles, which makes it difficult to have a clear perception of this. Given the operational requirements, i.e., the high flow rates to be treated (both in WWTPs and DWTPs), and the need for the photocatalysts to be economically viable, more than five cycles would be necessary to get an idea of the operational stability of these materials in real situations. It is also noteworthy that 2D and porous 3D structures frequently exhibit superior recyclability, which positions them as particularly promising for long-term applications. It would also be advisable to accompany with stability studies—under the same conditions—of unmodified gCN in order to obtain a clear idea of the effect of the modifications on the stability of these gCN derivatives. Also, accompanying these stability studies with characterizations (FTIR, XRD, etc.) of the modified photocatalyst would reinforce this perception.
- The lack of a standardized protocols for both the study and preliminary optimization of catalysts (generally with organic dyes) and for the degradation conditions of model pollutants—as exists for the evaluation of photocatalytic ceramic materials in aqueous media (ISO 10678) [146], for the evaluation of methods for air-purification performance of semiconductor photocatalytic materials (ISO 22197) [147], or for water purification performance of semiconductor photocatalytic materials by measurement of forming ability of active oxygen (ISO 10676) [148,149]—is a drawback. Standardization of these processes would be desirable, as it would facilitate comparability between materials.
- For the degradation of model EPs, experimental studies should also include assessments of the potential toxicity of both the materials and the degradation products of the target EPs. This approach would improve the positive perception of heterogeneous photocatalysis within industrial sectors.
- Finally, in the 21st century, and amid the planet’s environmental crisis, there is requirement for a commitment by researchers to align their work with Green Chemistry principles (the 12 tenets established in 1998 by Paul Anastas and John Warner).
- Recent advances in DFT and machine learning offer a powerful means to pre-screen and predict the performance of modified gCN, allowing for researchers to optimize band structure, charge dynamics, and catalytic behavior before experimental validation. Integrating these computational tools could greatly accelerate materials discovery while aligning with the principles of green chemistry. Few studies incorporate prior in silico analysis to guide and justify the selection of gCN modification methodologies, which would enhance the sustainability of experimental designs and processes.
- The improvement of photocatalytic capabilities by the combination of photocatalysts in the form of heterojunctions or homojunctions is also an important aspect for the improvement of the photodegradation efficiency of PEs that must be assessed by photocatalysis researchers. Although this review deliberately focuses on modifications of gCN itself, it is essential to highlight that the integration of gCN into heterojunction systems (e.g., type II, Z-scheme, Schottky) offers exciting avenues for synergistic performance improvements, meriting dedicated and systematic exploration in future studies.
- Immobilization of photocatalytic materials, although addressed in numerous studies, requires solutions and more investigations that do not compromise the inherent photocatalytic efficiency of gCN. Even though it is not an aim of this review, the fine and dispersed nature of gCN nanoarchitecture complicates its recovery after the photocatalytic process, as was detected in the reuse of the materials relying mostly on the loss of catalytic material. It is a challenge shared with other nano-photocatalysts, both metallic and those based on carbonaceous compounds.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO | anodic aluminum oxide |
| BET | Brunauer–Emmett–Teller |
| B-gCN | boron-doped graphitic carbon nitride |
| BPA | Bisphenol A |
| BZF | bezafibrate |
| CCDC | Cambridge Crystallographic Data Centre |
| CFO | cobalt ferrite |
| C-gCN | carbon-doped graphitic carbon nitride |
| CIP | ciprofloxacin |
| CN-B | untreated gCN |
| CQD | carbon quantum dot |
| CTC | chlortetracycline |
| CTO | cobalt titanate |
| CV | carbon vacancies |
| CVDTLA | Chemical Vapor Deposition Three Letter Acronym |
| DCN | pristine carbon nitride |
| DFT | density functional theory |
| DMF | dimethylformamide |
| DW | distilled water |
| ENR | enrofloxacin |
| EPDOAJ | Emerging Pollutants Directory of Open Access Journals |
| EtOH | ethanol |
| FCN | formaldehyde carbon nitride |
| FTIR | Fourier transform infrared spectroscopy |
| GCC | GCNQDs-CoTiO3/CoFe2O4 |
| gCN | graphitic carbon nitride |
| gCNNF | graphitic carbon nitride nanofiber |
| gCNNR | graphitic carbon nitride nanorod |
| gCNNT | graphitic carbon nitride nanotube |
| gCNNW | graphitic carbon nitride nanowire |
| gCNQD | graphitic carbon nitride quantum dot |
| IPA | isopropyl alcohol |
| MB | methylene blue |
| MCN | melamine-derived gCN |
| MeOH | Methanol |
| MGCN | microwave graphitic carbon nitride |
| MNCA | supramolecular aggregates melamine (MA) and cyanuric acid (CA) |
| MO | methyl orange |
| MUCN | homojunction (type II) between 0D and 1D structures of gCN |
| NDCN | nitrogen-doped carbon nitride |
| NFs | nanofibers |
| N-gCN | Nitrogen-doped graphitic carbon nitride |
| NMP | N-methyl-2-pyrrolidone |
| NOR | norfloxacin |
| NPX | naproxen |
| NR | nanorod |
| NTs | nanotubes |
| NV | nitrogen vacancies |
| NW | nanowire |
| OA | oxamide |
| ODH | oxalyl dihydrazide |
| O-gCN | oxygen-doped graphitic carbon nitride |
| OTC | oxytetracycline |
| PhOH | phenol |
| PL | photoluminescence |
| PPCPs | pharmaceuticals and personal care products |
| PR | phenol red |
| PVDLD | physical vapor deposition linear dichroism |
| QDs | quantum dots |
| RhB | Rhodamine B |
| SGCN | solvothermal graphitic carbon nitride |
| SHP | sodium hypophosphite |
| S-gCN | sulfur-doped graphitic carbon nitride |
| SMX | sulfamethoxazole |
| SPR | surface plasmon resonance |
| TC | tetracycline |
| TGCN | thermal graphitic carbon nitride |
References
- Thomas, S.; Anas, S.; Joy, J. (Eds.) Synthesis, Characterization, and Applications of Graphitic Carbon Nitride; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 9780128230381. [Google Scholar]
- Li, C.; Li, J.; Huang, Y.; Liu, J.; Ma, M.; Liu, K.; Zhao, C.; Wang, Z.; Qu, S.; Zhang, L.; et al. Recent Development in Electronic Structure Tuning of Graphitic Carbon Nitride for Highly Efficient Photocatalysis. J. Semicond. 2022, 43, 021701. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M. Comprehensive Review on Advanced Reusability of G-C3N4 Based Photocatalysts for the Removal of Organic Pollutants. Chemosphere 2022, 297, 134190. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, K.; He, T.; Zhao, Y.; Song, H.; Wang, H. Graphitic Carbon Nitride-Based Photocatalytic Materials: Preparation Strategy and Application. ACS Sustain. Chem. Eng. 2020, 8, 16048–16085. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Lin, K.C. Nanoscale Graphitic Carbon Nitride; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128230343. [Google Scholar]
- Liu, Y.; Mao, B.; Shi, W. Novel Carbon Materials and Composites; Jiang, X., Kang, Z., Guo, X., Zhuang, H., Eds.; Wiley: Hoboken, NJ, USA, 2019; ISBN 9781119313397. [Google Scholar]
- Qi, K.; Liu, S.; Zada, A. Graphitic Carbon Nitride, a Polymer Photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Antonietti, M.; Savateev, A.; Lotsch, B.V. Preface. In Carbon Nitrides; De Gruyter: Berlin, Germany, 2023; pp. V–VI. [Google Scholar]
- Kathiresan, M. Graphitic Carbon Nitrides: Synthesis and Properties. In Nanoscale Graphitic Carbon Nitride; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–16. [Google Scholar]
- Teter, D.M.; Hemley, R.J. Low-Compressibility Carbon Nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Fakhrul Ridhwan Samsudin, M.; Bacho, N.; Sufian, S. Recent Development of Graphitic Carbon Nitride-Based Photocatalyst for Environmental Pollution Remediation. In Nanocatalysts; IntechOpen: London, UK, 2019. [Google Scholar]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent Advances in G-C3N4-Based Materials and Their Application in Energy and Environmental Sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic Carbon Nitride Materials: Variation of Structure and Morphology and Their Use as Metal-Free Catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef]
- Kroke, E.; Schwarz, M.; Horath-Bordon, E.; Kroll, P.; Noll, B.; Norman, A.D. Tri-s-Triazine Derivatives. Part I. From Trichloro-Tri-s-Triazine to Graphitic C3N4 StructuresPart II: Alkalicyamelurates M3[C6N7O3], M = Li, Na, K, Rb, Cs, Manuscript in Preparation. New J. Chem. 2002, 26, 508–512. [Google Scholar] [CrossRef]
- Dong, Q.; Mohamad Latiff, N.; Mazánek, V.; Rosli, N.F.; Chia, H.L.; Sofer, Z.; Pumera, M. Triazine- and Heptazine-Based Carbon Nitrides: Toxicity. ACS Appl. Nano Mater. 2018, 1, 4442–4449. [Google Scholar] [CrossRef]
- Zhu, W.; Song, H.; Lv, Y. Triazine-Based Graphitic Carbon Nitride: Controllable Synthesis and Enhanced Cataluminescent Sensing for Formic Acid. Anal. Bioanal. Chem. 2018, 410, 7499–7509. [Google Scholar] [CrossRef] [PubMed]
- Algara-Siller, G.; Severin, N.; Chong, S.Y.; Björkman, T.; Palgrave, R.G.; Laybourn, A.; Antonietti, M.; Khimyak, Y.Z.; Krasheninnikov, A.V.; Rabe, J.P.; et al. Triazine-Based Graphitic Carbon Nitride: A Two-Dimensional Semiconductor. Angew. Chem. Int. Ed. 2014, 53, 7450–7455. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Zhao, Z.; Liu, J.; Luo, X.; Yuan, X.; Luo, K.; He, J.; Yu, D.; Zhao, Y. From Triazine to Heptazine: Origin of Graphitic Carbon Nitride as a Photocatalyst. ACS Omega 2020, 5, 12557–12567. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.P.; Gu, S.; Sun, J.H.; Xia, F.F.; Chen, G.H. First Principles Investigation of the Electronic Properties of Graphitic Carbon Nitride with Different Building Block and Sheet Staggered Arrangement. J. Alloys Compd. 2018, 735, 131–139. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Y.; Cao, Y.; Zhou, Y. The Favorable Surface Properties of Heptazine Based G-C3N4 (001) in Promoting the Catalytic Performance towards CO2 Conversion. Appl. Surf. Sci. 2019, 481, 604–610. [Google Scholar] [CrossRef]
- Melissen, S.; Le Bahers, T.; Steinmann, S.N.; Sautet, P. Relationship between Carbon Nitride Structure and Exciton Binding Energies: A DFT Perspective. J. Phys. Chem. C 2015, 119, 25188–25196. [Google Scholar] [CrossRef]
- Kaewsud, K.; Vchirawongkwin, V.; Ruangpornvisuti, V. Hydrogen Molecule Adsorption on Heptazine and Triazine Carbon Nitride Nanosheets and Their Selected-Elements (Periods 3 and 4)-Decorated Derivatives as Hydrogen Storage Materials. J. Energy Storage 2024, 84, 110926. [Google Scholar] [CrossRef]
- Tyborski, T.; Merschjann, C.; Orthmann, S.; Yang, F.; Lux-Steiner, M.-C.; Schedel-Niedrig, T. Crystal Structure of Polymeric Carbon Nitride and the Determination of Its Process-Temperature-Induced Modifications. J. Phys. Condens. Matter 2013, 25, 395402. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural Investigation of Graphitic Carbon Nitride via XRD and Neutron Diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Devthade, V.; Kulhari, D.; Umare, S.S. Role of Precursors on Photocatalytic Behavior of Graphitic Carbon Nitride. Mater. Today Proc. 2018, 5, 9203–9210. [Google Scholar] [CrossRef]
- Jiménez-Calvo, P.; Marchal, C.; Cottineau, T.; Caps, V.; Keller, V. Influence of the Gas Atmosphere during the Synthesis of G-C3N4 for Enhanced Photocatalytic H2 Production from Water on Au/g-C3N4 Composites. J. Mater. Chem. A Mater. 2019, 7, 14849–14863. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Díaz-Faes, E.; Barriocanal, C. Relationship between GCN Structure and Photocatalytic Water Splitting Efficiency. Carbon 2022, 187, 462–476. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Fan, J.; Xue, Y.; Chang, H.; Masubuchi, Y.; Yin, S. Synthesis of Graphitic Carbon Nitride from Different Precursors by Fractional Thermal Polymerization Method and Their Visible Light Induced Photocatalytic Activities. J. Alloys Compd. 2018, 735, 1297–1305. [Google Scholar] [CrossRef]
- Alwin, E.; Kočí, K.; Wojcieszak, R.; Zieliński, M.; Edelmannová, M.; Pietrowski, M. Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability. Materials 2020, 13, 2756. [Google Scholar] [CrossRef]
- Yang, W.; Jia, L.; Wu, P.; Zhai, H.; He, J.; Liu, C.; Jiang, W. Effect of Thermal Program on Structure–Activity Relationship of g-C3N4 Prepared by Urea Pyrolysis and Its Application for Controllable Production of g-C3N4. J. Solid State Chem. 2021, 304, 122545. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Li, C. A Comparison of Graphitic Carbon Nitrides Synthesized from Different Precursors through Pyrolysis. J. Photochem. Photobiol. A Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Leherte, L.; Datta, A. Molecular Mechanism for the Self-Supported Synthesis of Graphitic Carbon Nitride from Urea Pyrolysis. J. Phys. Chem. Lett. 2021, 12, 1396–1406. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric Graphitic Carbon Nitride as a Heterogeneous Organocatalyst: From Photochemistry to Multipurpose Catalysis to Sustainable Chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Saman, F.; Se Ling, C.H.; Ayub, A.; Rafeny, N.H.B.; Mahadi, A.H.; Subagyo, R.; Nugraha, R.E.; Prasetyoko, D.; Bahruji, H. Review on Synthesis and Modification of G-C3N4 for Photocatalytic H2 Production. Int. J. Hydrogen Energy 2024, 77, 1090–1116. [Google Scholar] [CrossRef]
- Guru, S.; Rao, G.R. Review—Strategic Design of Layered Double Hydroxides and Graphitic Carbon Nitride Heterostructures for Photoelectrocatalytic Water Splitting Applications. J. Electrochem. Soc. 2022, 169, 046515. [Google Scholar] [CrossRef]
- Kessler, F.K.; Zheng, Y.; Schwarz, D.; Merschjann, C.; Schnick, W.; Wang, X.; Bojdys, M.J. Functional Carbon Nitride Materials—Design Strategies for Electrochemical Devices. Nat. Rev. Mater. 2017, 2, 17030. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Yurdakal, S.; Garlisi, C.; Özcan, L.; Bellardita, M.; Palmisano, G. (Photo)Catalyst Characterization Techniques. In Heterogeneous Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–152. [Google Scholar]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of Functional Group Modifications on the Photocatalytic Performance of G-C3N4. Small 2023, 19, e2300109. [Google Scholar] [CrossRef]
- Liang, X.; Wang, G.; Dong, X.; Wang, G.; Ma, H.; Zhang, X. Graphitic Carbon Nitride with Carbon Vacancies for Photocatalytic Degradation of Bisphenol A. ACS Appl. Nano Mater. 2019, 2, 517–524. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Erakulan, E.S.; Thapa, R.; Ashokkumar, M.; Neppolian, B. Scrutinizing the Role of Tunable Carbon Vacancies in G-C3N4 Nanosheets for Efficient Sonophotocatalytic Degradation of Tetracycline in Diverse Water Matrices: Experimental Study and Theoretical Calculation. Chem. Eng. J. 2023, 452, 139437. [Google Scholar] [CrossRef]
- Molaei, M.J. Graphitic Carbon Nitride (g-C3N4) Synthesis and Heterostructures, Principles, Mechanisms, and Recent Advances: A Critical Review. Int. J. Hydrogen Energy 2023, 48, 32708–32728. [Google Scholar] [CrossRef]
- Hao, X.-Q.; Yang, H.; Jin, Z.-L.; Xu, J.; Min, S.-X.; Lü, G.-X. Quantum Confinement Effect of Graphene-Like C3N4 Nanosheets for Efficient Photocatalytic Hydrogen Production From Water Splitting. Acta Phys. Chim. Sin. 2016, 32, 2581–2592. [Google Scholar] [CrossRef]
- Wu, C.; Han, Q.; Qu, L. Functional Group Defect Design in Polymeric Carbon Nitride for Photocatalytic Application. APL Mater. 2020, 8, 120703. [Google Scholar] [CrossRef]
- Zhu, X.; Duan, C.; Wang, W.; Xin, G.; Song, J. Fabrication of Carboxylated G-C3N4 with Excellent Adsorption and Photocatalytic Properties. Mater. Lett. 2022, 317, 132045. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Li, B.; Sun, H.; Wang, S. Engineered Graphitic Carbon Nitride-Based Photocatalysts for Visible-Light-Driven Water Splitting: A Review. Energy Fuels 2021, 35, 6504–6526. [Google Scholar] [CrossRef]
- Iqbal, O.; Ali, H.; Li, N.; Al-Sulami, A.I.; Alshammari, K.F.; Abd-Rabboh, H.S.M.; Al-Hadeethi, Y.; Din, I.U.; Alharthi, A.I.; Altamimi, R.; et al. A Review on the Synthesis, Properties, and Characterizations of Graphitic Carbon Nitride (g-C3N4) for Energy Conversion and Storage Applications. Mater. Today Phys. 2023, 34, 101080. [Google Scholar] [CrossRef]
- Katsumata, H.; Sakakibara, K.; Tateishi, I.; Furukawa, M.; Kaneco, S. Structurally Modified Graphitic Carbon Nitride with Highly Photocatalytic Activity in the Presence of Visible Light. Catal. Today 2020, 352, 47–53. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Wang, D.; Hong, X.; Liang, B. Recent Advances in Heteroatom Doped Graphitic Carbon Nitride (g-C3N4) and g-C3N4/Metal Oxide Composite Photocatalysts. Curr. Org. Chem. 2020, 24, 673–693. [Google Scholar] [CrossRef]
- Huang, X.; Gu, W.; Ma, Y.; Liu, D.; Ding, N.; Zhou, L.; Lei, J.; Wang, L.; Zhang, J. Recent Advances of Doped Graphite Carbon Nitride for Photocatalytic Reduction of CO2: A Review. Res. Chem. Intermed. 2020, 46, 5133–5164. [Google Scholar] [CrossRef]
- Starukh, H.; Praus, P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts 2020, 10, 1119. [Google Scholar] [CrossRef]
- Blinder, S.M. Density Functional Theory. In Introduction to Quantum Mechanics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–244. [Google Scholar]
- Du, S.; Zhang, F. General Applications of Density Functional Theory in Photocatalysis. Chin. J. Catal. 2024, 61, 1–36. [Google Scholar] [CrossRef]
- Gusarov, S. Advances in Computational Methods for Modeling Photocatalytic Reactions: A Review of Recent Developments. Materials 2024, 17, 2119. [Google Scholar] [CrossRef]
- Masood, H.; Toe, C.Y.; Teoh, W.Y.; Sethu, V.; Amal, R. Machine Learning for Accelerated Discovery of Solar Photocatalysts. ACS Catal. 2019, 9, 11774–11787. [Google Scholar] [CrossRef]
- Silva, A.M.; Rojas, M.I. Electric and Structural Properties of Polymeric Graphite Carbon Nitride (g-C3N4): A Density Functional Theory Study. Comput. Theor. Chem. 2016, 1098, 41–49. [Google Scholar] [CrossRef]
- Wang, J.; Hao, D.; Ye, J.; Umezawa, N. Determination of Crystal Structure of Graphitic Carbon Nitride: Ab Initio Evolutionary Search and Experimental Validation. Chem. Mater. 2017, 29, 2694–2707. [Google Scholar] [CrossRef]
- Negro, P.; Cesano, F.; Casassa, S.; Scarano, D. Combined DFT-D3 Computational and Experimental Studies on g-C3N4: New Insight into Structure, Optical, and Vibrational Properties. Materials 2023, 16, 3644. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Lin, W.; Prezhdo, O.V.; Trivedi, D.J. Ab Initio Quantum Dynamics of Charge Carriers in Graphitic Carbon Nitride Nanosheets. J. Chem. Phys. 2020, 153, 054701. [Google Scholar] [CrossRef]
- Jing, B.; Ao, Z.; Teng, Z.; Wang, C.; Yi, J.; An, T. Density Functional Theory Study on the Effects of Oxygen Groups on Band Gap Tuning of Graphitic Carbon Nitrides for Possible Photocatalytic Applications. Sustain. Mater. Technol. 2018, 16, 12–22. [Google Scholar] [CrossRef]
- Ri, M.-H.; Ri, H.-M.; Ri, U.-S.; Kim, K.-I.; Kim, N.-H.; Sin, Y.-S. Ab Initio Study of Photocatalytic Characteristics of Graphitic Carbon Nitride Assisted by Oxalic Acid. J. Mol. Model. 2021, 27, 258. [Google Scholar] [CrossRef]
- Srinivasu, K.; Modak, B.; Ghosh, S.K. Porous Graphitic Carbon Nitride: A Possible Metal-Free Photocatalyst for Water Splitting. J. Phys. Chem. C 2014, 118, 26479–26484. [Google Scholar] [CrossRef]
- Chen, X.; Hu, R. DFT-Based Study of Single Transition Metal Atom Doped g-C3N4 as Alternative Oxygen Reduction Reaction Catalysts. Int. J. Hydrogen Energy 2019, 44, 15409–15416. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Gu, P.; Ma, R.; Wen, T.; Zhao, G.; Li, L.; Ai, Y.; Hu, C.; Wang, X. Enhanced Photodegradation of Toxic Organic Pollutants Using Dual-Oxygen-Doped Porous g-C3N4: Mechanism Exploration from Both Experimental and DFT Studies. Appl. Catal. B 2019, 248, 1–10. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Le, M.C.; Ha, N.N. Understanding the Influence of Single Metal (Li, Mg, Al, Fe, Ag) Doping on the Electronic and Optical Properties of g-C3N4: A Theoretical Study. Mol. Simul. 2021, 47, 10–17. [Google Scholar] [CrossRef]
- Lu, S.; Li, C.; Li, H.H.; Zhao, Y.F.; Gong, Y.Y.; Niu, L.Y.; Liu, X.J.; Wang, T. The Effects of Nonmetal Dopants on the Electronic, Optical and Chemical Performances of Monolayer g–C3N4 by First-Principles Study. Appl. Surf. Sci. 2017, 392, 966–974. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry; Oxford University Press: Oxford, UK, 2000; ISBN 9780198506980. [Google Scholar]
- Coronado, J.M. Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Green Energy and Technology; Coronado, J.M., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; ISBN 978-1-4471-5060-2. [Google Scholar]
- Naushad, M. (Ed.) A New Generation Material Graphene: Applications in Water Technology; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-75483-3. [Google Scholar]
- Durgalakshmi, D.; Ajay Rakkesh, R.; Rajendran, S.; Naushad, M. Green Photocatalysts; Environmental Chemistry for a Sustainable World; Naushad, M., Rajendran, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 34, ISBN 978-3-030-15607-7. [Google Scholar]
- Majdoub, M.; Sengottuvelu, D.; Nouranian, S.; Al-Ostaz, A. Graphitic Carbon Nitride Quantum Dots (G-C3N4 QDs): From Chemistry to Applications. ChemSusChem 2024, 17, e202301462. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nie, C.; Ao, Z.; Wang, S.; An, T. Recent Progress in G-C3N4 Quantum Dots: Synthesis, Properties and Applications in Photocatalytic Degradation of Organic Pollutants. J. Mater. Chem. A Mater. 2020, 8, 485–502. [Google Scholar] [CrossRef]
- Goren, A.Y.; Recepoglu, Y.K.; Vatanpour, V.; Yoon, Y.; Khataee, A. Insights into Engineered Graphitic Carbon Nitride Quantum Dots for Hazardous Contaminants Degradation in Wastewater. Environ. Res. 2023, 223, 115408. [Google Scholar] [CrossRef]
- Li, Y.; Lv, K.; Ho, W.; Dong, F.; Wu, X.; Xia, Y. Hybridization of Rutile TiO2 (RTiO2) with g-C3N4 Quantum Dots (CN QDs): An Efficient Visible-Light-Driven Z-Scheme Hybridized Photocatalyst. Appl. Catal. B 2017, 202, 611–619. [Google Scholar] [CrossRef]
- Feng, C.; Lu, Z.; Zhang, Y.; Liang, Q.; Zhou, M.; Li, X.; Yao, C.; Li, Z.; Xu, S. A Magnetically Recyclable Dual Z-Scheme GCNQDs-CoTiO3/CoFe2O4 Composite Photocatalyst for Efficient Photocatalytic Degradation of Oxytetracycline. Chem. Eng. J. 2022, 435, 134833. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Y.; Lu, Z.; Liang, Q.; Zhang, Y.; Li, Z.; Xu, S. Nanoflower Ni5P4 Coupled with GCNQDs as Schottky Junction Photocatalyst for the Efficient Degradation of Norfloxacin. Sep. Purif. Technol. 2022, 282, 120107. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Guo, X.; Zhang, W.; Wang, Y.; Zhang, M.; Li, R.; Peng, Z.; Xie, H.; Huang, Y. In-Situ Construction of Morphology-Controllable 0D/1D g-C3N4 Homojunction with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2021, 563, 150317. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Yan, Y.; Wang, W.; Ren, Y.; Li, X. Synthesis of Highly Porous G-C3N4 Nanotubes for Efficient Photocatalytic Degradation of Sulfamethoxazole. Mater. Today Commun. 2021, 27, 102288. [Google Scholar] [CrossRef]
- Xie, M.; Wei, W.; Jiang, Z.; Xu, Y.; Xie, J. Carbon Nitride Nanowires/Nanofibers: A Novel Template-Free Synthesis from a Cyanuric Chloride–Melamine Precursor towards Enhanced Adsorption and Visible-Light Photocatalytic Performance. Ceram. Int. 2016, 42, 4158–4170. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Aziz, M.; Youssef, T.E.; Alomair, N.A. Microwave Synthesized G-C3N4 Nanofibers with Modified Properties for Enhanced Solar Light Photocatalytic Performance. Inorg. Chem. Commun. 2024, 168, 112975. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, G.; Dang, M.; Dong, S.; Liu, Y.; Liu, T.; Ren, H.; Xia, A.; Lv, L. Study on Surface Modification of G-C3N4 Photocatalyst. J. Alloys Compd. 2022, 908, 164507. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Bao, S.-J.; Lu, S.; Xu, M.; Long, D.; Pu, S. Tuning and Thermal Exfoliation Graphene-like Carbon Nitride Nanosheets for Superior Photocatalytic Activity. Ceram. Int. 2016, 42, 18521–18528. [Google Scholar] [CrossRef]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile Synthesis of Exfoliated Graphitic Carbon Nitride for Photocatalytic Degradation of Ciprofloxacin Under Solar Irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Fan, F.; Li, G.; Duan, J.; Li, Y.; Jiang, G.; Yao, W. Enhanced Full-Spectrum Photocatalytic Activity of 3D Carbon-Coated C3N4 Nanowires via Giant Interfacial Electric Field. Appl. Catal. B 2022, 318, 121829. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Tian, T.; Liu, Y.; Chen, Y.; Xu, G.; Gu, L.; Li, H.; Yuan, Y. Chitosan-Assisted Synthesis of 1D g-C3N4 Nanorods for Enhanced Photocatalysis. Chem. Commun. 2023, 59, 10528–10531. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, D.; Wang, F.; Liu, H.; Chen, M. Enhanced Photocatalytic Performance of S/Cd Co-Doped g-C3N4 Nanorods for Degradation of Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130079. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Chen, H.; Hu, X.; Yang, P. Formation of G-C3N4 Nanotubes towards Superior Photocatalysis Performance. ChemCatChem 2019, 11, 4558–4567. [Google Scholar] [CrossRef]
- Joy, J.; Anas, S.; Thomas, S. Graphitic Carbon Nitride: An Uprising Carbonaceous Material. In Synthesis, Characterization, and Applications of Graphitic Carbon Nitride; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–14. [Google Scholar]
- Torres-Pinto, A.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. The Effect of Precursor Selection on the Microwave-Assisted Synthesis of Graphitic Carbon Nitride. Catal. Today 2023, 424, 113868. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs Thermal Exfoliation of G-C3N4 for NOx Removal under Visible Light Irradiation. Appl. Catal. B 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Zhao, J.; Bao, Y.; Hou, L. Exfoliation Method Matters: The Microstructure-Dependent Photoactivity of g-C3N4 Nanosheets for Water Purification. J. Hazard. Mater. 2022, 424, 127424. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. G-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2021, 12, 121. [Google Scholar] [CrossRef]
- Qian, X.; Meng, X.; Sun, J.; Jiang, L.; Wang, Y.; Zhang, J.; Hu, X.; Shalom, M.; Zhu, J. Salt-Assisted Synthesis of 3D Porous g-C3N4 as a Bifunctional Photo- and Electrocatalyst. ACS Appl. Mater. Interfaces 2019, 11, 27226–27232. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Meng, R.; Chen, M. Porous Graphitic Carbon Nitride Synthesized via Using Carbon Nanotube as a Novel Recyclable Hard Template for Efficient Visible Light Photocatalytic Organic Pollutant Degradation. ChemistrySelect 2019, 4, 6123–6129. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, C.; Zhang, L.; Hou, Y.; Wang, S.; Dong, P.; Lin, F.; Wang, Y. Facile Two-Step Synthesis of Porous Carbon Nitride with Enhanced Photocatalytic Activity Using a Soft Template. ACS Sustain. Chem. Eng. 2019, 7, 3866–3874. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, F.; Khaing, A.; Yang, D.; Jiang, Z. Acetic Acid-Assisted Supramolecular Assembly Synthesis of Porous g-C3N4 Hexagonal Prism with Excellent Photocatalytic Activity. Appl. Surf. Sci. 2019, 479, 757–764. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, B.; Wang, F.; Wang, S.; Liu, X.; Ao, Z.; Li, C. Mechanism Insight into Enhanced Photodegradation of Pharmaceuticals and Personal Care Products in Natural Water Matrix over Crystalline Graphitic Carbon Nitrides. Water Res. 2020, 180, 115925. [Google Scholar] [CrossRef]
- Dou, M.; Wang, J.; Gao, B.; Xu, C.; Yang, F. Photocatalytic Difference of Amoxicillin and Cefotaxime under Visible Light by Mesoporous G-C3N4: Mechanism, Degradation Pathway and DFT Calculation. Chem. Eng. J. 2020, 383, 123134. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, J.; Wang, Y.; Zhang, Y.; Zhou, Y.; Zhuo, S. Construction of Three-Dimensional Mesoporous Carbon Nitride with High Surface Area for Efficient Visible-Light-Driven Hydrogen Evolution. J. Colloid Interface Sci. 2020, 561, 601–608. [Google Scholar] [CrossRef]
- Liu, J.; Fu, W.; Liao, Y.; Fan, J.; Xiang, Q. Recent Advances in Crystalline Carbon Nitride for Photocatalysis. J. Mater. Sci. Technol. 2021, 91, 224–240. [Google Scholar] [CrossRef]
- Li, Z.; Ma, L.; Yu, M.; Chang, S.; Huang, Z.; Cheng, Z.; Li, Y.; Carabineiro, S.A.C.; Lv, K. Crystalline Graphitic Carbon Nitride in Photocatalysis. Surf. Interfaces 2024, 51, 104492. [Google Scholar] [CrossRef]
- Xu, T.; Hur, J.; Niu, P.; Wang, S.; Lee, S.; Chun, S.-E.; Li, L. Synthesis of Crystalline G-C3N4 with Rock/Molten Salts for Efficient Photocatalysis and Piezocatalysis. Green Energy Environ. 2024, 9, 890–898. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Pu, Y.; Sun, Z.; Huang, L. Progress of Crystalline Carbon Nitride in Synthesis, Atomic Structure Characterization and Photocatalysis. Nano Res. 2025, 18, 94907047. [Google Scholar] [CrossRef]
- Pu, W.; Zhou, Y.; Yang, L.; Gong, H.; Li, Y.; Yang, Q.; Zhang, D. High-Efficiency Crystalline Carbon Nitride Photocatalysts: Status and Perspectives. Nano Res. 2024, 17, 7840–7863. [Google Scholar] [CrossRef]
- Zhai, B.; Li, H.; Gao, G.; Wang, Y.; Niu, P.; Wang, S.; Li, L. A Crystalline Carbon Nitride Based Near-Infrared Active Photocatalyst. Adv. Funct. Mater. 2022, 32, 2207375. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Y.; Cen, W.; Luo, X. Functional Group Effects on the HOMO–LUMO Gap of g-C3N4. Nanomaterials 2018, 8, 589. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Li, Z.; Wu, C.; Zhao, H.; Zhong, N.; Wang, J.; Wang, H.; Yan, Y.; et al. Cyano-Rich G-C3N4 in Photochemistry: Design, Applications, and Prospects. Small 2024, 20, e2304404. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Fan, J.; Xiang, Q. Design and Application of Active Sites in G-C3N4-Based Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 69–88. [Google Scholar] [CrossRef]
- Shiravand, G.; Badiei, A.; Mohammadi Ziarani, G. Carboxyl-Rich g-C3N4 Nanoparticles: Synthesis, Characterization and Their Application for Selective Fluorescence Sensing of Hg2+ and Fe3+ in Aqueous Media. Sens. Actuators B Chem. 2017, 242, 244–252. [Google Scholar] [CrossRef]
- Teng, Z.; Yang, N.; Lv, H.; Wang, S.; Hu, M.; Wang, C.; Wang, D.; Wang, G. Edge-Functionalized g-C3N4 Nanosheets as a Highly Efficient Metal-Free Photocatalyst for Safe Drinking Water. Chem 2019, 5, 664–680. [Google Scholar] [CrossRef]
- Chuang, P.-K.; Wu, K.-H.; Yeh, T.-F.; Teng, H. Extending the π-Conjugation of g-C3N4 by Incorporating Aromatic Carbon for Photocatalytic H2 Evolution from Aqueous Solution. ACS Sustain. Chem. Eng. 2016, 4, 5989–5997. [Google Scholar] [CrossRef]
- Razavi-Esfali, M.; Mahvelati-Shamsabadi, T.; Fattahimoghaddam, H.; Lee, B.-K. Highly Efficient Photocatalytic Degradation of Organic Pollutants by Mesoporous Graphitic Carbon Nitride Bonded with Cyano Groups. Chem. Eng. J. 2021, 419, 129503. [Google Scholar] [CrossRef]
- Xu, R.; Li, J.; Sui, G.; Zhuang, Y.; Guo, D.; Luo, Z.; Liang, S.; Yao, H.; Wang, C.; Chen, S. Constructing Supramolecular Self-Assembled Porous g-C3N4 Nanosheets Containing Thiophene-Groups for Excellent Photocatalytic Performance Under Visible Light. Appl. Surf. Sci. 2022, 578, 152064. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Jeong, Y.J.; Cho, I.S.; Park, S.-J.; Lee, C.-G.; Alvarez, P.J.J. Facile Synthesis of N Vacancy G-C3N4 Using Mg-Induced Defect on the Amine Groups for Enhanced Photocatalytic •OH Generation. J. Hazard. Mater. 2023, 449, 131046. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, L.; Wang, P.; Guo, Y.; Shi, Z.; Guo, X.; Zhang, L. Synthesis of Nitrogen Vacancies G-C3N4 with Increased Crystallinity under the Controlling of Oxalyl Dihydrazide: Visible-Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2020, 505, 144576. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Qayum, A.; Zhao, X.; Xia, H.; Lu, F.; Hu, L. Enhanced Photocatalytic Degradation of 4-Chlorophenol under Visible Light over Carbon Nitride Nanosheets with Carbon Vacancies. Nanotechnology 2021, 32, 415704. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Huang, Y.; Ma, M.; Liu, K.; Dou, X.; Wang, Z.; Qu, S.; Wang, Z. Engineering the Photocatalytic Behaviors of G-C3N4-Based Metal-Free Materials for Degradation of a Representative Antibiotic. Adv. Funct. Mater. 2020, 30, 2002353. [Google Scholar] [CrossRef]
- Long, X.; Feng, C.; Yang, S.; Ding, D.; Feng, J.; Liu, M.; Chen, Y.; Tan, J.; Peng, X.; Shi, J.; et al. Oxygen Doped Graphitic Carbon Nitride with Regulatable Local Electron Density and Band Structure for Improved Photocatalytic Degradation of Bisphenol A. Chem. Eng. J. 2022, 435, 134835. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand New P-Doped g-C3N4: Enhanced Photocatalytic Activity for H2 Evolution and Rhodamine B Degradation under Visible Light. J. Mater. Chem. A Mater. 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Lin, Q.; Chen, Y.; Liao, X.; Yu, H.; Yu, C. Three-Dimensional P-Doped Porous g-C3N4 Nanosheets as an Efficient Metal-Free Photocatalyst for Visible-Light Photocatalytic Degradation of Rhodamine B Model Pollutant. J. Taiwan Inst. Chem. Eng. 2020, 114, 249–262. [Google Scholar] [CrossRef]
- Guan, K.; Li, J.; Lei, W.; Wang, H.; Tong, Z.; Jia, Q.; Zhang, H.; Zhang, S. Synthesis of Sulfur Doped G-C3N4 with Enhanced Photocatalytic Activity in Molten Salt. J. Mater. 2021, 7, 1131–1142. [Google Scholar] [CrossRef]
- Dou, Y.; Shen, X.; Zou, J.; Shi, R.; Yan, T.; Sun, Q.; Wang, L. Green Synthesis of Sulfur-doped G-C3N4 Nanosheets for Enhanced Removal of Oxytetracycline under Visible-light Irradiation and Reduction of Its N-Nitrosodimethylamine Formation Potential. J. Chem. Technol. Biotechnol. 2021, 96, 1580–1592. [Google Scholar] [CrossRef]
- Lei, L.; Wang, W.; Wang, C.; Zhang, M.; Zhong, Q.; Fan, H. In Situ Growth of Boron Doped G-C3N4 on Carbon Fiber Cloth as a Recycled Flexible Film-Photocatalyst. Ceram. Int. 2021, 47, 1258–1267. [Google Scholar] [CrossRef]
- Zou, J.; Yu, Y.; Yan, W.; Meng, J.; Zhang, S.; Wang, J. A Facile Route to Synthesize Boron-Doped g-C3N4 Nanosheets with Enhanced Visible-Light Photocatalytic Activity. J. Mater. Sci. 2019, 54, 6867–6881. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Shu, K.; Shi, W.; Lu, C. Facile Bottom-up Preparation of Cl-Doped Porous g-C3N4 Nanosheets for Enhanced Photocatalytic Degradation of Tetracycline under Visible Light. Sep. Purif. Technol. 2019, 228, 115770. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, D.K.; Selvaraj, R.; Kim, Y. Facile Synthesis of Br-Doped g-C3N4 Nanosheets via One-Step Exfoliation Using Ammonium Bromide for Photodegradation of Oxytetracycline Antibiotics. J. Ind. Eng. Chem. 2019, 79, 473–481. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. One-step Simple Green Method to Prepare Carbon-doped Graphitic Carbon Nitride Nanosheets for Boosting Visible-light Photocatalytic Degradation of Tetracycline. J. Chem. Technol. Biotechnol. 2021, 96, 3122–3133. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Hu, C.; Li, X.; Zhang, P.; Chen, Z. Porous Carbon-Doped g-C3N4 with Tunable Band Structure for Boosting Photocatalytic H2O2 Production with Simultaneous Pollutants Removal. J. Environ. Chem. Eng. 2022, 10, 107116. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen Self-Doped g-C3N4 Nanosheets with Tunable Band Structures for Enhanced Photocatalytic Tetracycline Degradation. J. Colloid. Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Nitrogen Doped G-C3N4 with the Extremely Narrow Band Gap for Excellent Photocatalytic Activities under Visible Light. Appl. Catal. B 2021, 281, 119474. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Li, R.; Zhang, Q.; Chen, T.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. An Efficient Metal-Free Phosphorus and Oxygen Co-Doped g-C3N4 Photocatalyst with Enhanced Visible Light Photocatalytic Activity for the Degradation of Fluoroquinolone Antibiotics. Chem. Eng. J. 2019, 374, 242–253. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Chen, X.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and Sulfur-Codoped g-C3N4: Facile Preparation, Mechanism Insight, and Application as Efficient Photocatalyst for Tetracycline and Methyl Orange Degradation under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Lei, L.; He, X.; Lin, X.; Zhao, Y.; Yang, C.; Cui, L.; Wu, G. Preparation of Carbon Self-Doped g-C3N4 for Efficient Degradation of Bisphenol A under Visible Light Irradiation. Environ. Sci. Pollut. Res. 2023, 30, 65328–65337. [Google Scholar] [CrossRef]
- Yan, W.; Yan, L.; Jing, C. Impact of Doped Metals on Urea-Derived g-C3N4 for Photocatalytic Degradation of Antibiotics: Structure, Photoactivity and Degradation Mechanisms. Appl. Catal. B 2019, 244, 475–485. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Liu, Z.; Zhu, Z.; Tang, X.; Wang, Y. Study on Optical Properties of Alkali Metal Doped G-C3N4 and Their Photocatalytic Activity for Reduction of CO2. Chem. Phys. Lett. 2020, 751, 137467. [Google Scholar] [CrossRef]
- Yu, X.; Song, Z.; Dong, X.; Li, H.; Liu, H.; Zhao, B.; Ye, T.; Jiang, Y.; Li, X.; Duan, L.; et al. Enhanced Photocatalytic Activity of Rare Earth (Yb, Nd and Ce)-Doped g-C3N4 Nanosheets for the Degradation of Organic Dyes Under Visible Light. J. Mater. Sci. Mater. Electron. 2022, 33, 13271–13289. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, F.; Wen, K.; Lu, J. Enhanced Photocatalytic Activity for Tetracyclines Degradation with Ag Modified G-C3N4 Composite under Visible Light. J. Photochem. Photobiol. A Chem. 2020, 389, 112217. [Google Scholar] [CrossRef]
- Yin, Z.; Tian, Y.; Gao, P.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Photodegradation Mechanism and Genetic Toxicity of Bezafibrate by Pd/g-C3N4 Catalysts under Simulated Solar Light Irradiation: The Role of Active Species. Chem. Eng. J. 2020, 379, 122294. [Google Scholar] [CrossRef]
- Nguyen Van, M.; Mai, O.; Pham Do, C.; Lam Thi, H.; Pham Manh, C.; Nguyen Manh, H.; Pham Thi, D.; Do Danh, B. Fe-Doped g-C3N4: High-Performance Photocatalysts in Rhodamine B Decomposition. Polymers 2020, 12, 1963. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Mao, J.N.; Peng, X.L.; Hong, B.; Xu, J.C.; Zeng, Y.X.; Han, Y.B.; Ge, H.L.; Wang, X.Q. Magnetically Separable Ni/g-C3N4 Nanocomposites for Enhanced Visible-Light Photocatalytic Degradation of Methylene Blue and Ciprofloxacin. Diam. Relat. Mater. 2022, 126, 109070. [Google Scholar] [CrossRef]
- Bao, J.; Bai, W.; Wu, M.; Gong, W.; Yu, Y.; Zheng, K.; Liu, L. Template-Mediated Copper Doped Porous g-C3N4 for Efficient Photodegradation of Antibiotic Contaminants. Chemosphere 2022, 293, 133607. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Khan, A.; Al-Assiri, M.S. Au Nanoparticles-Doped g-C3N4 Nanocomposites for Enhanced Photocatalytic Performance under Visible Light Illumination. Ceram. Int. 2020, 46, 22090–22101. [Google Scholar] [CrossRef]
- ISO 10678; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO 22197; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 10676; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Water Purification Performance of Semiconducting Photocatalytic Materials by Measurement of Forming Ability of Active Oxygen. International Organization for Standardization: Geneva, Switzerland, 2010.
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the Current ISO Tests for Photocatalytic Materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]




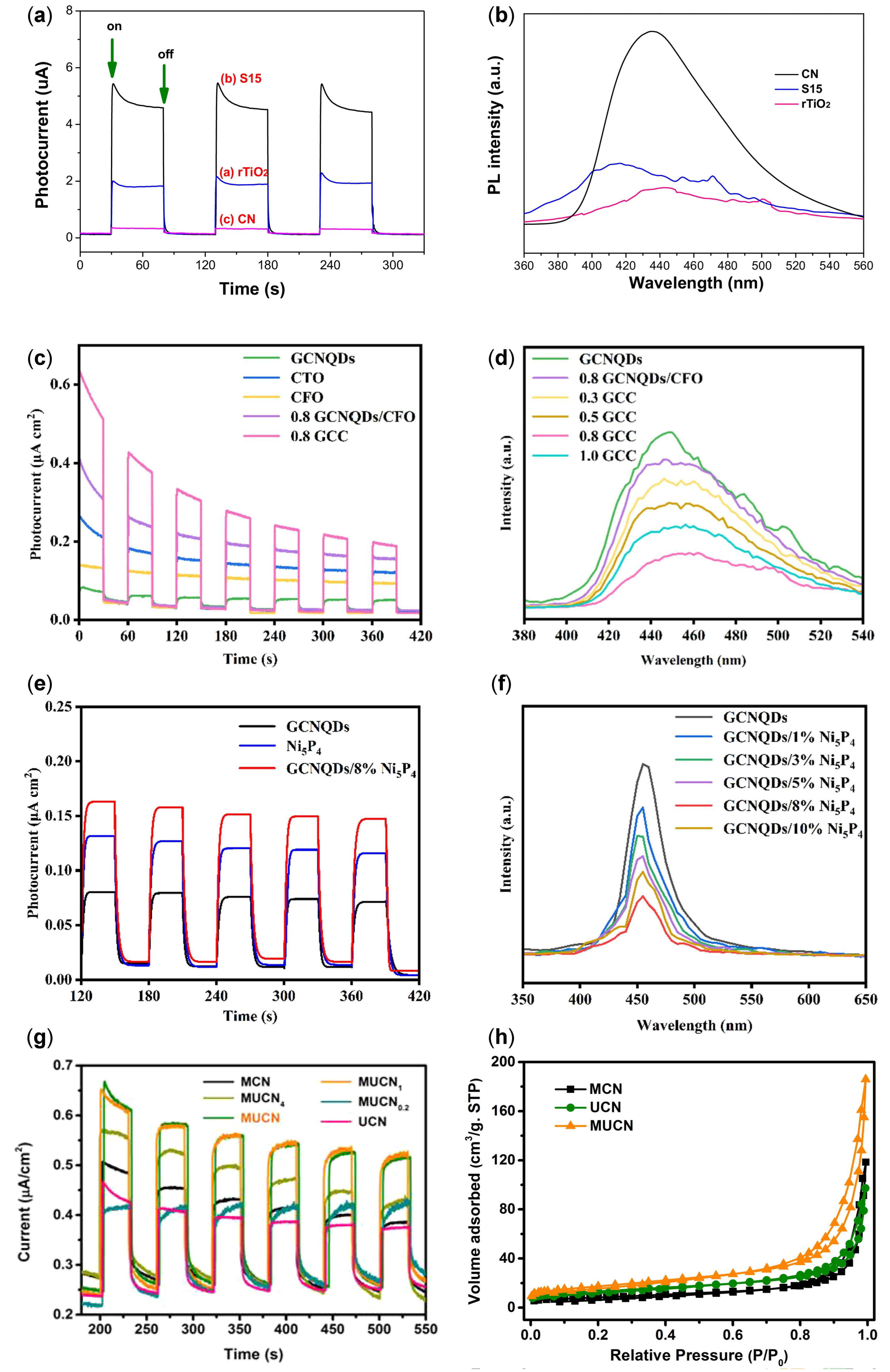
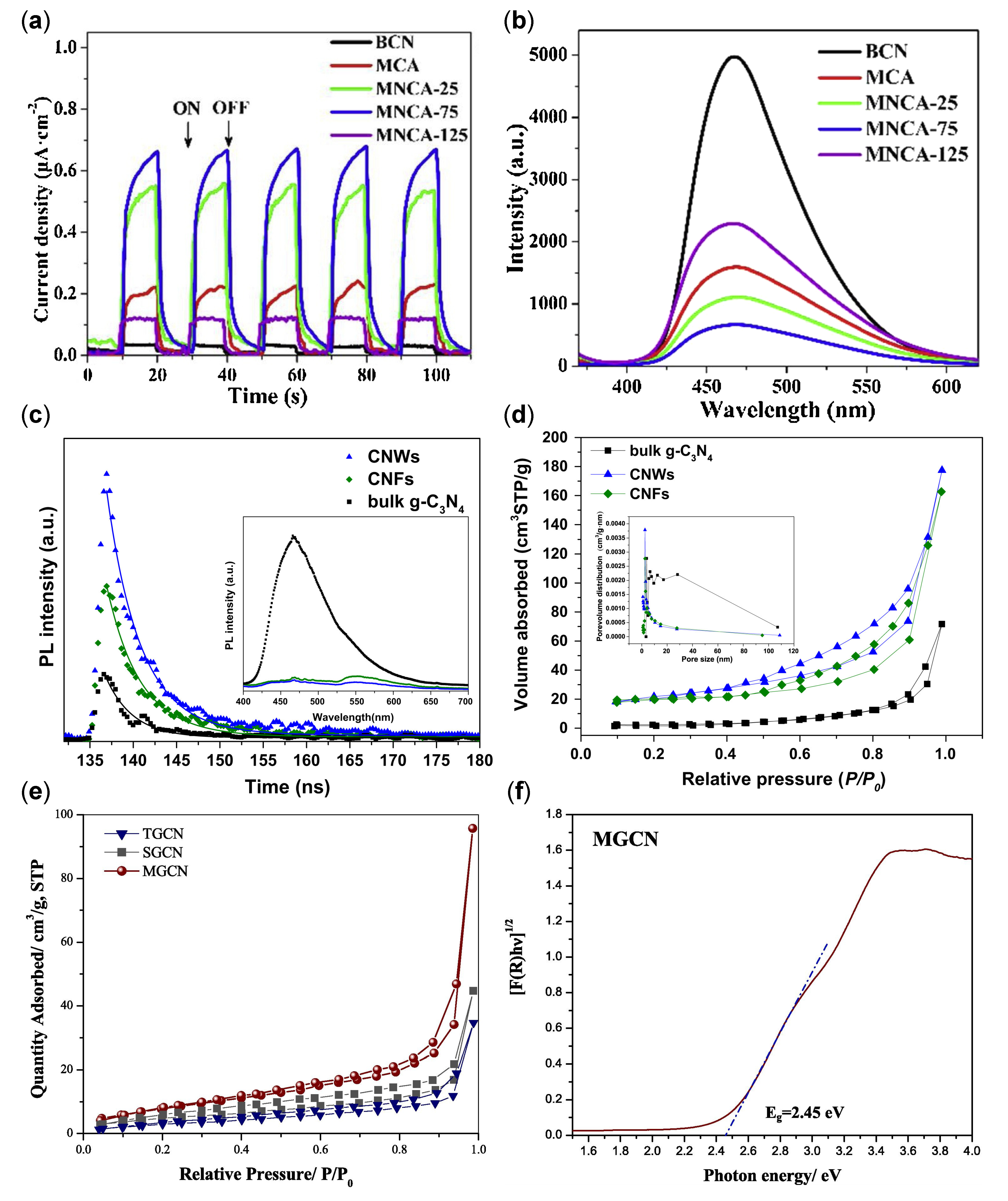



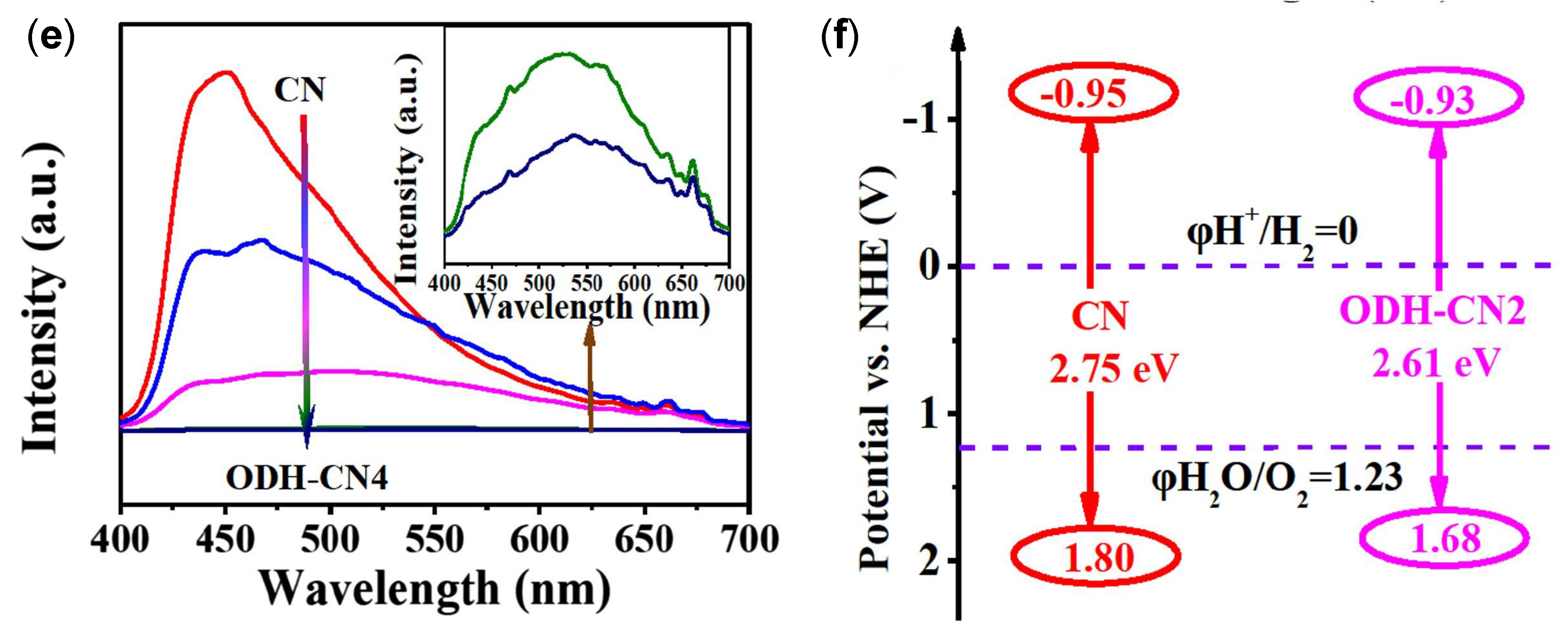
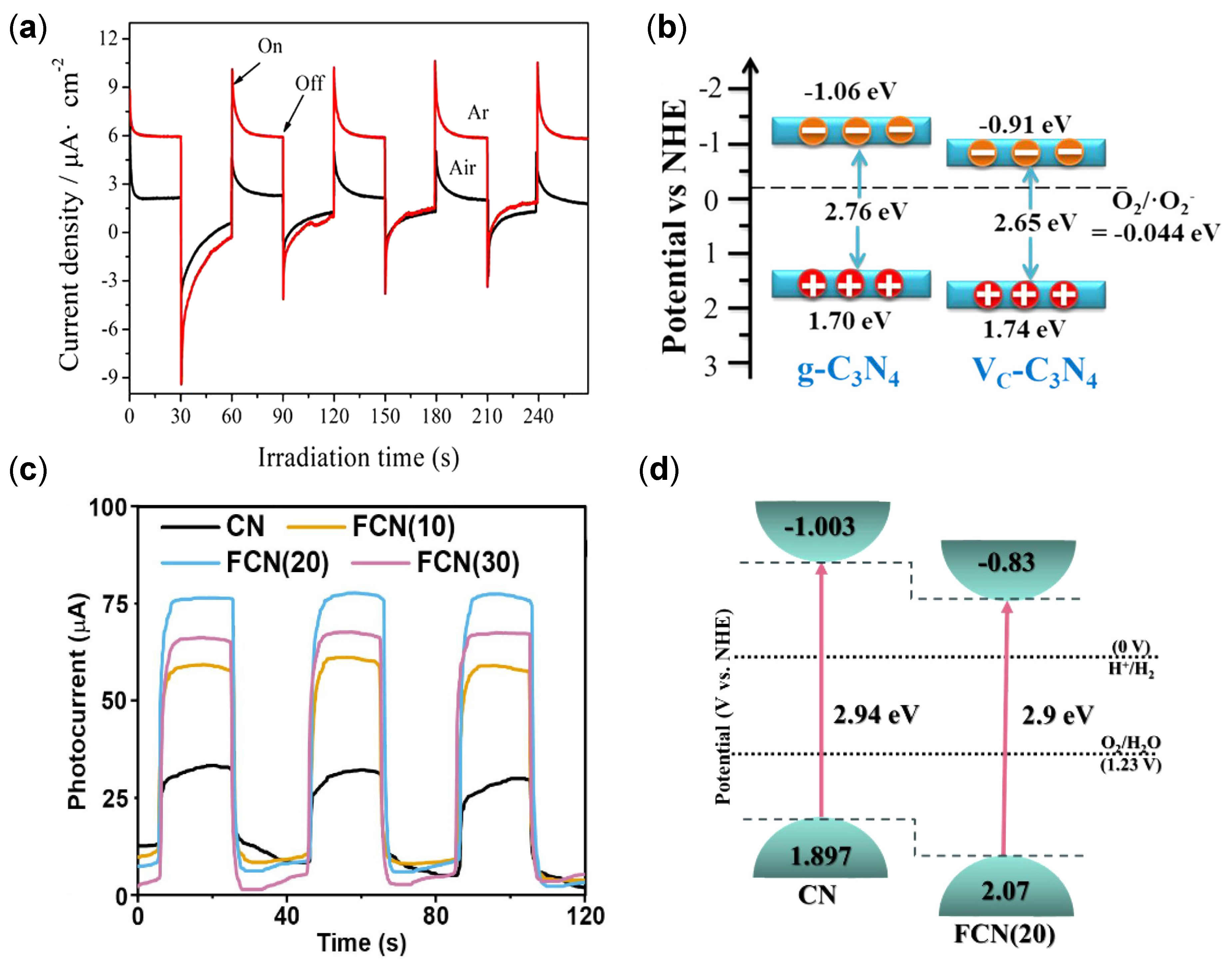
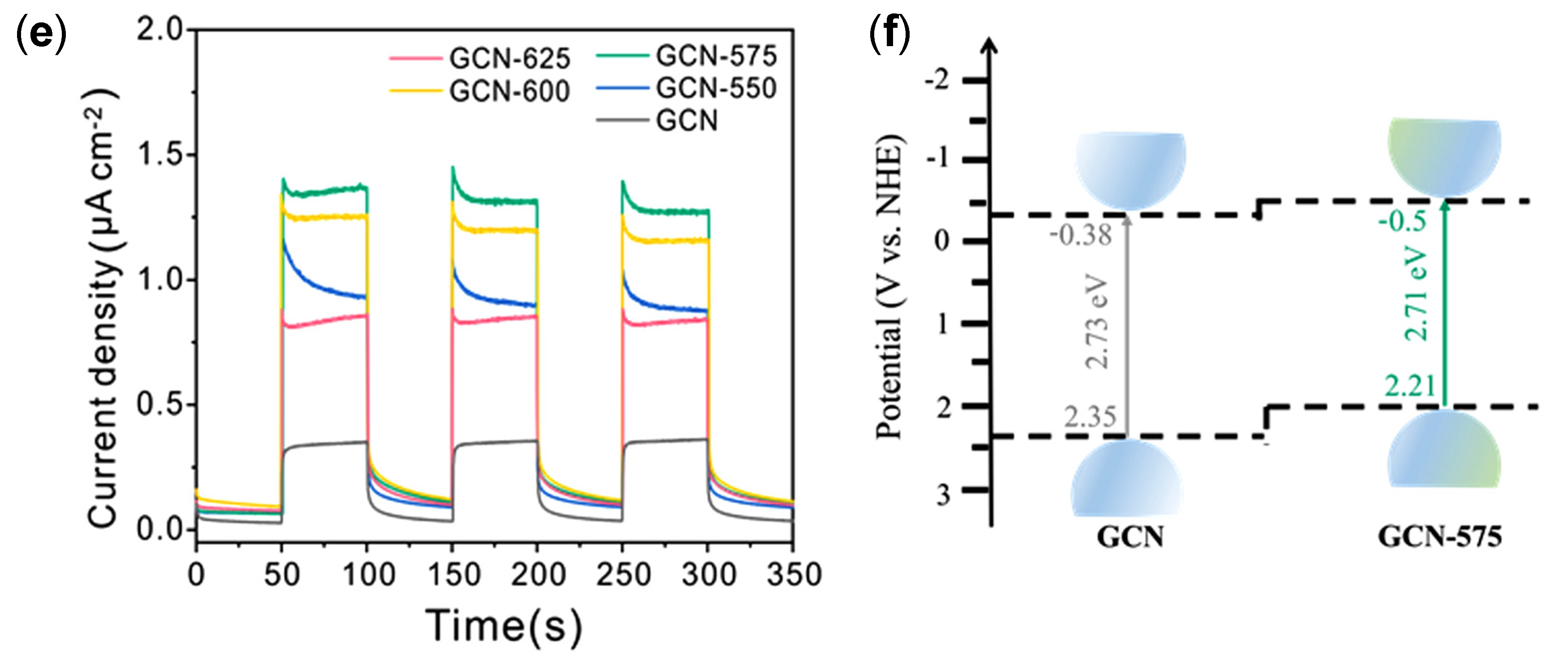
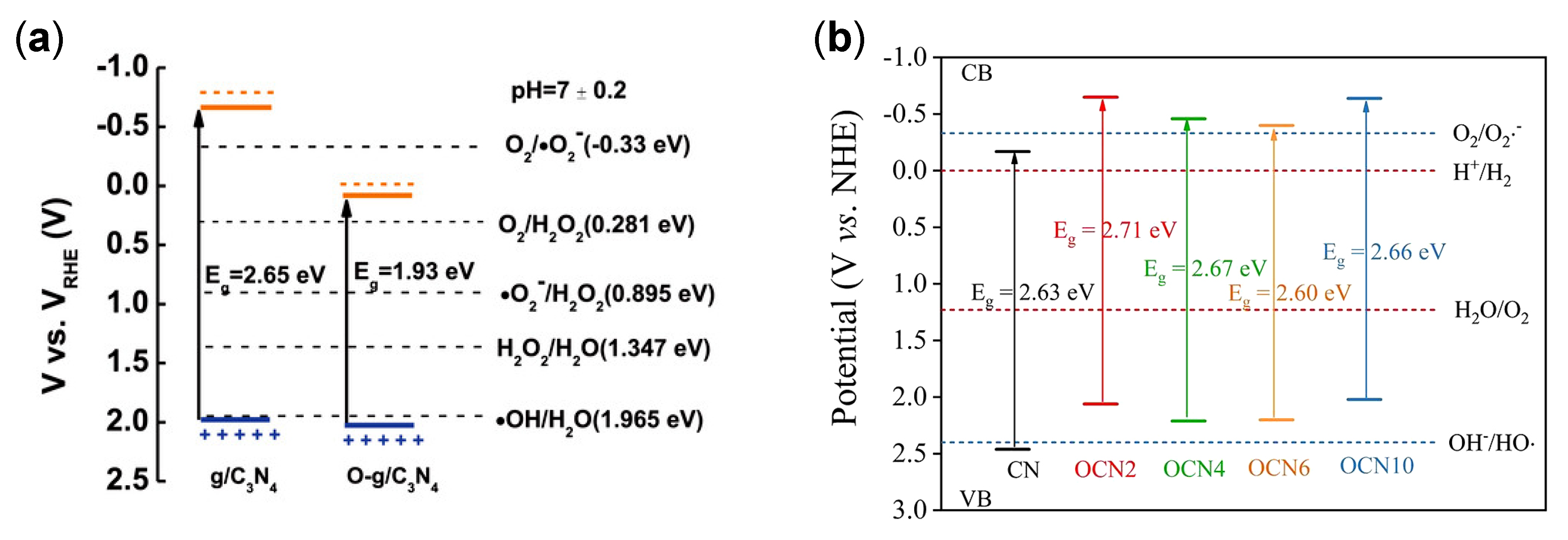

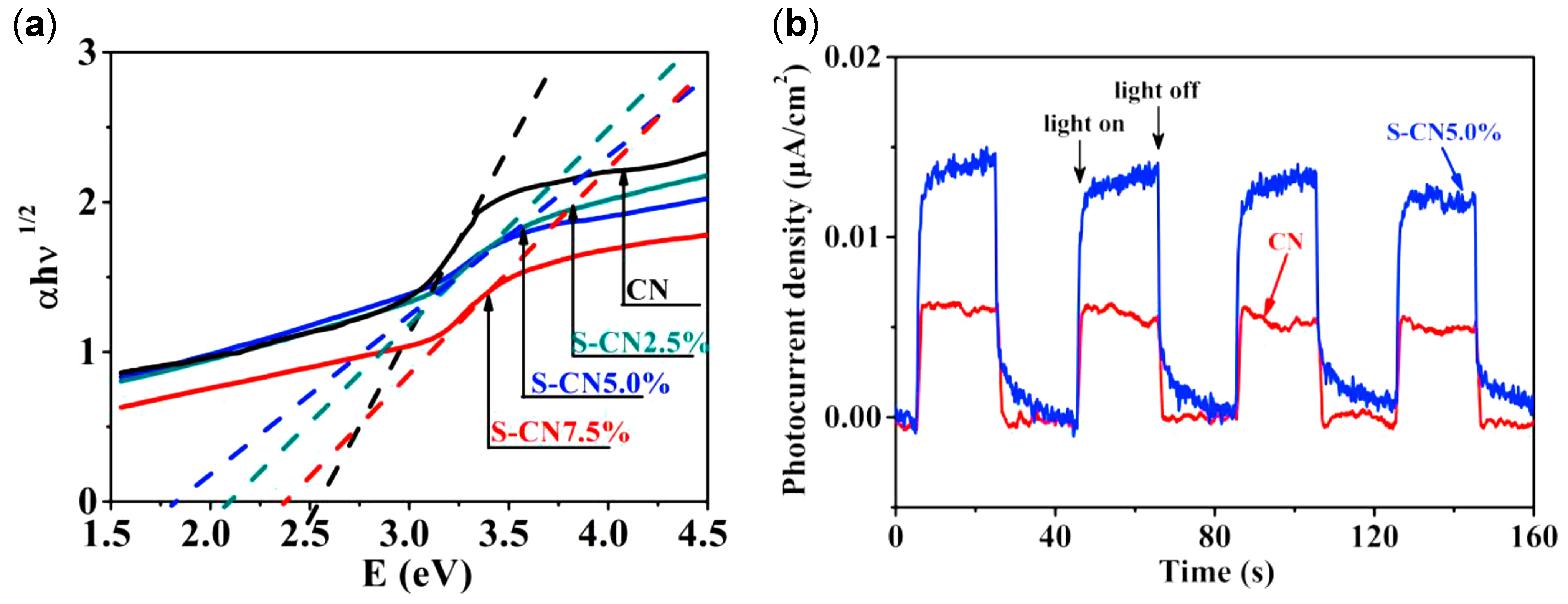
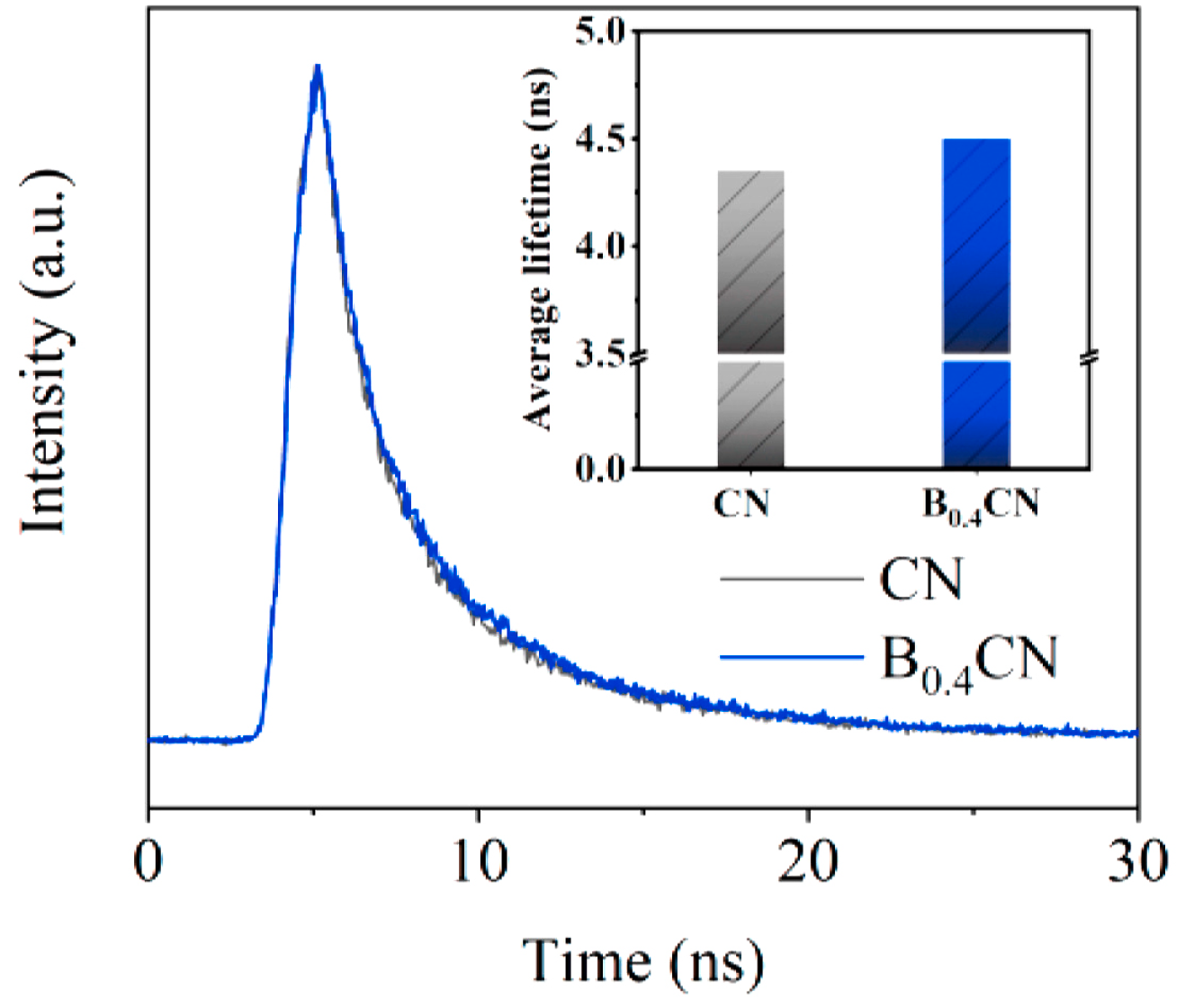
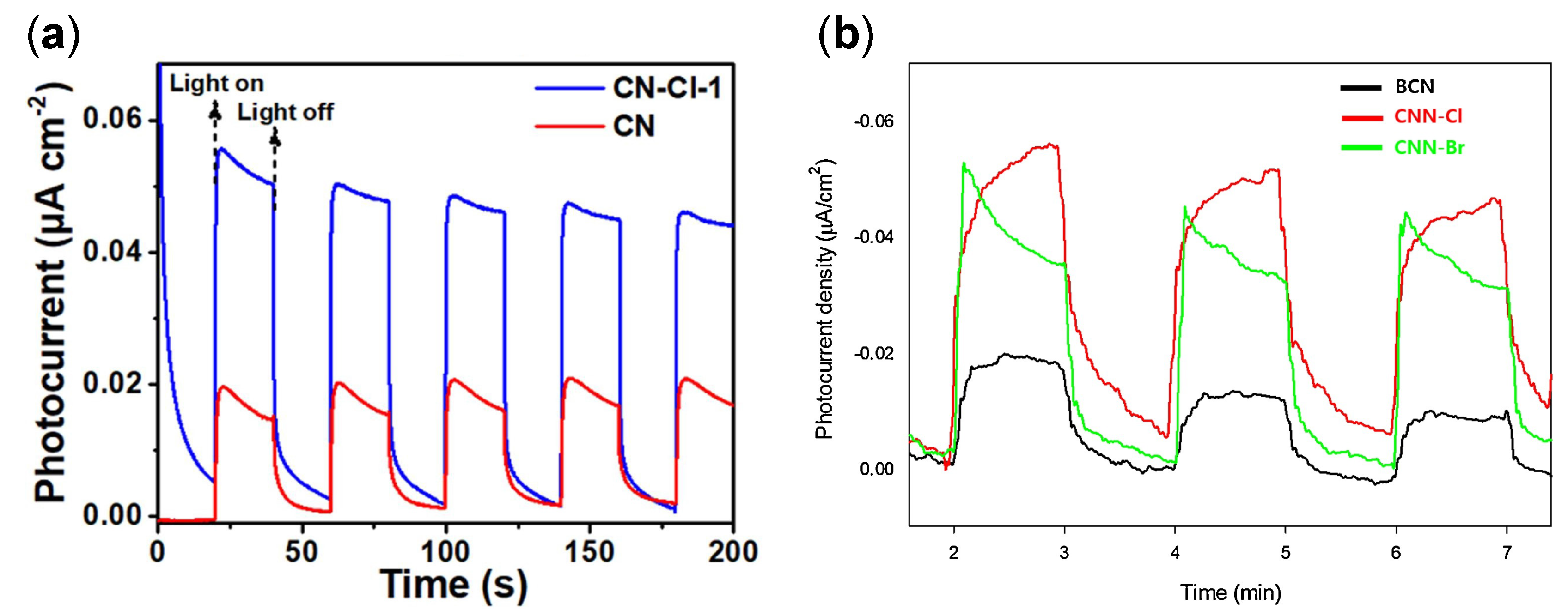
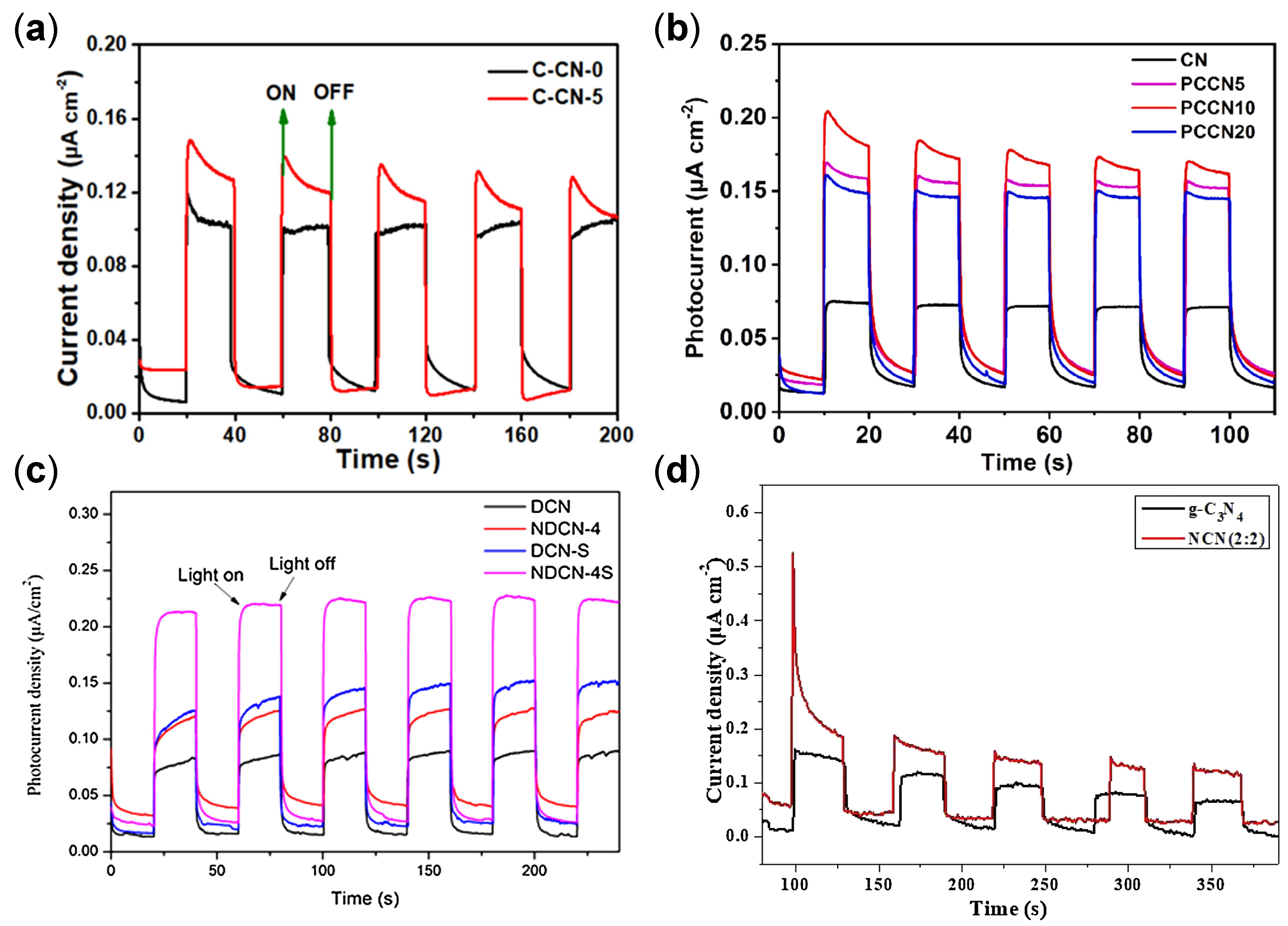
| Structure | Photocatalyst | Advantages | Refs. | Drawbacks | Refs. |
|---|---|---|---|---|---|
| 0D, 1D, 2D and porous 3D | 0D: gCNQDs; 1D: gCNNW, gCNNRs, gCNNF, or gCNNTs; 2D: nanosheets; or porous 3D | Favor the migration of charge carriers. Enlarge the active surface. Improve visible light absorption (3D porous). 0D, 1D, and 2D solubility in water. Non-toxic. | [4,6,13] | Quantum confinement effect by size reduction in 0D, 1D, and 2D structures. Gap broadening. | [6,13,14,45,46] |
| Functional groups | gCN with amino, imino, cyano, ureido, hydroxyl, carboxyl, and aromatic groups. | Amino and imino groups improve the anchoring of metal oxides during their support, as well as their dispersion on the surface. Improved visible light absorption. Improved separation of charges. Increased specific surface area and active sites number (-OH) may act as h+ trapping centers enhancing charge separation. | [4,41,42,47,48] | Cyano, ureido, carboxyl and aromatic groups often require toxic reagents that can cause contamination. Mobility of photogenerated charges restricted by the presence of these groups. | [1,42,49,50] |
| Vacancies | Cv-gCN | Serve as a reservoir for photogenerated electrons. Inhibit the recombination of h+ and e−. Serve as electron transfer centers for the adsorbed molecular oxygen favoring the production of superoxide radicals. Reduce the effective band gap. | [43,44] | Mobility of photogenerated loads is restricted by the presence of these vacancies. | [50] |
| Nv-gCN | Improve the separation of charges. Increase the lifetime of excitons. Reduce the effective band gap. | [4,6,50,51] | |||
| Doping | Non-metallic doping | Introduction of intermediate energy levels in the gap. Reduction in effective gap. Increased exciton lifetime. Corrects for the increased gap due to the quantum confinement effect. | [1,2,13,40] | Non-metallic species do not participate in the transport of charges; they act as exciton recombination centers. | [12] |
| Self-doping (C-gCN and N-gCN) | Introduction of intermediate energy levels in the gap; reduction in effective gap. Increased exciton lifetime. Corrects the increased gap due to quantum confinement effect. C-doping increases the number of delocalized π-bonds, improving conductivity, charge transfer, and their separation. Significant gap reduction. | [52,53,54] | |||
| Metal doping | Introduction of intermediate energy levels in the gap; reduction in effective gap (especially with alkalis). Increased exciton lifetime. Correction for the increased gap due to the quantum confinement effect. With noble metals, the effect of surface plasmon resonance (SPR) is introduced; the excitation of electrons to the conduction band is enhanced (greater number). | [1,13] | Causes secondary contamination due to leaching of metal ions. Excess metal ions act as exciton recombination centers. Serious pollution is associated with the mining of metals and their refining. | [12] |
| Morphology | Photocatalyst | Area (m2·g−1) | Gap (eV) | Pollutant (ppm) | Radiation | Efficiency (%) | Time (min) | k (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 0D | gCN (1000 ppm) | 10.9 | - | RhB: 5 | 500 W; Xe lamp (Vis.) | 35 | 240 | 0.002 | [77] |
| rTiO2/gCNQD 15% (1000 ppm) | 43.9 | - | 95 | 0.012 | |||||
| gCN (600 ppm) | - | - | OTC: 40 | 500 W; Xe lamp (UV) | 20 | 150 | 0.0017 | [78] | |
| gCNQDs-CoTiO3/CoFe2O4 (600 ppm) | - | - | 88 | 0.0141 | |||||
| gCNQDs (600 ppm) | 25.34 | - | NOR: 30 | 1000 W; Hg lamp (UV) | 35 | 120 | 0.0035 | [79] | |
| gCNQDs/Ni5P4 8% (600 ppm) | 83.61 | - | 92 | 0.022 | |||||
| 1D MCN (200 ppm) | 29.07 | - | RhB: 10 | 300 W; Xe lamp (Vis.) | 99.5 | 30 | 0.12 | [80] | |
| 0D UCN (200 ppm) | 44.27 | - | 70 | 30 | 0.036 | ||||
| 0D/1D MUCN (200 ppm) | 57.24 | - | 99.96 | 20 | 0.26 | ||||
| 1D | gCN (400 ppm) | 9.9 | 2.65 | SMX: 10 | 300 W; Xe lamp (Vis.) | 15 | 140 | 0.001 | [81] |
| gCNNT (400 ppm) | 100.4 | 2.31 | 100 | 120 | 0.035 | ||||
| gCN (1000 ppm) | 5.3 | 2.62 | MO: 10 | 350 W; Xe lamp (Vis.) | 71.1 | 120 | 0.009 | [82] | |
| gCNNWs (1000 ppm) | 74.25 | 1.52 | 98.5 | 0.02 | |||||
| gCNNFs (1000 ppm) | 60.16 | 1.61 | 90.9 | 0.01 | |||||
| TGCN (1000 ppm) | 8.52 | 2.8 | PhOH: 20 MO: 20 | SLB-300A, 300 W (Simulated sunlight) | PhOH: 50 MO: 62 | PhOH: 180 MO: 150 | 0.004 | [83] | |
| 0.006 | |||||||||
| SGCN (1000 ppm) | 21.13 | 2.4 | PhOH: 60 MO: 65 | 0.005 | |||||
| 0.007 | |||||||||
| MGCN (1000 ppm) | 31.84 | 2.45 | PhOH: 85 MO: 92 | 0.01 | |||||
| 0.013 | |||||||||
| 2D | Bulk (550 CN) | 25 | 2.54 | CIP: 5 RhB: 5 | 500 W; Xe lamp (Vis.) | CIP: 8 | CIP: 60 RhB: 40 | 0.001 | [84] |
| RhB: 58 | 0.017 | ||||||||
| Ultrasounds (550 ul CN) | 12 | 2.56 | CIP: - | _ | |||||
| RhB: 75 | 0.027 | ||||||||
| Thermic (475 CN) | 29 | 2.42 | CIP: - | _ | |||||
| RhB: 50 | 0.009 | ||||||||
| Acid (550 H CN) | 114 | 2.73 | CIP: 17 | 0.002 | |||||
| RhB: 85 | 0.023 | ||||||||
| Thermic + ultrasounds (475 ul CN) | 21 | 2.46 | CIP: 7 | 0.001 | |||||
| RhB: 43 | 0.012 | ||||||||
| Acid + ultrasounds (550 ul H CN) | 74 | 2.74 | CIP: - | _ | |||||
| RhB: 80 | 0.03 | ||||||||
| Thermic + acid (475 H CN) | 71 | 284 | CIP: - | _ | |||||
| RhB: 80 | 0.018 | ||||||||
| Thermic + acid + ultrasounds (475 ul H CN) | 57 | 2.67 | CIP: 57 | 0.006 | |||||
| RhB: 92 | 0.037 | ||||||||
| CN-B | 18 | 2.42 | Rh6G: 5 | 400 W; Xe lamp (Vis.) | 67 | 30 | 0.031 | [85] | |
| CN 500-4 | 31 | 2.56 | 78 | 0.046 | |||||
| CN 550-4 | 107 | 2.68 | 92 | 0.085 | |||||
| CN 550-5 | 165 | 2.73 | 96 | 0.102 | |||||
| CN 550-6 | 295 | 2.89 | 98 | 0.139 | |||||
| gCN | 22.2 | 2.8 | CIP: 20 | Sunlight | 45 | 60 | 0.009 | [86] | |
| E-gCN | 63.8 | 2.94 | 78 | 0.023 |
| Morphology | Template | Photocatalyst | Area (m2·g−1) | Pore (cm3·g−1) | Gap (eV) | Pollutant (ppm) | Radiation | Efficiency (%) | Time (min) | k (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3D | HARD | gCN (1000 ppm) | 10.5 | 0.091 | 2.79 | RhB: 10 | 300 W Xe lamp (Vis.) | 25 | 90 | 0.004 | [97] |
| Porous 3D gCN (1000 ppm) | 103.3 | 0.61 | 2.78 | 63 | 0.018 | ||||||
| gCN (500 ppm) | 9.75 | 0.061 | 2.7 | RhB: 10 | Visible | 25 | 40 | 0.007 | [98] | ||
| SOFT | Porous gCN (P123-6) (500 ppm) | 73.29 | 0.27 | 2.75 | 98.7 | 0.1 | |||||
| WITHOUT | gCN (1000 ppm) | 8.36 | 0.02 | 2.6 | RhB: 10 | 500 W Xe lamp (Vis.) | 40 | 80 | 0.004 | [99] | |
| Porous hexagonal gCN (1000 ppm) | 67.3 | 0.32 | 2.43 | 100 | 0.053 | ||||||
| High crystalline gCN | BCN (1000 ppm) | - | - | 2.67 | NPX: 8 | 350 W Xe lamp (Vis.) | 20.9 | 70 | 0.013 | [100] | |
| CCN (1000 ppm) | - | - | 2.72 | 98.4 | 0.092 |
| Group | Photocatalyst | Area (m2·g−1) | Gap (eV) | Pollutant (ppm) | Radiation Xe Lamp (Vis.) | Efficiency (%) | Time (min) | k (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| -COOH | gCN (200 ppm) | 38.2 | - | MB and RhB: 15 | 300 W | - | 180 | - | [48] |
| gCN-HNO3 (200 ppm) | 88.6 | 2.65 | MB: 79 RhB: 62 | RhB: 0.026; MB: 0.033 | |||||
| -C≡N | gCN (1000 ppm) | 21.49 | 2.72 | RhB and TC: 15 | 300 W | 100 | 30 | RhB: 0.025; TC: 0.029 | [115] |
| Cyano-gCN (1000 ppm) | 51.34 | 2.63 | 100 | RhB and TC: 0.099 | |||||
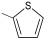 | gCN (500 ppm) | 16.6 | 2.61 | RhB: 5 | 500 W | 45 | 90 | 0.0072 | [116] |
| Thiophen-gCN (500 ppm) | 78.4 | 2.64 | 96 | 0.036 |
| Vacancy | Photocatalyst | Area (m2·g−1) | Gap (eV) | Pollutant (ppm) | Radiation | Efficiency (%) | Time (min) | k (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Nv | gCN (600 ppm) | 2.85 | 85 | BPA:10 | 300 W Xe lamp (Vis.) | 25 | 150 | 0.0021 | [51] |
| KOH-OA-gCN (600 ppm) | 2.6 | 29 | 90 | 0.0147 | |||||
| gCN (600 ppm) | 75.7 | 2.79 | OTC:20 | 24 W; LED (Vis.) | 45.8 | 135 | 0.0046 | [117] | |
| NvrCN (600 ppm) | 64.3 | 2.73 | 92.5 | 0.018 | |||||
| gCN (250 ppm) | 85.4 | 2.75 | TC: 15 SMX: 5 | 300 W Xe lamp (Vis.) | TC: 40 SMX: 52 | TC: 60 SMX: 120 | 0.009 0.025 | [118] | |
| ODH-CN-2 (250 ppm) | 108.2 | 2.61 | TC: 79.9 SMX: 91.5 | 0.007 0.020 | |||||
| Cv | gCN (300 ppm) | 30.1 | 2.76 | BPA: 10 | 350 W Xe lamp (Vis.) | 78 | 120 | 0.003 | [43] |
| Cv-gCN (300 ppm) | 14.7 | 2.65 | 90 | 0.006 | |||||
| gCN (250 ppm) | 272 | 2.94 | TC; 16 | 300 W halogen lamp (Vis.) + ul (600 W/40 kHz) | 30 | 60 | 0.0003 | [44] | |
| Cv-gCN-20 (250 ppm) | 331 | 2.9 | 96 | 0.0010 | |||||
| gCN (1000 ppm) | 79.7 | 2.73 | 4-clorophenol: 10 | 300 W Xe lamp (Vis.) | 33.8 | 120 | 0.003 | [119] | |
| Cv-gCN-575 (1000 ppm) | 64.2 | 2.71 | 60.1 | 0.008 |
| Dopant | Photocatalyst | Area (m2·g−1) | Gap (eV) | Pollutant (ppm) | Radiation | Efficiency (%) | Time (min) | k (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| O | gCN (300 ppm) | - | 2.65 | Lincomycin: 100 | 90 W LED (Vis.) | 45 | 180 | 0.005 | [120] |
| O-gCN (300 ppm) | - | 1.93 | 99 | 0.034 | |||||
| gCN (200 ppm) | 62.50 | 2.63 | BPA: 10 | 300 W Xe lamp (Vis.) | 14 | 120 | 0.001 | [121] | |
| O-gCN (200 ppm) | 70.32 | 2.60 | 99 | 0.032 | |||||
| P | gCN (1000 ppm) | 26.86 | 2.69 | RhB: 10 | 300 W, Xe lamp (Vis) | 100 | 30 | - | [122] |
| P-gCN (1000 ppm) | 40.5 | 2.84 | 10 | - | |||||
| gCN (100 ppm) | 73.8 | - | RhB: 20 | 300 W Xe lamp (Vis.) | 64.2 | 70 | 0.039 | [123] | |
| P-gCN (100 ppm) | 202.9 | - | 99.5 | 0.120 | |||||
| S | gCN (100 ppm for MB and 200 ppm for TC) | 11 | 2.55 | MB and TC: 20 | 300 W Xe lamp (Vis.) | MB: 4; TC: 10 | MB: 300 TC: 240 | MB: 0.00014 TC: 0.0003 | [124] |
| S-gCN (100 ppm for MB and 200 ppm for TC) | 15 | 1.83 | MB: 60; TC: 89 | MB: 0.0014 TC: 0.037 | |||||
| gCN (1000 ppm) | - | - | OTC: 10 | 300 W Xe lamp (Vis.) | 57.1 | 40 | - | [125] | |
| S-gCN (1000 ppm) | 31.2 | 2.83 | 93.3 | 0.133 | |||||
| B | gCN (68 mg gCN/62 mg CFs) | - | 2.71 | RhB: 5 | 8 W LED lamp (Vis.) | 82 | 120 | 0.015 | [126] |
| B-gCN (68 mg B-gCN/62 mg CFs) | - | 2.69 | 95 | 0.024 | |||||
| gCN (500 ppm) | 17.5 | 2.73 | RhB: 2 | 500 W Xe lamp (Vis.) | - | 30 | 0.026 | [127] | |
| B-gCN (500 ppm) | 105.1 | 2.70 | 97 | 0.086 | |||||
| Halogen | gCN (500 ppm) | 42.3 | 2.75 | TC: 10 | 300 W Xe lamp (Vis.) | 32 | 120 | 0.004 | [128] |
| Cl-gCN (500 ppm) | 114.4 | 2.7 | 92 | 0.02 | |||||
| gCN (1000 ppm) | - | 2.78 | 30 | 0.004 | |||||
| Br-gCN (1000 ppm) | - | 2.75 | OTC: 10 | 35 W LED lamp (Vis.) | 75 | 150 | 0.018 | [129] | |
| Cl-gCN (1000 ppm) | - | 2.73 | 75 | 0.017 | |||||
| C | gCN (400 ppm) | - | 2.77 | TC: 30 | 35 W LED lamp (Vis.) | 40 | 60 | 0.01 | [130] |
| C-gCN (400 ppm) | - | 2.71 | 77 | 0.03 | |||||
| gCN (1000 ppm) | 43 | 2.88 | BPA: 10 | 300 W Xe lamp (Vis.) | 25 | 60 | 0.005 | [131] | |
| C-gCN (1000 ppm) | 85 | 2.19 | 96 | 0.053 | |||||
| N | gCN (500 ppm) | 18.4 | 2.51 | TC: 10 | 300 W Xe lamp (Vis.) | 52.2 | 60 | 0.013 | [132] |
| N-gCN (500 ppm) | 74.79 | 2.47 | 81.7 | 0.026 | |||||
| gCN (1000 ppm) | 76.69 | 2.51 | PhOH: 10 | 300 W Xe lamp (Vis.) | 70.1 | 180 | 0.002 | [133] | |
| N-gCN (1000 ppm) | 72.26 | 1.82 | 37.6 | 0.006 |
| Dopant | Photocatalyst | Area (m2·g−1) | Gap (eV) | Pollutant (ppm) | Radiation | Efficiency (%) | Time (min) | k (min−1) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Alkaline and alkaline earth metals | gCN (400 ppm) | 177 | 2.57 | ENR, SMX, and TC: 5 | 300 W Xe lamp (Vis.) | ENR: 22 | 120 | 0.014 | [137] |
| TC: 42 | 0.036 | ||||||||
| SMX: 19 | 0.001 | ||||||||
| Mg-gCN (400 ppm) | 107 | 2.46 | ENR: 78 | 0.062 | |||||
| TC: 79 | 0.067 | ||||||||
| SMX: 40 | 0.003 | ||||||||
| Ca-gCN (400 ppm) | 100 | 2.29 | ENR: 58 | 0.046 | |||||
| TC: 78 | 0.067 | ||||||||
| SMX: 35 | 0.003 | ||||||||
| K-gCN (400 ppm) | 81 | 2.41 | ENR: 82 | 0.075 | |||||
| TC: 80 | 0.072 | ||||||||
| SMX: 67 | 0.006 | ||||||||
| Na-gCN (400 ppm) | 90 | 2.43 | ENR: 81 | 0.067 | |||||
| TC: 78 | 0.070 | ||||||||
| SMX: 65 | 0.006 | ||||||||
| Rare earths (Ln) | gCN | 46.33 | 2.73 | RhB, MB, and PR: 15 | 300 W Xe lamp (Vis.) | RhB: 54 | 150 | 0.0062 | [139] |
| MB: 52 | 0.0061 | ||||||||
| PR: 56 | 0.007 | ||||||||
| Yb-gCN | 52.56 | 2.5 | RhB: 88 | 0.017 | |||||
| MB: 91 | 0.019 | ||||||||
| PR: 88 | 0.018 | ||||||||
| Nd-gCN | 46.38 | 2.56 | RhB: 74 | 0.012 | |||||
| MB: 87 | 0.018 | ||||||||
| PR: 74 | 0.015 | ||||||||
| Ce-gCN | 58.09 | 2.47 | RhB: 91 | 0.019 | |||||
| MB: 93 | 0.023 | ||||||||
| PR: 91 | 0.020 | ||||||||
| Noble metals | gCN (1000 ppm) Ag-gCN (1000 ppm) | 59.5 | 2.66 | TC, OTC, and CTC: 20 | 300 W Xe lamp (Vis.) | TC: 35 | 120 | 0.0037 | [140] |
| OTC: 50 | 0.0053 | ||||||||
| CTC: 43 | 0.0039 | ||||||||
| 58.4 | 2.55 | TC: 83 | 0.0142 | ||||||
| OTC: 81 | 0.0130 | ||||||||
| CTC: 85 | 0.0139 | ||||||||
| gCN (1000 ppm) | - | 2.7 | BZF: 3 | 500 W Xe lamp (Vis.) | 27 | 90 | 0.012 | [141] | |
| Pd-gCN (1000 ppm) | - | 2.62 | 100 | 0.036 | |||||
| Transition metals | gCNNSs (600 ppm) | 91 | 2.7 | RhB: 20 | 300 W Xe lamp (Vis.) | 60 | 60 | 0.012 | [142] |
| Fe-gCNNs (600 ppm) | 132 | 2.5 | 100 | 30 | 0.117 | ||||
| gCN (1000 ppm) | 95.95 | 2.73 | MB; 10CIP; 15 | 300 W Xe lamp (Vis.) | MB: 67 | 150 | 0.013 | [143] | |
| CIP: 30 | 0.002 | ||||||||
| gCNNSs (1000 ppm) | 70.17 | 2.76 | MB: 81 | 0.014 | |||||
| CIP: 37 | 0.003 | ||||||||
| Ni-gCNNSs (1000 ppm) | 8.23 | 2.68 | MB: 93 | 0.017 | |||||
| CIP: 52 | 0.003 | ||||||||
| gCN (600 ppm) | 11.4 | 2.7 | TC: 30 | 300 W Xe lamp (Vis.) | 47 | 120 | - | [144] | |
| Cu-gCN (600 ppm) | 142.8 | 2.45 | 98 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Xardon, X.; Rosales, E.; Sanromán, M.Á. Current Strategies to Improve the Properties of Graphitic Carbon Nitride for Effective and Scalable Wastewater Pollutant Removal: A Critical Review. Catalysts 2025, 15, 523. https://doi.org/10.3390/catal15060523
Barreiro-Xardon X, Rosales E, Sanromán MÁ. Current Strategies to Improve the Properties of Graphitic Carbon Nitride for Effective and Scalable Wastewater Pollutant Removal: A Critical Review. Catalysts. 2025; 15(6):523. https://doi.org/10.3390/catal15060523
Chicago/Turabian StyleBarreiro-Xardon, Xan, Emilio Rosales, and María Ángeles Sanromán. 2025. "Current Strategies to Improve the Properties of Graphitic Carbon Nitride for Effective and Scalable Wastewater Pollutant Removal: A Critical Review" Catalysts 15, no. 6: 523. https://doi.org/10.3390/catal15060523
APA StyleBarreiro-Xardon, X., Rosales, E., & Sanromán, M. Á. (2025). Current Strategies to Improve the Properties of Graphitic Carbon Nitride for Effective and Scalable Wastewater Pollutant Removal: A Critical Review. Catalysts, 15(6), 523. https://doi.org/10.3390/catal15060523










