Photocatalytic Conversion of β-O-4 Lignin Model Dimers: The Effect of Benzylic Ketones on Reaction Pathway
Abstract
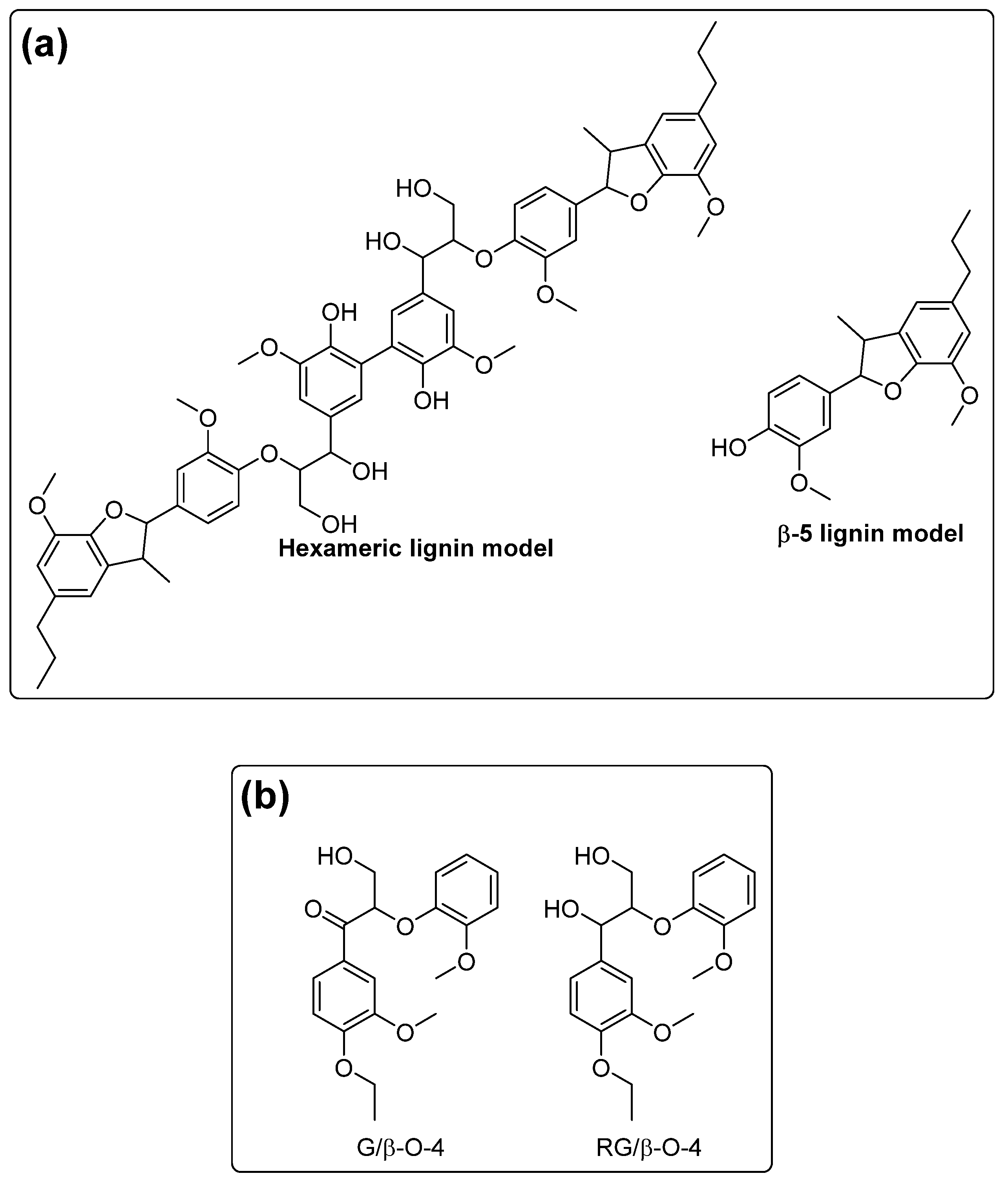
1. Introduction
2. Results
2.1. Photo-Degradation of G/β-O-4 and RG/β-O-4
2.2. Proposed Reaction Pathways for G/β-O-4 and RG/β-O-4
2.3. Role of Solvent
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, S.; Wang, X.; Li, W.; Zhang, Y.; Liu, S.; Xiao, W.; Yue, Z.; Lu, X.; Fan, X. A mini review on photocatalytic lignin conversion into monomeric aromatic compounds. Catal. Sci. Technol. 2025, 15, 962–987. [Google Scholar] [CrossRef]
- Huang, M.; Guo, H.; Zeng, Z.; Xiao, H.; Hu, H.; He, L.; Li, K.; Liu, X.; Yan, L. Selective Photocatalytic Transformation of Lignin to Aromatic Chemicals by Crystalline Carbon Nitride in Water–Acetonitrile Solutions. Int. J. Environ. Res. Public Health 2022, 19, 15690–15707. [Google Scholar] [CrossRef]
- Suo, C.; Li, W.; Luo, S.; Ma, C.; Liu, S. Multisite photocatalytic depolymerization of lignin model compound utilizing full-spectrum light over magnetic microspheres. iScience 2023, 26, 108167–108179. [Google Scholar] [CrossRef] [PubMed]
- Martínková, L.; Grulich, M.; Pátek, M.; Křístková, B.; Winkler, M. Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review. Biomolecules 2023, 13, 717–742. [Google Scholar] [CrossRef] [PubMed]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744–758762. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef]
- Murnaghan, C.W.J.; Skillen, N.; Hackett, B.; Lafferty, J.; Robertson, P.K.J.; Sheldrake, G.N. Toward the photocatalytic valorization of lignin: Conversion of a model lignin hexamer with multiple functionalities. ACS Sustain. Chem. Eng. 2022, 10, 12107–12116. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (• OH) generation processes in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Raja, P.; Bozzi, A.; Mansilla, H.; Kiwi, J. Evidence for superoxide-radical anion, singlet oxygen and OH-radical intervention during the degradation of the lignin model compound (3-methoxy-4-hydroxyphenylmethylcarbinol). J. Photochem. Photobiol. A Chem. 2005, 169, 271–278. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Alnashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Skillen, N.; Rice, C.; Pang, X.; Robertson, P.K.J.; McCormick, W.; McCrudden, D. Nanostructured Photocatalysts: From Fundamental to Practical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–118. [Google Scholar]
- Liu, I.; Lawton, L.A.; Robertson, P.K.J. Mechanistic studies of the photocatalytic oxidation of microcystin-LR: An investigation of byproducts of the decomposition process. Environ. Sci. Technol. 2003, 37, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, L.; Wang, S.; Zhu, N.; Li, Z.; Zhao, L.; Wang, Y. Recent advances of semiconductor photocatalysis for water pollutant treatment: Mechanisms, materials and applications. Phys. Chem. Chem. Phys. 2023, 25, 25899–25924. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Koiki, B.A.; Arotiba, O.A. Cu2O as an emerging semiconductor in photocatalytic and photoelectrocatalytic treatment of water contaminated with organic substances: A review. RSC Adv. 2020, 10, 36514–36525. [Google Scholar] [CrossRef]
- Li, X.; Wei, H.; Song, T.; Lu, H.; Wang, X. A review of the photocatalytic degradation of organic pollutants in water by modified TiO2. Water Sci. Technol. 2023, 88, 1495–1507. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, S. Photocatalytic Depolymerization of Lignin: C-O Bond Cleavage in β-O-4 Models Using S-Doped Ultra-Thin Bi3O4Cl Nanosheets. Molecules 2024, 29, 24. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Wu, L.; Tan, Y.; Kong, D.; Yimiti, M. Photocatalytic degradation of lignin by low content gC3N4 modified TiO2 under visible light. New J. Chem. 2022, 46, 8644–8652. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, Y.; Xu, S.; Li, Y. Dispersing agglomerated Zn4In2S7 on g-C3N4 nanosheets to form a 2D/2D S-scheme heterojunction for highly selective photocatalytic cleavage of lignin models. Catal. Sci. Technol. 2024, 14, 2294–2304. [Google Scholar] [CrossRef]
- Machado, A.E.H.; Furuyama, A.M.; Falone, S.Z.; Ruggiero, R.; Da Silva Perez, D.; Castellan, A. Photocatalytic degradation of lignin and lignin models, using titanium dioxide: The role of the hydroxyl radical. Chemosphere 2000, 40, 115–124. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, J. Aerobic oxidation of olefins and lignin model compounds using photogenerated phthalimide-N-oxyl radical. J. Org. Chem. 2016, 81, 9131–9137. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Wang, M.; Li, H.; Zhang, J.; Liu, H.; Wang, F. Photocatalytic oxidation–hydrogenolysis of lignin β-O-4 models via a dual light wavelength switching strategy. ACS Catal. 2016, 6, 7716–7721. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; Lu, J.; Zhang, J. Fine tuning the redox potentials of carbazolic porous organic frameworks for visible-light photoredox catalytic degradation of lignin β-O-4 models. ACS Catal. 2017, 7, 5062–5070. [Google Scholar] [CrossRef]
- Murnaghan, C.W.J.; Skillen, N.; Hardacre, C.; Bruce, J.; Sheldrake, G.N.; Robertson, P.K. Exploring lignin valorisation: The application of photocatalysis for the degradation of the β-5 linkage. J. Phys. Energy 2021, 3, 035002. [Google Scholar] [CrossRef]
- Forsythe, W.G.; Garrett, M.D.; Hardacre, C.; Nieuwenhuyzen, M.; Sheldrake, G.N. An efficient and flexible synthesis of model lignin oligomers. Green Chem. 2013, 15, 3031. [Google Scholar] [CrossRef]
- Liu, J.; Ralphs, K.; Murnaghan, C.W.J.; Skillen, N.; Sheldrake, G.N.; McCarron, P.; Robertson, P.K. Exploring the Photocatalytic Cleavage Pathway of the β-5 Linkage Lignin Model Compound on Carbon Nitride. ChemSusChem 2025, 18, e202400955. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; He, J.; Zhang, Y. Redox-neutral photocatalytic strategy for selective C–C bond cleavage of lignin and lignin models via PCET process. Sci. Bull. 2019, 64, 1658–1666. [Google Scholar] [CrossRef]
- Han, G.; Yan, T.; Zhang, W.; Zhang, Y.C.; Lee, D.Y.; Cao, Z.; Sun, Y. Highly selective photocatalytic valorization of lignin model compounds using ultrathin metal/CdS. ACS Catal. 2019, 9, 11341–11349. [Google Scholar] [CrossRef]
- Norrish, R.G.W.; Appleyard, M.E.S. 191. Primary photochemical reactions. Part IV. Decomposition of methyl ethyl ketone and methyl butyl ketone. J. Chem. Soc. 1934, 874, 455–459. [Google Scholar] [CrossRef]
- Saltmarsh, O.D.; Norrish, R.G. Primary photochemical reactions. Part VI. The photochemical decomposition of certain cyclic ketones. J. Chem. Soc. 1935, 98, 455–459. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Li, M.; Ren, Y.L. Catalyst-Free Hydrogenolysis of Lignin β-O-4 Ketone Models with Water as an H-transfer Reagent. ChemistrySelect 2023, 8, e202204417. [Google Scholar] [CrossRef]
- Fumoto, E.; Sato, S.; Kawamata, Y.; Koyama, Y.; Yoshikawa, T.; Nakasaka, Y.; Tago, T.; Masuda, T. Determination of carbonyl functional groups in lignin-derived fraction using infrared spectroscopy. Fuel 2022, 318, 123530. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bianco, A.; Vazquez, J.C.; Garcıa-Lopez, E.; Irico, A.; Loddo, V.; Rodrıguez, S.M.; Marcı, G.; Palmisano, L.; Pramauro, E. Photocatalytic oxidation of acetonitrile in aqueous suspension of titanium dioxide irradiated by sunlight. Adv. Environ. Res. 2004, 8, 329–335. [Google Scholar] [CrossRef]
- Furukawa, S.; Shishido, T.; Teramura, K.; Tanaka, T. Photocatalytic oxidation of alcohols over TiO2 covered with Nb2O5. ACS Catal. 2012, 2, 175–179. [Google Scholar] [CrossRef]
- Guo, H.; Miles-Barrett, D.M.; Zhang, B.; Wang, A.; Zhang, T.; Westwood, N.J.; Li, C. Is oxidation–reduction a real robust strategy for lignin conversion? A comparative study on lignin and model compounds. Green Chem. 2019, 21, 803–811. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Xia, H.; Zhou, M.; Zhao, J.; Sharma, B.K.; Jiang, J. Photocatalytic cleavage of β-O-4 ether bonds in lignin over Ni/TiO2. Molecules 2020, 25, 2109. [Google Scholar] [CrossRef]
- Tyndall, G.S.; Orlando, J.J.; Wallington, T.J.; Hurley, M.D.; Goto, M.; Kawasaki, M. Mechanism of the reaction of OH radicals with acetone and acetaldehyde at 251 and 296 K. Phys. Chem. Chem. Phys. 2002, 4, 2189–2193. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Coluccia, S.; Di Paola, A.; García-López, E.; Loddo, V.; Marcì, G.; Martra, G.; Palmisano, L. The role of water in the photocatalytic degradation of acetonitrile and toluene in gas-solid and liquid-solid regimes. Int. J. Photoenergy 2006, 2006, 039182. [Google Scholar] [CrossRef]
- Addamo, M.; Augugliaro, V.; Coluccia, S.; Faga, M.G.; García-López, E.; Loddo, V.; Marcì, G.; Martra, G.; Palmisano, L. Photocatalytic oxidation of acetonitrile in gas–solid and liquid–solid regimes. J. Catal. 2005, 235, 209–220. [Google Scholar] [CrossRef]





| Reaction of G/β-O-4 | Rate of Consumption (First 15 min) | Overall Consumption 1 |
|---|---|---|
| Photocatalytic | 1.22 × 10−3 mg mL−1 min−1 | 0.0832 mg mL−1 |
| Photolytic | 2.70 × 10−3 mg mL−1 min−1 | 0.0905 mg mL−1 |
| Reaction of RG/β-O-4 | Rate of Consumption (First 15 min) | Overall Consumption 1 |
| Photocatalytic | 1.92 × 10−3 mg mL−1 min−1 | 0.0948 mg mL−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheldrake, G.N.; Skillen, N.; Robertson, P.K.J.; Murnaghan, C.W.J. Photocatalytic Conversion of β-O-4 Lignin Model Dimers: The Effect of Benzylic Ketones on Reaction Pathway. Catalysts 2025, 15, 525. https://doi.org/10.3390/catal15060525
Sheldrake GN, Skillen N, Robertson PKJ, Murnaghan CWJ. Photocatalytic Conversion of β-O-4 Lignin Model Dimers: The Effect of Benzylic Ketones on Reaction Pathway. Catalysts. 2025; 15(6):525. https://doi.org/10.3390/catal15060525
Chicago/Turabian StyleSheldrake, Gary. N., Nathan Skillen, Peter. K. J. Robertson, and Christopher W. J. Murnaghan. 2025. "Photocatalytic Conversion of β-O-4 Lignin Model Dimers: The Effect of Benzylic Ketones on Reaction Pathway" Catalysts 15, no. 6: 525. https://doi.org/10.3390/catal15060525
APA StyleSheldrake, G. N., Skillen, N., Robertson, P. K. J., & Murnaghan, C. W. J. (2025). Photocatalytic Conversion of β-O-4 Lignin Model Dimers: The Effect of Benzylic Ketones on Reaction Pathway. Catalysts, 15(6), 525. https://doi.org/10.3390/catal15060525








