Catalytic Deoxygenation of Lipids for Bio-Jet Fuel: Advances in Catalyst Design and Reaction Pathways
Abstract
1. Introduction
2. Construction of Efficient Multifunctional Catalysts
2.1. Design of Active Sites
2.1.1. Noble Metal Catalysts
2.1.2. Non-Noble Metal Catalysts
2.1.3. Non-Noble Metal Compound Catalysts
2.2. Morphology and Texture Properties
2.3. Metal Support Interaction
2.4. Synergetic Effects Between Metal and Acid Sites
2.5. Synergetic Effects Between Metal and Oxygen Vacancy
2.6. Catalyst Stability
3. Reaction Regulation of Lipid Conversion to Alternative Jet Fuels
3.1. Cleavage of Ester Bond
3.2. Deoxygenation
3.3. Cracking, Isomerization, and Aromatization
3.4. Side Reactions
3.5. The Influence of Reactants
4. Summary and Outlook
- It is necessary to accurately design the active center of the catalyst to improve performance. The rational design of the electronic properties and modulation of the coordination environment at active centers significantly enhances their intrinsic catalytic activity. Synergistic catalysis between active sites is pivotal for achieving efficient transformations, where structural engineering can be strategically employed to tailor reaction pathways. The distance between the two active centers (metal acid and metal oxygen vacancies) may be important parameters, but there are few pieces of research regarding that, which might be caused by the lack of effective and feasible in situ characterization methods.
- The stability and regeneration of catalysts require attention. Some strategies have been used to enhance the catalyst life, such as utilizing strong metal support interactions to improve metal dispersion and avoid catalyst sintering. Modification technologies for optimizing surface properties, especially for the catalysts of hydrothermal reactions, can maintain the hydrothermal stability of the catalyst while keeping the accessibility of the active site. Carbon deposition (coke) is usually the dominated reasons for catalyst deactivation during the bio-refining of lipids to produce bio-jet fuel. Accordingly, promoters such as both metal or non-metal species can be introduced to realize the in situ conversion and removal of carbon deposition, or integrated regeneration technology to oxidatively remove coke and then reduce the catalyst. It is necessary to study the recovery ability of regeneration technology on the physical and chemical properties of the catalyst.
- With the help of advanced in situ characterization methods and theoretical calculations, the structure–activity relationship of catalysts is constructed. The production of alternative jet fuels from bio fats involves complex reaction mechanism, while the persuasion of only indirectly inferring from the macroscopic properties of the catalyst and the distribution of products needs to be improved. For example, in situ FT-IR could be used to explore the functional group changes after the adsorption of reactants on specific structural sites on the catalyst surface, so as to speculate the adsorption surface reaction desorption behavior of raw materials. Furthermore, the combination of various in situ characterization techniques will contribute to monitoring the reaction process in real time, capture key intermediates, and finally clarify the reaction mechanism. A thorough understanding of the mechanism of the deoxidation, cracking, isomerization, and aromatization reactions involved in the process of oil conversion can provide theoretical guidance for the design of catalysts and the control of reaction conditions.
- The catalytic deoxygenation reaction under mild reaction conditions is always the goal of researchers. Other types of reaction have gradually attracted more attention from researchers. For example, photocatalytic conversion technology is favored due to its mild reaction conditions and low energy consumption. In particular, it is very attractive because it is expected to use natural sunlight as the final energy. Research on various reaction types in the process of bio lipid deoxygenation will further contribute to achieving the goal of net zero emissions. On the other hand, coupling thermal catalysis with both enzyme or photocatalysis is possibly promising, which would upset the thermal dynamic equilibrium to achieve high yields of targeted biofuels.
- The production of bio jet fuel from lipids is considered as a complex reaction system. Real bio-oils (animal and vegetable oils, microalgae oils, waste cooking oils, etc.) contain complex raw materials. There are significant differences between various lipid sources, which requires catalysts to have strong adaptability to different raw materials. During the reaction process, multiple intermediates coexist and involve various reaction types (deoxygenation, cracking, isomerization, and aromatization) and different reaction mechanisms. These together form a complex reaction network for the catalytic conversion of lipids. The deoxygenation of model compounds often resulted in one or two components of alkanes as products, while we hope to obtain hydrocarbon mixtures that meet strict fuel standards through the simple conversion of complex biomass. It is necessary to conduct in-depth research on the interactions between different substances and reactions in the complex reaction network of lipid deoxygenation reactions in order to develop more suitable catalytic systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAF | Sustainable Aviation Fuel |
| HEFA | Hydroprocessed Esters and-Fatty Acids |
| F-T | Fischer–Tropsch |
| FT-SKA | Fischer–Tropsch containing aromatics |
| SIP | Direct sugars to hydrocarbons producing Synthetic Iso-Paraffins |
| ATJ | Alcohol-to-Jet |
| CHJ | Catalytic Hydrothermolysis Jet Fuel |
| HH-SPK | Hydroprocessed Hydrocarbons |
| DO | Deoxygenation |
| HIS | Hydroisomerization |
| HC | Hydrocracking |
| HDO | Hydrodeoxygenation |
| DCO | Decarbonylation |
| DCX | Decarboxylation |
References
- Copernicus. Global Climate Highlights 2023. Available online: https://climate.copernicus.eu/global-climate-highlights-2023 (accessed on 9 January 2024).
- IEA. World Energy Outlook 2024. Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 15 October 2024).
- IEA. Renewables 2024. Available online: https://www.iea.org/reports/renewables-2024 (accessed on 29 October 2024).
- Gonzalez-Garay, A.; Heuberger-Austin, C.; Fu, X.; Klokkenburg, M.; Zhang, D.; van der Made, A.; Shah, N. Unravelling the potential of sustainable aviation fuels to decarbonise the aviation sector. Energy Environ. Sci. 2022, 15, 3291–3309. [Google Scholar] [CrossRef]
- Jasiński, R.; Przysowa, R. Evaluating the Impact of Using HEFA Fuel on the Particulate Matter Emissions from a Turbine Engine. Energies 2024, 17, 1077. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Y.; Zheng, Y.; Ding, S.; Zhu, M.; Li, G.; Wang, M.; Yu, Z.; Song, Y.; Chang, L.; et al. Emission reduction characteristics of heavy-fuel aircraft piston engine fueled with 100% HEFA sustainable aviation fuel. Environ. Pollut. 2025, 368, 125661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, M.; Zhou, L.; Gao, M.; Xu, Z.; Zhong, S.; Pan, K.; Chen, L. Evaluating high-resolution aviation emissions using real-time flight data. Sci. Total Environ. 2024, 951, 175429. [Google Scholar] [CrossRef]
- ITIA. Global Outlook for Air Transport. Available online: https://www.iata.org/en/publications/economics/reports/global-outlook-december-2024/ (accessed on 10 December 2024).
- ITIA. Net Zero 2050: Sustainable Aviation Fuels. Available online: https://www.iata.org/en/programs/sustainability/flynetzero/ (accessed on 9 December 2024).
- IEA. Oil 2024. Available online: https://www.iea.org/reports/oil-2024 (accessed on 11 June 2024).
- ITIA. Sustainable Aviation Fuel: Technical Certification. Available online: https://www.iata.org/contentassets/d13875e9ed784f75bac90f000760e998/saf-technical-certifications.pdf (accessed on 30 July 2020).
- Watson, M.J.; Machado, P.G.; da Silva, A.V.; Saltar, Y.; Ribeiro, C.O.; Nascimento, C.A.O.; Dowling, A.W. Sustainable aviation fuel technologies, costs, emissions, policies, and markets: A critical review. J. Clean. Prod. 2024, 449, 141472. [Google Scholar] [CrossRef]
- Tabandeh, M.; Cheng, C.K.; Centi, G.; Show, P.L.; Chen, W.-H.; Ling, T.C.; Ong, H.C.; Ng, E.-P.; Juan, J.C.; Lam, S.S. Recent advancement in deoxygenation of fatty acids via homogeneous catalysis for biofuel production. Mol. Catal. 2022, 523, 111207. [Google Scholar] [CrossRef]
- Yao, X.; Strathmann, T.J.; Li, Y.; Cronmiller, L.E.; Ma, H.; Zhang, J. Catalytic hydrothermal deoxygenation of lipids and fatty acids to diesel-like hydrocarbons: A review. Green Chem. 2021, 23, 1114–1129. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Martínez-Klimov, M.; Murzin, D.Y. Hydroconversion of fatty acids and vegetable oils for production of jet fuels. Fuel 2021, 306, 121673. [Google Scholar] [CrossRef]
- Žula, M.; Grilc, M.; Likozar, B. Hydrocracking, hydrogenation and hydro-deoxygenation of fatty acids, esters and glycerides: Mechanisms, kinetics and transport phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of Jet Biofuels by Catalytic Hydroprocessing of Esters and Fatty Acids: A Review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; da Silva, S.S.O.; Cavalcante, C.L.; de Luna, F.M.T.; Bolivar, J.M.; Vieira, R.S.; Fernandez-Lafuente, R. Biosynthesis of alkanes/alkenes from fatty acids or derivatives (triacylglycerols or fatty aldehydes). Biotechnol. Adv. 2022, 61, 108045. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-S.; Zeng, Y.-Y.; Liu, L.; Chen, L.; Duan, P.; Luque, R.; Ge, R.; Zhang, W. Advances in catalytic decarboxylation of bioderived fatty acids to diesel-range alkanes. Renew. Sustain. Energy Rev. 2022, 158, 112178. [Google Scholar] [CrossRef]
- Cheah, K.W.; Yusup, S.; Loy, A.C.M.; How, B.S.; Skoulou, V.; Taylor, M.J. Recent advances in the catalytic deoxygenation of plant oils and prototypical fatty acid models compounds: Catalysis, process, and kinetics. Mol. Catal. 2022, 523, 111469. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Chen, Y.; Zhang, Q.; Chen, L.; Liu, J.; Ma, L. Hydroprocessing of lipids: An effective production process for sustainable aviation fuel. Energy 2023, 283, 129107. [Google Scholar] [CrossRef]
- Alkhoori, S.; Khaleel, M.; Vega, L.F.; Polychronopoulou, K. Deoxygenation of vegetable oils and fatty acids: How can we steer the reaction selectivity towards diesel range hydrocarbons? J. Ind. Eng. Chem. 2023, 127, 36–61. [Google Scholar] [CrossRef]
- Xing, S.; Fu, J.; Li, M.; Yang, G.; Lv, P. Emerging catalysis in solvent-free hydrodeoxygenation of waste lipids under mild conditions: A review. Renew. Sustain. Energy Rev. 2024, 200, 114459. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Chu, Y.; Fu, J.; Yang, L. Dilemma and strategies for production of diesel-like hydrocarbons by deoxygenation of biomass-derived fatty acids. Green Energy Environ. 2024. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Ma, Y.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. A mini review on catalytic hydrodeoxygenation for biofuels production: Catalyst, mechanism and process. Appl. Catal. A Gen. 2025, 699, 120278. [Google Scholar] [CrossRef]
- Fu, J.; Lu, X.; Savage, P.E. Catalytic hydrothermal deoxygenation of palmitic acid. Energy Environ. Sci. 2010, 3, 311–317. [Google Scholar] [CrossRef]
- Na, J.-G.; Yi, B.E.; Han, J.K.; Oh, Y.-K.; Park, J.-H.; Jung, T.S.; Han, S.S.; Yoon, H.C.; Kim, J.-N.; Lee, H.; et al. Deoxygenation of microalgal oil into hydrocarbon with precious metal catalysts: Optimization of reaction conditions and supports. Energy 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Yang, L.; Carreon, M.A. Deoxygenation of Palmitic and Lauric Acids over Pt/ZIF-67 Membrane/Zeolite 5A Bead Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 31993–32000. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.N.; Fortes, I.C.P.; de Sousa, F.P.; Pasa, V.M.D. Biokerosene and green diesel from macauba oils via catalytic deoxygenation over Pd/C. Fuel 2016, 164, 329–338. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, J.; Long, F.; Zhang, X.; Xu, J.; Jiang, J. Al-modified Pd@mSiO2 core-shell catalysts for the selective hydrodeoxygenation of fatty acid esters: Influence of catalyst structure and Al atoms incorporation. Appl. Catal. B Environ. 2022, 305, 121068. [Google Scholar] [CrossRef]
- Mondal, S.; Singuru, R.; Chandra Shit, S.; Hayashi, T.; Irle, S.; Hijikata, Y.; Mondal, J.; Bhaumik, A. Ruthenium Nanoparticle-Decorated Porous Organic Network for Direct Hydrodeoxygenation of Long-Chain Fatty Acids to Alkanes. ACS Sustain. Chem. Eng. 2018, 6, 1610–1619. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, Z.; Zhang, C.; Lu, J.; Liu, H.; Luo, N.; Zhang, J.; Wang, F. Enhanced photocatalytic alkane production from fatty acid decarboxylation via inhibition of radical oligomerization. Nat. Catal. 2020, 3, 170–178. [Google Scholar] [CrossRef]
- Yang, H.; Tian, L.; Grirrane, A.; García-Baldoví, A.; Hu, J.; Sastre, G.; Hu, C.; García, H. Enhanced Fatty Acid Photodecarboxylation over Bimetallic Au–Pd Core–Shell Nanoparticles Deposited on TiO2. ACS Catal. 2023, 13, 15143–15154. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Lu, X.; Savage, P.E. Hydrothermal Decarboxylation and Hydrogenation of Fatty Acids over Pt/C. ChemSusChem 2011, 4, 481–486. [Google Scholar] [CrossRef]
- Jiraroj, D.; Jirarattanapochai, O.; Anutrasakda, W.; Samec, J.S.M.; Tungasmita, D.N. Selective decarboxylation of biobased fatty acids using a Ni-FSM-16 catalyst. Appl. Catal. B Environ. 2021, 291, 120050. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, K.M.; Luyao, Z.; Lup, A.N.K.; Patah, M.F.A.; Chuah, C.Y.; Wan Daud, W.M.A. Jet fuel production via palm oil hydrodeoxygenation over bifunctional zeolite mixture supported Ni catalyst: Effect of Si/Al ratio. Biomass Bioenergy 2024, 185, 107237. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Li, W.; Luo, F.; Li, S.; Li, X.; Huang, Y.; Zhang, A.; Xiao, Z.; Wang, D.; et al. Production of diesel-like hydrocarbons via hydrodeoxygenation of palmitic acid over Ni/TS-1 catalyst. Biomass Bioenergy 2021, 149, 106081. [Google Scholar] [CrossRef]
- Wang, J.; Ren, D.; Zhang, N.; Lang, J.; Du, Y.; He, W.; Norinaga, K.; Huo, Z. Boosting in-situ hydrodeoxygenation of fatty acids over a fine and oxygen-vacancy-rich NiAl catalyst. Renew. Energy 2023, 202, 952–960. [Google Scholar] [CrossRef]
- Ma, B.; Yi, X.; Chen, L.; Zheng, A.; Zhao, C. Interconnected hierarchical HUSY zeolite-loaded Ni nano-particles probed for hydrodeoxygenation of fatty acids, fatty esters, and palm oil. J. Mater. Chem. A 2016, 4, 11330–11341. [Google Scholar] [CrossRef]
- Yang, H.; Zeng, Y.; Zhou, Y.; Du, X.; Li, D.; Hu, C. One-step synthesis of highly active and stable Ni-ZrO2 catalysts for the conversion of methyl laurate to alkanes. J. Catal. 2022, 413, 297–310. [Google Scholar] [CrossRef]
- Xin, H.; Chen, X.; Xiao, C.; Chao, J.; Zhang, H.; Tan, J.; Hu, C.; Wu, L.; Li, D. The CeO2 morphology modulates the density and catalytic performance of dual-active site for enhancing the palmitic acid conversion. Fuel 2024, 378, 132890. [Google Scholar] [CrossRef]
- Yu, P.; Xu, J.; Liang, R.; Cai, Z.; Ma, Y.; Zhang, H.; Liu, F.; Cao, Y.; Huang, K.; Jiang, L. Solvent-free deoxygenation of biolipid into liquid alkanes over bifunctional Ni/B2O3-ZrO2 catalyst. Fuel 2024, 375, 132649. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Al Maksoud, W.; Abou-Hamad, E.; Charisiou, N.D.; Constantinou, A.; Harkou, E.; Latsiou, A.I.; AlKhoori, S.; Hinder, S.J.; Baker, M.A.; et al. Tuning of the acid sites in the zeolite-alumina composite Ni catalysts and their impact on the palm oil hydrodeoxygenation reaction. Chem. Eng. J. 2024, 493, 152351. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Yu, P.; Hu, C. Temperature-tuned selectivity to alkanes or alcohol from ethyl palmitate deoxygenation over zirconia-supported cobalt catalyst. Fuel 2020, 278, 118295. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, X.; Zhan, L.; Li, X.; Song, X.; Wu, Y. Product distribution-tuned and excessive hydrocracking inhibiting in fatty acid deoxygenation over amorphous Co@SiO2 porous nanorattles. Fuel 2022, 318, 123605. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, J.; Zhu, W.; Li, Y.; Liang, C. Efficient hydrodeoxygenation of methyl palmitate over NiMo/ZrO2 catalyst with electron transfer between Ni and Mo. Catal. Today 2024, 437, 114780. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Zhang, T.; Li, D.; Tang, X.; Chen, M.; Zhou, Y.; Zhang, M.; Liu, Y.; Zhou, X.; et al. Promoting catalytic transfer hydrodecarbonylation of methyl stearate over bimetallic CoNi/HAP catalysts with strong electronic coupling effect. Appl. Catal. B Environ. 2022, 306, 121138. [Google Scholar] [CrossRef]
- Xin, H.; Yang, H.; Lei, X.; Du, X.; Zhou, K.; Li, D.; Hu, C. Ni–Fe Catalysts Supported on γ-Al2O3/HZSM-5 for Transformation of Palmitic Acid into Hydrocarbon Fuel. Ind. Eng. Chem. Res. 2020, 59, 17373–17386. [Google Scholar] [CrossRef]
- Yang, H.; Du, X.; Zhou, L.; Li, D.; Hu, C. Enhanced deoxygenation performance and coke resistance of Ni-based catalysts for jatropha oil conversion by rare earth elements. Fuel 2023, 334, 126779. [Google Scholar] [CrossRef]
- Guo, X.; Wang, W.; Liu, P.; Lu, Y.; Zhao, P.; Xu, J.; Jiang, J. Re metal addition promotes the stability of catalytic fatty acid conversion over Ni-Fe catalysts. Fuel 2024, 364, 131115. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547. [Google Scholar] [CrossRef]
- Çakan, A.; Kiren, B.; Ayas, N. Hydrodeoxygenation of safflower oil over cobalt-doped metal oxide catalysts for bio-aviation fuel production. Mol. Catal. 2023, 546, 113219. [Google Scholar] [CrossRef]
- Chen, S.; Miao, C.; Xie, H.; Jiao, Z.; Zhang, X.; Zhou, G. Synergistic effect between acidity and metallicity on the methyl laurate hydrodeoxygenation performance for Ni-based catalyst. Biomass Bioenergy 2024, 180, 107002. [Google Scholar] [CrossRef]
- Yıldız, A.; Goldfarb, J.L.; Ceylan, S. Sustainable hydrocarbon fuels via “one-pot” catalytic deoxygenation of waste cooking oil using inexpensive, unsupported metal oxide catalysts. Fuel 2020, 263, 116750. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Phimsen, S.; Kiatkittipong, K.; Wongsakulphasatch, S.; Laosiripojana, N.; Assabumrungrat, S. Diesel-like hydrocarbon production from hydroprocessing of relevant refining palm oil. Fuel Process. Technol. 2013, 116, 16–26. [Google Scholar] [CrossRef]
- Xin, H.; Guo, K.; Li, D.; Yang, H.; Hu, C. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B Environ. 2016, 187, 375–385. [Google Scholar] [CrossRef]
- Zhou, W.; Xin, H.; Yang, H.; Du, X.; Yang, R.; Li, D.; Hu, C. The Deoxygenation Pathways of Palmitic Acid into Hydrocarbons on Silica-Supported Ni12P5 and Ni2P Catalysts. Catalysts 2018, 8, 153. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Du, X.; Hu, C. Regulating the Hydrodeoxygenation Activity of Molybdenum Carbide with Different Diamines as Carbon Sources. Catalysts 2024, 14, 138. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Li, C.; Liang, C. Engineering the structural formula of N-doped molybdenum carbide nanowires for the deoxygenation of palmitic acid. Sustain. Energy Fuels 2020, 4, 2370–2379. [Google Scholar] [CrossRef]
- Du, X.; Zhou, K.; Zhou, L.; Lei, X.; Yang, H.; Li, D.; Hu, C. Efficient catalytic conversion of jatropha oil to high grade biofuel on Ni-Mo2C/MCM-41 catalysts with tuned surface properties. J. Energy Chem. 2021, 61, 425–435. [Google Scholar] [CrossRef]
- Du, X.; Liu, J.; Li, D.; Xin, H.; Lei, X.; Zhang, R.; Zhou, L.; Yang, H.; Zeng, Y.; Zhang, H.; et al. Structural and electronic effects boosting Ni-doped Mo2C catalyst toward high-efficiency C–O/C–C bonds cleavage. J. Energy Chem. 2022, 75, 109–116. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Stellwagen, D.R.; Bitter, J.H. Tungsten-Based Catalysts for Selective Deoxygenation. Angew. Chem. Int. Ed. 2013, 52, 5089–5092. [Google Scholar] [CrossRef]
- Zhou, K.; Du, X.; Zhou, L.; Yang, H.; Lei, X.; Zeng, Y.; Li, D.; Hu, C. The Deoxygenation of Jatropha Oil to High Quality Fuel via the Synergistic Catalytic Effect of Ni, W2C and WC Species. Catalysts 2021, 11, 469. [Google Scholar] [CrossRef]
- Lei, X.; Xin, H.; Du, X.; Yang, H.; Zeng, Y.; Zhou, L.; Juan, C.; Zhang, H.; Li, D.; Hu, C. Efficiency conversion of jatropha oil into high-quality biofuel over the innovative Ni-Mo2N based catalyst. Fuel 2022, 324, 124548. [Google Scholar] [CrossRef]
- Du, X.; Lei, X.; Zhou, L.; Peng, Y.; Zeng, Y.; Yang, H.; Li, D.; Hu, C.; Garcia, H. Bimetallic Ni and Mo Nitride as an Efficient Catalyst for Hydrodeoxygenation of Palmitic Acid. ACS Catal. 2022, 12, 4333–4343. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A real support effect on the hydrodeoxygenation of methyl oleate by sulfided NiMo catalysts. Catal. Today 2017, 298, 181–189. [Google Scholar] [CrossRef]
- Şenol, O.İ.; Viljava, T.R.; Krause, A.O.I. Hydrodeoxygenation of aliphatic esters on sulphided NiMo/γ-Al2O3 and CoMo/γ-Al2O3 catalyst: The effect of water. Catal. Today 2005, 106, 186–189. [Google Scholar] [CrossRef]
- Kovács, S.; Kasza, T.; Thernesz, A.; Horváth, I.W.; Hancsók, J. Fuel production by hydrotreating of triglycerides on NiMo/Al2O3/F catalyst. Chem. Eng. J. 2011, 176–177, 237–243. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Porsin, A.A.; Aleksandrov, P.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Co-processing of rapeseed oil—Straight run gas oil mixture: Comparative study of sulfide CoMo/Al2O3-SAPO-11 and NiMo/Al2O3-SAPO-11 catalysts. Catal. Today 2021, 378, 119–125. [Google Scholar] [CrossRef]
- Thongkumkoon, S.; Kiatkittipong, W.; Hartley, U.W.; Laosiripojana, N.; Daorattanachai, P. Catalytic activity of trimetallic sulfided Re-Ni-Mo/γ-Al2O3 toward deoxygenation of palm feedstocks. Renew. Energy 2019, 140, 111–123. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Ni2P/SBA-15 As a Hydrodeoxygenation Catalyst with Enhanced Selectivity for the Conversion of Methyl Oleate Into n-Octadecane. ACS Catal. 2012, 2, 592–598. [Google Scholar] [CrossRef]
- Hollak, S.A.W.; Gosselink, R.W.; van Es, D.S.; Bitter, J.H. Comparison of Tungsten and Molybdenum Carbide Catalysts for the Hydrodeoxygenation of Oleic Acid. ACS Catal. 2013, 3, 2837–2844. [Google Scholar] [CrossRef]
- Deng, Y.; Ge, Y.; Xu, M.; Yu, Q.; Xiao, D.; Yao, S.; Ma, D. Molybdenum Carbide: Controlling the Geometric and Electronic Structure of Noble Metals for the Activation of O–H and C–H Bonds. Acc. Chem. Res. 2019, 52, 3372–3383. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, L.; Chen, H.; Zeng, Y.; Li, D.; Hu, C. Efficient hydrogenation of aliphatic acyclic amides to amines by bimetallic NiMo nitrides via heterogeneous catalysis. Chem. Eng. J. 2023, 473, 145374. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Chavarría-Escamilla, H.G.; Ángeles-Chávez, C.; Zuriaga-Monroy, C.; Martínez-Magadán, J.M.; Cortés-Jácome, M.A.; López-Salinas, E.; Cedeño-Caero, L.; Toledo-Antonio, J.A. Ethyl palmitate decarboxylation using colloidal SiO2-templated mesoporous Ni-ZrO2 catalysts. Appl. Catal. A Gen. 2024, 670, 119547. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Li, G.; Hu, C. Insights into the Influence of ZrO2 Crystal Structures on Methyl Laurate Hydrogenation over Co/ZrO2 Catalysts. ACS Catal. 2021, 11, 7099–7113. [Google Scholar] [CrossRef]
- Cai, Z.; Liang, R.; Yu, P.; Liu, Y.; Ma, Y.; Cao, Y.; Huang, K.; Jiang, L.; Bao, X. Improving conversion of methyl palmitate to diesel-like fuel through catalytic deoxygenation with B2O3-modified ZrO2. Fuel Process. Technol. 2022, 226, 107091. [Google Scholar] [CrossRef]
- Feng, F.; Wang, L.; Zhang, X.; Wang, Q. Selective Hydroconversion of Oleic Acid into Aviation-Fuel-Range Alkanes over Ultrathin Ni/ZSM-5 Nanosheets. Ind. Eng. Chem. Res. 2019, 58, 5432–5444. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Xia, Q.; Jia, H.; Hong, X.; Liu, G. Tailoring of Surface Acidic Sites in Co–MoS2 Catalysts for Hydrodeoxygenation Reaction. J. Phys. Chem. Lett. 2021, 12, 5668–5674. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhang, W.; Li, F.; Fan, L.; Fu, J.; Liu, X.; Lyu, Y. Role of Acid Centers over Ni/ZSM-5 Catalysts for Hydrodeoxygenation of Methyl Laurate to Biojet Fuels. Ind. Eng. Chem. Res. 2023, 62, 16513–16520. [Google Scholar] [CrossRef]
- Liu, A.; Liu, X.; She, Y.; Hu, X.; Hu, M.; Zhang, Z.; Wang, X.; Liu, B. An alternative reaction pathway triggered by oxygen vacancies for boosting selective hydrodeoxygenation reactions. Green Chem. 2023, 25, 8633–8644. [Google Scholar] [CrossRef]
- Chen, C.; Ji, X.; Xiong, Y.; Jiang, J. Ni/Ce co-doping metal–organic framework catalysts with oxygen vacancy for catalytic transfer hydrodeoxygenation of lignin derivatives vanillin. Chem. Eng. J. 2024, 481, 148555. [Google Scholar] [CrossRef]
- Cao, X.; Long, F.; Zhang, G.; Xu, J.; Jiang, J. Selective Hydrogenation of Methyl Palmitate to Cetyl Alcohol via Ternary Synergistic Catalysis of Ni, Oxygen Vacancies, and Lewis Acid Sites under Mild Reaction Conditions. ACS Sustain. Chem. Eng. 2021, 9, 9789–9801. [Google Scholar] [CrossRef]
- Lee, W.-S.; Wang, Z.; Wu, R.J.; Bhan, A. Selective vapor-phase hydrodeoxygenation of anisole to benzene on molybdenum carbide catalysts. J. Catal. 2014, 319, 44–53. [Google Scholar] [CrossRef]
- Yu, C.; Yu, S.; Li, L. Upgraded methyl oleate to diesel-like hydrocarbons through selective hydrodeoxygenation over Mo-based catalyst. Fuel 2022, 308, 122038. [Google Scholar] [CrossRef]
- Sun, Q.-Q.; Liu, C.; Zhang, G.-Q.; Liu, Z.-Q.; Wang, M.-Y.; Wang, A.-M.; Liu, Y.; Shen, R.; Ying, A. Synergistic enhancement of catalytic hydrodeoxygenation performance by oxygen vacancies and frustrated Lewis pairs. Fuel 2025, 382, 133748. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, H.; Yang, H.; Juan, C.; Li, D.; Wen, X.; Zhang, F.; Zou, J.-J.; Peng, C.; Hu, C. Ni nanoparticle coupled surface oxygen vacancies for efficient synergistic conversion of palmitic acid into alkanes. Chin. J. Catal. 2023, 47, 229–242. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Zhang, Q.; Chen, L.; Chen, Y.; Liu, J.; Ma, L. Hydrodeoxygenation of Fatty Acid Esters over Nitrogen-Functionalized Bimetallic Oxides Catalyst. Ind. Eng. Chem. Res. 2024, 63, 10584–10595. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Zhang, B.; Hu, M. Improvement of hydrodeoxygenation stability of nickel phosphide based catalysts by silica modification as structural promoter. Fuel 2017, 204, 144–151. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Marliza, T.S.; Taufiq-Yap, Y.H. Pyrolytic-deoxygenation of triglycerides model compound and non-edible oil to hydrocarbons over SiO2-Al2O3 supported NiO-CaO catalysts. J. Anal. Appl. Pyrolysis 2018, 129, 221–230. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, K.; Kim, M.Y.; Chang, Y.K.; Choi, M. Effects of Fatty Acid Compositions on Heavy Oligomer Formation and Catalyst Deactivation during Deoxygenation of Triglycerides. ACS Sustain. Chem. Eng. 2018, 6, 17168–17177. [Google Scholar] [CrossRef]
- Ojagh, H.; Creaser, D.; Salam, M.A.; Grennfelt, E.L.; Olsson, L. The effect of rosin acid on hydrodeoxygenation of fatty acid. J. Energy Chem. 2019, 28, 85–94. [Google Scholar] [CrossRef]
- Xiao, Y.; Ramanathan, A.; Subramaniam, B.; Varma, A. Guaiacol Hydrodeoxygenation and Hydrogenation over Bimetallic Pt-M (Nb, W, Zr)/KIT-6 Catalysts with Tunable Acidity. ACS Sustain. Chem. Eng. 2022, 10, 4831–4838. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhang, S.; Wang, Y.; Hu, S.; Xiang, J.; Wei, T.; Niu, S.; Hu, X. Correlations of Lewis acidic sites of nickel catalysts with the properties of the coke formed in steam reforming of acetic acid. J. Energy Inst. 2022, 101, 277–289. [Google Scholar] [CrossRef]
- Yang, H.; Du, X.; Lei, X.; Zhou, K.; Tian, Y.; Li, D.; Hu, C. Unraveling enhanced activity and coke resistance of Pt-based catalyst in bio-aviation fuel refining. Appl. Energy 2021, 301, 117469. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, H.; Yang, Z.; Li, Y. Boosting bio-lipids hydrodeoxygenation via highly dispersed and coking-resistance bimetallic Ni-La/SiO2 catalyst. J. Environ. Chem. Eng. 2025, 13, 114968. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, J.-K.; Lee, M.-E.; Lee, S.; Choi, M. Maximizing Biojet Fuel Production from Triglyceride: Importance of the Hydrocracking Catalyst and Separate Deoxygenation/Hydrocracking Steps. ACS Catal. 2017, 7, 6256–6267. [Google Scholar] [CrossRef]
- Lee, K.; Lee, M.-E.; Kim, J.-K.; Shin, B.; Choi, M. Single-step hydroconversion of triglycerides into biojet fuel using CO-tolerant PtRe catalyst supported on USY. J. Catal. 2019, 379, 180–190. [Google Scholar] [CrossRef]
- Rabaev, M.; Landau, M.V.; Vidruk-Nehemya, R.; Goldbourt, A.; Herskowitz, M. Improvement of hydrothermal stability of Pt/SAPO-11 catalyst in hydrodeoxygenation–isomerization–aromatization of vegetable oil. J. Catal. 2015, 332, 164–176. [Google Scholar] [CrossRef]
- Valencia, D.; García-Cruz, I.; Uc, V.H.; Ramírez-Verduzco, L.F.; Amezcua-Allieri, M.A.; Aburto, J. Unravelling the chemical reactions of fatty acids and triacylglycerides under hydrodeoxygenation conditions based on a comprehensive thermodynamic analysis. Biomass Bioenergy 2018, 112, 37–44. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Phillips, C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2011, 397, 1–12. [Google Scholar] [CrossRef]
- Agarwal, P.; Al-Khattaf, S.S.; Klein, M.T. Molecular-Level Kinetic Modeling of Triglyceride Hydroprocessing. Energy Fuels 2019, 33, 7377–7384. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Chen, X.; Zhao, C.; Ling, Y.; Liang, C. Co3Mo3N as an alternative for noble-metal catalysts in hydrodeoxygenation of methyl palmitate to diesel range hydrocarbons. Sustain. Energy Fuels 2022, 6, 3025–3034. [Google Scholar] [CrossRef]
- Oyeyemi, V.B.; Dieterich, J.M.; Krisiloff, D.B.; Tan, T.; Carter, E.A. Bond Dissociation Energies of C10 and C18 Methyl Esters from Local Multireference Averaged-Coupled Pair Functional Theory. J. Phys. Chem. A 2015, 119, 3429–3439. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, W.; Zhang, Y.; Zhang, L.; Meng, C.; Liu, C.; Tang, T. Deep dive into the underlying cause of the carbon loss and the associated chemical processes in fatty acid hydrodeoxygenation over Ni@Hβ catalyst. Fuel Process. Technol. 2024, 255, 108062. [Google Scholar] [CrossRef]
- Jeništová, K.; Hachemi, I.; Mäki-Arvela, P.; Kumar, N.; Peurla, M.; Čapek, L.; Wärnå, J.; Murzin, D.Y. Hydrodeoxygenation of stearic acid and tall oil fatty acids over Ni-alumina catalysts: Influence of reaction parameters and kinetic modelling. Chem. Eng. J. 2017, 316, 401–409. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Z.-M.; Liu, L.-J.; Liu, T.-H.; Li, D.; Yang, H.-Q.; Hu, C.-W. Theoretical insight into the deoxygenation molecular mechanism of butyric acid catalyzed by a Ni12P6 cluster. Catal. Sci. Technol. 2021, 11, 6425–6437. [Google Scholar] [CrossRef]
- Fu, S.; Li, D.; Liu, T.; Liu, L.; Yang, H.; Hu, C. Mechanism Insight into Catalytic Performance of Ni12P5 over Ni2P toward the Catalytic Deoxygenation of Butyric Acid. Catalysts 2022, 12, 569. [Google Scholar] [CrossRef]
- Wagenhofer, M.F.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Carbon–Carbon Bond Scission Pathways in the Deoxygenation of Fatty Acids on Transition-Metal Sulfides. ACS Catal. 2017, 7, 1068–1076. [Google Scholar] [CrossRef]
- Rao, K.R.; Pace, R.B.; Suarez, H.; Santillan-Jimenez, E.; Risko, C. Carboxylic Acid Decarbonylation on Nickel: The Critical Role of the Acid Binding Geometry. ACS Catal. 2023, 13, 9102–9112. [Google Scholar] [CrossRef]
- Zhao, L.; Li, B.; Zhao, C. Selective Decarbonylation of Fatty Acids to Long-Chain Alkenes via PtSn/SnOx-Induced C–O Activation. ACS Sustain. Chem. Eng. 2021, 9, 12970–12977. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, W.; Chen, C.; Wang, J.; Li, B.; Fu, J. Dehydrative decarbonylation of fatty acids into long-chain olefins over CoNx/NC catalysts. Fuel 2024, 362, 130721. [Google Scholar] [CrossRef]
- Zhong, J.; Deng, Q.; Cai, T.; Li, X.; Gao, R.; Wang, J.; Zeng, Z.; Dai, G.; Deng, S. Graphitic carbon embedded FeNi nanoparticles for efficient deoxygenation of stearic acid without using hydrogen and solvent. Fuel 2021, 292, 120248. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Briones, L.; Arroyo, M. Selective hydrodecarboxylation of fatty acids into long-chain hydrocarbons catalyzed by Pd/Al-SBA-15. Microporous Mesoporous Mater. 2019, 280, 88–96. [Google Scholar] [CrossRef]
- Arroyo, M.; Briones, L.; Hernando, H.; Escola, J.M.; Serrano, D.P. Selective Decarboxylation of Fatty Acids Catalyzed by Pd-Supported Hierarchical ZSM-5 Zeolite. Energy Fuels 2021, 35, 17167–17181. [Google Scholar] [CrossRef]
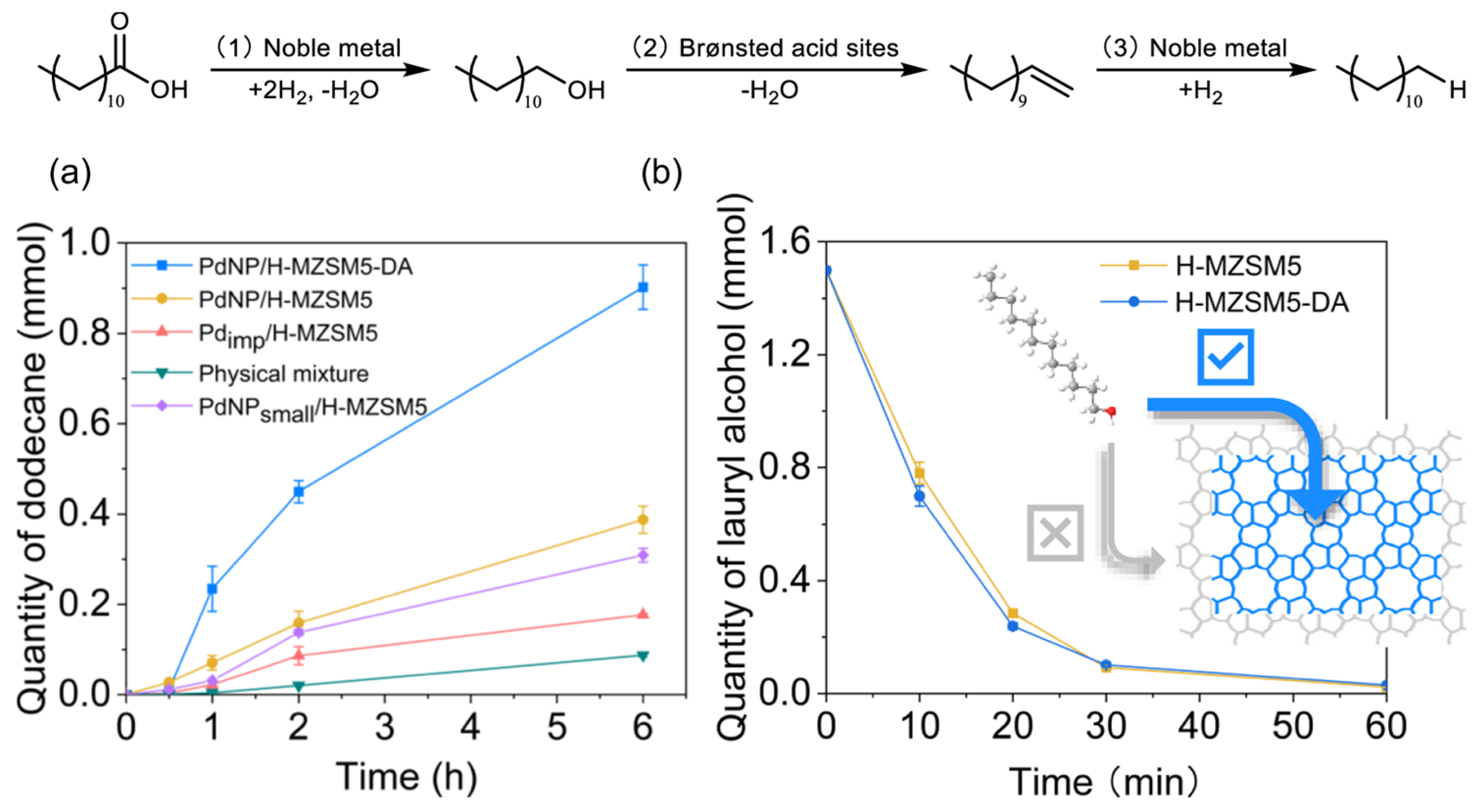
- Ding, S.; Fernandez Ainaga, D.L.; Hu, M.; Qiu, B.; Khalid, U.; D’Agostino, C.; Ou, X.; Spencer, B.; Zhong, X.; Peng, Y.; et al. Spatial segregation of catalytic sites within Pd doped H-ZSM-5 for fatty acid hydrodeoxygenation to alkanes. Nat. Commun. 2024, 15, 7718. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Xia, S. Highly Efficient Catalytic Hydrodeoxygenation for Aliphatic Acid to Liquid Alkane: The Role of Molybdenum. Catalysts 2023, 13, 1329. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Duan, J.; Chen, J.; Ye, Y.; Wang, D.; Li, S.; Zheng, Z. Rationally control the path of hydrodeoxygenation of palmitic acid over Ni/red-mud catalysts by surface decoration of oxophilic MoOx species. Fuel 2022, 329, 125447. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Deng, Z.; Dasgupta, A.; Guo, Y. Hydrothermal hydrodeoxygenation of palmitic acid over Pt/C catalyst: Mechanism and kinetic modeling. Chem. Eng. J. 2021, 407, 126332. [Google Scholar] [CrossRef]
- Verma, V.; Mishra, A.; Anand, M.; Farooqui, S.A.; Sinha, A.K. Catalytic hydroprocessing of waste cooking oil for the production of drop-in aviation fuel and optimization for improving jet biofuel quality in a fixed bed reactor. Fuel 2023, 333, 126348. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; Xin, H.; Wang, G.; Li, D.; Hu, C. The production of diesel-like hydrocarbons from palmitic acid over HZSM-22 supported nickel phosphide catalysts. Appl. Catal. B Environ. 2015, 174–175, 504–514. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Y.; Zhao, Y.; Zhou, H.; Liu, X.; Guo, Y.; Wang, Y. Hydrotreating of Palm Oil into Bio Jet Fuel through a One-Step Process over Nb Doping Pt/AlSiO-Nb Catalyst. Ind. Eng. Chem. Res. 2024, 63, 8216–8227. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, J.; Yu, P.; Zhang, H.; Ma, Y.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. An in-situ combo Mo-based ionic liquid and SAPO-11 catalyst for efficient biolipids hydrodeoxygenation and isomerization. Fuel 2024, 362, 130827. [Google Scholar] [CrossRef]
- Ishihara, A.; Takemoto, K.; Hashimoto, T. Aromatics formation by dehydrocyclization-cracking of methyl oleate using ZnZSM-5-alumina composite-supported NiMo sulfide catalysts. Fuel 2021, 289, 119885. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, K.M.; Kay Lup, A.N.; Patah, M.F.A.; Wan Daud, W.M.A. Role of Ni–Fe/ZSM-5/SAPO-11 bifunctional catalyst on hydrodeoxygenation of palm oil and triolein for alternative jet fuel production. Biomass Bioenergy 2022, 164, 106563. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Mastuli, M.S.; Salmiaton, A.; Azuwa Mohamed, M.; Lee, H.V.; Taufiq-Yap, Y.H. Single-step catalytic deoxygenation-cracking of tung oil to bio-jet fuel over CoW/silica-alumina catalysts. Fuel 2022, 325, 124917. [Google Scholar] [CrossRef]
- Hellier, P.; Ladommatos, N.; Yusaf, T. The influence of straight vegetable oil fatty acid composition on compression ignition combustion and emissions. Fuel 2015, 143, 131–143. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A model compound (methyl oleate, oleic acid, triolein) study of triglycerides hydrodeoxygenation over alumina-supported NiMo sulfide. Appl. Catal. B Environ. 2017, 201, 290–301. [Google Scholar] [CrossRef]
- Hachemi, I.; Murzin, D.Y. Kinetic modeling of fatty acid methyl esters and triglycerides hydrodeoxygenation over nickel and palladium catalysts. Chem. Eng. J. 2018, 334, 2201–2207. [Google Scholar] [CrossRef]
- Zeng, D.; Li, Y.; Ma, H.; Cui, F.; Zhang, J. CuO@NiO Nanoparticles Derived from Metal–Organic Framework Precursors for the Deoxygenation of Fatty Acids. ACS Sustain. Chem. Eng. 2021, 9, 15612–15622. [Google Scholar] [CrossRef]
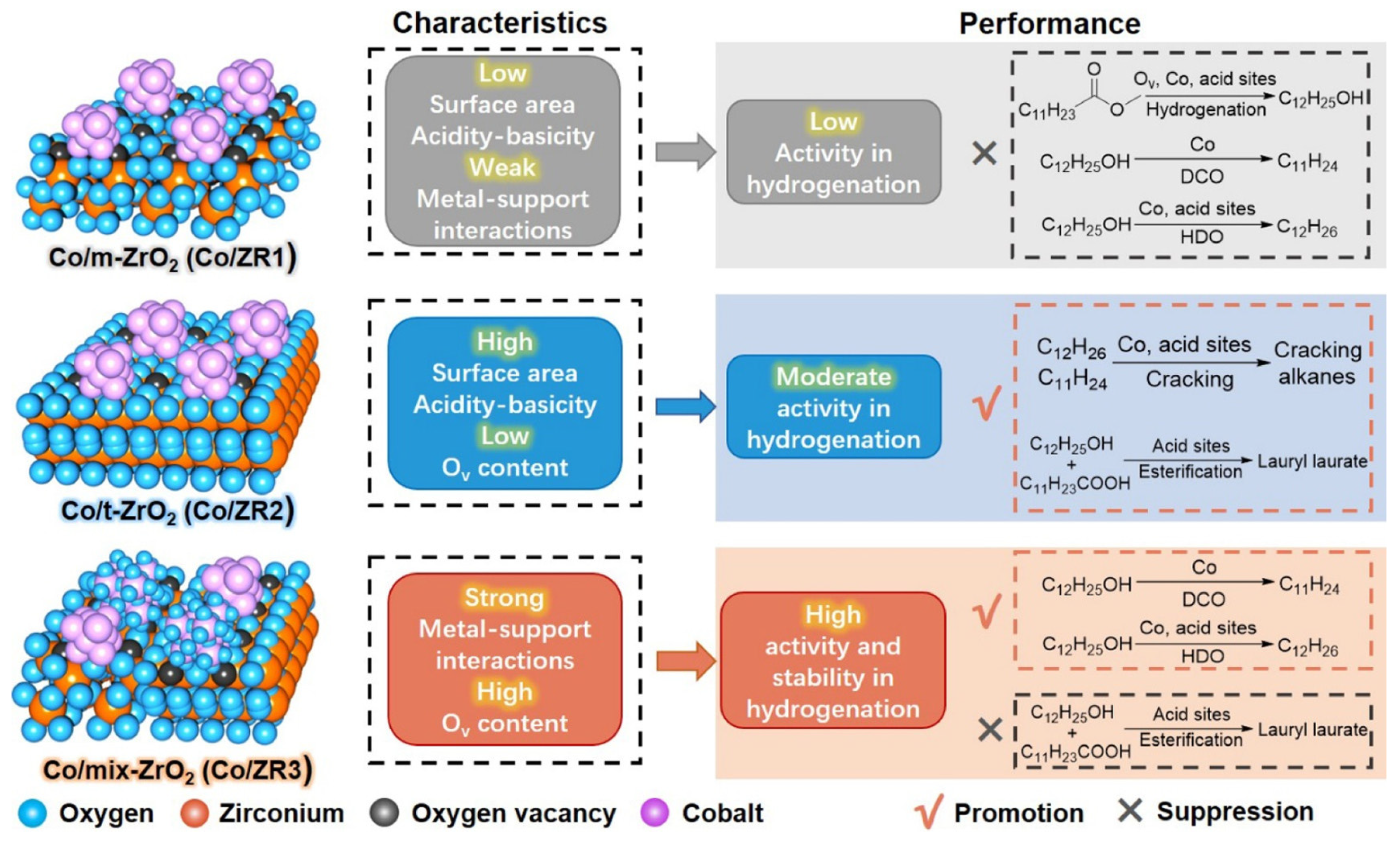
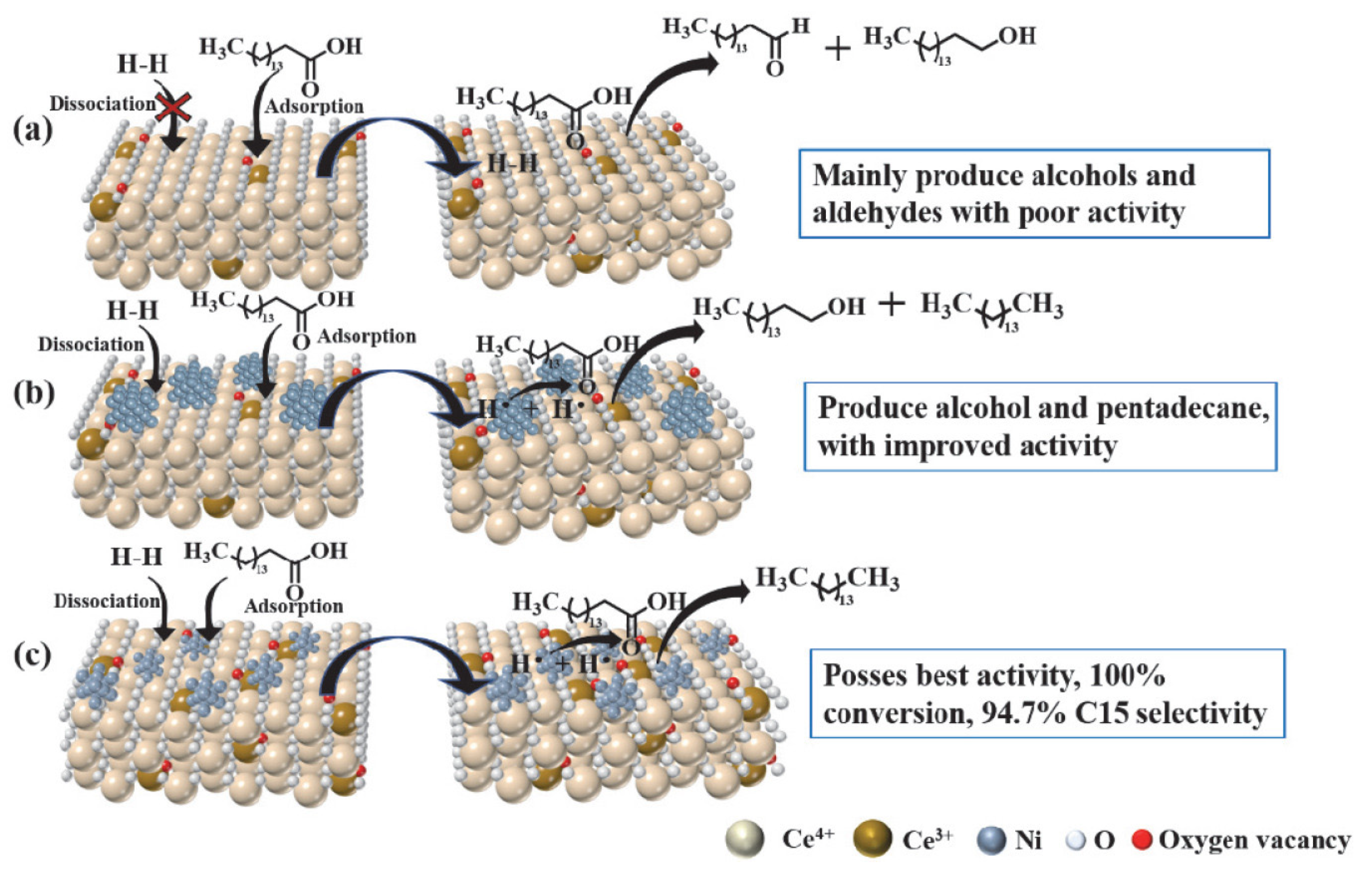

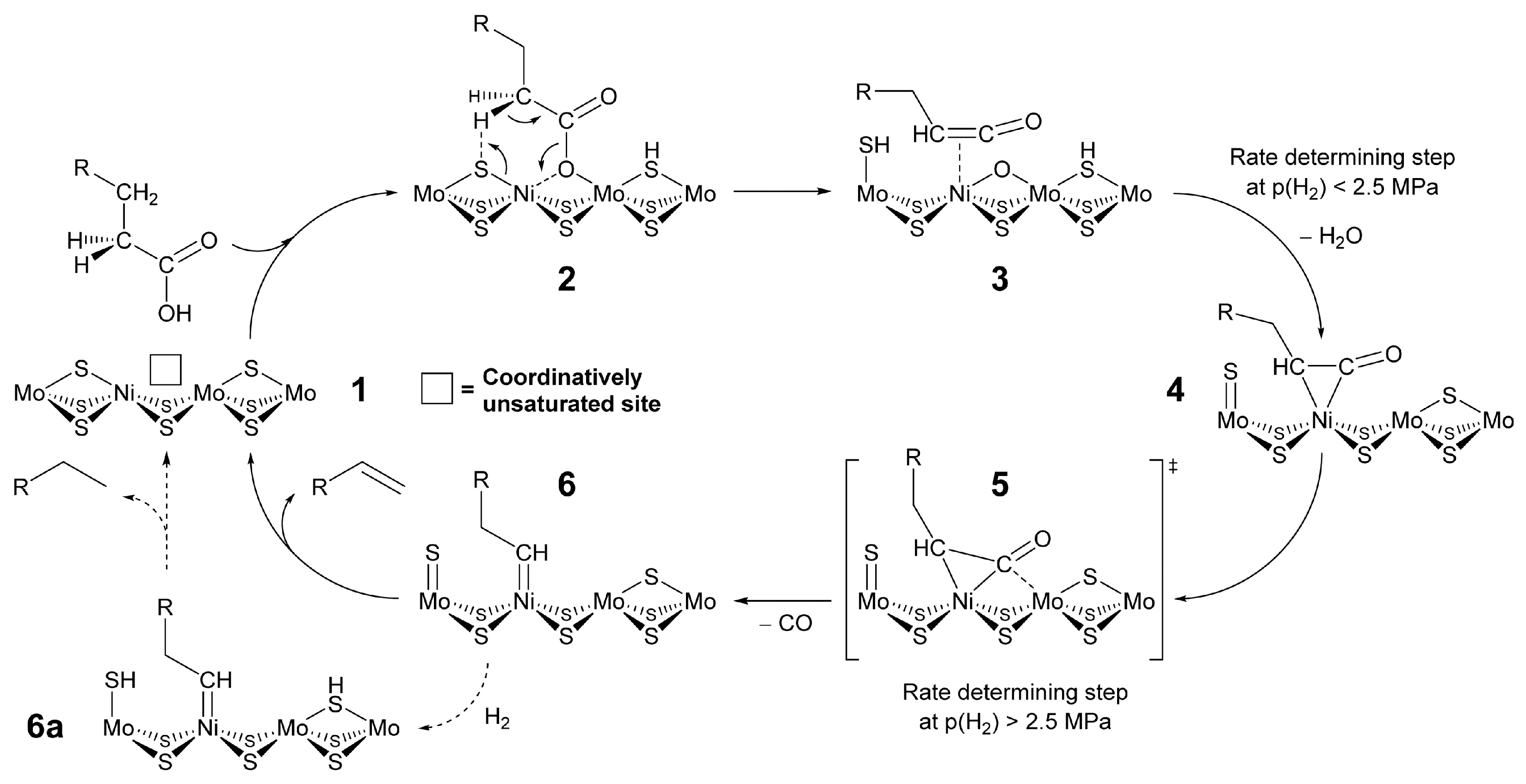

| Year | Review Title | Ref. |
|---|---|---|
| 2020 | Recent advancement in deoxygenation of fatty acids via homogeneous catalysis for biofuel production | [13] |
| 2021 | Catalytic hydrothermal deoxygenation of lipids and fatty acids to diesel-like hydrocarbons: a review | [14] |
| 2021 | Hydroconversion of fatty acids and vegetable oils for production of jet fuels | [15] |
| 2022 | Hydrocracking, hydrogenation, and hydro-deoxygenation of fatty acids, esters, and glycerides: Mechanisms, kinetics, and transport phenomena | [16] |
| 2022 | Production of jet biofuels by catalytic hydroprocessing of esters and fatty acids: A review | [17] |
| 2022 | Biosynthesis of alkanes/alkenes from fatty acids or derivatives (triacylglycerols or fatty aldehydes) | [18] |
| 2022 | Advances in catalytic decarboxylation of bioderived fatty acids to diesel-range alkanes | [19] |
| 2022 | Recent advances in the catalytic deoxygenation of plant oils and prototypical fatty acid models compounds: Catalysis, process, and kinetics | [20] |
| 2023 | Hydroprocessing of lipids: An effective production process for sustainable aviation fuel | [21] |
| 2023 | Deoxygenation of vegetable oils and fatty acids: How can we steer the reaction selectivity towards diesel range hydrocarbons? | [22] |
| 2024 | Emerging catalysis in solvent-free hydrodeoxygenation of waste lipids under mild conditions: A review | [23] |
| 2024 | Dilemma and strategies for production of diesel-like hydrocarbons by deoxygenation of biomass-derived fatty acids | [24] |
| 2025 | A mini review on catalytic hydrodeoxygenation for biofuels production: catalyst, mechanism, and process | [25] |
| Catalysts | Feed | Reaction Conditions | Activity Performance | Ref. |
|---|---|---|---|---|
| Pt/C | Palmitic acid | Batch reactor, T = 370 °C, | 76% conversion, 90% C15 selectivity | [26] |
| P = 0.1 MPa N2, t = 1 h | ||||
| Rea/Cat (wt/wt) = 5/3 | ||||
| Pt/C | Stearic acid | Batch reactor, T = 350 °C, | 100% conversion, 90% alkanes selectivity | [27] |
| P = 0.1 MPa N2, t = 3 h, | ||||
| Rea/Cat (wt/wt) = 20 | ||||
| Pt/ZIF-67/zeolite 5A | Palmitic acid | Batch reactor, | 95% conversion, 91.7% C15 selectivity | [28] |
| T = 300 °C, P = 2 MPa CO2, t = 5 h, | ||||
| Rea/Cat (wt/wt) = 1 | ||||
| Pt/ZIF-67/zeolite 5A | Lauric acid | Batch reactor, | 95% conversion, 93.5% C11 selectivity | [28] |
| T = 320 °C, P = 2 MPa CO2, t = 2 h, | ||||
| Rea/Cat (wt/wt) = 1 | ||||
| Pd/C | Palmitic acid | Batch reactor, | 85 wt% Hydrocarbons yield | [29] |
| T = 270 °C, P = 2 MPa H2, t = 10 h, | ||||
| Rea/Cat (wt/wt) = 5 | ||||
| Pd@Al3-mSiO2 | Methyl palmitate | Batch reactor, | 95.6% conversion, 99% alkanes selectivity | [30] |
| T = 260 °C, P = 3 MPa H2, t = 5 h, | ||||
| Rea/Cat (wt/wt) = 10/3 | ||||
| Ru@TpPON | Stearic acid | Batch reactor, | 100% conversion, 96.0% alkanes selectivity | [31] |
| T = 180 °C, P = 3 MPa H2, t = 8 h, | ||||
| Rea/Cat (wt/wt) = 5 | ||||
| Pt/TiO2 | Stearic acid | LED photoreactor, | 96% conversion, 92% C17 yield | [32] |
| 365 nm, 18 W, | ||||
| T = 30 °C, P = 0.1 MPa H2, t = 2 h, | ||||
| Rea/Cat (wt/wt) = 1.5 | ||||
| 1.5Au-0.8Pd/TiO2 | Hexanoic acid | Xe lamp photoreactor, 300 W, | 94.7% conversion, 100% pentane selectivity | [33] |
| T = 20 °C, P = 0.5 MPa H2, t = 4 h, | ||||
| Rea/Cat (wt/wt) = 1.5 |
| Catalysts | Feed | Reaction Conditions | Activity Performance | Ref. |
|---|---|---|---|---|
| Ni-FSM-16 | Oleic acid | Round-bottom glass flask | 85.7% conversion, 87% hydrocarbons selectivity | [35] |
| T = 350 °C, t = 0.5 h, | ||||
| Rea/Cat (wt/wt) = 10 | ||||
| Ni/ZSM-5.SAPO-11 | Palmitic oil | Batch reactor, | 51% jet fuel yield | [36] |
| T = 350 °C, P = 2.7 MPa H2, t = 2 h, | ||||
| Rea 10 mL, Cat 2 g | ||||
| Ni/TS-1 | Palmitic acid | Batch reactor, | 100% conversion, 91.6% C15 selectivity | [37] |
| T = 260 °C, P = 4 MPa H2, t = 10 h, | ||||
| Rea/Cat (wt/wt) = 5 | ||||
| Ni-Al0.33Ox | Stearic acid | Batch reactor, T = 250 °C, t = 8 h, | 99% conversion, 93.2% C18 selectivity | [38] |
| Rea/Cat (wt/wt) = 2.5 | ||||
| isopropanol as hydrogen source | ||||
| Ni/HUSY-4 | Stearic acid | Batch reactor, | 100% conversion, 96% C18 selectivity | [39] |
| T = 260 °C, P = 4 MPa H2, t = 1 h, | ||||
| Rea/Cat (wt/wt) = 10 | ||||
| Ni-ZrO2 | Methyl laurate | Batch reactor, | 100% alkanes yield, 87.6% C11 selectivity | [40] |
| T = 280 °C, P = 2 MPa H2, t = 8 h, | ||||
| Rea/Cat (wt/wt) = 2 | ||||
| Ni/CeO2-NR | Palmitic acid | Batch reactor, | 99.5% conversion, 84.0% C15 selectivity | [41] |
| T = 270 °C, P = 2 MPa H2, t = 10 h, | ||||
| Rea/Cat (wt/wt) = 5 | ||||
| Ni/B2O3-ZrO2 | Methyl palmitate | Batch reactor, | 100% conversion, 84.4% biofuel yield 65% C15 selectivity | [42] |
| T = 375 °C, P = 4 MPa H2, | ||||
| Rea/Cat (wt/wt) = 10 | ||||
| Ni/BZ-Al50 | Palmitic oil | Trickle bed, | 75% conversion, 52% n-C16-15 selectivity | [43] |
| T = 375 °C, P = 3 MPa H2, t = 6 h, | ||||
| LHSV = 1.2 h−1, H2/oil = 400 (v/v) | ||||
| 15Co/ZrO2 | Ethyl palmitate | Batch reactor, | 100% conversion, 82% alkanes selectivity | [44] |
| T = 240 °C, P = 2 MPa H2, t = 8 h, | ||||
| Rea/Cat (wt/wt) = 2 | ||||
| Co@SiO2 | Palmitic acid | Batch reactor, | 100% conversion, 100% alkanes selectivity | [45] |
| T = 300 °C, P = 2 MPa H2, t = 4 h, | ||||
| Rea/Cat (wt/wt) = 3 | ||||
| Ni1Mo1/ZrO2 | Methyl palmitate | Trickle bed, | 99.4% conversion, 95.0% alkanes selectivity | [46] |
| T = 270 °C, P = 3 MPa H2, | ||||
| H2/oil = 400 (v/v), | ||||
| contact time = 71.3 min | ||||
| Co5Ni5/HAP | Methyl stearate | Batch reactor, | 99.4% conversion, 98.2% C17 selectivity | [47] |
| T = 290 °C, P = 0.1 MPa N2, t = 8 h, | ||||
| Rea/Cat (wt/wt) = 1.5, | ||||
| methanol as hydrogen source | ||||
| 10%Ni-5%Fe/γ-Al2O3 | Palmitic acid | Batch reactor, | 100% conversion, 83.7% C16 + C15 selectivity | [48] |
| T = 270 °C, P = 1.5 MPa H2, t = 6 h, | ||||
| Rea/Cat (wt/wt) = 2 | ||||
| Ni-Er/50S-Al | Jatropha oil | Trickle bed, | 64.7% biofuel yield 99.3% deoxygenation ratio | [49] |
| 340 °C, LHSV = 0.8 h−1, P = 3 MPa | ||||
| Flow = 200 mL/min (H2/N2 = 1:1) | ||||
| Ni3Fe1Re/HZSM-5 | Stearic acid | Batch reactor, T = 260 °C, | 100% conversion, 94.8% C17 selectivity | [50] |
| P = 3 MPa H2, t not mentioned, | ||||
| Rea/Cat (wt/wt) = 10/3 |
| Catalysts | Feed | Reaction Conditions | Activity Performance | Ref. |
|---|---|---|---|---|
| CaO | Waste cooking oil | Batch reactor, | 20.9% acid selectivity, 45.4% alkane selectivity, 18.9% alkene selectivity, 3.0% aromatic selectivity, | [54] |
| T = 300 °C, P = 2.5 MPa N2, t = 1 h, | ||||
| Rea/Cat (wt/wt) = 20 | ||||
| TiO2 | Waste cooking oil | Batch reactor, | 5.4% acid selectivity, 32.8% alkane selectivity, 3.2% alkene selectivity, 48.5% aromatic selectivity, | [54] |
| T = 300 °C, P = 2.5 MPa N2, t = 1 h, | ||||
| Rea/Cat (wt/wt) = 20 | ||||
| Sulfide NiMo/γ-Al2O3 | Degummed palmitic oil | Batch reactor, | 70% diesel yield | [55] |
| T = 260 °C, P = 3 MPa H2, t = 0.5 h, | ||||
| Rea 2 mL, Cat 0.1 g | ||||
| Ni1.5P/AC | Palmitic acid | Trickle bed, | 100% conversion, 56% oil yield, 64% C11-15 selectivity | [56] |
| T = 350 °C, P = 0.1 MPa, 5% H2/Ar, | ||||
| gas/feed = 15 (v/v), WHSV = 0.25 h−1 | ||||
| Ni12P5/SiO2 | Palmitic acid | Batch reactor, | 100% conversion, 59.8% C15 yield 33.7% C16 yield | [57] |
| T = 270 °C, P = 1.2 MPa H2, t = 6 h, | ||||
| Rea/Cat (wt/wt) = 2 | ||||
| Mo2C | Palmitic acid | Batch reactor, | 100% conversion, 96.6% n-C16 selectivity | [58] |
| T = 275 °C, P = 2 MPa H2, t = 8 h, | ||||
| Rea/Cat (wt/wt) = 5 | ||||
| Mo2.56CN0.50 | Palmitic acid | Trickle bed, | 99.6% conversion, 99.2% alkanes selectivity | [59] |
| T = 300 °C, P = 4 MPa H2, | ||||
| H2/feed= 600 (v/v), | ||||
| contact time = 1.18 min | ||||
| Ni-Mo2C/MCM-41 | Palmitic oil | Trickle bed, | 83.3% biofuel yield 95.2% C15-18 selectivity | [60] |
| T = 340 °C, LHSV = 1.4 h−1, P = 3 MPa | ||||
| Flow = 200 mL/min, (H2/N2 = 1) | ||||
| Ni-Mo2C/MCM-41 | Palmitic acid | Batch reactor, | 100% conversion, 96.8% alkanes selectivity | [61] |
| T = 270 °C, P = 2 MPa H2, t = 7 h, | ||||
| Rea/Cat (wt/wt) = 5 | ||||
| W-1000 (W2C) | Stearic acid b | Batch reactor, | 81% conversion, 83% deoxygenation products selectivity | [62] |
| T = 350 °C, P = 0.5 MPa H2, t = 5 h, | ||||
| Rea/Cat (wt/wt) = 4 | ||||
| Ni-W2C-WC/AC | Jatropha oil | Trickle bed, T = 340 °C, | 99.7% deoxygenation rate 94.5% C15-18 selectivity | [63] |
| P = 3 MPa, WHSV = 55.2 h−1, | ||||
| Flow = 200 mL/min (H2/N2 = 1) | ||||
| Ni-Mo2N/γ-Al2O3 | Jatropha oil | Trickle bed, | 100% conversion, 80.1% C15-18 selectivity | [64] |
| T = 320 °C, LHSV = 0.8 h−1, P = 3 MPa | ||||
| Flow = 200 mL/min (H2/N2 = 1) | ||||
| Ni3Mo3N@600 | Palmitic acid | Batch reactor, | 100% conversion, 90% alkanes selectivity | [65] |
| T = 270 °C, P = 2 MPa H2, t = 10 h, | ||||
| Rea/Cat (wt/wt) = 10 |
| Catalyst Type | Advantages | Disadvantages |
|---|---|---|
| Noble metal catalysts | Wide application and excellent activity. The potential for developing photocatalysts is enormous | Expensive and not environmentally friendly Sensitive to impurities of feedstocks |
| Non-noble metal catalysts | Most of them are cheap and easy to obtain. High catalytic activity | Stability is insufficient for industrialization |
| Non-noble metal compound catalysts | Most of them are cheap and easy to obtain. Metal sulfides, phosphides, carbides, and nitrides exhibit excellent activity. | The activity of metal oxides is limited There is a risk of contamination in sulfurized catalysts Metal carbides and nitrides are prone to oxidation and deactivation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Yang, H.; Hu, C. Catalytic Deoxygenation of Lipids for Bio-Jet Fuel: Advances in Catalyst Design and Reaction Pathways. Catalysts 2025, 15, 518. https://doi.org/10.3390/catal15060518
Zhou L, Yang H, Hu C. Catalytic Deoxygenation of Lipids for Bio-Jet Fuel: Advances in Catalyst Design and Reaction Pathways. Catalysts. 2025; 15(6):518. https://doi.org/10.3390/catal15060518
Chicago/Turabian StyleZhou, Linyuan, Huiru Yang, and Changwei Hu. 2025. "Catalytic Deoxygenation of Lipids for Bio-Jet Fuel: Advances in Catalyst Design and Reaction Pathways" Catalysts 15, no. 6: 518. https://doi.org/10.3390/catal15060518
APA StyleZhou, L., Yang, H., & Hu, C. (2025). Catalytic Deoxygenation of Lipids for Bio-Jet Fuel: Advances in Catalyst Design and Reaction Pathways. Catalysts, 15(6), 518. https://doi.org/10.3390/catal15060518




_Xu.png)




