Abstract
In the present study, cellulose and lignin with different weight ratios were mixed and pyrolyzed to prepare biochars for organic dye pollutant removal in water via Fenton-like catalysis. The results indicated that a higher cellulose content in a biomass precursor could result in a lower biochar yield with a lower carbon content in the biochar. Moreover, with the increase in cellulose content, the resulting biochar had a higher graphitization degree with higher levels of crystallinity, as well as a richer porosity. When using Rhodamine B (RhB) as the dye probe, the biochar derived from a higher cellulose/lignin ratio precursor exhibited better adsorptive performance. It was further found that the biochar could act as a Fenton-like catalyst to activate peroxydisulfate (PDS) and accelerate RhB removal via a degradation route, in which single oxygen (1O2) was identified as the active species. Therefore, the biochar/PDS catalytic system exhibited prominent RhB removal stability in various water matrices with a wide pH application range. This study develops a new approach to prepare biomass-derived biochar with high organic removal capacity via Fenton-like catalysis assisted with adsorption synergy.
1. Introduction
Organic dye pollution in the textile industry has received considerable attention in recent years since it can pose a serious threat to ecosystems and human body health []. Many studies have indicated that synthetic dyes with aromatic structures usually have the characteristics of non-biodegradability and high structural and thermal stability []. The effluents containing these compounds are highly toxic which can cause an anaphylactic reaction, nervous system damage, respiratory system injury, and reproductive system and blood system damage and can be a carcinogenic risk to both humans and animals [,]. Therefore, it is important to properly treat the dye effluent before it is discharged into the water.
The adsorptive removal of organic pollutants has been regarded as a promising approach for the treatment of synthetic dye contamination due to its advantages of low processing cost, simple operation flow, high removal rate, and high effluent processing flux []. However, most available adsorbents have many defects including limited adsorption capacity, low universality, poor recyclability, etc. Many recent studies attempted to synergize catalytic degradation with adsorptive removal to reach complete dye effluent decontamination, in which advanced oxidation processes are considered as a promising replenishment [,]. Amongst all applicable adsorbents, biochar prepared from pyrolyzing wood biomass precursors at high temperatures in an oxygen-deficient environment has always been frequently selected due to the advantages of abundant availability, high surface area, low biotoxicity, high stability, and strong affinity to organic molecules [,]. Many previous studies have illustrated that wood-derived biochars were capable of adsorbing a variety of organic pollutants in water []. Most woods contain cellulose, hemi-cellulose, and lignin as their main biomass components [,]. Since these lignocellulosic substances exhibit different decomposition and structure conversion behaviors during the pyrolysis process, the resulting biochar samples often contained different constitutions of oxygen-containing functional groups, which significantly influence the adsorptive capability of the corresponding biochar towards organic pollutants []. Moreover, recent studies have also shown that biochar could also act as Fenton/Fenton-like catalysts to active peroxide with its inherent oxygen-containing functional groups and generate highly active species for organic pollutant degradations []. This means biochar can play a dual role as both an adsorbent and a catalyst for prompt synthetic dye removal in water. Additionally, compared with traditional transition metal-based catalysts, biochar catalysts do not cause secondary pollution in the water media after the catalytic removal process []. Although many previous studies have tried to clarify the influence of different cellulose/lignin ratios in the wood biomass precursor on the adsorptive performance of the resulting biochar [], few studies focused on the influence of cellulose/lignin ratios on the adsorptive/catalytic synergy of the biochar towards organic pollutant removals by Fenton/Fenton-like processes [].
Therefore, in this study, we mixed cellulose and lignin and made a series of biomass precursors with different cellulose/lignin ratios, which were used to prepare biochar Fenton-like catalysts by pyrolysis for Rhodamine B (RhB) dye removal in water. The RhB removal efficiency in different biochar/persulfate systems were studied in detail to elucidate the adsorptive/catalytic synergistic mechanisms in the reaction system.
2. Results and Discussion
2.1. Characterization of Biochar
The morphology of the prepared CL-biochar samples was investigated first in terms of SEM with the results shown in Figure 1. It was clearly illustrated that all three samples exhibited ribbon-like structures in which small spheres were adhered to the ribbons. This was because cellulose and lignin precursors had characteristic tubular and spherical appearances []. With the increase in the lignin content in the biomass precursor, more spheres could be observed. It was also seen that all the samples exhibited clear hierarchical pore structures. This was because the biomass procures released abundant gaseous materials during the pyrolysis process which expanded the pores of the resulting biochar samples. Table 1 shows the biochar yield and EDS elemental concentrations of three biochar samples. It was first found that the biochar sample with higher lignin content had a high biochar yield. This was because cellulose was more prone to decomposing at lower pyrolysis temperatures (200–400 °C) and releasing more volatile contents during pyrolysis []. Conversely, lignin with highly crosslinked conjugated aromatic structures often had a thermal degradation temperature of higher than 400 °C []. Therefore, the higher thermal stability of lignin can lead to a higher biochar yield when the pyrolysis temperature is as high as 800 °C like the present study. As shown in Table 2, with the increase in the lignin content, the carbon content also increased based on the EDS elemental analysis, which was also in accordance with the previous studies [].

Figure 1.
SEM images of (a) C2L1-biochar, (b) C1L1-biochar, and (c) C1L2-biochar.

Table 1.
Biochar yield and elemental concentrations based on EDS elemental analysis of CL-biochar samples.

Table 2.
Specific surface area and pore volume values of C2L1-biochar, C1L1-biochar, and C1L2-biochar.
Figure 2 shows the XRD patterns of the prepared CL-biochar samples. It was illustrated that all three samples had characteristic peaks located at ~23° and 42°, which corresponded to the (002) and (100) lattice planes of a classic carbonaceous material with graphitic structures []. It was reported that cellulose tended to be converted to graphitic carbon under high-temperature treatment, whereas lignin was inclined to be converted to amorphous carbon after pyrolysis []. Therefore, it can be further observed from Figure 2 that the peaks of C2L1 were the sharpest amongst all three samples, manifesting the highest degree of graphitization. Additionally, it was found that the (002) plane peaks shifted to a higher degree with the increase in the cellulose content in the precursor. Since cellulose released more volatile gases and forms graphitic carbon with higher crystallinity, the resulting biochar sample should have fewer oxygen-containing groups between graphitic carbon layers. As a result, the blue shift of the XRD peaks was observed in the biochar with higher cellulose content based on Bragg’s equation (nλ = 2dsinθ) [].
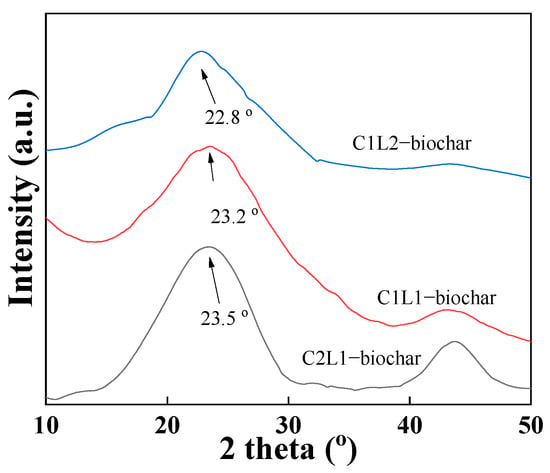
Figure 2.
XRD patterns of C2L1-biochar, C1L1-biochar, and C1L2-biochar.
The graphitic structure could be also investigated with Raman spectra with the results shown in Figure 3. It was presented that all three biochar samples exhibited two characteristic peaks which were located at ~1380 cm−1 and 1560 cm−1 in the figure. These two peaks usually are associated with the defective structure and graphitic structure of a carbonaceous material, respectively, and are named as D band and G band []. As well as this, the graphitic degree of the carbonaceous materials could be quantitively represented by the intensity ratio of these two peaks (ID/IG). If a carbonaceous material had a higher content of sp2 hybridized carbon, it often had a lower ID/IG value, and vice versa. Figure 3 exhibited that with the increase in lignin content in the biomass precursor, the resulting biochar had a higher ID/IG value, indicating a higher content of structural defects in its carbon skeleton, which was in accordance with the XRD results.
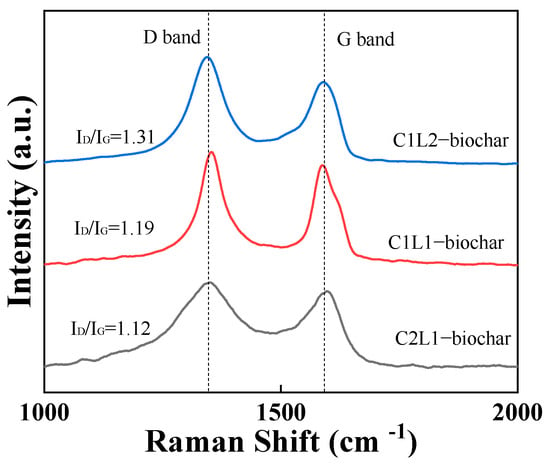
Figure 3.
Raman spectra of C2L1-biochar, C1L1-biochar, and C1L2-biochar.
Figure 4 shows the N2 adsorption/desorption curves and the resulting pore distributions of three biochar samples. The specific values are also listed in Table 2. All the samples exhibited a typical Type IV curve []. It was further demonstrated that C2L1-biochar had the richest porosity amongst all three prepared samples. As discussed above, cellulose was likely to release more volatile gaseous substances during the pyrolysis process due to low thermal stability at high temperatures. These gaseous substances could contribute to the construction of a more developed pore structure of the resulting biochar sample. Therefore, C2L1-biochar had a higher specific surface area and pore volume values than the other two.
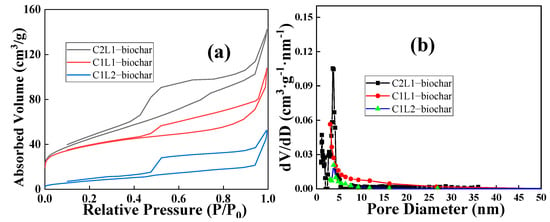
Figure 4.
(a) Nitrogen adsorption/desorption test and (b) corresponding pore distributions of C2L1-biochar, C1L1-biochar, and C1L2-biochar.
2.2. Removal of RhB by Biochar via Adsorptive/Catalytic Synergy
The removal capacity comparison between C2L1-biochar, C1L1-biochar, and C1L2-biochar was investigated by adsorbing RhB in water at room temperature (25 °C) and a pH value of 7.0. As shown in Figure 5a, all three biochar samples had RhB removal capability, in which C2L1-biochar presented the highest RhB adsorptive capacity. Specifically, all the RhB could be removed within 30 min by C2L1-biochar. Based on the calculated pseudo-first-order kinetic value shown in Figure 5b, it was illustrated that the removal rate of C2L1-biochar (k = 0.15 min−1) was nearly 10 times higher than that of C1L2-biochar (k = 0.015 min−1). Having the richest porosity, C2L1-biochar could provide more active sites for RhB adsorption, which resulted in its optimal RhB removal rate. Additionally, since C1L2-biochar contained the most integrated graphitic structure within its skeleton, it could provide the highest adhesion force towards aromatic structured RhB through p-π and π–π conjugations [].
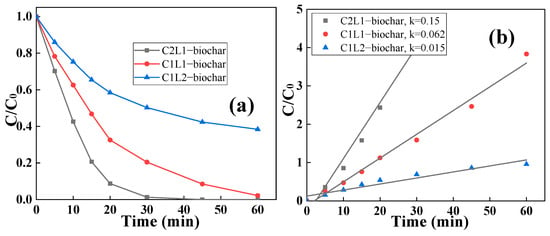
Figure 5.
(a) The adsorption of RhB by C2L1-biochar, C1L1-biochar, and C1L2-biochar; (b) corresponding pseudo-first-order kinetics. ([biochar]0 = 0.5 g/L, [RhB]0 = 0.005 g/L).
The C2L1-biochar was then selected for the following tests due to its optimal performance. The adsorption kinetics of the sample were then investigated and analyzed with pseudo-first-order and pseudo-second-order models. It was found from Figure 6a that the adsorption of RhB by C2L1-biochar fitted both pseudo-first-order kinetics (R2 = 0.985) and pseudo-first-order kinetics (R2 = 0.961) well. This indicated that both chemical adsorption and physical adsorption might co-exist in the solution, while physical interactions were more likely to be built between C2L1-biochar and RhB []. As discussed above, since C2L1-biochar had a more conjugated structure with a higher graphitization degree, it contained fewer oxygen-containing groups within its skeleton. Therefore, it was not likely to build chemical interactions between the adsorbent and adsorbate, and physical bonding like p-π and π–π conjugations were the predominant driving force to trigger RhB adsorption.
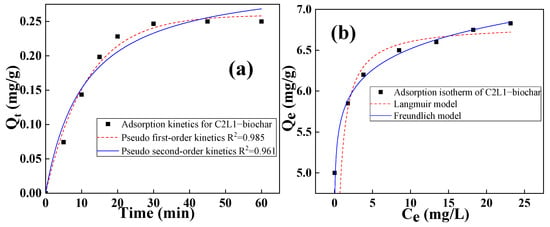
Figure 6.
(a) Adsorption kinetics and (b) adsorption isotherms of C2L1-biochar.
The adsorption isotherm of RhB on C2L1-biochar was then investigated by fitting Langmuir and Freundlich models with the results shown in Figure 6b []. Therefore, 50 mg of C2L1-biochar was put into 50 mL of RhB solution, and the RhB concentration increased from 5 to 30 mg/L. The data were fitted by the model equations as follows:
where qm is the theoretical maximal adsorption amount when the monolayer absorbent is fully covered with the absorbate (mg/g) and KL is the Langmuir constant (L/g) which relates to the adsorption energy.
where is the Freundlich constant (mg/g) related to the adsorption capacity and is also the Freundlich constant related to the adsorption intensity.
It was found that the results fitted both Langmuir and Freundlich models, indicating a homogeneous adsorption of RhB on the C2L1-biochar surface.
It has been intensively reported that carbonaceous biochar could act as the Fenton or Fenton-like catalyst to active peroxide for high-speed organic pollutant degradations []. Therefore, peroxydisulfate (PDS) was introduced to analyze the catalytic removal performance of C2L1-biochar towards RhB. Figure 7 shows the RhB removal by C2L1-biochar with or without the existence of PDS. It was clearly observed from Figure 7a that at the existence of PDS, the RhB removal rate boosted dramatically. As revealed in Figure 7b, the RhB removal rate enhanced nearly 2.5 times when PDS was in the solution, which indicated that degradation besides adsorption also played a significant role in RhB removal. The C2L1-biochar/PDS catalytic system also had equivalent RhB removal capability compared with many previously reported biochar/persulfate systems (Table 3). This means a wood-based biomass was suitable for preparing non-metallic catalyst with high Fenton-like activity through rationally choosing wood precursor with a proper cellulose/lignin ratio. Without the incorporation of transition metal compounds, C2L1-biochar prepared in this study would have higher practical application potentials.
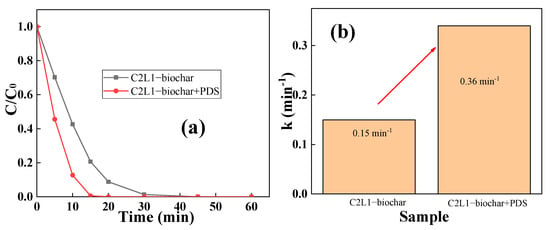
Figure 7.
(a) The impact of PDS introduction on the removal performance of C2L1-biochar; (b) corresponding pseudo-first-order kinetics value comparison. ([biochar]0 = 0.5 g/L, [RhB]0 = 0.005 g/L, [PDS]0 = 0.5 mM).

Table 3.
Performances of biochar/persulfate systems for RhB removal.
Therefore, the active species which participated in RhB degradation were then detected by introducing various scavengers including methanol (MeOH), p-benzoquinone (p-BQ), and L-histidine (L-his) into the reaction solution []. As can be seen in Figure 8a, the existence of both MeOH and p-BQ did not interfere with the performance of C2L1-biochar []. On the contrary, when L-his was introduced, RhB seemed to be only removed by adsorption. Since L-his was a classical singlet oxygen (1O2) scavenger, the results indicated that the introduction of PDS could generate 1O2, which contributed to RhB removal, and 1O2 was generated by the activation of PDS by C2L1-biochar []. ESR shown in Figure 8b also could reveal the type of active species that existed in the C2L1-biochar/PDS catalytic system. It was illustrated that a triplet signal with equal intensity ratios was observed when TMP was introduced as the trapping agent. The peaks were ascribed to the characteristic signal of TMP-1O2 [], indicating the existence of the non-radical species.
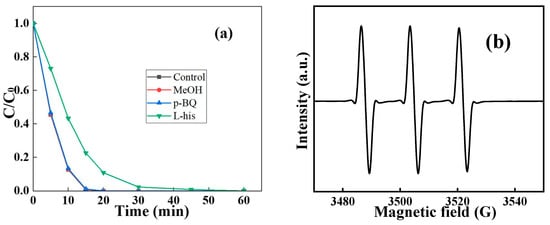
Figure 8.
(a) The impact of various scavenger introductions on the removal performance of C2L1-biochar ([biochar]0 = 0.5 g/L, [RhB]0 = 0.005 g/L, [PDS]0 = 0.5 mM, [scavenger]0 = 10 mM); (b) ESR spectrum of C2L1-biochar/PDS system in the presence of TMP.
The impact of the RhB concentration on the performance of the C2L1-biochar/PDS catalytic system was then investigated alongside the results shown in Figure 9a. It was shown that with the increase in RhB concentration, pristine C2L1-biochar could not completely remove all RhB via a pure adsorption process after all the adsorptive active sites were occupied by RhB molecules. When PDS was introduced into the reaction solution, the RhB removal rate was dramatically accelerated, meaning that RhB with a concentration of 10 mg/L could be completely removed within 30 min. In the meantime, only ~60% of RhB could be removed by a pure adsorption process and even the contact time between C2L1-biochar and RhB was extended to 60 min. The results indicated that adsorptive/catalytic synergy was established in the C2L1-biochar/PDS system for fast RhB removal, in which C2L1-biochar enriched RhB molecules to the surrounding of the active sites, and the generated 1O2 by C2L1-biochar-activated PDS quickly degraded the RhB []. As shown in Figure 9b, the increase in C2L1-biochar dosage in the reaction solution could lead to a higher RhB removal rate by the adsorptive/catalytic synergy. When the dosage of C2L1-biochar reached 1.0g/L, 5 mg/L of RhB could be completely removed within 10 min.
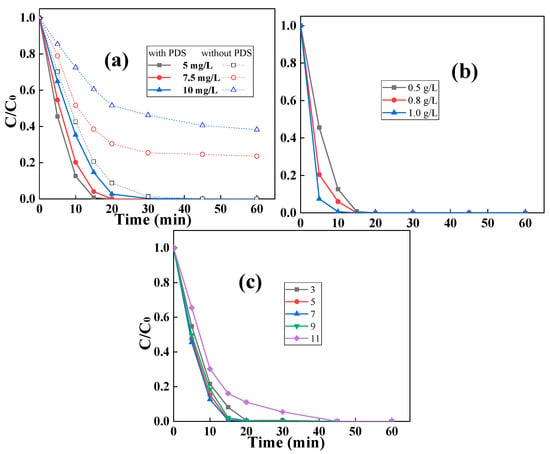
Figure 9.
The impact of (a) RhB concentration ([biochar]0 = 0.5 g/L, [PDS]0 = 0.5 mM), (b) catalyst dosage ([RhB]0 = 0.005 g/L, [PDS]0 = 0.5 mM), and (c) the pH value on the removal performance of C2L1-biochar ([biochar]0 = 0.5 g/L, [RhB]0 = 0.005 g/L, [PDS]0 = 0.5 mM).
Since non-radical 1O2 was the predominant active species responsible for RhB degradation, the C2L1-biochar/PDS catalytic system should have high stability []. The influence of pH fluctuation on the RhB removal rate was then investigated with the results shown in Figure 9c. As illustrated, C2L1-biochar exhibited high adaptability in a wide range of pH values from 3 to 11. The performance of the C2L1-biochar/PDS catalytic system only suffered a little in a strong basic environment, since the negatively charged C2L1-biochar struggled to contact negatively charged PDS when the pH value of the reaction solution reached to 11.
The high adaptability of C2L1-biochar could be further revealed by testing its performance at the existence of foreign interferences. Figure 10a shows the impact of anion on the performance of the C2L1-biochar/PDS system. It was seen that 20 mM of all anions including Cl−, HCO3−, NO3−, and HSO4− had a negligible impact on the performance of the catalytic system. Additionally, when Cl− was introduced, the RhB degradation rate was even enhanced, which might be because the generated active species could also react with Cl− and produce more chlorine-based radicals []. Besides inorganic matter, organic matter existence could also not impede the performance of the catalytic system. Figure 10b illustrates that RhB could still be completely removed in either natural tap water or natural lake water, manifesting the high practical application potential of the prepared biochar. The apparent performance decline should be ascribed to the occupation of adsorptive sites by foreign matters in natural water. Furthermore, since C2L1-biochar exhibited high adsorptive capability, the C2L1-biochar/PDS catalytic system could still maintain its high RhB removal performance for more than three consecutive catalytic cycles even when some of its oxygen-containing functional groups have been consumed during PDS activation (Figure 10c).
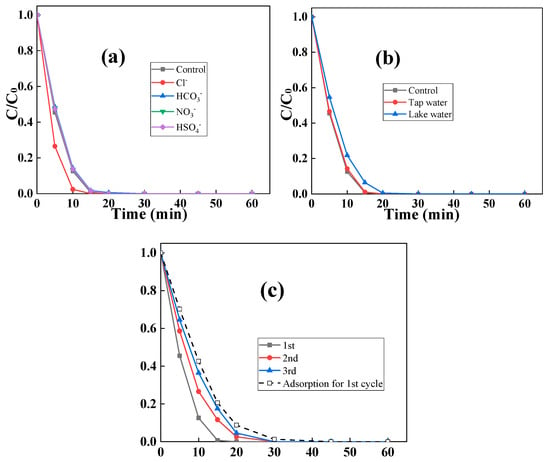
Figure 10.
The impact of (a) foreign anions; (b) natural water matrix and three consecutive runs on the removal performance of C2L1-biochar, (c) recyclability of C2L1-biochar ([biochar]0 = 0.5 g/L, [RhB]0 = 0.005 g/L, [PDS]0 = 0.5 mM, [anion]0 = 20 mM).
3. Materials and Methods
3.1. Reagents and Chemicals
Cellulose powder (particle size= 180 μm), Rhodamine B (RhB, ≥99%), L-histidine (L-his, 99%), sodium peroxydisulfate (PDS, ≥99%), and 4-hydroxy-2,2,6-tetramethylpiperidine (TEP, ≥98%) are purchased from Aladdin (Shanghai, China). Enzymatic hydrolysis lignin (EHL) is purchased from Shandong Gaotang Poly lignin Co., Ltd. (Liaocheng, China). Other reagents and solvents were of analytical grade and used as received. Double-distilled deionized water (H2O) is used throughout the study.
3.2. Preparation of Cellulose/Lignin-Derived Biochar (CL-Biochar)
In order to simulate different species of plants with different cellulose/lignin ratios (wood with high cellulose content, shell with equal cellulose/lignin ratio, and bark with higher lignin ratio), the cellulose/EHL weight ratio was set as 2/1, 1/1, and 1/2 in the biomass precursor. Generally, 2 g of cellulose/EHL mixture was pyrolyzed in a tubular furnace with a heating rate of 5 °C/min under a N2 atmosphere from room temperature to 800 °C, then staying at 800 °C for 3 h. After being cooled to room temperature, the resulting biochar was washed with H2O and ethanol 3 times, respectively, and dried in an oven at 60 °C for 24 h. The obtained CL-biochar samples were named C2L1-biochar, C1L1-biochar, and C1L2 biochar based on their cellulose/EHL in the biomass precursor.
3.3. Characterizations
The microstructure of biochar was observed by a scanning electron microscope (SEM, SEM 6490, JEOL, Tokyo, Japan) after being sprayed by a thin layer of gold. An X-ray diffraction diffractometer (XRD, Rigaku Smartlab, Tokyo, Japan) with Cu-Kα as the radiation source (1.54 A) was employed to analyze the crystalline structure. The graphitic structure of CL-biochar was studied using Raman spectroscopy (Raman, Horiba Jobin Yvon HR800, Kyoto, Japan) equipped with an Ar laser (532 nm, 180 mW) as the excitation light source and a microscope. The N2 adsorption–desorption isotherms of the catalysts were determined using the specific surface area and pore size analyzer (ASAP2020 HD88, Micromeritics, Norcross, USA) and their specific surface area and pore size were analyzed using the Brunauer–Emmett–Teller (BET) model. Electron spin resonance (ESR) was detected by the EMX-10/12 device (Bruker, Billerica, USA) with X-band field scanning. The concentration of the RhB was analyzed by an ultraviolet and visible spectrophotometer (UV1800PC, Jinghua, Shanghai, China).
3.4. Removal of RhB in CL-Biochar/PDS Catalytic System
If not specified, all the experiments were conducted in a 150 mL beaker with magnetic stirring containing 100 mL RhB solution with a certain concentration at an ambient temperature (25 °C). In a typical experiment, 0.5 g/L of CL-biochar was first added to the RhB solution. In the RhB degradation tests, PDS was simultaneously dosed into the solution with CL-biochar. After that, 1 mL of the solution was taken out of the solution at pre-determined time intervals and filtered with a 0.22 μm polytetrafluoroethylene membrane. In the RhB degradation tests, the filtered solution was further injected into 0.5 mL of furfuryl alcohol to cease the reaction.
4. Conclusions
To conclude, biochar samples with different cellulose/lignin ratios in biomass precursor were prepared to remove RhB via Fenton-like catalysis assisted with adsorption synergy in water. The results indicated that cellulose/lignin ratios significantly influenced the structure and RhB removal capability of the resulting biochar. Since cellulose could release more volatile substances during pyrolysis and convert them to graphitic carbon after pyrolysis, C2L1-biochar had the richest porosity with the optimal RhB adsorptive performance. When PDS was added, C2L1-biochar could further act as a Fenton-like catalyst to activate PDS and generate 1O2 for RhB degradation. The C2L1-biochar/PDS catalytic system exhibited high stability in a variety of water matrices due to the existence of adsorptive/catalytic synergy. Compared with many previously reported biochar/persulfate systems containing metal elements, the metal-free C2L1-biochar/PDS catalytic system had equivalent or even superior RhB removal capability without introducing metal ion pollution. Moreover, by rationally choosing a wood precursor with a proper cellulose/lignin ratio, a highly active biochar Fenton-like catalyst could be prepared, which can provide a new suggestion for the treatment of synthetic dye water contamination in the textile industry.
Author Contributions
Investigation, X.Y. and W.X.; data curation, X.Y. and W.X.; formal analysis, X.Y.; writing—original draft preparation, X.Y.; writing—review and editing, L.G.; supervision, L.G.; project administration, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (2023YFD2201405).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The Advanced Analysis and Testing Center of Nanjing Forestry University is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gan, L.; Xu, L.; Qian, K. Preparation of core-shell structured CoFe2O4 incorporated Ag3PO4 nanocomposites for photocatalytic degradation of organic dyes. Mater. Des. 2016, 109, 354–360. [Google Scholar] [CrossRef]
- Turhan, K.; Durukan, I.; Ozturkcan, S.A.; Turgut, Z. Decolorization of textile basic dye in aqueous solution by ozone. Dye. Pigment. 2012, 92, 897–901. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Xu, L.; Chen, M.; Wang, L.; Liu, J.; Han, S.; Mei, C.; Zhong, Q. The fabrication of bio-renewable and recyclable cellulose based carbon microspheres incorporated by CoFe2O4 and the photocatalytic properties. J. Clean. Prod. 2018, 196, 594–603. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Shi, S.; Pan, Y.; Qin, R.; Zhang, Y. The influence of substituent effects of polyphenols on the removal of Cr(VI): The significance of electron-donating and electron-withdrawing groups. Sep. Purif. Technol. 2025, 364, 132500. [Google Scholar] [CrossRef]
- Gan, L.; Xu, L.; Pan, Z.; Jiang, F.; Shang, S. Alginic acid/graphene oxide hydrogel film coated functional cotton fabric for controlled release of matrine and oxymatrine. RSC Adv. 2016, 6, 76420–76425. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Puyang, C.; Tang, S.; Guo, H. Periodate activation by plasma coupled with Fe N C for contaminant removal: Machine learning assisted catalyst optimization and electron shuttle mechanism. J. Colloid Interface Sci. 2025, 685, 975–987. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Guo, H.; Zhang, Z.; Wang, Y.; Pang, P.; Gao, Y.; Guo, H. Enhancing the synergistic effect by introducing Cu(II)-PMS into the underwater bubble plasma treatment process for efficient degradation of emerging contaminants in water. Chem. Eng. J. 2025, 507, 160480. [Google Scholar] [CrossRef]
- Veksha, A.; McLaughlin, H.; Layzell, D.B.; Hill, J.M. Pyrolysis of wood to biochar: Increasing yield while maintaining microporosity. Bioresour. Technol. 2014, 153, 173–179. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Chen, S.; Ling, Z.; Zhang, X.; Kim, Y.S.; Xu, F. Towards a multi-scale understanding of dilute hydrochloric acid and mild 1-ethyl-3-methylimidazolium acetate pretreatment for improving enzymatic hydrolysis of poplar wood. Ind. Crop. Prod. 2018, 114, 123–131. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Gao, S.; Yu, M.; Fu, L.; Zhang, Y.; Sun, H.; Pan, Y. Lignosulfonate modified zero-valent iron enhanced activation of peroxymonosulfate for sulfamethazine removal: Performance and mechanism. Chem. Eng. J. 2024, 504, 159054. [Google Scholar] [CrossRef]
- Geng, A.; Xu, L.; Gan, L.; Mei, C.; Wang, L.; Fang, X.; Li, M.; Pan, M.; Han, S.; Cui, J. Using wood flour waste to produce biochar as the support to enhance the visible-light photocatalytic performance of BiOBr for organic and inorganic contaminants removal. Chemosphere 2020, 250, 126291. [Google Scholar] [CrossRef]
- Shi, J.; Dai, B.; Fang, X.; Xu, L.; Wu, Y.; Lu, H.; Cui, J.; Han, S.; Gan, L. Waste preserved wood derived biochar catalyst for promoted peroxymonosulfate activation towards bisphenol A degradation with low metal ion release: The insight into the mechanisms. Sci. Total. Environ. 2022, 813, 152673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Zhao, C.; Ge, L.; Wang, R.; Chu, H.; Mai, L.; Zha, W.; Wang, Y.; Xu, C. Effects of cellulose addition on the physicochemical properties, pore structure and iodine adsorption of lignin-based biochar. Fuel 2023, 352, 129061. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wu, Y.; Zheng, M. A comparison of biochars from lignin, cellulose and wood as the sorbent to an aromatic pollutant. J. Hazard. Mater. 2014, 280, 450–457. [Google Scholar] [CrossRef]
- Chen, C.; Sun, K.; Huang, C.; Yang, M.; Fan, M.; Wang, A.; Zhang, G.; Li, B.; Jiang, J.; Xu, W.; et al. Investigation on the mechanism of structural reconstruction of biochars derived from lignin and cellulose during graphitization under high temperature. Biochar 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Lédé, J. Cellulose pyrolysis kinetics: An historical review on the existence and role of intermediate active cellulose. J. Anal. Appl. Pyrolysis 2012, 94, 17–32. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. Effect of the Temperature on the Composition of Lignin Pyrolysis Products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Z.; Pan, Y.; Zhang, Y.; Gong, H.; Mei, X.; Qiao, W.; Gan, L. Effect of lignocellulosic biomass composition on the performance of biochar for the activation of peroxymonosulfate to degrade diclofenac. Sep. Purif. Technol. 2023, 311, 123312. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Dai, Z.; Zhang, A.-Y.; Yin, J.; Peng, S.; Liang, H. Recycling chestnut shell for superior peroxymonosulfate activation in contaminants degradation via the synergistic radical/non-radical mechanisms. J. Hazard. Mater. 2022, 430, 128471. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Liu, X.; Xiong, W.; Wang, H.; Ji, X.; Kong, F.; Yang, G.; Xu, J. Converting amorphous kraft lignin to hollow carbon shell frameworks as electrode materials for lithium-ion batteries and supercapacitors. Ind. Crop. Prod. 2021, 174, 114184. [Google Scholar] [CrossRef]
- Yoo, S.; Kelley, S.S.; Tilotta, D.C.; Park, S. Structural Characterization of Loblolly Pine Derived Biochar by X-ray Diffraction and Electron Energy Loss Spectroscopy. ACS Sustain. Chem. Eng. 2018, 6, 2621–2629. [Google Scholar] [CrossRef]
- Shi, J.; Dai, B.; Shen, X.; Xu, L.; Zhang, Y.; Gan, L. Wood induced preparation of Fe3C decorated biochar for peroxymonosulfate activation towards bisphenol a degradation with low ion leaching. J. Environ. Manag. 2023, 340, 117978. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of biochar production from preservative-treated wood with detailed analysis of biochar characteristics, heavy metals behaviors, and their ecotoxicity. J. Hazard. Mater. 2020, 384, 121356. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surfaces Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef]
- Ouyang, F.; Liu, Y.; Chen, J.; Tang, C.; Wang, A.; Lu, Y.; Yuan, Y. Study on Preparation of Rabbit Manure Biochar and Activation of Peroxymonosulfate for Rhodamine B Degradation. Water 2023, 15, 2015. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, Y.; Ding, Y.; Ge, S. Iron-modified granular sludge biochar-based catalysts for improved Rhodamine B degradation by activating peroxymonosulfate. Biomass-Convers. Biorefinery 2022, 14, 27755–27765. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, J.; Tan, W.; Tang, H.; Li, H.; Li, Y. Enhanced degradation of Rhodamine B through activating peroxydisulfate by humic acid-Fe incorporated biochar: Kinetics and mechanism studies. J. Mol. Liq. 2024, 396, 123962. [Google Scholar] [CrossRef]
- Deng, S.; Yang, M.; An, Q.; Li, Z.; Zhao, B.; Ran, B. Efficient rhodamine B dye degradation by red mud-grapefruit peel biochar catalysts activated persulfate in water. Environ. Sci. Pollut. Res. 2023, 30, 119034–119049. [Google Scholar] [CrossRef]
- Mustafa, F.S.; Aziz, K.H.H. Heterogeneous catalytic activation of persulfate for the removal of rhodamine B and diclofenac pollutants from water using iron-impregnated biochar derived from the waste of black seed pomace. Process. Saf. Environ. Prot. 2022, 170, 436–448. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Pan, Y.; Qin, R.; Wang, W.; Zhou, M.; Zhang, Y. Detection methodologies and mechanisms of reactive oxygen species generated in Fenton/Fenton-like processes. Sep. Purif. Technol. 2024, 355, 129578. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, W.; Li, Y.; Zhu, D.; Guo, H. Enhanced degradation of sulfamethoxazole in water using non-thermal plasma-activated persulfate: Effects of heat and radical synergies. J. Environ. Chem. Eng. 2025, 13, 115416. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-D.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Huang, F.; Liu, Y.; Feng, L.; Jiang, J.; Zhang, L.; Qi, F.; Liu, C. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation. Appl. Catal. B Environ. 2021, 286, 119921. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, J. CoMn2O4 embedded hollow activated carbon nanofibers as a novel peroxymonosulfate activator. Chem. Eng. J. 2021, 406, 127158. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, X.; Feng, W.; Lyu, C.; Zhang, Y. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water. J. Hazard. Mater. 2020, 384, 121350. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Gan, L.; Wang, L.; Gong, H.; Xu, L.; Wu, Y.; Lu, H.; Han, S.; Cui, J.; Xia, C. Enhanced degradation of bisphenol A by mixed ZIF derived CoZn oxide encapsulated N-doped carbon via peroxymonosulfate activation: The importance of N doping amount. J. Hazard. Mater. 2021, 419, 126363. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).