Natural Pyrolusite-Catalyzed Ozonation for Nanoplastics Degradation
Abstract
:1. Introduction
2. Results and Discussion
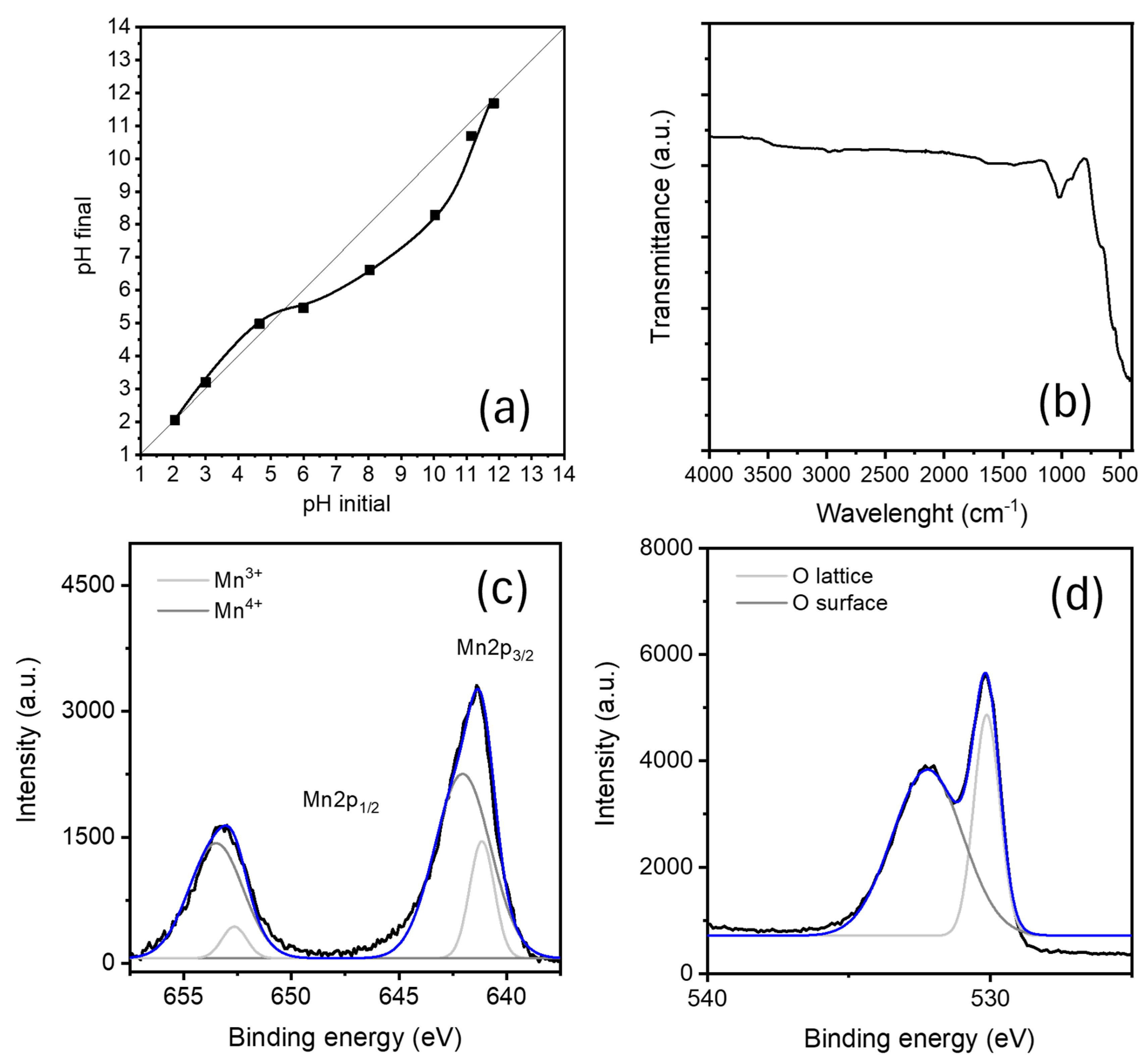
2.1. Pyrolusite Characterization
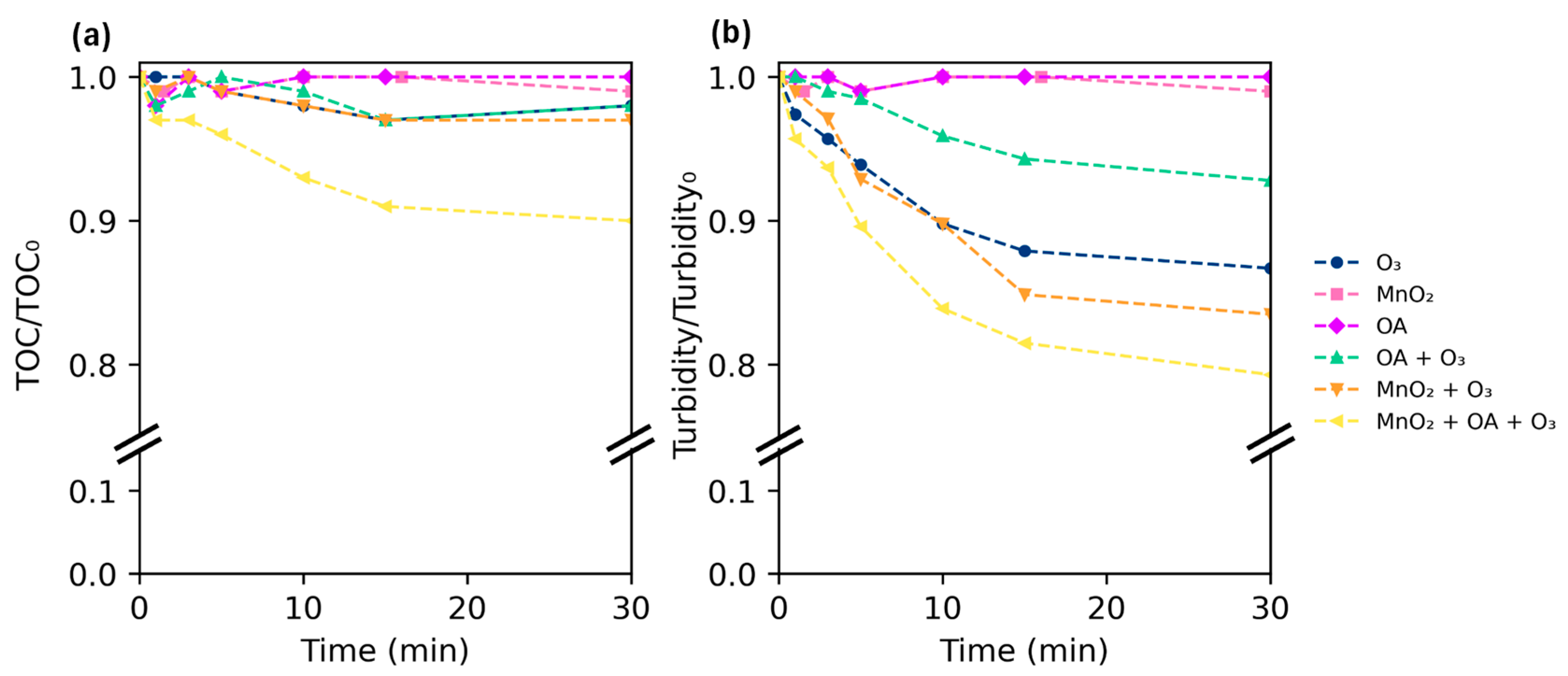
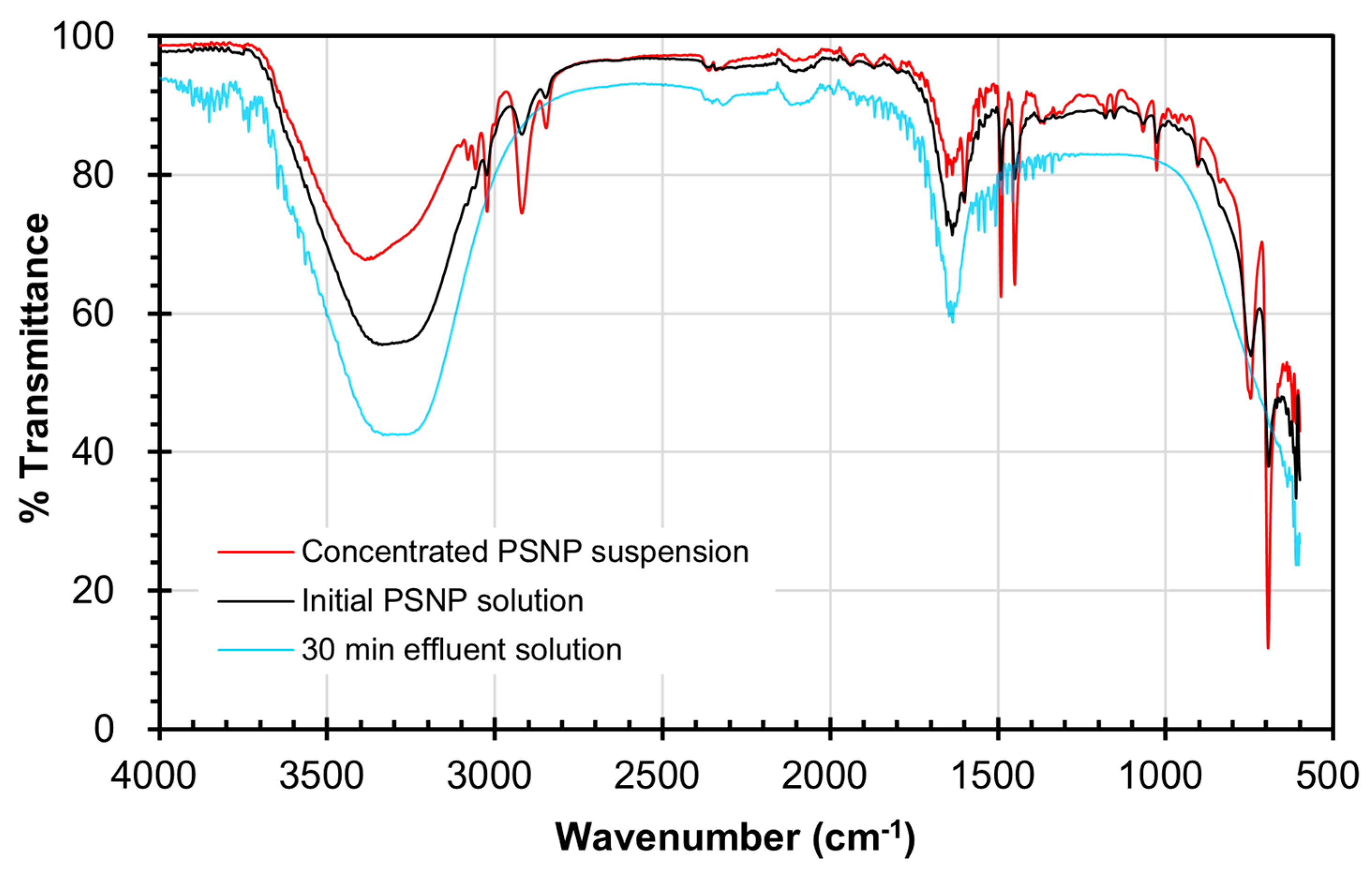
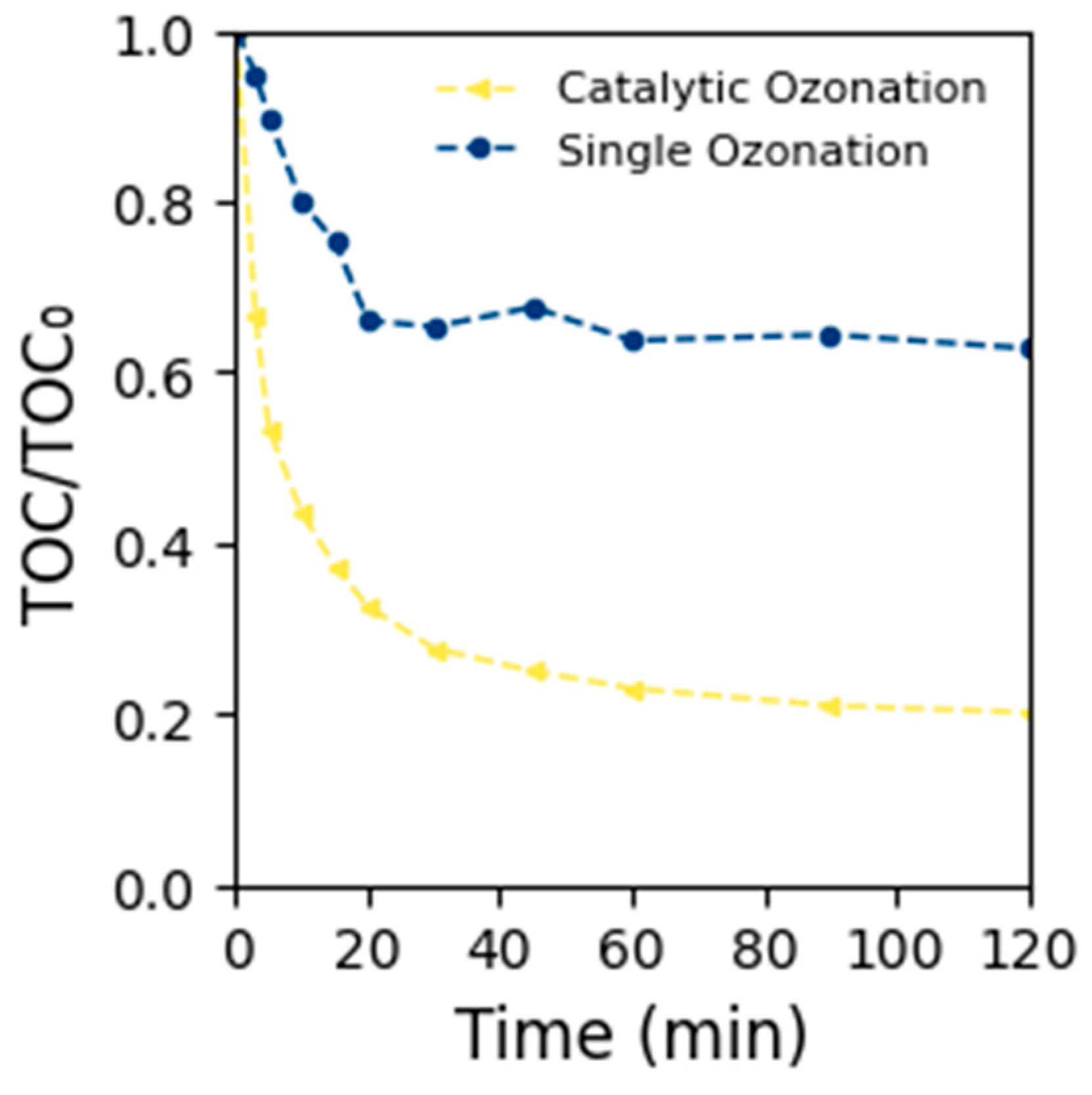
2.2. Catalytic Ozonation of Polystyrene Nanoplastics
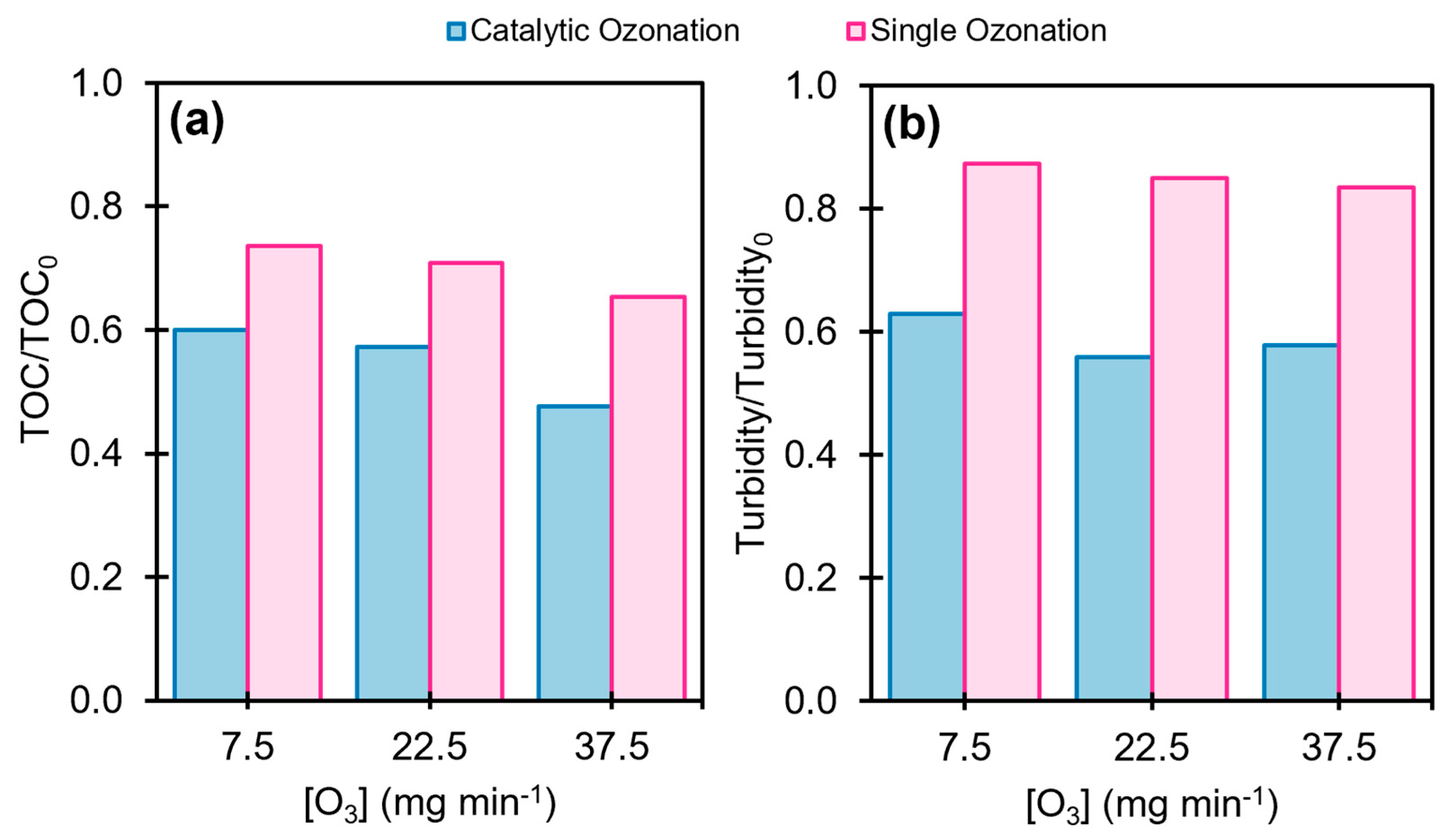
2.3. Effects of pH and Ozone Dosage
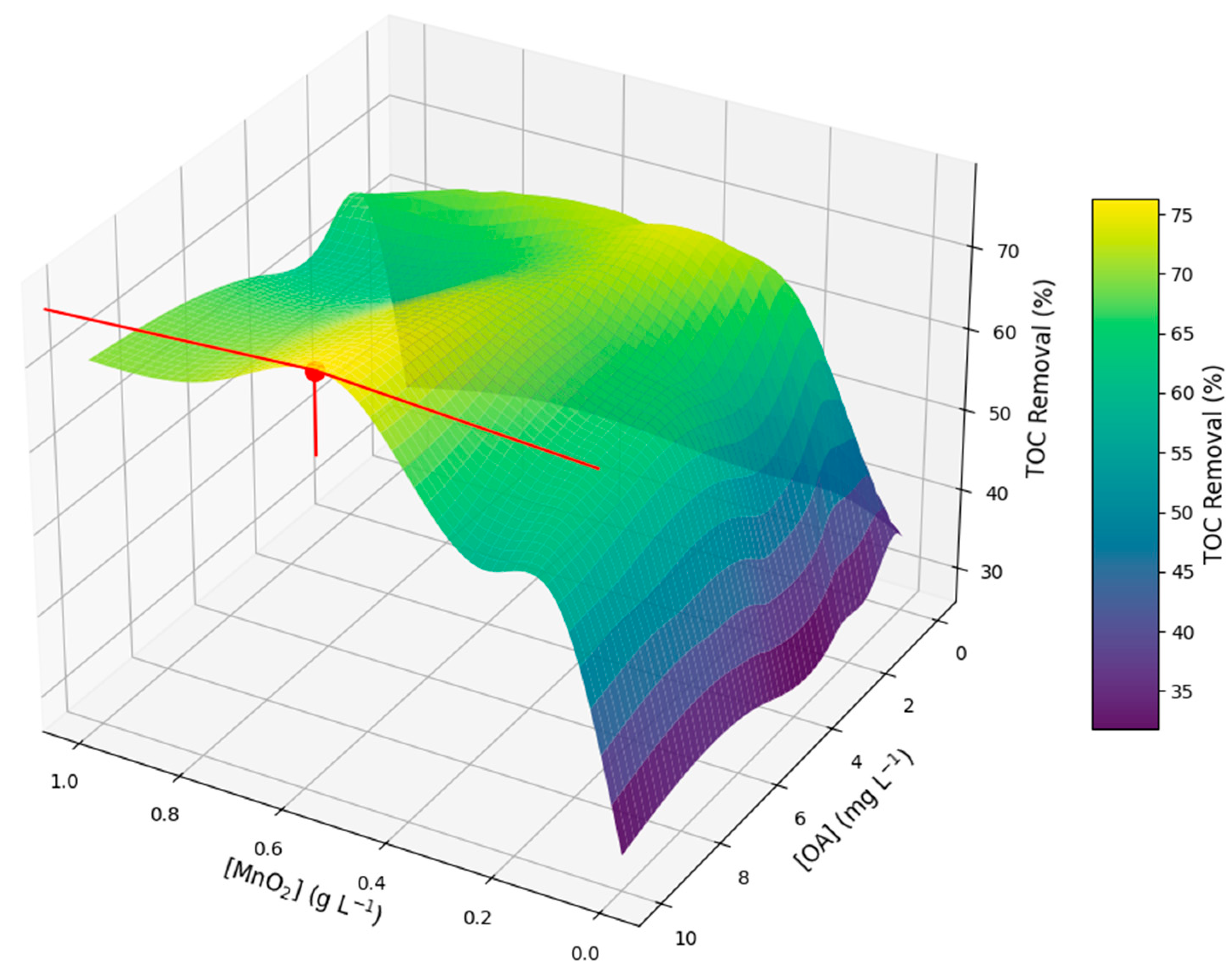
2.4. Effect of Pyrolusite and Oxalic Acid Doses
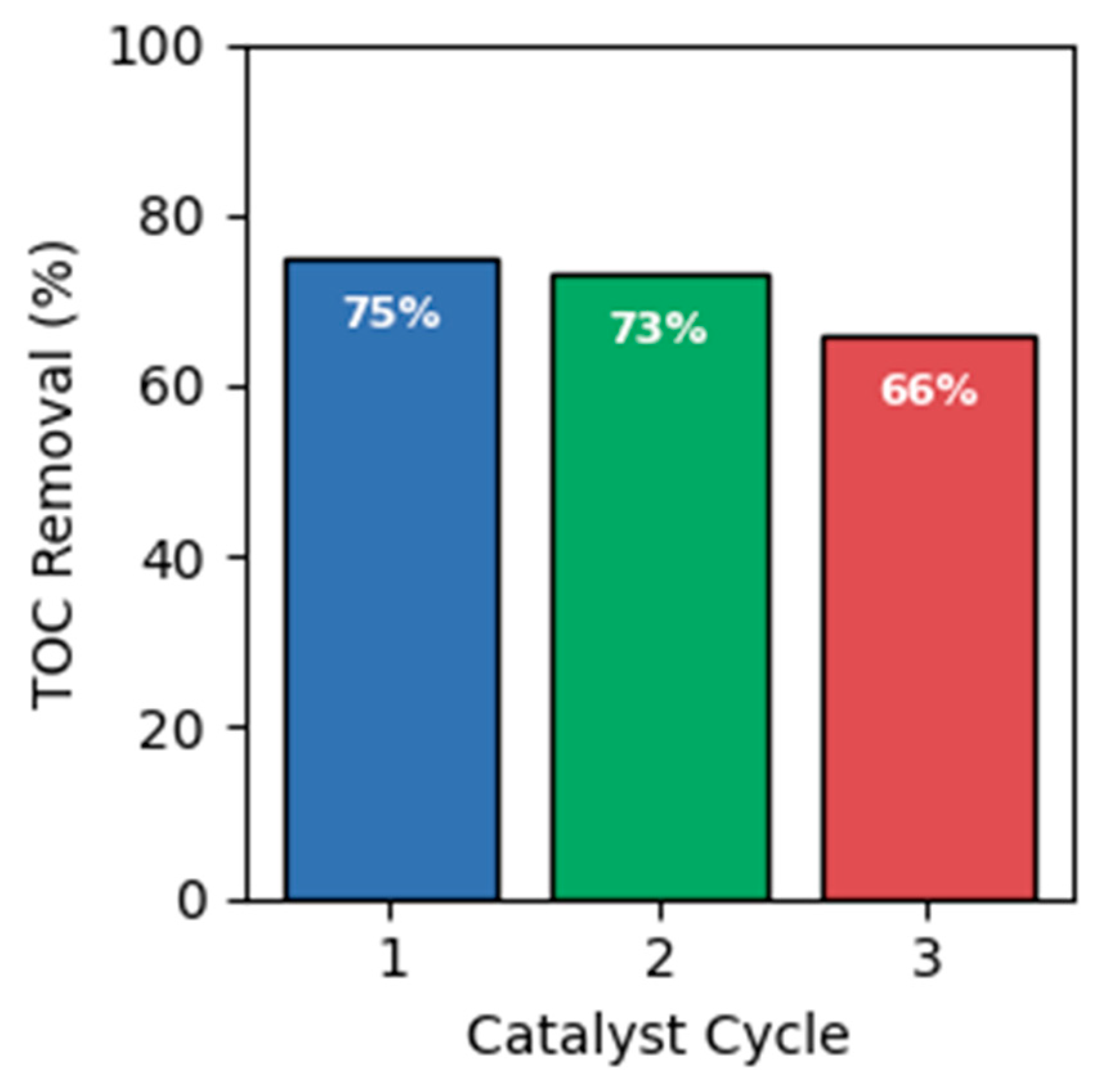
2.5. Catalyst Reusability
3. Materials and Methods
3.1. Materials and Reagents
3.2. Experimental Setup and Procedure
3.3. Analytical Methods
3.4. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation process |
| FTIR | Fourier-transform infrared spectroscopy |
| GPC | Gel permeation chromatography |
| MnO2 | Manganese dioxide |
| MP | Microplastic |
| NP | Nanoplastic |
| n-MnO2 | Natural manganese dioxide (pyrolusite) |
| OA | Oxalic acid |
| O3 | Ozone |
| ●OH | Hydroxyl radical |
| O2•− | Superoxide radical |
| pHPZC | Point of zero charge |
| PS | Polystyrene |
| PSNPs | Polystyrene nanoplastics |
| ROS | Reactive oxygen species |
| TOC | Total organic carbon |
| XPS | X-ray photoelectron spectroscopy |
References
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; Van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directive (EU). 2020/2184 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Union 2020, L435, 1–62. [Google Scholar]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Kang, J.H.; Kwon, O.Y.; Han, G.M.; Shim, W.J. Large Accumulation of Micro-Sized Synthetic Polymer Particles in the Sea Surface Microlayer. Environ. Sci. Technol. 2014, 48, 9014–9021. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. Freshwater Microplastics: Emerging Environmental Contaminants? In The Handbook of Environmental Chemistry; Barceló, D., Kostianoy, A.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 58, ISBN 978-3-319-61614-8. [Google Scholar] [CrossRef]
- von Gunten, U. Ozonation of Drinking Water: Part I. Oxidation Kinetics and Product Formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, R.; Zhao, X.; Qu, J. Enhanced Degradation of 2,4-Dinitrotoluene by Ozonation in the Presence of Manganese(II) and Oxalic Acid. J. Mol. Catal. A Chem. 2008, 286, 149–155. [Google Scholar] [CrossRef]
- Issaka, E.; AMU-Darko, J.N.O.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced Catalytic Ozonation for Degradation of Pharmaceutical Pollutants―A Review. Chemosphere 2022, 289, 133208. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater—A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Issaka, E.; Baffoe, J.; Adams, M. Exploring Heterogeneous Catalytic Ozonation: Catalyst Types, Reaction Mechanisms, Applications, Challenges, and Future Outlook. Sustain. Chem. Environ. 2024, 8, 100185. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shen, C.; Jiang, G.; Guan, B. Enhanced Ozonation of Polystyrene Nanoplastics in Water with CeOx@MnOx Catalyst. Environ. Res. 2023, 220, 115220. [Google Scholar] [CrossRef] [PubMed]
- Isibor, P.O.; Devi, G.; Enuneku, A.A. (Eds.) Environmental Nanotoxicology Combatting the Minute Contaminants; Springer: Berlin/Heidelberg, Germany, 2024; ISBN 978-3-031-54153-7. [Google Scholar]
- Huang, J.; Dai, Y.; Singewald, K.; Liu, C.C.; Saxena, S.; Zhang, H. Effects of MnO2 of Different Structures on Activation of Peroxymonosulfate for Bisphenol A Degradation under Acidic Conditions. Chem. Eng. J. 2019, 370, 906–915. [Google Scholar] [CrossRef]
- Andreozzi, R.; Insola, A.; Caprio, V.; D’Amore, M.G. The Kinetics of Mn(II)-Catalysed Ozonation of Oxalic Acid in Aqueous Solution. Water Res. 1992, 26, 917–921. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Giovanna D’amore, M.; Insola, A. Manganese Catalysis in Water Pollutants Abatement by Ozone. Environ. Technol. 1995, 16, 885–891. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The Efficiency and Mechanisms of Catalytic Ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Sans, C. Insights into the Role of β-MnO2 and Oxalic Acid Complex Expediting Ozonation: Structural Properties and Mechanism. Sep. Purif. Technol. 2024, 341, 126904. [Google Scholar] [CrossRef]
- Omorogie, M.O.; Agbadaola, M.T.; Olatunde, A.M.; Helmreich, B.; Babalola, J.O. Surface Equilibrium and Dynamics for the Adsorption of Anionic Dyes onto MnO2/Biomass Micro-Composite. Green Chem. Lett. Rev. 2022, 15, 49–58. [Google Scholar] [CrossRef]
- Dubal, D.P.; Kim, W.B.; Lokhande, C.D. Galvanostatically Deposited Fe: MnO2 Electrodes for Supercapacitor Application. J. Phys. Chem. Solids 2012, 73, 18–24. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Zhang, R.; Guo, W. Insights into the Morphology-Dependent Adsorption of Aged Polystyrene Nanoplastics on Manganese Oxides. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 679, 132565. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y. Rapid Synthesis of Graphene/Amorphous α-MnO2 Composite with Enhanced Electrochemical Performance for Electrochemical Capacitor. Mater. Sci. Eng. B 2015, 194, 41–47. [Google Scholar] [CrossRef]
- Kumar, Y.; Chopra, S.; Gupta, A.; Kumar, Y.; Uke, S.J.; Mardikar, S.P. Low Temperature Synthesis of MnO2 Nanostructures for Supercapacitor Application. Mater. Sci. Energy Technol. 2020, 3, 566–574. [Google Scholar] [CrossRef]
- Malvestiti, J.A.; Cavalcante, R.P.; Tornisielo, V.L.; Dantas, R.F. Metals as Catalysts for Ozonation. In Heavy Metals–Recent Advances; Almayyahi, B., Ed.; IntechOpen: London, UK, 2023; ISBN 978-1-83768-515-8. [Google Scholar]
- Chen, S.; Shu, X.; Wang, H.; Zhang, J. Thermally Driven Phase Transition of Manganese Oxide on Carbon Cloth for Enhancing the Performance of Flexible All-Solid-State Zinc-Air Batteries. J. Mater. Chem. A 2019, 7, 19719–19727. [Google Scholar] [CrossRef]
- Jiang, Y.; Ba, D.; Li, Y.; Liu, J. Noninterference Revealing of “Layered to Layered” Zinc Storage Mechanism of δ-MnO2 toward Neutral Zn–Mn Batteries with Superior Performance. Adv. Sci. 2020, 7, 1902795. [Google Scholar] [CrossRef]
- De la Cruz, I.J.; Rodríguez, S.J.L.; Fuentes, I.; Tiznado, H.; Vazquez-Arce, J.L.; Romero-Ibarra, I.; Guzmán, C.J.I.; Gutiérrez, H.M. Effect of Crystalline Phase of MnO2 on the Degradation of Bisphenol A by Catalytic Ozonation. J. Environ. Chem. Eng. 2023, 11, 110753. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, M.; Wang, Y.; Sun, P.; Zeng, D.; Wang, X.; Liang, P. Effect of PH on Microstructure and Catalytic Oxidation of Formaldehyde in MnO2 Catalyst. Catalysts 2023, 13, 490. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, Z.; Wang, J.; Zhu, Y.; Zhang, Q. Surface Oxygen Vacancy Induced A-MnO2nanofiber for Highly Efficient Ozone Elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Cao, H.; Chi, Z.; Chen, C.; Duan, X.; Xie, Y.; Qi, F.; Song, W.; Liu, J.; et al. Role of Oxygen Vacancies and Mn Sites in Hierarchical Mn2O3/LaMnO3-Δ Perovskite Composites for Aqueous Organic Pollutants Decontamination. Appl. Catal. B Environ. 2019, 245, 546–554. [Google Scholar] [CrossRef]
- Xia, L.; Liang, W.; Chen, G.; Li, W.; Gao, M. Catalytic Ozonation of Quinoline Utilizing Manganese-Based Catalyst with Abundant Oxygen Vacancies. Catal. Letters 2022, 152, 1669–1677. [Google Scholar] [CrossRef]
- Radinger, H.; Connor, P.; Stark, R.; Jaegermann, W.; Kaiser, B. Manganese Oxide as an Inorganic Catalyst for the Oxygen Evolution Reaction Studied by X-Ray Photoelectron and Operando Raman Spectroscopy. ChemCatChem 2021, 13, 1175–1185. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, S.X.; Wang, Y.F.; Zhao, S.J.; Zhang, X.H. Synthesis of Petal-like δ-MnO2 and Its Catalytic Ozonation Performance. New J. Chem. 2018, 42, 6770–6777. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Chen, Z.; Shen, B.; Wei, J.; Zeng, P.; Wen, X. Catalytic Ozonation for Metoprolol and Ibuprofen Removal over Different MnO2 Nanocrystals: Efficiency, Transformation and Mechanism. Sci. Total Environ. 2021, 785, 147328. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, D.; Wang, Z.; Liu, G.; Liu, H.; Chen, Y. Oxalate Route for Promoting Activity of Manganese Oxide Catalysts in Total VOCs’ Oxidation: Effect of Calcination Temperature and Preparation Method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic Ozonation and Methods of Enhancing Molecular Ozone Reactions in Water Treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ding, J.; Song, Z.; Yang, B.; Zhang, C.; Guan, B. Degradation of Nano-Sized Polystyrene Plastics by Ozonation or Chlorination in Drinking Water Disinfection Processes. Chem. Eng. J. 2022, 427, 131690. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Ammar, R.; Sans, C. Enhancing Nanoplastics Removal by Metal Ion-Catalyzed Ozonation. Chem. Eng. J. Adv. 2024, 19, 100621. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone-Based Technologies in Water and Wastewater Treatment. Handb. Environ. Chem. Vol. 5 Water Pollut. 2008, 5 (Suppl. S2), 127–175. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R.; Tufano, V. The Ozonation of Pyruvic Acid in Aqueous Solutions Catalyzed by Suspended and Dissolved Manganese. Water Res. 1998, 32, 1492–1496. [Google Scholar] [CrossRef]
- Wang, J.; Yue, W.; Teng, Y.; Zhai, Y.; Zhu, H. Degradation Kinetics and Transformation Pathway of Methyl Parathion by δ-MnO2/Oxalic Acid Reaction System. Chemosphere 2023, 320, 138054. [Google Scholar] [CrossRef]
- Psaltou, S.; Mitrakas, M.; Zouboulis, A. Heterogeneous Catalytic Ozonation: Solution PH and Initial Concentration of Pollutants as Two Important Factors for the Removal of Micropollutants from Water. Separations 2022, 9, 413. [Google Scholar] [CrossRef]
- Fallah, N.; Bloise, E.; Santoro, D.; Mele, G. State of Art and Perspectives in Catalytic Ozonation for Removal of Organic Pollutants in Water: Influence of Process and Operational Parameters. Catalysts 2023, 13, 324. [Google Scholar] [CrossRef]
- Wu, Z.; Abramova, A.; Nikonov, R.; Cravotto, G. Sonozonation (Sonication/Ozonation) for the Degradation of Organic Contaminants—A Review. Ultrason. Sonochem. 2020, 68, 105195. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Ren, Y.; Wei, T. Elevated Removal of Di-n-Butyl Phthalate by Catalytic Ozonation over Magnetic Mn-Doped Ferrospinel ZnFe2O4 Materials: Efficiency and Mechanism. Appl. Surf. Sci. 2020, 505, 144476. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers V: Epoxies. Spectroscopy 2022, 37, 17–19. [Google Scholar] [CrossRef]
- di Luca, C.; Garcia, J.; Munoz, M.; Hernando-Pérez, M.; de Pedro, Z.M.; Casas, J.A. Strategies for the Quantification and Characterization of Nanoplastics in AOPs Research. Chem. Eng. J. 2024, 493, 152490. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Cao, H.; Xie, Y.; Wang, Y.; Xiao, J. (Eds.) Advanced Ozonation Processes for Water and Wastewater Treatment: Active Catalysts and Combined Technologies; Royal Society of Chemistry: Cambridge, UK, 2022; ISBN 978-1-83916-389-0. [Google Scholar]
- Nawaz, F.; Xie, Y.; Cao, H.; Xiao, J.; Wang, Y.; Zhang, X.; Li, M.; Duan, F. Catalytic Ozonation of 4-Nitrophenol over an Mesoporous α-MnO2 with Resistance to Leaching. Catal. Today 2015, 258, 595–601. [Google Scholar] [CrossRef]
- Yang, K.; Yu, J.; Guo, Q.; Wang, C.; Yang, M.; Zhang, Y.; Xia, P.; Zhang, D.; Yu, Z. Comparison of Micropollutants’ Removal Performance between Pre-Ozonation and Post-Ozonation Using a Pilot Study. Water Res. 2017, 111, 147–153. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Manganese Oxides at Different Oxidation States for Heterogeneous Activation of Peroxymonosulfate for Phenol Degradation in Aqueous Solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the Degradation of Microplastics by Fenton Oxidation: From Surface Modification to Mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef]
- ISO. ISO 7027:2016; Water Quality—Determination of Turbidity. International Organization for Standardization: Geneva, Switzerland, 2016.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mello, V.; Nieto-Sandoval, J.; Dezotti, M.; Sans, C. Natural Pyrolusite-Catalyzed Ozonation for Nanoplastics Degradation. Catalysts 2025, 15, 502. https://doi.org/10.3390/catal15050502
Mello V, Nieto-Sandoval J, Dezotti M, Sans C. Natural Pyrolusite-Catalyzed Ozonation for Nanoplastics Degradation. Catalysts. 2025; 15(5):502. https://doi.org/10.3390/catal15050502
Chicago/Turabian StyleMello, Victor, Julia Nieto-Sandoval, Márcia Dezotti, and Carmen Sans. 2025. "Natural Pyrolusite-Catalyzed Ozonation for Nanoplastics Degradation" Catalysts 15, no. 5: 502. https://doi.org/10.3390/catal15050502
APA StyleMello, V., Nieto-Sandoval, J., Dezotti, M., & Sans, C. (2025). Natural Pyrolusite-Catalyzed Ozonation for Nanoplastics Degradation. Catalysts, 15(5), 502. https://doi.org/10.3390/catal15050502









