Cobalt-Decorated Carbonized Wood as an Efficient Electrocatalyst for Water Splitting
Abstract
1. Introduction
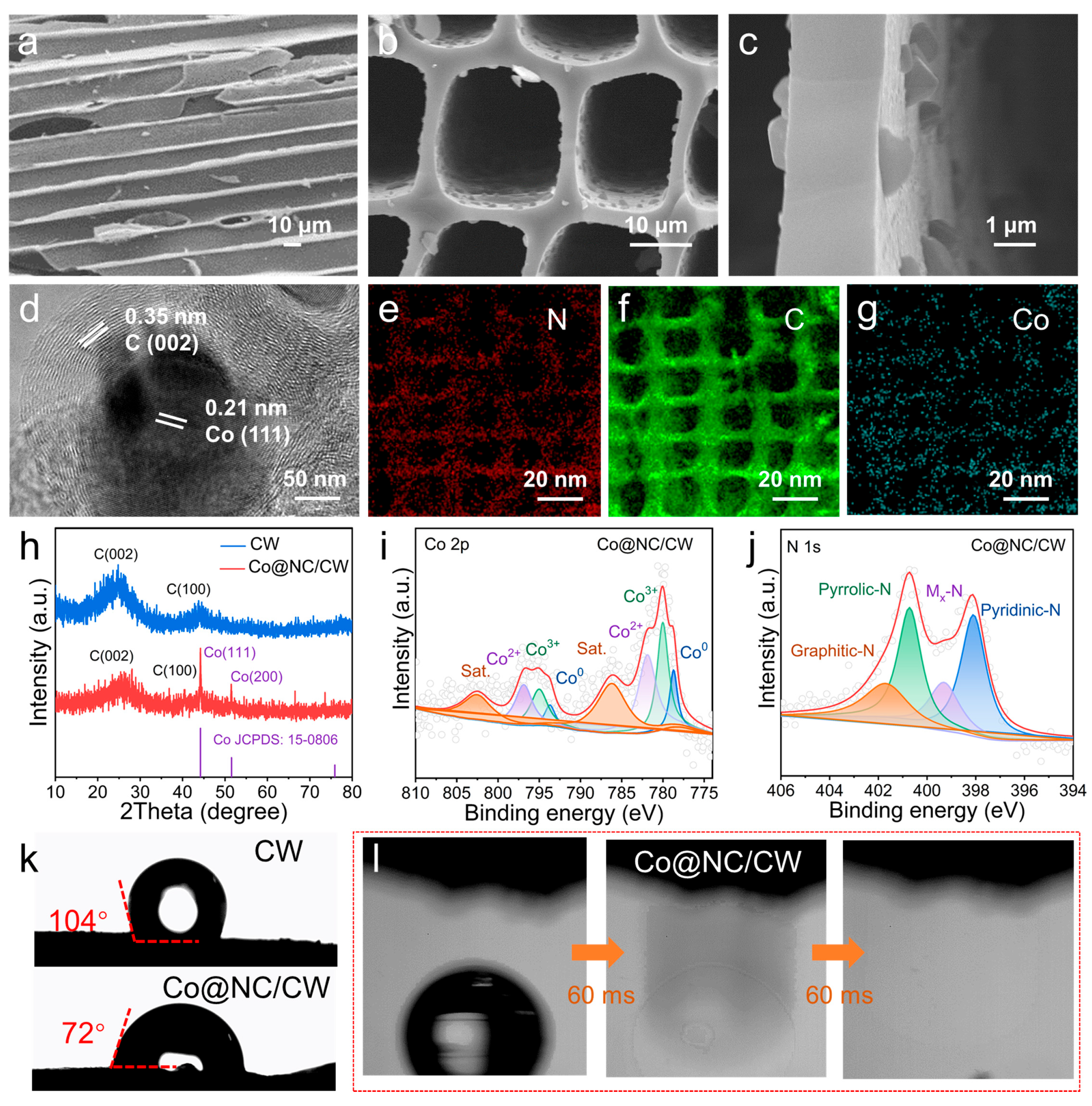
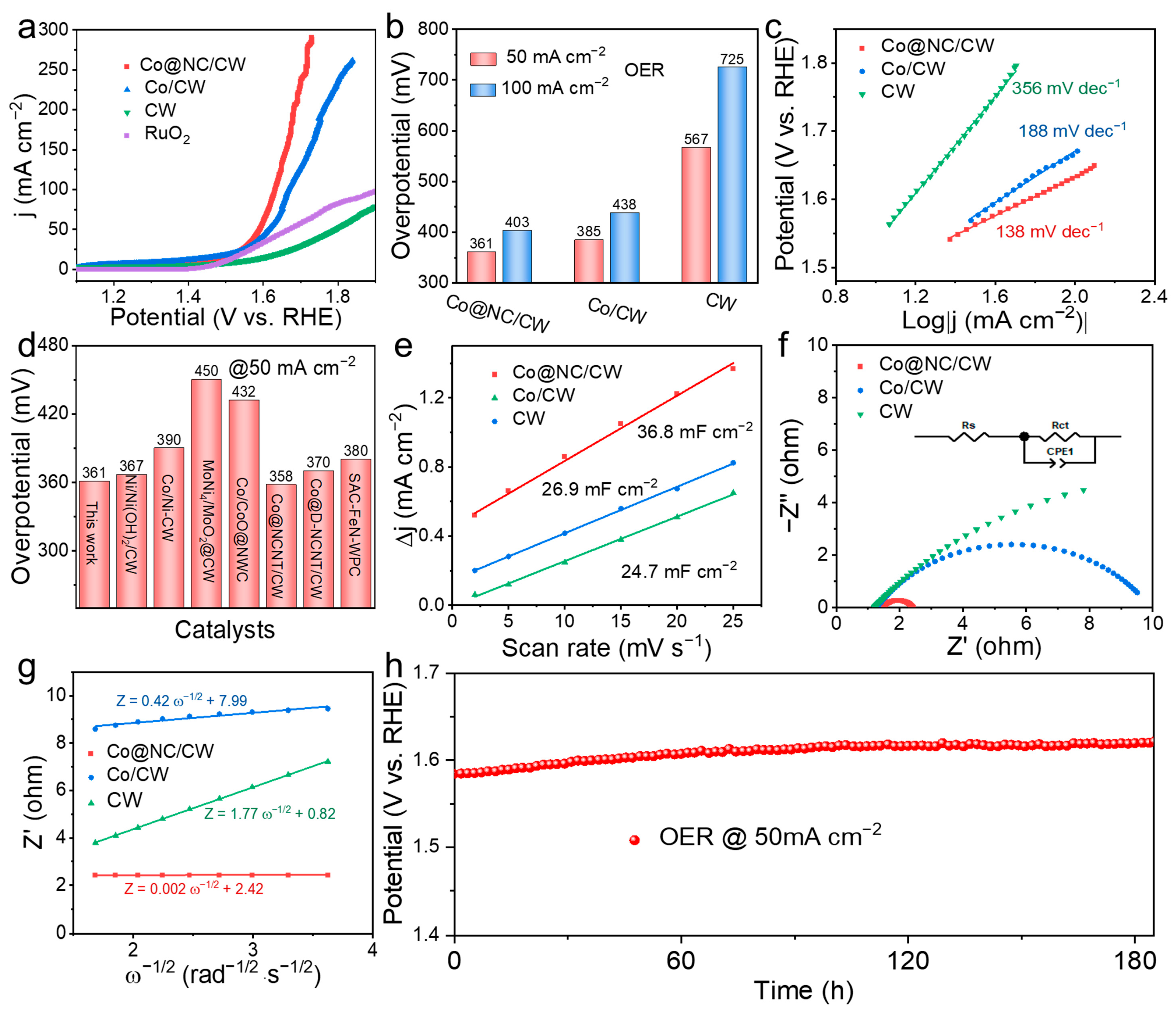
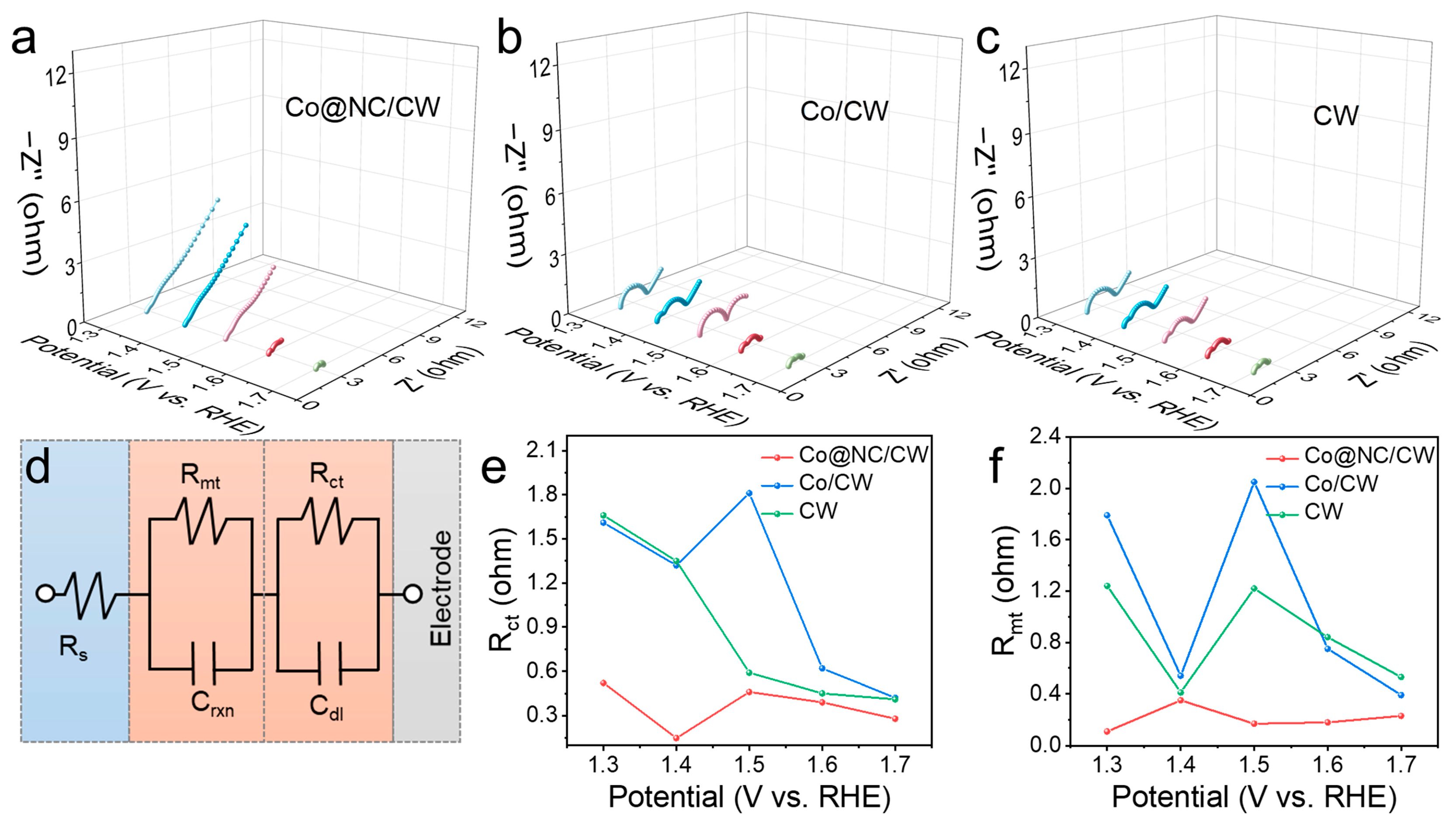
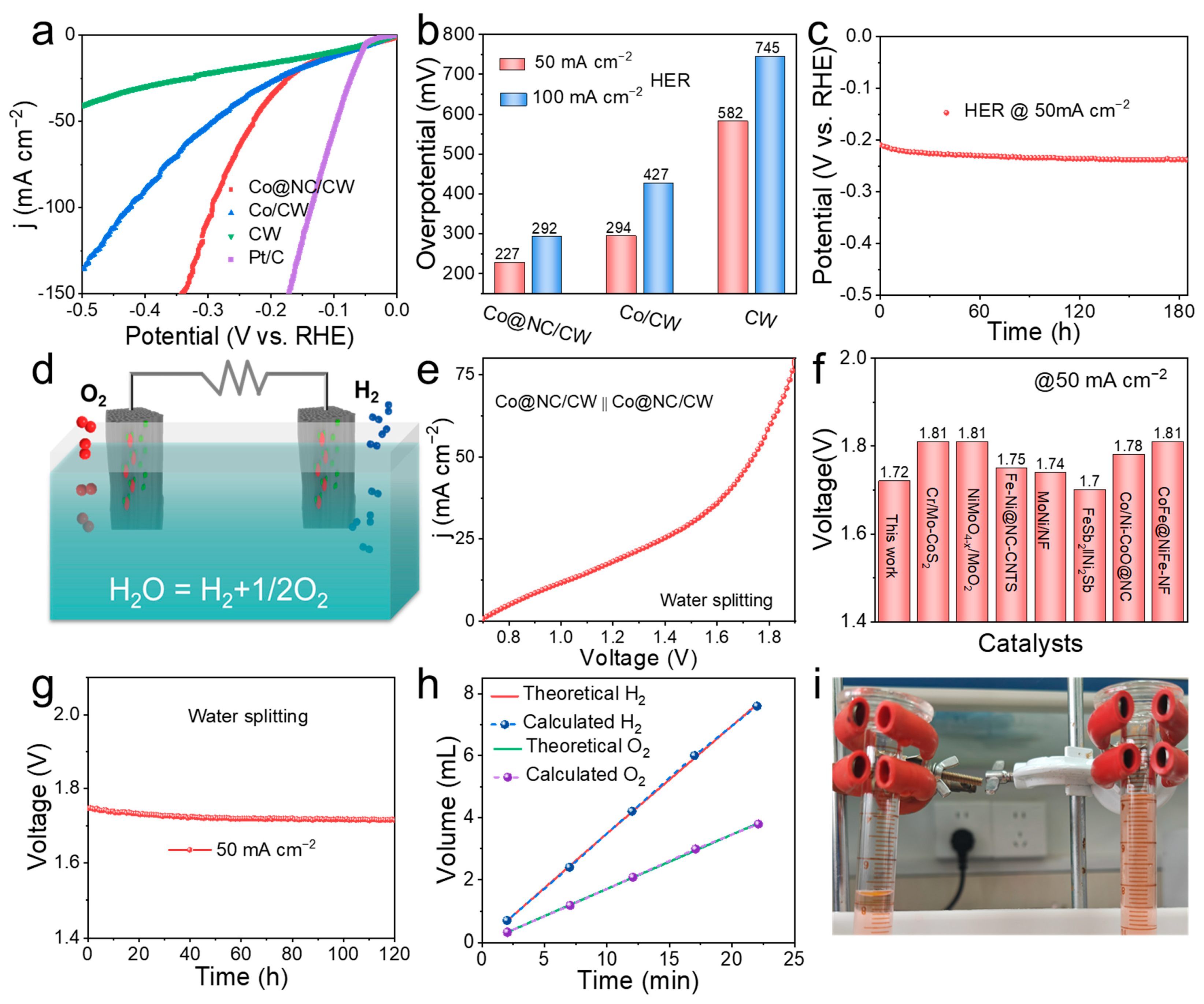
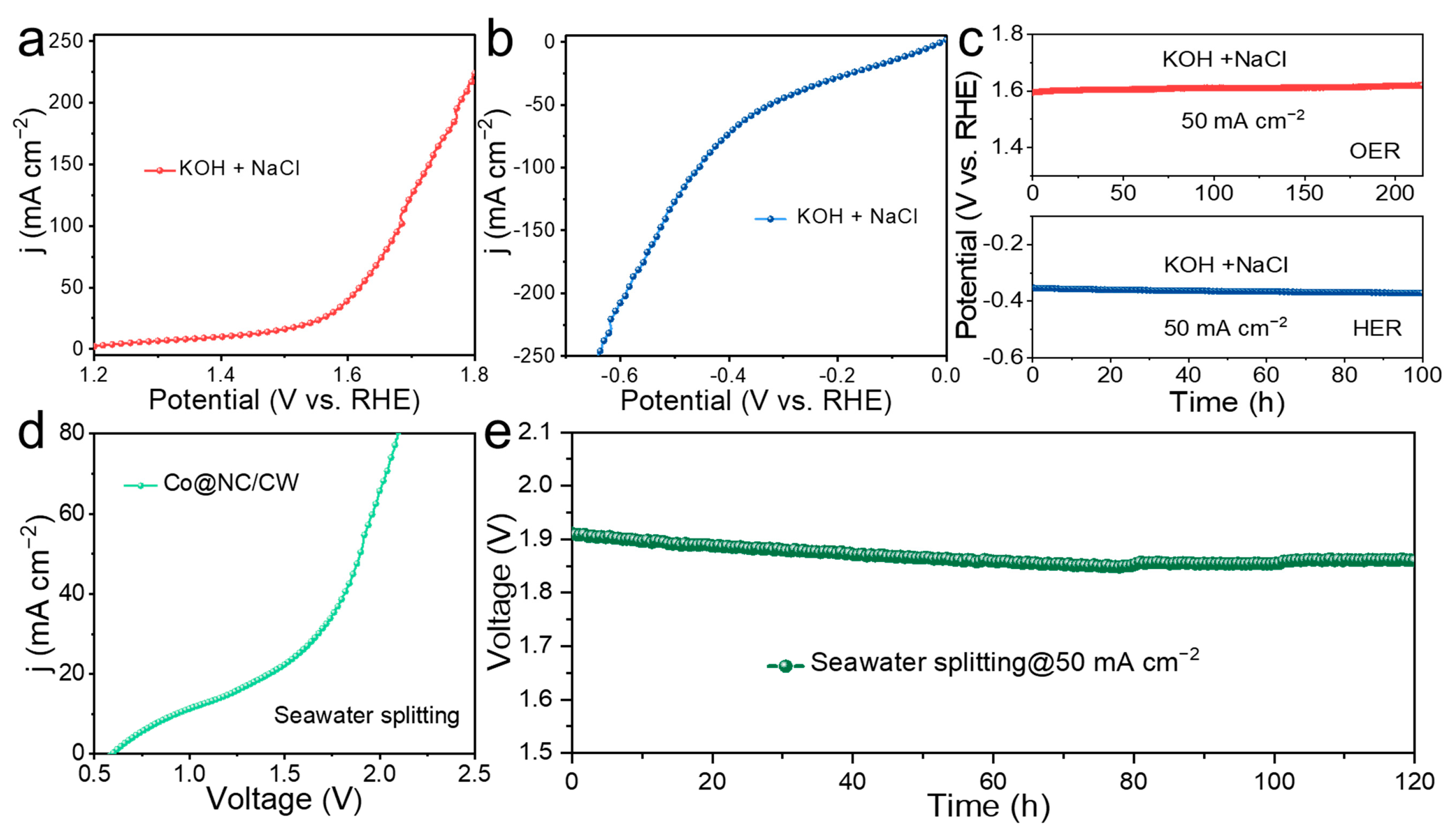
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Co@NC/CW
3.3. Synthesis of Co/CW and CW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OER | Oxygen evolution reaction |
| HER | Hydrogen evolution reaction |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
| BET | Brunauer–Emmett–Teller |
| XPS | X-ray photoelectron spectroscopy |
| LSV | Linear sweep voltammetry |
| Cdl | Double-layer capacitance |
| ECSA | Electrochemically active surface area |
| EIS | Electrochemical impedance spectroscopy |
| Rs | Solution resistance |
| Rmt | Mass transport resistance |
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Yu, Y.; Wang, S.; Zhou, Y.; Wu, X.; Li, K.; Qi, A.; Deng, P.; Cheng, Y.; Li, J.; et al. Understanding the improvement mechanism of plasma etching treatment on oxygen reduction reaction catalysts. Exploration 2024, 4, 20230034. [Google Scholar] [CrossRef]
- Ye, P.; Fang, K.; Wang, H.; Wang, Y.; Huang, H.; Mo, C.; Ning, J.; Hu, Y. Lattice oxygen activation and local electric field enhancement by co-doping Fe and F in CoO nanoneedle arrays for industrial electrocatalytic water oxidation. Nat. Commun. 2024, 15, 1012. [Google Scholar] [CrossRef]
- Sun, S.; Dang, J.; Pan, Q.; Zhang, C.; Liu, S. pH-responsive flexible copper electrode with switchable wettability for electrocatalytic hydrogen/oxygen evolution reactions and urea oxidation reaction. ACS Appl. Mater. Interfaces 2023, 15, 27357–27368. [Google Scholar] [CrossRef]
- Sun, R.; Zhu, M.; Chen, J.; Yan, L.; Bai, L.; Ning, J.; Zhong, Y.; Hu, Y. Tuning the formation kinetics of *OOH intermediate with hollow bowl-like carbon by pulsed electroreduction for enhanced H2O2 Production. ACS Nano 2025, 19, 13414–13426. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, P. Fundamentals of water electrolysis. In Water Electrolysis for Hydrogen Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–60. [Google Scholar]
- Jia, Y.; Yao, X. Defects in carbon-based materials for electrocatalysis: Synthesis, recognition, and advances. Acc. Chem. Res. 2023, 56, 948–958. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, W.; Li, J.; Yan, Q.; Chen, Z.; Zhou, X.; Li, J.; Gao, R.; Wu, Y.; Li, G.D. Recent development of non-iridium-based electrocatalysts for acidic oxygen evolution reaction. Carbon Neutral. 2024, 3, 1101–1130. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Zhang, T.; Lu, H.; Yan, L.; Ning, J.; Hu, Y. Enhancing efficiency and durability of alkaline Zn-Co/air hybrid batteries with self-reconstructed Co/Co2P heterojunctions. Adv. Energy Mater. 2024, 15, 2402839. [Google Scholar] [CrossRef]
- Park, M.G.; Hwang, J.; Deng, Y.P.; Lee, D.U.; Fu, J.; Hu, Y.; Jang, M.J.; Choi, S.M.; Feng, R.; Jiang, G.; et al. Longevous cycling of rechargeable Zn-air battery enabled by “raisin-bread” cobalt oxynitride/porous carbon hybrid electrocatalysts. Adv. Mater. 2023, 36, e2311105. [Google Scholar] [CrossRef]
- Yan, L.; Xie, B.; Yang, C.; Wang, Y.; Ning, J.; Zhong, Y.; Hu, Y. Engineering self-supported hydrophobic–aerophilic air cathode with CoS/Fe3S4 nanoparticles embedded in S, N co-doped carbon plate arrays for long-life rechargeable Zn–air batteries. Adv. Energy Mater. 2023, 13, 2204245. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, S.; Zhang, R.; Wang, L.; Wang, Y.; Yang, L.; Li, J.; Guan, F.; Duan, J.; Hou, B. Regulating N species in N-doped carbon electro-catalysts for high-efficiency synthesis of hydrogen peroxide in simulated seawater. Adv. Sci. 2023, 10, 2302446. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Fan, J.G.; Pan, J.M.; Wang, H.; Liu, S.; Zhan, Y.; Yan, X. Research and progress in mitigating carbon oxidation in air electrodes. Adv. Funct. Mater. 2025, 35, 2417580. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Li, H.; Zeng, S.; Li, R.; Yao, Q.; Chen, H.; Qu, K. N, S codoped porous carbon with trace single-atom Fe for enhanced oxygen reduction with robust poison resistance and efficient rechargeable zinc–air battery. Carbon Neutral. 2025, 4, e196. [Google Scholar] [CrossRef]
- Huang, C.; Wang, F.; Chen, X.; Li, J.; Shao, M.; Wei, Z. Innovative strategies for designing and constructing efficient fuel cell electrocatalysts. Chem. Commun. 2025, 61, 2658–2683. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, D.; Li, W.; Zheng, Y.; Wang, L.; Shao, G.; Liu, Q.; Yang, W. Highly dispersive Co@N-C catalyst as freestanding bifunctional cathode for flexible and rechargeable zinc–air batteries. Energy Environ. Mater. 2022, 5, 543–554. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Chen, W.; Hu, Y.; Chen, D.; He, M.; Zhou, M.; Lei, T.; Zhang, Y.; Xiong, J. Designing reliable cathode system for high-performance inorganic solid-state pouch cells. Adv. Sci. 2024, 11, 2401889. [Google Scholar] [CrossRef]
- Abu Nayem, S.M.; Hardianto, Y.P.; Shuaibu, A.D.; Shah, S.S.; Islam, S.; Jafar Mazumder, M.A.; Aziz, M.A.; Saleh Ahammad, A.J. Biomass derived amino acid assisted synthesis of FeNi layered double hydroxide for efficient oxygen evolution reaction. Inorg. Chem. Commun. 2025, 171, 113574. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Azam, A.; Liu, Y.; Yang, J.; Wang, D.; Sorrell, C.C.; Zhao, C.; Li, S. Unlocking efficiency: Minimizing energy loss in electrocatalysts for water splitting. Adv. Mater. 2024, 36, 2404658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Huang, Z.; Yang, J. A new class of electronic devices based on flexible porous substrates. Adv. Sci. 2022, 9, e2105084. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Chen, Z.; Shi, G.; Wang, L.; Chen, Z.; Tu, W.; Xia, R.; Iwuoha, E.I.; Liu, C.; et al. Wood-derived, monolithic chainmail electrocatalyst for biomass-assisted hydrogen production. Adv. Energy Mater. 2023, 13, 2300427. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, S.; He, S.; Song, M.; Xia, B.Y.; You, B. Heterogeneous electrocatalysts from nanostructures to single atoms for biomass-derived feedstocks upgrading. Coord. Chem. Rev. 2025, 527, 216399. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, L.; Wang, B.; Yang, G.; Lucia, L.; Li, F.; Chen, J. Wood based quasi-solid-state Zn-air battery with dual honeycomb-like porous carbon and cationic nanocellulose film. Ind. Crop. Prod. 2022, 186, 115242. [Google Scholar] [CrossRef]
- Alemu, M.A.; Assegie, A.A.; Ilbas, M.; Al Afif, R.; Getie, M.Z. Biomass-derived metal-free nanostructured carbon electrocatalysts for high-performance rechargeable zinc–air batteries. Adv. Energy Sust. Res. 2025, 6, 2400414. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.C.; Liu, C.; Chen, W.; Yu, G.; Zhang, D.; Li, Z.; Mei, C. A flexible Zn-ion capacitor based on wood derived porous carbon and polyacrylamide/cellulose nanofiber hydrogel. Ind. Crop. Prod. 2023, 193, 116216. [Google Scholar] [CrossRef]
- Yu, L.; Shang, Z.; Lin, X.; Li, M.; Liu, Y.; Ren, L.; Deng, S.; Zhang, F.; Luo, L.; Duan, H.; et al. Bio-derived wood-based gas diffusion electrode for high-performance aluminum–air batteries: Insights into pore structure. Adv. Mater. Interfaces 2023, 11, 2300355. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Shrivastava, K.; Gill, F.S.; Juyal, S.; Lal, M.; Jain, A. Green carbon for clean energy: Biomass-derived hierarchical structures in energy storage. WIREs Energy Environ. 2025, 14, e70007. [Google Scholar] [CrossRef]
- Segmehl, J.S.; Studer, V.; Keplinger, T.; Burgert, I. Characterization of wood derived hierarchical cellulose scaffolds for multifunctional applications. Materials 2018, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Liu, Y.; Yang, L.; Wang, X.; Xu, C.; Wang, C. Engineering biomass into advanced carbon-based materials for Zn-air batteries. Sci. China Mater. 2025, 68, 1509–1529. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.Y.; Hu, W.K.; Ning, J.Q.; Zhong, Y.J.; Hu, Y. Formation of sandwiched leaf-like CNTs-Co/ZnCo2O4@NC-CNTs nanohybrids for high-power-density rechargeable Zn-air batteries. Nano Energy 2021, 82, 105710. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, K.; Tu, B.; Shi, Y.; Lu, K.; Qian, X.; Shi, C. Fire-retardant, smoke-suppressive and sustainable diatomite-based auxiliaries for highly fire-resistant epoxy resin. Adv. Powder Technol. 2024, 35, 104639. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Liu, X.; Mahmood, S.; Shen, J.; Ning, J.; Li, S.; Zhong, Y.; Hu, Y. Integrating trifunctional Co@NC-CNTs@NiFe-LDH electrocatalysts with arrays of porous triangle carbon plates for high-power-density rechargeable Zn-air batteries and self-powered water splitting. Chem. Eng. J. 2022, 446, 137049. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Murugananthan, M.; Sun, P.; Dionysiou, D.D.; Zhang, K.; Khan, A.; Zhang, Y. Glucose and melamine derived nitrogen-doped carbonaceous catalyst for nonradical peroxymonosulfate activation. Carbon 2020, 156, 399–409. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, Y.; Cao, Z.; Zeng, K.; Xie, Y.; Li, J.; Yao, Y.; Gan, W. Highly efficient, field-assisted water splitting enabled by a bifunctional Ni3Fe magnetized wood carbon. Chem. Eng. J. 2022, 439, 135722. [Google Scholar] [CrossRef]
- Yan, L.; Wang, H.Y.; Shen, J.L.; Ning, J.Q.; Zhong, Y.J.; Hu, Y. Formation of mesoporous Co/CoS/Metal-N-C@S, N-codoped hairy carbon polyhedrons as an efficient trifunctional electrocatalyst for Zn-air batteries and water splitting. Chem. Eng. J. 2021, 403, 126385. [Google Scholar] [CrossRef]
- Jing, L.; Tian, Q.; Li, X.; Sun, J.; Wang, W.; Yang, H.; Chai, X.; Hu, Q.; He, C. Dual-engineering of porous structure and carbon edge enables highly selective H2O2 electrosynthesis. Adv. Funct. Mater. 2023, 33, 2305795. [Google Scholar] [CrossRef]
- Gan, W.; Wu, L.; Wang, Y.; Gao, H.; Gao, L.; Xiao, S.; Liu, J.; Xie, Y.; Li, T.; Li, J. Carbonized wood decorated with cobalt-nickel binary nanoparticles as a low-cost and efficient electrode for water splitting. Adv. Funct. Mater. 2021, 31, 2010951. [Google Scholar] [CrossRef]
- Douka, A.I.; Xu, Y.; Yang, H.; Zaman, S.; Yan, Y.; Liu, H.; Salam, M.A.; Xia, B.Y. A Zeolitic-imidazole frameworks-derived interconnected macroporous carbon matrix for efficient oxygen electrocatalysis in rechargeable zinc-air batteries. Adv. Mater. 2020, 32, e2002170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jia, Y.; Wei, F.; Zhuang, L.; Yang, D.; Liu, J.; Wang, X.; Lin, S.; Yuan, P.; Yao, X. Understanding the activity of Co-N4-xCx in atomic metal catalysts for oxygen reduction catalysis. Angew. Chem. Int. Ed. 2020, 59, 6122–6127. [Google Scholar] [CrossRef]
- Yan, L.; Sun, R.; Mahmood, S.; Bai, L.; Zhong, Y.; Ning, J.; Hu, Y. Facilitating reactant accessibility to heterometallic phosphide with superhydrophilic interfaces for efficient electrochemical production of ammonia and lactic acid. Appl. Catal. B Environ. 2024, 358, 124390. [Google Scholar] [CrossRef]
- Yang, J.Y.; Ahn, J.G.; Ko, B.; Park, T.; Hong, S.J.; Han, D.K.; Lee, D.; Li, C.A.; Song, S.H. Green and sustainable bifunctional carbonized wood electrodes decorated with controlled nickel/α(β)-nickel(ii) hydroxide to boost overall water splitting. J. Mater. Chem. A 2023, 11, 26672–26680. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Han, G.; Cao, M.; Han, L.; Zhou, B.; Mehdi, S.; Wu, X.; Li, B.; Jiang, J. Wood-derived integral air electrode for enhanced interfacial electrocatalysis in rechargeable zinc–air battery. Small 2021, 17, 2101607. [Google Scholar] [CrossRef]
- Mao, C.; Shi, Z.; Peng, J.; Ou, L.; Chen, Y.; Huang, J. Hierarchically porous carbonized wood decorated with MoNi4-embedded MoO2 nanosheets: An efficient electrocatalyst for water splitting. Adv. Funct. Mater. 2024, 34, 2308337. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Li, T.; Tian, X.; Zhang, K.; Miao, Y.; Xia, C.; Cai, L.; Hui, B.; Chen, C. Defective wood-based chainmail electrocatalysts boost performances of seawater-medium Zn-air batteries. J. Energy Chem. 2025, 102, 134–143. [Google Scholar] [CrossRef]
- Zhong, L.; Jiang, C.; Zheng, M.; Peng, X.; Liu, T.; Xi, S.; Chi, X.; Zhang, Q.; Gu, L.; Zhang, S. Wood carbon based single-atom catalyst for rechargeable Zn–air batteries. ACS Energy Lett. 2021, 6, 3624–3633. [Google Scholar] [CrossRef]
- Huang, J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond. Electrochim. Acta 2018, 281, 170–188. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Wang, H.; Ye, P.; Ping, Z.; Ning, J.; Zhong, Y.; Hu, Y. Two-step nitrogen and sulfur doping in porous carbon dodecahedra for Zn-ion hybrid supercapacitors with long term stability. Chem. Eng. J. 2022, 431, 133250. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Li, H.; Zhang, D.; Tao, L.; Fu, X.Z.; Liu, M.; Wang, S. Tip-like Fe-N4 sites induced surface microenvironments regulation boosts the oxygen reduction reaction. Angew. Chem. Int. Ed. 2024, 63, e202319370. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Shimizu, K.; Khatun, S.; Singha, S.; Watanabe, S.; Roy, P. Electrolyte engineering for effective seawater splitting based on manganese iron chromium layered triple hydroxides as novel bifunctional electrocatalysts. J. Mater. Chem. A 2023, 11, 12151–12163. [Google Scholar] [CrossRef]
- Lu, S.Y.; Dou, W.; Wu, C.; Zhang, J.; Wang, L.; Hu, T.; Wang, R.; Liu, Y.; Yang, Q.; Jin, M. Strong electronic interaction in chromium-molybdenum dual incorporated few-layer cobalt sulfide nanosheets with lattice distortion for efficient bifunctional water splitting. Fuel 2024, 377, 132780. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Tang, J. Porous NiMoO4−x/MoO2 hybrids as highly effective electrocatalysts for the water splitting reaction. J. Mater. Chem. A 2018, 6, 12361–12369. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Simke, J.R.J.; Schmidt, J.; Thomas, A. Bifunctional electrocatalysts for overall water splitting from an iron/nickel-based bimetallic metal–organic framework/dicyandiamide composite. Angew. Chem. Int. Ed. 2018, 57, 8921–8926. [Google Scholar] [CrossRef]
- Nairan, A.; Zou, P.; Liang, C.; Liu, J.; Wu, D.; Liu, P.; Yang, C. NiMo solid solution nanowire array electrodes for highly efficient hydrogen evolution reaction. Adv. Funct. Mater. 2019, 29, 1903747. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, J.; Chen, Z.; Zhao, L.; Qiu, F.; Li, W.; Zhang, W.; Miao, S. High crystalline MxSby (M=Fe, Co, and Ni) nanocrystals tuned by antimony for boosting overall water splitting catalysis. Adv. Energy Mater. 2025, 65, 2405275. [Google Scholar] [CrossRef]
- Sang, Y.; Li, G.; Xue, J.; Hu, J.; Tong, Y.; Liang, B.; Li, L. Engineering of metal Co/Ni-CoO nanoparticles embedded in N-doped unclosed hollow carbon nanoboxes as a highly efficient bifunctional electrocatalyst for overall water splitting. Surf. Interfaces 2024, 44, 103729. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Li, Z.; Huang, S.; Pan, J.; Mei, J.; Zhang, S.; Peng, X.; Lu, W.; Yan, L. Cobalt-Decorated Carbonized Wood as an Efficient Electrocatalyst for Water Splitting. Catalysts 2025, 15, 503. https://doi.org/10.3390/catal15050503
Cheng Z, Li Z, Huang S, Pan J, Mei J, Zhang S, Peng X, Lu W, Yan L. Cobalt-Decorated Carbonized Wood as an Efficient Electrocatalyst for Water Splitting. Catalysts. 2025; 15(5):503. https://doi.org/10.3390/catal15050503
Chicago/Turabian StyleCheng, Zichen, Zekun Li, Shou Huang, Junfan Pan, Jiaxian Mei, Siqi Zhang, Xingyu Peng, Wen Lu, and Lei Yan. 2025. "Cobalt-Decorated Carbonized Wood as an Efficient Electrocatalyst for Water Splitting" Catalysts 15, no. 5: 503. https://doi.org/10.3390/catal15050503
APA StyleCheng, Z., Li, Z., Huang, S., Pan, J., Mei, J., Zhang, S., Peng, X., Lu, W., & Yan, L. (2025). Cobalt-Decorated Carbonized Wood as an Efficient Electrocatalyst for Water Splitting. Catalysts, 15(5), 503. https://doi.org/10.3390/catal15050503







