Supported TiO2 Photocatalysis of Spiked Contaminants in Water and Municipal Wastewater
Abstract
1. Introduction
2. Results and Discussion
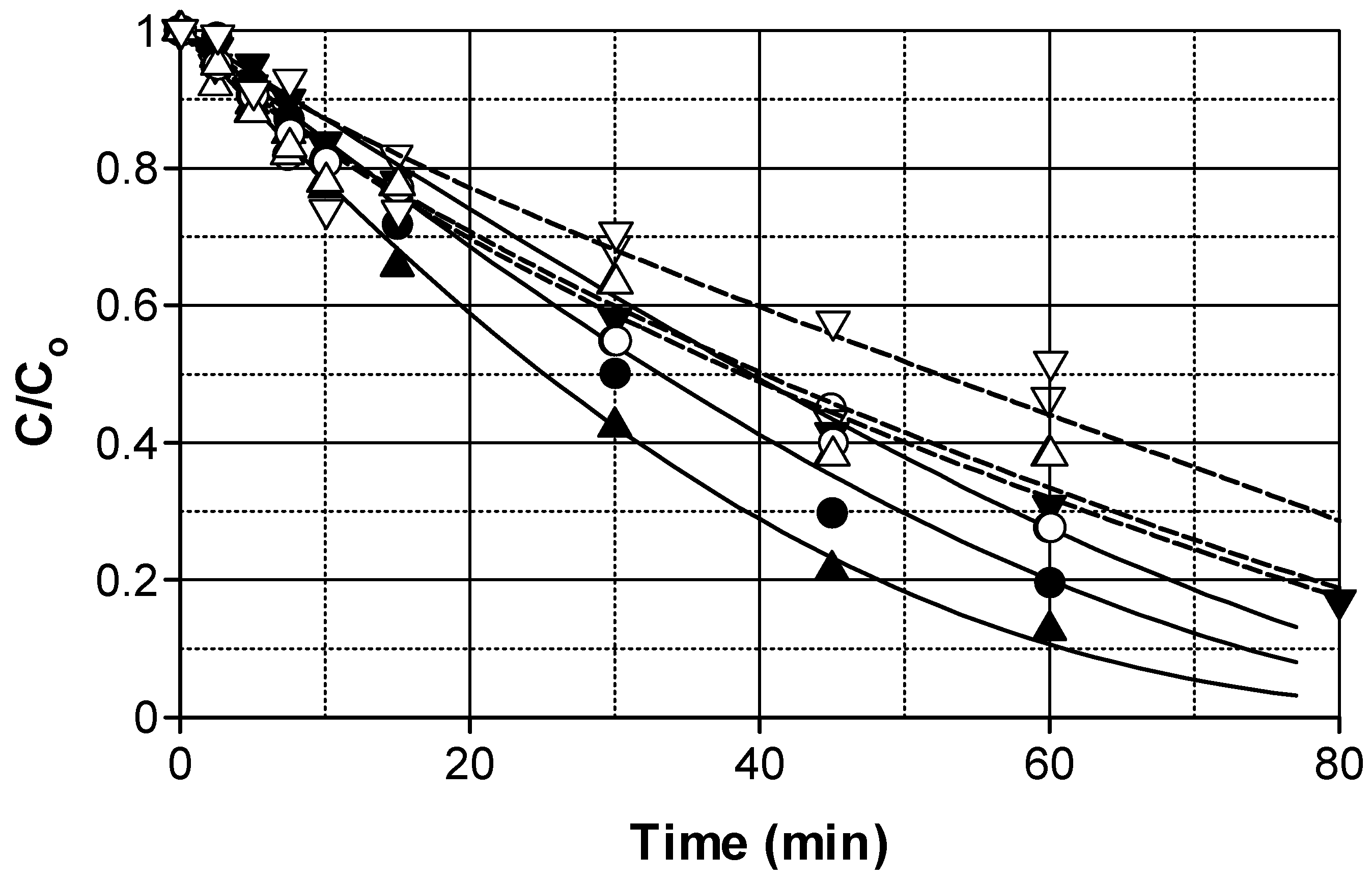
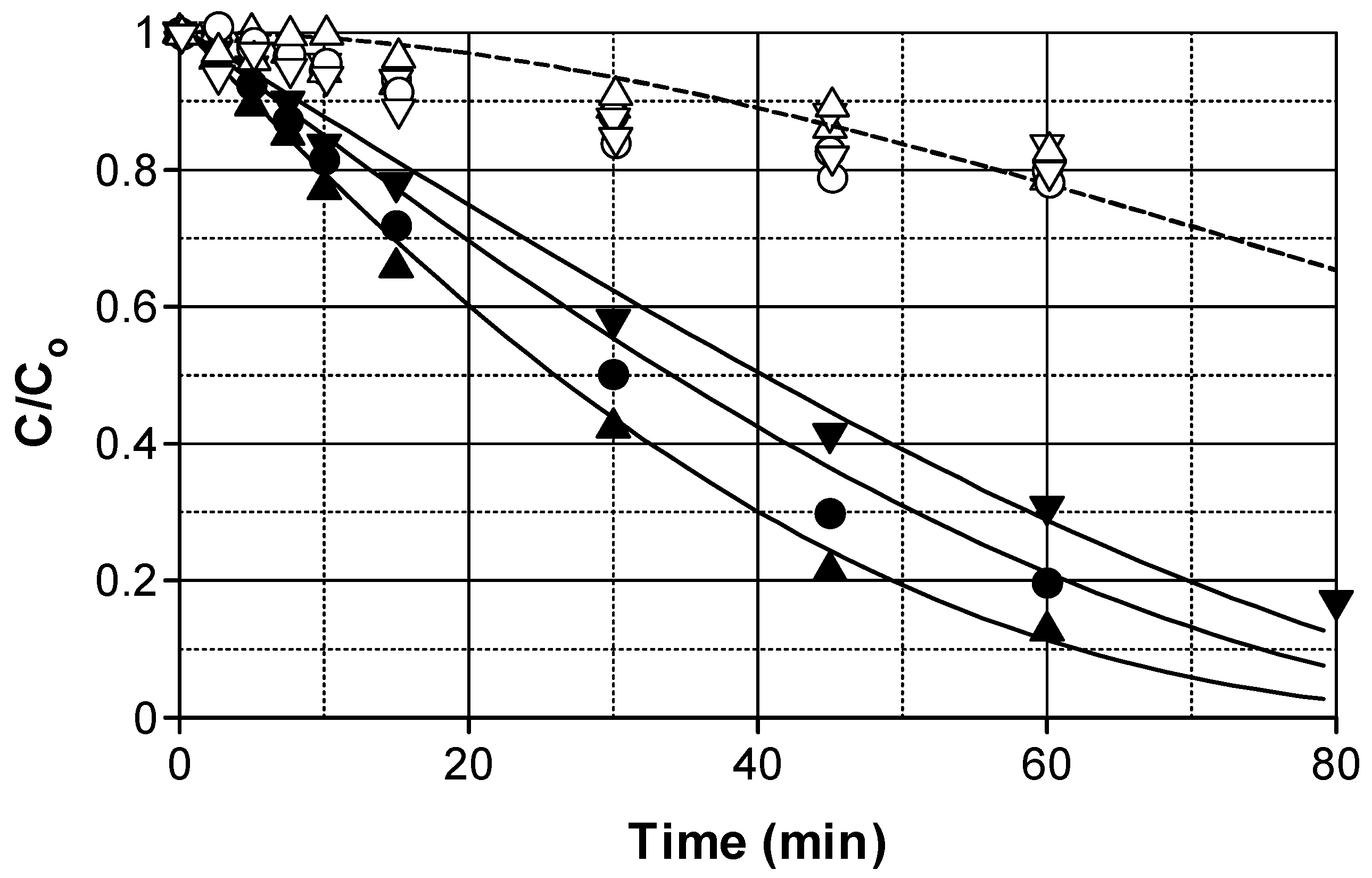
2.1. Photocatalytic Degradation Influence of a Water Matrix
2.2. Influence of Volumetric Photon Flux
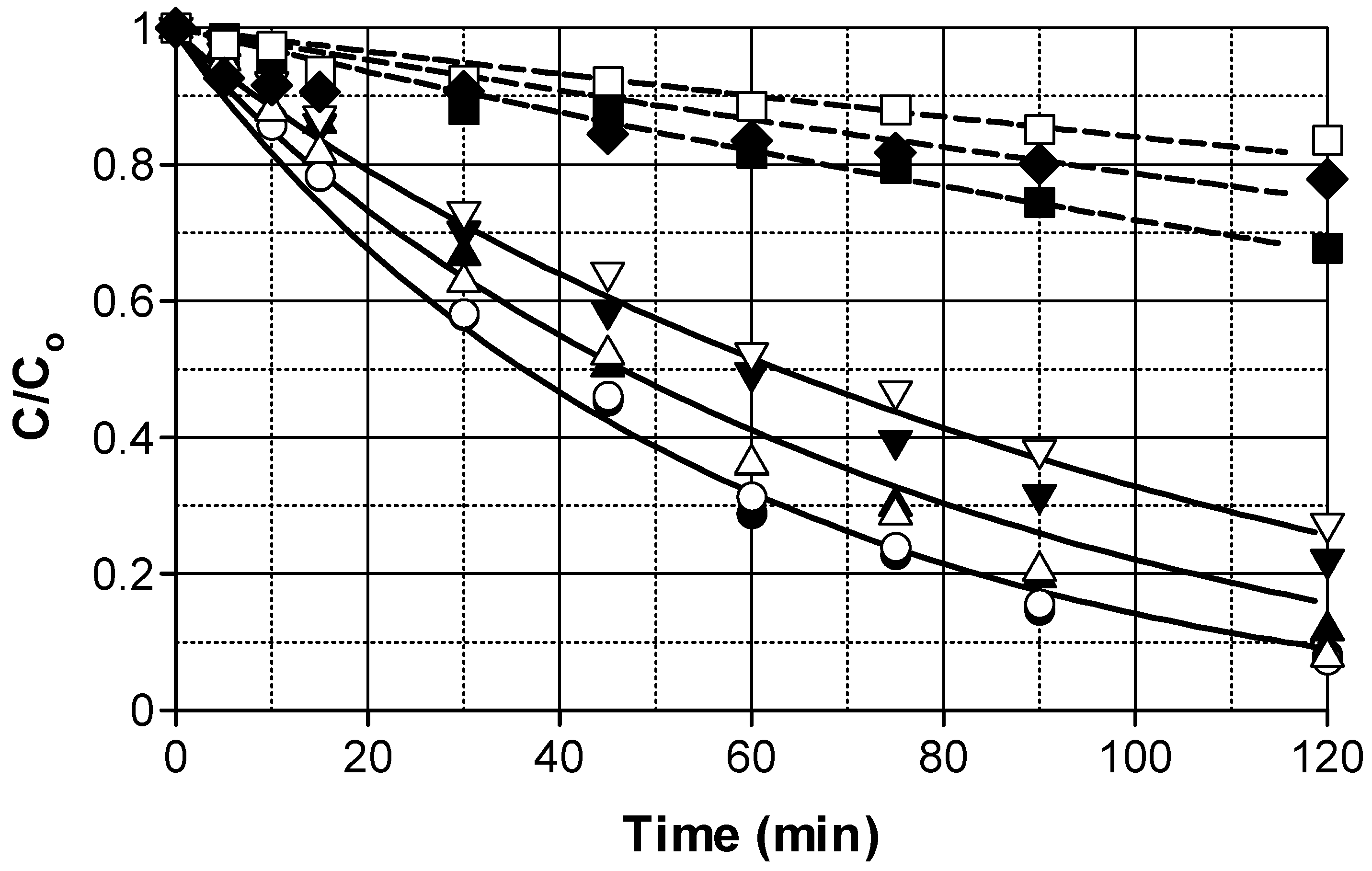
2.3. Effect of Radical Scavengers Addition
2.3.1. Phosphates Addition
2.3.2. Carbonates Addition
2.3.3. Tert-Butyl Alcohol (t-BuOH) Addition
2.3.4. Removal of Alkalinity from Wastewater
3. Materials and Methods
3.1. Reagents
3.2. Photocatalyst Synthesis and Characterization
3.3. Experimental Setup and Procedure
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Wastewater Chemical Contaminants: Remediation by Advanced Oxidation Processes. Photochem. Photobiol. Sci. 2018, 17, 1573–1598. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Mohapatra, S.; Zhang, J.; Tran, N.H.; You, L.; He, Y.; Gin, K.Y.-H. Source, Fate, Transport and Modelling of Selected Emerging Contaminants in the Aquatic Environment: Current Status and Future Perspectives. Water Res. 2022, 217, 118418. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N. Organic Contaminants in African Aquatic Systems: Current Knowledge, Health Risks, and Future Research Directions. Sci. Total Environ. 2018, 619–620, 1493–1514. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y.; Sun, J.; Ding, S. Developing a Molecularly Imprinted Channels Catalyst Based on Template Effect for Targeted Removal of Organic Micropollutants from Wastewaters. Chem. Eng. J. 2022, 445, 136755. [Google Scholar] [CrossRef]
- Matoh, L.; Žener, B.; Kovačić, M.; Kušić, H.; Arčon, I.; Levstek, M.; Lavrenčič Štangar, U. Photocatalytic Sol-Gel/P25 TiO2 Coatings for Water Treatment: Degradation of 7 Selected Pharmaceuticals. Ceram. Int. 2023, 49, 24395–24406. [Google Scholar] [CrossRef]
- Liangdy, A.; Lee, W.J.; Bao, Y.; Oh, W.-D.; Lim, T.-T. Hybrid Process of Persulfate-Based Advanced Oxidation with MeOx-Functionalized Catalytic Ceramic Membrane for Synergistic Removal of Micropollutants: Recent Developments, New Insights, and Prospects. Chem. Eng. J. 2023, 466, 143280. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent Advances in Photo-Activated Sulfate Radical-Advanced Oxidation Process (SR-AOP) for Refractory Organic Pollutants Removal in Water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Wang, X.; Ao, X.; Zhang, T.; Li, Z.; Cai, R.; Chen, Z.; Wang, Y.; Sun, W. Ultraviolet-Light-Emitting-Diode Activated Monochloramine for the Degradation of Carbamazepine: Kinetics, Mechanisms, by-Product Formation, and Toxicity. Sci. Total Environ. 2022, 806, 151372. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Cai, J.; Gendy, E.A.; Chen, Z. Impact of Chloride Ions on Activated Persulfates Based Advanced Oxidation Process (AOPs): A Mini Review. Chemosphere 2021, 280, 130949. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, B.; Goutham, R.; Badri Narayan, R.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent Advancements in Supporting Materials for Immobilised Photocatalytic Applications in Waste Water Treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef]
- Giannakis, T.; Zervou, S.-K.; Triantis, T.M.; Christophoridis, C.; Bizani, E.; Starinskiy, S.V.; Koralli, P.; Mousdis, G.; Hiskia, A.; Kandyla, M. Enhancing the Photocatalytic Activity of Immobilized TiO2 Using Laser-Micropatterned Surfaces. Appl. Sci. 2024, 14, 3033. [Google Scholar] [CrossRef]
- Figueredo, M.; Rodríguez, E.M.; Cordero, E.M.; Beltrán, F.J. UVA LEDs and Solar Light Photocatalytic Oxidation/Ozonation as a Tertiary Treatment Using Supported TiO2: With an Eye on the Photochemical Properties of the Secondary Effluent. J. Environ. Chem. Eng. 2022, 10, 107371. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Lee, H.-Y. Preparation of TiO2-Deposited Silica-Based Catalysts for Photocatalytic Decomposition of Chloro-Pesticide to Environmentally Less Toxic Species. Chemosphere 2022, 290, 133300. [Google Scholar] [CrossRef]
- Rodrigues-Silva, F.; Masceno, G.P.; Panicio, P.P.; Imoski, R.; Prola, L.D.T.; Vidal, C.B.; Xavier, C.R.; Ramsdorf, W.A.; Passig, F.H.; Liz, M.V. de Removal of Micropollutants by UASB Reactor and Post-Treatment by Fenton and Photo-Fenton: Matrix Effect and Toxicity Responses. Environ. Res. 2022, 212, 113396. [Google Scholar] [CrossRef]
- Kaprara, E.; Belesakos, C.; Kollis, K.; Psaltou, S.; Zouboulis, A.; Mitrakas, M. Evaluation of Heterogeneous Catalytic Ozonation Process for the Removal of Micropollutants from Water/Wastewater: Application of a Novel Pilot-Scale Continuous Flow System. Catalysts 2023, 13, 899. [Google Scholar] [CrossRef]
- Danfá, S.; Oliveira, C.; Santos, R.; Martins, R.C.; Quina, M.M.J.; Gomes, J. Development of TiO2-Based Photocatalyst Supported on Ceramic Materials for Oxidation of Organic Pollutants in Liquid Phase. Appl. Sci. 2022, 12, 7941. [Google Scholar] [CrossRef]
- Martín-González, M.A.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Susial, P.; Doña-Rodríguez, J.M. Open-Cell Ceramic Foams Covered with TiO2 for the Photocatalytic Treatment of Agro-Industrial Wastewaters Containing Imazalil at Semi-Pilot Scale. J. Taiwan Inst. Chem. Eng. 2023, 147, 104902. [Google Scholar] [CrossRef]
- Pellegrino, F.; De Bellis, N.; Ferraris, F.; Prozzi, M.; Zangirolami, M.; Petriglieri, J.R.; Schiavi, I.; Bianco-Prevot, A.; Maurino, V. Evaluation of the Photocatalytic Activity of a Cordierite-Honeycomb-Supported TiO2 Film with a Liquid–Solid Photoreactor. Molecules 2019, 24, 4499. [Google Scholar] [CrossRef]
- McNeill, K.; Canonica, S. Triplet State Dissolved Organic Matter in Aquatic Photochemistry: Reaction Mechanisms, Substrate Scope, and Photophysical Properties. Environ. Sci. Process. Impacts 2016, 18, 1381–1399. [Google Scholar] [CrossRef]
- Faust, B.; Hoigne, J. Sensitized Photooxidation of Phenols by Fulvic Acid and in Natural Waters. Environ. Sci. Technol. 1987, 21, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Beltran, F.J.; García-Araya, J.F.; Acedo, B. Advanced Oxidation of Atrazine in Water—I. Ozonation. Water Res. 1994, 28, 2153–2164. [Google Scholar] [CrossRef]
- Balci, B.; Oturan, N.; Cherrier, R.; Oturan, M.A. Degradation of Atrazine in Aqueous Medium by Electrocatalytically Generated Hydroxyl Radicals. A Kinetic and Mechanistic Study. Water Res. 2009, 43, 1924–1934. [Google Scholar] [CrossRef]
- Li, H.; Wang, L. Degradation of P-Chlorobenzoic Acid by Hydroxyl Radicals: Kinetic Modeling and Analysis. J. Hazard. Mater. 2011, 185, 124–130. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Zhang, T. Kinetics and Mechanisms of P-Chlorobenzoic Acid Oxidation by Hydroxyl Radicals. Environ. Pollut. 2009, 157, 1211–1218. [Google Scholar] [CrossRef]
- Díaz, G.A.; Quijano, J.C. Kinetics and Mechanism of the Reaction of Hydroxyl Radicals with Atrazine: A Theoretical and Experimental Study. Environ. Toxicol. Chem. 2007, 26, 277–282. [Google Scholar] [CrossRef]
- Acero, J.L.; Stemmler, K.; von Gunten, U. Degradation Kinetics of Atrazine and Its Degradation Products with Ozone and OH Radicals: A Predictive Tool for Drinking Water Treatment. Environ. Sci. Technol. 2000, 34, 591–597. [Google Scholar] [CrossRef]
- Luo, C.; Ma, J.; Jiang, J.; Liu, Y.; Song, Y.; Yang, Y.; Guan, Y.; Wu, D. Simulation and Comparative Study on the Oxidation Kinetics of Atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−. Water Res. 2015, 80, 99–108. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Zhou, X.; Lu, X.; Gu, H.; Ma, J. Insight into the Performance of UV/Chlorine/TiO2 on Carbamazepine Degradation: The Crucial Role of Chlorine Oxide Radical (ClO•). Sci. Total Environ. 2022, 853, 158345. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, J.; Luo, Z.; Zeng, W.; Wei, Z.; Spinney, R.; Hu, W.; Dionysiou, D.D. Experimental and Theoretical Insight into Hydroxyl and Sulfate Radicals-Mediated Degradation of Carbamazepine. Environ. Pollut. 2020, 257, 113498. [Google Scholar] [CrossRef]
- Calisto, V.; Domingues, M.R.M.; Erny, G.L.; Esteves, V.I. Direct Photodegradation of Carbamazepine Followed by Micellar Electrokinetic Chromatography and Mass Spectrometry. Water Res. 2011, 45, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhuang, X. Photocatalytic Degradation of Carbamazepine by TiO2 and Its Interaction with Hydroxyl Radicals. Chem. Eng. J. 2014, 242, 26–33. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Gil, J.V. Oxidation of Carbamazepine in Aqueous Solution Using Ozone and UV Radiation: Kinetics and by-Products. J. Hazard. Mater. 2008, 152, 101–107. [Google Scholar] [CrossRef]
- Psaltou, S.; Kaprara, E.; Mitrakas, M.; Zouboulis, A.I. Performance of Heterogeneous Catalytic Ozonation with Minerals in Degradation of P-Chlorobenzoic Acid (p-CBA) from Aqueous Solutions. Proceedings 2020, 48, 12. [Google Scholar]
- Zhao, D.; Chen, C.; Wang, Y.; Ji, H.; Ma, W.; Zang, L.; Zhao, J. Surface Modification of TiO2 by Phosphate: Effect on Photocatalytic Activity and Mechanism Implication. J. Phys. Chem. C 2008, 112, 5993–6001. [Google Scholar] [CrossRef]
- Kosmulski, M. The Significance of the Difference in the Point of Zero Charge between Rutile and Anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef]
- Ameta, R.; Ameta, S.C. Photocatalysis: Principles and Applications; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2016; ISBN 978-0367870638. [Google Scholar]
- Gao, X.; Guo, Q.; Tang, G.; Peng, W.; Luo, Y.; He, D. Effects of Inorganic Ions on the Photocatalytic Degradation of Carbamazepine. J. Water Reuse Desalin. 2019, 9, 301–309. [Google Scholar] [CrossRef]
- Zhang, G.; He, X.; Nadagouda, M.N.; O’Shea, K.E.; Dionysiou, D.D. The Effect of Basic PH and Carbonate Ion on the Mechanism of Photocatalytic Destruction of Cylindrospermopsin. Water Res. 2015, 73, 353–361. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Xiao, R.; Meng, Y.; Fu, Y.; Wacławek, S.; Wei, Z.; Spinney, R.; Dionysiou, D.D.; Zeng, W.; Hu, W. The Overlooked Carbonate Radical in Micropollutant Degradation: An Insight into Hydration Interaction. Chem. Eng. J. 2023, 474, 145245. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, C.; Guo, K.; Wu, Z.; Wang, L.; Hua, Z.; Fang, J. Kinetics and Pathways of the Degradation of PPCPs by Carbonate Radicals in Advanced Oxidation Processes. Water Res. 2020, 185, 116231. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Qureshi, A.; Cohen, G. Production of Formaldehyde and Acetone by Hydroxyl-Radical Generating Systems during the Metabolism of Tertiary Butyl Alcohol. Biochem. Pharmacol. 1983, 32, 3517–3524. [Google Scholar] [CrossRef]
- Rivas, J.; Solis, R.R.; Gimeno, O.; Sagasti, J. Photocatalytic Elimination of Aqueous 2-Methyl-4-Chlorophenoxyacetic Acid in the Presence of Commercial and Nitrogen-Doped TiO2. Int. J. Environ. Sci. Technol. 2015, 12, 513–526. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Chávez, A.M.; Dionysiou, D.D. Peroxymonosulfate/Solar Radiation Process for the Removal of Aqueous Microcontaminants. Kinetic Modeling, Influence of Variables and Matrix Constituents. J. Hazard. Mater. 2020, 400, 123118. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, S.; Wang, D.; Zabalegui, N.; Li, D.; Lamkaddam, H.; Bachmeier, F.; Vogel, A.; Monge, M.E.; Perrier, S.; Baltensperger, U.; et al. Structures and Reactivity of Peroxy Radicals and Dimeric Products Revealed by Online Tandem Mass Spectrometry. Nat. Commun. 2021, 12, 300. [Google Scholar] [CrossRef]
- Zhang, Q.; Anastasio, C. Chemistry of Fog Waters in California’s Central Valley—Part 3: Concentrations and Speciation of Organic and Inorganic Nitrogen. Atmos. Environ. 2001, 35, 5629–5643. [Google Scholar] [CrossRef]
- Neta, P.; Grodkowski, J.; Ross, A.B. Rate Constants for Reactions of Aliphatic Carbon-Centered Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1996, 25, 709–1050. [Google Scholar] [CrossRef]
- Rivas, F.J. Monopersulfate in Water Treatment: Kinetics. J. Hazard. Mater. 2022, 430, 128383. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Rey, A.; Mena, E.; Beltrán, F.J. Application of Solar Photocatalytic Ozonation in Water Treatment Using Supported TiO2. Appl. Catal. B Environ. 2019, 254, 237–245. [Google Scholar] [CrossRef]





| Parameter (Units) | Value |
|---|---|
| pH | 8.2–8.4 |
| Conductivity (µS/Cm) (25 °C) | 670 |
| Turbidity (NTU) | 5 |
| COD (mg O2 L−1) | 36 |
| BOD5 (mg O2 L−1) | 11 |
| Total Organic Carbon, TOC (mg L−1) | 12 |
| Inorganic Carbon, IC (mg L−1) | 25 |
| Absorbance 254 nm | 0.24 |
| Chloride, Cl− (mg L−1) | 90 |
| Nitrate, NO3− (mg L−1) | 30 |
| Sulfate SO42− (mg L−1) | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajah, Z.; Dhibi, H.; Abdelkader, M.; Rodriguez, E.; Guiza, M.; Rivas, F.J. Supported TiO2 Photocatalysis of Spiked Contaminants in Water and Municipal Wastewater. Catalysts 2025, 15, 495. https://doi.org/10.3390/catal15050495
Rajah Z, Dhibi H, Abdelkader M, Rodriguez E, Guiza M, Rivas FJ. Supported TiO2 Photocatalysis of Spiked Contaminants in Water and Municipal Wastewater. Catalysts. 2025; 15(5):495. https://doi.org/10.3390/catal15050495
Chicago/Turabian StyleRajah, Zouhour, Houda Dhibi, Mariem Abdelkader, Eva Rodriguez, Monia Guiza, and Francisco Javier Rivas. 2025. "Supported TiO2 Photocatalysis of Spiked Contaminants in Water and Municipal Wastewater" Catalysts 15, no. 5: 495. https://doi.org/10.3390/catal15050495
APA StyleRajah, Z., Dhibi, H., Abdelkader, M., Rodriguez, E., Guiza, M., & Rivas, F. J. (2025). Supported TiO2 Photocatalysis of Spiked Contaminants in Water and Municipal Wastewater. Catalysts, 15(5), 495. https://doi.org/10.3390/catal15050495








