Growth of Two-Dimensional Edge-Rich Screwed WS2 with High Active Site Density for Accelerated Hydrogen Evolution
Abstract
1. Introduction
2. Results and Discussion
2.1. Growth Mechanism and Characterization
2.2. Optical Properties of Screwed Nanomaterials
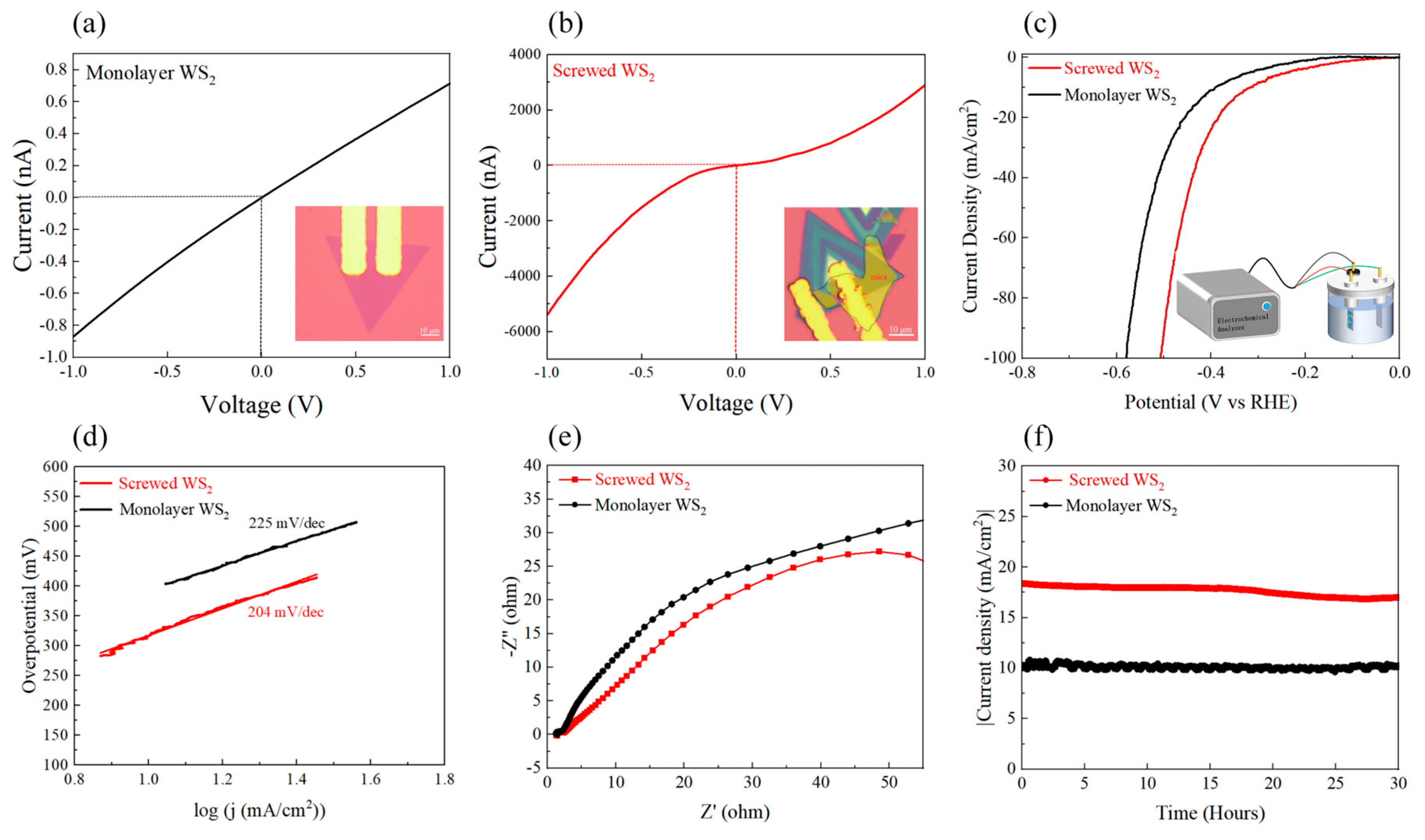
2.3. Electrocatalytic Testing
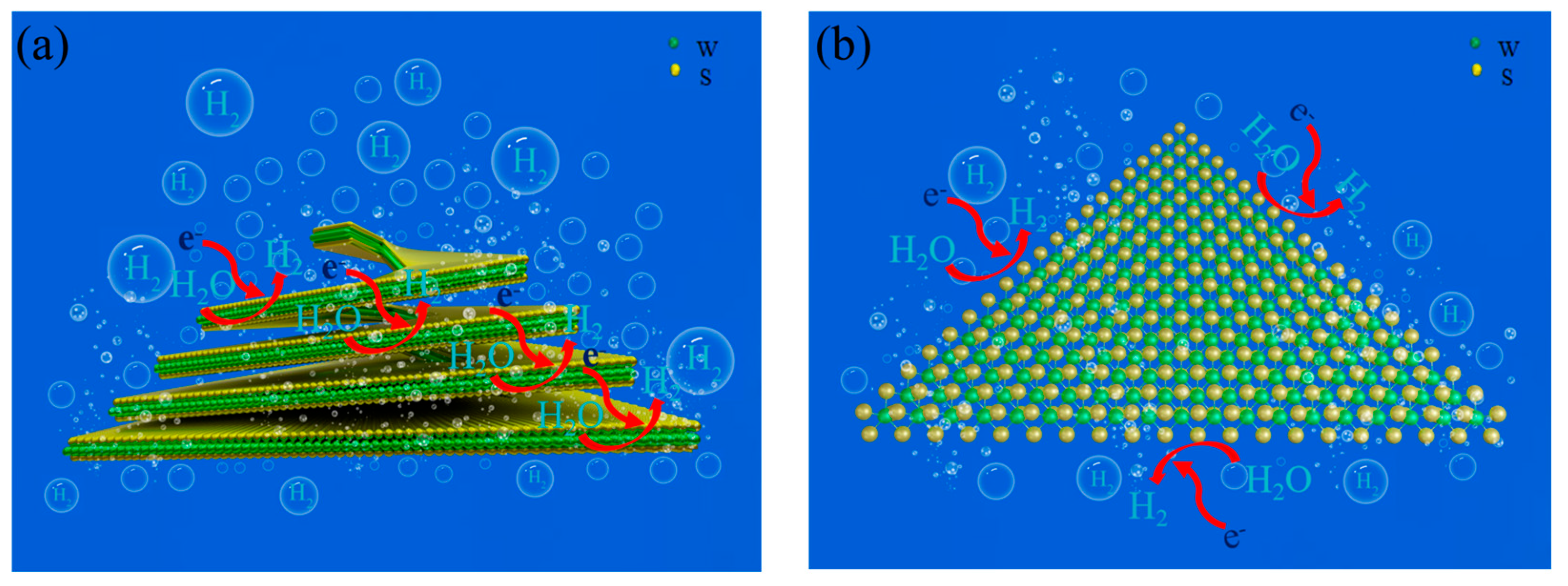
2.4. Electrocatalytic Mechanism Analysis
3. Experimental Section
3.1. Synthesis of Screwed WS2 Nanosheets
3.2. Catalytic Sample Preparation
3.3. Material Characterization
3.4. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiong, G.W.; Chen, Y.K.; Zhou, Z.Q.; Liu, F.; Liu, X.Y.; Yang, L.J.; Liu, Q.L.; Sang, Y.H.; Liu, H.; Zhang, X.L.; et al. Rapid synthesis of various electrocatalysts on Ni foam using a universal and facile induction heating method for efficient water splitting. Adv. Funct. Mater. 2021, 31, 2009580. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, J.J.; Chen, Y.; Wu, J.Y.; Wang, K.; Chen, L.J.; Wang, Y.; Jiang, X.W.; Liu, Y.Y.; Wu, Y.L.; et al. Magneto-electrically enhanced intracellular catalysis of FePt-FeC heterostructures for chemodynamic therapy. Adv. Mater. 2021, 33, 2100472. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 2019, 4, 430–433. [Google Scholar] [CrossRef]
- Dotan, H.; Landman, A.; Sheehan, S.W.; Malviya, K.D.; Shter, G.E.; Grave, D.A.; Arzi, Z.; Yehudai, N.; Halabi, M.; Gal, N.; et al. Decoupled hydrogen and oxygen evolution by a two-step electrochemical-chemical cycle for efficient overall water splitting. Nat. Energy 2019, 4, 786–795. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.W.; Wang, T.; Kang, Q.; Ouyang, S.X.; Ye, J.H. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Karunadasa, H.I.; Montalvo, E.; Sun, Y.J.; Majda, M.; Long, J.R.; Chang, C.J. A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 2012, 335, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides—Efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution. Energy. Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Li, Z.H.; Jiang, Z.Z.; Zhou, W.D.; Chen, M.Y.; Su, M.X.; Luo, X.F.; Yu, T.; Yuan, C.L. MoS2 nanoribbons with a prolonged photoresponse lifetime for enhanced visible light photoelectrocatalytic hydrogen evolution. Inorg. Chem. 2021, 60, 1991–1997. [Google Scholar] [CrossRef]
- Voiry, D.; Fullon, R.; Yang, J.; de Carvalho Castro e Silva, C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G.; et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Parsons, R.; Rosenberg, H. The kinetics of hydrogen evolution. J. Chem. Phys. 1950, 18, 762–763. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.Y.; Zhang, X.; Liang, J.X.; Yu, Q.; Xiao, H.; Li, J. Theoretical understandings of graphene-based metal single-atom catalysts: Stability and catalytic performance. Chem. Rev. 2020, 120, 12315–12341. [Google Scholar] [CrossRef]
- Lin, X.Q.; Liu, Y.Y.; Wang, K.; Wei, C.; Zhang, W.; Yan, Y.L.; Li, Y.J.; Yao, J.N.; Zhao, Y.S. Two-Dimensional Pyramid-Like WS2 Layered Structures for Highly Efficient Edge Second-Harmonic Generation. ACS Nano 2018, 12, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wu, W.Z.; Li, H.; Lin, X.C.; Wu, T.; Zhang, Y.D.; Xu, Q.; Zhang, L.P.; Zhu, Y.H.; Yang, X.; et al. Atomic plane-vacancy engineering of transition-metal dichalcogenides with enhanced hydrogen evolution capability. ACS Appl. Mater. Interfaces 2019, 11, 25264–25270. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.; Qin, W.J.; Jiang, J.Y.; Li, M.X.; Wei, C.; Xu, Q. Selective sulfuration of two-dimensional nonlayered MoO2 nanosheets for high-current-density hydrogen evolution. ACS Appl. Energy Mater. 2022, 5, 10483–10489. [Google Scholar] [CrossRef]
- Li, W.F.; Liu, J.H.; Wu, R.R.; Qiao, A.; Li, S.H.; Wei, C. Ultrasensitive and Light Erasable Surface-Enhanced Raman Scattering Substrates Based on Au-MoO2 Heterostructures. Talanta 2025, 287, 127669. [Google Scholar] [CrossRef]
- Li, W.F.; Wu, R.R.; Shang, X.Q.; Li, S.H.; Tian, Q.Y.; Xu, Q.; Wei, C. Light erasable surface-enhanced Raman scattering substrates based on the metallic molybdenum dioxide nanospheres. Sens. Actuators B Chem. 2024, 409, 135576. [Google Scholar] [CrossRef]
- Garrett, J.E.; Geisert, R.D.; Zavy, M.T.; Morgan, G.L. Evidence for maternal regulation of early conceptus growth and development in beef cattle. Reproduction 1988, 84, 437–446. [Google Scholar] [CrossRef]
- Bollinger, M.V.; Lauritsen, J.V.; Jacobsen, K.W.; Nørskov, J.K.; Helveg, S.; Besenbacher, F. One-dimensional metallic edge states in MoS2. Phys. Rev. Lett. 2001, 87, 196803. [Google Scholar] [CrossRef]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.M.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Zhou, W.D.; Chen, M.Y.; Guo, M.M.; Hong, A.J.; Yu, T.; Luo, X.F.; Yuan, C.L.; Lei, W.; Wang, S.G. Magnetic enhancement for hydrogen evolution reaction on ferromagnetic MoS2 catalyst. Nano Lett. 2020, 20, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.L.; Unwin, P.R.; Bentley, C.L. Nanoscale variations in the electrocatalytic activity oflayered transition-metal dichalcogenides. J. Phys. Chem. C 2019, 124, 789–798. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Nørskov, J.K.; Abild-Pedersen, F. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 2015, 640, 133–140. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Dyck, O.; Balke, N.; Neumayer, S.; Tsai, W.Y.; Vasudevan, R.; Lingerfelt, D.; Ahmadi, M.; Ziatdinov, M.; McDowell, M.T.; et al. Toward Electrochemical Studies on the Nanometer and Atomic Scales: Progress, Challenges, and Opportunities. ACS Nano 2019, 13, 9735–9780. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Yu, F.; Sun, J.Y.; He, R.; Wang, Y.M.; Guo, C.F.; Wang, F.; Lan, Y.C.; Ren, Z.F.; Chen, S. Highly active and durable self-standing WS2/graphene hybrid catalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 9472–9476. [Google Scholar] [CrossRef]
- Yu, S.H.; Chen, W.Z.; Wang, H.Y.; Pan, H.; Chua, D.H.C. Highly stable tungsten disulfide supported on a self-standing nickel phosphide foam as a hybrid electrocatalyst for efficient electrolytic hydrogen evolution. Nano Energy 2019, 55, 193–202. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Zhou, W.D.; Hong, A.J.; Guo, M.M.; Yuan, C.L. MoS2 Moiré superlattice for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 2830–2835. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Zhao, W.; Liu, S.; Huang, W.; Zhao, Q. WS2 moiré superlattices derived from mechanical flexibility for hydrogen evolution reaction. Nat. Commun. 2021, 12, 5070. [Google Scholar] [CrossRef]
- Liu, L.L.; Sun, Y.H.; Cui, X.Q.; Qi, K.; He, X.; Bao, Q.L.; Ma, W.L.; Lu, J.; Fang, H.Y.; Zhang, P.; et al. Bottom-up growth of homogeneous Moiré superlattices in bismuth oxychloride spiral nanosheets. Nat. Commun. 2019, 10, 4472. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.V.; Kayal, A.; Sharma, C.H.; Thalakulam, M.; Mitra, J.; Shaijumon, M.M. Electrocatalysis on edge-rich spiral WS2 for hydrogen evolution. ACS Nano 2019, 13, 10448–10455. [Google Scholar] [CrossRef]
- Fan, X.P.; Jiang, Y.; Zhuang, X.J.; Liu, H.J.; Xu, T.; Zheng, W.H.; Fan, P.; Li, H.L.; Wu, X.P.; Zhu, X.L.; et al. Broken symmetry induced strong nonlinear optical effects in spiral WS2 nanosheets. ACS Nano 2017, 11, 4892–4898. [Google Scholar] [CrossRef]
- Barman, P.K.; Sarma, P.V.; Shaijumon, M.M.; Kini, R.N. High degree of circular polarization in WS2 spiral nanostructures induced by broken symmetry. Sci. Rep. 2019, 9, 2784. [Google Scholar] [CrossRef]
- Xu, F.B.; Yu, H.; Sadrzadeh, A.; Yakobson, B.I. Riemann surfaces of carbon as graphene nanosolenoids. Nano Lett. 2016, 16, 34–39. [Google Scholar] [CrossRef]
- Parzinger, E.; Mitterreiter, E.; Stelzer, M.; Kreupl, F.; Ager, J.W.; Holleitner, A.W.; Wurstbauer, U. Hydrogen evolution activity of individual mono-, bi-, and few-layer MoS2 towards photocatalysis. Appl. Mater. Today 2017, 8, 132–140. [Google Scholar] [CrossRef]
- Su, M.; Zhou, W.D.; Liu, L.; Chen, M.Y.; Jiang, Z.Z.; Luo, X.F.; Yang, Y.; Yu, T.; Lei, W.; Yuan, C.L. Micro eddy current facilitated by screwed MoS2 structure for enhanced hydrogen evolution reaction. Adv. Funct. Mater. 2022, 32, 2111067. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, L.; Belabbas, I.; Kioseoglou, J.; Chen, J. First-principles calculations of threading screw dislocations in AlN and InN. Phys. Rev. Mater. 2018, 2, 064607. [Google Scholar] [CrossRef]
- Zhan, H.F.; Zhang, G.; Yang, C.H.; Gu, Y.T. Breakdown of Hooke’s law at the nanoscale-2D material-based nanosprings. Nanoscale 2018, 10, 18961–18968. [Google Scholar] [CrossRef]
- Sun, H.B.; Kong, X.; Park, H.; Liu, F.N.; Lee, Z.H.; Ding, F. Spiral growth of adlayer graphene. Adv. Mater. 2022, 34, 2107587. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.Y.; Park, H.J.; Lin, J.J.; Ng, Z.K.; Jing, L.; Li, H.L.; Zhu, M.M.; Tsang, S.H.; Lee, Z.H.; Teo, E.H.T. Concentric and Spiral Few-Layer Graphene: Growth Driven by Interfacial Nucleation vs Screw Dislocation. Chem. Mater. 2018, 30, 6858. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sasaki, S.; Mori, S.; Hibino, H.; Liu, Z.; Watanabe, K.; Taniguchi, T.; Suenaga, K.; Maniwa, Y.; Miyata, Y. Growth and optical properties of high-quality monolayer WS2 on graphite. ACS Nano 2015, 9, 4056–4063. [Google Scholar] [CrossRef]
- Cain, J.D.; Shi, F.; Wu, J.; Dravid, V.P. Growth mechanism of transition metal dichalcogenide monolayers: The role of self-seeding fullerene nuclei. ACS Nano 2016, 10, 5440–5445. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.; Samad, L.; Zhang, Y.; Zhao, Y.Z.; Puretzky, A.; Eliceiri, K.W.; Wright, J.C.; Hamers, R.J.; Jin, S. Complex and noncentrosymmetric stacking of layered metal dichalcogenide materials created by screw dislocations. J. Am. Chem. Soc. 2017, 139, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Zhang, C.Y.; Kohler, D.D.; Scheeler, J.M.; Wright, J.C.; Voyles, P.M.; Jin, S. Supertwisted spirals of layered materials enabled by growth on non-Euclidean surfaces. Science 2020, 370, 442–445. [Google Scholar] [CrossRef]
- Zhang, L.M.; Liu, K.H.; Wong, A.B.; Kim, J.; Hong, X.P.; Liu, C.; Cao, T.; Louie, S.G.; Wang, F.; Yang, P.D. Three-dimensional spirals of atomic layered MoS2. Nano Lett. 2014, 14, 6418–6423. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, S. Stacking and twisting of layered materials enabled by screw dislocations and non-Euclidean surfaces. Acc. Mater. Res. 2022, 3, 369–378. [Google Scholar] [CrossRef]
- Han, W.Q.; Liu, Z.H.; Pan, Y.B.; Guo, G.N.; Zou, J.X.; Xia, Y.; Peng, Z.M.; Li, W.; Dong, A.G. Designing champion nanostructures of tungsten dichalcogenides for electrocatalytic hydrogen evolution. Adv. Mater. 2020, 32, 2002584. [Google Scholar] [CrossRef]
- Ouyang, Y.L.; Li, W.; Zhang, C.W.; Wang, X.Y.; Ma, L.; Wang, H.; Lin, H.; Zou, G.F.; Zhu, J.T. Hydroxide Modified Synthesis of Atomically-Doped Photoluminescent WS2 Monolayers. Adv. Opt. Mater. 2024, 12, 2302596. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhai, L.; Zhang, Q.H.; Zhai, W.; Li, P.; Chen, B.; Chen, C.S.; Yao, Y.; Ge, Y.Y.; Yang, H.; et al. 1T′-transition metal dichalcogenide monolayers stabilized on 4H-Au nanowires for ultrasensitive SERS detection. Nat. Mater. 2024, 23, 1355–1362. [Google Scholar] [CrossRef]
- Zhang, S.H.; Liu, H.; Zhang, F.; Zheng, X.M.; Zhang, X.Z.; Zhang, B.H.; Zhang, T.; Ao, Z.K.; Zhang, X.Y.; Liu, H.; et al. Controllable Synthesis of WSe2–WS2 Lateral Heterostructures via Atomic Substitution. ACS. Nano. 2024, 18, 30321–30331. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Pendurthi, R.; Choudhury, T.H.; Redwing, J.M.; Das, S. Benchmarking monolayer MoS2 and WS2 field-effect transistors. Nat. Commun. 2021, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.W.; Cai, S.; Wei, X.; Zheng, T.; Xu, X.; Zafar, A.; Liu, H.W.; Yu, T.; Lu, J.P.; Chen, Y.F. The thinnest light disk: Rewritable data storage and encryption on WS2 monolayers. Adv. Funct. Mater. 2021, 31, 2103140. [Google Scholar] [CrossRef]
- Zeng, Z.X.S.; Sun, X.X.; Zhang, D.L.; Zheng, W.H.; Fan, X.P.; He, M.; Xu, T.; Sun, L.T.; Wang, X.; Pan, A.L. Controlled vapor growth and nonlinear optical applications of large-area 3R phase WS2 and WSe2 atomic layers. Adv. Funct. Mater. 2019, 29, 1806874. [Google Scholar] [CrossRef]
- Aoki, S.; Dong, Y.; Wang, Z.Q.; Huang, X.S.W.; Itahashi, Y.M.; Ogawa, N. Giant Modulation of the Second Harmonic Generation by Magnetoelectricity in Two-Dimensional Multiferroic CuCrP2S6. Adv. Mater. 2024, 36, 2312781. [Google Scholar] [CrossRef]
- Guo, S.Q.; Wang, Y.Y.; Zhang, J.Y. Realization of valley polarization in monolayer WS2 via 3d transition metal atom adsorption. J. Phys. D Appl. Phys. 2020, 53, 384001. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Hou, L.L.; Shautsova, V.; Warner, H. Ultrathin all-2D lateral diodes using top and bottom contacted laterally spaced graphene electrodes to WS2 semiconductor monolayers. ACS Appl. Mater. Interfaces. 2023, 15, 18012–18021. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Sun, C.; Wang, Y.; Zhao, F.; Li, Y.; Song, L.; Lv, C.; Zheng, W.; Li, H. Growth of Two-Dimensional Edge-Rich Screwed WS2 with High Active Site Density for Accelerated Hydrogen Evolution. Catalysts 2025, 15, 496. https://doi.org/10.3390/catal15050496
Hu D, Sun C, Wang Y, Zhao F, Li Y, Song L, Lv C, Zheng W, Li H. Growth of Two-Dimensional Edge-Rich Screwed WS2 with High Active Site Density for Accelerated Hydrogen Evolution. Catalysts. 2025; 15(5):496. https://doi.org/10.3390/catal15050496
Chicago/Turabian StyleHu, Dengchao, Chaocheng Sun, Yida Wang, Fade Zhao, Yubao Li, Limei Song, Cuncai Lv, Weihao Zheng, and Honglai Li. 2025. "Growth of Two-Dimensional Edge-Rich Screwed WS2 with High Active Site Density for Accelerated Hydrogen Evolution" Catalysts 15, no. 5: 496. https://doi.org/10.3390/catal15050496
APA StyleHu, D., Sun, C., Wang, Y., Zhao, F., Li, Y., Song, L., Lv, C., Zheng, W., & Li, H. (2025). Growth of Two-Dimensional Edge-Rich Screwed WS2 with High Active Site Density for Accelerated Hydrogen Evolution. Catalysts, 15(5), 496. https://doi.org/10.3390/catal15050496






