Abstract
The use of fibers or fabrics as frameworks for loading photocatalysts is beneficial in solving the problems of photocatalytic nanomaterials, which tend to agglomerate and are difficult to recycle. In this study, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb photocatalytic fibers rich in oxygen vacancies were prepared using carbon fibers as the framework by the crystal seed attachment method and in situ growth method by using the self-polymerization and strong adhesion properties of dopamine. The results of SEM, TEM and XRD tests showed that Bi2WO6 and Bi2WO6/Bi2S3 nanosheets were uniformly and completely encapsulated on the surface of the carbon fibers. The results of XPS and EPR tests showed that Bi2WO6 nanosheets were rich in oxygen vacancies. The PL, transient photocurrent responses and EIS results showed that the introduction of Bi2S3 significantly improved the migration efficiency of the photogenerated carriers of Bi2WO6/Bi2S3/CFb, which effectively hindered the recombination of photogenerated electron–hole pairs. By conducting degradation experiments on p-nitrophenol and analyzing the bandgap structure, it was postulated that the heterojunction structure of Bi2WO6/Bi2S3/CFb in the Bi2WO6/Bi2S3 material was not Type-II but Z-scheme. As analyzed by the active species assay, the active species that played a major role in the degradation process were O2− and h+. The incorporation of a small amount of Bi2S3 resulted in enhanced photocatalytic degradation activity of Bi2WO6/Bi2S3/CFb toward tetracycline hydrochloride compared to Bi2WO6/CFb. The excellent photocatalytic performance of Bi2WO6/Bi2S3/CFb photocatalytic fibers can be attributed to the rapid transmission and separation performance and the high oxidation and reduction capacities of photogenerated electron–hole pairs formed by direct Z-scheme heterojunctions.
1. Introduction
With the development of the industrial economy, the problem of water pollution has been a focus of social concern, especially the uncontrolled discharge of a large amount of industrial wastewaters, such as those containing antibiotics, dye wastewater, inorganic heavy metal ions and so on, which has become the main factor of environmental pollution and urgently needs to be solved [1,2,3,4]. Photocatalytic technology is an environmentally friendly water pollution control method that is increasingly attracting the attention of researchers, as it is able to completely mineralize many organic pollutants that are difficult to biodegrade by using sunlight energy through catalysts. Among many photocatalytic materials, TiO2, ZnO and SrTiO3 are commonly used catalytic materials with superior performance, but their bandgap widths are large, and they can only absorb ultraviolet light. Bi2WO6, an Aurivillius-phase compound with a bandgap of 2.7–3.0 eV, is a visible-light-responsive catalyst that has received much attention in recent years [5,6]. The reaction sites of this layered structure are located on the surface and edges, promoting rapid hole migration to the lamellar surface where they are trapped by interlayer hydroxide groups, thereby effectively inhibiting photogenerated electron–hole recombination. However, improving the photocatalytic performance of Bi2WO6 while preventing nanoparticle agglomeration remains a challenge.
To address the challenges of agglomeration and difficulty in recycling associated with powdered nano-photocatalysts, researchers have developed a series of semiconductor photocatalytic carriers, primarily including silicon-based carriers, ceramic-based carriers and carbon-based carriers, among others. In this context, a photocatalytic fiber material based on carbon fiber as a carrier has emerged [7].
Carbon fibers are considered to be one of the most promising materials at present due to their excellent mechanical properties such as good electrical conductivity, flexibility and corrosion resistance. Carbon fiber exhibits high electrical conductivity, enabling efficient capture and transfer of photogenerated electron–hole pairs to suppress carrier recombination, thereby enhancing photocatalytic performance. Additionally, its large specific surface area facilitates π–π stacking interactions with organic pollutants, improving adsorption capacity and photocatalytic degradation rates [8]. For example, BiVO4/carbon fiber was prepared by activation treatment of carbon fiber followed by a direct hydrothermal method [9], BiOI/Bi2WO6/ACF [10] was prepared by activated carbon cloth as a substrate, and Bi2WO6/carbon fiber was prepared by the hydrothermal method [8]. However, if we take a closer look at the SEM images (Figure S1) in the above literature, it is evident that the photocatalysts such as BiVO4, Bi2WO6 and BiOI/Bi2WO6 are not completely encapsulated on the surface of carbon fiber. This is mainly due to the smooth surface of carbon fiber, which has few active groups, making it difficult to load semiconductor photocatalysts or causing them to easily fall off.
A review of the literature revealed that dopamine, as a biological neurotransmitter, could generate tightly adherent polydopamine layers on the surface of a range of materials through self-polymerization and strong adhesion properties [11,12], which can provide an effective carrier for the deposition of photocatalysts. Whether photocatalysts or electrocatalysts can be loaded onto carbon support materials through the self-polymerization and strong adhesion properties of dopamine deserves further investigation.
In this work, Bi2WO6 crystal species (Bi, W ions) were adhered to the surface of carbon fibers (CFbs) using the bonding effect of polydopamine, and then through the hydrothermal process, the in situ growth of Bi2WO6 and Bi2WO6/Bi2S3 on the surface of carbon fibers was achieved to obtain the Bi2WO6/CFb. Bi2WO6/Bi2S3/Cfb with a fully encapsulated core–shell structure. Tetracycline hydrochloride (TC), a widely used antibiotic, was selected as the target pollutant in this study due to its stable aromatic molecular structure that resists degradation under natural conditions. The photocatalytic degradation experiments showed that Bi2WO6/Bi2S3/Cfb had excellent photocatalytic degradation performance for tetracycline hydrochloride. More importantly, by analyzing the experimental results of photocatalytic degradation of p-nitrophenol, the heterojunction composite of Bi2WO6/Bi2S3 was postulated as a Z-scheme structure.
2. Results and Discussion
2.1. Analysis of Catalyst Morphology and Physical Structure
Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb composite photocatalytic materials were synthesized by a two-step synthesis method. In the first step, the Bi2WO6 crystal species (Bi ions and W ions) were loaded onto the surface of the carbon fiber using the strong adhesion and self-polymerization of polydopamine. As shown in Figure 1a, the carbon fibers prepared from bamboo pulp fibers are elongated fibers with a diameter of about 5 µm, and the longitudinal surface of the fibers is very smooth with many grooves. From Figure 1b–d, it can be observed that the surface of the carbon fibers became rough, with many particles adhering to the fiber surface, and some of the particles showed a laminar structure after immersing the carbon fibers in a mixture of dopamine, Bi(NO3)3·5H2O and (NH4)6H2W12O40). Figure S3 showed the SEM-EDS diagram of the carbon fibers loaded with Bi2 WO6 crystal species (Bi ions and W ions), from which it can be observed that Bi and W elements were uniformly distributed on the surface of the carbon fiber, indicating that the Bi2WO6 crystal species (Bi ions and W ions) were uniformly adhered to the surface of the carbon fiber through the polymerization and adhesion of polydopamine.
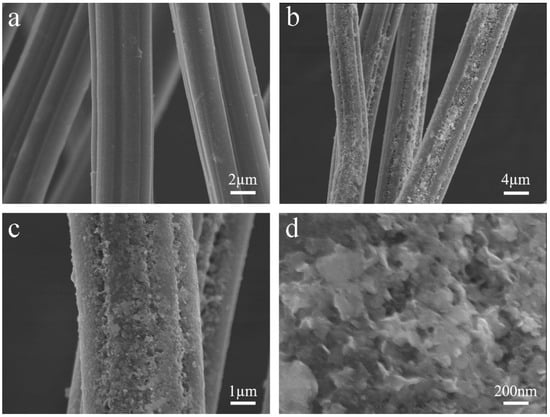
Figure 1.
The SEM images of (a) the carbon fiber (CFb) and (b–d) CFb with Bi2WO6 seeds under different magnifications.
In the second step of the two-step synthesis method, carbon fibers loaded with Bi2WO6 crystalline species were used as substrates to form CFb/Bi2WO6 and Bi2WO6/Bi2S3/CFb composite photocatalytic fibers on the surface of carbon fibers by the solvent–thermal method via an in situ growth method, and the SEM images of the specimens are shown in Figure 2. Figure 2a–c show the SEM images of CFb/Bi2WO6 specimens with different magnifications, from which it can be seen that the Bi2WO6 nanoflake layer was flower-like, encapsulated on the surface of the carbon fiber, and the encapsulation was relatively uniform and complete. From Figure 2c, it can be observed that the thickness of the Bi2WO6 nanoflake layer on the surface of CFb/Bi2WO6 was about 10 nm, which grew uniformly and perpendicularly on the surface of the carbon fiber. Figure 2d–f show SEM images of Bi2WO6/Bi2S3/CFb specimens with different ratios, whose surface morphology was similar to that of CFb/Bi2WO6. The Bi2S3 material was not clearly observed from Figure 2d–f, which may be due to the low content of Bi2S3 and the limitation of resolution, which made it difficult to distinguish the Bi2S3 material. To further confirm the presence of Bi2S3 material, the Bi2WO6/Bi2S3/CFb specimens were analyzed by SEM-EDS. The elemental mapping in Figure S4 shows that the elements Bi, W, S and O were uniformly distributed on the surface of the carbon fiber, but the content of the S element was relatively low.
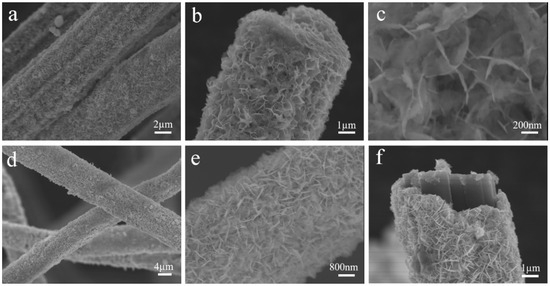
Figure 2.
The SEM images (a–c) of Bi2WO6/CFb and the SEM images (d–f) of Bi2WO6/Bi2S3/CFb.
XRD was applied to detect and analyze the crystal structure of the samples. As shown in Figure 3a, the pure Bi2WO6 specimen showed significant peaks at 28.0°, 32.5°, 46.6°, 47.0° and 55.9°, which corresponded to the (131), (060), (260), (202) and (331) facets of Bi2WO6 (JCPDS#39-0256) [13]. The pure Bi2S3 specimen showed strong diffraction peaks at 15.7°, 17.4°, 22.2°, 23.6°, 24.8°, 28.5° and 31.7°, which were attributed to the (020), (120), (220), (101), (130), (211) and (221) facets of Bi2S3 (JCPDS#17-0320) [14]. The remarkable characteristic peaks of CFb/Bi2WO6 corresponded to those of Bi2WO6 (JCPDS#17-0320), indicating the successful loading of the aureoviris-structured Bi2WO6 on the surface of carbon fibers. The XRD spectra of Bi2WO6/Bi2S3/CFb were similar to that of CFb/Bi2WO6, but no obvious diffraction characteristic peaks of Bi2S3 were observed, which could be attributed to the relatively low content of Bi2S3. The microstructure of the Bi2WO6/Bi2S3/CFb composite fibers was further examined and analyzed by TEM and HRTEM, and the presence or absence of Bi2S3 was determined. Figure 3b showed Bi2WO6/Bi2S3/CFb composite fibers that fell off from Bi2WO6/Bi2S3 nanomaterials, consisting of nanosheets and consistent with the previous SEM images. Figure 3c shows the HRTEM image, which shows that the lattice spacing of 0.315 nm corresponded to the (131) crystallographic surface of Bi2WO6 [15]. In addition, the lattice spacing of 0.356 nm and 0.231 nm could be attributed to the (130) and (041) crystal planes of Bi2S3 [16], respectively, and this result suggested the presence of Bi2S3 material in Bi2WO6/Bi2S3/CFb.
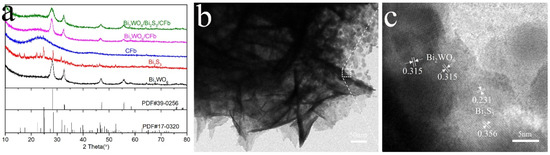
Figure 3.
XRD patterns of samples (a), including Bi2WO6/Bi2S3/CFb, Bi2WO6/CFb, CFb, Bi2WO6 and Bi2S3. TEM image (b) and HRTEM image (c) of Bi2WO6/Bi2S3/CFb.
In summary, the strong adhesion and self-polymerization properties of polydopamine were utilized to enable the uniform and complete encapsulation of Bi2WO6 and Bi2WO6/Bi2S3 nanosheets on the carbon fiber surface through a two-step method.
2.2. Surface and Valence Bond Analysis of Materials
The electronic states and surface chemical bonding of the Bi2WO6/Bi2S3/CFb composite fiber materials were analyzed by XPS measurements, and the results are detailed in Figure 4a–e. Figure 4a shows the full spectrum of Bi2WO6/Bi2S3/CFb, in which the characteristic signals of the elements C, Bi, W and O were more significant, while the signal of the element S was weaker. As shown in Figure 4b, the high-resolution spectrum of the C 1s spectrum fitted to three characteristic peaks, and the fitted peak at 284.6 eV was attributed to the C–C bond of graphitic carbon, while the fitted peaks at 286.5 eV and 287.9 eV corresponded to the characteristic peaks of the C–O and C=O bonds. As shown in Figure 4c, there were three fitted peaks in the spectrum of O 1s at 530.8 eV, 531.5 eV and 533.1 eV, where the peak at 530.8 eV corresponded to the lattice oxygen in Bi2WO6, and the peak at the binding energy of 531.5 eV corresponded to the surface adsorbed oxygen, while the peak at the binding energy of 533.1 eV corresponded to the adsorbed oxygen of oxygen vacancies, which suggested that there may be oxygen vacancies in the Bi2WO6 of Bi2WO6/Bi2S3/CFb photocatalytic fibers. As shown in Figure 4d, the fitted peaks located at the binding energies of 159.3 eV and 164.6 eV in the spectrum of Bi 4f (with a difference of 5.3 eV between the two peaks) corresponded to Bi 4f7/2 and Bi 4f5/2 in Bi2WO6 [17], whereas the fitted peaks located at 159.2 eV and 164.5 eV with weaker peak strength should belong to Bi 4f7/2 and Bi 4f5/2 in Bi2S3. In the spectra of S-2p, the weakly fitted peaks located at 160.9 eV and 162.1 eV were attributed to S 2p3/2 and S 2p3/2, with a distance of 1.2 eV between the two peaks, which suggested that the predominant state of the S atoms in Bi2S3 is S2− [17,18]. In addition, the fitted peak located at 166.6 eV may represent the S–O bond [19], which was attributed to the interaction of Bi2S3 nanoparticles and Bi2WO6 nanosheets. The weak intensity of the S-2p fitted peak may be attributed to the weaker content of Bi2S3 in the composites. As shown in Figure 4e, the fitted peaks of W4f in Bi2WO6 were located at 35.5 eV and 37.6 eV, the spacing between the two peaks was 2.1 eV, and the main state of W atom in Bi2WO6 was W6+ [17,18].
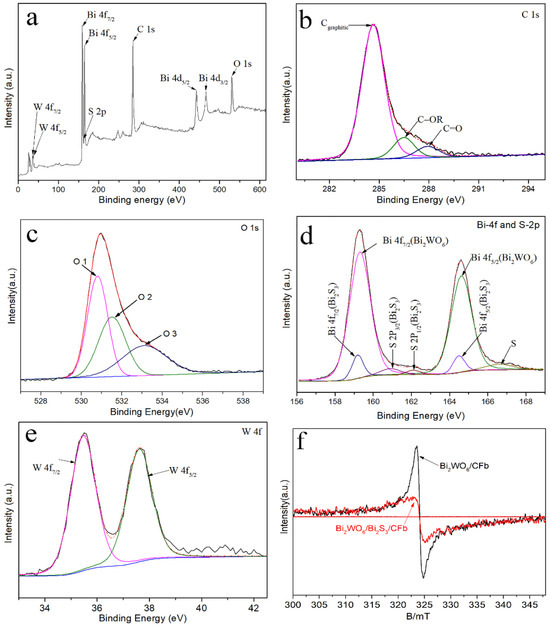
Figure 4.
XPS survey spectra (a) and high-resolution XPS spectra in C 1s (b), O 1s (c), Bi 4f and S 2p (d) and W 4f (e) of Bi2WO6/Bi2S3/CFb. (f) EPR oxygen vacancy test for Bi2WO6/Bi2S3/CFb and Bi2WO6/CFb.
The presence of oxygen vacancies in Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb materials was analyzed by EPR testing. As shown in Figure 4f, there were oxygen vacancy signal peaks at g = 2.003 in Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb, and the oxygen vacancy signal peaks of Bi2WO6/Bi2S3/CFb were stronger than those of Bi2WO6/CFb, suggesting that both Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb materials had oxygen vacancies, but the number of oxygen vacancies decreased with the introduction of Bi2S3. In the photocatalyst synthesis process, ethylene glycol and methanol were used as solvents to load Bi2WO6 and Bi2WO6/Bi2S3 materials onto carbon fibers via an anhydrous solvothermal method. Ethylene glycol, with its higher viscosity and density and weaker polarity, was prone to forming oxygen vacancies in Bi2WO6 [20]. However, the introduction of S ions during the preparation of Bi2WO6/Bi2S3/CFb could easily fill the oxygen vacancies.
2.3. Analysis of Specific Surface and Thermogravimetric of Materials
The fibers are generally considered to have a relatively large specific surface area, as shown in Figure S5, the specific surface area of Bi2WO6/Bi2S3/CFb material is 18.058 cm2/g, and the average pore size is about 3.797 nm [21]. The content of the carbon fibers in the Bi2WO6/Bi2S3/CFb specimen was investigated by thermogravimetric analysis, as shown in Figure S6, and it is estimated that the content of Bi2WO6/Bi2S3 in the Bi2WO6/Bi2S3/CFb composite photocatalyst was about 47 wt%, the content of carbon fiber was about 53 wt%, and the content of Bi2S3 accounted for about 4 wt%.
2.4. Optical and Photoelectrochemical Properties of Materials
The activity of photocatalysts is closely related to the light absorption properties of the catalysts, and the optical absorption properties of Bi2WO6, Bi2S3, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb were tested and investigated by using the UV-vis DRS. As shown in Figure 5a, the optical absorption edge of Bi2WO6 was located around 420 nm, which was at the edge of the visible light absorption, and Bi2WO6/CFb had a certain optical absorption performance in the visible region compared with Bi2WO6, which is attributed to the black carbon fiber having good optical absorption performance. Bi2S3 showed excellent light absorption in the visible region and even in the near-infrared region. Therefore, the introduction of a small amount of Bi2S3 into the Bi2WO6/Bi2S3/CFb composite photocatalytic fibers significantly improved the light-absorbing properties of the composite fibers. According to Tauc’s law, the semiconductor forbidden bandwidth can be determined according to the absorption spectra. As shown in Figure 5b, the bandgap widths of pure Bi2WO6 and Bi2S3 are about 2.70 and about 0.94 eV, respectively.
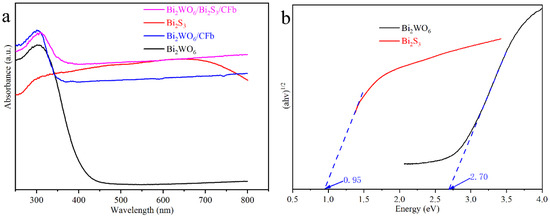
Figure 5.
(a) UV-vis spectra of Bi2WO6/Bi2S3/CFb, Bi2WO6/CFb, Bi2WO6 and Bi2S3 sample, (b) plots of (αhν)1/2 vs. photon energy (hν) for Bi2WO6 and Bi2S3.
Here, the semiconductor types and energy band potentials of Bi2WO6 and Bi2S3 were further investigated by electrochemical Mott–Schottky experiments. As shown in Figure S7, based on the Mott–Schottky equation and test curves, the slopes of the Mott–Schottky curves for Bi2WO6 and Bi2S3 were positive, which indicated that the Bi2WO6 and Bi2S3 catalysts were typical n-type semiconductors. By fitting, the flat-band potentials of pure Bi2WO6 and Bi2S3 can be inferred to be −0.16 eV and −0.52 eV (glymercury electrode). The saturated calomel electrode (SCE) potential (25 °C) is about 0.24 eV with respect to the standard hydrogen electrode (NHE) potential [22]. For n-type semiconductors, the flat-band potential is 0.1 eV more positive than the CB potential [23]. Therefore, the CB of Bi2WO6 and Bi2S3 should be about −0.02 and −0.38 eV with respect to the NHE by calculation. Combined with the bandgap widths, the VBs of Bi2WO6 and Bi2S3 can be determined to be about 2.68 eV and 0.57 eV, respectively.
The Interfacial charge separation and migration of Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb were analyzed by means of electrochemical impedance and transient photocurrent. As shown in Figure 6a, the Nyquist plots of Bi2WO6/CFb, Bi2WO6/Bi2S3/CFb and CFb exhibit a single semicircle in the high-frequency region. The semicircle radius reflects the charge transfer resistance, where a smaller Nyquist radius corresponds to faster carrier migration rates [24,25]. Notably, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb demonstrate significantly smaller semicircle radii than CFb, with Bi2WO6/Bi2S3/CFb exhibiting the smallest radius. These results indicate that the incorporation of Bi2S3 substantially enhances the migration rate of photogenerated electrons and holes in Bi2WO6/Bi2S3/CFb. As shown in Figure 6b, the transient photocurrent of Bi2WO6/CFb is about 3.5 nA under the same light intensity, while the transient photocurrent of Bi2WO6/Bi2S3/CFb is about 11 nA, which is about three times that of Bi2WO6/CFb. Carbon fibers have no photocurrent, but they have strong electrical conductivity. Therefore, the construction of a Bi2WO6/Bi2S3 heterojunction improved the separation rate of photogenerated carriers in Bi2WO6/Bi2S3/CFb materials. Photoluminescence (PL) spectroscopy can be used to study the photogenerated electron–hole pair recombination of the photocatalysts. The charge complexation of Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb composites was investigated by PL at an excitation wavelength of 300 nm, as shown in Figure 6c. Compared with Bi2WO6/CFb, with the complexation of Bi2S3, the Bi2WO6/Bi2S3/CFb underwent a substantial decrease in PL intensity, and the photogenerated electron–hole pair recombination was inefficient, which suggested that the construction of the Bi2WO6/Bi2S3 heterojunction enhanced the photogenerated carrier mobility efficiency, which effectively hindered photogenerated electron–hole pair recombination; thus, the photocatalytic performance was improved.
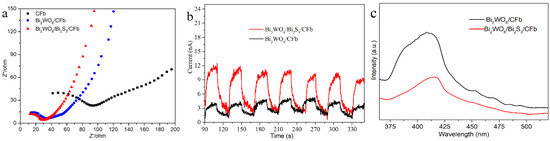
Figure 6.
(a) EIS Nyquist plots, (b) photocurrent response of different photocatalysts and (c) steady-state PL emission of Bi2WO6/Bi2S3/CFb and Bi 2WO6/CFb.
2.5. Results and Analyses of Degradation Experiments
To evaluate the photocatalytic activity of Bi2WO6/Bi2S3/CFb and Bi2WO6/CFb fiber materials, photocatalytic degradation experiments were performed using tetracycline hydrochloride (TC) from antibiotics as a target pollutant, as shown in Figure 7a. In the TC degradation experiments, the degradation curves of CFb and control experiments (without catalyst) were basically the same, which indicated that carbon fiber had no photocatalytic activity for TC. The degradation efficiency of TC in the presence of Bi2WO6/CFb reached about 74% after 50 min. Comparatively, the degradation efficiency of TC by Bi2WO6/Bi2S3/CFb was about 88%, which was significantly higher than that of Bi2WO6/CFb. It was clear that the introduction of a small amount of Bi2S3 nanoparticles improved the photocatalytic performance, which was attributed to the synergistic effect between Bi2WO6 and Bi2S3. As shown in Figure 7b, the photocatalytic degradation process of TC followed a quasi-primary reaction kinetic equation, and it was obvious that the Bi2WO6/Bi2S3/CFb photocatalytic fibers presented the fastest efficiency for degrading TC. As shown in Figure S8a, the degradation rate of Bi2WO6/Bi2S3/CFb only weakly decayed after four cycling experiments and the XRD spectra before and after the cycling experiments. As shown in Figure S8b, the XRD spectrum before and after the cyclic experiment showed that there was no obvious structural change in Bi2WO6/Bi2S3/CFb. The above results demonstrated that Bi2WO6/Bi2S3/CFb had excellent stability and potential practical applications.
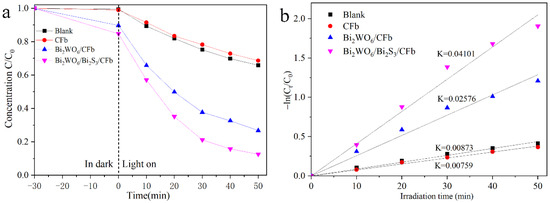
Figure 7.
The photocatalytic degradation rate of TC by different catalysts in dark and light (a) and the corresponding degradation kinetics curves (b).
2.6. Identification of Active Species for Photocatalytic Degradation
In this paper, the active radical species generated during photocatalyst degradation were determined by ESR technique. As shown in Figure 8a, Bi2WO6 was detected with significant hydroxyl radical signals after 5 min of light exposure, which was consistent with the findings of many scholars [26,27]. A very interesting finding was that Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb failed to be detected with obvious hydroxyl radical signals under the same testing conditions, that is to say, the composite of Bi2WO6 with carbon fiber failed to be detected with obvious hydroxyl radical signals, which was consistent with the test results in the research report of Chen Jian’s research team [28]. As shown in Figure 8b, superoxide radical signals were detected for Bi2WO6, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb under the effect of light [29], which may be attributed to the combination of electrons on the catalyst conduction band with the oxygen adsorbed on the surface of the material to generate superoxide radicals. Close observation revealed that the superoxide radical signals of Bi2WO6, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb were sequentially enhanced, suggesting that the stepwise introduction of the carbon fibers and Bi2S3 enhanced the photocatalytic activity. To further identify the photocatalytic degradation active species, capture experiments were performed by adding inhibitors to the photocatalytic degradation experiments of TC. Among them, isopropanol (IPA), p-benzoquinone (BQ) and ethylenediaminetetraacetic acid (EDTA) were used as inhibitors of −OH, ‧O2− and h+, respectively. As shown in Figure 8c, the degradation rate of TC changed after the addition of the inhibitors, with the trend of no inhibitor > IPA > EDTA > BQ. After the addition of IPA, the degradation rate was close to that in the case of no inhibitor, indicating that fewer active -OH species was generated during the degradation of TC by Bi2WO6/Bi2S3/CFb, which was consistent with the previous ESR assay results. The experimental results indicate that the active species produced in this photocatalytic degradation process acted in the following order, ‧O2− > h+ > −OH. ‧O2−, and h+ were the most dominant active species [8].
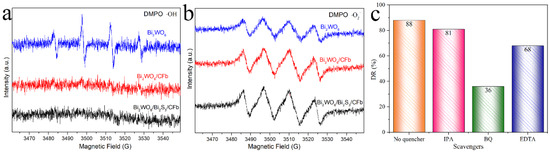
Figure 8.
EPR free-radical detection in different samples: (a) hydroxyl radicals and (b) superoxide radicals. (c) The reaction rate constants’ values intervened.
2.7. Z-Scheme Heterojunction Structure Analysis and Photocatalytic Degradation Mechanism Study
To study and analyze the photocatalytic degradation mechanism of Bi2WO6/Bi2S3/CFb composite photocatalytic fibers in the removal of TC, it is firstly necessary to determine the type of heterojunction structure as well as the charge transfer path of Bi2WO6/Bi2S3. Through a Web of Science search, the literature reports on the heterojunction structure of Bi2WO6/Bi2S3 show discrepancies in terms of two types of structures, that is, 15 papers reported that the heterojunction structure of Bi2WO6/Bi2S3 was Type-II, whereas 9 papers reported that the heterojunction structure of Bi2WO6/Bi2S3 was a direct Z-scheme heterojunction. The Type-II and direct Z-scheme heterojunction structures of the Bi2WO6/Bi2S3 heterojunction are shown in Figure 9. Based on the previous ESR detection and analysis, the type of heterojunction structure of Bi2WO6/Bi2S3 in Bi2WO6/Bi2S3/CFb could not be determined simply by the type of radicals.
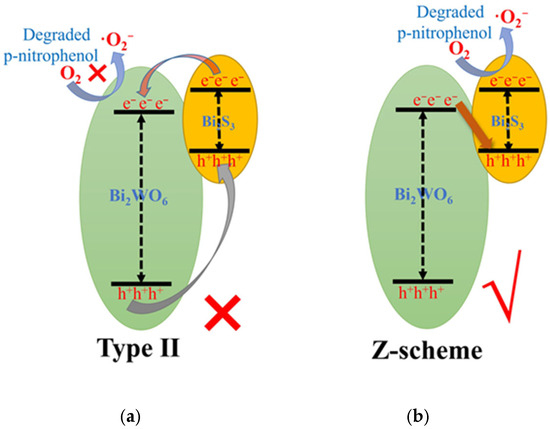
Figure 9.
Schematic diagrams of Bi2WO6/Bi2S3 heterojunction structures: (a) Type-II and (b) Z-scheme.
Yi-Jun Xu’s research team confirmed that the heterojunction structure of the prepared Bi2WO6/TiO2 material was a direct Z-scheme heterojunction through the inability of Bi2WO6 to degrade 4-nitroaniline [30]. Here, the heterojunction structure of Bi2WO6/Bi2S3 in Bi2WO6/Bi2S3/CFb composite photocatalytic fibers was determined by experiments using Bi2WO6, Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb for the photocatalytic degradation of p-nitrophenol. As shown in Figure S9, in the degradation test of p-nitrophenol, the CFb, Bi2WO6 and Bi2WO6/CFb materials were essentially incapable of photocatalytic degradation of p-nitrophenol. Comparatively, the removal of p-nitrophenol by Bi2WO6/Bi2S3/CFb photocatalytic fibers was about 23% after 80 min of light, indicating that the complexation of a small amount of Bi2S3 improved the photocatalytic activity of Bi2WO6/Bi2S3/CFb photocatalytic fibers. Bi2WO6 cannot degrade p-nitrophenol, while Bi2S3 can degrade p-nitrophenol [31,32]. Assuming that the heterojunction structure of Bi2WO6/Bi2S3 in Bi2WO6/Bi2S3/CFb composite photocatalytic fibers was Type-II type, the electrons on the conduction band of the Bi2S3 material will migrate to the conduction band of the Bi2WO6, while the valence band holes of Bi2WO6 will migrate to the lower valence band of Bi2S3, and both electrons and holes will be shifted to the low-energy positions. However, electron aggregation at the conduction band position of Bi2WO6 cannot drive the degradation of p-nitrophenol and should not enhance the photocatalytic activity of Bi2WO6/Bi2S3/CFb composite photocatalytic fibers for the degradation of p-nitrophenol, which is obviously inconsistent with the experimental results (Figure S9). Therefore, it can reasonably be assumed that the heterojunction structure of the Bi2WO6/Bi2S3 heterojunction in Bi2WO6/Bi2S3/CFb composite photocatalytic fibers is not Type-II but a direct Z-scheme heterojunction (Figure 9b).
According to the previous results and analysis, the degradation mechanism of Bi2WO6/Bi2S3/CFb composite fibers was proposed as shown in Figure 10. Bi2WO6 in Bi2WO6/Bi2S3/CFb contains oxygen vacancies, which will generate a new sodding energy level underneath the conduction band position of Bi2WO6 to form the oxygen defective states (VOs). Based on the previous energy band structure analysis, compared with the VB positions of Bi2WO6, the VB positions of Bi2S3 were closer to the VOs and CB positions of Bi2WO6, which made it easier for the electrons on the VOs and CB of Bi2WO6 to migrate towards the VB of Bi2S3. Therefore, the heterojunction structure of Bi2WO6/Bi2S3 in Bi2WO6/Bi2S3/CFb composite photocatalytic fibers should be a direct Z-scheme. The Z-scheme heterojunction makes photocatalytic materials have stronger redox capabilities, which is consistent with the previous conclusion that the superoxide radical signal is strongest in Bi2WO6/Bi2S3/CFb (Figure 8b). Bi2WO6/Bi2S3/CFb is a core–shell-structured photocatalyst with the carbon fiber in the center, and some of the photogenerated electrons on Bi2WO6 migrate towards the surface of the carbon fiber, which promotes the separation and migration of the photogenerated electrons and holes.
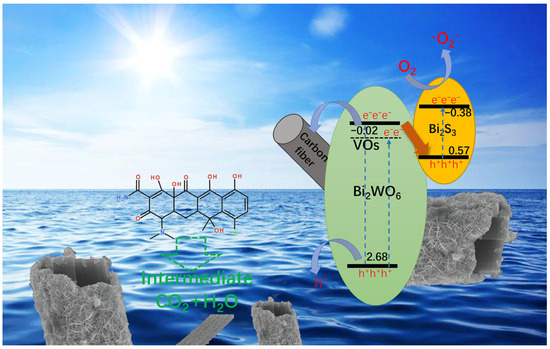
Figure 10.
Proposed mechanism for TC photodegradation by Bi2WO6/Bi2S3/CFb.
Based on the previous analysis, it was known that ‧O2− and h+ were the main active species produced during the degradation of TC by Bi2WO6/Bi2S3/CFb photocatalytic fibers, and the reaction mechanism processes were as follows:
3. Experimental Section
The characterizations are provided in Supporting Information.
3.1. Preparation of Materials
In this paper, carbon fibers (CFbs) were obtained from bamboo pulp fibers by pre-oxidation, carbonization and activation processes. The Bi2WO6 crystal species (Bi, W ions) were loaded onto the carbon fiber surface using the strong adhesion of polydopamine, and then Bi2WO6 and Bi2WO6/Bi2S3 composite photocatalysts were successfully loaded onto the carbon fiber surface by solvent-heated in situ growth method, as illustrated in Figure S2.
Preparation of activated carbon fibers. The bamboo pulp fibers were placed into a tube furnace and heated at 200 °C for 2 h for air pre-oxidation. Then, it was heated up to 800 °C at a heating rate of 5 °C/min under Ar atmosphere, and the heat was maintained for 2 h. After cooling to room temperature, carbon fibers were obtained through carbonization [33]. To activate the carbon fibers, they were soaked in concentrated nitric acid for 12 h, followed by soaking in ethanol and deionized water for 12 h each and finally dried in an oven.
Bi2WO6 crystal species loaded onto the surface of carbon fiber. Firstly, 180 mL of deionized water was heated to 80 °C, and 0.485 g of (NH4)6H2W12O40 was dissolved in the hot water with vigorous stirring to obtain a clear solution (Solution A). Meanwhile, 1.94 g of Bi(NO3)3·5H2O was added to 20 mL of ethylene glycol and stirred for 20 min to obtain solution B. Under stirring conditions, solution B was added to solution A to obtain a mixed solution containing Bi2WO6 crystal species (Bi, W ions). After the temperature of the mixed solution was reduced to room temperature, 0.4 g of dopamine hydrochloride and 0.24 g of tris (hydroxymethyl) aminomethane were dissolved in the mixed solution, respectively, and stirring was continued for 10 min to make the dopamine hydrochloride dispersed evenly. Finally, the activated carbon fibers were immersed into the above mixed solution, and the Bi and W ions were uniformly adhered to the surface of the carbon fibers by artificial intermittent stirring, taking advantage of the self-polymerization and strong adhesion of polydopamine. After removing the carbon fiber, it was dried at 60 °C for 12 h. The carbon fiber coated with Bi2WO6 crystal species was obtained.
Preparation of Bi2WO6/CFb. Typically, 0.388 g of Bi(NO3)3·5H2O was added to 5 mL of ethylene glycol and stirred for 30 min to obtain solution C. Simultaneously, 0.132 g of Na2WO4·2H2O was added to 30 mL of methanol and stirred vigorously for 30 min to obtain solution D. Both solutions were transferred to a polytetrafluoroethylene (PTEE)-lined autoclave, and carbon fibers loaded with Bi2WO6 crystal species were immersed into the reactor solution. The above autoclave was placed into an oven, heated to 180 °C and kept there for 10 h. After the autoclave was cooled to room temperature, the fibers in the PTFE-lined autoclave were taken out, washed with deionized water three times and then dried at 60 °C for 12 h; thus, Bi2WO6/CFb photocatalytic fibers were obtained.
Preparation of Bi2WO6/Bi2S3/CFb. First, 0.119 g of Na2WO4·2H2O and 0.029 g of Na2S·5H2O were added to 30 mL of methanol and stirred for 30 min to obtain analogous solution D. Following the method for the preparation of Bi2WO6/CFb photocatalytic fibers, Bi2WO6/Bi2S3/CFb photocatalytic fibers were obtained.
Preparation of pure Bi2WO6. First, 0.776 g of Bi(NO3)3·5H2O was dissolved in 5 mL of ethylene glycol and stirred for 30 min to obtain solution E. Then, 0.264 g of Na2WO4·2H2O was dissolved in 30 mL of methanol and stirred for 30 min to obtain solution F. Solution E and solution F were added to a PTFE-lined autoclave and placed in an oven and heated at 180 °C for 10 h. After the autoclave was cooled to room temperature, the solution in the PTFE-lined autoclave was removed, centrifuged five times with deionized water and anhydrous ethanol and then dried at 60 °C for 12 h to obtain pure Bi2WO6.
Preparation of pure Bi2S3. First, 0.776 g of Bi(NO3)3·5H2O was dissolved in 5 mL of ethylene glycol solution and sonicated for 10 min, and 30 mL of methanol was added to this solution. Then, 0.576 g of Na2S-5H2O was added to the mixed solution while it was stirred vigorously for 30 min. Subsequently, the mixed solution was transferred to a 50 mL PTFE-lined stainless-steel autoclave and heated at 180 °C for 10 h. After the autoclave was cooled to room temperature, the solution in the PTFE-lined autoclave was removed and centrifuged five times with deionized water and anhydrous ethanol, respectively, and then dried at 60 °C for 12 h to obtain pure Bi2S3.
3.2. Photocatalytic Tests
In this experiment, the photocatalytic activity of Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb specimens was evaluated by degrading different pollutants such as tetracycline hydrochloride (TC) and p-nitrophenol (4-NP) under simulated sunlight conditions. In this test, 30 mg of catalyst specimens was firstly put into 50 mL of TC (40 mg/L) and 4-NP (10 mg/L) solution, respectively, and stirred for 30 min under dark conditions to achieve the equilibrium of pollutant adsorption and desorption on the surface of photocatalytic materials. The photocatalytic degradation experiments were carried out using a xenon lamp with a power of 250 W to simulate sunlight conditions, and 3 mL of the aqueous solution of the degradants was taken out at different time points for UV–visible absorption tests. The variation in absorbance of TC aqueous solution was measured at a wavelength of 357 nm. The photocatalytic degradation performance of the material was evaluated based on the linear relationship between the absorbance and concentration of TC.
In order to further investigate and analyze the photocatalytic degradation mechanism, the main active species for photocatalytic degradation were identified by free radical and hole trapping experiments and electron spin resonance analysis (ESR) detection techniques. Capture experiments were performed by adding 1 mM inhibitor to the photocatalytic degradation of TC test. In the trapping experiments, isopropanol (IPA), ethylenediaminetetraacetic acid (EDTA) and p-benzoquinone (BQ) were used as the trapping agents for −OH, h+ and ‧O2−, respectively.
4. Conclusions
In this paper, Bi2WO6 and Bi2WO6/Bi2S3 nanosheets were successfully encapsulated on the surface of carbon fibers by using the self-polymerization and strong attachment properties of polydopamine, and Bi2WO6/CFb and Bi2WO6/Bi2S3/CFb photocatalytic fibers were prepared through the crystalline seed attachment method and in situ growth method. It is foreseeable that the seed adhesion method through polydopamine will be a good method for loading other photocatalysts or electrocatalysts onto carbon carrier materials, which is worthy of further exploration and research. The photocatalytic degradation experiments showed that the introduction of a small amount of Bi2S3 significantly improved the photocatalytic degradation performance of tetracycline hydrochloride by Bi2WO6/Bi2S3/CFb composite fibers. Through the detection and identification of active species, ‧O2− and h+ were the active species that played a major role in the degradation process. The heterojunction structure of Bi2WO6/Bi2S3 in Bi2WO6/Bi2S3/CFb material was postulated to not be Type-II but a Z-scheme structure by the degradation of p-nitrophenol. Therefore, the excellent photocatalytic performance of Bi2WO6/Bi2S3/CFb photocatalytic fibers can be attributed to the rapid transmission and separation performance and the high oxidation and reduction capacities of photogenerated electron–hole pairs formed by direct Z-scheme heterojunctions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15040350/s1. Refs. [8,9,10,21] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, C.L.; Methodology, J.N., J.P. and J.Q.; Formal analysis, J.N.; Investigation, C.L.; Resources, J.P.; Data curation, J.N., J.P. and J.Q.; Writing—original draft, J.N.; Writing—review & editing, J.N.; Project administration, C.L.; Funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Natural Science Foundation of Jiangsu Province (BK20231205) and in part by the “Qinglan Project” of Jiangsu Province in China under Grant 2024.
Data Availability Statement
No data were used for the research described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wang, J.; Zhou, A.; Zhang, Y.; Si, C.; Chen, Z.; Qian, H.; Zhao, Z. Research on the adsorption and migration of sulfa antibiotics in underground environment. Environ. Earth Sci. 2016, 75, 1252. [Google Scholar]
- Chen, D.; Liu, S.; Zhang, M.; Li, S.; Wang, J. Comparison of the occurrence of antibiotic residues in two rural ponds: Implication for ecopharmacovigilance. Environ. Monit. Assess. 2018, 190, 539. [Google Scholar]
- Gothwal, R.; Thatikonda, S. Role of environmental pollution in prevalence of antibiotic resistant bacteria in aquatic environment of river: Case of Musi river, South India. Water Environ. J. 2017, 31, 456–462. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar]
- Morita, K.; Park, J.-S.; Kim, S.; Yasuoka, K.; Walsh, A. Crystal engineering of Bi2WO6 to polar aurivillius-phase oxyhalides. J. Phys. Chem. C 2019, 123, 29155–29161. [Google Scholar]
- Chen, T.; Liu, L.; Hu, C.; Huang, H. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Catal. 2021, 42, 1413–1438. [Google Scholar]
- Wang, W.; Yang, R.; Li, T.; Komarneni, S.; Liu, B. Advances in recyclable and superior photocatalytic fibers: Material, construction, application and future perspective. Compos. Part B-Eng. 2021, 205, 108512. [Google Scholar] [CrossRef]
- Balta, Z.; Simsek, E.B.; Berek, D. Facile synthesis of flake-like Bi2WO6/carbon fiber heterojunction catalysts with enhanced photoactivity under visible light illumination. Optik 2019, 183, 38–46. [Google Scholar]
- Ma, J.; Chen, J.; Wang, B.; Cai, S. The in-situ growth of BiVO4 coatings on carbon fibers and their photocatalytic performance. Mater. Res. Bull. 2016, 77, 253–257. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Liu, F.; Zhao, C.; Zhao, D.; Li, X. Study on preparation and toluene removal of BiOI/Bi2WO6/ACF photocatalyst. Appl. Surf. Sci. 2019, 488, 161–169. [Google Scholar]
- Shi, Y.; Jiang, R.; Liu, M.; Fu, L.; Zeng, G.; Wan, Q.; Mao, L.; Deng, F.; Zhang, X.; Wei, Y. Facile synthesis of polymeric fluorescent organic nanoparticles based on the self-polymerization of dopamine for biological imaging. Mater. Sci. Eng. C 2017, 77, 972–977. [Google Scholar]
- Xu, Y.; Hu, J.; Hu, J.; Cheng, Y.; Chen, X.; Gu, Z.; Li, Y. Bioinspired polydopamine hydrogels: Strategies and applications. Prog. Polym. Sci. 2023, 101740. [Google Scholar]
- Zhu, Z.; Yan, Y.; Li, J. Synthesis of flower-like WO3/Bi2WO6 heterojunction and enhanced photocatalytic degradation for Rhodamine B. Micro Nano Lett. 2015, 10, 460–464. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L. Synthesis and characterization of Bi2S3 nanorods by solvothermal method in polyol media. Mater. Lett. 2007, 61, 1667–1670. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Gao, E.; Ren, J.; Wang, L. Bi2WO6/Cu0: A novel coupled system with enhanced photocatalytic activity by Fenton-like synergistic effect. Catal. Commun. 2011, 12, 834–838. [Google Scholar] [CrossRef]
- Vadivel, S.; Kamalakannan, V.P.; Keerthi; Balasubramanian, N. D-Pencillamine assisted microwave synthesis of Bi2S3 microflowers/RGO composites for photocatalytic degradation—A facile green approach. Ceram. Int. 2014, 40, 14051–14060. [Google Scholar] [CrossRef]
- Adhikari, S.; Kim, D.-H. Synthesis of Bi2S3/Bi2WO6 hierarchical microstructures for enhanced visible light driven photocatalytic degradation and photoelectrochemical sensing of ofloxacin. Chem. Eng. J. 2018, 354, 692–705. [Google Scholar] [CrossRef]
- Huang, T.; Li, Y.; Wu, X.; Lv, K.; Li, Q.; Li, M.; Du, D.; Ye, H. In-situ transformation of Bi2WO6 to highly photoreactive Bi2WO6@Bi2S3 nanoplate via ion exchange. Chin. J. Catal. 2018, 39, 718–727. [Google Scholar] [CrossRef]
- Ren, X.; Chang, L.; Li, F.; Xie, K. Study of intrinsic sulfidation behavior of Fe2O3 for high temperature H2S removal. Fuel 2010, 89, 883–887. [Google Scholar] [CrossRef]
- Qing, L.; Luo-Guang, H.; Yi-Lin, C.; Bi-Fen, G.; Bi-Zhou, L. Preparation and Property of Oxygen-deficient Bi2WO6−x Photocatalyst Active in Visible Light. J. Inorg. Mater. 2015, 30, 413–419. [Google Scholar]
- Albiss, B.; Abu-Dalo, M. Photocatalytic Degradation of Methylene Blue Using Zinc Oxide Nanorods Grown on Activated Carbon Fibers. Sustainability 2021, 13, 4729. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, X.; Wang, G.; Liu, Y.; Li, Y.; Jin, Z. Dodecahedron ZIF-67 anchoring ZnCdS particles for photocatalytic hydrogen evolution. Mol. Catal. 2020, 485, 110832. [Google Scholar] [CrossRef]
- Nasir, S.N.S.; Mohamed, N.A.; Tukimon, M.A.; Noh, M.F.M.; Arzaee, N.A.; Teridi, M.A.M. Direct extrapolation techniques on the energy band diagram of BiVO4 thin films. Phys. B Condens. Matter 2021, 604, 412719. [Google Scholar] [CrossRef]
- Xu, P.; Shen, X.; Luo, L.; Shi, Z.; Liu, Z.; Chen, Z.; Zhu, M.; Zhang, L. Preparation of TiO2/Bi2WO6 nanostructured heterojunctions on carbon fibers as a weaveable visible-light photocatalyst/photoelectrode. Environ. Sci.-Nano 2018, 5, 327–337. [Google Scholar] [CrossRef]
- Shen, X.; Song, L.; Luo, L.; Zhang, Y.; Zhu, B.; Liu, J.; Chen, Z.; Zhang, L. Preparation of TiO2/C3N4 heterojunctions on carbon-fiber cloth as efficient filter-membrane-shaped photocatalyst for removing various pollutants from the flowing wastewater. J. Colloid Interface Sci. 2018, 532, 798–807. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, B.; Xiong, T.; Jiang, D.; Ren, X.; Lu, P.; Fu, M. Enhanced visible light driven photocatalytic performance of Bi2WO6 nano-catalysts by introducing oxygen vacancy. J. Alloys Compd. 2021, 887, 161297. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Lv, K.; Lu, Y.; Li, Q.; Wu, X.; Li, Y.; Li, X.; Fan, J.; Li, M. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement. Chin. J. Catal. 2019, 40, 755–764. [Google Scholar]
- Lei, S.; Qin, C.; Tang, X.; Zhong, J.; Li, J.; Chen, J. Spiral carbon fibers modified Bi2WO6 with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 141, 109430. [Google Scholar]
- Li, F.; Tang, J.; Ke, Q.; Guo, Y.; Ha, M.N.; Wan, C.; Lei, Z.; Gu, J.; Ling, Q.; Nguyen, V.N. Investigation into Enhanced Catalytic Performance for Epoxidation of Styrene over LaSrCoxFe2–xO6 Double Perovskites: The Role of Singlet Oxygen Species Promoted by the Photothermal Effect. ACS Catal. 2021, 11, 11855–11866. [Google Scholar] [CrossRef]
- Yuan, L.; Weng, B.; Colmenares, J.C.; Sun, Y.; Xu, Y.-J. Multichannel Charge Transfer and Mechanistic Insight in Metal Decorated 2D-2D Bi2WO6-TiO2 Cascade with Enhanced Photocatalytic Performance. Small 2017, 13, 1702253. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.P.; Liu, Y.H.; Zhang, Y.; Chen, R.F. Rational construction of defect-enriched α-Fe2O3/Bi2S3 composites for high-effective photocatalytic degradation of p-nitrophenol. J. Alloys Compd. 2024, 985, 174059. [Google Scholar] [CrossRef]
- Yetim, N.K.; Aslan, N.; Koç, M.M. Structural and catalytic properties of Fe3O4 doped Bi2S3 novel magnetic nanocomposites: P-Nitrophenol case. J. Environ. Chem. Eng. 2020, 8, 104258. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Zhao, J.; Li, Q.; Ao, C.; Xia, T.; Zhang, W.; Lu, C. Ultra-lightweight and highly porous carbon aerogels from bamboo pulp fibers as an effective sorbent for water treatment. Results Phys. 2017, 7, 2919–2924. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).